Introduction
Osteonecrosis of the femoral head (ONFH) is a
debilitating and progressive disease which can lead to femoral head
collapse and subsequent osteoarthritis. This disease is the major
cause of total hip arthroplasty in young adults (1). It is reported that more than 1
million new patients are affected with this disease annually and
the annual incidence is 15 to 20 million according to a nationwide
survey (2). Accumulating evidence
has alluded to several etiologic and pathogenic mechanisms for ONFH
(3). The principal mechanism
involves interruption of osteogenic differentiation of bone
marrow-derived mesenchymal stem cells (BMSCs) (4). Thus, investigation of the disordered
mechanism of osteogenic differentiation in BMSCs and development of
effective methods of prevention and early therapy are crucial.
A number of previous studies have reported that
osteogenic differentiation-related genes are expressed periodically
(5,6). Brain and muscle ARNT-like 1 (BMAL1)
is the most important component of the molecular biological clock,
expression of which has been found to have 24-h periodicity in
bone. The circadian system rhythmically regulates cellular
proliferation, physiology, and behavior (7,8).
Moreover, it is implicated in drug efficacy, metabolism,
detoxification and toxicity (9–11).
The core transcriptional activator of the clock network is BMAL1
which is regulated by delicate systems in mammals (7). Increasing numbers of studies have
demonstrated that BMAL1 and other circadian clock genes could be
regulated by drugs and toxicants. Miranda et al (12) showed that resveratrol could reverse
circadian disruption induced by a high-fat diet in rats. Chen et
al (13) demonstrated that
carbon tetrachloride altered circadian rhythms of liver clock genes
in a mouse model of hepatic fibrosis, while Gabás-Rivera et
al (14) reported that dietary
oleanolic acid supplementation mediated circadian clock gene
expression in an animal model.
Icariin is extracted from Herba Epimedii which is
widely used as a major active ingredient to prevent osteonecrosis
induced by glucocorticoids in patients with severe acute
respiratory syndrome. Our previous study revealed that icariin
regulated cell proliferation and osteogenic differentiation in
MC3T3-E1 cells (15) and acted as
an effective ingredient for preventing the progression of ONFH
induced by glucocorticoids in a rat model (16). However, the mechanism underlying
the association between icariin and BMAL1 in osteogenic
differentiation of BMSCs remains unclear.
In the present study, it was demonstrated that
icariin could alter BMAL1 and induce osteogenic differentiation of
BMSCs in vivo. Furthermore, it was also shown that icariin
enhanced the bone morphogenetic protein 2 (BMP2)/RUNX family
transcription factor 2 (RUNX2) signaling pathway through
upregulation of BMAL1 expression.
Materials and methods
Cell culture and treatment
According to the method described by Gong et
al (17), a total of 20
3-week-old female Sprague-Dawley rats (Laboratory Animal Center of
Huazhong University of Science and Technology) were sacrificed by
the intraperitoneal injection of 100 mg/kg sodium pentobarbital and
BMSCs were extracted from femurs and tibias by aseptic
manipulation. Cells were expanded in minimum essential medium
(α-MEM; Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin G-streptomycin and cultured in 5% CO2 at 37°C. BMSCs
at a density of 80–90% were passaged and cells at passage three to
six were used in the following experiments. For osteogenic
induction, basic medium was supplemented with 10−7 mol/l
dexamethasone, 10−2 mol/l sodium β-glycerophosphate and
50 µg/ml L-ascorbic acid. The medium was changed every 3 days for
BMSC differentiation. Each experiment was performed in triplicate.
All the experimental protocols involving animals were approved by
the Institutional Animal Care and Use Committee of Tongji Medical
College, Huazhong University of Science and Technology (IACUU no.
S496; date of permission, October, 31, 2017).
Icariin (purity >98%; Abcam) was dissolved in
dimethyl sulfoxide (DMSO) and then stored at −20°C without light.
Subsequent experiments used a DMSO concentration of ≤0.1%. All
experiments were performed in triplicate.
Cell Counting Kit-8 (CCK-8) assay
The effect of icariin on cell proliferation was
evaluated using the CCK-8 assay. Briefly, BMSCs (5×103
cells/well) were plated in 96-well plates and treated with icariin
(10−10−10−3 mol/l) for 48 h at 37°C.
Subsequently, cell proliferation was assessed using the CCK-8
reagent (Nanjing Jiancheng Bioengineering Institute), according to
the manufacturer's instructions, at a wavelength of 450 nm using an
ELx800 multifunctional microplate reader (BioTek Instruments,
Inc.). The concentration of icariin used to treat the cells that
were analyzed by RT-qPCR and western blotting was based on the
results of the CCK-8 assay.
Flow cytometry
After reaching confluence, BMSCs at passage 3–4 were
used for flow cytometric analysis. First, the cells were incubated
with trypsin for 1 min at 37°C, and then the cell pellet was
collected by transient centrifugation at 400 × g at room
temperature for 5 min. Next, the cell pellet was washed with
ice-cold PBS and then centrifuged at 400 × g at room temperature
for 5 min. Subsequently, the cell pellet was resuspended in 100 µl
ice-cold PBS supplemented with 0.5% BSA (Invitrogen; Thermo Fisher
Scientific, Inc.) for 20 min at 4°C. Additionally, the cells were
treated with Rat Fc block (cat. no. 550271; BioLegend, Inc.) for 20
min at 4°C. Finally, the cells were identified by negative
expression of CD34 [phycoerythrin (PE)-labeled; cat. no. 128611;
BioLegend, Inc.] and CD45 (FITC-labeled; cat. no. 202205;
BioLegend, Inc.) and positive expression of CD73 (FITC-labeled;
cat. no. 127219; BioLegend, Inc.), CD90 (PE-labeled; cat. no.
205903; BioLegend, Inc.) and CD105 (PE-labeled; cat. no. 120407;
BioLegend, Inc.) using a FACSscan flow cytometer (Beckton
Dickinson) and analyzed by FlowJo software (version X; FlowJo
LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) from BMSCs and the reverse
transcription RT reaction (starting with 1 µg of total RNA) was
performed using the EasyScript One-Step gDNA Removal and cDNA
Synthesis Supermix (Beijing TransGen Biotech Co., Ltd.) according
to the manufacturer's instructions. The SYBR Green/ROX RT-qPCR mix
(Takara Bio, Inc.) and the StepOnePlus™ Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) were used for
mRNA quantification, according to the manufacturer's protocol. The
primer sequences used for PCR were as follows: BMAL1 forward,
5′-AACCTTCCCGCAGCTAACAG-3′ and reverse, 5′-AGTCCTCTTTGGGCCACCTT-3′;
BMAL1−/−, forward 5′-CCACCAAGCCCAGCAACTCA-3′ and
reverse, 5′-TCGCCTTCTATGGCCTTCTTGACG-3′; BMAL1 (overexpression)
forward,
5′-AGGTCGACTCTAGAGGATCCCGCCACCATGGCGGACCAGAGAATGGACATTTC-3′ and
reverse, 5′-TCCTTGTAGTCCATACCCAGCGGCCATGGCAAGTCACTAAAG-3′; BMP2
forward, 5′-AGGATGCTGGGAAGTCCG-3′ and reverse,
5′-AGGTGCCACGATCCAGTCAT-3′; RUNX2, forward,
5′-AGCGGACGAGGCAAGAGTTT-3′ and reverse,
5′-AGGCGGGACACCTACTCTCATA-3′; alkaline phosphatase (ALP) forward,
5′-CAAGGATGCTGGGAAGTCCG-3′ and reverse, 5′-CTCTGGGCGCATCTCATTGT-3′;
osteocalcin (OC) forward, 5′-TCAACAATGGACTTGGAGCCC-3′ and reverse,
5′-AGCTCGTCACAATTGGGGTT-3′; and β-actin forward,
5′-GAGACCTTCAACACCCCAGC-3′ and reverse, 5′-ATGTCACGCACGATTTCCC-3′.
The reaction conditions were as follows: 95°C for 15 sec, 60°C for
20 sec and 75°C for 10 sec after denaturing DNA templates at 95°C
for 10 min; 40 cycles. The 2−ΔΔCq method (18) was used to determine the relative
expression levels.
Western blotting
Total protein was extracted with RIPA buffer
(Sigma-Aldrich; Thermo Fisher Scientific, Inc.) according to
standard protocols. Cell lysates with 50 µg of total protein
(quantified using the bicinchoninic acid assay) were separated by
10% SDS-PAGE, transferred onto PVDF membranes, and then blocked in
TBST with 5% non-fat dried milk for 1 h at room temperature. The
membranes were incubated with primary monoclonal mouse antibodies
targeted against: BMAL1 (1:1,000; cat. no. ab93806; Abcam), BMP2
(1:1,000; cat. no. ab14933; Abcam), RUNX2 (1:1,000; cat. no.
ab23981; Abcam), ALP (1:1,000; cat. no. ab95462; Abcam), OC
(1:1,000; cat. no. ab34710; Abcam) and β-actin (1:1,000; cat. no.
ab8227; Abcam) at 4°C overnight, and then followed by secondary
antibodies conjugated with horseradish peroxidase (1:10,000; cat.
no. ASP00001; Dako; Agilent Technologies, Inc.) for 1 h at room
temperature after washing. The immunoblots were visualized and
detected using the ECL system (Pierce; Thermo Fisher Scientific,
Inc.). Protein expression was quantified using Fusion Solo software
(version 4; Vilber Lourmat Deutschland GmbH).
Transient transfection and luciferase
assay
BMAL1 short hairpin RNA (shRNA) interference
lentiviral vector was constructed and synthesized by Shanghai
Genechem Co., Ltd. The BMAL1 shRNA interference target sequence was
5′-ACACGCAATAGATGGGAAA-3′, and a scramble sequence
5′-TTCAAGATCCTCAATTATA-3′ was used as a negative control. BMSCs
were cultured in 6-well plates with specific media to appropriate
confluence and then transfected with the virus assisted with 6
µg/ml polybrene for 12 h at 37°C. The viral supernatants were
removed and the culture was continued in complete medium. BMSCs
were grown to 30–70% confluence and then transfected with BMAL1,
BMAL1-shRNAs or empty vector overnight. The following day, the
cells were co-transfected with pRL-SV40 Renilla luciferase
reporter plasmids (10 ng; Promega Corporation) and firefly
luciferase reporter vectors (50 ng), using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as the delivery system and a control. Cells were
lysed after a 48-h transfection and firefly and Renilla
luciferase activities were measured using the Dual Luciferase
Reporter Assay system (Promega Corporation).
ALP and Alizarin red S (ARS)
staining
BMSCs were seeded at a density of 1×105
cells/well in 6-well plates and incubated with osteogenic medium.
According to the manufacturer's instructions, ALP staining was
assessed using an ALP staining kit (Nanjing Jiancheng
Bioengineering Institute) on day 7.
After osteogenic induction for 14 days, the cells
were washed three times with PBS and fixed in 4% paraformaldehyde
for 30 min at room temperature. Then paraformaldehyde was removed
and the cells were washed three times with ddH2O.
Finally, the cells were stained with 0.04 M ARS for 30 min at room
temperature, and then rinsed twice with ddH2O and
visualized under a light microscope (magnification, ×40). For
quantitative analysis of ARS staining, the absorbance at 540 nm was
measured using a UV spectrophotometer. Results of ARS are expressed
as µg/mg protein with average and standard deviation.
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed using SPSS software (version 17.0; SPSS,
Inc.). Differences between groups were analyzed using an unpaired
Student's t-test or one-way ANOVA followed by Dunnett's post hoc
test. P<0.05 was considered to indicate statistical
significance.
Results
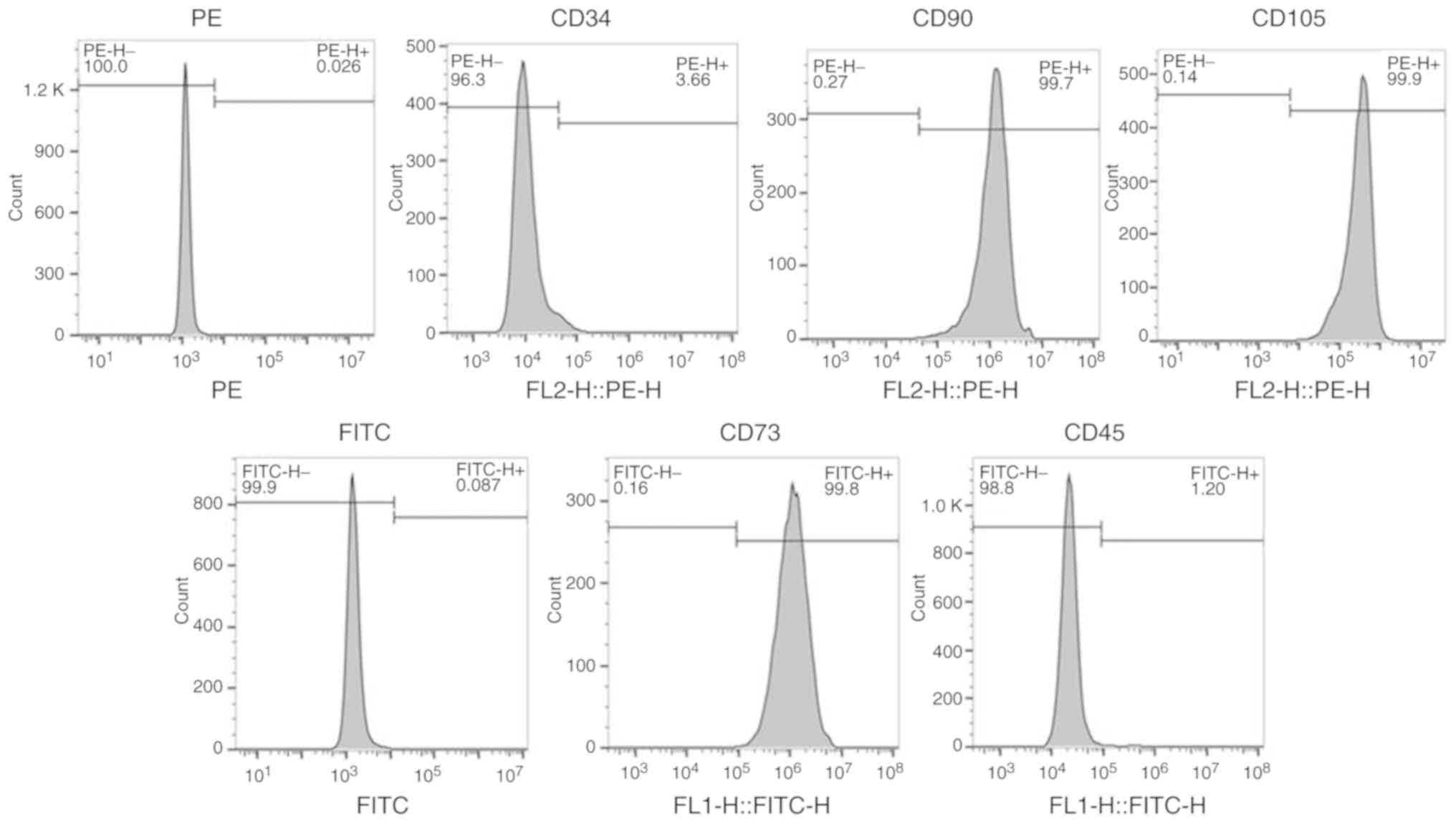
Identification of BMSCs using stem
cell markers
Flow cytometry was performed to identify BMSCs by
expression of CD34, CD45, CD73, CD90 and CD105 in the isolated
cells. The percentages of CD73-positive cells, CD90-positive cells
and CD105-positive cells were 99.8, 99.7 and 99.9%, respectively;
while the percentages of CD34-positive cells and CD45-positive
cells were 3.66 and 1.2%, respectively, indicating that the
isolated cells were BMSCs (Fig.
1).
Icariin induces expression of
osteoblastic genes in BMSCs
The proliferative activity of BMSCs in the presence
of different concentrations of icariin
(10−10−10−3 M) was determined using the CCK-8
assay (Fig. 2A). The results
showed that cell proliferation was significantly increased by lower
concentrations of icariin (10−9−10−5 M;
P<0.05), with 10−7 M being the most effective.
RT-qPCR and western blotting demonstrated that
icariin significantly increased the expression of BMP2, RUNX2, ALP
and OC (Fig. 2B and C).
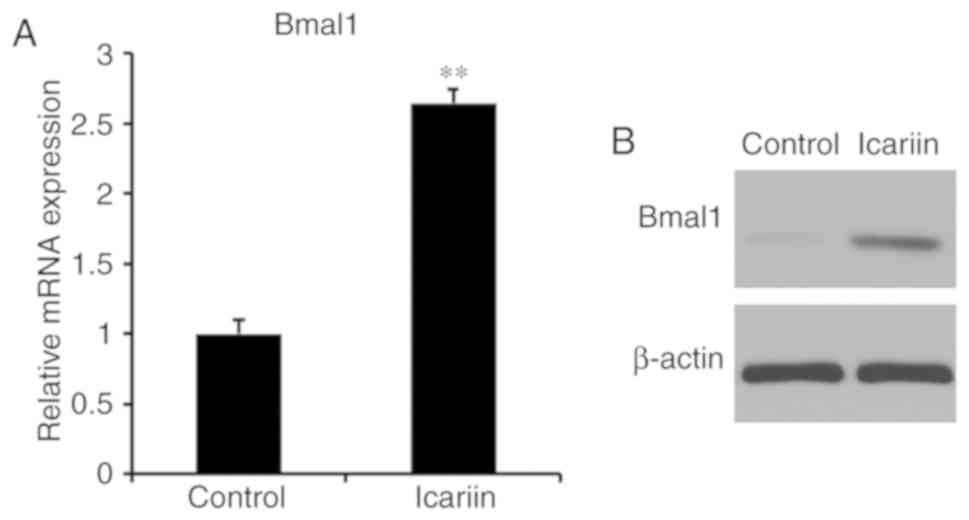
Icariin regulates BMAL1 in BMSCs
To investigate whether icariin induces BMAL1
expression in BMSCs, BMAL1 expression of 10−7 M
icariin-treated cells was evaluated by RT-qPCR and western
blotting. Icariin significantly increased BMAL1 mRNA and protein
levels (Fig. 3). These results
suggested that icariin induced BMAL1 expression in BMSCs.
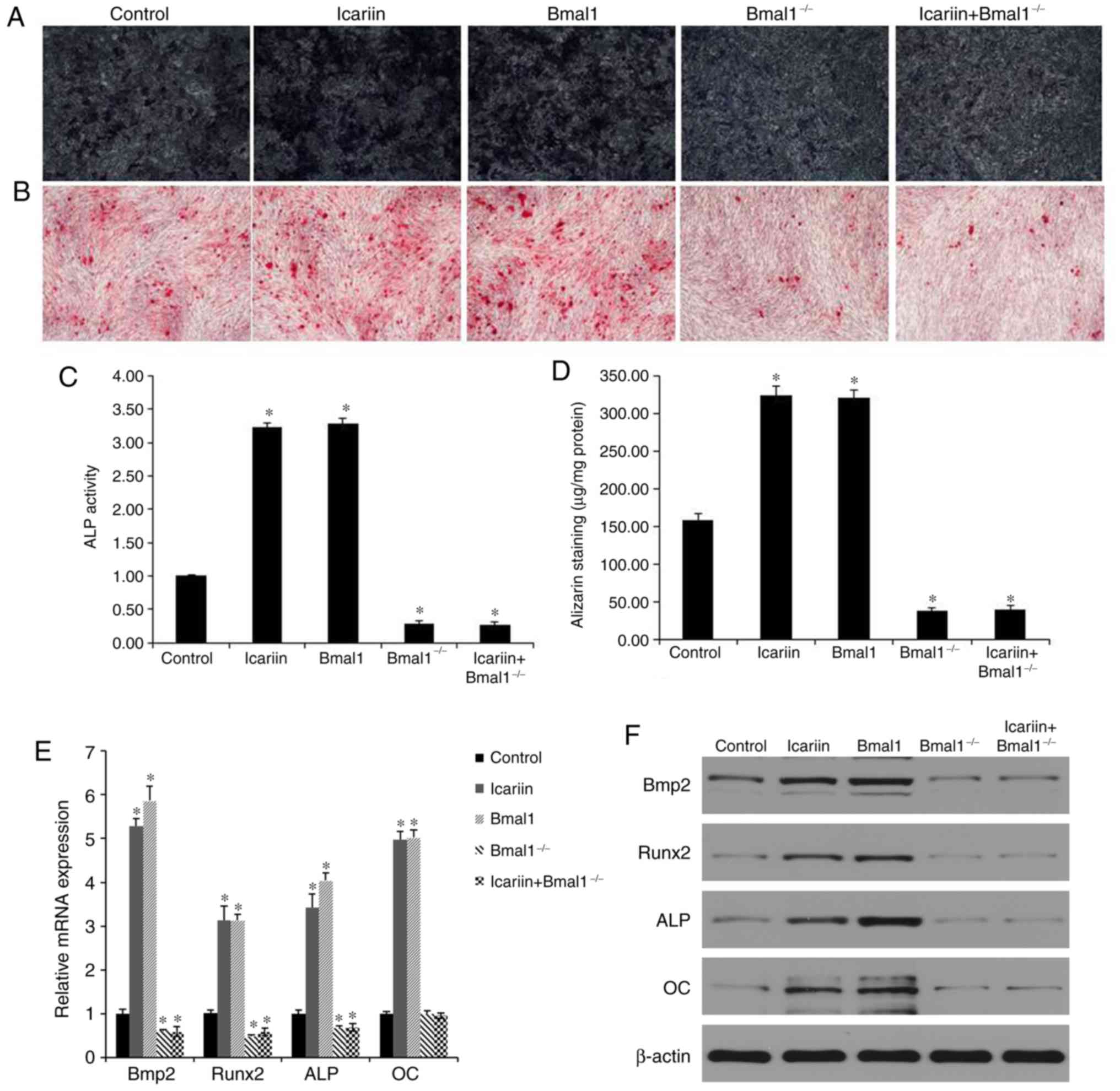
BMAL1 induces osteogenic
differentiation via BMP2 in BMSCs
To evaluate whether BMAL1 regulates osteogenic
differentiation via BMP2, BMSCs were transduced with BMAL1. BMAL1
overexpression increased expression of osteogenic
differentiation-associated genes. Moreover, BMAL1 overexpression
enhanced both mRNA expression and the promoter activity of BMP2
(Fig. 4E and F).
BMAL1 deficiency suppresses osteogenic
differentiation in BMSCs
In order to determine whether BMAL1 is essential for
osteogenic differentiation induced by icariin, the osteogenic
differentiation-inducing effects of 10−7 M icariin
treatment were further investigated in BMAL1-knockdown
(BMAL1−/−) BMSCs. Compared with the effects of icariin
in wild-type (WT) BMSCs, expression of osteogenic genes was reduced
in the BMAL1−/− BMSCs evaluated by PCR analysis
(Fig. 4E). In accordance with the
PCR results, western blotting also showed that levels of BMP2,
RUNX2, ALP and OC were lower in the BMAL1−/− BMSCs
compared to the WT BMSCs (Fig.
4F). Furthermore, ARS of BMAL1−/− BMSCs indicated
that BMAL1 was required for mineralized nodule formation during
osteogenic differentiation (Fig. 4B
and D). In addition, less positive staining was detected in
BMAL1−/− BMSCs (Fig. 4A and
C).
Taken together, icariin increased the BMAL1, BMP2
and RUNX2 expression in BMSCs. BMAL1 overexpression also
upregulated BMP2 and RUNX2 expression compared with the control
group. However, these levels of expression were significantly
downregulated after BMAL1 knockdown whether icariin treatment was
administered or not. The results suggested the icariin could
enhance the BMP2 signaling pathway by upregulating BMAL1 expression
in vitro.
Discussion
In the present study, it was demonstrated that
icariin promoted osteogenic differentiation by upregulating BMAL1
expression through BMP signaling in BMSCs. These findings suggested
that BMAL1 plays a critical role in icariin-mediated osteogenic
differentiation.
In fact, a circadian clock exists in every cell of
the human body. The central players in these are BMAL1 encoded by
the gene ARNTL and clock circadian regulator (CLOCK) by the gene
CLOCK (19). BMSCs are stem cells
with the ability to differentiate in vitro into adipocytes,
osteoblasts and cartilage-forming chondroblasts (20). Regardless of its exact
developmental origin, the function of the circadian clock gene is
related to the behavior of various stem cells involved in
homeostasis and repair of bone and adipose tissue (21). A recent report revealed impairment
of osteogenic differentiation of BMSCs from BMAL1−/−
mice (5). This finding was in line
with our present study as well as an observational study that
showed that the numbers of active osteocytes and osteoblasts were
reduced in BMAL1−/− mice in vivo (22). However, a previous study showed
that the amount of osteoblastic activity was increased in
BMAL1−/− mice (21).
These differences may due to the different ages of the mice. In
this study, we clearly evaluated the positive regulatory mechanism
of BMAL1 on osteogenic differentiation. Our results indicated that
BMAL1 could accelerate osteogenic differentiation through BMP2
signaling. BMP2, a member of the transforming growth factor (TGF)-β
superfamily, is considered to be a key regulator of cell growth and
differentiation (23–25). A number of studies have suggested
that the activity of clock genes is closely correlated with BMPs.
One study reported that regulation of clock genes controlled BMP2
expression in osteoblasts (26).
Meanwhile, BMAL1 regulation of TGF-β and BMP signaling in the
control of fat adipogenesis has also been reported (27). A recent study showed that BMAL1
silencing-induced BMP2 was found in rat uterus endometrium stromal
cells (28). Samsa et al
(5) recently reported that
BMAL1−/− mice had reduced osteoblast differentiation
in vitro and they proposed some potential molecular
mechanism of BMAL1-dependent control of osteogenic differentiation
including oxidative stress response, the mTOR signaling pathway and
translation. However, the precise mechanism via which BMAL1 induces
osteogenic differentiation of BMSCs remains unclear. In the present
study, it was demonstrated that BMAL1 deficiency decreased BMP2
expression during osteogenic differentiation of BMSCs.
The disorder and downregulation of clock genes may
be the cause of disease, and in some cases could also be used as a
target of drug action to improve disease symptoms and treat
disease. Recently, in order to alleviate circadian clock-related
disease, there has been significant progress in the development of
chemical compounds which are capable of manipulating clock genes as
novel preventive and therapeutic agents (29). For example, resveratrol, which is
considered a natural antioxidant polyphenol compound, has also been
demonstrated to ameliorate circadian disorders of lipid metabolism
(30). Moreover, a recent study
revealed that glucocorticoids altered the circadian clock in the
iris-ciliary body in a mouse model (31). However, few studies have yet
demonstrated the potential mechanism by which osteogenic
differentiation is mediated between icariin and BMAL1.
The results of the present study suggested that
icariin at a concentration range between 10−9 and
10−5 mol/l significantly promoted the proliferation of
BMSCs, especially at 10−7 mol/l. A number of previous
studies have reported that icariin increased cell proliferation at
different concentrations. Yang et al (32) showed enhanced proliferation of
human neural stem cells in vitro at a concentration of
10−5 mol/l. In addition, Mok et al (33) demonstrated that icariin stimulated
the proliferation of UMR 106 cells at concentrations ranging from
10−14 to 10−6 mol/l. These differences may be
attributable to the variety of cells used in the experiments.
Icariin has been demonstrated to have therapeutic
properties, with reports of antitumor, anti-hepatotoxic,
immune-enhancing, anti-inflammatory, neurite outgrowth activity and
erection dysfunction-improving effects over the past decades among
Chinese researchers (34–36). Furthermore, the bone-strengthening
activity of icariin has attracted much recent attention. However,
there have been few reports on the effect of icariin on the
circadian clock in BMSCs. The present study suggested that icariin
increased BMAL1 expression. Furthermore, reduced expression of
BMP2/RUNX2 was observed in the present study during osteogenic
differentiation of BMAL1-deficient cells. In addition, mineralized
nodule formation was suppressed both in BMAL1−/− BMSCs
and in icariin-mediated BMAL1−/− BMSCs. Taken together;
these findings demonstrated that icariin promoted osteogenic
differentiation by upregulating BMAL1 expression through BMP
signaling in BMSCs. However, as the effects of icariin on BMSCs in
the present study were mainly observed in vitro, further
studies will be needed to confirm these results in animal
models.
In conclusion, to the best of our knowledge, the
present study is the first to provide evidence that icariin
promoted osteogenic differentiation through BMAL1-BMP2/RUNX2
signaling.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Hubei Province (grant. nos. 2013CFB376 and
2018CFB095).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZH and SZ conceived and designed the study. HW, ZL,
JX and ZW performed the experiments. ZH and HW wrote the paper. XW,
YH, YX and XL analyzed the data, and reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research.
Ethics approval and consent to
participate
All the experimental protocols involving animals
were approved by the Institutional Animal Care and Use Committee of
Tongji Medical College, Huazhong University of Science and
Technology (IACUU no. S496; date of permission, October, 31,
2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Mankin HJ: Nontraumatic necrosis of bone
(osteonecrosis). N Engl J Med. 326:1473–1479. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu Y, Zhang Y, Wu J, Sun Y, Xiong Z, Niu
F, Lei L, Du S, Chen P and Yang Z: Genetic polymorphisms in IL1B
predict susceptibility to steroid-induced osteonecrosis of the
femoral head in Chinese Han population. Osteoporos Int. 30:871–877.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mont MA, Cherian JJ, Sierra RJ, Jones LC
and Lieberman JR: Nontraumatic osteonecrosis of the femoral head:
Where do we stand today? A ten-year update. J Bone Joint Surg Am.
97:1604–1627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shui C, Spelsberg TC, Riggs BL and Khosla
S: Changes in Runx2/Cbfa1 expression and activity during
osteoblastic differentiation of human bone marrow stromal cells. J
Bone Miner Res. 18:213–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samsa WE, Vasanji A, Midura RJ and
Kondratov RV: Deficiency of circadian clock protein BMAL1 in mice
results in a low bone mass phenotype. Bone. 84:194–203. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He Y, Chen Y, Zhao Q and Tan Z: Roles of
brain and muscle ARNT-like 1 and Wnt antagonist Dkk1 during
osteogenesis of bone marrow stromal cells. Cell Prolif. 46:644–653.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohawk JA, Green CB and Takahashi JS:
Central and peripheral circadian clocks in mammals. Annu Rev
Neurosci. 35:445–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richards J and Gumz ML: Mechanism of the
circadian clock in physiology. Am J Physiol Regul Integr Comp
Physiol. 304:R1053–R1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeBruyne JP, Weaver DR and Dallmann R: The
hepatic circadian clock modulates xenobiotic metabolism in mice. J
Biol Rhythms. 29:277–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zmrzljak UP and Rozman D: Circadian
regulation of the hepatic endobiotic and xenobitoic detoxification
pathways: The time matters. Chem Res Toxicol. 25:811–824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bailey SM, Udoh US and Young ME: Circadian
regulation of metabolism. J Endocrinol. 222:R75–R96. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miranda J, Portillo MP, Madrid JA, Arias
N, Macarulla MT and Garaulet M: Effects of resveratrol on changes
induced by high-fat feeding on clock genes in rats. Br J Nutr.
110:1421–1428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen P, Kakan X and Zhang J: Altered
circadian rhythm of the clock genes in fibrotic livers induced by
carbon tetrachloride. FEBS Lett. 584:1597–1601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabás-Rivera C, Martínez-Beamonte R, Ríos
JL, Navarro MA, Surra JC, Arnal C, Rodríguez-Yoldi MJ and Osada J:
Dietary oleanolic acid mediates circadian clock gene expression in
liver independently of diet and animal model but requires
apolipoprotein A1. J Nutr Biochem. 24:2100–2109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Z, Cheng C, Wang J, Liu X, Wei H,
Han Y, Yang S and Wang X: Icariin regulates the osteoblast
differentiation and cell proliferation of MC3T3-E1 cells through
microRNA-153 by targeting Runt-related transcription factor 2. Exp
Ther Med. 15:5159–5166. 2018.PubMed/NCBI
|
|
16
|
Huang Z, Cheng C, Cao B, Wang J, Wei H,
Liu X, Han Y, Yang S and Wang X: Icariin protects against
glucocorticoid-induced osteonecrosis of the femoral head in rats.
Cell Physiol Biochem. 47:694–706. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong H, Wang X, Wang L, Liu Y, Wang QL,
Pang H, Zhang Q and Wang Z: Inhibition of IGF-1 receptor kinase
blocks the differentiation into cardiomyocyte-like cells of BMSCs
induced by IGF-1. Molecular medicine reports. 16:787–793. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dierickx P, Van Laake LW and Geijsen N:
Circadian clocks: From stem cells to tissue homeostasis and
regeneration. EMBO Rep. 19:18–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murray IR, West CC, Hardy WR, James AW,
Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C and Péault B:
Natural history of mesenchymal stem cells, from vessel walls to
culture vessels. Cell Mol Life Sci. 71:1353–1374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weger M, Diotel N, Dorsemans AC, Dickmeis
T and Weger BD: Stem cells and the circadian clock. Dev Biol.
431:111–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu L, Patel MS, Bradley A, Wagner EF and
Karsenty G: The molecular clock mediates leptin-regulated bone
formation. Cell. 122:803–815. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gosselet FP, Magnaldo T, Culerrier RM,
Sarasin A and Ehrhart JC: BMP2 and BMP6 control p57(Kip2)
expression and cell growth arrest/terminal differentiation in
normal primary human epidermal keratinocytes. Cell Signal.
19:731–739. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rogers MB, Shah TA and Shaikh NN: Turning
bone morphogenetic protein 2 (BMP2) on and off in mesenchymal
cells. J Cell Biochem. 116:2127–2138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shu B, Zhang M, Xie R, Wang M, Jin H, Hou
W, Tang D, Harris SE, Mishina Y, O'Keefe RJ, et al: BMP2, but not
BMP4, is crucial for chondrocyte proliferation and maturation
during endochondral bone development. J Cell Sci. 124:3428–3440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirai T, Tanaka K and Togari A:
α1-adrenergic receptor signaling in osteoblasts regulates clock
genes and bone morphogenetic protein 4 expression through
up-regulation of the transcriptional factor nuclear factor IL-3
(Nfil3)/E4 promoter-binding protein 4 (E4BP4). J Biol Chem.
289:17174–17183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nam D, Guo B, Chatterjee S, Chen MH,
Nelson D, Yechoor VK and Ma K: The adipocyte clock controls brown
adipogenesis through the TGF-β and BMP signaling pathways. J Cell
Sci. 128:1835–1847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tasaki H, Zhao L, Isayama K, Chen H,
Yamauchi N, Shigeyoshi Y, Hashimoto S and Hattori MA: Inhibitory
role of REV-ERBα in the expression of bone morphogenetic protein
gene family in rat uterus endometrium stromal cells. Am J Physiol
Cell Physiol. 308:C528–C538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gloston GF, Yoo SH and Chen ZJ:
Clock-enhancing small molecules and potential applications in
chronic diseases and aging. Front Neurol. 8:1002017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun L, Wang Y, Song Y, Cheng XR, Xia S,
Rahman MR, Shi Y and Le G: Resveratrol restores the circadian
rhythmic disorder of lipid metabolism induced by high-fat diet in
mice. Biochem Biophys Res Commun. 458:86–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuchiya S, Sugiyama K and Van Gelder RN:
Adrenal and glucocorticoid effects on the circadian rhythm of
murine intraocular pressure. Invest Ophthalmol Vis Sci.
59:5641–5647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang P, Guan YQ, Li YL, Zhang L, Zhang L
and Li L: Icariin promotes cell proliferation and regulates gene
expression in human neural stem cells in vitro. Mol Med Rep.
14:1316–1322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mok SK, Chen WF, Lai WP, Leung PC, Wang
XL, Yao XS and Wong MS: Icariin protects against bone loss induced
by oestrogen deficiency and activates oestrogen receptor-dependent
osteoblastic functions in UMR 106 cells. Br J Pharmacol.
159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SR, Xu XZ, Wang YH, Chen JW, Xu SW,
Gu LQ and Liu PQ: Icariin derivative inhibits inflammation through
suppression of p38 mitogen-activated protein kinase and nuclear
factor-kappaB pathways. Biol Pharm Bull. 33:1307–1313. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou J, Wu J, Chen X, Fortenbery N,
Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY and Wei S: Icariin
and its derivative, ICT, exert anti-inflammatory, anti-tumor
effects, and modulate myeloid derived suppressive cells (MDSCs)
functions. Int Immunopharmacol. 11:890–898. 2011. View Article : Google Scholar : PubMed/NCBI
|