A coordination system has been formed under the
interaction of various cells in the skin. For instance, the
cutaneous neuron-immune-endocrine system consists of interaction
and coordination between keratinocytes, melanocytes and dendritic
Langerhans cells in the epidermis and the components of the dermis
such as mast cells, macrophages, fibroblasts and nerve cells
(1–3). Allergens, pathogens, chemical
stimuli, and physical damage can all lead to skin inflammation
(4–7), which is a defense response to
exogenous or endogenous stimuli (8). Skin inflammation plays a crucial role
in the body, such as resisting the invasion of bacteria and other
pathogens and promoting the repair of wounds. Recent studies have
revealed that inflammatory cytokines are closely related to skin
pigmentation (9,10).
Skin hyperpigmentation or hypopigmentation after
inflammation is a clinically common symptom. Various acute or
chronic inflammatory skin reactions may cause changes in skin
pigmentation (11), such as
psoriasis, eczema, or laser surgery. Recent studies have confirmed
that interleukin (IL)-1, IL-4, IL-6 and other inflammatory
mediators can regulate the proliferation and differentiation of
human epidermal melanocytes directly or indirectly and participate
in the regulation of melanogenesis in melanocytes (11–13).
Treatments that modulate these inflammatory mediators may have
great clinical utility in the treatment of some dyschromatosis
(14). This review will focus on
the role of inflammatory factors in melanogenesis and the
mechanisms involved.
Melanocytes originate from the ectodermal neural
crest, migrate to the mesenchyme as the embryo develops, and then
further migrate to the skin, eye uveal, stria vascularis,
vestibular organ, endolymphatic sac and pia mater (15,16).
The migration, proliferation, and differentiation of melanoblasts
are mainly regulated by regulatory factors secreted by the dorsal
neural tube, ectoderm, and keratinocytes such as the fmily of
Wingless-type protein (WNT), endothelin 3 (EDN3), and stem cell
factor (SCF) (17). Melanogenesis
in mature melanocytes occurs in melanosomes. Melanosomes are unique
organelles located in the cytoplasm of melanocytes, which contain
key enzymes regulating the production of pigments such as
tyrosinase (TYR), tyrosinase-related protein-1 (TYRP-1) and
tyrosinase-related protein-2 (TYRP-2) (17,18).
Activation of the transcription factor microphthalmia-associated
transcription factor (MITF) (19–21)
results in the upregulation of the expression of key genes such as
TYR, TYRP-1 and TYRP-2 (16,22,23),
and promotes melanogenesis in melanocytes (17). Mature melanosomes can migrate from
the perinuclear region to the dendrites of melanocyte under the
regulation of tubulin (kinesin, dynein) (17). In the epidermis, melanocytes are
associated with 30 to 40 keratinocytes through dendrites,
transferring mature melanosomes into the cytoplasm of keratinocytes
(15,24).
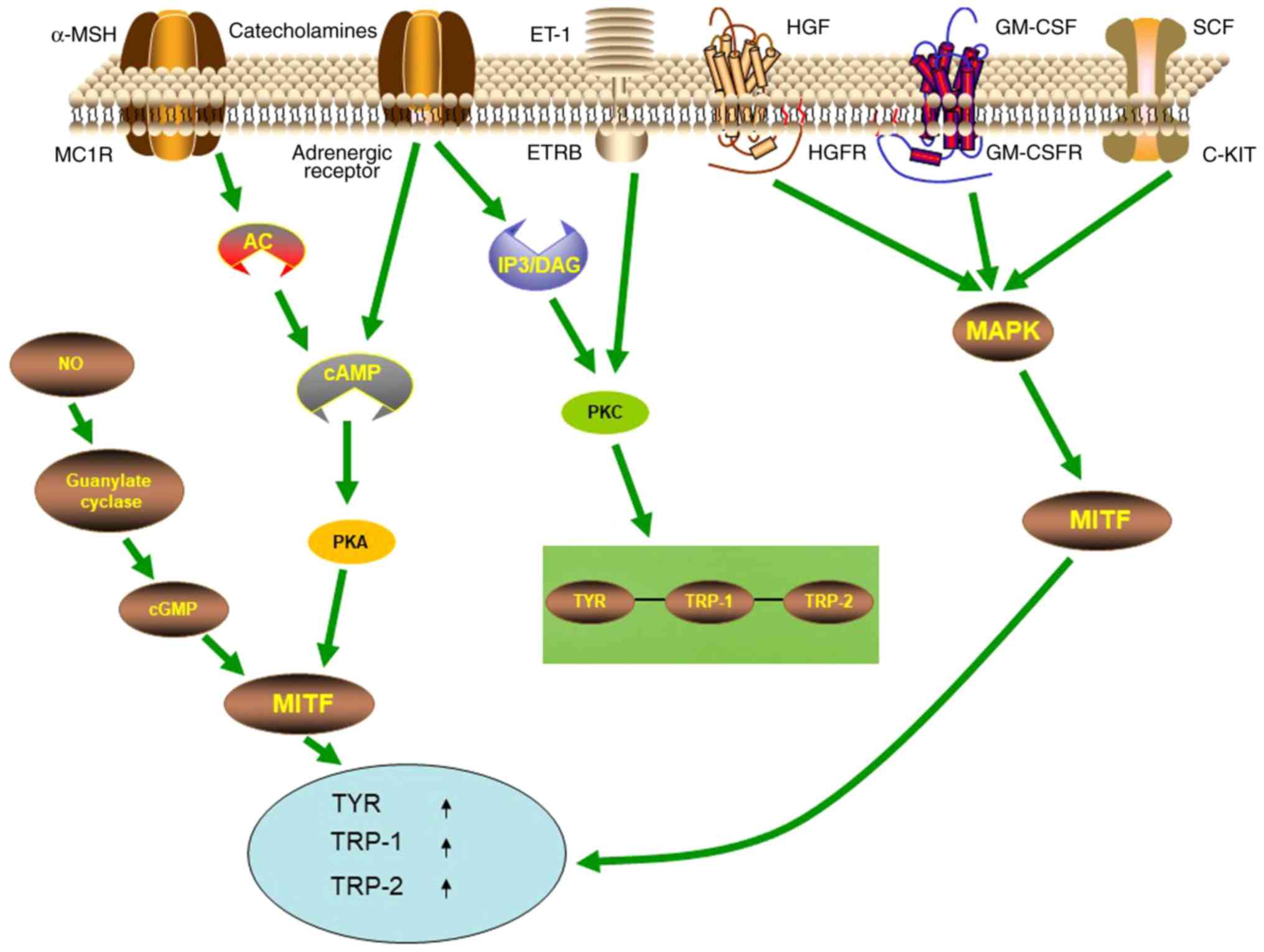
Multiple signaling pathways are involved in the
regulation of melanogenesis, with the cyclic AMP (cAMP)/protein
kinase A (PKA) signaling pathway being one of the most important
signaling pathways (Fig. 1). The
most well-known receptor on melanocytes that modulates their
function is the melanocortin-1 receptor (MC1R). When
α-melanocyte-stimulating hormone (α-MSH) binds to MC1-R on the
membrane of melanocytes, it activates adenylate cyclase, increases
intracellular cAMP, activates PKA-cAMP response element-binding
protein (CREB) pathway, and then increases MITF, promoting
melanogenesis (25–28). MC1R is also a major regulator of
human pigmentation and is also a melanoma susceptibility gene
(28). In addition, signaling
pathways such as mitogen activated protein kinase (MAPK), inositol
trisphosphate/diacylglycerol (IP3/DAG), WNT, and protein kinase C
(PKC) have also been revealed to participate in melanogenesis. The
α1 adrenergic receptor can activate the IP3/DAG pathway and
increase the intracellular levels of PKC-β and activate tyrosinase
(29). SCF, GM-SCF and hepatocyte
growth factor (HGF) can activate signaling pathways mediated by the
corresponding receptor c-KIT, GM-CSFR, and HGFR, leading to
autophosphorylation and activation of MAP kinase, thereby
phosphorylating MITF, upregulating the expression of
melanogenesis-related enzymes (30–32).
The WNT signaling pathway can activate MITF-M promoter (33–35),
thereby resulting in upregulation of MITF expression to further
regulate melanogenesis. Catecholamines can promote melanogenesis
through the cAMP/PKA pathway, while catecholamines also mediate
melanogenesis through the activation of PKC-β pathways by α1 and β2
adrenergic receptors (29,36).
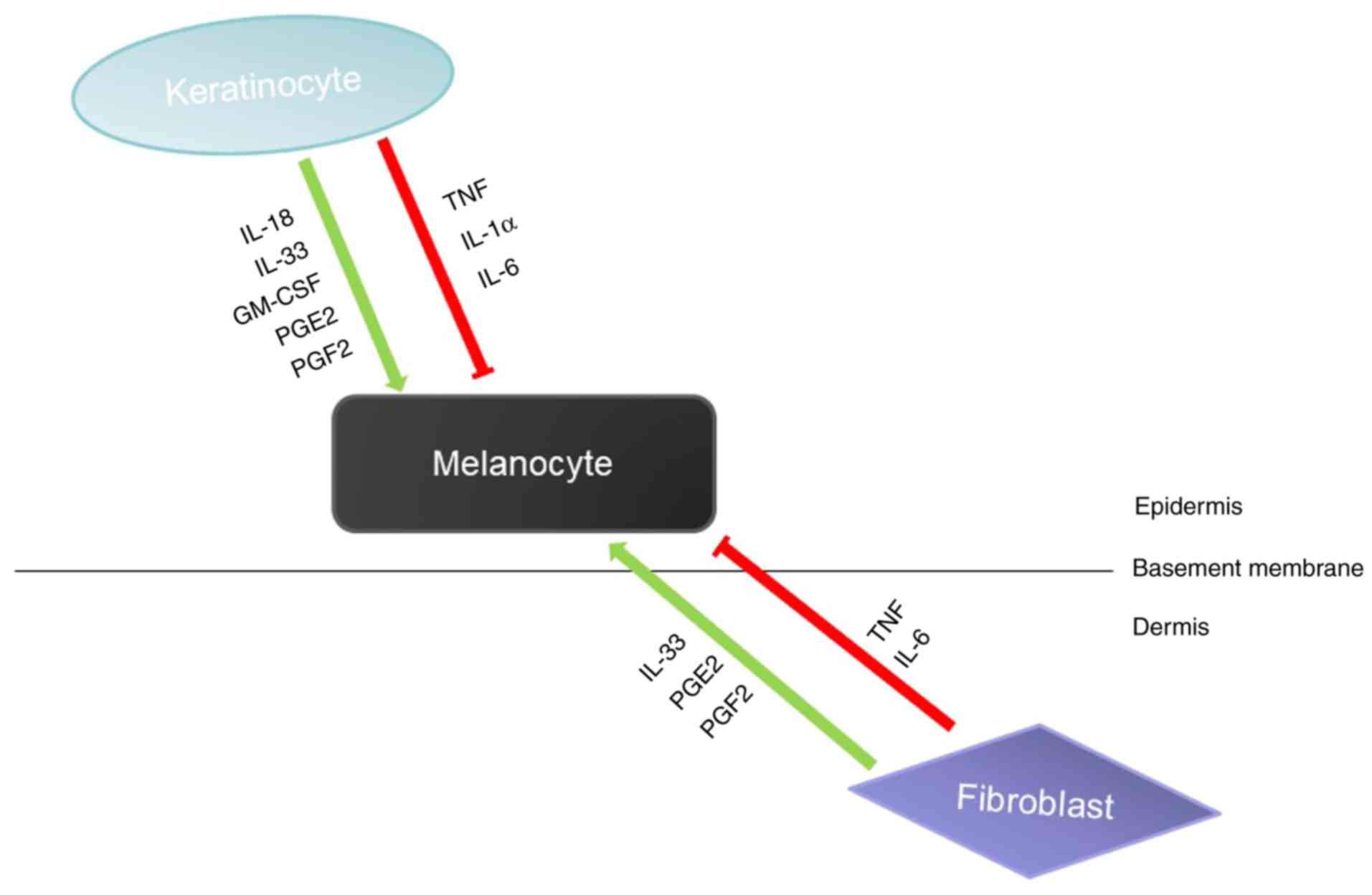
Skin melanogenesis is affected by the epidermal
melanin unit, which is mainly composed of keratinocytes and
melanocytes. Many of the paracrine factors secreted by
keratinocytes can act on melanocytes to promote or inhibit
melanogenesis. For example, IL-18, IL-33, GM-CSF can promote
melanogenesis, and TNF, IL-1 and IL-6 can inhibit melanogenesis
(37,38). In addition to keratinocytes, other
types of cells in the skin, such as fibroblasts, also participate
in the regulation of melanocytes by producing paracrine factors
(Fig. 2). Melanocytes interact
with these surrounding cells by expressing corresponding receptors
on the cell surface (27). In
addition, studies have revealed that paracrine factors can provide
a variety of mechanisms to activate DNA repair mechanisms by
activating different receptors and signaling pathways to maintain
melanocyte homeostasis and prevent UV mutagenesis (28).
Inflammation is a basic pathological process mainly
involving defensive reactions of living tissues with a vascular
system in response to the stimulation of various damage factors.
The chemical factors involved in mediating inflammatory reactions
are called chemical mediators or inflammatory mediators. The
inflammatory mediators in the skin are mainly secreted by Th cells,
lymphocytes, monocytes-macrophages, dendritic cells, and the like.
Th cells are mainly classified as Th1 and Th2 cells (39). Th1 cells play an important role in
cellular immune responses, secreting cytokines such as interferon-γ
(IFN-γ), tumor necrosis factor (TNF), IL-2, IL-3, GM-CSF; Th2 cells
play a key part in humoral immune responses, secreting IL-4, IL-5,
IL-10, IL-13, IL-3, GM-CSF as well as other cytokines (40,41).
In a normal body, Th1 cytokines and Th2 cytokines are in
equilibrium. When the body suffers from a certain disease, the
balance between Th1 and Th2 is impaired, and there is a drift
toward Th1 or Th2 (39). T helper
cell 17 (Th17) is a newly discovered T cell subset that secretes
IL-17, IL-6, IL-21 and IL-22 and participates in the occurrence of
innate immunity and certain inflammations by secreting IL-17, IL-6
and TNF-α. Studies have revealed that keratinocytes can secrete
IL-18, TNF, IL-1, GM-CSF, INF-γ, and IL-3, fibroblasts can secrete
IL-33, TNF, IL-6, and IL-8, and melanocytes can secrete INF-β,
IL-1, IL-8, IL-10 and TNF-α (37,38,42).
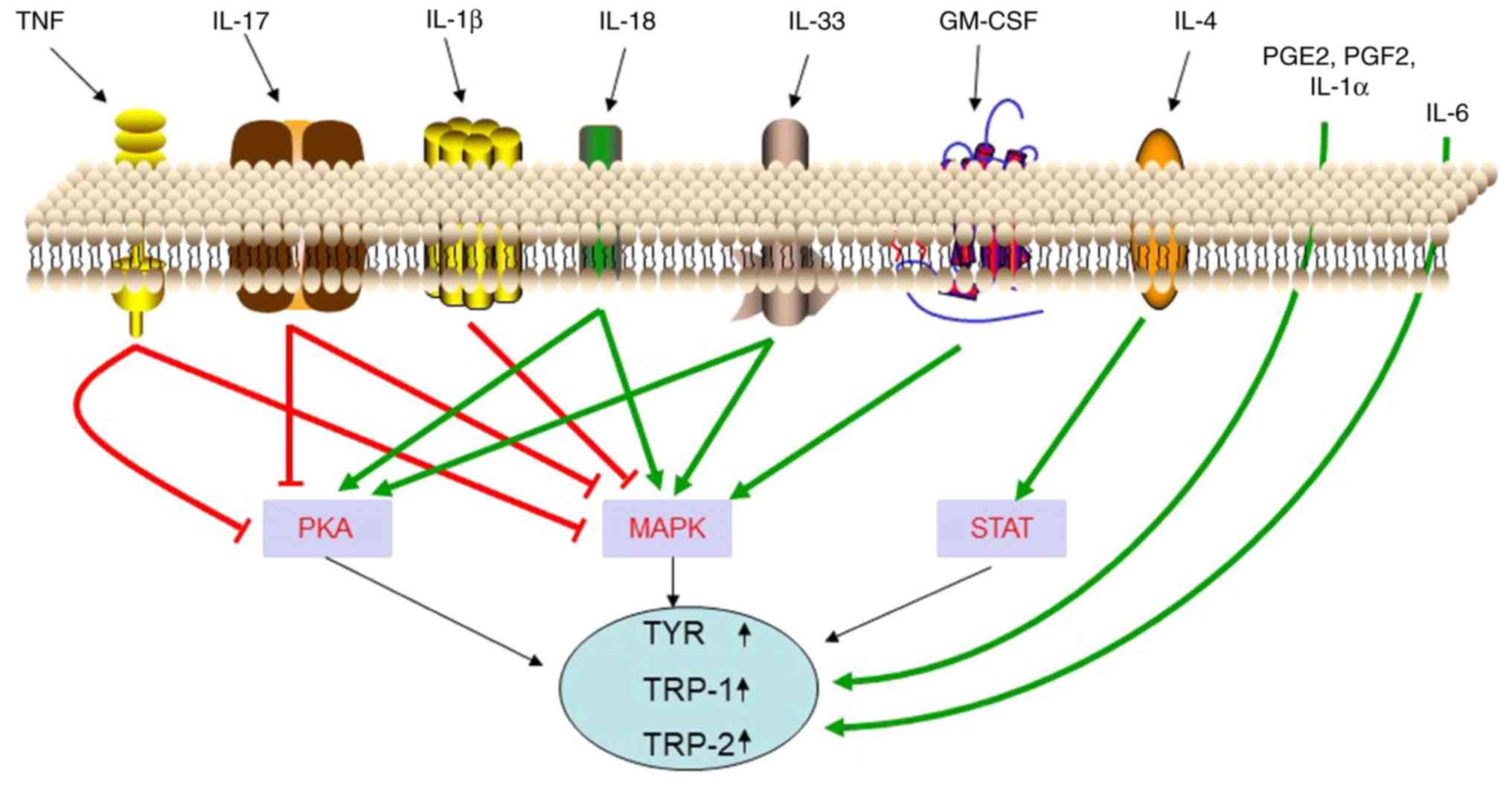
The main inflammatory mediators that are secreted by various types
of cells in the skin are presented in Table I. Recent studies have revealed that
local inflammatory factors of the skin may be involved in the
regulation of skin pigmentation (Fig.
3). The function and mechanisms of these inflammatory factors
in regulating melanogenesis are presented in Table II.
IL-18 is produced by inflammatory stimuli in
Langerhans cells (LC), dendritic cells (DC), Kupffer cells,
activated monocytes/macrophages, and keratinocytes in the epidermis
(43–45). IL-18 has been revealed to increase
the cascade expression of MITF and downstream enzymes by activating
the p38/MAPK and PKA pathways, and thus promote melanogenesis and
upregulate TYRP-1 and TYRP-2 expression (43,46).
These results suggest that IL-18 may participate in the regulation
of pigmentation by regulating melanocytes.
IL-33 can induce mast cells to produce
pro-inflammatory cytokines and chemokines (47–51),
thereby activating macrophages (52–54),
CD4+T cells, basophils, dendritic cells and neutrophils
(47,55–58),
and promoting skin inflammation. It has been revealed that IL-33
mRNA is expressed in multiple organs in humans (including the
skin), and in particular, relatively abundant IL-33 mRNA is found
in keratinocytes and fibroblasts (59,60).
Research has revealed that IL-33 can improve melanin biosynthesis
in NHEM and promote the expression of MITF and its
downstream-regulated tyrosine, TYRP-1, and TYRP-2 through the
activation of MAPK and PKA pathways (14), thereby promoting melanogenesis.
In addition, granulocyte-macrophage
colony-stimulating factor (GM-CSF) which is produced by mononuclear
macrophages, keratinocytes and Th cells, has been revealed to
promote melanocyte proliferation and melanin synthesis (17). Wu et al revealed that
increased serum levels of GM-CSF may be used as the serum
biomarkers to predict the prognosis of TCAM (transplantation of
cultured autologous melanocytes) when vitiligo patients are treated
(61).
Prostaglandin E2 (PGE2) and PGF2α which are produced
by fibroblasts and keratinocytes have been revealed to stimulate
dendritic cell formation and activate tyrosinase in melanocytes
through their dependence on the cAMP signaling pathway and
phospholipase C (PLC) (62,63).
Ma et al revealed that PGE2 is important in melanosome
transfer by promoting filopodia delivery (including miniaturization
of melanosome, filopodia formation, and broadening diameter of
filopodia) and the number of shedding spheroid granules in primary
melanocytes (MCs), but has no effects on morphological observation
of KCs (64).
As one of the most important endogenous mediators of
immunity and inflammation, IFN-γ is also a common secretory
cytokine in the skin (46). As a
pro-inflammatory cytokine, IFN-γ is mainly secreted by Th1
lymphocytes, CD8+ cytotoxic T lymphocytes and NK cells
(65). Other cells, including
antigen-presenting cells, B cells and NKT cells, can also secrete
IFN-γ (66–68). Recent studies have demonstrated
that the local accumulation of IFN-γ through melanocyte-specific
CD8+ T cells plays an important role in skin
discoloration spots in various mouse models of vitiligo (69,70).
Yang et al reported that increased IFN-γ is essential for
the pathogenesis of vitiligo by inducing apoptosis of melanocytes
(71). Natarajan et al
revealed that IFN-γ signaling blocks maturation of melanosomes by
regulating pigmentation genes (72). Moreover, IFN-γ has been revealed to
regulate melanogenesis by upregulating STAT1 phosphorylation, and
its inhibiting effect can be restrained by JAK1 inhibitors. Studies
have also revealed that IFN-γ inhibits IL-18-induced melanogenesis
(46).
TNF is a homotrimeric cytokine, secreted mainly by
monocytes and macrophages, and also by keratinocytes, dendritic
cells, Th1, Th17 and Th22. It functions by binding to two different
receptors: TNFR1/p55 and TNFR2/p75 (9). TNF not only induces inflammation
through the activation of vascular endothelial cells and immune
cells, but also acts as an important regulator of lymphoid tissue
development by controlling apoptosis (9). Elevated levels of TNF have been
revealed at sites of inflammation in several autoimmune diseases,
and inflammatory symptoms have generally decreased after
neutralization of TNF. For instance, higher expression levels of
TNF, TNFR1 and TNFR2 are observed in psoriasis (73). Studies have revealed that after
treatment of melanocytes with both IL-17 and TNF for 24–48 h, the
levels of c-KIT, MC1-R, MITF, and TYRP-2 were on the decrease, and
the levels of tyrosinase and melanin were significantly reduced
(10). It has been revealed that,
through the combination with IL-17, TNF can inhibit melanogenesis
by PKA and MAPK signaling pathways (9,10).
Blocking TNF can lead to rapid restoration of pigmentation gene
expression in psoriatic lesions. This suggests that anti-TNF has
the potential of treating pigmented dermatosis (10).
IL-1 is an important pro-inflammatory cytokine in
innate immunity that stimulates the differentiation and function of
immune surveillance cells and contributes to increased tumor
invasiveness, metastasis, and angiogenesis under chronic
inflammatory conditions (74).
IL-1α is an inflammatory mediator mainly produced by Langerhans
cells, and is also secreted by melanocytes and keratinocytes. Its
signal transduction is initiated by binding to IL-1 receptor type I
(IL-1Rα chain) (75), which can
inhibit tyrosinase activity and melanogenesis (12,74).
Of its many activities, IL-1α also stimulates human fibroblasts to
produce keratinocyte growth factor (KGF) (76). Keratinocytes store a large amount
of active IL-1α, express IL-1 receptors (77) and produce more IL-1α upon
ultraviolet B (UVB) exposure (78). KGF is thought to induce TYR
expression in primary melanocytes (79). The combination of KGF and IL-1α
increases melanin deposition and they may be involved in the
initial stage of human Solar lentigines lesion formation (79). Although they share only 24%
identity in protein sequence, IL-1β and IL-1α fold in a highly
similar manner and recognize the same receptor, the type I IL-1
receptor (IL-1RI) (80). After
treatment of a panel of melanoma cell lines with IL-1β, it was
observed that most of the MITF-M was inhibited and was NF-κB- and
JNK-dependent. The inactivation of these two pathways could
eliminate the inhibitory effects of IL-1β on melanin, which
indicated that IL-1β could downregulate MITF-M through NF-kB and
JNK pathways, thereby inhibiting melanogenesis (74).
IL-4 is a cytokine mainly secreted by Th2 cells and
can also be produced by CD8-positive cytotoxic T cells, basophils,
eosinophils, and mast cells in chronic inflammation (81,82).
IL-4 plays a key role in the generation of the major mediator IgE
in hypersensitivity as well as in the induction of inflammation,
contributing to the autoimmunity of the body (83). IL-4 is involved in the maintenance
of Th2 lymphocytes and acts as an autocrine growth factor of
differentiated Th2 cells (84). It
is hypothesized that vitiligo development is directly affected by
the imbalance of the Th1/Th2 response (85). Nouri-Koupaee et al revealed
the Th1 and Th2 response profiles in vitiligo by assessing IFN-γ
and IL-4. This study revealed significant increases in IFN-γ and
marked decreases of IL-4 in patients when compared to controls
(86). It has also been revealed
that IL-4 downregulates the expression of MITF, TYRP-1, and TYRP-2
through the JAK2/STAT6 signaling pathway and thus inhibits
melanogenesis (13).
IL-6 is secreted by keratinocytes, epidermal cells,
fibroblasts and dermal endothelial cells and is involved in the
regulation of various biological responses including immune
response, inflammation, hematopoiesis, and tumorigenesis by
regulating cell growth, survival, and differentiation (87). Research has revealed that IL-6
decreases tyrosinase activity and melanogenesis (12).
IL-17 is a pro-inflammatory cytokine produced mainly
by Th17 cells, and also by other immune cells, including
neutrophils, natural killer cells, mast cells, αβ and γδT cells
(88). The most well-known
function of IL-17 is to prevent bacterial and fungal infections
(88). IL-17 has a variety of
inflammatory effects, resulting in the release of large amounts of
cytokines from a variety of cells, such as epithelial cells,
endothelial cells, and fibroblasts (89). Studies have revealed that IL-17 can
bind to TNF to inhibit the signaling pathway for melanogenesis,
thereby inhibiting melanogenesis (10). The function and mechanisms of these
inflammatory factors in regulating melanogenesis are presented in
Table II.
It should be noted that the IFN-γ-related data were
acquired from a murine melanoma model (B16F10) and IL-1α-related
data were based on observations from porcine skin. Therefore,
whether their effects on melanogenesis in human melanocytes are the
same still requires confirmation by subsequent experiments.
In clinical practice, various treatments can be
effective for post-inflammatory hyperpigmentations and
hypopigmentations by influencing inflammatory factors. For example,
chloasma is a postinflammatory hyperpigmented disease caused by
many factors such as heredity, ultraviolet radiation, pregnancy,
hormone therapy, cosmetics, and phototoxic drugs (90). Kojic acid, hydroquinone, and
tranexamic acid are commonly used to treat melasma (91). It is well-known that their
inhibitory effect on tyrosine activity or melanocyte-specific
cytotoxicity is the decolorization mechanism (92,93).
In recent years, it has been revealed that kojic acid also inhibits
the melanogenesis of melanocytes by promoting the expression of
IL-6 in keratinocytes. Resveratrol was revealed to play an
important role in ameliorating inflammation, including skin
inflammation and reducing inflammatory injury in HaCaT cells
(94). Studies have also reported
that resveratrol inhibits melanin synthesis to treat hyperpigmented
diseases (95). Therefore,
resveratrol may also affect melanogenesis by regulating
inflammatory factors.
Although the causes of vitiligo are not completely
clear, inflammation has been revealed to play a role in its
pathogenesis (96). Certain
studies revealed that higher expression of pro-inflammatory
cytokines had an inhibitory effect on pigmentation in vitiligo
lesions (97,98). For example, Kim et al
(99) revealed that increased
expression of TNF-α in keratinocytes of the lesion area in vitiligo
patients inhibited the secretion of melanocyte growth factor from
KCs. Barygina et al suggested that low-dose IL-4,
β-endorphin, bFGF and IL-10 may be considered as new therapeutic
tools for vitiligo treatment (100). Studies have revealed that 308 nm
excimer laser can significantly reduce the level of TNF-α in
lesions (101), thereby promoting
MC function. Various studies reported that the expression of IL-4,
TNF-α and other inflammatory cytokines was downregulated after
topical application of tacrolimus in lesions of vitiligo (102,103). Methotrexate (MTX) is used in the
treatment of autoimmune diseases to decrease T cells that produce
TNF-α, which is a key step in the development of vitiligo (104). A study by Alghamdi and Khurrum
revealed that oral MTX was a safe and effective therapeutic
approach for vitiligo, however, due to the fact that this was a
small uncontrolled pilot study, further research needs to be
carried out (105). Afamelanotide
is a potent and longer-lasting synthetic analogue of naturally
occurring α-MSH, which is decreased in vitiligo. Grimes et
al (106) found that NB-UVB
combined with afamelanotide is safe and effective and that
afamelanotide represents a potentially effective treatment for
vitiligo, however this still requires further studies. The
aforementioned findings indicated that the external use of
medications, light therapy and other treatments may serve to treat
inflammation related-hyperpigmentations or hypopigmentations by
regulating the expression of inflammatory factors associated with
melanin production.
Studies have revealed that a variety of inflammatory
factors can promote or inhibit the melanogenesis of melanocytes
through different mechanisms, suggesting that the development of
medicine or therapies from the perspective of inflammation
regulation can provide new ideas and new targets for the treatment
of pigmented dermatosis. It is widely considered that the
regulatory network of inflammation is very complex, since all types
of inflammatory cells are involved in the activation and release of
inflammatory mediators. The imbalance of inflammatory factors
related to T-cell subsets plays an important role in the
development of various skin diseases, however, the relationship
between imbalance or changes of T-cell subsets and melanogenesis
has yet to be confirmed by further experiments.
Not applicable.
The present study was supported by the Fundamental
Research Funds for the Central Universities of Central South
University (no. 2017zzts890), the National Natural Science
Foundation of China (no. 81703101) and the Natural Science
Foundation of Hunan Province (nos. 2018JJ3788 and 2018JJ3793).
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
CF and JC designed and wrote the paper. JH and QZ
designed and supervised the study. JL, LY, XT, LK, SP, YO, LJ, YD,
XZ, SL and YY analyzed and interpreted the data. All authors have
read and approved the final manuscript and agreed to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no interests.
|
1
|
Gröne A: Keratinocytes and cytokines. Vet
Immunol Immunopathol. 88:1–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC
and Slominski A: Cutaneous hypothalamic-pituitary-adrenal axis
homolog: Regulation by ultraviolet radiation. Am J Physiol
Endocrinol Metab. 301:E484–E493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weiss E, Mamelak AJ, La Morgia S, Wang B,
Feliciani C, Tulli A and Sauder DN: The role of interleukin 10 in
the pathogenesis and potential treatment of skin diseases. J Am
Acad Dermatol. 50:657–678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin SF: Contact dermatitis: From
pathomechanisms to immunotoxicology. Exp Dermatol. 21:382–389.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller LS and Cho JS: Immunity against
Staphylococcus aureus cutaneous infections. Nat Rev Immunol.
11:505–518. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Behrends U, Peter RU, Hintermeier-Knabe R,
Eissner G, Holler E, Bornkamm GW, Caughman SW and Degitz K:
Ionizing radiation induces human intercellular adhesion molecule-1
in vitro. J Invest Dermatol. 103:726–730. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuchs J and Kern H: Modulation of
UV-light-induced skin inflammation by D-alpha-tocopherol and
L-ascorbic acid: A clinical study using solar simulated radiation.
Free Radic Biol Med. 25:1006–1012. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basler K and Brandner JM: Tight junctions
in skin inflammation. Pflugers Arch. 469:3–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grine L, Dejager L, Libert C and
Vandenbroucke RE: An inflammatory triangle in psoriasis: TNF, type
I IFNs and IL-17. Cytokine Growth Factor Rev. 26:25–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CQF, Akalu YT, Suarez-Farinas M,
Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P and Krueger JG:
IL-17 and TNF synergistically modulate cytokine expression while
suppressing melanogenesis: Potential relevance to psoriasis. J
Invest Dermatol. 133:2741–2752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swope VB, Abdel-Malek Z, Kassem LM and
Nordlund JJ: Interleukins 1 alpha and 6 and tumor necrosis
factor-alpha are paracrine inhibitors of human melanocyte
proliferation and melanogenesis. J Invest Dermatol. 96:180–185.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi H, Choi H, Han J, Jin SH, Park JY,
Shin DW, Lee TR, Kim K, Lee AY and Noh M: IL-4 inhibits the
melanogenesis of normal human melanocytes through the JAK2-STAT6
signaling pathway. J Invest Dermatol. 133:528–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Song J, Ping F and Shang J:
Enhancement of the p38 MAPK and PKA signaling pathways is
associated with the pro-melanogenic activity of Interleukin 33 in
primary melanocytes. J Dermatol Sci. 73:110–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsatmali M, Ancans J and Thody AJ:
Melanocyte function and its control by melanocortin peptides. J
Histochem Cytochem. 50:125–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Videira IF, Moura DF and Magina S:
Mechanisms regulating melanogenesis. An Bras Dermatol. 88:76–83.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi Y, Brenner M and Hearing VJ: The
regulation of skin pigmentation. J Biol Chem. 282:27557–27561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seong ZK, Lee SY, Poudel A, Oh SR and Lee
HK: Constituents of cryptotaenia japonica inhibit melanogenesis via
CREB- and MAPK-associated signaling pathways in murine B16 melanoma
cells. Molecules. 21(pii): E12962016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campos PM, Prudente AS, Horinouchi CD,
Cechinel-Filho V, Fávero GM, Cabrini DA and Otuki MF: Inhibitory
effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J
Ethnopharmacol. 174:224–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsao YT, Huang YF, Kuo CY, Lin YC, Chiang
WC, Wang WK, Hsu CW and Lee CH: Hinokitiol inhibits melanogenesis
via AKT/mTOR signaling in B16F10 mouse melanoma cells. Int J Mol
Sci. 17:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirobe T: Role of keratinocyte-derived
factors involved in regulating the proliferation and
differentiation of mammalian epidermal melanocytes. Pigment Cell
Res. 18:2–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schallreuter KU, Kothari S, Chavan B and
Spencer JD: Regulation of melanogenesis-controversies and new
concepts. Exp Dermatol. 17:395–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin JY and Fisher DE: Melanocyte biology
and skin pigmentation. Nature. 445:843–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park HY, Kosmadaki M, Yaar M and Gilchrest
BA: Cellular mechanisms regulating human melanogenesis. Cell Mol
Life Sci. 66:1493–1506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schiaffino MV: Signaling pathways in
melanosome biogenesis and pathology. Int J Biochem Cell Biol.
42:1094–1104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan XH and Jin ZH: Paracrine regulation
of melanogenesis. Br J Dermatol. 178:632–639. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Swope VB and Abdel-Malek ZA: MC1R: Front
and center in the bright side of dark eumelanin and DNA repair. Int
J Mol Sci. 19(pii): E26672018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grando SA, Pittelkow MR and Schallreuter
KU: Adrenergic and cholinergic control in the biology of epidermis:
Physiological and clinical significance. J Invest Dermatol.
126:1948–1965. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonaventure J, Domingues MJ and Larue L:
Cellular and molecular mechanisms controlling the migration of
melanocytes and melanoma cells. Pigment Cell Melanoma Res.
26:316–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Besmer P, Murphy JE, George PC, Qiu FH,
Bergold PJ, Lederman L, Snyder HW Jr, Brodeur D, Zuckerman EE and
Hardy WD: A new acute transforming feline retrovirus and
relationship of its oncogene v-kit with the protein kinase gene
family. Nature. 320:415–421. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yarden Y, Kuang WJ, Yang-Feng T, Coussens
L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U and
Ullrich A: Human proto-oncogene c-kit: A new cell surface receptor
tyrosine kinase for an unidentified ligand. EMBO J. 6:3341–3351.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dorsky RI, Raible DW and Moon RT: Direct
regulation of nacre, a zebrafish MITF homolog required for pigment
cell formation, by the Wnt pathway. Genes Dev. 14:158–162.
2000.PubMed/NCBI
|
|
34
|
Flaherty KT, Hodi FS and Fisher DE: From
genes to drugs: Targeted strategies for melanoma. Nat Rev Cancer.
12:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Widlund HR, Horstmann MA, Price ER, Cui J,
Lessnick SL, Wu M, He X and Fisher DE: Beta-catenin-induced
melanoma growth requires the downstream target
Microphthalmia-associated transcription factor. J Cell Biol.
158:1079–1087. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung E, Lee J, Huh S, Lee J, Kim YS, Kim G
and Park D: Phloridzin-induced melanogenesis is mediated by the
cAMP signaling pathway. Food Chem Toxicol. 47:2436–2440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Satomi H, Wang B, Fujisawa H and Otsuka F:
Interferon-beta from melanoma cells suppresses the proliferations
of melanoma cells in an autocrine manner. Cytokine. 18:108–115.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mattei S, Colombo MP, Melani C, Silvani A,
Parmiani G and Herlyn M: Expression of cytokine/growth factors and
their receptors in human melanoma and melanocytes. Int J Cancer.
56:853–857. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mosmann TR and Sad S: The expanding
universe of T-cell subsets: Th1, Th2 and more. Immunol Today.
17:138–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Garra A: Cytokines induce the
development of functionally heterogeneous T helper cell subsets.
Immunity. 8:275–283. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reiner SL and Seder RA: Dealing from the
evolutionary pawnshop: How lymphocytes make decisions. Immunity.
11:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bennicelli JL and Guerry D VI: Production
of multiple cytokines by cultured human melanomas. Exp Dermatol.
2:186–190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou J, Shang J, Song J and Ping F:
Interleukin-18 augments growth ability of primary human melanocytes
by PTEN inactivation through the AKT/NF-κB pathway. Int J Biochem
Cell Biol. 45:308–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yun W and Li C: JNK pathway is required
for TNCB-induced IL-18 expression in murine keratinocytes. Toxicol
In Vitro. 24:1064–1069. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wittmann M, Macdonald A and Renne J: IL-18
and skin inflammation. Autoimmun Rev. 9:45–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou J, Ling J, Wang Y, Shang J and Ping
F: Cross-talk between interferon-gamma and interleukin-18 in
melanogenesis. J Photochem Photobiol B. 163:133–143. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ali S, Huber M, Kollewe C, Bischoff SC,
Falk W and Martin MU: IL-1 receptor accessory protein is essential
for IL-33-induced activation of T lymphocytes and mast cells. Proc
Natl Acad Sci USA. 104:18660–18665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Allakhverdi Z, Smith DE, Comeau MR and
Delespesse G: Cutting edge: The ST2 ligand IL-33 potently activates
and drives maturation of human mast cells. J Immunol.
179:2051–2054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moulin D, Donze O, Talabot-Ayer D, Mezin
F, Palmer G and Gabay C: Interleukin (IL)-33 induces the release of
pro-inflammatory mediators by mast cells. Cytokine. 40:216–225.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Theoharides TC, Zhang B, Kempuraj D, Tagen
M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi
S, Stavrianeas N, et al: IL-33 augments substance P-induced VEGF
secretion from human mast cells and is increased in psoriatic skin.
Proc Natl Acad Sci USA. 107:4448–4453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pushparaj PN, Tay HK, H'ng SC, Pitman N,
Xu D, McKenzie A, Liew FY and Melendez AJ: The cytokine
interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA.
106:9773–9778. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kurowska-Stolarska M, Stolarski B, Kewin
P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B,
van Rooijen N, et al: IL-33 amplifies the polarization of
alternatively activated macrophages that contribute to airway
inflammation. J Immunol. 183:6469–6477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ohno T, Oboki K, Kajiwara N, Morii E,
Aozasa K, Flavell RA, Okumura K, Saito H and Nakae S: Caspase-1,
caspase-8, and calpain are dispensable for IL-33 release by
macrophages. J Immunol. 183:7890–7897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schmieder A, Multhoff G and Radons J:
Interleukin-33 acts as a pro-inflammatory cytokine and modulates
its receptor gene expression in highly metastatic human pancreatic
carcinoma cells. Cytokine. 60:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hueber AJ, Alves-Filho JC, Asquith DL,
Michels C, Millar NL, Reilly JH, Graham GJ, Liew FY, Miller AM and
McInnes IB: IL-33 induces skin inflammation with mast cell and
neutrophil activation. Eur J Immunol. 41:2229–2237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suzukawa M, Iikura M, Koketsu R, Nagase H,
Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K and
Yamaguchi M: An IL-1 cytokine member, IL-33, induces human basophil
activation via its ST2 receptor. J Immunol. 181:5981–5989. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rank MA, Kobayashi T, Kozaki H, Bartemes
KR, Squillace DL and Kita H: IL-33-activated dendritic cells induce
an atypical TH2-type response. J Allergy Clin Immunol.
123:1047–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Arend WP, Palmer G and Gabay C: IL-1,
IL-18, and IL-33 families of cytokines. Immunol Rev. 223:20–38.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Byrne SN, Beaugie C, O'Sullivan C,
Leighton S and Halliday GM: The immune-modulating cytokine and
endogenous Alarmin interleukin-33 is upregulated in skin exposed to
inflammatory UVB radiation. Am J Pathol. 179:211–222. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu XG, Hong WS and Xu A: GM-CSF: A
possible prognostic serum biomarker of vitiligo patients'
considered for transplantation treatment with cultured autologous
melanocytes: A pilot study. J Eur Acad Dermatol Venereol.
30:1409–1411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Scott G, Leopardi S, Printup S, Malhi N,
Seiberg M and Lapoint R: Proteinase-activated receptor-2 stimulates
prostaglandin production in keratinocytes: Analysis of
prostaglandin receptors on human melanocytes and effects of PGE2
and PGF2alpha on melanocyte dendricity. J Invest Dermatol.
122:1214–1224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Scott G, Jacobs S, Leopardi S, Anthony FA,
Learn D, Malaviya R and Pentland A: Effects of PGF2alpha on human
melanocytes and regulation of the FP receptor by ultraviolet
radiation. Exp Cell Res. 304:407–416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ma HJ, Ma HY, Yang Y, Li PC, Zi SX, Jia CY
and Chen R: a-Melanocyte stimulating hormone (MSH) and
prostaglandin E2 (PGE2) drive melanosome transfer by promoting
filopodia delivery and shedding spheroid granules: Evidences from
atomic force microscopy observation. J Dermatol Sci. 76:222–230.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bach EA, Aguet M and Schreiber RD: The IFN
gamma receptor: A paradigm for cytokine receptor signaling. Annu
Rev Immunol. 15:563–591. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Carnaud C, Lee D, Donnars O, Park SH,
Beavis A, Koezuka Y and Bendelac A: Cutting edge: Cross-talk
between cells of the innate immune system: NKT cells rapidly
activate NK cells. J Immunol. 163:4647–4650. 1999.PubMed/NCBI
|
|
67
|
Frucht DM, Fukao T, Bogdan C, Schindler H,
O'Shea JJ and Koyasu S: IFN-gamma production by antigen-presenting
cells: Mechanisms emerge. Trends Immunol. 22:556–560. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Flaishon L, Hershkoviz R, Lantner F, Lider
O, Alon R, Levo Y, Flavell RA and Shachar I: Autocrine secretion of
interferon gamma negatively regulates homing of immature B cells. J
Exp Med. 192:1381–1388. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Harris JE, Harris TH, Weninger W, Wherry
EJ, Hunter CA and Turka LA: A mouse model of vitiligo with focused
epidermal depigmentation requires IFN-γ for autoreactive
CD8+ T-cell accumulation in the skin. J Invest Dermatol.
132:1869–1876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gregg RK, Nichols L, Chen Y, Lu B and
Engelhard VH: Mechanisms of spatial and temporal development of
autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J
Immunol. 184:1909–1917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L
and Li M: Interferon-gamma inhibits melanogenesis and induces
apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T
lymphocytes in vitiligo. Acta Derm Venereol. 95:664–670. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Natarajan VT, Ganju P, Singh A, Vijayan V,
Kirty K, Yadav S, Puntambekar S, Bajaj S, Dani PP, Kar HK, et al:
IFN-γ signaling maintains skin pigmentation homeostasis through
regulation of melanosome maturation. Proc Natl Acad Sci USA.
111:2301–2306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kristensen M, Chu CQ, Eedy DJ, Feldmann M,
Brennan FM and Breathnach SM: Localization of tumour necrosis
factor-alpha (TNF-alpha) and its receptors in normal and psoriatic
skin: Epidermal cells express the 55-kD but not the 75-kD TNF
receptor. Clin Exp Immunol. 94:354–362. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kholmanskikh O, van Baren N, Brasseur F,
Ottaviani S, Vanacker J, Arts N, van der Bruggen P, Coulie P and De
Plaen E: Interleukins 1alpha and 1beta secreted by some melanoma
cell lines strongly reduce expression of MITF-M and melanocyte
differentiation antigens. Int J Cancer. 127:1625–1636. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Martin MU and Wesche H: Summary and
comparison of the signaling mechanisms of the Toll/interleukin-1
receptor family. Biochim Biophys Acta. 1592:265–280. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tang A and Gilchrest B: Regulation of
keratinocyte growth factor gene expression in human skin
fibroblasts. J Dermatol Sci. 11:41–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Grewe M, Gyufko K, Budnik A, Ruzicka T,
Olaizola-Horn S, Berneburg M and Krutmann J: Interleukin-1
receptors type I and type II are differentially regulated in human
keratinocytes by ultraviolet B radiation. J Invest Dermatol.
107:865–870. 1996.PubMed/NCBI
|
|
78
|
Kondo S, Sauder DN, Kono T, Galley KA and
McKenzie RC: Differential modulation of interleukin-1 alpha (IL-1
alpha) and interleukin-1 beta (IL-1 beta) in human epidermal
keratinocytes by UVB. Exp Dermatol. 3:29–39. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen N, Hu Y, Li WH, Eisinger M, Seiberg M
and Lin CB: The role of keratinocyte growth factor in
melanogenesis: A possible mechanism for the initiation of solar
lentigines. Exp Dermatol. 19:865–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sims J, March C, Cosman D, Widmer MB,
MacDonald HR, McMahan CJ, Grubin CE, Wignall JM, Jackson JL, Call
SM, et al: cDNA expression cloning of the IL-1 receptor, a member
of the immunoglobulin superfamily. Science. 241:585–589. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Barata LT, Ying S, Meng Q, Barkans J,
Rajakulasingam K, Durham SR and Kay AB: IL-4- and IL-5-positive T
lymphocytes, eosinophils, and mast cells in allergen-induced
late-phase cutaneous reactions in atopic subjects. J Allergy Clin
Immunol. 101:222–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Min B, Prout M, Hu-Li J, Zhu J, Jankovic
D, Morgan ES, Urban JF Jr, Dvorak AM, Finkelman FD, LeGros G and
Paul WE: Basophils produce IL-4 and accumulate in tissues after
infection with a Th2-inducing parasite. J Exp Med. 200:507–517.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Imran M, Laddha N, Dwivedi M, Mansuri MS,
Singh J, Rani R, Gokhale RS, Sharma VK, Marfatia YS and Begum R:
Interleukin-4 genetic variants correlate with its transcript and
protein levels in patients with vitiligo. Br J Dermatol.
167:314–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Salgame P, Abrams JS, Clayberger C,
Goldstein H, Convit J, Modlin RL and Bloom BR: Differing lymphokine
profiles of functional subsets of human CD4 and CD8 T cell clones.
Science. 254:279–282. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Basak PY, Adiloglu AK, Ceyhan AM, Tas T
and Akkaya VB: The role of helper and regulatory T cells in the
pathogenesis of vitiligo. J Am Acad Dermatol. 60:256–260. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Nouri-Koupaee A, Mansouri P, Jahanbini H,
Sanati MH and Jadali Z: Differential expression of mRNA for T-bet
and GATA-3 transcription factors in peripheral blood mononuclear
cells of patients with vitiligo. Clin Exp Dermatol. 40:735–740.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hirano T, Ishihara K and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Speeckaert R, Lambert J, Grine L, Van Gele
M, De Schepper S and van Geel N: The many faces of interleukin-17
in inflammatory skin diseases. Br J Dermatol. 175:892–901. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Volpe E, Servant N, Zollinger R, Bogiatzi
SI, Hupé P, Barillot E and Soumelis V: A critical function for
transforming growth factor-beta, interleukin 23 and proinflammatory
cytokines in driving and modulating human T(H)-17 responses. Nat
Immunol. 9:650–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kang WH, Yoon KH, Lee ES, Kim J, Lee KB,
Yim H, Sohn S and Im S: Melasma: Histopathological characteristics
in 56 Korean patients. Br J Dermatol. 146:228–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Nakajima M, Shinoda I, Fukuwatari Y and
Hayasawa H: Arbutin increases the pigmentation of cultured human
melanocytes through mechanisms other than the induction of
tyrosinase activity. Pigment Cell Res. 11:12–17. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Palumbo A, d'Ischia M, Misuraca G and
Prota G: Mechanism of inhibition of melanogenesis by hydroquinone.
Biochim Biophys Acta. 1073:85–90. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Smith CJ, O'Hare KB and Allen JC:
Selective cytotoxicity of hydroquinone for melanocyte-derived cells
is mediated by tyrosinase activity but independent of melanin
content. Pigment Cell Res. 1:386–389. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang X and Zhang Y: Resveratrol alleviates
LPS-induced injury in human keratinocyte cell line HaCaT by
up-regulation of miR-17. Biochem Biophys Res Commun. 501:106–112.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kim ES, Chang H, Choi H, Shin JH, Park SJ,
Jo YK, Choi ES, Baek SY, Kim BG, Chang JW, et al: Autophagy induced
by resveratrol suppresses a-MSH-induced melanogenesis. Exp
Dermatol. 23:204–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Salzes C, Abadie S, Seneschal J, Whitton
M, Meurant JM, Jouary T, Ballanger F, Boralevi F, Taieb A, Taieb C
and Ezzedine K: The vitiligo impact patient scale (VIPs):
Development and validation of a vitiligo burden assessment tool. J
Invest Dermatol. 136:52–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Moretti S, Spallanzani A, Amato L,
Hautmann G, Gallerani I, Fabiani M and Fabbri P: New insights into
the pathogenesis of vitiligo: Imbalance of epidermal cytokines at
sites of lesions. Pigment Cell Res. 15:87–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Moretti S, Fabbri P, Baroni G, Berti S,
Bani D, Berti E, Nassini R, Lotti T and Massi D: Keratinocyte
dysfunction in vitiligo epidermis: Cytokine microenvironment and
correlation to keratinocyte apoptosis. Histol Histopathol.
24:849–857. 2009.PubMed/NCBI
|
|
99
|
Kim NH, Jeon S, Lee HJ and Lee AY:
Impaired PI3K/Akt activation-mediated NF-kappaB inactivation under
elevated TNF-alpha is more vulnerable to apoptosis in vitiliginous
keratinocytes. J Invest Dermatol. 127:2612–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Barygina V, Becatti M, Lotti T, Moretti S,
Taddei N and Fiorillo C: Treatment with low-dose cytokines reduces
oxidative-mediated injury in perilesional keratinocytes from
vitiligo skin. J Dermatol Sci. 79:163–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Debbaneh MG, Levin E, Sanchez Rodriguez R,
Leon A, Koo J and Rosenblum MD: Plaque-based sub-blistering
dosimetry: Reaching PASI-75 after two treatments with 308-nm
excimer laser in a generalized psoriasis patient. J Dermatolog
Treat. 26:45–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Grimes P, Morris R, Avaniss-Aghajani E,
Soriano T, Meraz M and Metzger A: Topical tacrolimus therapy for
vitiligo: Therapeutic responses and skin messenger RNA expression
of proinflammatory cytokines. J Am Acad Dermatol. 51:52–61. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sakuma S, Higashi Y, Sato N, Sasakawa T,
Sengoku T, Ohkubo Y, Amaya T and Goto T: Tacrolimus suppressed the
production of cytokines involved in atopic dermatitis by direct
stimulation of human PBMC system. (Comparison with steroids). Int
Immunopharmacol. 1:1219–1226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Birol A, Kisa U, Kurtipek GS, Kara F,
Kocak M, Erkek E and Caglayan O: Increased tumor necrosis factor
alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the
lesional skin of patients with nonsegmental vitiligo. Int J
Dermatol. 45:992–993. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Alghamdi K and Khurrum H: Methotrexate for
the treatment of generalized vitiligo. Saudi Pharm J. 21:423–424.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Grimes PE, Hamzavi I, Lebwohl M, Ortonne
JP and Lim HW: The efficacy of afamelanotide and narrowband UV-B
phototherapy for repigmentation of vitiligo. JAMA Dermatol.
149:68–73. 2013. View Article : Google Scholar : PubMed/NCBI
|