Introduction
According to functional difference, the cytokine
system can be divided into two distinct phenotypes: Proinflammatory
cytokines and anti-inflammatory cytokines. Generally,
proinflammatory cytokines include interleukin (IL)-1β, IL-2,
interferon (IFN)-γ, tumor necrosis factor-α (TNF-α), IL-6, IL-9
IL-12, IL-18, IL-17 and IL-23, which are produced predominantly by
Th1, Th9, Th17 cells and M1 macrophages. Anti-inflammatory
cytokines include IL-4, IL-10, IL-13 and transforming growth factor
(TGF)-β, which are represented by Th2, Th3 cells and M2
macrophages. Type 1 (IL-2, IFN-γ and TNF-α) cytokines have been
identified to play a role in organ-specific autoimmune diseases,
such as multiple sclerosis, type 1 diabetes, rheumatoid arthritis
and autoimmune hepatitis (1). As
an alarm molecule in the IL-1 family (2), IL-33 has attracted increasing
attention in recent years due to its immunoregulatory role in
inducing type 2 immune responses. Higher expression of IL-33 and
soluble spliced variant of suppression of tumorigenicity 2 (sST2)
was identified in the sera and endobronchial biopsies of asthmatic
patients, as well as in mouse models of asthma induced by ovalbumin
through increased Th2 cytokine production, such as IL-4, IL-5 and
IL-13 (3,4). In addition, IL-33 promoted eosinophil
infiltration and pathogenic Th2 immune responses, leading to
chronic experimental ileitis (5).
In dextran sulfate sodium-induced experimental colitis, IL-33
played a protective role via goblet cell induction and also
exhibited proinflammatory properties as a Th2 cytokine (6). In addition, male-specific IL-33
expression regulates sex-dimorphic experimental autoimmune
encephalomyelitis susceptibility as attenuators of the pathogenic
Th1/Th17 response (7).
Including endothelial cells, fibroblasts, basophils,
dendritic cells, macrophages and mast cells can produce IL-33 in
response to local or systemic balance disorders, such as cell
damage, stress, inflammation or microbial invasion (8). IL-33 has also been reported to
promote mast cell maturation, activation and survival (9). Through binding to the IL-1R/Toll-like
receptor (TLR) superfamily member ST2 receptor, IL-33 stimulates
target cells and induces subsequent activation of NF-κB and
mitogen-activated protein kinase (MAPK) pathways via identical
signaling events to those observed for IL-1β, resulting in the
production of cytokines and chemokines (10). Although it is a weak inducer of
mast cell degranulation, IL-33 can augment the amplitude of cell
degeneration in response to cross-linking stimulation triggered by
antigens and immunoglobulin E (IgE) receptors (11). In addition, IL-33 is also a nuclear
factor that is abundantly expressed in high endothelial venules
from lymphoid organs that is associated with chromatin, exhibiting
transcriptional regulatory properties (12).
Resveratrol (RSV), a natural polyphenol found in
grapes and other herbal plants, has been reported to be beneficial
in allergic diseases (13),
fibrogenetic disorders (14) and
immunoinflammatory pathologies characterized by upregulated type 2
cytokine production, including some forms of inflammatory bowel
disease, ileitis and systemic lupus erythematosus (8). A previous study demonstrated that RSV
inhibited the release of IgE-associated mediators from bone
marrow-derived mouse mast cells in vitro, such as histamine,
TNF-α, leukotrienes and prostaglandin D2 (15). Another study reported that RSV
suppressed IgE-mediated basophilic mast cell degranulation in
vitro, including β-hexosaminidase and histamine. In addition,
RSV decreased IgE-mediated passive cutaneous anaphylaxis and
alleviated allergic inflammation, such as monocyte chemotactic
protein (MCP)-1 and macrophage inflammatory protein (MIP)-2
(16). A recent study indicated
that RSV-curcumin hybrids exhibited anti-inflammatory efficacy as
potential therapeutic agents for inflammatory lung diseases,
decreasing lipopolysaccharide (LPS)-induced TNF-α, IL-6, IL-12 and
IL-33 mRNA expression (17).
Although these studies suggest that RSV can inhibit the mast cell
activation involved in inflammatory responses, no direct evidence
has yet demonstrated the effect of RSV on IL-33-induced
inflammatory responses in mast cells and the detailed underlying
molecular mechanisms should be elucidated.
In the present study, the effect and underlying
molecular mechanisms of RSV on IL-33-induced mast cell inflammation
were investigated. The data revealed that RSV decreased
IL-33-stimulated inflammatory cytokine production in vivo
and in vitro and also inhibited IL-33-induced enhancement of
IgE-mediated responses in mast cells, at least partly, contributing
to inhibition of NF-κB activation and P38 signaling. These findings
indicate the potential application of RSV as an immunoregulator in
allergic disorders associated with mast cell inflammation.
Materials and methods
Cell culture
Rat basophilic leukemia (RBL)-2H3 cells were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences. The cells were grown in DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with penicillin (100
IU/ml), streptomycin (100 µg/ml) and 10% heat-inactivated fetal
bovine serum (Hyclone; GE Healthcare Life Sciences) at 37°C in a
humified incubator with 5% CO2.
Toluidine Blue Staining
RBL-2H3 cells (1×105) were washed three
times with PBS and subsequently fixed with 4% paraformaldehyde at
4°C. After 24 h, the slides were washed with distilled water for 5
min and stained using 1% toluidine blue solution for 2 h at room
temperature. Subsequently, excess solution was removed and three
fields of view were observed under a light microscope
(magnification, ×200).
RSV treatment
RSV (Sigma-Aldrich; Merck KGaA) was dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) as a stock
solution. Various concentrations of RSV (0–100 µM) were then used
as a working solution in the cell culture for the indicated time
with or without IL-33 (50 ng/ml; BioLegend, Inc.)
administration.
Cell viability assay
RBL-2H3 cells (4×104) were seeded in
96-well plates and treated with RSV at the indicated concentrations
(0–100 µM) and times (0–72 h). Cell viability was assessed using an
MTT assay (Sigma-Aldrich; Merck KGaA). Briefly, MTT (5 mg/ml) was
added to the plates and incubated at 37°C for 4 h. DMSO was used to
dissolve the formazan crystals. The absorbance was measured at 590
nm using a microplate reader (Bio-Rad Laboratories, Inc.). Unless
stated otherwise, 4×105 RBL-2H3 cells were used for the
subsequent experiments.
Cytokine measurement by ELISA
Rat IL-6 (cat. no. BGK20607), IL-13 (cat. no.
BGK42203), TNF-α (cat. no. BGK16599) and MCP-1 (cat. no. 900-M59)
ELISA kits were purchased from PeproTech, Inc. Cytokine measurement
was performed according to the manufacturer's protocol.
IgE-mediated mast cell activation
RBL-2H3 cells were first sensitized to IgE (500
ng/ml) (Sigma-Aldrich; Merck KGaA) overnight at 37°C prior to
antigen (Ag) stimulation in the presence or absence of RSV (10 µM).
The cells were then stimulated with 500 ng/ml DNP-HSA
(Sigma-Aldrich; Merck KGaA) for 6 h at 37°C. Where indicated, IL-33
(50 ng/ml) was added at the same time as the Ag. IL-33 stimulation
(for 6 h at 37°C) with or without RSV treatment (24 h prior to
IL-33 application) was used as the control. Cytokine production in
the resultant supernatant was assayed using an ELISA.
Reverse transcription-quantitative PCR
(RT-qPCR)
RBL-2H3 cells were harvested and total RNA was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The RNA concentration (406 ng/µl) was calculated and 1 µg RNA was
reverse transcribed (42°C for 10 min and 80°C for 10 min) into
complementary DNA (cDNA) using a Reverse Transcription kit (Takara
Bio Inc.), according to the manufacturer's protocol. cDNA was used
for qPCR with SYBR Green Supermix kit (Bio-Rad Laboratories),
according to the manufacturer's protocol. The primers used in the
present study included: ST2 forward, 5′-CGCCTGTTCAGTGGTTTA-3′ and
reverse, 5′-TGGTTCCGTTCTCCGTGT-3′; β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTAGC-3′ and reverse,
5′-GGCCGGACTCATCGTACTCCTGCTT-3′, which were synthesized by Sangon
Biotech, Co., Ltd. The amplification conditions for the PCR
consisted of an initial incubation at 50°C for 2 min and
denaturation for 10 min at 95°C; followed by 40 cycles of 95°C for
15 sec, 55°C for 30 sec and 60°C for 1 min; final extension at 72°C
for 5 min; and storage at 4°C. All melting curve analyses were
performed between 50–95°C. mRNA expression was quantified using the
2−ΔΔCq method and normalized to the internal reference
gene β-actin (18).
Western blot analysis
RBL-2H3 cells were collected and lysed with RIPA
lysis buffer containing 1 mM inhibitor PMSF (Wuhan Boster
Biological Technology, Ltd.). The cytoplasmic and nuclear proteins
were extracted via their subcellular structure with a cytoplasm
protein and nucleoprotein extraction kit (grant no. AR0106; Wuhan
Boster Biological Technology, Ltd.), according to the
manufacturer's protocol. Protein concentration was determined using
the bicinchoninic protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Samples (20 µg per lane) were separated via
SDS-PAGE (10% gel) and transferred onto PVDF membranes (EMD
Millipore), which were then blocked with 5% non-fat powdered milk
for 2 h at room temperature. Membranes were incubated overnight at
4°C with primary rabbit anti-rat antibodies against: IκBα (cat. no.
4812; 1:1,000), phosphorylated (p)-IκBα (cat. no. 2859; 1:1,000),
NF-κB (p65; cat. no. 8242; 1:1,000), p-ERK (cat. no. 4370;
1:2,000), ERK (cat. no. 4695; 1:1,000), p-JNK (cat. no. 4668;
1:1,000), JNK (cat. no. 9252; 1:1,000), p-P38 (cat. no. 4511;
1:1,000), P38 (cat. no. 8690; 1:1,000), β-actin (cat. no. 4970;
1:1,000) and Lamin B1 (cat. no. 13435; 1:1,000; all purchased from
Cell Signaling Technology, Inc.). On the next day, the membranes
were washed with TBST and incubated with a horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody (cat. no.
7074; 1:5,000; Cell Signaling Technology, Inc.) for 2 h at room
temperature. Peroxidase-labeled protein bands were detected using
the Immobilon Western Chemiluminescent HRP substrate (EMD
Millipore) and the protein intensity was analyzed using ImageJ
software (version 1.52; National Institutes of Health) with β-actin
and Lamin B1 as the loading controls.
Signal transduction inhibitors
assessment
BAY 11-7082 (NF-κB inhibitor; 5 µM) was obtained
from Santa Cruz Biotechnology (cat. no. sc-200615). PD98059 (ERK
inhibitor; 10 µM; cat. no. 9900), SP600125 (JNK inhibitor; 10 µM;
cat. no. 8177) and SB203580 (P38 inhibitor; 10 µM; cat. no. 5633),
were purchased from Cell Signaling Technology, Inc. Signal
transduction inhibitors were added to the culture medium 2 h at
37°C prior to activation with IL-33 (50 ng/ml). After 6 h of
stimulation at 37°C, supernatants were collected for ELISA.
In vivo evaluation
Male 6-week-old Sprague-Dawley rats (200–220 g;
n=32) were purchased from the Hubei Research Center of Laboratory
Animals (no. 42000600024858) and housed in an air-conditioned room
(22±1°C; 12-h light/dark cycles) with free access to food and
water. The care and use of the animals in the present study were
approved by the Animal Care and Use Committee of Hubei University
of Chinese Medicine (no. SYXK2017-0067). Rats were treated with RSV
(5 mg/kg) or PBS (5 mg/kg) via intraperitoneal injection for 7 days
consecutively (n=8 per group). On the eighth day, IL-33 (5 µg) was
administered into rats via intraperitoneal injection to induce
inflammatory cytokine production. After 6 h, rats were euthanized
and blood samples were collected for cytokine detection via
ELISA.
Statistical analysis
Results were presented as the mean ± standard
deviation and data were analyzed using GraphPad Prism software
(version 6.0; GraphPad Software, Inc.). Comparisons among multiple
groups were performed using one-way ANOVA followed by Tukey's
post-hoc test. Comparisons between two groups were performed using
an unpaired Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
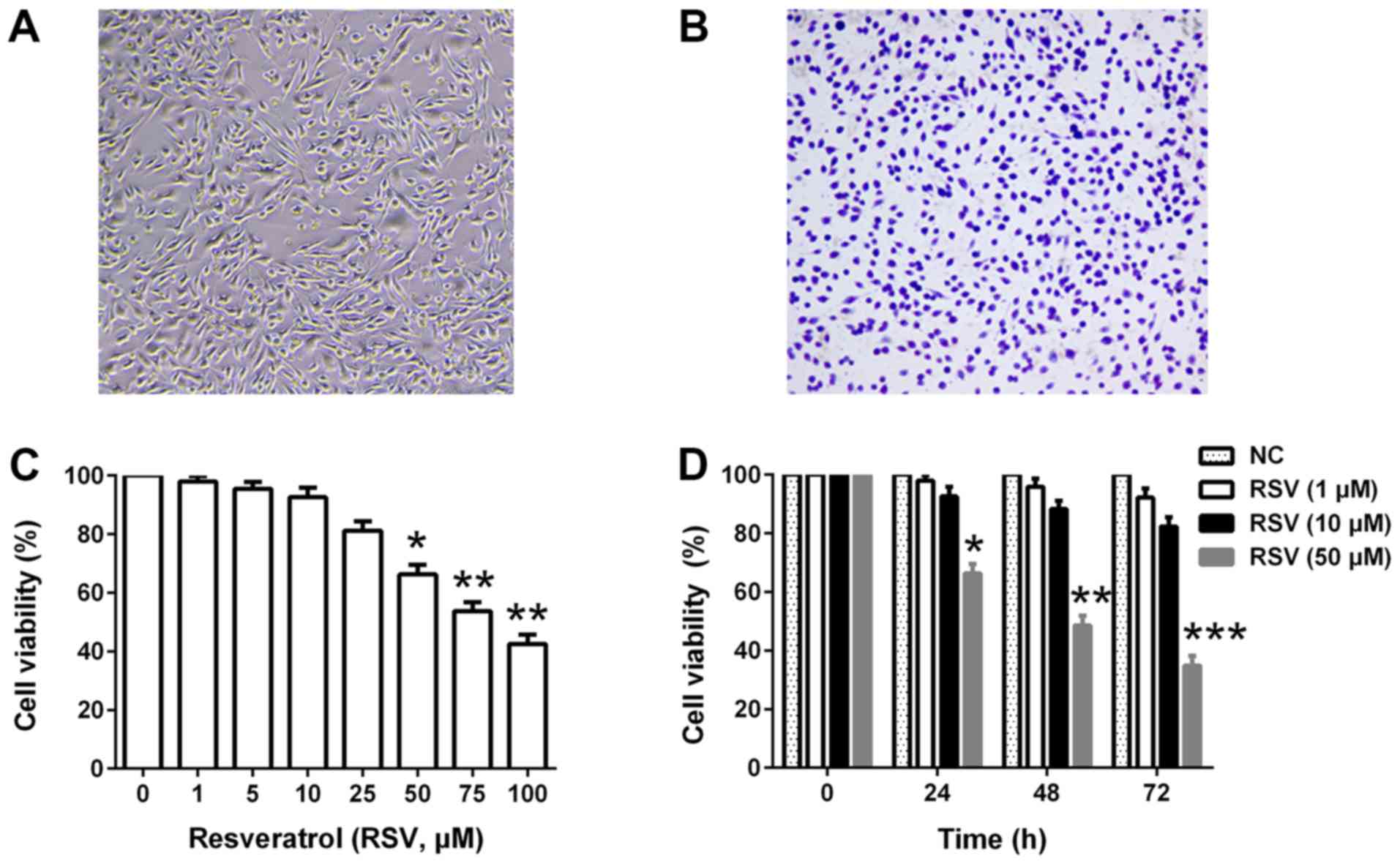
Effects of RSV on cell viability of
RBL-2H3 cells
RBL-2H3 cells were used in the present study to
mimic mast cells in vitro. Fig.
1A and B present the morphological features of RBL-2H3 cells in
the culture medium and toluidine blue staining at resting state,
respectively. First, the present study investigated the cytotoxic
effects of RSV in RBL-2H3 cells. As presented in Fig. 1A, RSV inhibited cell viability in a
dose-dependent manner (≥50 µM). Furthermore, the cells were treated
with 1, 10 or 50 µM RSV for up to 72 h and cell viability was
determined at each 24 h interval. Although a low concentration of
RSV (1 µM) had no effect on cell viability, a high concentration of
RSV (50 µM) resulted in significant cytotoxicity following the
extension of cell culture time (P<0.05; Fig. 1B). Based on these findings, 10 µM
RSV was used for further experiments.
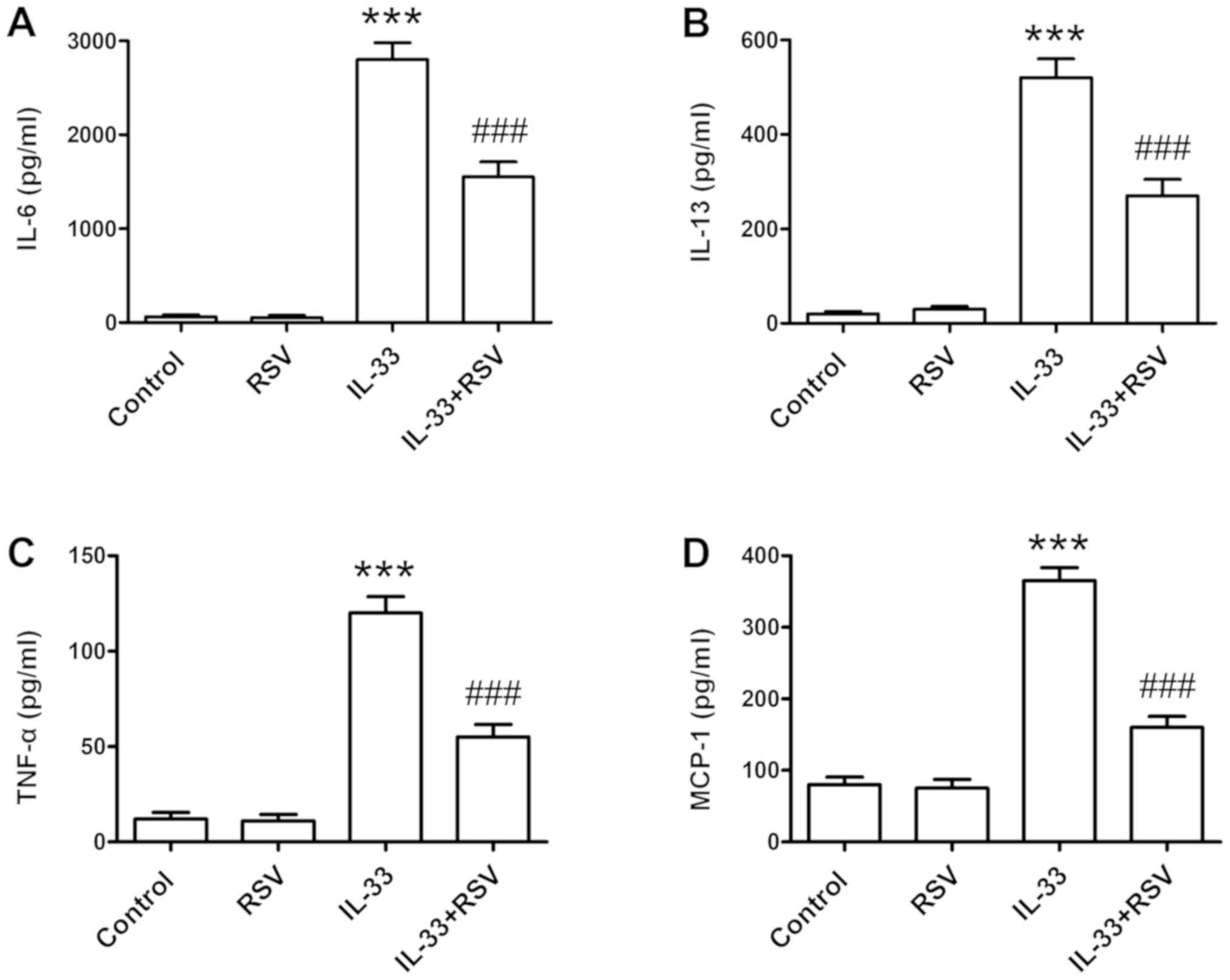
RSV attenuates IL-33-induced
inflammatory cytokine production
RBL-2H3 cells were pretreated with RSV for 24 h and
the cells were then stimulated with IL-33 (50 ng/ml) for 6 h.
Subsequently, inflammatory cytokine production, such as IL-6,
IL-13, TNF-α and MCP-1, were detected by ELISA. IL-33 stimulation
significantly increased the release of inflammatory cytokines
(P<0.001). In contrast, RSV treatment significantly attenuated
IL-33-induced inflammatory cytokine production in RBL-2H3 cells
(P<0.001; Fig. 2).
RSV restrains IL-33 and IgE-regulated
synergistic effect in RBL-2H3 cells
Due to the synergy of IL-33 combined with IgE on the
cytokine production in mast cells, the present study further
investigated the effect of RSV on IL-33-induced enhancement of
IgE-mediated responses in RBL-2H3 cells. As presented in Fig. 3, an overt additive effect was
observed in the presence of IL-33 alongside Ag stimulation,
resulting in amplification of cytokine production. RSV restrained
cytokine secretion with a similar trend under all conditions,
decreasing IL-33 and IgE-regulated synergistic responses in RBL-2H3
cells.
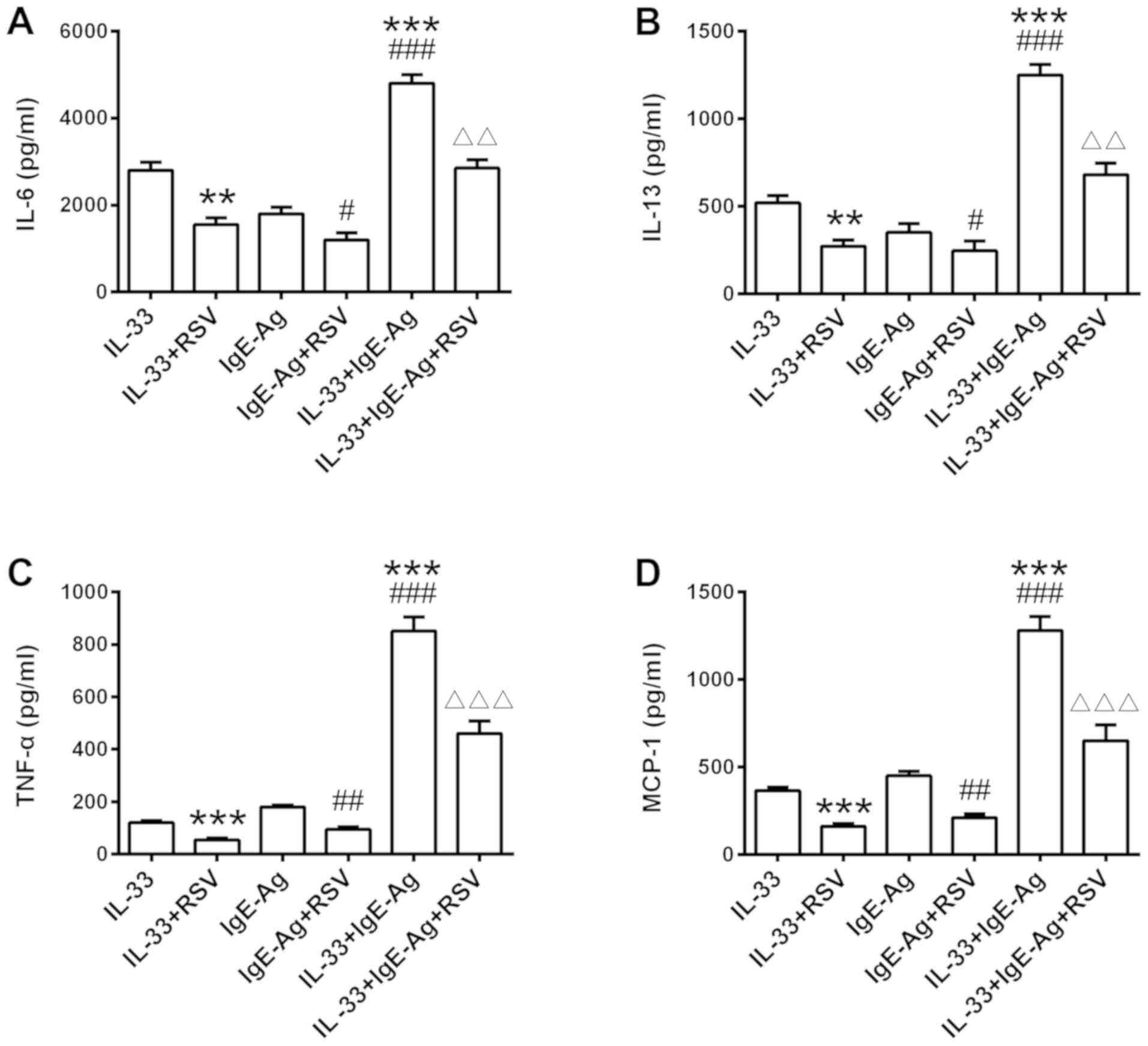 | Figure 3.Effects of RSV on IgE-induced
cytokine secretion. RBL-2H3 cells were sensitized with IgE (500
µg/ml) overnight at 37°C with RSV treatment. Cells were then
stimulated with 500 ng/ml DNP-HSA, IL-33, or both together for 6 h
at 37°C. (A) IL-6, (B) IL-13, (C) TNF- and (D) MCP-1 secretion was
measured by ELISA. **P<0.01 and ***P<0.001 vs. IL-33 only
stimulated group. #P<0.05, ##P<0.01 and
###P<0.001 vs. IgE-Ag group. ΔΔP<0.01
and ΔΔΔP<0.001 vs. IL-33 + IgE-Ag group. RSV,
resveratrol; RBL, rat basophilic leukemia; IL, interleukin; TNF-α,
tumor necrosis factor; MCP-1, monocyte chemotactic protein; Ag,
antigen; Ig, immunoglobulin. |
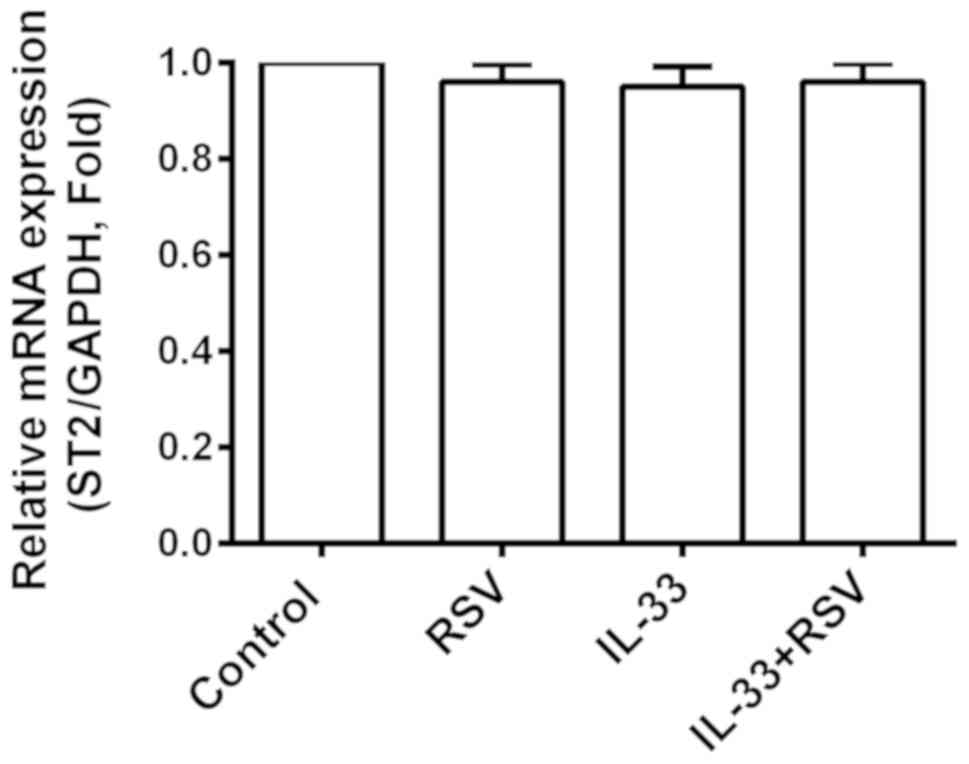
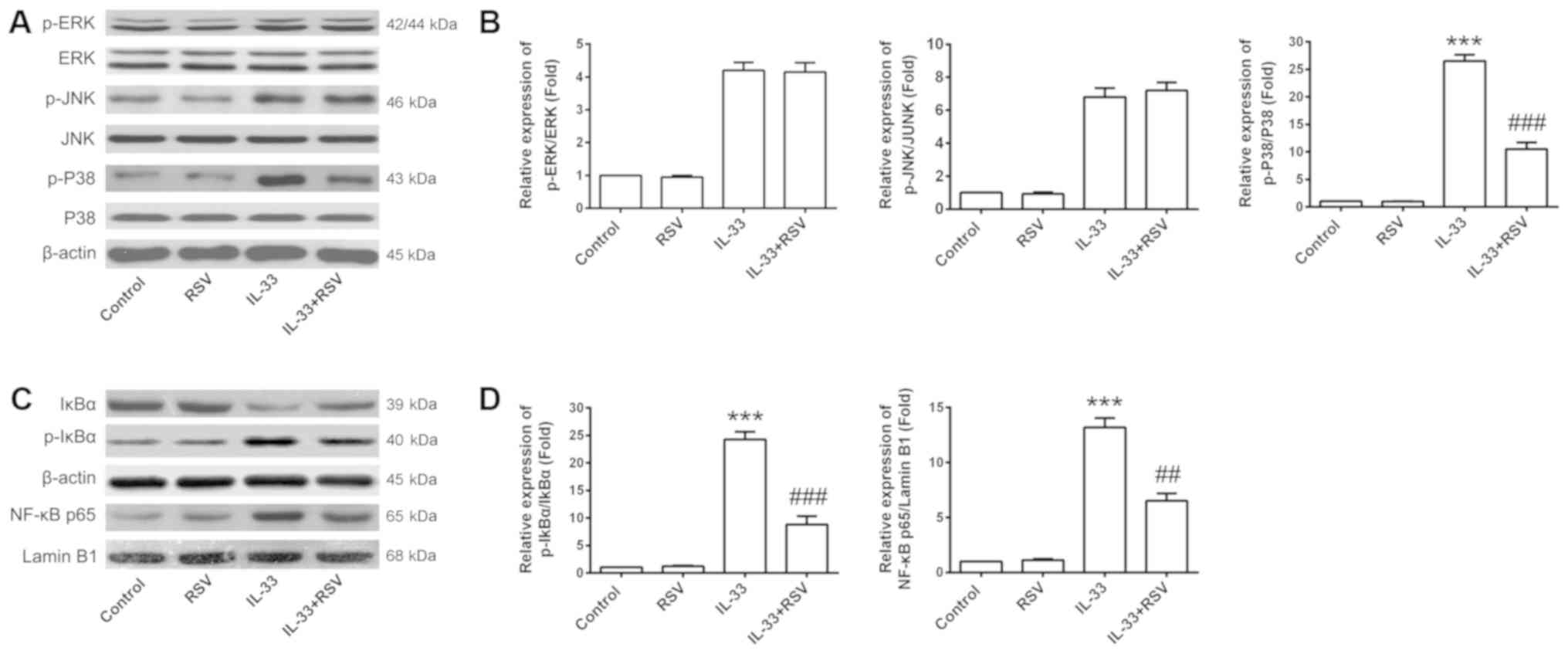
RSV inhibits IL-33-mediated P38 MAPK
signaling and NF-κB activation
In order to investigate the potential molecular
mechanisms involved in the suppressive effects of RSV on
IL-33-induced inflammation in mast cells, the present study
assessed ST2 receptor expression promoted by IL-33 activity. RSV
had no distinct effect on ST2 expression compared with the other
three groups (Fig. 4). The effects
of RSV on IL-33-mediated MAPK signaling and NF-κB activation were
then investigated. RSV significantly inhibited both NF-κB-mediated
transcription and P38 phosphorylation in response to IL-33
stimulation (P<0.001; Fig. 5).
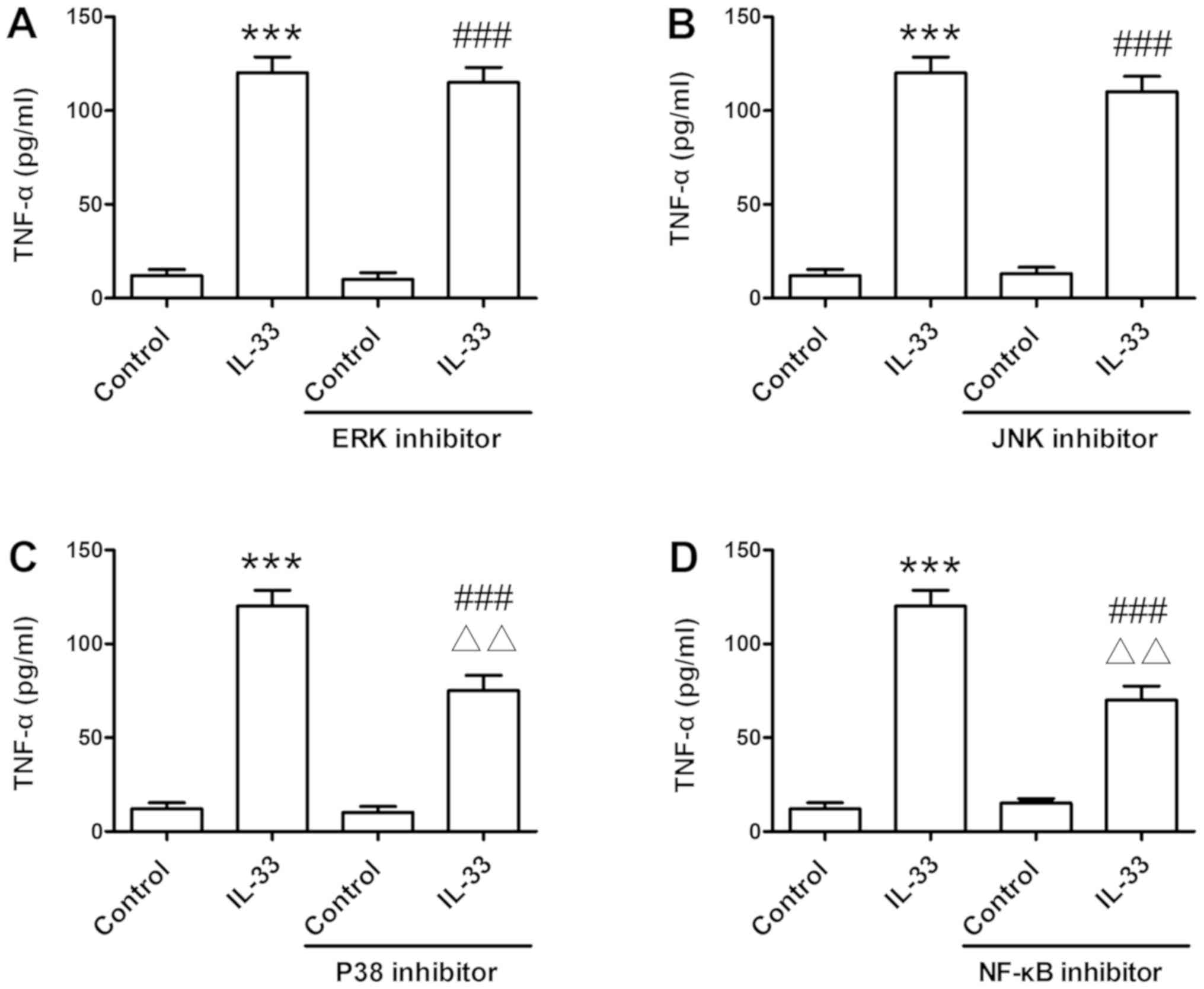
Furthermore, the application of signal transduction suppressor,
including NF-κB and P38 inhibitor (Fig. 6C and D), significantly reversed
IL-33-induced TNF-α release (P<0.01). This was not true for the
ERK and JNK inhibitors, which demonstrated no marked effect on
TNF-α production (Fig. 6A and
B).
RSV suppresses IL-33-induced
inflammation in rats
Finally, the present study assessed the effect of
RSV on IL-33-induced inflammation in vivo (n=8 rats per
group). Similar to the in vivo results, IL-33 injection
significantly increased production of circulating inflammatory
cytokines, such as IL-6, IL-13, TNF-α and MCP-1 (P<0.001;
Fig. 7). However, RSV-treated rats
exhibited lower levels of IL-6, IL-13, TNF-α and MCP-1 compared
with the rats that received PBS injection alone (Fig. 7), suggesting RSV is a potent
inhibitor of IL-33-mediated inflammation.
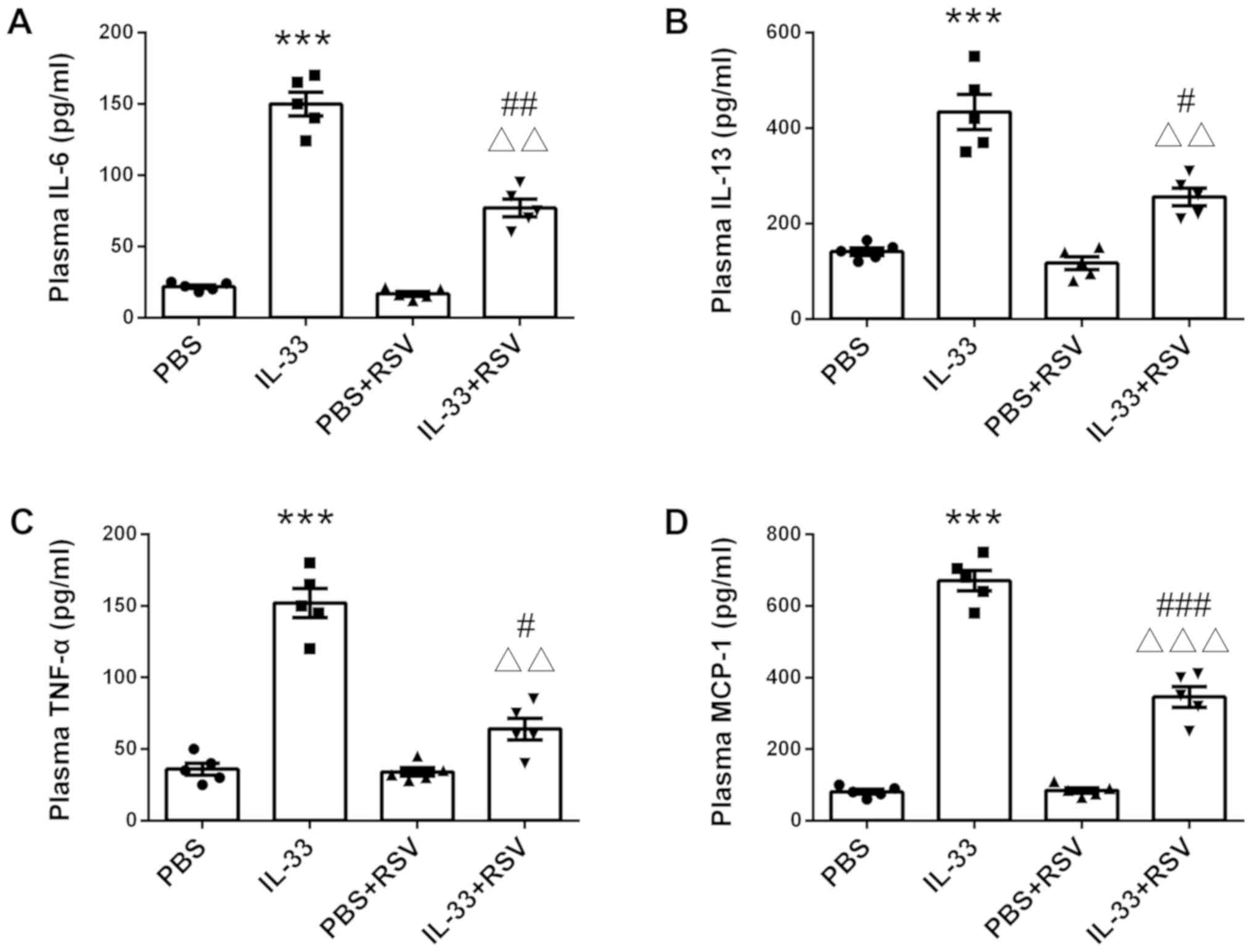 | Figure 7.Effects of RSV on IL-33-mediated
inflammation in vivo. Rats were injected intraperitoneally
with either RSV (5 mg/kg) or PBS (5 mg/kg) for 7 days
consecutively. On the eighth day, 5 µg IL-33 was administrated via
intraperitoneal injection. After 6 h, rats were euthanized and
blood samples were collected for cytokine detection via ELISA,
including (A) IL-6, (B) IL-13, (C) TNF-α and (D) MCP-1. Each of the
symbols (including dots, squares, up and down-facing triangles) in
the different groups represent a rat (n=5 per group). ***P<0.001
vs. PBS. #P<0.05, ##P<0.01 and
###P<0.001 vs. PBS + RSV group.
ΔΔP<0.01 and ΔΔΔP<0.01 vs. single
IL-33-injected group. RSV, resveratrol; RBL, rat basophilic
leukemia; IL, interleukin; TNF, tumor necrosis factor; MCP-1,
monocyte chemotactic protein. |
Discussion
To the best of our knowledge, the data obtained in
the present study demonstrates for the first time that RSV can
effectively attenuate IL-33-induced inflammatory responses in mast
cells in vitro and inflammatory cytokine production in
vivo. The potential beneficial effects of RSV offer an
alternative and promising treatment strategy for allergic
inflammation associated with mast cells.
Mast cells are attracting increasing attention due
to their roles in regulating a broad spectrum of immune responses,
particularly allergic and hypersensitivity responses (19). RBL-2H3 cells are a basophilic
leukemia cell line with high affinity IgE receptors and can be
activated to secrete histamine and other mediators. As a result of
this, RBL-2H3 cells have been used extensively to mimic the
activation and characteristics of mast cells involved in allergic
disease in vitro. Activation of mast cells results in the
release of a diverse panel of inflammatory mediators, including
histamine, leukotriene and potent inflammatory cytokines (e.g.,
IL-6 and TNF-α), depending on the type and strength of the stimuli
(20). The most characterized
pathway associated with mast cell activation is Ag-mediated
crosslinking of IgE and FcεRI (21). However, a previous study reported
that mast cell-produced IL-33 could regulate IgE-dependent
inflammation in marrow-derived mast cells via the IL-33/ST2 axis,
indicating the potency of IL-33 on IgE-mediated mast cell
activation (22). Another study
demonstrated that mast cells and the IL-33/ST2 axis were involved
in pulmonary and cardiovascular diseases, which was associated with
the decrease of neutrophil infiltration and IL-6 expression at the
mRNA level (23).
3,4-dihydroxybenzohydroxamic acid suppressed IL-33-induced cytokine
production in primary mouse mast cells, such as IL-6, IL-13, TNF
and MIP-1α (24). These studies
suggest that inflammatory cytokines induced by IL-33 participate in
the immune responses associated with mast cell activation. In line
with these findings, moderate concentrations of RSV treatment
significantly inhibited the production of inflammatory cytokines
induced by IL-33 or the synergistic effect of IL-33 and IgE in the
present study, including IL-6, IL-13, TNF-α and MCP-1. Notably, the
suppressive effects of RSV on IL-33-stimulated inflammatory
cytokine production was also confirmed in rats in the present
study.
The bioactivity of IL-33 is controlled by the
regulation of IL-33 binding to the receptor, IL-1R/TLR superfamily
member ST2. On the one hand, IL-33 combines with the ST2 receptor
and results in subsequent signaling cascades, contributing to
allergic diseases, such as asthma and atopic dermatitis (25). On the other hand, sST2 directly
binds to IL-33 and curbs the biological activation of IL-33 as a
decoy receptor produced by mast cells and Th2 cells in asthmatic
patients (26). Cytokines that are
produced due to activation of the IL-33-ST2 axis, such as IL-5,
IL-6, IL-8, IL-13, TNF-α, MCP-1, MIP-1α, chemokines and
prostaglandins, participate in inflammatory responses (27). In addition, ST2 was also important
for the development of peripheral airway hyper-responsiveness and
inflammation in a house dust mite mouse model of asthma (28). The levels of the inflammatory
cytokines IL-1β, IL-5, IL-13, IL-33, granulocyte-macrophage-colony
stimulating factor, thymic stromal lymphopoietin and mast cell
protease MCP-1 were decreased in house dust mite-treated ST2(−/-)
mice compared with wild-type controls (29). In the present study, RSV exhibited
little effect on ST2 receptor expression, although it inhibited the
synergistic effect of IL-33 and IgE in cytokine production. The
results also promoted further investigations into the molecular
mechanisms underlying IL-33-mediated signaling in mast cells
accompanying RSV administration.
As is already established, MAPK signaling pathways
are responsible for and involved in a variety of cellular
activities, such as cell proliferation, differentiation, apoptosis
and autophagy. There are three major MAPK pathways that have been
identified thus far, including ERK, JNK and P38 MAPK (30). Accumulating evidence demonstrates
the anti-inflammatory properties of RSV as an immunoregulator
(31–33). Due to its potential as a new drug
candidate for the treatment of inflammation, the anti-inflammatory
properties of RSV have always been of interest. In LPS-treated
RAW264.7 murine macrophages, RSV inhibited the activation of MAPK
and NF-κB signaling, decreasing proinflammatory cytokine levels,
such as TNF-α, IL-6 and IL-1β (34). Using an LPS-mediated acute
inflammation rat model, RSV treatment decreased the production of
IL-1β, TNF-α, IL-6 and cyclooxygenase-2, which is associated with
inhibition of the TLR4/NF-κB p65/MAPKs signaling cascade (35). These results indicate that MAPK and
NF-κB signaling is involved in the biological effects of RSV. It is
well known that NF-κB is a protein complex that plays a central
role in the transcription of DNA, cytokine production and cell
survival. In the classical pathway, following the degradation of
IκB, the NF-κB complex is then free to enter the nucleus and induce
target gene expression (36). In
the present study, RSV restrained IL-33-mediated P38 MAPK signaling
and NF-κB activation, at least in part, contributing to the
suppression of inflammatory responses in mast cells. Other
molecular mechanisms have also been proposed that attempt to
explain the inhibitory effects of RSV on inflammatory and allergic
diseases, including the activation of AMP-activated protein kinase,
FcεRI-MAPK or JAK1-signal transducer and activator of transcription
3 signaling, as well as the inhibition of phosphoinositide
dependent kinase 1 kinase, phosphoinositide 3 kinase-protein kinase
B or TGF-β1/mothers against decapentaplegic homolog 9 pathways
(37–41). As the present study did not assess
the effects of RSV on all the key proteins involved in
intracellular signaling pathways, additional studies are required
in order to elucidate the details.
In conclusion, the results from the present study
demonstrate that RSV administration both in vitro and in
vivo effectively attenuated IL-33-induced inflammatory cytokine
production associated with mast cell inflammation, which were
mediated by the inhibition of NF-κB activation and P38 signaling.
These results at least partially provide a theoretical basis for
RSV as a potential therapeutic agent against the development of
allergic diseases.
Acknowledgements
The authors would like to thank Dr Zhigang Wang
(School of Basic Medical Sciences, Hubei University of Chinese
Medicine, Wuhan, China) for assisting in the editing of the
manuscript before submission.
Funding
The present study was supported by the Science and
Technology Project of Hubei Provincial Department of Education
(grant no. 2017ZTZ033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ conceived the study. YDX and GZ designed the
study. QL, YDX and XHG performed the experiments (including cell
culture, ELISA analysis and western blotting), analyzed the data
and wrote the manuscript. LX contributed to the animal breeding and
treatment. XHG and GZ revised the manuscript.
Ethics approval and consent to
participate
Animal care and use were performed according to the
guidelines of the Animal Care and Use Committee of Hubei University
of Chinese Medicine (No. SYXK2012-0067).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh VK, Mehrotra S and Agarwal SS: The
paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity
and allergy. Immunol Res. 20:147–161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liew FY, Pitman NI and McInnes IB:
Disease-associated functions of IL-33: The new kid in the IL-1
family. Nat Rev Immunol. 10:103–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Préfontaine D, Lajoie-Kadoch S, Foley S,
Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG and
Hamid Q: Increased expression of IL-33 in severe asthma: Evidence
of expression by airway smooth muscle cells. J Immunol.
183:5094–5103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayakawa H, Hayakawa M, Kume A and
Tominaga S: Soluble ST2 blocks interleukin-33 signaling in allergic
airway inflammation. J Biol Chem. 282:26369–26380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Salvo C, Wang XM, Pastorelli L,
Mattioli B, Omenetti S, Buela KA, Chowdhry S, Garg RR, Goodman WA,
Rodriguez-Palacios A, et al: IL-33 drives eosinophil infiltration
and pathogenic type 2 helper T-cell immune responses leading to
chronic experimental ileitis. Am J Pathol. 186:885–898. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imaeda H, Andoh A, Aomatsu T, Uchiyama K,
Bamba S, Tsujikawa T, Naito Y and Fujiyama Y: Interleukin-33
suppresses Notch ligand expression and prevents goblet cell
depletion in dextran sulfate sodium-induced colitis. Int J Mol Med.
28:573–578. 2011.PubMed/NCBI
|
|
7
|
Russi AE, Ebel ME, Yang Y and Brown MA:
Male-specific IL-33 expression regulates sex-dimorphic EAE
susceptibility. Proc Natl Acad Sci USA. 115:E1520–E1529. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palmer G and Gabay C: Interleukin-33
biology with potential insights into human diseases. Nat Rev
Rheumatol. 7:321–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balato A, Lembo S, Mattii M, Schiattarella
M, Marino R, De Paulis A, Balato N and Ayala F: IL-33 is secreted
by psoriatic keratinocytes and induces pro-inflammatory cytokines
via keratinocyte and mast cell activation. Exp Dermatol.
21:892–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Yin N, Tao W, Wang Q, Fan H and Wang
Z: Berberine suppresses IL-33-induced inflammatory responses in
mast cells by inactivating NF-κB and p38 signaling. Int
Immunopharmacol. 66:82–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Silver MR, Margulis A, Wood N, Goldman SJ,
Kasaian M and Chaudhary D: IL-33 synergizes with IgE-dependent and
IgE-independent agents to promote mast cell and basophil
activation. Inflamm Res. 59:207–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cayrol C and Girard JP: Interleukin-33
(IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev.
281:154–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takatori H, Makita S, Ito T, Matsuki A and
Nakajima H: Regulatory mechanisms of IL-33-ST2-mediated allergic
inflammation. Front Immunol. 9:20042018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Wang L, Szklarz G, Bi Y and Ma Q:
Resveratrol inhibits paraquat-induced oxidative stress and
fibrogenic response by activating the nuclear factor erythroid
2-related factor 2 pathway. J Pharmacol Exp Ther. 342:81–90. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baolin L, Inami Y, Tanaka H, Inagaki N,
Iinuma M and Nagai H: Resveratrol inhibits the release of mediators
from bone marrow-derived mouse mast cells in vitro. Planta Med.
70:305–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han SY, Bae JY, Park SH, Kim YH, Park JH
and Kang YH: Resveratrol inhibits IgE-mediated basophilic mast cell
degranulation and passive cutaneous anaphylaxis in mice. J Nutr.
143:632–639. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan J, Xu T, Xu F, Zhang Y, Liu Z, Chen W,
Fu W, Dai Y, Zhao Y, Feng J and Liang G: Development of
resveratrol-curcumin hybrids as potential therapeutic agents for
inflammatory lung diseases. Eur J Med Chem. 125:478–491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thangam EB, Jemima EA, Singh H, Baig MS,
Khan M, Mathias CB, Church MK and Saluja R: The role of histamine
and histamine receptors in mast cell-mediated allergy and
inflammation: The hunt for new therapeutic targets. Front Immunol.
9:18732018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sibilano R, Frossi B and Pucillo CE: Mast
cell activation: A complex interplay of positive and negative
signaling pathways. Eur J Immunol. 44:2558–2566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitaura J, Song J, Tsai M, Asai K,
Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas
BG, et al: Evidence that IgE molecules mediate a spectrum of
effects on mast cell survival and activation via aggregation of the
FcepsilonRI. Proc Natl Acad Sci USA. 100:12911–12916. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu CL, Neilsen CV and Bryce PJ: IL-33 is
produced by mast cells and regulates IgE-dependent inflammation.
PLoS One. 5:e119442010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katwa P, Wang X, Urankar RN, Podila R,
Hilderbrand SC, Fick RB, Rao AM, Ke PC, Wingard CJ and Brown JM: A
carbon nanotube toxicity paradigm driven by mast cells and the
IL-33/ST2 axis. Small. 8:2904–2912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caslin HL, McLeod JJA, Spence AJ, Qayum
AA, Kolawole EM, Taruselli MT, Paranjape A, Elford HL and Ryan JJ:
Didox (3,4-dihydroxybenzohydroxamic acid) suppresses IL-33-induced
cytokine production in primary mouse mast cells. Cell Immunol.
319:10–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Milovanovic M, Volarevic V, Radosavljevic
G, Jovanovic I, Pejnovic N, Arsenijevic N and Lukic ML: IL-33/ST2
axis in inflammation and immunopathology. Immunol Res. 52:89–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bandara G, Beaven MA, Olivera A, Gilfillan
AM and Metcalfe DD: Activated mast cells synthesize and release
soluble ST2-a decoy receptor for IL-33. Eur J Immunol.
45:3034–3044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Velez TE, Bryce PJ and Hulse KE: Mast cell
interactions and crosstalk in regulating allergic inflammation.
Curr Allergy Asthma Rep. 18:302018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zoltowska AM, Lei Y, Fuchs B, Rask C,
Adner M and Nilsson GP: The interleukin-33 receptor ST2 is
important for the development of peripheral airway
hyperresponsiveness and inflammation in a house dust mite mouse
model of asthma. Clin Exp Allergy. 46:479–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saglani S, Lui S, Ullmann N, Campbell GA,
Sherburn RT, Mathie SA, Denney L, Bossley CJ, Oates T, Walker SA,
et al: IL-33 promotes airway remodeling in pediatric patients with
severe steroid-resistant asthma. J Allergy Clin Immunol.
132:676–685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee M, Kim S, Kwon OK, Oh SR, Lee HK and
Ahn K: Anti-inflammatory and anti-asthmatic effects of resveratrol,
a polyphenolic stilbene, in a mouse model of allergic asthma. Int
Immunopharmacol. 9:418–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmad SF, Ansari MA, Nadeem A, Bakheet SA,
Alzahrani MZ, Alshammari MA, Alanazi WA, Alasmari AF and Attia SM:
Resveratrol attenuates pro-inflammatory cytokines and activation of
JAK1-STAT3 in BTBR T+ Itpr3tf/J autistic mice. Eur J Pharmacol.
829:70–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou ZX, Mou SF, Chen XQ, Gong LL and Ge
WS: Anti-inflammatory activity of resveratrol prevents inflammation
by inhibiting NF-κB in animal models of acute pharyngitis. Mol Med
Rep. 17:1269–1274. 2018.PubMed/NCBI
|
|
34
|
Byun EB, Sung NY, Park JN, Yang MS, Park
SH and Byun EH: Gamma-irradiated resveratrol negatively regulates
LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages.
Int Immunopharmacol. 25:249–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang G, Hu Z, Fu Q, Song X, Cui Q, Jia R,
Zou Y, He C, Li L and Yin Z: Resveratrol mitigates
lipopolysaccharide-mediated acute inflammation in rats by
inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade. Sci Rep.
7:450062017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kauppinen A, Suuronen T, Ojala J,
Kaarniranta K and Salminen A: Antagonistic crosstalk between NF-κB
and SIRT1 in the regulation of inflammation and metabolic
disorders. Cell Signal. 25:1939–1948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Z, Jiang C, Zhang J, Liu B and Du Q:
Resveratrol inhibits inflammation and ameliorates insulin resistant
endothelial dysfunction via regulation of AMP-activated protein
kinase and sirtuin 1 activities. J Diabetes. 8:324–335. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han SY, Choi YJ, Kang MK, Park JH and Kang
YH: Resveratrol suppresses cytokine production linked to FcεRI-MAPK
activation in IgE-antigen complex-exposed basophilic mast cells and
mice. Am J Chin Med. 43:1605–1623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hossen MJ, Cho JY and Kim D: PDK1 in NF-κB
signaling is a target of Xanthium strumarium methanolic
extract-mediated anti-inflammatory activities. J Ethnopharmacol.
190:251–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aich J, Mabalirajan U, Ahmad T, Khanna K,
Rehman R, Agrawal A and Ghosh B: Resveratrol attenuates
experimental allergic asthma in mice by restoring inositol
polyphosphate 4 phosphatase (INPP4A). Int Immunopharmacol.
14:438–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee HY, Kim IK, Yoon HK, Kwon SS, Rhee CK
and Lee SY: Inhibitory effects of resveratrol on airway remodeling
by transforming growth factor-β/Smad signaling pathway in chronic
asthma model. Allergy Asthma Immunol Res. 9:25–34. 2017. View Article : Google Scholar : PubMed/NCBI
|