Introduction
Arctigenin (ATG) is a bioactive natural lignan found
in the seeds of Arctium lappa (Burdock) in the family
Asteraceae (1). Burdock has long
been used as a folk medicine to treat various infectious symptoms
such as inflammation and sore throat (2), and several scientific studies have
demonstrated that arctigenin has various physiological activities,
which include anti-viral, -oxidative, -inflammatory, and anti-tumor
effects (2–12). Furthermore, several recent
investigations have reported that ATG exhibits anti-cancer activity
in various human cancer cells, including those of breast,
pancreatic, hepatic, and colon cancer (1,2,12–18).
Breast cancer is the one of the most common causes
of female mortality worldwide (19). Breast cancer may be classified as
progesterone receptor (PR) and estrogen receptor (ER) positive,
HER2 (human epidermal growth factor 2) overexpressing, and
triple-negative breast cancer (TNBC). In particular, TNBC cannot
treated using a selective target therapy because it lacks HER2, ER,
and PR and has a poor prognosis caused by its high metastatic
potential (20–22).
Metastasis is a complex process that results in
secondary tumor formation and is caused by the detachment,
migration, invasion, and attachment of cells at secondary sites.
This process requires the participations of many proteases to
degrade extracellular matrix (ECM) and basement membrane (BM), and
the matrix metalloproteinases (MMPs) are known play important roles
in development, progression, and in the invasion and migration of
breast cancers (23). MMPs are
classified into 23 types of proteases [e.g., collagenase,
stromelysin, gelatinase, and matrilysin) (24)], and MMP-9 (a gelatinase B type) is
known to degrade ECM and BM by breaking down gelatin, and to be an
important player during invasion and migration. Notably, MMP-9 has
been reported to be an important predictor of cancer invasion,
metastasis, prognosis, and angiogenesis in breast cancer (24–26).
MMP-3 (a stromelysin-1 type) also plays an important role during
metastasis and enhances metastasis by activating of MMPs (e.g.,
MMP-1, MMP-7, and MMP-9) and degrading collagen types II, IV, and
IX, proteoglycans, laminin, fibronectin, gelatin, and elastin in
ECM and BM (22,23,25,26).
In addition, MMP-3 activates MMP-9 via the proteolytic removal of
the pro-domain in pro-MMP-9 (27).
Flores-Pliego et al showed increased MMP-3 secretion in
placental leukocytes was closely linked with MMP-9 secretion and
that the activity of MMP-9 was diminished by treating cells with
MMP-3 inhibitor (28), which
adequately demonstrated MMP-9 and MMP-3 secretions and activities
are closely linked.
Furthermore, cyclooxygenase-2 (COX-2) is another
known metastasis-enhancing factor and catalyzes the synthesis of
prostaglandin E2 (PGE2) from arachidonic
acid, and thus, enhances metastasis and angiogenesis (23). In one notable study conducted in a
COX-2-silenced MDA-MB-231 TNBC xenograft model tumor growth and
metastasis to lung were found to be inhibited (29). In another, transfection of siCOX-2
into pancreatic cancer tumors significantly downregulated MMP-9
expression (30). Therefore, it
appears the downregulations of MMP-9, MMP-3 and COX-2 are needed to
prevent metastatic potential in breast cancer.
The mitogen-activated protein kinases (MAPKs) are
typical serine/threonine protein kinases that participate in the
regulations of many cellular processes (e.g., growth,
proliferation, differentiation, migration, and death) (31–33),
and several studies have revealed that MAPKs such as extracellular
signal-regulated kinases (ERKs), c-Jun amino-terminal kinases
(JNKs), and P38 play important roles during tumor development,
progression, metastasis, invasion, and angiogenesis (34,35).
MMPs and COX-2 contain promoter sites that bind to transcription
factors, such as activating protein-1 (AP-1), nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-κB), and signal
transducer and activator of transcription 3 (STAT3), and the
activities of these transcription factors are regulated by MAPK,
Akt, and STAT signaling pathways, respectively (36–40).
AP-1 is formed by a homodimeric or heterodimeric interactions with
Jun, Fos, or ATF subunits and the formed complexes bind to AP-1
binding sites on DNA (39–42). Jun/Fos heterodimers are more stable
than other AP-1 complexes and have greater DNA binding activity
(41,42). Therefore, we evaluated the effect
of arctigenin on cancer metastatic potential and investigated
whether MAPK/AP-1 signaling is involved in suppression of
metastatic potential by arctigenin in 4T-1 mouse TNBC cells.
Materials and methods
Materials
Arctigenin and bovine serum albumin (BSA) were
bought from Santa Cruz Biotechnology (Dallas, TX, USA) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and Pierce™ BCA Protein Assay Kits were purchased from Thermo
Scientific (Waltham, MA, USA). Dimethyl sulfate (DMSO) was obtained
from Duksan Pure Chemicals (Ansan, Korea) and protease inhibitor
cocktail and phosphatase inhibitor cocktail were from GenDEPOT
(Barker, TX, USA). Dulbecco's modified Εagles medium (DMEM),
antimycotic/antibiotic solution and fetal bovine serum (FBS) were
obtained from Welgene (Daegu, Korea), Tris-base and glycine were
from BioShop Canada Inc. (Burlington, ON, Canada).
Polyvinylidenefluoride (PVDF) membranes were purchased from Pall
Life Sciences (Port Washington, NY, USA). Antibodies for ERK1/2
(cat. no. 4695), p-ERK1/2 (cat. no. 4370), JNK1/2 (cat. no. 9258),
p-JNK1/2 (cat. no. 4668), P38 MAPK (cat. no. 8690), p-P38 MAPK
(cat. no. 4511), nuclear factor kappa-light-chain-enhancer of
activated B cells (NF-κB; cat. no. 8242), p-NF-κB (cat. no. 3033),
c-Jun (cat. no. 9265), c-Fos (cat. no. 2250), COX-2 (cat. no.
12282), GAPDH (cat. no. 5174), and histone H3 (cat. no. 14269) were
obtained from Cell Signaling Technology (Beverly, MA, USA). β-actin
(cat. no. sc-69879) was from Santa Cruz Biotechnology (Dallas, TX,
USA); HRP-conjugated anti-rabbit IgG (cat. no. NCI1460KR) and
-mouse IgG (cat. no. NCI1430OKR) from Thermo Scientific Fisher
Scientific, Inc (Rockford, IL, USA); sodium dodecyl sulfate (SDS)
from Amresco (VWR Life Science, Radnor, PA, USA), and 30%
polyacrylamide solution from SERVA (Heidelberg, Germany). Collagen
type I and matrigel were bought from Corning Life Sciences
(Bedford, MA, USA), and hematoxylin and eosin were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell culture and viability assay
4T-1 mouse TNBC cells were purchased from the Korean
Cell Line Bank (Seoul, Korea) and routinely cultured in DMEM
containing 10% FBS and 1% antibiotic-antimycotic solution at 37°C
in a 5% CO2 incubator. The effect of arctigenin on cell
viability was evaluated using an MTT assay. Briefly,
2×103 cells/well were seeded into 96-well plates
incubated for 24 h at 37°C, treated with 0, 25, 50, 100 or 200 µM
arctigenin and cultured for an additional 24, 48 or 72 h. Cell
viabilities were determined by measuring absorbances at 570 nm
using a Spectramax M2e (Molecular Devices, Sunnyvale, CA,
USA).
Wound healing assay
Cells were seeded in 6-well plates coated with
collagen type I (Corning Life Sciences, Bedford, MA, USA), grown
until confluent, and scratched with a blue tip to create wounds.
They were then treated with culture media containing 0, 50, 100,
150 or 200 µM arctigenin. Optical microscopic images were captured
from two different areas of each well at 0 and 48 h after
wounding.
Transwell invasion assay
The effect of arctigenin on the invasiveness of 4T-1
mouse TNBC cells was determined using transwell chambers (Corning
Life Sciences) inserts in 24-well plates. Lower faces of
polycarbonate filters (transwell inserts) were coated with matrigel
for 1 h at 37°C, and then, 3×104 cells were seeded into
matrigel-coated transwell chambers and 750 µl of culture media was
added to lower chambers. After 24 h, cells were treated with
conditioned media containing 2 or 10% FBS and 0, 50, 100, 150 or
200 µM arctigenin and incubated at 37°C in 5% CO2
atmosphere for 24 h. Cells that migrated across membranes were then
fixed and stained using hematoxylin and eosin (H&E) and
photographed under an inverted microscope at ×200.
Gelatin zymography
The effect of arctigenin on MMP-9 activity was
evaluated by gelatin zymography. Briefly 2×105
cells/well were seeded into 6-well plates and allowed to attach for
24 h. Cells were then serum-starved for 4 h and treated with
serum-free media supplemented with various concentrations of
arctigenin (0, 50, 100, 150 or 200 µM) for 24 h. Conditioned media
were then transferred to new conical tubes and centrifuged to
remove cell debris. The supernatants were objected by 8%
SDS-polyacrylamide gel electrophoresis (PAGE) containing 0.1% (v/v)
gelatin under non-reducing conditions. The gel was then washed with
2.5% Triton X-100 for 1 h at room temperature to remove SDS and
gelatinase reactions were performed in reaction buffer (50 mM
Tris-HCl, pH 7.5, 10 mM CaCl2, 0.04% NaN3) at
37°C for 24 h. The gel was then stained with Coomassie staining
solution (0.05% Coomassie brilliant blue R, 45% methanol, and 10%
acetic acid) and destained at room temperature. Densitometric
analysis was performed using ImageJ.
RNA extraction, cDNA synthesis, and
RT-qPCR
4T-1 mouse TNBC cells (2×105 cells/well)
were seeded into 6-well plates, allowed to attach for 24 h, and
cultured in serum-free DMEM containing 0, 25, 50, 100, 150 or 200
µM arctigenin for an additional 24 h. The cells were then collected
by trypsinization for RNA extraction, which was performed using the
easy-BLUE™ Total RNA extraction kit (iNtRON Biotechnology, Inc.,
Sungnam, Korean). Extracted total RNA was quantified using a
NanoDrop spectrophotometer (Schimazu Scientific Instruments, Kyoto,
Japan), and cDNA was synthesized from 1 µg of total RNA in 1X
Goscript reaction buffer containing 2 mM MgCl2, 0.5 mM
and Goscript™ Reverse Transcriptase (all from Promega, Madison, WI,
USA). RT-q PCR was conducted using Q Green SYBR Green Master Mix
Kits (Cellsafe, Suwon, Korea) using an Eco™ Real-Time PCR machine
(Illumina, San Diego, CA, USA). cDNA amplification reactions were
performed as follows: Pre-heating for 5 min at 95°C, 45 cycles at
95°C for 10 sec, 60°C for 15 sec and 72°C for 20 sec. Relative mRNA
expressions were calculated automatically using the
2−ΔΔCq method and Eco™ Software v3.1.7 (Illumina, Inc.)
(43). The primer sequences used
for RT-q PCR were as follows: MMP-9, forward,
5′-TGTCTGGAGATTCGACTTCA-3′ and reverse, 5′-TGAGTTCCAGGGCACACCA-3′;
MMP-3, forward, 5′-CTTTGAAGCATTTGGGTTTCTCTAC-3′ and reverse,
5′-AGCTATTGCTCTTCAATATGTGGGT-3′; COX-2, forward,
5′-CCTGCTGCCCGACACCTTCA-3′ and reverse, 5′-AGCAACCCGGCCAGCAATCT-3′;
β-actin, forward, 5′-CATCCGTAAAGACCTCTATGCCAAC and reverse,
5′-ATGGAGCCACCGATCCACA-3′.
Nuclear fractionation
4T-1 mouse TNBC cells were seeded into 6-well plates
at 2×105 cells/well and allowed to attach for 24 h. The
cells were then serum-starved for 4 h, treated with
conditioned-media containing various concentration of arctigenin
(0, 25, 50, 100, 150 or 200 µM) for 24 h, and washed twice with
ice-cold phosphate buffered saline. Hypertonic buffer (20 mM
Tris-HCl (pH 7.4), 10 mM NaCl, 3 mM MgCl2] containing
protease inhibitor cocktail and phosphatase inhibitor cocktail
(GenDEPOT, Barker, TX, USA) was then added to each well. The cells
were detached with a rubber policeman (SPL Life Sciences, Pocheon,
Korea), transferred to 1.5 ml microtubes, kept on ice for 15 min,
treated with 10% NP-40 (final NP-40 concentration 0.125%) with
vortex-mixing for 10 sec at the highest setting, and left on ice
for 10 min. Cell mixtures were then centrifuged at 3,000 rpm for 10
min at 4°C, supernatants (cytosolic fractions) were removed and
pellets were lysed with Cell Extraction Buffer (Invitrogen,
Carlsbad, CA, USA) containing phosphatase and protease inhibitor
cocktail for 30 min on ice, lysates were centrifuged at 14,000 × g
for 30 min at 4°C, and supernatants (nuclear fractions) were
collected. Cytosolic and nuclear fractions were stored at −80°C
until required.
Western blotting
After treating 4T-1 mouse TNBC cells for 24 h with
various concentration of arctigenin (0, 25, 50, 100, 150 or 200
µM), they were lysed with RIPA lysis buffer [50 mM Tris-HCl (pH
7.5), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS and 2 mM ethylenediaminetetraacetic acid] (Biosesang, Seongnam,
Korea) containing protease inhibitor cocktail and phosphatase
inhibitor cocktail (GenDEPOT, Barker, TX, USA), and centrifuged at
13,000 rpm for 10 min at 4°C. Supernatants (whole cell lysates)
were transferred to microtubes and stored at −80°C until required.
Total protein in whole cell lysates was quantified using the BCA
method, and same amounts of total protein in whole cell lysates
were subjected to SDS-PAGE. After transferring proteins to PVDF
membranes, membranes were blocked with 1% BSA in Tris-buffered
saline (TBS)-Tween (50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20) for
1 h at room temperature, probed with primary antibodies diluted
1:3,000 with 1% bovine serum albumin in TBS-Tween solution
overnight at 4°C, washed three times in TBS-Tween, and reacted with
secondary antibodies (dilution 1:5,000) for 1 h at room temperature
in TBS-Tween. Target proteins were visualized using a homemade
chemiluminescent substrate and photographed using a Luminescent
Image Analyzer LAS-4000 (Fujifilm, Tokyo).
Statistical analysis
One-way analysis of variance followed by the Tukey's
post hoc test was used to determine the significances of
differences. The analysis was performed using SPSS Ver. 20.0
software (SPSS, Inc., Chicago, IL, USA), and results are presented
as means ± SDs. Statistical significance was accepted for P-values
of <0.05.
Results
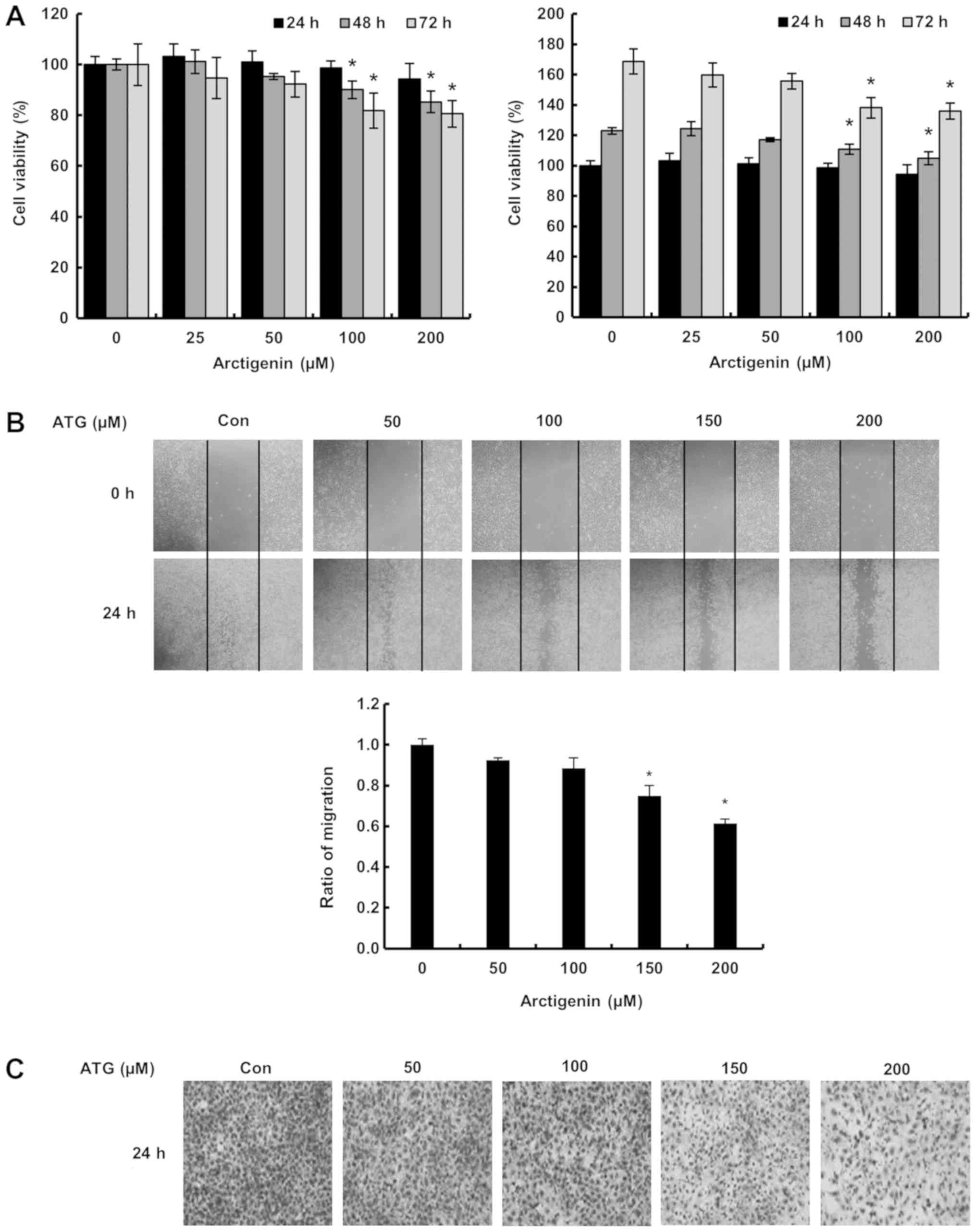
The effect of arctigenin on the cell
viability, migration, and invasion of 4T-1 mouse TNBC cells
We firstly evaluated the effect of arctigenin on the
viability of 4T-1 mouse TNBC cells using an MTT assay. As shown in
Fig. 1A, arctigenin (100 and 200
µM at 48 and 72 h) slightly reduced cell viability. Furthermore,
arctigenin inhibited cell migration and invasiveness as determined
by the matrigel invasion and wound healing assays in a
concentration-dependent manner, respectively. Lignans should enters
into cells with simple diffusion or a low affinity transporter
(44). Therefore, these results
suggest arctigenin should inhibit invasion and migration by 4T-1
mouse TNBC cells and we postulated the effect was mediated via the
regulation of signaling molecules by arctigenin entered into the
cells with simple diffusion or a low affinity transporter.
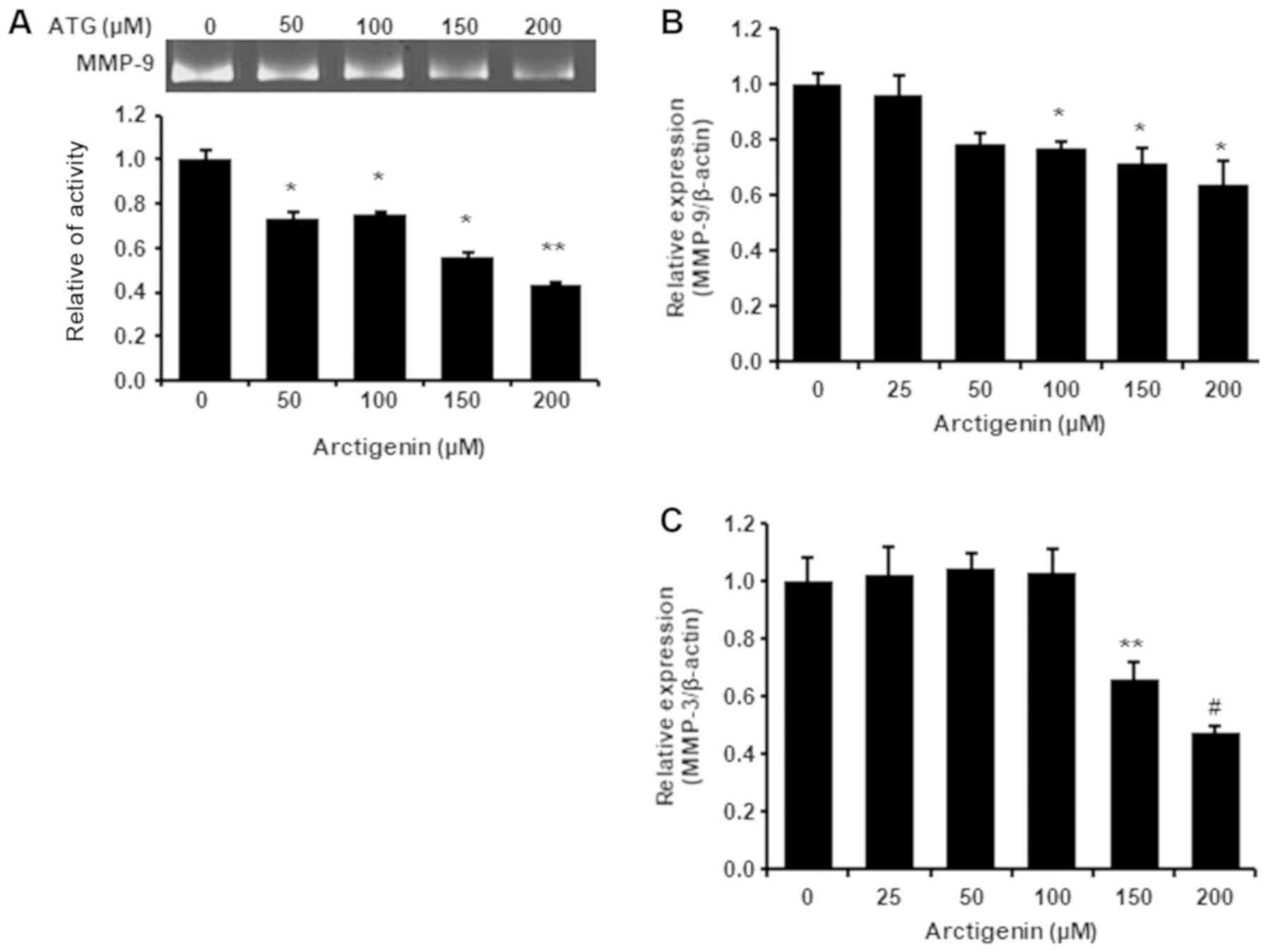
Arctigenin inhibited MMP-9 activity
and its gene expression in 4T-1 mouse TNBC cells
Due to its important role on metastasis in breast
cancer and the associations between MMP-9 activity and cell
migration and invasiveness, we evaluated the effects of arctigenin
on MMP-9 activity and its mRNA level. Arctigenin was found to
reduce both the protein and mRNA levels of MMP-9 dose-dependently
(Fig. 2A and B), which suggested
that the suppression of MMP-9 expression by arctigenin was
responsible for its inhibition of cell migration and invasion.
Arctigenin inhibited MMP-3 mRNA
expression in 4T-1 TNBC cells
Active MMP-9 is produced by cleavage of the
prodomain in pro-MMP-9 mediated by MMP-3 and MMP-9 and as mentioned
above, its activity is also positively associated with MMP-3
activity. We found arctigenin at 150 or 200 µM suppressed MMP-3
transcription (Fig. 2C). Mehner
et al showed metastatic potential and MMP-3 expression are
associated in breast carcinoma (45), and Chu et al found breast
cancer tumorigenesis and metastasis were prevented by MMP-3
knockdown by mir-519d (46). These
reports indicate MMP-3 activity is closely linked with its gene
expression. Therefore, our observation suggest arctigenin might
inhibit MMP-9 activity by downregulating MMP-3 expression in 4T-1
mouse TNBC cells.
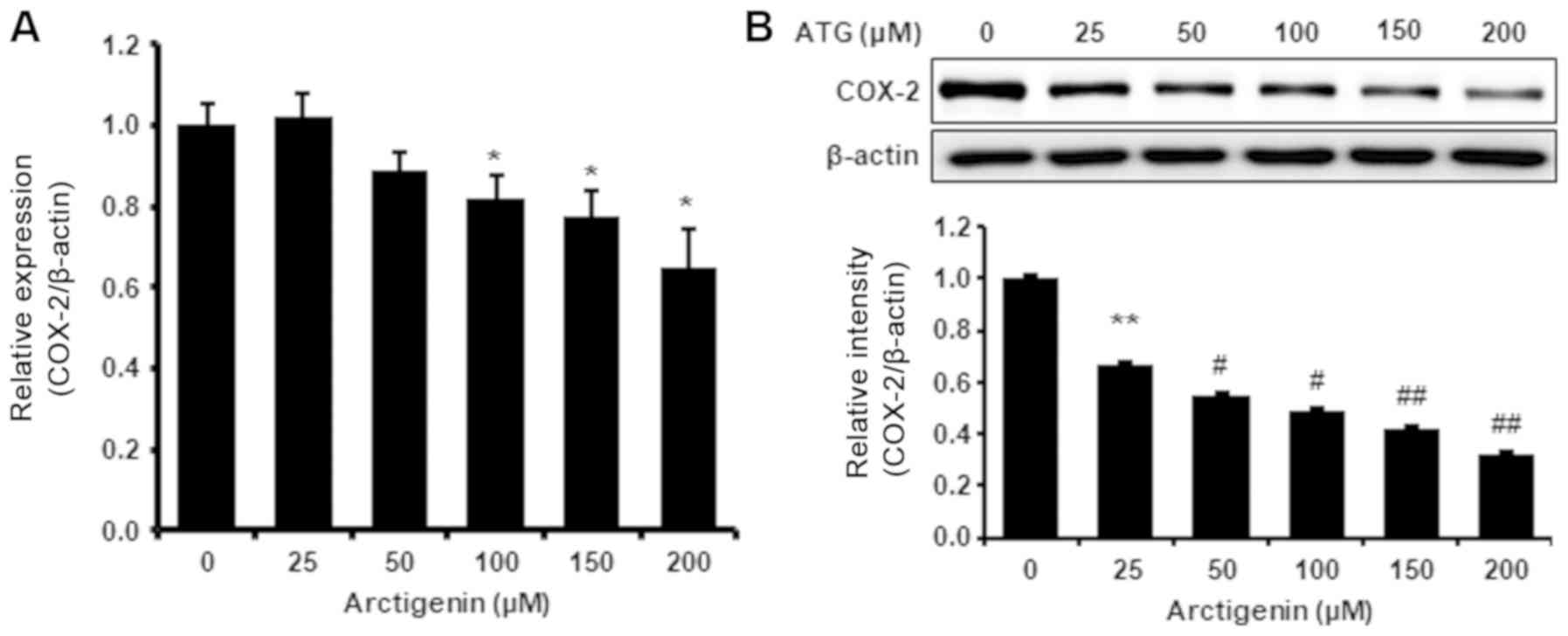
Arctigenin inhibited COX-2 protein and
mRNA levels in 4T-1 mouse TNBC cells
COX-2 is another important factor of metastasis in
breast cancer and MMP-9 activity is positively associated with
COX-2 expression. We found arctigenin dose-dependently
downregulated COX-2 protein and mRNA levels (Fig. 3), which suggests that arctigenin
might also reduce cancer cell growth and metastatic potential by
inhibiting COX-2 expression in 4T-1 mouse TNBC cells.
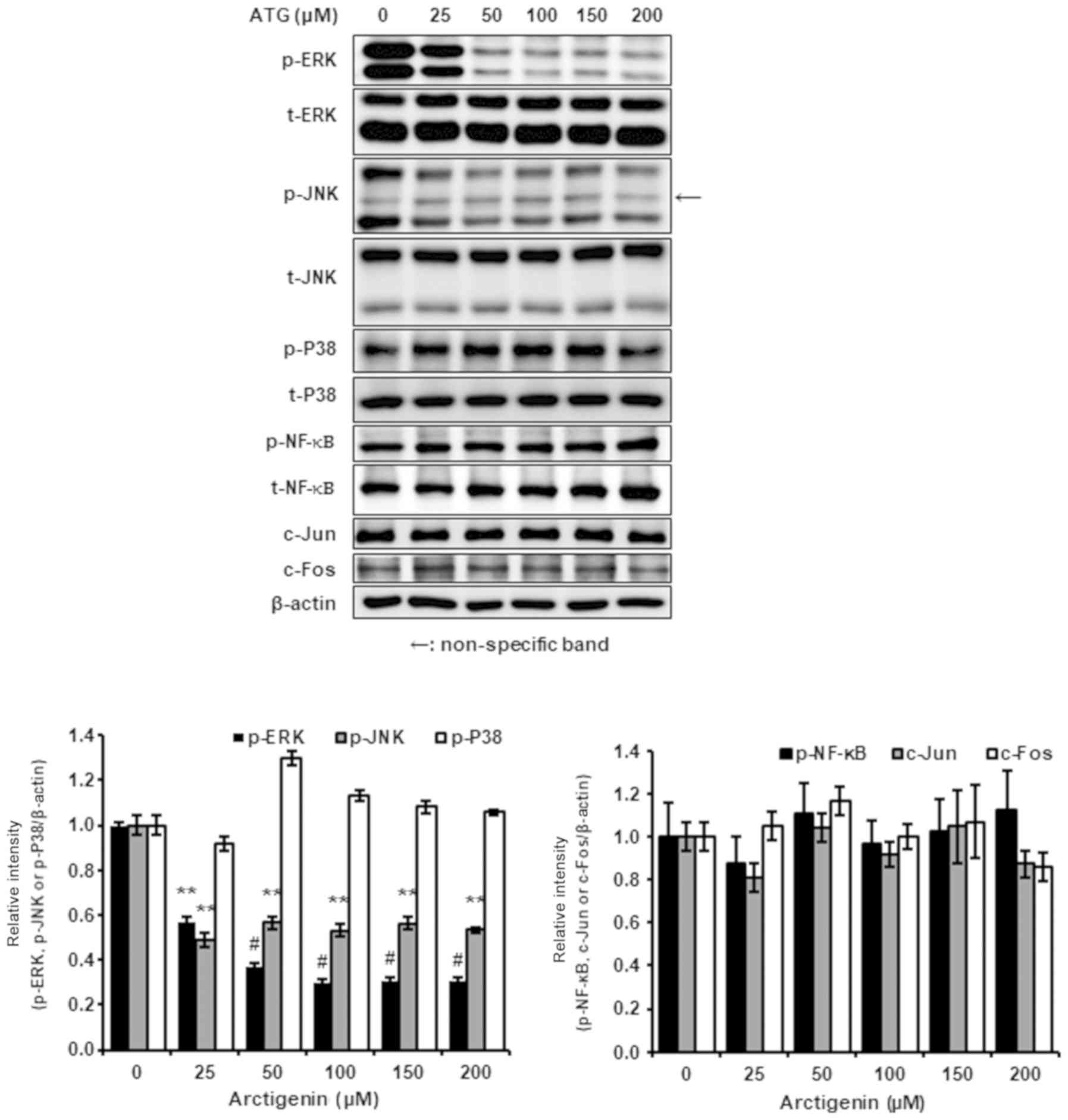
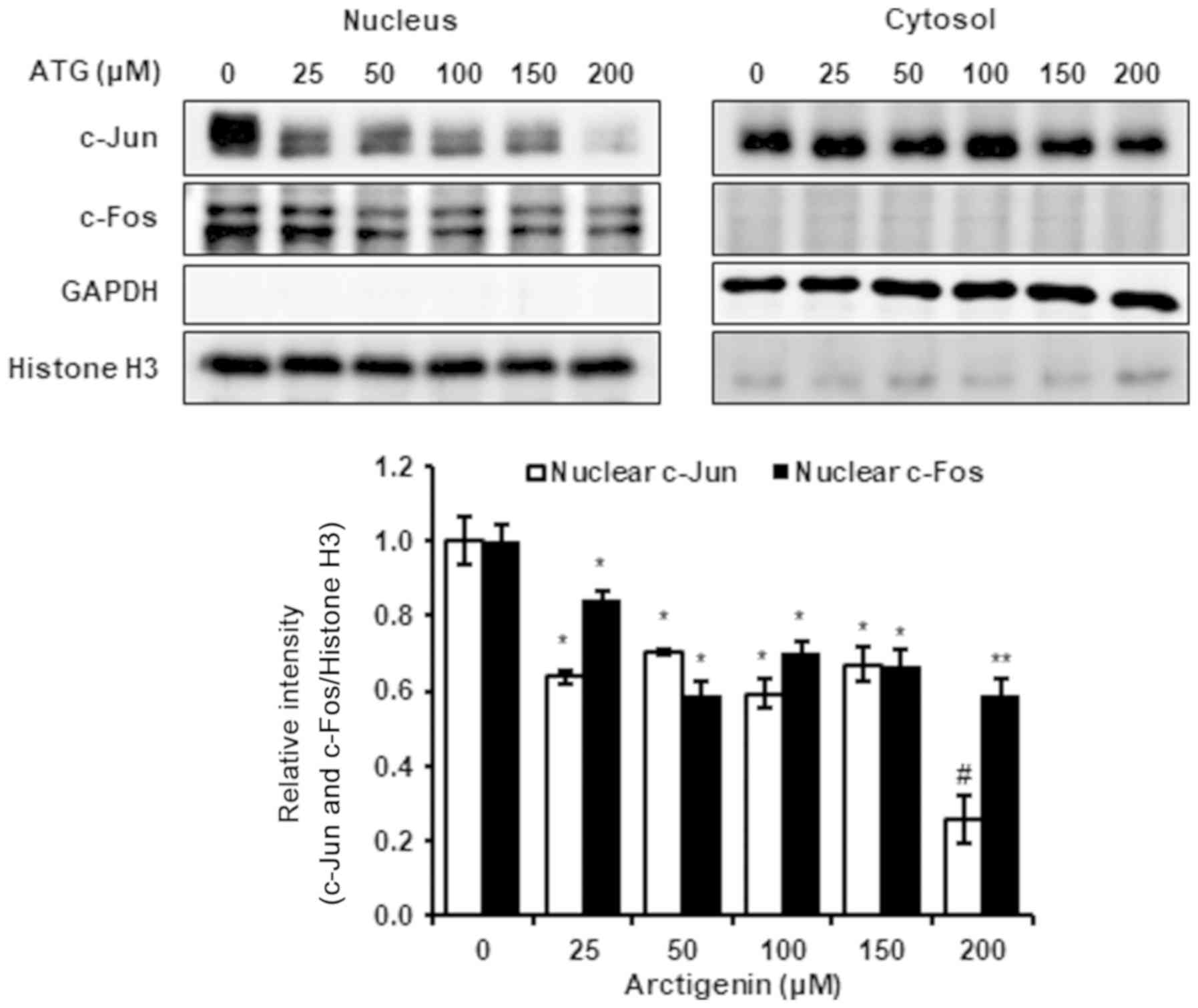
Arctigenin inhibited nuclear c-Jun and
c-Fos levels via the ERK1/2 and JNK1/2 signaling pathways
Because arctigenin appeared to inhibit 4T-1 TNBC
cell migration and invasion by suppressing MMP-9, MMP-3, and COX-2.
Therefore, we investigated the effect of arctigenin on ERK1/2 and
JNK1/2 signaling pathways, which are key regulators of the
expressions of MMP-9, MMP-3, and COX-2. The results obtained showed
that arctigenin inhibited the phosphorylations of ERK1/2 and JNK1/2
but not p38 MAPK (Fig. 4).
Arctigenin did not change the whole cell expressions of c-Jun and
c-Fos (AP-1 subunits), but attenuated their nuclear expressions and
reduced AP-1 transcriptional activity (Fig. 5). Consequently, our results suggest
that anti-metastatic activity of arctigenin is governed by
reduction in nuclear c-Jun and c-Fos levels and associated
inhibitions of the phosphorylations of ERK1/2 and JNK1/2.
Discussion
Although various studies have reported arctigenin
has anti-cancer effects on some types of cancer cells,
comparatively little is known of its effects on metastasis
(2,13). In this study, we evaluated the
effect of arctigenin on metastatic potential in 4T-1 mouse TNBC
cells. We found arctigenin suppressed the migration and
invasiveness of these cells (Fig. 1B
and C) but did not significantly decrease cell viability in 24
h (Fig. 1A). These results suggest
arctigenin has therapeutic potential for preventing the invasion
and migration that are important roles in metastasis in
triple-negative breast cancer.
Cell migration and invasiveness are closely
associated with the activity of MMP-9 in breast cancer and MMP-9 is
activated by proteolytic cleavage of the prodomain in pro-MMP-9 by
MMP-3. Furthermore, MMP-9 gene expression is known to be closely
associated with that of MMP-3 (26,28).
It has also been well-established that the activity and expression
of MMP-9 importantly contribute to breast cancer metastasis.
Several authors have demonstrated that reductions in MMP-9 activity
and expression in breast cancer cells are associated with reduced
metastatic potential (22,47,48).
In the present study, arctigenin decreased MMP-9 activity and
suppressed its gene expression (Fig.
2A and B) and also downregulated MMP-3 mRNA expression
(Fig. 2C). Furthermore, arctigenin
also inhibited COX-2 at the protein and mRNA levels (Fig. 3), and as mentioned above, COX-2
also affects metastatic potential and MMP-9 activity and expression
in cancer cells (29,30). Therefore, our results indicate
arctigenin inhibits the migration and invasion of 4T-1 mouse TNBC
cells by suppressing the activity and mRNA levels of MMP-9,
transcription of MMP-3, and the protein and mRNA expression of
COX-2.
The MAPK/AP-1 signaling pathway plays an important
role in the regulations of various metastasis-associated gene
expressions. AP-1 is a transcription factor regulated by MAPKs,
such as ERK1/2, JNK1/2, and p38 MAPK, and the transcriptional
activity of AP-1 is determined by the nuclear levels of AP-1
subunits, which are in turn, governed by the regulation of MAPK
phosphorylation. In the present study, we found that arctigenin
suppressed the gene expressions of MMP-9, MMP-3 and COX-2 and the
phosphorylations of ERK1/2 and JNK1/2, which were associated with
reductions in the nuclear levels of c-Jun and c-Fos (AP-1 subunits)
(Figs. 2B and C and 3–5).
Furthermore, down-regulations of the gene expressions of MMP-9,
MMP-3, and COX-2 corresponded to diminished phosphorylations of
ERK1/2 and JNK1/2 and decreased nuclear c-Jun and c-Fos. However,
p38 MAPK phosphorylation did not affected by arctigenin (Fig. 4). The promotor sites on MMP-9,
MMP-3 and COX-2 genes contain AP-1 binding site, and thus, their
gene expressions are closely linked with the nuclear levels of
c-Jun and c-Fos (36,37,41,42,49,50).
Consequently, our results suggest that the inhibitions of MMP-9,
MMP-3, and COX-2 by arctigenin are mediated via partial suppression
of MAPK/AP-1 signaling pathway. Also, these investigations implies
that the inhibitory effects of arctigenin are not associated with
the direct inhibition of protein kinase C.
Our findings suggest arctigenin reduces the
metastatic potential of 4T-1 mouse TNBC cells by reducing cell
motility, invasiveness and MMP-9 activity and that these effects
are linked with suppression of the gene expressions of MMP-9,
MMP-3, and COX-2 via the partial suppression of MAPK/AP-1 signaling
pathway. Taken together, our observations suggest arctigenin be
considered a potential means of preventing metastatic potential in
triple negative breast cancer.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Korean Ministry of Education (grant no.
2015R1D1A1A01058841).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MGL, KSL and KSN designed the experiments. MGL
performed experiments. MGL, KSL and KSN analyzed the data. MGL and
KSL wrote the manuscript. KSN reviewed the manuscript. All authors
confirmed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He Y, Fan Q, Cai T, Huang W, Xie X, Wen Y
and Shi Z: Molecular mechanisms of the action of arctigenin in
cancer. Biomed Pharmacother. 108:403–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maxwell T, Chun SY, Lee KS, Kim S and Nam
KS: The anti-metastatic effects of the phytoestrogen arctigenin on
human breast cancer cell lines regardless of the status of ER
expression. Int J Oncol. 50:727–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Li W, Jin E, He Q, Yan W, Yang H,
Gong S, Guo Y, Fu S, Chen X, et al: The antiviral activity of
arctigenin in traditional Chinese medicine on porcine circovirus
type 2. Res Vet Sci. 106:159–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen YF, Liu L, Chen WC, Hu Y, Zhu B and
Wang GX: Evaluation on the antiviral activity of arctigenin against
spring viraemia of carp virus. Aquaculture. 483:252–262. 2018.
View Article : Google Scholar
|
|
5
|
Hayashi K, Narutaki K, Nagaoka Y, Hayashi
T and Uesato S: Therapeutic effect of arctiin and arctigenin in
immunocompetent and immunocompromised mice infected with influenza
A virus. Biol Pharm Bull. 33:1199–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang CZ, Wu SC, Chang CM, Lin CL and Kwan
AL: Arctigenin, a potent ingredient of arctium Lappa L., induces
endothelial nitric oxide synthase and attenuates subarachnoid
hemorrhage-induced vasospasm through PI3K/Akt pathway in a rat
model. Biomed Res Int. 2015:4902092015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu RM, Sun YY, Zhou TT, Zhu ZY, Zhuang JJ,
Tang X, Chen J, Hu LH and Shen X: Arctigenin enhances swimming
endurance of sedentary rats partially by regulation of antioxidant
pathways. Acta Pharmacol Sin. 35:1274–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong YH, Park JS, Kim DH and Kim HS:
Arctigenin increases hemeoxygenase-1 gene expression by modulating
PI3K/AKT signaling pathway in rat primary astrocytes. Biomol Ther
(Seoul). 22:497–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Q, Yang M and Zuo Z: Overview of the
anti-inflammatory effects, pharmacokinetic properties and clinical
efficacies of arctigenin and arctiin from Arctium lappa L.
Acta Pharmacol Sin. 39:787–801. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao F, Wang L and Liu K: In vitro
anti-inflammatory effects of arctigenin, a lignan from Arctium
lappa L., through inhibition on NOS pathway. J Ethnopharmacol.
122:457–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ,
Jang SE, Han MJ and Kim DH: Arctigenin ameliorates inflammation in
vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing
M1 macrophages to M2-like macrophages. Eur J Pharmacol. 708:21–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maxwell T, Lee KS, Kim S and Nam KS:
Arctigenin inhibits the activation of the mTOR pathway, resulting
in autophagic cell death and decreased ER expression in ER-positive
human breast cancer cells. Int J Oncol. 52:1339–1349.
2018.PubMed/NCBI
|
|
13
|
Lou CH, Zhu Z, Zhao Y, Zhu R and Zhao H:
Arctigenin, a lignan from Arctium lappa L., inhibits
metastasis of human breast cancer cells through the downregulation
of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep.
37:179–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng T, Cao W, Shen W, Zhang L, Gu X, Guo
Y, Tsai HI, Liu X, Li J, Zhang J, et al: Arctigenin inhibits STAT3
and exhibits anticancer potential in human triple-negative breast
cancer therapy. Oncotarget. 8:329–344. 2017.PubMed/NCBI
|
|
15
|
Hsieh CJ, Kuo PL, Hsu YC, Huang YF, Tsai
EM and Hsu YL: Arctigenin, a dietary phytoestrogen, induces
apoptosis of estrogen receptor-negative breast cancer cells through
the ROS/p38 MAPK pathway and epigenetic regulation. Free Radical
Bio Med. 67:159–170. 2014. View Article : Google Scholar
|
|
16
|
Awale S, Lu J, Kalauni SK, Kurashima Y,
Tezuka Y, Kadota S and Esumi H: Identification of arctigenin as an
antitumor agent having the ability to eliminate the tolerance of
cancer cells to nutrient starvation. Cancer Res. 66:1751–1757.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Tan YJ, Lu ZZ, Li BB, Sun CH, Li T,
Zhao LL, Liu Z, Zhang GM, Yao JC and Li J: Arctigenin inhibits
liver cancer tumorigenesis by inhibiting gankyrin expression via
C/EBP and PPARα. Front Pharmacol. 9:2682018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li QC, Liang Y, Tian Y and Hu GR:
Arctigenin induces apoptosis in colon cancer cells through
ROS/p38MAPK pathway. J Buon. 21:87–94. 2016.PubMed/NCBI
|
|
19
|
Tungsukruthai S, Petpiroon N and
Chanvorachote P: Molecular mechanisms of breast cancer metastasis
and potential anti-metastatic compounds. Anticancer Res.
38:2607–2618. 2018.PubMed/NCBI
|
|
20
|
Campone M, Valo I, Jezequel P, Moreau M,
Boissard A, Campion L, Loussouarn D, Verriele V, Coqueret O and
Guette C: Prediction of recurrence and survival for triple-negative
breast cancer (TNBC) by a protein signature in tissue samples. Mol
Cell Proteomics. 14:2936–2946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gautam P, Karhinen L, Szwajda A, Jha SK,
Yadav B, Aittokallio T and Wennerberg K: Identification of
selective cytotoxic and synthetic lethal drug responses in triple
negative breast cancer cells. Mol Cancer. 15:342016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehner C, Hockla A, Miller E, Ran S,
Radisky DC and Radisky ES: Tumor cell-produced matrix
metalloproteinase 9 (MMP-9) drives malignant progression and
metastasis of basal-like triple negative breast cancer. Oncotarget.
5:2736–2749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee KS, Shin JS and Nam KS: Effect of
proton beam irradiation on the regulation of metastasis-enhancing
factors in MCF-7 human breast cancer cells. J Korean Phys Soc.
63:1373–1378. 2013. View Article : Google Scholar
|
|
24
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: Changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brinckerhoff CE and Matrisian LM:
Timeline-Matrix metalloproteinases: A tail of a frog that became a
prince. Nat Rev Mol Cell Bio. 3:207–214. 2002. View Article : Google Scholar
|
|
26
|
Ye S, Eriksson P, Hamsten A, Kurkinen M,
Humphries SE and Henney AM: Progression of coronary atherosclerosis
is associated with a common genetic variant of the human
stromelysin-1 promoter which results in reduced gene expression. J
Biol Chem. 271:13055–13060. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steenport M, Khan KM, Du B, Barnhard SE,
Dannenberg AJ and Falcone DJ: Matrix metalloproteinase (MMP)-1 and
MMP-3 induce macrophage MMP-9: Evidence for the Role of TNF-alpha
and Cyclooxygenase-2. J Immunol. 183:8119–8127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flores-Pliego A, Espejel-Nuñez A,
Castillo-Castrejon M, Meraz-Cruz N, Beltran-Montoya J,
Zaga-Clavellina V, Nava-Salazar S, Sanchez-Martinez M,
Vadillo-Ortega F and Estrada-Gutierrez G: Matrix
metalloproteinase-3 (MMP-3) is an endogenous activator of the MMP-9
secreted by placental leukocytes: Implication in human labor. PLoS
One. 10:e01453662015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stasinopoulos I, O'Brien DR, Wildes F,
Glunde K and Bhujvalla ZM: Silencing of cyclooxygenase-2 inhibits
metastasis and delays tumor onset of poorly differentiated
metastatic breast cancer cells. Mol Cancer Res. 5:435–442. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bu X, Zhao C and Dai X: Involvement of
COX-2/PGE2 pathway in the upregulation of MMP-9 expression in
pancreatic cancer. Gastroenterol Res Prac. 2011:2142692011.
|
|
31
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Low HB and Zhang Y: Regulatory roles of
MAPK phosphatases in cancer. Immune Netw. 16:85–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang M and Huang CZ: Mitogen-activated
protein kinase signaling pathway and invasion and metastasis of
gastric cancer. World J Gastroenterol. 21:11673–11679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benbow U and Brinckerhoff CE: The AP-1
site and MMP gene regulation: What is all the fuss about? Matrix
Biol. 15:519–526. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mishra M, Flaga J and Kowluru RA:
Molecular mechanism of transcriptional regulation of matrix
metalloproteinase-9 in diabetic retinopathy. J Cell Physiol.
231:1709–1718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang F, Cao J, Liu Q, Zou Y, Li H and Yin
T: MAPK/ERK signal pathway involved expression of COX-2 and VEGF by
IL-1β induced in human endometriosis stromal cells in vitro. Int J
Clin Exp Pathol. 6:2129–2136. 2013.PubMed/NCBI
|
|
39
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weekes D, Kashima TG, Zandueta C, Perurena
N, Thomas DP, Sunters A, Vuillier C, Bozec A, El-Emir E, Miletich
I, et al: Regulation of osteosarcoma cell lung metastasis by the
c-Fos/AP-1 target FGFR1. Oncogene. 35:2852–2861. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Pu X, Shi M, Chen L, Qian L, Song
Y, Yuan G, Zhang H, Yu M, Hu M, et al: c-Jun, a crucial molecule in
metastasis of breast cancer and potential target for biotherapy.
Oncol Rep. 18:1207–1212. 2007.PubMed/NCBI
|
|
42
|
Malnou CE, Brockly F, Favard C,
Moquet-Torcy G, Piechaczyk M and Jariel-Encontre I:
Heterodimerization with different Jun proteins controls c-Fos
intranuclear dynamics and distribution. J Biol Chem. 285:6552–6562.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
During A, Debouche C, Raas T and
Larondelle Y: Among plant lignans, pinoresinol has the strongest
antiinflammatory properties in human intestinal Caco-2 cells. J
Nutr. 142:1798–1805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mehner C, Miller E, Nassar A, Bamlet WR,
Radisky ES and Radisky DC: Tumor cell expression of MMP3 as a
prognostic factor for poor survival in pancreatic, pulmonary, and
mammary carcinoma. Genes Cancer. 6:480–489. 2015.PubMed/NCBI
|
|
46
|
Chu C, Liu X, Bai X, Zhao T, Wang M, Xu R,
Li M, Hu Y, Li W, Yang L, et al: MiR-519d suppresses breast cancer
tumorigenesis and metastasis via targeting MMP3. Int J Biol Sci.
14:228–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nalla AK, Gorantla B, Gondi CS, Lakka SS
and Rao JS: Targeting MMP-9, uPAR, and cathepsin B inhibits
invasion, migration and activates apoptosis in prostate cancer
cells. Cancer Gene Ther. 17:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu DM, Mckee CM, Cao YH, Ding YC, Kessler
BM and Muschel RJ: Matrix metalloproteinase-9 regulates tumor cell
invasion through cleavage of protease nexin-1. Cancer Res.
70:6988–6998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim
KH, Eun HC and Chung JH: Heat shock-induced matrix
metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and
JNK activation and via an autocrine interleukin-6 loop. J Invest
Dermatol. 123:1012–1019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hannemann N, Jordan J, Paul S, Reid S,
Baenkler HW, Sonnewald S, Bäuerle T, Vera J, Schett G and Bozec A:
The AP-1 Transcription factor c-Jun promotes arthritis by
regulating cyclooxygenase-2 and arginase-1 expression in
macrophages. J Immunol. 198:3605–3614. 2017. View Article : Google Scholar : PubMed/NCBI
|