Introduction
The porcine reproductive physiology is a clear and
valuable model for studying and developing the knowledge in
follicle growth and maturation of the oocyte. Moreover, it gives
lots of information that might be implemented in human research,
taking into account the relevant similarity of reproduction between
the species. The past and recent animal research gave rise to the
basics of embryology and implemented many techniques in assisted
reproduction. The oocyte development and ovulation are one of the
most important processes in mammalian reproduction, though it gives
the opportunity to fertilize and transfer genes to a new entity.
Nevertheless, growth, differentiation and maturation of the oocyte
and surrounding structures still remains a subject of inquiring
debate. Literature suggests, that oocyte itself, plays an essential
role in regulatory mechanisms of its growth and development,
influencing and being influenced by surrounding granulosa cells via
specific gap junctions. These mechanisms are regulated by
expression of particular genes and their biochemical signaling
pathways, presence of specific molecules and growth and
differentiation factors (1–5).
Oocyte maturation consists of numerous rearranges in cell nucleus
and cytoplasm, which are essential to finalize its competence to
fertilize. Nuclear maturity is strictly conjoined with resume and
finish of first meiotic division and entrance into second one,
arrested in metaphase II, until contact with spermatozoon. The
initiation of final maturation is present in antral-dominant
follicles and is based on the mid-cyclic LH surge or administration
of human chorionic gonadotropin (hCG). However, as mentioned,
mechanisms of oocyte maturation in vivo are still under
investigation, therefore an animal in vitro models provide
insight into these complicated and sensitive cross-linked actions,
comprising regulation of gene expression, transcription and
macromolecule metabolic processes. The adequate gene expression and
storage of macromolecules seems to be crucial for protein
biosynthesis during pre- and periimplantation stages of embryo
development (6).
The mentioned, bi-directional communication between
the oocyte and accompanying cumulus cells is necessary for growth,
development and function of the whole cumulus-oocyte complex (COC),
but it has also been published, that the oocyte is the crucial cell
determining the direction of differentiation and the function of
the granulosa cells surrounding it (1). It secretes factors, such as growth
and differentiation factor 9 (GDF9), bone morphogenetic protein 15
(BMP15) and possibly many others, regulating proliferation,
apoptosis, expansion luteinisation and metabolism of GCs (7). However, transcriptomic profiles of
exclusively expressed either in granulosa cells or oocytes have
been hardly studied. We investigated transcriptome profile of
porcine oocytes before and after in vitro culture, assuming,
that oocyte itself plays a crucial role in self-development and
early embryo evolution. The results obtained are advancing our
knowledge about oocyte transcriptome changes during in vitro
culture (8–10).
Materials and methods
Part of the materials and methods section is based
on our previous publications of the same research team, presenting
results from the same cycle of studies related to porcine oocytes
(11–13).
Animals
In the present study, pubertal crossbred Landrace
gilts (median age of 170, ranging from 150–180 days; median weight
of 98 kg, ranging from 95–110 kg) in a total number of 45 were
used. The animals were bred under the same conditions, also feeding
and housing state of all the animals was identical. The specimen
were obtained from a commercial slaughterhouse and the methods of
anaesthesia, euthanasia and death verification were compliant to
the law of the European Union (European Union Parliament directives
853/2004, 854/2004 and 1099/2009, on the hygiene, quality control,
worker qualification and slaughter methods in the meat industry)
and Local Laws [Directives of the Polish Ministry of Agriculture
and Countryside Development from 24th of September 2009 (Protection
of Slaughtered Animals) and 19th of May 2010 (Qualification of
Small-scale Slaughterhouse Working Conditions and Hygiene)]. All
experiments employed in this study were approved by the Local
Bioethical Committee of Poznań University of Medical Sciences
(resolution no. 32/2012, issued 01.06.2012).
Collection of porcine ovaries and
cumulus-oocyte-complexes (COCs)
After slaughter, the ovaries and reproductive tracts
of the animal specimen were recovered and transported to the
laboratory within 30 min at 38°C. in 0.9% NaCl, to provide material
integrity through transport time. Ovaries of each animal were
placed in phosphate buffered saline (PBS) supplemented with 5%
fetal bovine serum (FBS; Sigma-Aldrich Co.; Merck KGaA, Darmstadt,
Germany) to ensure optimal environment for subsequent oocyte in
vitro maturation and fertilization. In the next step, single
preovulatory large follicles (an estimated diameter greater than 5
mm) were opened (in the number of 300) in a sterile Petri dish by
puncturing (a 5-ml syringe and 20-G needle were employed to
puncture selected follicles). This operation allows to obtain the
cumulus-oocyte complexes (COCs). The COCs extracted from follicles
were washed thrice in PBS supplemented with 36 µg/ml pyruvate, 50
µg/ml gentamicin, and 0.5 mg/ml Bovine Serum Albumin (BSA; all
ingredients provided by Sigma-Aldrich Co.; Merck KGaA). After
collection and washing, an inverted microscope Zeiss, Axiovert 35
(Zeiss, Lübeck, Germany) were used for COCs selection, counting,
and morphological evaluation. Subsequently, the scale suggested by
Pujol et al (14) and Le
Guienne et al (15) and
also described in our previous study were used. Only grade I COCs
that exhibited homogeneous cytoplasm and uniform, compact cumulus
cells were qualified for the following steps of study, which
resulted in a total of 300 grade I oocytes. From the total number
of 300 grade I oocytes that were determined BCB+. 150 of
those were used for analysis as ‘Before IVM’ with the other
directed for in vitro maturation (‘After IVM’).
Ovaries from ten animals were collected and fixed in
the Bouin's solution for 48 h. Subsequently, tissues were
dehydrated and embedded in paraffin wax. Sections of ovaries (3–4
µm) were cut with a semi-automatic rotary microtome (Leica RM 2145,
Leica Microsystems, Nussloch, Germany). Then, the paraffin sections
were stained with hematoxylin and eosin (H&E) routine
procedure, following the steps: Deparaffinization and rehydration,
H and staining, and finally, dehydration. Histological sections
were evaluated by light microscope and selected pictures were taken
with a use of high-resolution scanning technique and Olympus BX61VS
microscope scanner (Olympus, Tokyo, Japan).
Assessment of oocyte developmental
competence by BCB test
Measurement of the activity of the
glucose-6-phosphate (G6PDH) enzyme, which is responsible for
conversion of Brilliant Cresyl Blue (BCB) stain from blue to
colourless, was employed to assess oocyte developmental competence.
In oocytes that completed their growth, activity of the enzyme
decreases significantly, which causes the stain to be immobilized,
resulting in blue oocytes (marked BCB+). To perform the
BCB staining test oocytes were washed twice in modified Dulbecco
PBS (DPBSm) (Sigma-Aldrich; Merck KGaA) additionally supplemented
with 50 IU/ml penicillin, 50 µg/ml streptomycin (Sigma-Aldrich;
Merck KGaA), 0.4% [w/v] BSA, 0.34 mM pyruvate, and 5.5 mM glucose.
After the initial preparation of oocytes, cells were treated with
13 µM BCB (Sigma-Aldrich; Merck KGaA) diluted in DPBSm at 38.5°C,
5% CO2 for 90 min. In the next step oocyte were twice
washed in DPBSm. During the washing procedure, the oocytes were
examined under an inverted microscope, which allowed for their
classification as stained blue (BCB+) or colourless
(BCB−). Immature oocytes, characterized by the presence
of a cumulus cells layer, give a negative result in this test, and
were used for the downstream analyses as ‘Before IVM’ group.
Whereas, the BCB+ COCs to remove cumulus and granulosa
cells layer, were incubated with hyaluronidase solution
(Sigma-Aldrich; Merck KGaA) for 2 min at 38°C. After removing these
additional cells in the 1% sodium citrate buffer, oocytes for
further cell culture procedures were obtained.
In vitro maturation (IVM) of porcine
COCs
The oocytes that gave a positive result after the
first BCB test were used during IVM procedure. Oocytes were
cultured in 500 µl standard porcine IVM culture medium, TCM-199
(tissue culture medium) with Earle's salts and L-glutamine, (Gibco
BRL Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 2.2 mg/ml sodium bicarbonate (Nacalai
Tesque, Inc., Kyoto, Japan), 0.1 mg/ml sodium pyruvate
(Sigma-Aldrich; Merck KGaA), 10 mg/ml Bovine Serum Albumin (BSA),
(Sigma-Aldrich; Merck KGaA), 0.1 mg/ml cysteine (Sigma-Aldrich;
Merck KGaA), 10% (v/v) filtered porcine follicular fluid and
gonadotropin supplements at final concentrations of 2.5 IU/ml hCG
(human Chorionic Gonadotropin; Ayerst Laboratories, Inc.,
Philadelphia, PA, USA) and 2.5 IU/ml eCG (equine Chorionic
Gonadotropin; Intervet, Whitby, ON, Canada). Each well in Nunclon™Δ
4-well dishes (Thermo Fisher Scientific, Inc.) was covered with
mineral oil overlay and placed for the first 22 h of maturation in
38°C under 5% CO2. Subsequently, in the same culture
conditions for 22 h, a new portion of the maturation medium has
been used, however now without hormone supplementation. After
maturation, second BCB staining test was performed, with
BCB+ oocytes used for downstream analyses. Based on
visual analysis under inverted microscope, around 70% from the
initial pool of cells were determined as BCB+. This
contributed to obtain the final number around 105 out of the
initial 150 of oocytes marked as ‘After IVM’.
RNA extraction from porcine oocytes
and reverse transcription
Before the extraction, COCs from both of the
experimental groups were denuded with the use of hyaluronidase and
glass micropipette to eliminate all of the surrounding somatic
cells. Total RNA from all of the samples (both before and after
IVM) was isolated according to the method published by Chomczyński
and Sacchi (16) employing TRI
reagent (Sigma-Aldrich; Merck KGaA). RNA integrity was determined
by denaturing agarose gel (2%) electrophoresis, and then, the RNA
was quantified by measuring the optical density (OD) at 260 nm
(NanoDrop spectrophotometer; Thermo Scientific, Inc.). The RNA
samples were re-suspended in 20–40 µl of RNase-free water and
stored at −80°C. RNA samples were treated with DNase I and
reverse-transcribed into cDNA using RT2 First Strand kit (Qiagen,
Hilden, Germany), according to the manufacturer's protocol. From
each RNA sample, 100 ng of RNA was taken for the following
molecular analyses (Microarray and RT-qPCR).
Microarray expression analysis and
statistics
Experiments were performed in three replicates. cDNA
obtained was used for biotin labelling and fragmentation using
Affymetrix GeneChip® WT Terminal Labeling and
Hybridization (Affymetrix, Santa Clara, CA, USA). Biotin-labelled
fragments of cDNA (5.5 µg) were hybridized to
Affymetrix® Porcine Gene 1.1 ST Array Strip (48°C/20 h).
Microarrays were then washed and stained according to the technical
protocol using the Affymetrix GeneAtlas Fluidics Station. The array
strips were scanned employing Imaging Station of the GeneAtlas
System. Preliminary analysis of the scanned chips was performed
using Affymetrix GeneAtlas™ Operating Software. The quality of gene
expression data was confirmed according to the quality control
criteria provided by the software. The obtained CEL files were
imported into downstream data analysis software.
To prepare all presented in the manuscript analyses
and graphs Bioconductor, based on the statistical R programming
language were used. Each CEL file was merged with a description
file. In order to correct background, normalize and summarize the
raw data, we used Robust Multiarray Averaging (RMA) algorithm.
To determine the statistical significance of the
analyzed genes, moderated t-statistics from the empirical Bayes
method were performed. The obtained P-value was corrected for
multiple comparisons using Benjamini and Hochberg's false discovery
rate. The selection of significantly altered genes was based on a
P<0.05 and expression higher than two-fold. The differentially
expressed gene list (separated for up- and downregulated genes) was
uploaded to the DAVID software (Database for Annotation,
Visualization and Integrated Discovery). In our work we focused on
27 genes belonging to ‘positive regulation of transcription,
DNA-dependent’, ‘positive regulation of gene expression’, ‘positive
regulation of macromolecule metabolic process’ and ‘positive
regulation of transcription from RNA polymerase II promoter’ Gene
Ontology Biological Process (GO BP) terms.
Subsequently, sets of differentially expressed genes
from selected GO BP terms were applied to STRING10 software (Search
Tool for the Retrieval of Interacting Genes/Proteins) for
interaction prediction. STRING is a huge database containing
information about protein/gene interactions, including experimental
data, computational prediction methods and public text
collections.
In the next step of microarray data analysis, we
employed ReactomeFIViz application to the Cytoscape 3.6.0 software.
Through the use of this analytical tool the functional interaction
between genes that belongs to the chosen GO BP terms were
examined.
The ReactomeFIViz app used for location pathways and
network patterns that are connected to cancer and other disease
types. It allows to run pathway enrichment analysis for a specified
gene group, visualizes found pathways with the use of manually
laid-out pathway diagrams, as well as to investigate the functional
relations between the genes of those pathways, through accessing
the Reactome database.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The RT-qPCR method was performed to confirm and
quantitatively validate the results obtained in the analysis of
expression microarrays. LightCycler 96 real-time PCR detection
system (Roche Diagnostics GmbH, Mannheim, Germany) was used to
perform the RT-qPCR reactions. SYBR® Green I detection
dye was used during the analyzes, with target cDNA quantified using
the relative quantification method. The relative abundance of FOS,
VEGFA, ESR1, AR, CCND2, EGR2, ENDRA, GJA1, INHBA transcripts in
each sample was standardized to the glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) mRNA level as an internal control. For
efficient amplification, 2 µl of previously received cDNA was mixed
with 18 µl of RT2 SYBR® Green ROX™ qPCR
Master Mix (Qiagen Sciences, Inc., Gaithersburg, MD, USA) and
sequence-specific primers (Table
I). One RNA repeat from each preparation was processed without
the RT-reaction to provide a negative control for subsequent PCR.
For relative quantification, we applied the 2-ΔΔCq method. Agarose
gel electrophoresis was used to confirm the specificity of the
amplified PCR products. To quantify mRNA levels of specific genes
in the oocyte, transcript levels were calculated in relation to
porphobilinogen deaminase (PBGD) and β-actin (ACTB). To ensure
result integrity, an additional housekeeping gene, 18S rRNA, was
used as an internal standard.
 | Table I.Oligonucleotide sequences of the
primers used for reverse transcription-quantitative PCR
analysis. |
Table I.
Oligonucleotide sequences of the
primers used for reverse transcription-quantitative PCR
analysis.
| Gene | Accession
number | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| APP | P79307 | F:
CATCGCTTACAAACTCGCCA | 160 |
|
|
| R:
TGCCAAGAAGTCTACCCTGA |
|
| AR | NP_999479 | F:
TGGTGCAATCATTTCTGCTG | 153 |
|
|
| R:
ACTTTCCACCCCAGAAGACC |
|
| CCND2 | Q8WNW2 | F:
ACGTCGGTGTTGGTGATCC | 154 |
|
|
| R:
CCACTGACTTCAAGTTCGCC |
|
| ECE1 | HGNC:3146 | F:
GTAATGTTAGCAGCGCCGTT | 110 |
|
|
| R:
GAGAAGGTGCTGACGGGATA |
|
| EDNRA | NP_999394 | F:
AGGTGTTCACTGAGGGCAAT | 168 |
|
|
| R:
TGCCACGTCAAAATTCATGGA |
|
| EGR1 | HGNC:3238 | F:
CCACTAGAACCTTCTCGTTATTCA | 103 |
|
|
| R:
AGCGCCTTCAACCCTCAG |
|
| EGR2 | NP_001090957 | F:
GCTGGCACCAGGGTACTG | 154 |
|
|
| R:
CGGAGATGGCATGATCAAC |
|
| ESR1 | Q29040 | F:
TTCCTGTCCAGGAGCAAGTT | 155 |
|
|
| R:
AAGAGGGTGCCAGGATTTTT |
|
| FOS | NP_001116585 | F:
CAGATCGGTGCAGAAGTCCT | 159 |
|
|
| R:
ATGATGTTCTCCGGCTTCAA |
|
| GJA1 | NP_001231141 | F:
GGGCAGGGATCTCTTTTGC | 150 |
|
|
| R:
CAAGGTGAAAATGCGAGGGG |
|
| IHH | NP_001231399 | F:
CACACGTGCCCCACTCTC | 134 |
|
|
| R:
GCTTCGACTGGGTGTATTACG |
|
| IKZF2 | HGNC:13177 | F:
AGGAGGTACATGGTGACTCA | 174 |
|
|
| R:
CTCACAGGACACCTCAGGAC |
|
| INHBA | P03970 | F:
TCAGGAAGAGCCAGATTTCG | 151 |
|
|
| R:
AGGGCGGAAATGAATGAACT |
|
| INSR | HGNC:6091 | F:
GTCGGTGACTCTCTCTGGAC | 160 |
|
|
| R:
CACTTCTTCCGACATGTGGT |
|
| KIT | NP_001037990 | F:
ACAGCCTAATCTCATCGCCA | 152 |
|
|
| R:
CGCCTGGGATTTTCTCTTCG |
|
| MAP3K1 | HGNC:6848 | F:
CTACATTCTTCTGCCCAGATTG | 150 |
|
|
| R:
TTCAGAAAACAGTATAAAAGATGAAGA |
|
| MEF2C | NP_001038005 | F:
TTTTGCTTGCATATTCTTGTTCA | 168 |
|
|
| R:
CCTGGTGTAACACATCGACCT |
|
| MITF | NM_001315782 | F:
CTGTTCCCGTTGCAACTTTC | 163 |
|
|
| R:
CAATCACAACTTGATTGAACGAA |
|
| NFAT5 | HGNC:7774 | F:
GTTGGCCTGGCTGACTTATG | 157 |
|
|
| R:
GGAGGTACAATGAACCAGCTACA |
|
| NR5A1 | P79387 | F:
GGTCAGCTCCACCTCCTG | 150 |
|
|
| R:
GTCTTCAAGGAGCTGGAGGT |
|
| PSMB4 | NP_001231384 | F:
ACGTAACCCAGGAAGCTCTC | 165 |
|
|
| R:
GGTGATTGATGAGGAGCTGC |
|
| RORA | HGNC:10258 | F:
CATGAGCGATCTGCTGACAT | 152 |
|
|
| R:
TATGCTAGCCCCGATGTCTT |
|
| SH3D19 | HGNC:30418 | F:
GCTCTGTTATTATGTCTCCAGCC | 154 |
|
|
| R:
ATCTTCCCCGCAGTCTTCG |
|
| SMAD4 | Q9GKQ9 | F:
TGTTTTAATTCATTTTTGTGAAGATCA | 150 |
|
|
| R:
AACCACAAATGGAGCTCACC |
|
| SMARCA1 | HGNC:11097 | F:
TTCCTTGGGACCTTATAGCC | 164 |
|
|
| R:
AGAGAGGCATTACGTGTCAGC |
|
| VEGFA | NP_999249 | F:
TCTCGATTGGACGGCAGTAG | 154 |
|
|
| R:
CTCTCTTGGGTGCATTGGAG |
|
| WWTR1 | HGNC:24042 | F:
ATTGATGTTCATGGGTGTGG | 152 |
|
|
| R:
ATGGGGGACCATATCATTCA |
|
| ACTB | DQ845171 | F:
GGGAGATCGTGCGGGACAT | 141 |
|
|
| R:
CGTTGCCGATGGTGATGAC |
|
| PBGD | NM_001097412 | F:
GAGAGTGCCCCTATGATGCTAT | 214 |
|
|
| R:
GATGGCACTGAACTCCT |
|
| 18S rRNA | AB117609 | F:
GTGAAACTGCGAATGGCTC | 105 |
|
|
| R:
CCGTCGGCATGTATTAGCT |
|
Results
We observed upregulation of all genes involved in
‘positive regulation of transcription, DNA-dependent’, ‘positive
regulation of transcription from RNA polymerase II promoter’,
‘positive regulation of gene expression’, ‘positive regulation of
macromolecule metabolic process’ before IVM as compared to
transcriptional profile analyzed after IVM.
Microarray
Whole transcriptome profiling by Affymetrix
microarray allowed us to analyze the oocyte gene expression changes
after in vitro maturation in relation to freshly isolated
oocyte, before in vitro procedure (in vivo). By
Affymetrix® Porcine Gene 1.1 ST Array we examined
expression of 12258 porcine transcripts. Genes with fold change
higher than abs (2) and with
corrected P-value lower than 0.05 were considered as differentially
expressed. This set of genes consisted of 419 different
transcripts.
DAVID (Database for Annotation, Visualization and
Integrated Discovery) software was used for extraction of enriched
GO BP terms. Up and downregulated gene sets were subjected to DAVID
searching separately and only gene sets where adj. P-value were
lower than 0.05 were selected, as previously described (17). We find that 28 genes that belong to
‘positive regulation of transcription, DNA-dependent’, ‘positive
regulation of gene expression’, ‘positive regulation of
macromolecule metabolic process’ and ‘positive regulation of
transcription from RNA polymerase II promoter’ GO BP terms were
significantly represented in downregulated gene set. This set of
genes was subjected to hierarchical clusterization procedure and
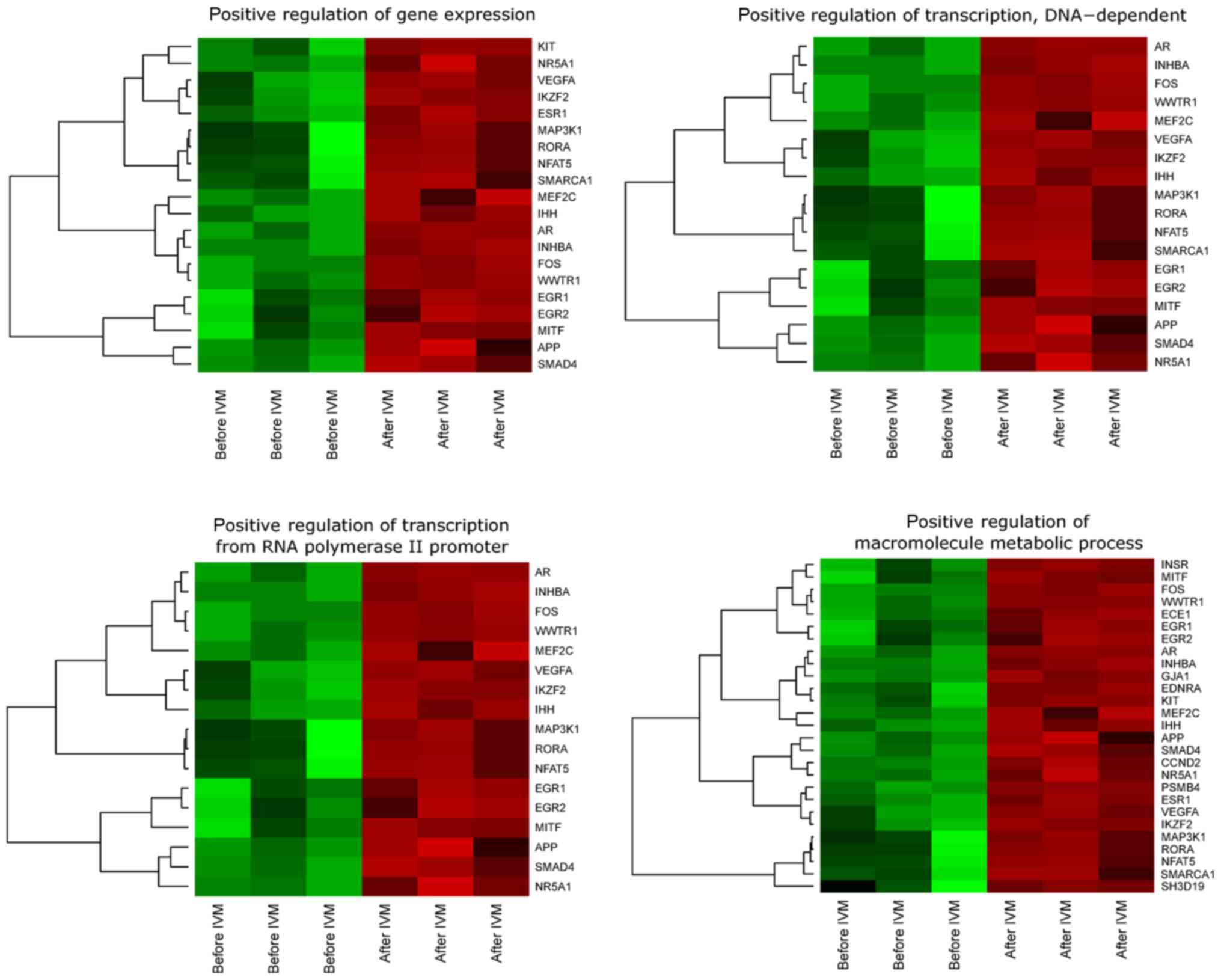
presented as heatmaps (Fig. 1).
Fold changes in expression, Entrez gene IDs and adjusted P. values
of genes belonging to selected GO BP were shown (Table II).
 | Table II.Gene symbols, fold changes on
expression, Entrez gene IDs and adjusted P-values of the genes
studied. |
Table II.
Gene symbols, fold changes on
expression, Entrez gene IDs and adjusted P-values of the genes
studied.
| Gene | Fold-change | Adjusted
P-value | Entrez Gene ID |
|---|
| APP | 0.324139 | 0.005602 | 351 |
| AR | 0.105986 | 0.000138 | 367 |
| CCND2 | 0.121809 | 0.000179 | 894 |
| ECE1 | 0.395388 | 0.001178 | 1889 |
| EDNRA | 0.166939 | 0.001854 | 1909 |
| EGR1 | 0.376128 | 0.005477 | 1958 |
| EGR2 | 0.165504 | 0.00795 | 1959 |
| ESR1 | 0.08163 | 0.000522 | 2099 |
| FOS | 0.052794 | 4.74E-05 | 2353 |
| GJA1 | 0.206907 | 0.000108 | 2697 |
| IHH | 0.304996 | 0.000551 | 3549 |
| IKZF2 | 0.431872 | 0.002983 | 22807 |
| INHBA | 0.24126 | 0.000148 | 3624 |
| INSR | 0.316016 | 0.001913 | 3643 |
| KIT | 0.430444 | 0.002556 | 3815 |
| MAP3K1 | 0.368765 | 0.024748 | 4214 |
| MEF2C | 0.453593 | 0.003964 | 4208 |
| MITF | 0.49166 | 0.00633 | 4286 |
| NFAT5 | 0.344889 | 0.013145 | 10725 |
| NR5A1 | 0.425153 | 0.001889 | 2516 |
| PSMB4 | 0.493702 | 0.000939 | 5692 |
| RORA | 0.383924 | 0.021554 | 6095 |
| SH3D19 | 0.488128 | 0.046513 | 152503 |
| SMAD4 | 0.367802 | 0.001239 | 4089 |
| SMARCA1 | 0.329141 | 0.014759 | 6594 |
| VEGFA | 0.069689 | 0.001913 | 7422 |
| WWTR1 | 0.327202 | 0.000254 | 25937 |
To further investigate the changes within chosen GO
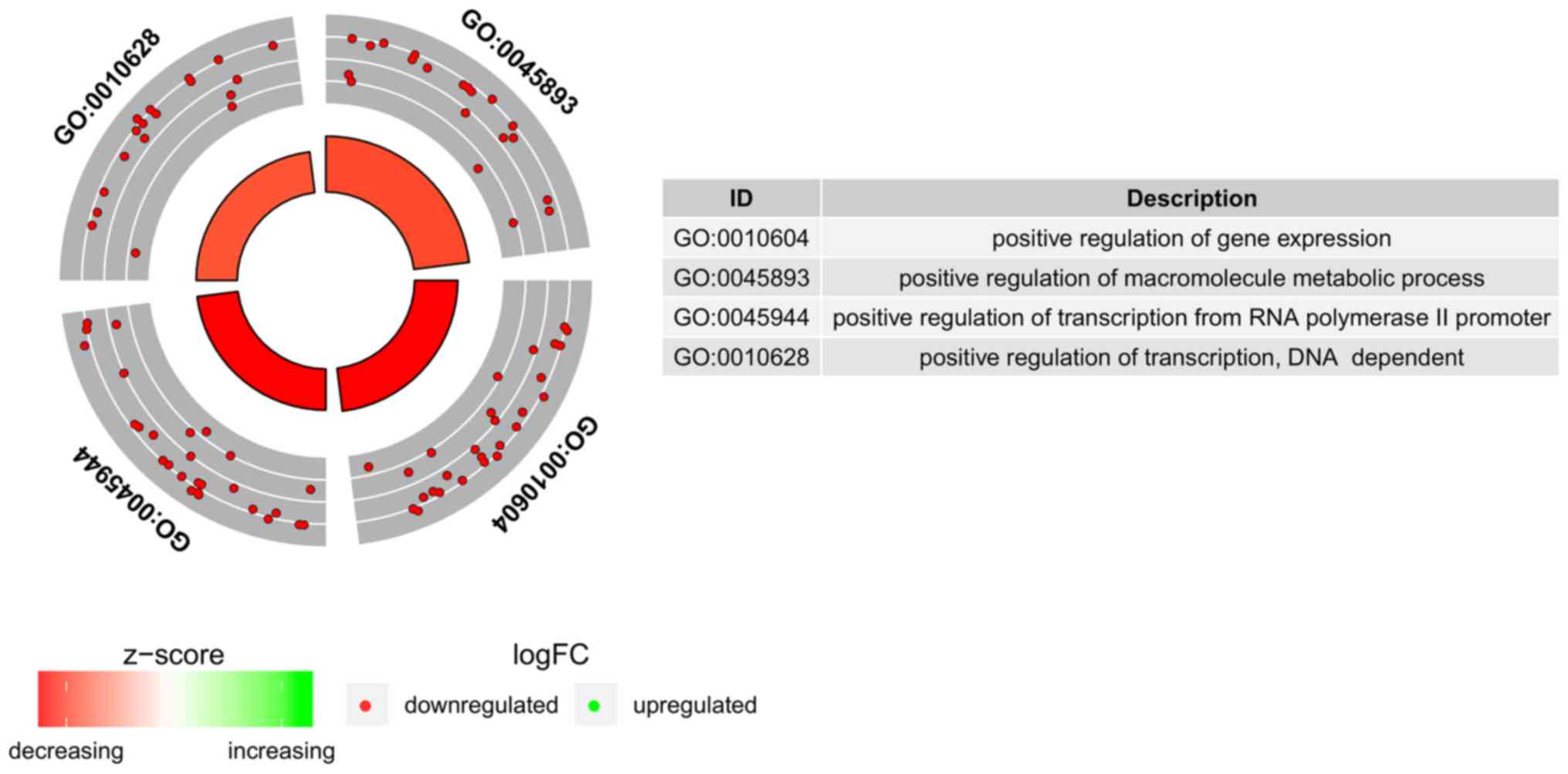
BP terms we measured the enrichment levels of each selected GO BP
terms. The enrichment levels were expressed as z-score and
presented as circular visualization (Fig. 2).
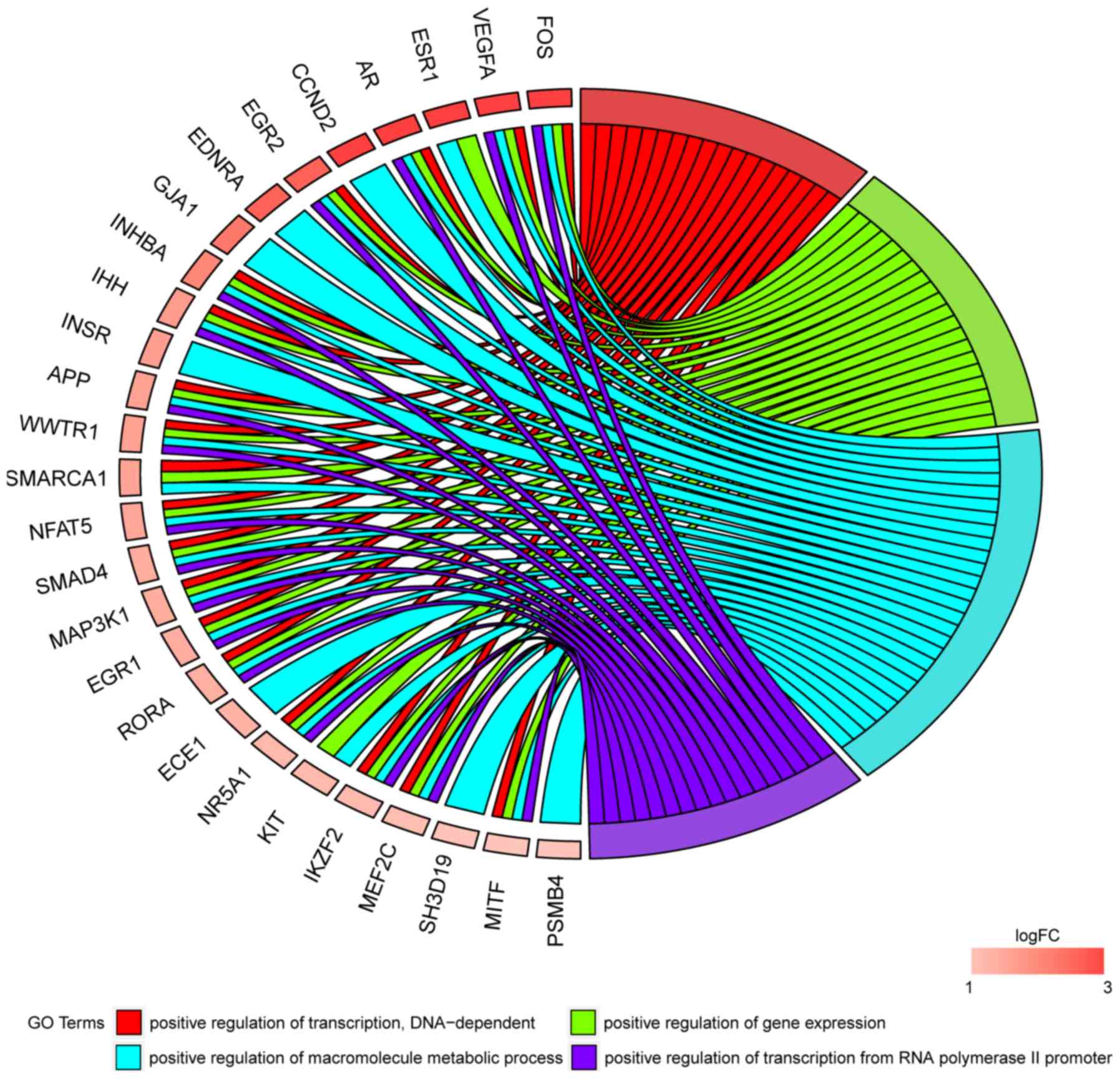
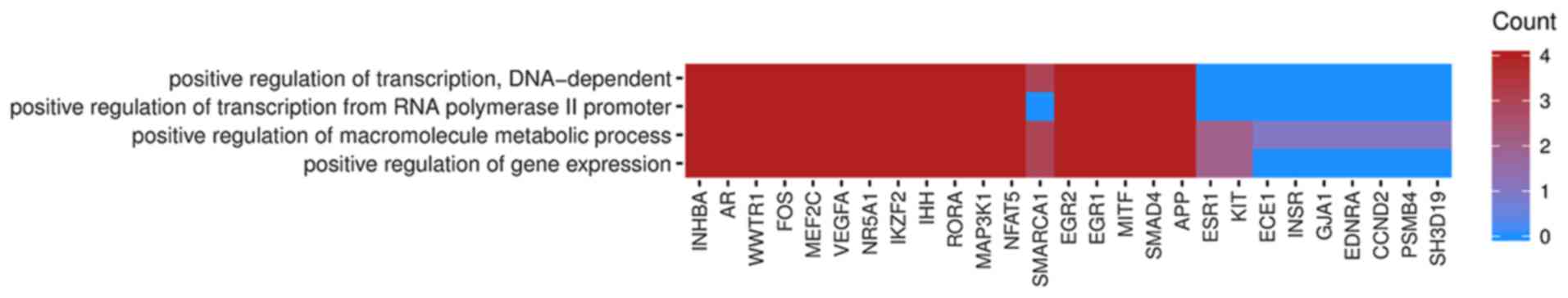
In Gene Ontology database genes that formed one
particular GO group can also belong to other different GO term
categories. For this reason, we have explored the gene
intersections between selected GO BP terms. The relation between
those GO BP terms was presented as circle plot (Fig. 3) as well as heatmap (Fig. 4).
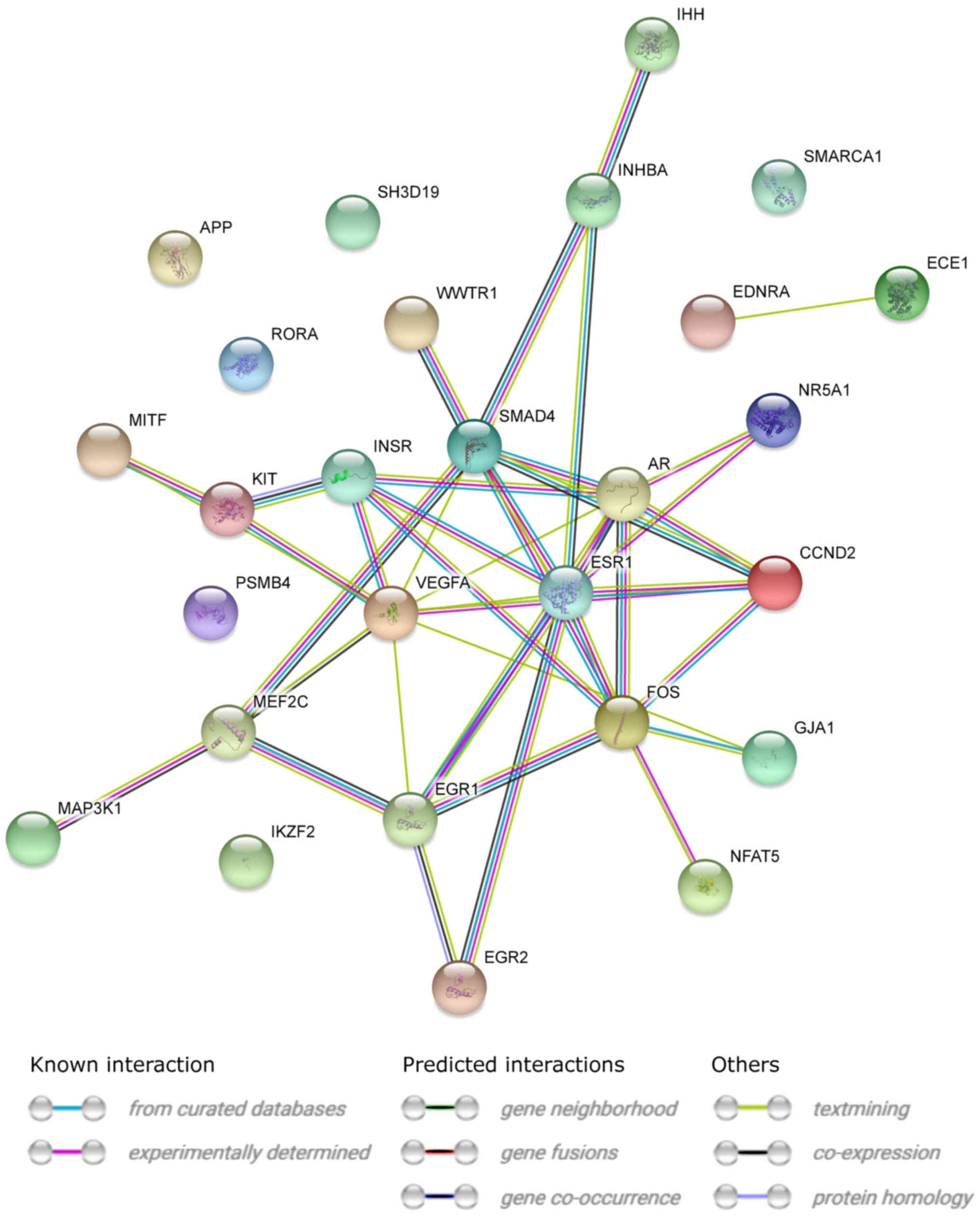
STRING interaction network was generated among
chosen differentially expressed genes belonging to each of the
selected GO BP terms. Using such prediction method provided us with
a molecular interaction network formed between protein products of
studied genes (Fig. 5). Finally,
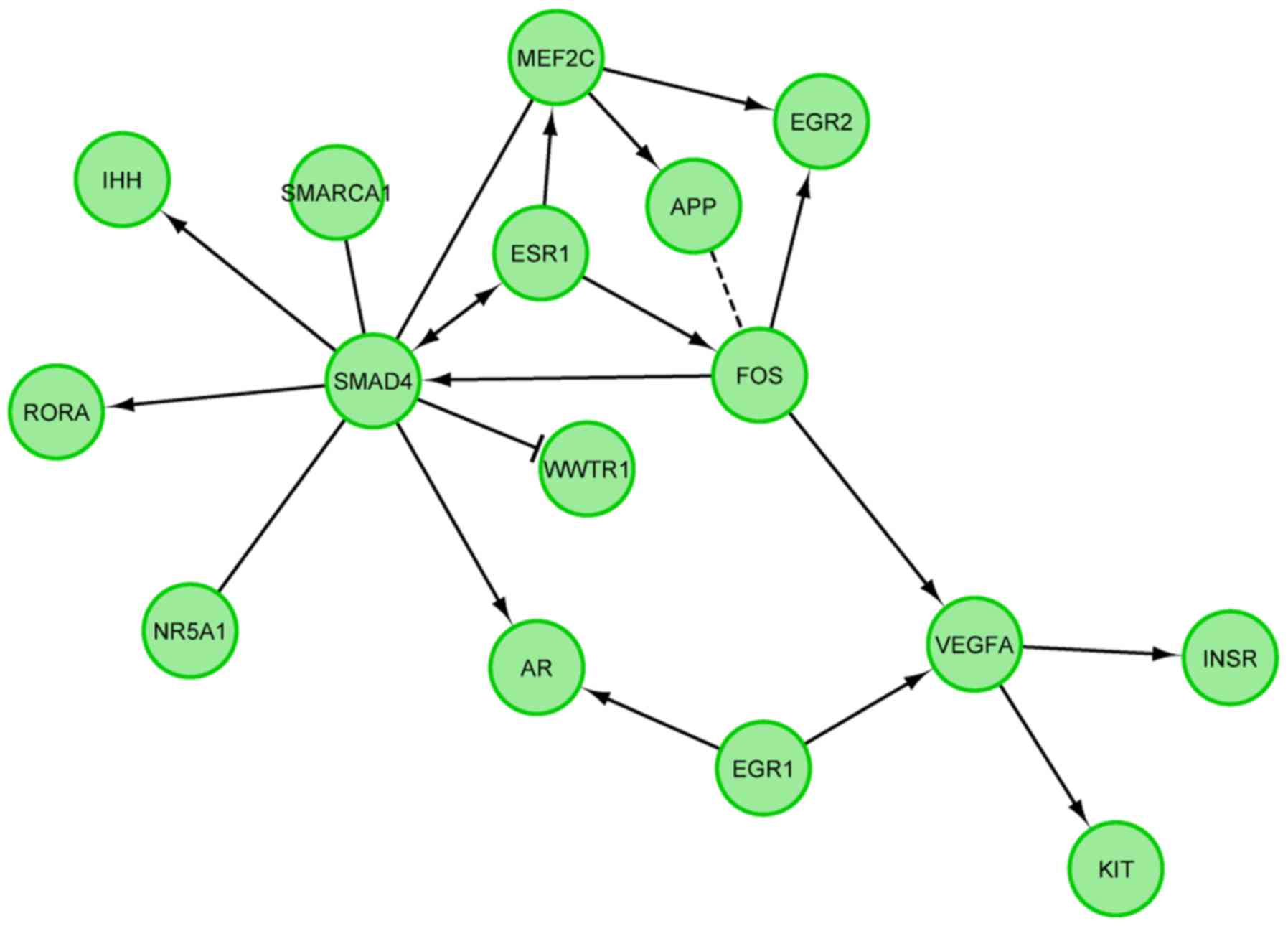
we investigated the functional interactions between chosen genes
with REACTOME FIViz app to Cytoscape 3.6.0 software. The results
were shown in Fig. 6.
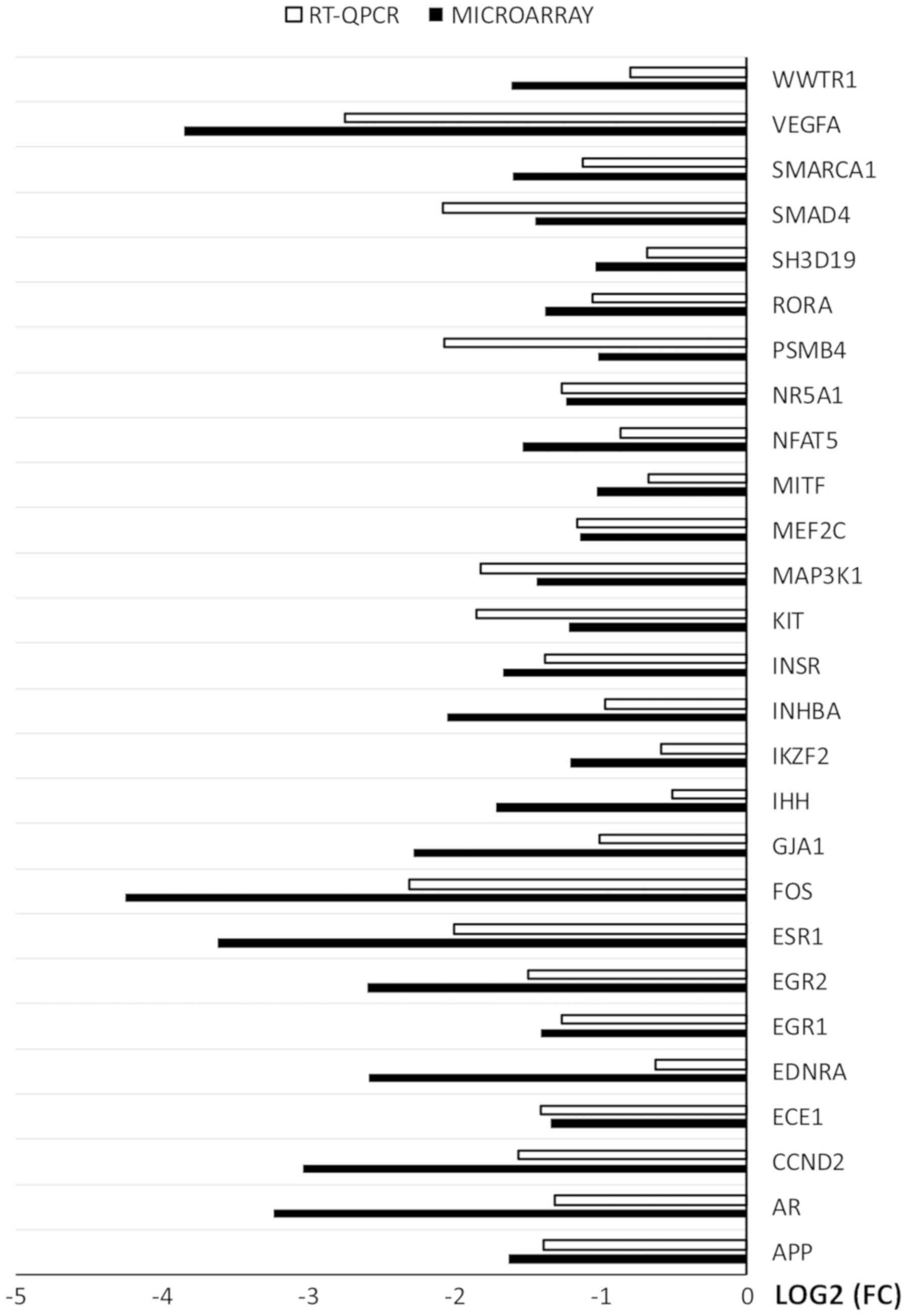
RT-qPCR was used to validate the results of
microarray analysis. The results were presented in a form of a bar
graph (Fig. 7).
Overall, the direction of changes was confirmed in
every example. However, the scale of changes in levels of gene
expression varied between the methods. In examples such as: APP,
NR5A1, MEF2C, INSR, EGR1 and ECE1 the difference was
rudimental. However, in some genes (WWTR1, VEGFA, PSMB4, INGBA,
IHH, FOS, ESR1, EGR2, ENDRA, CCND2, AR) the variation between
the methods was major. The rest of the genes presented moderate
variation between the microarray and RT-qPCR results.
Light microscope observation of H&E stained
sections revealed the normal histological structure of ovaries
(Fig. 8). The follicles in
different stages of development were present in each of analyzed
sections.
 | Figure 8.Photomicrograph representing the
sections of ovaries stained with hemotoxylin and eoesin. All images
present follicles in various stages of development. The description
of each arrow and number is represented in brackets. (A) Primordial
and primary follicles. Tissue sections exhibiting: (B) Primordial
follicles and primary follicles, and (C) primary oocytes, zona
pellucida, granulosa cells, theca cells, follicular cells, cortical
stroma and tunica albuginea. (D) Secondary follicle. Tissue
sections exhibiting: (E) secondary follicles, and (F) primary
oocytes, zona pellucida, antrum, granulosa cells, theca cells and
theca externa. (G) Graafian follicle. Tissue sections exhibiting:
(H) Graafian follicles, and (I) secondary oocytes, zona pellucida,
corona radiata, cumulus oophorus, antrum, granulosa cells and theca
externa. 1, primordial follicle; 2, primary follicle; 3, 7 and 12,
primary oocyte; 4, 13 and 20, zona pellucida; 5, 15 and 24,
granulosa cells; 6 and 16, theca cells; 8, follicular cells; 9,
corticol stroma; 10, tunica albuginea; 11, secondary follicle; 14
and 23, antrum; 17 and 25, theca externa; 18, Graafian follicle;
19, secondary oocyte; 21, corona radiata; 22, cumulus oophorus. |
Discussion
Heat maps show the expression of genes which belong
to the category of ‘positive regulation of transcription,
DNA-dependent’, ‘positive regulation of transcription from RNA
polymerase II promoter’, ‘positive regulation of gene expression’
and ‘positive regulation of macromolecule metabolic process’ from
GO. BP database. We have found abundant differences in the
expression of genes: FOS, VEGFA, ESR1, AR, CCND2, EGR2, ENDRA,
GJA1, INHBA. These genes were upregulated in porcine oocytes
before IVM as compared with post-IVM expression analysis.
In the complexity of genes up- and downregulated in
mammalian cumulus-oocyte-complex cross-talk, we have tried to
distinguish these, which are differentially expressed in the in
vitro oocyte culture in comparison to development in
vivo. Investigation of transcriptomic profiles of oocyte and
CCs separately is a very demanding challenge, requiring deep
knowledge of factors acting bi-directionally and expressed
exceptionally in particular cell type. Regassa et al
(7) analyzed transcriptome profile
from oocyte and CCs samples in bovine ovaries. They detected
multiple genes in germinal vesicle and in metaphase II oocytes,
reflecting their different expression patterns, in accordance to
presence, or absence of accompanying cumulus cells. This study
revealed, that a total of 265 transcripts expressed differentially
between oocytes cultured with and without CCs, of which 217 and 48
were over expressed in the former and the later groups,
respectively. Authors concluded, that the presence or absence of
cumulus cells' factors during bovine oocyte maturation can have a
significant influence on transcript abundance, showing the dominant
importance of molecular cross-talk between oocytes and their
corresponding CCs. This leads us to the conclusion, that ‘bare’
oocyte, even matured in vitro in cumulus-like conditions,
can express differently than one encapsulated in cumulus complex.
Moreover, it might express some GCs specific factors in conditions
of granulosa crosstalk deficiency. Therefore, it is a very
challenging request and limit of our study, to differentiate
cell-specific transcriptome and to conclude, that particular gene
is expressed in oocyte exceptionally, especially in in vitro
culture of isolated oocyte.
Moreover, considering the above, in the present
study we performed a microarray approach-analyzed porcine oocyte
transcriptome and found abundant expression of multiple genes,
including FOS, VEGFA, ESR1, AR, CCND2, EGR2, ENDRA, GJA1, INHBA,
IHH, INSR, APP, WWTR1, SMARCA1, NFAT5, SMAD4, MAP3K1, EGR1, RORA,
ECE1, NR5A1, KIT, IKZF2, MEF2C, SH3D19, MITF and
PSMB4.
These genes were downregulated after in vitro
oocyte maturation, what might indicate decreased potential of the
cell to develop in an environment different than physiological. We
will discuss only the most abundant differences in expression of
oocyte genes before and after IVM procedure.
The strongest inhibitory effect after in
vitro maturation of oocytes referred to transcription factor
FBJ-murine osteosarcoma viral oncogene homolog (FOS). It refers to
all ontology groups analyzed in recent study. Defined also, as Fos
Proto-Oncogene or AP-1 Transcription Factor Subunit, it belongs to
the Fos family, comprising of c-Fos, FosB and its smaller splice
variants, Fra-1 and Fra-2. It is the part of the
SMAD3/SMAD4/JUN/FOS complex and it forms AP1 promoter site due to
response to TGF-β (18). It binds
to the regulatory regions of numerous genes (19,20).
Specific functions of Jun-Fos proteins depend on posttranslational
modifications, selective dimerization between family members, and
protein-protein interactions with other regulatory molecules
(21). It plays a critical role in
regulating the development of cells destined to form and maintain
the skeleton. It was proved by Johnson et al (22), that the c-fos proto-oncogene has
been admitted as a central regulatory component of the nuclear
response to mitogens and other extracellular stimuli in mice. The
homozygous mutants showed diminished placental and fetal weights
and significant loss of viability at birth. There were multiple
defects observed, like foreshortening of the long bones,
ossification of the marrow space, and absence of tooth eruption,
delayed or absent gametogenesis, lymphopenia, and altered behavior.
The above indicated that c-Fos was involved in the development and
function of several distinct tissues. Moreover, in humans, c-Fos
gene transcripts were localized significantly more abundant in
fetal amniotic and chorionic cells, what suggested that these gene
may be related to embryo-derived cells, whose primary functions are
protection and nourishment of the fetus (23). The analysis of porcine ovaries,
obtained by Rusovici et al (21), suggests that although most AP-1
factors are present throughout the follicular cycle, specific
Jun/Fos proteins are more characteristic for a particular stage of
development than for another. The authors found c-Fos, FosB and
Fra-1, Fra-2 in all follicular cell compartments, also in oocyte
nucleus, in which being present throughout whole follicle
development, but the last staining could be nonspecific due to
technical limitations. Nevertheless, a study conducted by Hattori
et al (24), showed the
presence of c-Fos mRNA in porcine oocytes as well. There is limited
data concerning AP1/FOS in folliculogenesis and in ovulation,
though it may be involved in many cellular processes, e.g.
regulation of steroidogenesis or granulosa cell proliferation. It
has been found to be induced by FSH in pig oocyte culture after 1
h, what was consistent with rat studies, but progressively fell
over the whole culture period (25).
The second most significant expression decline was
observed in VEGFA. This gene is downregulated in all of the
ontology groups in our study, after IVM procedure. VEGF gene codes
a heparin-binding growth factor specific for vascular endothelial
cells and it induces angiogenesis in vivo (26). It has been purified from bovine
pituitary follicular cells in 1989 and proved to be mitogenic
factor for capillary endothelial cells (27). The gene itself, is a member of
platelet-derived growth factor (PDGF)/vascular endothelial growth
factor (VEGF) family. This potent factor occurs in five isoforms
that rise from alternative splicing of the same gene, and the above
have various metabolic effects (28).
The known, basic functions of vascular endothelial
growth factor A (VEGFA) are: The regulation of cell growth and
differentiation; the stimulation of angiogenesis and
neovascularization in tissues and organs, inhibition of apoptosis,
promotion of cell migration and vascular permeability (29). It is essential for both
physiological and pathological vessel formation. This gene is
upregulated in many known tumors and its expression is correlated
with tumor stage and progression. VEGFA has also been widely
studied because of its important role in follicle development. It
has been proven, that the growth of antral follicles is associated
with increased density of blood vessels within the theca cell
layers (30). Moreover, the
injection of VEGF gene directly into gilts' ovaries, increased the
levels of mRNA expression of VEGF 120 and VEGF 164 isoforms in the
granulosa cells and VEGF protein level in the follicular fluid.
This resulted in the increase in number of preovulatory follicles,
indicating, that angiogenesis is an important factor in the
development of ovulatory follicles (30). However, since the expression of
VEGFA has also been localized in nonvascular follicles and cells,
influence on follicular development through nonangiogenic
mechanisms is debated (31). It is
unknown, if oocyte originated VEGFA has an influence on surrounding
cells, but Bui et al (32)
published data confirming, that VEGF supplementation improves the
meiotic and developmental competence and fertilization ability of
oocytes derived from 0.3–5 mm porcine follicles, at adequate
concentrations during IVM. This might indicate, that VEGF has to be
secreted locally (by GCs or oocyte), before vascularization process
occurs, to increase growth and developmental competence of the
oocyte. In addition, its levels do not increase, until antrum
formation. Consistent with previous studies (33,34),
in the culture of monkey follicles, Xu et al (35) revealed that VEGF is increased
markedly in activated, growing follicles (theca and GCs close to
the oocyte), but not in primordial and preantral ones. The
hypothesis concludes, that VEGF-dependent antrum formation is a
result of hypoxic state of the growing follicle, and increasing
need for oxygen and substrates for developing oocyte (36).
Besides FOS and VEGFA, there was significant
reduction in expression of AR, EGR2 and INHBA in all four ontology
groups observed.
It has been distinctly proven, that androgen
receptor (AR) is one of the essential players in reproductive
system in mammals (37). AR is
expressed in various reproductive tissues, including, various
ovarian cells and neuroendocrine ovarian-pituitary-hypothalamus
axis. Reproductive phenotypes examined in androgen receptor knock
out mice (ARKO) showed disturbances explainable by granulosa cells
AR expression, including premature ovarian failure (POF),
subfertility with longer estrous cycles, fewer ovulated oocytes,
more preantral and atretic follicles, fewer antral follicles and
fewer corpora lutea. In addition, in vitro growth of
follicles was slower than in control wild-type animals (38). Lenie and Smitz analyzed mouse in
vitro folliculogenesis model and proved the importance of AR to
normal follicle maturation. Treatment with anti-androgenic
compounds reduced follicle growth during the preantral phase and
oocyte meiotic maturation in response to human hCG was arrested
(39). Therefore, interestingly,
oocytes also appear to benefit from androgens. Li et al
(40) revealed that testosterone
positively contributes to porcine oocyte meiotic resumption in
culture system containing a low dose of hypoxanthine. AR apparently
significantly contributes to testosterone-induced mitogen-activated
protein kinase activation and germinal vesicle breakdown (38,40).
In human model AR has been widely investigated in polycystic ovary
syndrome (PCOS) patients, in whom there are high androgen levels
observed and diminished oocyte developmental competence. Despite
the fact, that PCOS is a heterogenous condition, that has different
diagnostic criteria worldwide, the AR gene is the X-linked
candidate for follicle arrest and maturation disorders. Van
Nieuwerburgh et al (41)
suggested that the gene's CAG repeat polymorphism is associated
with a PCOS phenotype. They reported, shorter CAG repeats in the AR
gene, that appear to enhance androgenicity in PCOS.
The next significantly downregulated expression
concerns an EGR2 gene-protein also termed Krox20. The early growth
response (EGR) family of zinc finger transcription-regulatory
factors influences critical genetic programs in cellular growth,
differentiation, and function (42,43).
There are four EGR members, EGR1, EGR2, EGR3, and EGR4 and each of
them have specific biological functions, for example inflammation,
immune tolerance and glucose homeostasis (44). It was extensively examined in the
context of autoimmunity, being a potent negative regulator of
adaptive immune responses (45).
The gene products are induced in response to multiple extracellular
signals, such as growth factors, hypoxia, cytokines and mechanical
forces associated with injury and cellular stress (46). EGR2 gene is highly expressed in a
population of migrating neural crest cells in mice and is critical
for peripheral nerve myelination in Schwann cells (47). It also has a specific function in
the regulation of hindbrain segmentation, stabilization of
long-term potentiation, neural plasticity, learning and memory
(48), and EGR2-null mice died
early, in the embryonic stage, due to defective myelination
(45,49). The interesting research by Fang
et al (46), showed, that
it also may take part in tissue remodeling and wound healing
processes, due to upregulation of multiple genes associated with
these above. They revealed EGR2, as a functionally distinct
transcription factor that was both necessary and sufficient for
TGF-β-induced profibrotic responses. These findings suggested that
EGR2 plays a role in the pathogenesis of fibrosis. Moreover EGR2
has been suggested to participate in multiple extracellular
signals, including adipogenesis and carcinogenesis (50) and it was shown to control adipocyte
differentiation, which potentially links to insulin resistance
(51). In ovary, EGR2 was
investigated, as an intra-ovarian factor, part of a novel signaling
axis comprising of gonadotropins-EGR2-IER3, regulating the survival
of granulosa cells during folliculogenesis in rats and humans
(52). It was also found in the
article above, to regulate the expression of myeloid cell leukemia
1 (MCL-1), which belongs to the BCL-2 family of proteins regulating
cell survival. It is rather EGR3, that localizes to chromosomes
during meiotic progression and it seems to be dispensable during
oocyte maturation in mice (43).
INHBA is the protein also known as inhibin beta A or
follicle-stimulating hormone-releasing protein (FRP), and is
encoded by the INHBA gene (53).
The resultant preprotein is a member of the transforming growth
factor β (TGF-β) superfamily and is proteolytically modified to
build a subunit of the dimeric activin and inhibin protein
complexes. Inhibin β A (INHBA) forms a disulfide-linked homodimer
known as activin A, which is also a well-known factor in the
hypothalamic-pituitary-gonadal axis (54). Activin A is also proved to play
role during embryogenesis and in specific differentiation
processes, such as hematopoiesis and osteogenesis (55). INHBA gene is overexpressed in
various types of neoplasms, including colorectal, pancreatic, skin
and lung cancer (56), but also
presents cytostatic and proapoptotic signaling in human melanoma
cells (57). According to Locci
et al (58), it is proven,
that activin A programs the differentiation of human follicular
helper T cells, which play a role in protective antibody responses
and autoimmune diseases, using SMAD2 and SMAD3 signaling pathways.
High expression of INHBA has also been correlated with
developmental competence of oocytes and proposed, among others, as
predictor of embryo quality in women and in animal models (7,59,60).
Little is known about specific roles of inhibins (activin A as
well) in early stages of folliculogenesis, but mouse and human
models present it, as an important factor for cell proliferation
(germ and somatic) and follicular assembly as well (61). Myers et al (62) showed, that the absence of inhibin
(but in this paper-particularly inhibin A) induced more granulosa
cell-derived activin signaling and oocyte-derived GDF9, reducing
Kitl expression in granulosa cells, which in turn influence oocyte
growth and contributes to the small oocyte phenotype.
ENDRA and GJA1 expression was markedly reduced in
our post-IVM expression analysis. They belong to only one ontology
group-the positive regulation of macromolecule metabolic
process.
ENDRA is a endothelin-1 receptor type A, also
indexed as ETA, is a peptide that plays a role in potent and
long-lasting vasoconstriction. There are two receptors, ETA and ETB
(the endothelin-1 receptor type B), which are members of the family
A G-protein-coupled receptors (63). ENDRA signaling is known to be
involved in cardiovascular and craniofacial development, and mice
deficient in Endothelin1/Ednra signaling present malformation of an
aortic arch and outflow anomalies, which are attributed to cardiac
neural crest mesenchymal cell defects (64). Kawamura et al (65) demonstrated, that in periovulatory
mice ovaries, there is an increase in the expression of
endothelin-1 and one of its receptors endothelin receptor type A
(EDNRA). They also presented the ability of endothelin-1 to promote
GVBD of preovulatory oocytes by using in vitro and in
vivo models, analyzing communication between cumulus cells and
oocytes. In addition, the experimental study by Cui et al
(66) on supplementation of
endothelin-1 to culture medium with human oocytes, presented
significant promotion of oocyte maturation via connexin 26 (Cx26)
gene expression.
GJA1-Gap junction alpha-1 protein, also known as
connexin 43 (Cx43)-is a component of gap junctions, which allow
cell to cell communications, providing a route for the diffusion of
low molecular weight materials from cell to cell, regulating
proliferation, differentiation and cell death (67). It is detected in most cell types,
and a major protein responsible for synchronized heart contraction
(68). It is also expressed in in
cumulus cells, participating in connexons with other cumulus
granulosa cells or the oocyte. It regulates oocyte meiosis
resumption, and lower levels of GJA1 in cumulus cells are
beneficial for oocyte maturation (69). Li et al (70) evaluated cumulus GJA1 gene
expression levels according to maturation of the oocyte,
fertilization and embryo development. They found GJA1 to be a
promising oocyte maturation predictor, but not a fertilization and
embryo morphology marker, as indicated in previous studies
(71,72).
ESR1 gene, coding estrogen receptor alpha is highly
conserved among the vertebrates, and is nuclear, ligand-activated
transcription factor composed of several domains important for
hormone binding, DNA binding, and activation of transcription
(73). The 5′ region of the gene
contains multiple promoters responsible for tissue-specific gene
expression. ERs form homo- and heterodimers and, when activated,
can bind to specific estrogen response elements (EREs) in genes, to
mediate transcription of various developmental and homeostatic
pathways (74). During sex
differentiation in human, when ESR1 is bound by its main ligand,
estradiol, the whole complex converts to a form binding to nuclear
components, starting the activation or repression of numerous genes
involved in development, organization and other functions. It is
fundamental in maintaining female phenotype of the endocrine
somatic cells of the ovary and development of interstitial
compartment cells, keeping the integrity of female sex
differentiation (75). ESR1 female
knockout homozygous mice appear to develop testis-like features
with Leydig cell-like changes to interstitial cells with symptoms
of sex reversal in the aspect of morphology and function (76). In the human ovary immunoreactivity
for ESR1 was found in thecal, interstitial, and germinal epithelium
cells, moreover it is also noticeable in adrenal cortices, kidneys
mammary gland, bone, heart, hypothalamus, pituitary gland, liver,
lung, spleen, adipose tissue and significantly in epithelial,
stromal, and muscle uterine cells (77). ESR1 plays an important role in a
variety of tissues and structures, like reproductive, central
nervous, skeletal, and cardiovascular systems (78,79).
In addition, there are two splice variants resulted from
alternative splicing of an ESR1, presenting different
tissue-specific manners. ESR1 splice variants were detected in
nuclei of granulosa cells of growing follicles at all stages from
primary to mature follicles, interstitial gland, theca cells and
germinal epithelium cells (80).
It is widely known, that ESR1 is extensively disputed in relation
to breast cancer therapy and valuable use of selective estrogen
receptor modulators (81). Artini
et al (82), interestingly
analyzed human and mouse cumulus cells samples and concluded, that
the downregulation of ESR1 could be related to oocyte competence
and is likely to be the driver of expression changes highlighted in
the PI3K/AKT pathway.
Cyclin D2 (CCND2) is also downregulated after IVM
procedure in porcine oocytes. In general, D-type cyclins are
considered as ultimate recipients of mitogenic and oncogenic
signals and present specific over-expression in some malignancies
in humans. They promote G1/S transition during cell cycle
progression, and the action is based on binding to cyclin-dependent
kinases, increasing retinoblastoma protein phosphorylation and
relieving inhibition of the transcription factor E2F (83). CCND2 is expressed mainly in
granulosa cells in response to FSH in the ovary, and cyclin D2
deficient mice present sterility due to lack of proliferative
action of GCs and resulting in follicle arrest (84). Han et al (85) reveled, that in rat ovaries, FSH
rapidly increased CCND2 expression through both the cAMP/PKA and
PI3K/PDK1/Akt signaling pathways, but also enhanced cAMP-mediated
CCND2 degradation through a ubiquitin-proteasome pathway. Moreover,
the inquiring study on infant mice ovaries published by François
et al (86), showed, that
cyclin D2 expression is influenced by FSH, but in particular
concentrations. Low concentrations were inducing CCND2, resulting
in GCs proliferations, while high levels of FSH were unable to
stimulate cyclin D2-dependent follicular growth, but supporting
steroidogenesis. In the study above, it was suggested, that since
the paracrine oocyte action plays a significant role in development
of ovarian follicles, it would be of interest to determine the
possible contribution of the oocyte in the function of the
infantile ovary. Cyclin D2 could be one of the factors explaining
this process.
Overall, the study has provided us with a plethora
of genes that could potentially become new markers of oocyte in
vitro maturation associated with regulation of transcription
and macromolecule metabolism. However, it needs to be noted that it
is an entry level transcriptomic study, which will need further
validation on protein level, followed by further in vivo
studies to gain clinical significance. Despite that, this research
might serve as a point of reference for future studies and possible
clinical approaches.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants obtained
from the Polish National Centre of Science (grant no.
UMO-2016/21/B/NZ9/03535).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MBra designed and performed the experiments,
selected the models and wrote the manuscript. KO designed the
experiments and provided editorial assistance. WK revised the
medical methodology and performed the experiments. MJN wrote the
manuscript, performed the experiments and analyzed the data. PSK
performed histological experiments and analyzed the data. MJa
analyzed the data and corrected the language. AK performed
histological experiements. IK revised the medical methodology and
performed the experiments. PC analyzed the data, prepared the
figures and wrote the manuscript. MJe revised the medical
methodology and analyzed the models. PA revised the medical
methodology and analyzed the models. DB revised the medical
methodology, provided editorial supervision and interpreted the
data. MTS provided editorial supervision and interpreted the data.
MBru supervised the study, interpreted the data and approved the
manuscript. HPK supervised the project and interpreted the data. MN
designed and supervised the project, and drafted/revised the
manuscript. LP revised the medical methodology, provided editorial
supervision and interpreted the data. MZ designed the study,
revised the methodology and wrote the manuscript. BK designed and
supervised the project, revised the methodology, provided editorial
supervision and wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Experiments were approved by the Local Ethics
Committee in Poznan (resolution no. 32/2012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eppig JJ: Oocyte control of ovarian
follicular development and function in mammals. Reproduction.
122:829–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilchrist RB, Ritter LJ and Armstrong DT:
Oocyte-somatic cell interactions during follicle development in
mammals. Anim Reprod Sci. 82-83:431–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matzuk MM, Burns KH, Viveiros MM and Eppig
JJ: Intercellular communication in the mammalian ovary: Oocytes
carry the conversation. Science. 296:2178–2180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rybska M, Knap S, Jankowski M, Jeseta M,
Bukowska D, Antosik P, Nowicki M, Zabel M, Kempisty B and Jaśkowski
JM: Cytoplasmic and nuclear maturation of oocytes in mammals-living
in the shadow of cells developmental capability. Med J Cell Biol.
6:13–17. 2018. View Article : Google Scholar
|
|
5
|
Rybska M, Knap S, Stefańska K, Jankowski
M, Gliszczyńska AC, Popis M, Jeseta M, Bukowska D, Antosik P,
Kempisty B and Jaśkowski JM: Transforming growth factor (TGF)-is it
a key protein in mammalian reproductive biology? Med J Cell Biol.
6:125–130. 2018. View Article : Google Scholar
|
|
6
|
Rybska M, Knap S, Jankowski M, Jeseta M,
Bukowska D, Antosik P, Nowicki M, Zabel M, Kempisty B and Jaśkowski
JM: Characteristic of factors influencing the proper course of
folliculogenesis in mammals. Med J Cell Biol. 6:33–38. 2018.
View Article : Google Scholar
|
|
7
|
Regassa A, Rings F, Hoelker M, Cinar U,
Tholen E, Looft C, Schellander K and Tesfaye D: Transcriptome
dynamics and molecular cross-talk between bovine oocyte and its
companion cumulus cells. BMC Genomics. 12:572011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borys-Wójcik S, Kocherova I, Celichowski
P, Popis M, Jeseta M, Bukowska D, Antosik P, Nowicki M and Kempisty
B: Protein oligomerization is the biochemical process highly
up-regulated in porcine oocytes before in vitro maturation (IVM).
Med J Cell Biol. 6:155–162. 2018. View Article : Google Scholar
|
|
9
|
Budna J, Celichowski P, Bryja A, Jeseta M,
Jankowski M, Bukowska D, Antosik P, Nowicki A, Brüssow KP, Bruska
M, et al: Expression changes in fatty acid metabolic processrelated
genes in porcine oocytes during in vitro maturation. Med J Cell
Biol. 6:48–54. 2018. View Article : Google Scholar
|
|
10
|
Kranc W, Brązert M, Ożegowska K,
Budna-Tukan J, Celichowski P, Jankowski M, Bryja A, Nawrocki MJ,
Popis M, Jeseta M, et al: Response to abiotic and organic
substances stimulation belongs to ontologic groups significantly
up-regulated in porcine immature oocytes. Med J Cell Biol.
6:91–100. 2018. View Article : Google Scholar
|
|
11
|
Chermuła B, Brązert M, Jeseta M, Ożegowska
K, Sujka-Kordowska P, Konwerska A, Bryja A, Kranc W, Jankowski M,
Nawrocki MJ, et al: The unique mechanisms of cellular
proliferation, migration and apoptosis are regulated through oocyte
maturational development-A complete transcriptomic and
histochemical study. Int J Mol Sci. 20:E842018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ożegowska K, Dyszkiewicz-Konwińska M,
Celichowski P, Nawrocki MJ, Bryja A, Jankowski M, Kranc W, Brązert
M, Knap S, Jeseta M, et al: Expression pattern of new genes
regulating female sex differentiation and in vitro maturational
status of oocytes in pigs. Theriogenology. 121:122–133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borys S, Brązert M, Jankowski M, Kocherova
I, Ożegowska K, Celichowski P, Nawrocki MJ, Kranc W, Bryja A, Kulus
M, et al: Enzyme linked receptor protein signaling pathway is one
of the ontology groups that are highly up-regulated in porcine
oocytes before in vitro maturation. J Biol Regul Homeost Agents.
32:1089–1103. 2018.PubMed/NCBI
|
|
14
|
Pujol M, López-Béjar M and Paramio MT:
Developmental competence of heifer oocytes selected using the
brilliant cresyl blue (BCB) test. Theriogenology. 61:735–744. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Guienne B: Small atlas of bovine
oocyte. Elevage et Insemination (France). 24–30. 1998.[In
French].
|
|
16
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawrocki MJ, Celichowski P, Jankowski M,
Kranc W, Bryja A, Borys-Wójcik S, Jeseta M, Antosik P, Bukowska D,
Bruska M, et al: Ontology groups representing angiogenesis and
blood vessels development are highly up-regulated during porcine
oviductal epithelial cells long-term real-time proliferation-a
primary cell culture approach. Med J Cell Biol. 6:186–194. 2018.
View Article : Google Scholar
|
|
18
|
Budna J, Celichowski P, Karimi P, Kranc W,
Bryja A, Ciesiółka S, Rybska M, Borys S, Jeseta M, Bukowska D, et
al: Does porcine oocytes maturation in vitro is regulated by genes
involved in transforming growth factor beta receptor signaling
pathway? Adv Cell Biol. 5:1–14. 2017. View Article : Google Scholar
|
|
19
|
Curran T and Franza BR Jr: Fos and jun:
The AP-1 connection. Cell. 55:395–397. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Angel P and Karin M: The role of Jun, Fos
and the AP-1 complex in cell-proliferation and transformation.
Biochim Biophys Acta. 1072:129–157. 1991.PubMed/NCBI
|
|
21
|
Rusovici R and LaVoie HA: Expression and
distribution of AP-1 transcription factors in the porcine ovary.
Biol Reprod. 69:64–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson RS, Spiegelman BM and Papaioannou
V: Pleiotropic effects of a null mutation in the c-fos
proto-oncogene. Cell. 71:577–586. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müller R, Tremblay JM, Adamson ED and
Verma IM: Tissue and cell type-specific expression of two human
c-onc genes. Nature. 304:454–456. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hattori MA, Kato Y and Fujihara N:
Retinoic acid suppression of endothelial nitric oxide synthase in
porcine oocyte. Can J Physiol Pharmacol. 80:777–782. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blaha M, Nemcova L, Kepkova KV, Vodicka P
and Prochazka R: Gene expression analysis of pig cumulus-oocyte
complexes stimulated in vitro with follicle stimulating hormone or
epidermal growth factor-like peptides. Reprod Biol Endocrinol.
13:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrara N and Henzel WJ: Pituitary
follicular cells secrete a novel heparin-binding growth factor
specific for vascular endothelial cells. Biochem Biophys Res
Commun. 161:851–858. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sousa LM, Campos DB, Fonseca VU, Viau P,
Kfoury JR Jr, Oliveira CA, Binelli M, Buratini J Jr and Papa PC:
Vascular endothelial growth factor A (VEGFA) modulates bovine
placenta steroidogenesis in vitro. Placenta. 33:788–794. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimizu T: Promotion of ovarian follicular
development by injecting vascular endothelial growth factor (VEGF)
and growth differentiation factor 9 (GDF-9) genes. J Reprod Dev.
52:23–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McFee RM, Rozell TG and Cupp AS: The
balance of proangiogenic and antiangiogenic VEGFA isoforms regulate
follicle development. Cell Tissue Res. 349:635–647. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bui TMT, Nguyễn KX, Karata A, Ferré P,
Trần MT, Wakai T and Funahashi H: Presence of vascular endothelial
growth factor during the first half of IVM improves the meiotic and
developmental competence of porcine oocytes from small follicles.
Reprod Fertil Dev. 29:1902–1909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ravindranath N, Little-Ihrig L, Phillips
HS, Ferrara N and Zeleznik AJ: Vascular endothelial growth factor
messenger ribonucleic acid expression in the primate ovary.
Endocrinology. 131:254–260. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamamoto S, Konishi I, Tsuruta Y, Nanbu K,
Mandai M, Kuroda H, Matsushita K, Hamid AA, Yura Y and Mori T:
Expression of vascular endothelial growth factor (VEGF) during
folliculogenesis and corpus luteum formation in the human ovary.
Gynecol Endocrinol. 11:371–381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu J, Xu M, Bernuci MP, Fisher TE, Shea
LD, Woodruff TK, Zelinski MB and Stouffer RL: Primate follicular
development and oocyte maturation in vitro. Adv Exp Med Biol.
761:43–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Silva CM, Matos MH, Rodrigues GQ, Faustino
LR, Pinto LC, Chaves RN, Araújo VR, Campello CC and Figueiredo JR:
In vitro survival and development of goat preantral follicles in
two different oxygen tensions. Anim Reprod Sci. 117:83–89. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walters KA, Simanainen U and Handelsman
DJ: Molecular insights into androgen actions in male and female
reproductive function from androgen receptor knockout models. Hum
Reprod Update. 16:543–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gleicher N, Weghofer A and Barad DH: The
role of androgens in follicle maturation and ovulation induction:
Friend or foe of infertility treatment? Reprod Biol Endocrinol.
9:1162011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lenie S and Smitz J: Functional AR
signaling is evident in an in vitro mouse follicle culture bioassay
that encompasses most stages of folliculogenesis. Biol Reprod.
80:685–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li M, Ai JS, Xu BZ, Xiong B, Yin S, Lin
SL, Hou Y, Chen DY, Schatten H and Sun QY: Testosterone potentially
triggers meiotic resumption by activation of intra-oocyte SRC and
MAPK in porcine oocytes. Biol Reprod. 79:897–905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Van Nieuwerburgh F, Stoop D, Cabri P,
Dhont M, Deforce D and De Sutter P: Shorter CAG repeats in the
androgen receptor gene may enhance hyperandrogenicity in polycystic
ovary syndrome. Gynecol Endocrinol. 24:669–673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Donovan KJ, Tourtellotte WG, Millbrandt
J and Baraban JM: The EGR family of transcription-regulatory
factors: Progress at the interface of molecular and systems
neuroscience. Trends Neurosci. 22:167–173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shin H, Seol DW, Nam M, Song H, Lee DR and
Lim HJ: Expression of Egr3 in mouse gonads and its localization and
function in oocytes. Asian-Australas J Anim Sci. 30:781–787. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thiel G, Müller I and Rössler OG:
Expression, signaling and function of Egr transcription factors in
pancreatic β-cells and insulin-responsive tissues. Mol Cell
Endocrinol. 388:10–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Safford M, Collins S, Lutz MA, Allen A,
Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH
and Powell JD: Egr-2 and Egr-3 are negative regulators of T cell
activation. Nat Immunol. 6:472–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang F, Ooka K, Bhattachyya S, Wei J, Wu
M, Du P, Lin S, Del Galdo F, Feghali-Bostwick CA and Varga J: The
early growth response gene Egr2 (alias Krox20) is a novel
transcriptional target of transforming growth factor-β that is
up-regulated in systemic sclerosis and mediates profibrotic
responses. Am J Pathol. 178:2077–2090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nagarajan R, Svaren J, Le N, Araki T,
Watson M and Milbrandt J: EGR2 mutations in inherited neuropathies
dominant-negatively inhibit myelin gene expression. Neuron.
30:355–368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu TM, Chen CH, Chuang YA, Hsu SH and
Cheng MC: Resequencing of early growth response 2 (EGR2) gene
revealed a recurrent patient-specific mutation in schizophrenia.
Psychiatry Res. 228:958–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Le N, Nagarajan R, Wang JY, Araki T,
Schmidt RE and Milbrandt J: Analysis of congenital hypomyelinating
Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of schwann cell
differentiation and myelination. Proc Natl Acad Sci USA.
102:2596–2601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Zhang Z, Yu M, Li L, Du G, Xiao W
and Yang H: Involvement of miR-20a in promoting gastric cancer
progression by targeting early growth response 2 (EGR2). Int J Mol
Sci. 14:16226–16239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barbeau DJ, La KT, Kim DS, Kerpedjieva SS,
Shurin GV and Tamama K: Early growth response-2 signaling mediates
immunomodulatory effects of human multipotential stromal cells.
Stem Cells Dev. 23:155–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jin H, Won M, Shin E, Kim HM, Lee K and
Bae J: EGR2 is a gonadotropin-induced survival factor that controls
the expression of IER3 in ovarian granulosa cells. Biochem Biophys
Res Commun. 482:877–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Burger HG: Inhibin: Definition and
nomenclature, including related substances. J Endocrinol.
117:159–160. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Seder CW, Hartojo W, Lin L, Silvers AL,
Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB and
Beer DG: Upregulated INHBA expression may promote cell
proliferation and is associated with poor survival in lung
adenocarcinoma. Neoplasia. 11:388–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Howley BV, Hussey GS, Link LA and Howe PH:
Translational regulation of inhibin βa by TGFβ via the RNA-binding
protein hnRNP E1 enhances the invasiveness of epithelial-to-
mesenchymal transitioned cells. Oncogene. 35:1725–1735. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Katayama Y, Oshima T, Sakamaki K, Aoyama
T, Sato T, Masudo K, Shiozawa M, Yoshikawa T, Rino Y, Imada T and
Masuda M: Clinical significance of INHBA gene expression in
patients with gastric cancer who receive curative resection
followed by adjuvant s-1 chemotherapy. In Vivo. 31:565–571. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Donovan P, Dubey OA, Kallioinen S, Rogers
KW, Muehlethaler K, Müller P, Rimoldi D and Constam DB: Paracrine
activin-A signaling promotes melanoma growth and metastasis through
immune evasion. J Invest Dermatol. 137:2578–2587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Locci M, Wu JE, Arumemi F, Mikulski Z,
Dahlberg C, Miller AT and Crotty S: Activin A programs the
differentiation of human TFH cells. Nat Immunol. 17:976–984. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McKenzie LJ, Pangas SA, Carson SA, Kovanci
E, Cisneros P, Buster JE, Amato P and Matzuk MM: Human cumulus
granulosa cell gene expression: A predictor of fertilization and
embryo selection in women undergoing IVF. Hum Reprod. 19:2869–2874.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Assidi M, Dufort I, Ali A, Hamel M,
Algriany O, Dielemann S and Sirard MA: Identification of potential
markers of oocyte competence expressed in bovine cumulus cells
matured with follicle-stimulating hormone and/or phorbol myristate
acetate in vitro. Biol Reprod. 79:209–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bristol-Gould SK, Kreeger PK, Selkirk CG,
Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE and Woodruff TK:
Postnatal regulation of germ cells by activin: The establishment of
the initial follicle pool. Dev Biol. 298:132–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Myers M, Middlebrook BS, Matzuk MM and
Pangas SA: Loss of inhibin alpha uncouples oocyte-granulosa cell
dynamics and disrupts postnatal folliculogenesis. Dev Biol.
334:458–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Maguire JJ and Davenport AP: Endothelin
receptors and their antagonists. Semin Nephrol. 35:125–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Asai R, Kurihara Y, Fujisawa K, Sato T,
Kawamura Y, Kokubo H, Tonami K, Nishiyama K, Uchijima Y,
Miyagawa-Tomita S and Kurihara H: Endothelin receptor type A
expression defines a distinct cardiac subdomain within the heart
field and is later implicated in chamber myocardium formation.
Development. 137:3823–3833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kawamura K, Ye Y, Liang CG, Kawamura N,
Gelpke MS, Rauch R, Tanaka T and Hsueh AJ: Paracrine regulation of
the resumption of oocyte meiosis by endothelin-1. Dev Biol.
327:62–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cui L, Shen J, Fang L, Mao X, Wang H and
Ye Y: Endothelin-1 promotes human germinal vesicle-stage oocyte
maturation by downregulating connexin-26 expression in cumulus
cells. Mol Hum Reprod. 24:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cheng JC, Chang HM, Fang L, Sun YP and
Leung PC: TGF-β1 up-regulates connexin43 expression: A potential
mechanism for human trophoblast cell differentiation. J Cell
Physiol. 230:1558–1566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Salameh A, Haunschild J, Bräuchle P, Peim
O, Seidel T, Reitmann M, Kostelka M, Bakhtiary F, Dhein S and
Dähnert I: On the role of the gap junction protein Cx43 (GJA1) in
human cardiac malformations with fallot-pathology. A study on
paediatric cardiac specimen. PLoS One. 9:e953442014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Edry I, Sela-Abramovich S and Dekel N:
Meiotic arrest of oocytes depends on cell-to-cell communication in
the ovarian follicle. Mol Cell Endocrinol. 252:102–106. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li SH, Lin MH, Hwu YM, Lu CH, Yeh LY, Chen
YJ and Lee RK: Correlation of cumulus gene expression of GJA1,
PRSS35, PTX3, and SERPINE2 with oocyte maturation, fertilization,
and embryo development. Reprod Biol Endocrinol. 13:932015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hasegawa J, Yanaihara A, Iwasaki S,
Mitsukawa K, Negishi M and Okai T: Reduction of connexin 43 in
human cumulus cells yields good embryo competence during ICSI. J
Assist Reprod Genet. 24:463–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang HX, Tong D, El-Gehani F, Tekpetey FR
and Kidder GM: Connexin expression and gap junctional coupling in
human cumulus cells: Contribution to embryo quality. J Cell Mol
Med. 13:972–984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Amberger JS, Bocchini CA, Schiettecatte F,
Scott AF and Hamosh A: OMIM.org: Online mendelian inheritance in
man (OMIM®), an online catalog of human genes and
genetic disorders. Nucleic Acids Res. 43:D789–D798. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Calatayud NE, Pask AJ, Shaw G, Richings
NM, Osborn S and Renfree MB: Ontogeny of the oestrogen receptors
ESR1 and ESR2 during gonadal development in the tammar wallaby,
Macropus eugenii. Reproduction. 139:599–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jameson JL, DeGroot LJ, De Kretser DM,
Giudice LC, Grossman AB, Melmed S, Potts JT Jr and Weir GC:
Endocrinology: Adult & Pediatric. 7th. Elsevier; Philadelphia,
PA: 2016
|
|
76
|
Labrie F, Luu-The V, Lin SX, Simard J and
Labrie C: Role of 17β-hydroxysteroid dehydrogenases in sex steroid
formation in peripheral intracrine tissues. Trends Endocrinol
Metab. 11:421–427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kuiper GG, Carlsson B, Grandien K, Enmark
E, Häggblad J, Nilsson S and Gustafsson JA: Comparison of the
ligand binding specificity and transcript tissue distribution of
estrogen receptors alpha and beta. Endocrinology. 138:863–870.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bondesson M, Hao R, Lin CY, Williams C and
Gustafsson JÅ: Estrogen receptor signaling during vertebrate
development. Biochim Biophys Acta. 1849:142–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Paterni I, Granchi C, Katzenellenbogen JA
and Minutolo F: Estrogen receptors alpha (ERα) and beta (ERβ):
Subtype-selective ligands and clinical potential. Steroids.
90:13–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pelletier G and El-Alfy M:
Immunocytochemical localization of estrogen receptors alpha and
beta in the human reproductive organs. J Clin Endocrinol Metab.
85:4835–4840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nathan MR and Schmid P: A review of
fulvestrant in breast cancer. Oncol Ther. 5:17–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Artini PG, Tatone C, Sperduti S, D'Aurora
M, Franchi S, Di Emidio G, Ciriminna R, Vento M, Di Pietro C,
Stuppia L, et al: Cumulus cells surrounding oocytes with high
developmental competence exhibit down-regulation of phosphoinositol
1, 3 kinase/protein kinase B (PI3K/AKT) signalling genes involved
in proliferation and survival. Hum Reprod. 32:2474–2484. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Robker RL and Richards JS: Hormone-induced
proliferation and differentiation of granulosa cells: A coordinated
balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol
Endocrinol. 12:924–940. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Han Y, Xia G and Tsang BK: Regulation of
cyclin D2 expression and degradation by follicle-stimulating
hormone during rat granulosa cell proliferation in vitro. Biol
Reprod. 88:572013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
François CM, Petit F, Giton F, Gougeon A,
Ravel C, Magre S, Cohen-Tannoudji J and Guigon CJ: A novel action
of follicle-stimulating hormone in the ovary promotes estradiol
production without inducing excessive follicular growth before
puberty. Sci Rep. 7:462222017. View Article : Google Scholar : PubMed/NCBI
|