Introduction
Adult mesenchymal stem cells (MSCs) are
fibroblast-like, multipotent cells that can be isolated from bone
marrow (BM), adipose tissue, umbilical cord, skeletal muscle, liver
and tumoral tissues (1–3). MSCs display unique characteristics
that enable them to develop into several different cell types,
including osteoblasts, adipocytes, chondrocytes and
hematopoiesis-supportive stroma (4); they can be mobilized from BM, and
other tissues, to sites of inflammation, such as areas of injury
and tumors (5–8), respond to the local microenvironment,
and exert immunosuppressive and anti-inflammatory activities
(9,10). Previous studies have reported that
MSCs can promote the growth, metastasis and invasion of cancers
(11–13); for example, Zhu et al
(14) demonstrated that the
inhibition of microRNA (miR)-155-5p promoted the transition of
BM-MSCs into gastric cancer-MSCs through the activation of the
NF-κB p65-signaling pathway. MSCs also reportedly induce the
expression of discoidin domain-containing receptor 2 to mediate the
growth and metastasis of breast cancer (8). MSC senescence influences the growth,
metastasis and angiogenesis of colon cancer by secreting galectin-3
(15), and MSCs are reported to
represent promising potential for their use in cancer therapy; with
Zhang et al (16)
demonstrating that MSCs have potential beneficial effects for
breast cancer therapy through the targeting of fibronectin 1, CD44
and nerve growth factor.
p53 is a prominent transcription factor and tumor
suppressor gene that regulates the homeostasis of cells (17), as well as several cellular
processes, such as cell cycle control and growth, differentiation
and DNA repair; therefore, p53 is often referred to as the guardian
of the genome (18). A mutation or
loss of p53 expression occurs in ~50% of human cancers (19,20),
and p53 mutations can lead to genome instability, functional
alterations in cell proliferation, migration, differentiation and
the cell cycle, and the aberrant transformation of MSCs. For
example, the absence of p53 can increase the osteogenic
differentiation of BM-MSCs (21–23),
and the inactivation of p53 skews MSCs towards an osteogenic fate
and impairs hematopoiesis-supporting activity (24). p53 abnormality is correlated with
the transformation of MSCs, which promotes mesodermal tumor
formation (18,25,26).
The differential characteristics of mouse (m)BM-MSCs
exhibiting distinct p53 statuses has not been thoroughly
investigated. In the present study, the characteristics of mBM-MSCs
obtained from p53 wild-type (p53+/+), p53 knockdown
(p53+/−) and p53 knockout (p53−/−) mice were
analyzed to investigate their abilities to grow, differentiate and
target stemness-related proteins, in addition to their ability to
target miRNA and protein expression, as well as inflammatory
cytokine secretion, to provide novel evidence for the role of
stromal p53.
Materials and methods
Animal studies and the isolation and
culture of mBM-MSCs
All experimental procedures involving animals were
conducted in accordance with the Guide for the Care and Use of
Laboratory Animals and were approved by the Animal Use Ethics
Committee of Jiangsu University (Zhenjiang, China). A total of 18
C57BL/6 mice (sex, male; weight, 15–20 g; age, 6–8 weeks;
n=6/group) with a p53+/+, p53+/− or
p53−/− genotype were obtained from Nanjing Medical
University (Nanjing, China), and were housed under standard
conditions at 20–26°C and 40–70% humidity, in a 12-h light/dark
cycle with free access to food and water. Mice were euthanized by
CO2 inhalation; mice were placed in an enclosed box and
CO2 was released at a flow rate of 2.5 l/min, with a
displacement rate of 28% volume/min. Death was ensured following
confirmation that the mice exhibited no breathing, pupil dilation
and no heartbeat. The BM was collected from mice by flushing the
femurs. Cells from the BM were cultured in DMEM with low glucose
(Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 15%
FBS (Invitrogen; Thermo Fisher Scientific, Inc.) and 50 U/ml
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
and maintained in a humidified atmosphere at 37°C with 5%
CO2 for 4 days to facilitate attachment. Non-adherent
cells were removed after 4 days incubation by changing the culture
medium. Cells were trypsinized with 0.25% trypsin/0.1% EDTA
(Sigma-Aldrich, Merck KGaA) and re-plated at 8×103
cells/cm2 (approximately 1:3), and the medium was
changed every 3 days. Homogeneous fibroblast-like cell populations
appeared after five passages, and mBM-MSCs obtained at passage five
were used for subsequent experimentation.
Morphology detection
mBM-MSCs were cultured in DMEM with low glucose
(Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 15%
FBS (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were
trypsinized with 0.25% trypsin/0.1% EDTA (Sigma-Aldrich; Merck
KGaA) and re-plated at 8×103 cells/cm2 for 12
h at 37°C. Cells were subsequently visualized using an Olympus
CKX41 inverted phase contrast light microscope (magnification,
×20).
Flow cytometry
mBM-MSCs were trypsinized with 0.25% trypsin/0.1%
EDTA and washed twice with 10.2 g/l PBS (pH=7.2). Cells were
subsequently incubated on ice with the following monoclonal
antibodies (1:250): FITC-conjugated CD29 (cat. no. 561796; BD
Pharminogen; BD Biosciences), FITC-conjugated CD34 (cat. no.
560238; BD Pharminogen; BD Biosciences), FITC-conjugated CD90 (cat.
no. 561973; BD Pharminogen; BD Biosciences), phycoerythrin
(PE)-conjugated CD44 (cat. no. 553134; BD Pharminogen; BD
Biosciences), PE-conjugated CD45 (cat. 553081; BD Pharminogen; BD
Biosciences) or PE-conjugated CD11b (cat. no 12-0112-82;
eBioscience; Thermo Fisher Scientific, Inc.). PE- and
FITC-conjugated IgM and IgG were used as controls. Labeled cells
were analyzed by flow cytometry using a BD FACSCaliburä flow
cytometer (BD Biosciences) and FlowJo version 10 software (Tree
Star, Inc.).
Growth curves
p53+/+, p53+/− and
p53−/− mBM-MSCs were seeded in 24-well plates
(5×103 cells/well) during the logarithmic growth phase,
and the number of cells/well was counted on 12 consecutive days
manually. Briefly, the cells in the three duplicated wells were
digested and the number of cells were counted using an abalone
counting board.
Cell cycle analysis
To determine DNA synthesis, the cells were
trypsinized and harvested by centrifugation (25°C; 92 × g; 5 min).
The pellets were resuspended in 0.1% PBS and subsequently fixed
with 75% ethanol at −20°C for 12 h, and then permeabilized with
0.1% Triton X-100 at room temperature for 20 min. Following
treatment with RNase at 37°C for 30 min, the cells were incubated
with propidium iodide (50 µg/ml) at 25°C for 15 min. Cells were
analyzed by flow cytometry using a BD FACSCalibur™ flow cytometer
(BD Biosciences). The percentage of cells in the G1, S and G2
phases were quantified using BD CellQuestä version 5.1 software (BD
Biosciences).
Tumor formation assay
For the tumor formation assay, BALB/c nu/nu mice
(age, 4 weeks; sex, male; weight, 15–20 g; n=6/group) obtained from
The Shanghai Animal Laboratory Center of the Chinese Academy of
Sciences, were injected subcutaneously under the armpit with
1×106 and 5×106 p53+/+,
p53+/− or p53−/− mBM-MSCs (passage no. 9, 11,
13 or 15) suspended in 200 µl 0.1% PBS, and the incidence of tumor
formation was observed for 3 months. Mice were euthanized as
previously described above at 3 months prior to measuring the tumor
size.
Hematoxylin & eosin staining
Tumor sections were fixed in formalin for 24 h at
room temperature and embedded in paraffin. Paraffin-embedded
tissues were cut into 4–6-µm thick sections. The tissue sections
were subsequently deparaffinized in xylene for 12 h at 75°C,
rehydrated using a descending ethanol series (100, 95, 85 and 75%
for 2 min each) and then boiled for 30 min in 10 mM citrate buffer
(pH 6.0) for antigen retrieval. Endogenous peroxidase activity was
inhibited by exposing the sections to 3% hydrogen peroxide for 10
min at room temperature. Sections were stained with 0.2%
hematoxylin for 5 min at room temperature and then 0.5% eosin for
1–3 min at room temperature. Stained cells were visualized using an
Olympus CKX41 inverted phase contrast light microscope
(magnification, ×100; Olympus Corporation).
Adipogenic and osteogenic
differentiation in vitro
p53+/+, p53+/− and
p53−/− mBM-MSCs were seeded at 5×103
cells/cm2 in 35-mm plates and cultured in L-DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) with 15% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.), and either adipogenic
[1 µM dexamethasone and 10 µg/ml insulin (Sigma-Aldrich, Merck
KGaA)] or osteogenic [0.1 µM dexamethasone, 10 µM
β-glycerophosphate, 50 µg/l ascorbic acid, and 4 µg/ml basic
fibroblast growth factor (Sigma-Aldrich, Merck KGaA)] supplements
for a total induction period of 1–2 weeks; with the medium being
changed three times/week. Following induction, intracellular lipid
accumulation was visualized using Oil Red O staining. Briefly,
cells were fixed with 10% neutral formaldehyde for 30 min at room
temperature and were subsequently incubated with 0.5% Oil Red O
solution for 10 min at room temperature. Osteogenic differentiation
was assessed by examining alkaline phosphatase activity through
alkaline phosphatase staining. Briefly, the cells were fixed in 10%
formalin methanol solution for 10 min at 0.5°C and subsequently
washed in distilled water. Cells were stained with a solution
containing 35 mg α-phosphate naphthol sodium, 35 mg Fast Garnet and
35 ml 0.05 M acrylamide for 5–10 min at room temperature. The cells
were subsequently washed with tap water for 10 min and the
cytoplasm of the cells could be viewed as light red granules. Cells
cultured in basic medium were stained with the two staining
reagents, as described above, to serve as the negative controls.
Stained cells were visualized using an Olympus CKX41 inverted phase
contrast light microscope (magnification, ×100; Olympus
Corporation).
Colony formation assay
p53+/+, p53+/− and
p53−/− mBM-MSCs were harvested, seeded at
1×103 cells/well in 35-mm plates, and incubated in a
humidified atmosphere at 37°C and 5% CO2 for 14 days;
with the L-DMEM, supplemented with 15% FBS (Invitrogen; Thermo
Fisher Scientific, Inc.) being replaced every 3 days. Following
incubation, colonies were subsequently fixed with 4%
paraformaldehyde for 20 min at room temperature prior to being
stained with 0.5% crystal violet at room temperature for 20 min.
Stained colonies of 0.3–1.0 mm were counted using a Vernier Caliper
and the formation of colonies was semi-quantified using ImageJ
version 1.8.0 software (National Institutes of Health).
Transwell migration assay
A total of 1×105 p53+/+,
p53+/− and p53−/− mBM-MSCs/well were plated
in the upper chambers of Transwell plates (Corning Inc.) with
serum-free DMEM, and DMEM, supplemented with 15% FBS was plated in
the lower chambers. Following incubation for 12 h at 37°C, the
cells remaining on the upper surface of the membrane were removed
with a cotton swab. The cells that migrated through the 8-mm pores
and adhered to the lower surface of the membrane were fixed with 4%
paraformaldehyde at room temperature for 20 min, and subsequently
stained with 0.5% crystal violet at room temperature for 20 min.
Stained cells were visualized using an Olympus CKX41 inverted phase
contrast light microscope (magnification, ×20; Olympus
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from p53+/+,
p53+/− and p53−/− mBM-MSCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the miScriptIIRT kit (cat. no.
218161; Qiagen China Co., Ltd.), according to the manufacturer's
protocol. The following RT temperature protocol was used: 37°C for
1 h and 95°C for 5 min. qPCR was subsequently performed using the
miScript SYBR Green PCR kit (cat. no. 218073; Qiagen China Co.,
Ltd.), according to the manufacturer's protocol, and the CFX96
Touch Real-Time PCR detection system (Bio-Rad Laboratories, Inc.).
The primer pairs used for the qPCR were obtained from Qiagen (cat.
no. 218073; Qiagen China Co., Ltd.). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
10 min; and 40 cycles of 95°C for 10 sec, 55°C for 30 sec and 72°C
for 32 sec. miRNA expression levels were quantified using the
2−ΔΔCq method (27) and
normalized to the internal reference gene U6.
Western blotting
Total protein was extracted from p53+/+,
p53+/− and p53−/− mBM-MSCs using RIPA buffer
supplemented with protease inhibitors (Santa Cruz Biotechnology,
Inc.). Total protein concentration was quantified using a BCA assay
kit (Thermo Fisher Scientific, Inc.) and 40 µg protein/lane was
separated by 10% SDS-PAGE. Following electrophoresis, the separated
proteins were subsequently transferred onto a PVDF membrane and
blocked in 5% (w/v) non-fat milk for 1 h at room temperature. The
membranes were incubated with the following primary antibodies:
Anti-GAPDH (1:1,000; cat. no. KC-5G5; Kangchen BioTech Co., Ltd.),
anti-p53 (1:1,000; cat. no. abs130596; Absin Bioscience, Inc.),
anti-Sal-like protein 4 (SALL4); (1:500; cat. no. ab29112; Abcam),
anti-protein lin-28 homolog B (LIN28B; 1:500; cat. no. 21626;
Signalway Antibody LLC), anti-Sox2 (1:500; cat. no. ab5603; EMD
Millipore), anti-octamer-binding protein 4 (Oct4; 1:400; cat. no.
21424; Signalway Antibody LLC), anti-c-Myc (1:200; cat. no.
10057-1-AP; ProteinTech Group, Inc.), anti-ubiquitin protein ligase
E3 component n-recognin 2 (UBR2; 1:500; cat. no. BS60150; Bioworld
Technology, Inc.), anti-matrix metalloproteinase 19 (MMP19; 1:500;
cat. no. BS1235; Bioworld Technology, Inc.) and anti-RING-finger
protein 31 (RNF31; 1:500; cat. no ab46322; Abcam). Following the
primary incubation, membranes were incubated for 1 h at room
temperature with horseradish peroxidase-conjugated secondary
antibodies: Goat anti-rabbit IgG (1:2,000; cat. no. CW0103; CoWin
Biosciences) and goat anti-mouse IgG (1:2,000; cat. no. CW0102;
CoWin Biosciences). Protein bands were visualized using the
Immobilon Western Chemiluminescent HRP substrate (EMD Millipore).
GAPDH was used as the loading control. Expression levels were
quantified using ImageJ version 1.8.0 software (National Institutes
of Health).
Luminex assay
Supernatants from p53+/+,
p53+/− and p53−/− mBM-MSCs were collected by
centrifugation (500 × g; 10 min; 25°C) to remove cellular debris
following 6–8 h of cell culture. The MILLIPLEX MAP Mouse
Cytokine/Chemokine Magnetic Bead Panel kit (cat. no.
MCYTOMAG-70K-12; Sigma-Aldrich; Merck KGaA) was used to assess
cytokine levels of tumor necrosis factor (TNF)-α and
interferon-γ-inducible protein (IP)-10 in the supernatants,
according to the manufacturer's protocol. The final detection and
analysis were performed using the Luminex 200™ system (Merck
KGaA).
Statistical analysis
All data are expressed as the mean ± SD from ≥3
independent experimental repeats. The statistical differences
between groups were determined using an one-way ANOVA, followed by
Tukey's range test using GraphPad Prism version 5 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphology of mBM-MSCs and p53 protein
expression
Following the initial 8–14 days (passage 5) in
culture, mBM-MSCs adhered to a plastic surface and presented as a
mixture of fibroblastic and hematopoietic cell types, as determined
by the expression of surface markers (Figs. S1 and S2). Following 20 days from initial
plating, the cells demonstrated a long, spindle-shaped fibroblast
phenotype, began to form colonies and become confluent. After being
re-plated for 20 days, the fibroblast-like cells appeared polygonal
or spindly, with a long process and an orderly pattern at
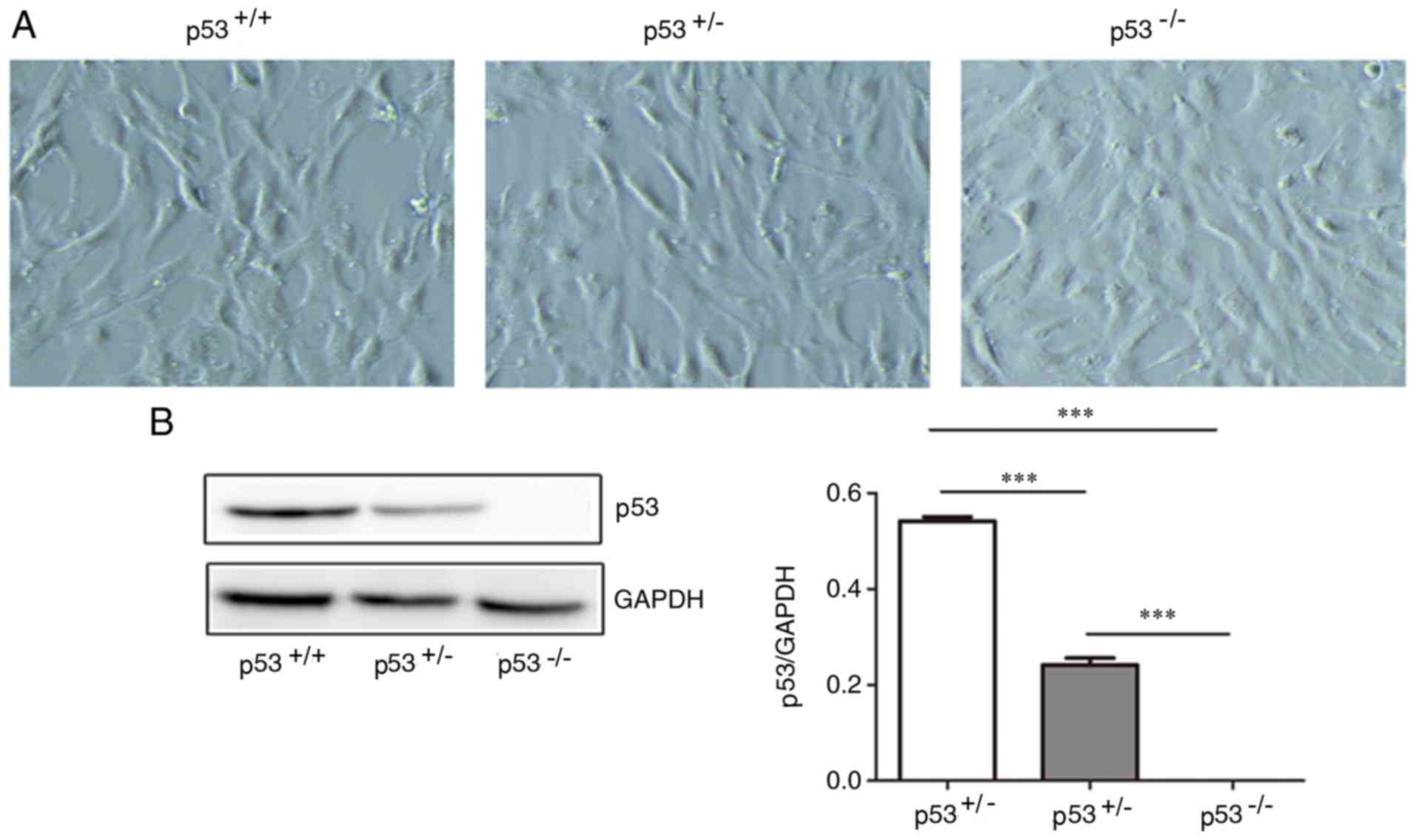
confluence (Fig. 1A). The
p53+/+, p53+/− and p53−/− mBM-MSCs
were observed to share similar phenotypes on the basis of typical
morphology. p53+/+ mBM-MSCs expressed the highest levels
of p53 protein, whilst p53+/− mBM-MSCs displayed
intermediate expression levels of p53 protein and p53−/−
mBM-MSCs expressed very little levels of p53 protein (Fig. 1B).
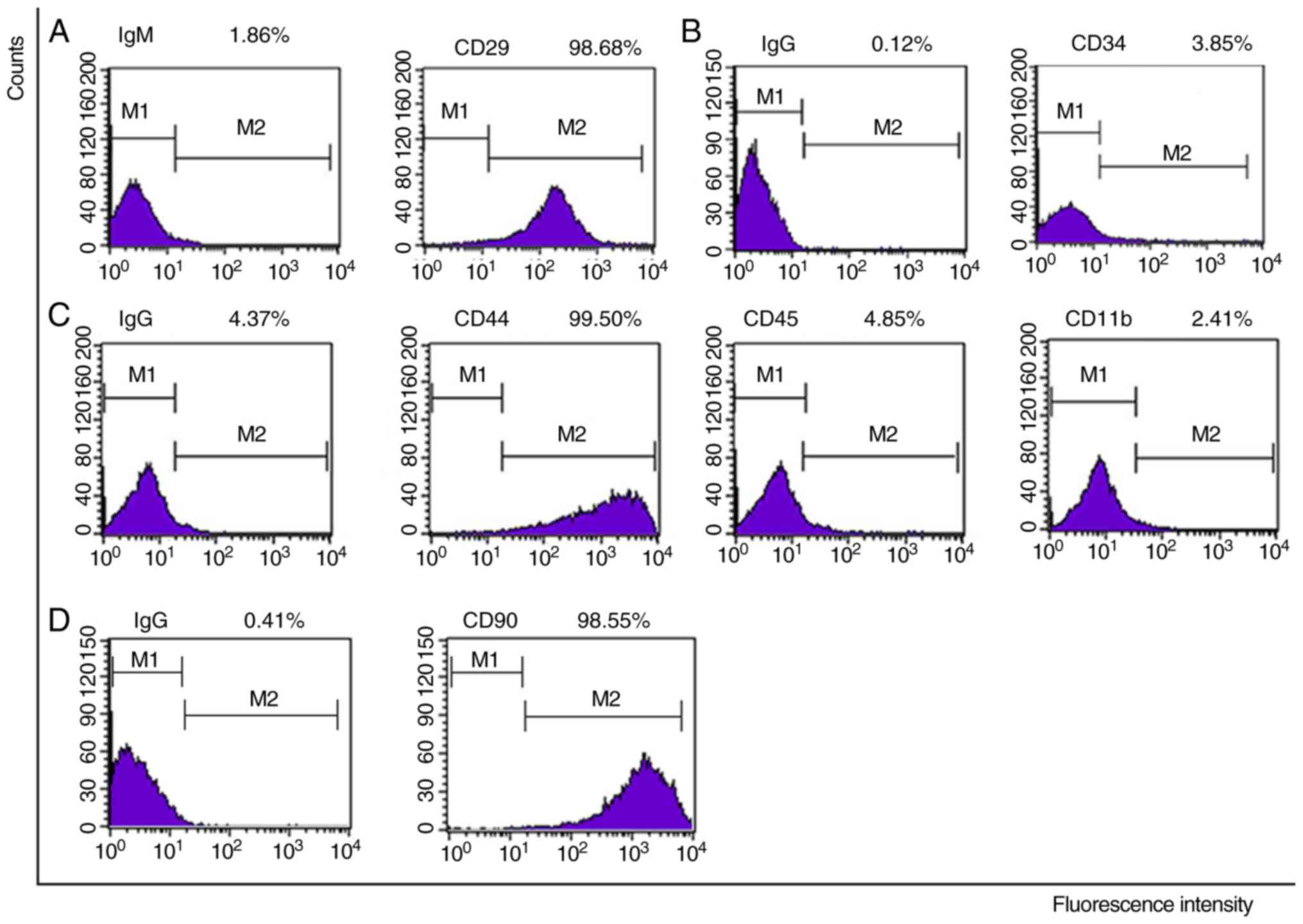
Surface antigens
Following five cell passages, p53+/+,
p53+/− and p53−/− mBM-MSCs were characterized
by determining the expression of stem cell markers, CD29 (98.68,
99.74 and 99.72%, respectively), CD44 (99.50, 99.06 and 99.43%,
respectively) and CD90 (98.55, 97.43 and 98.71%, respectively),
hemocyte markers, CD34 (3.85, 2.67 and 4.99%, respectively) and
CD45 (4.85, 0.42 and 0.95%, respectively), and the macrophage
marker, CD11b (2.41, 2.72 and 2.58%, respectively; Figs. 2, S1 and S2). The three types of mBM-MSCs were all
positive for CD29, CD44 and CD90, but were negative for CD45, CD34,
and CD11b.
Cell cycle analysis of mBM-MSCs
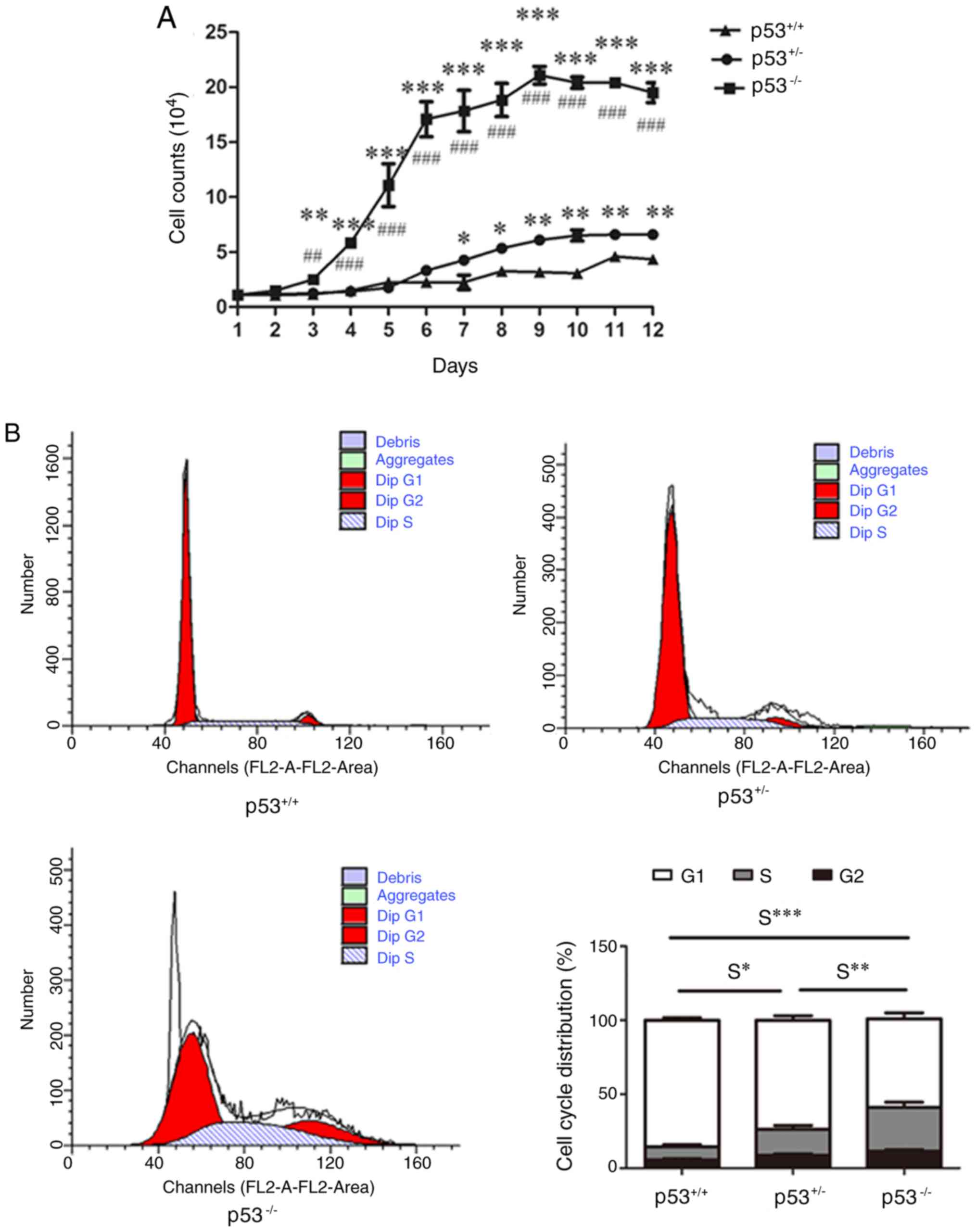
p53+/+ mBM-MSCs demonstrated slow growth
rate throughout the 12-day culture (Fig. 3A); however, 3 days post-seeding,
p53−/− mBM-MSCs began to expand rapidly and move into
the logarithmic phase of growth. At day 9, cell counts reached
their highest levels in p53−/− mBM-MSCs, before
subsequently entering the plateau phase. At 3 days post-seeding,
the number of p53−/− mBM-MSCs was ~2 times that of
p53+/+ mBM-MSCs. From day 6, the number of
p53+/− mBM-MSCs was greater than that of
p53+/+ mBM-MSCs. p53−/− mBM-MSCs were
observed to grow faster than p53+/− mBM-MSCs from day 3
until day 12 (Fig. 3A). Cell cycle
analysis indicated that the S phase rate of p53−/− and
p53+/− mBM-MSCs was significantly higher compared with
p53+/+ mBM-MSCs (Fig.
3B), and p53−/− mBM-MSCs demonstrated an increased S
phase rate compared with p53+/− mBM-MSCs (Fig. 3B).
mBM-MSCs are unable to form
spontaneous tumors in mice regardless of p53 status
To study the ability of p53+/+,
p53+/− and p53−/− mBM-MSCs to induce tumor
formation, different numbers of cells from different passages were
subcutaneously injected into BALB/c nu/nu mice. Three months after
injection, no tumors were observed to have formed in mice from any
group, regardless of the number of cells injected or the cell
passage number used. Thus, p53+/+, p53+/− and
p53−/− mBM-MSCs were unable to form spontaneous
transformation in mice within a three-month period.
Differentiation of mBM-MSCs into
adipocytes and osteocytes
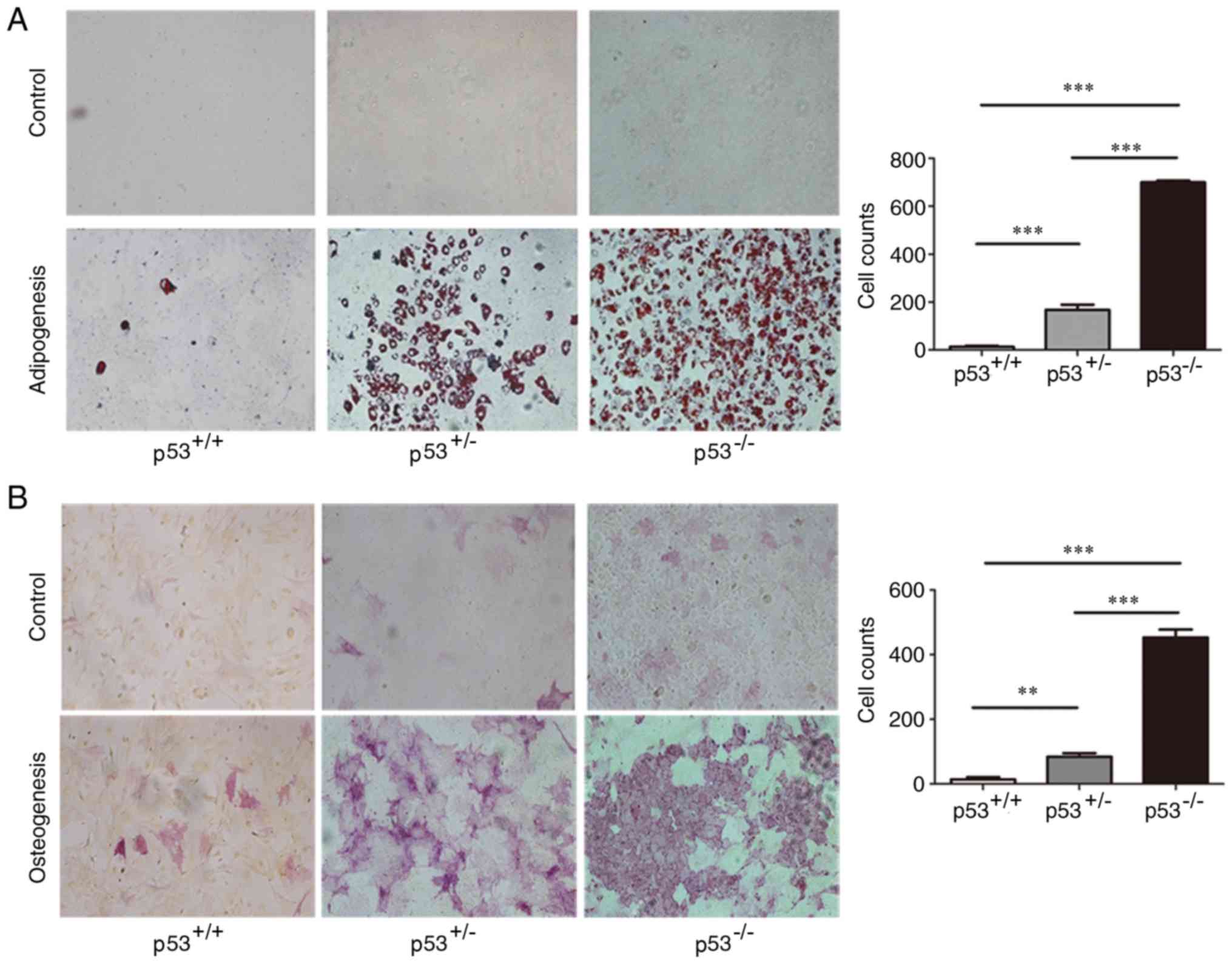
p53+/− and p53−/− mBM-MSCs
induced with adipogenic medium for 2 weeks were observed to contain
a significant number of Oil Red O-positive lipid droplets compared
with p53+/+ mBM-MSCs (Fig.
4A); in addition, p53−/− mBM-MSCs exhibited
significantly more positive cells compared with p53+/−
mBM-MSCs. Following osteogenic supplementation, p53−/−
mBM-MSCs had significantly more alkaline phosphatase-positive cells
compared with p53+/− mBM-MSCs, and both demonstrated
significantly enhanced osteogenic differentiation levels compared
with p53+/+ mBM-MSCs (Fig.
4B). Thus, overall, the p53+/+ mBM-MSCs displayed
very little adipogenic or osteogenic differentiation.
Colony formation and migratory ability
of mBM-MSCs
Colony formation assay analysis demonstrated that
p53+/− and p53−/− mBM-MSCs formed
significantly more colonies compared with p53+/+
mBM-MSCs (Fig. 5A); in addition,
p53−/− mBM-MSCs were observed to form significantly more
colonies compared with p53+/− mBM-MSCs. The role of p53
in regulating mBM-MSC motility was determined using the Transwell
migration assay. Compared with p53+/+ mBM-MSCs, the
migratory rate of p53+/− and p53−/− mBM-MSCs
was significantly increased (Fig.
5B); however, there was no significant difference between the
migration rate of p53+/− and p53−/− mBM-MSCs
(Fig. 5B).
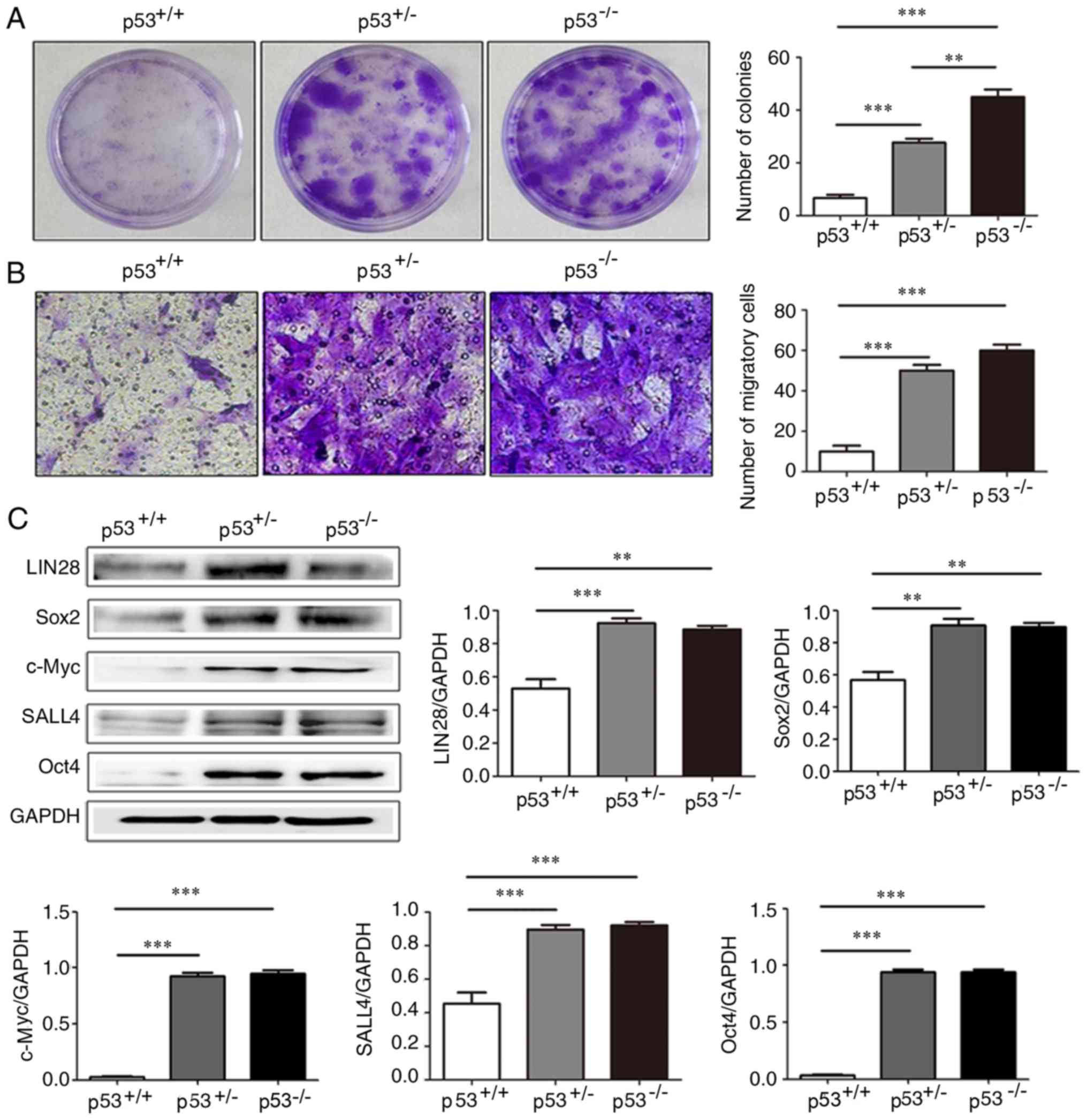 | Figure 5.Colony forming and migratory ability,
and the expression of stemness-related proteins in, mBM-MSCs with
differential levels of p53 expression. (A) Colony formation assay
of p53+/+, p53+/− and p53−/−
mBM-MSCs. (B) Migration assay of p53+/+,
p53+/− and p53−/− mBM-MSCs. (C) Western blot
assay and densitometric analysis for the expression of LIN28B,
Sox2, c-Myc, SALL4 and Oct4 in p53+/+, p53+/−
and p53−/− mBM-MSCs. **P<0.01 and ***P<0.001.
LIN28B, protein lin-28 homolog B; mBM-MSCs, mouse bone marrow
mesenchymal stem cells; Oct4, octamer binding protein 4; SALL4,
Sal-like protein 3. |
Expression of stem cell-associated
proteins in mBM-MSCs
Western blot analysis revealed that stem
cell-related proteins LIN28B, Sox2, c-Myc, SALL4 and Oct4 were
expressed at significantly higher expression levels in
p53+/− and p53−/− mBM-MSCs compared with
p53+/+ mBM-MSCs; however, no significant differences in
the protein expression levels were observed between
p53+/− and p53−/− mBM-MSCs (Fig. 5C).
Differential miRNA expression in
mBM-MSCs
To establish whether p53 gene exerted its activity
through microRNAs (miRNAs), RT-qPCR was performed to determine the
levels of miRNAs in mBM-MSCs with differential p53 expression, that
have previously been reported to serve vital roles in cancer
progression, including miR-221, miR-155, miR-1288, miR-4669,
miR-3152 and miR-337 (Fig. 6A)
(28–39). The expression levels of miR-3152
and miR-337 were significantly increased in p53+/− and
p53−/− mBM-MSCs compared with p53+/+
mBM-MSCs, whereas the expression levels of miR-221, miR-155,
miR-1288 and miR-4669 were significantly decreased in
p53+/− and p53−/− mBM-MSCs compared with
p53+/+ mBM-MSCs (Fig.
6A). Among these miRNAs, miR-3152, miR-337 and miR-221 were
expressed at significantly higher levels in the p53−/−
mBM-MSCs compared with p53+/− (Fig. 6A).
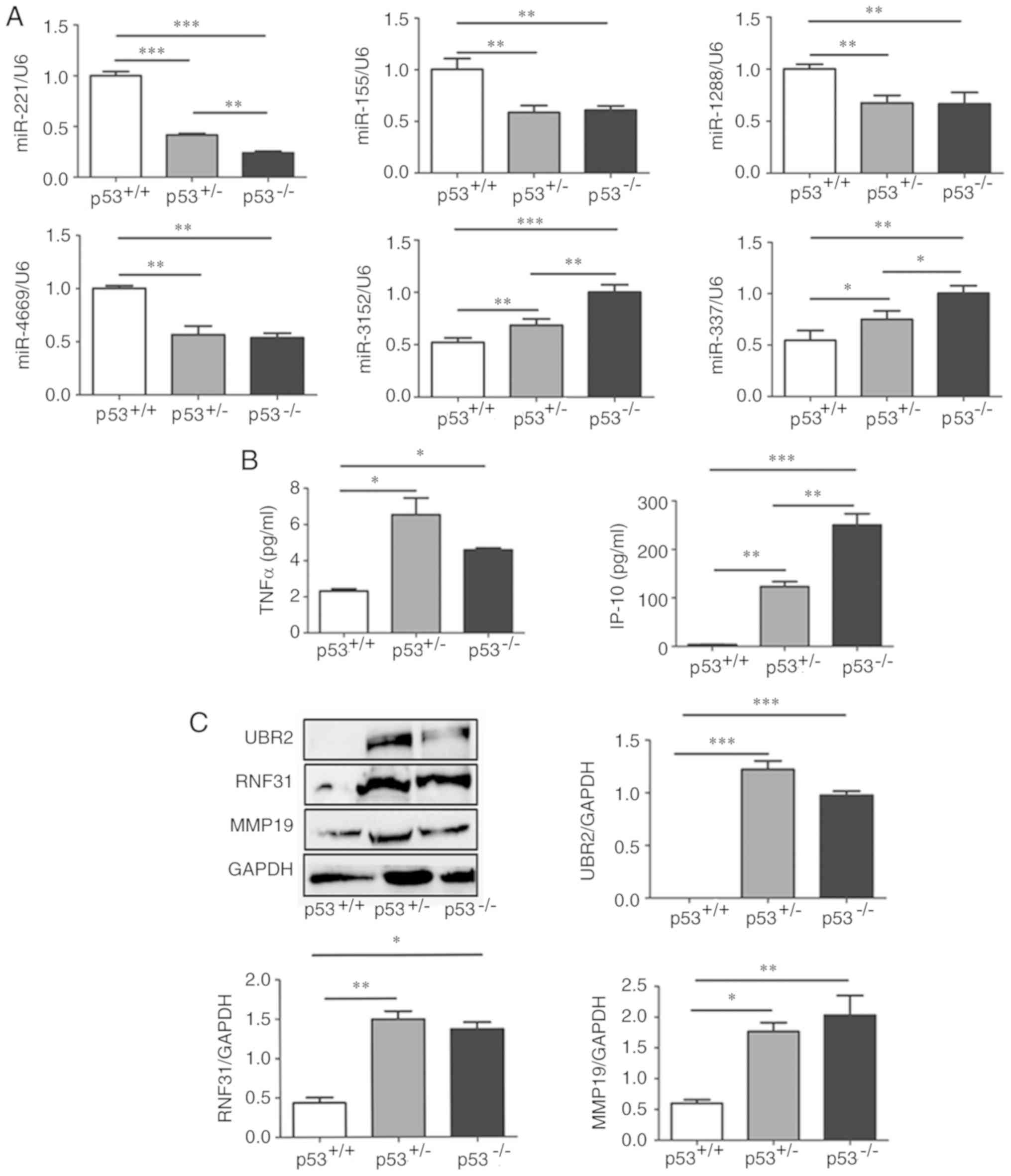 | Figure 6.Expression of miRNAs, cytokines and
proteins in mBM-MSCs with differential p53 statuses. (A) Reverse
transcription-quantitative PCR analysis of miRNAs expression levels
of in p53+/+, p53+/− and p53−/−
mBM-MSCs; expressions were normalized to U6. (B) Luminex assay of
TNFα and IP-10 secretion in the supernatant obtained from
p53+/+, p53+/− and p53−/−
mBM-MSCs. (C) Western blotting and densitometric analysis of
protein expression levels of UBR2, RNF31 and MMP19 in
p53+/+, p53+/− and p53−/−
mBM-MSCs; GAPDH was used as a loading control. *P<0.05,
**P<0.01 and ***P<0.001. IP-10, interferon-γ-inducible
protein; mBM-MSCs, mouse bone marrow mesenchymal stem cells;
miR/miRNA, microRNA; MMP19, matrix metalloproteinase 19; RNF31,
RING-finger protein 31; TNFα, tumor necrosis factor α; UBR2,
ubiquitin protein ligase E3 component n-recognin. |
Inflammatory cytokine secretion
To determine whether p53 status affected
inflammatory cytokine secretion from mBM-MSCs, the Luminex analysis
system was used to determine the content of several inflammation-
and cancer-related cytokines in the cell culture supernatant. The
expression levels of TNF-α and IP-10 were significantly upregulated
in the supernatant of p53+/− and p53−/−
mBM-MSCs compared with p53+/+ mBM-MSCs (Fig. 6B); however, whilst
p53+/− mBM-MSCs secreted significantly less IP-10
compared with p53−/− mBM-MSCs, they secreted higher
levels of TNFα compared with p53−/− mBM-MSCs, but no
statistical difference was observed (Fig. 6B).
Differential protein expression of
mBM-MSCs
Previous proteomic analysis has confirmed that UBR2,
RNF31 and MMP19 are enriched in human umbilical cord MSC-derived
exosomes (40); the three proteins
were reported to promote cellular proliferation and metastasis in
tumor progression (41–43). The protein expression levels of the
three proteins in p53+/− and p53−/− mBM-MSCs
were significantly higher compared with p53+/+ mBM-MSCs
(Fig. 6C); however, no significant
differences in the protein expression levels of the three proteins
were observed between p53+/− and p53−/−
mBM-MSCs (Fig. 6C).
Discussion
The tumor suppressor gene p53 has numerous functions
in biological processes, such as bone homeostasis, organogenesis
and neoplasia (24). However, the
role of MSCs exhibiting differential p53 statuses remains poorly
described. In the present study, p53 wild-type (p53+/+),
p53 knockdown (p53+/−) and p53 knockout
(p53−/−) mBM-MSCs were analyzed to determine the effect
of p53 on the biology of MSCs. Successful isolation, purification
and culture of mBM-MSCs revealed that p53+/+,
p53+/− and p53−/− mBM-MSCs all presented with
a fibroblast-like appearance; they all positively expressed the
typical surface antigens, CD29, CD44, CD90, and negatively
expressed CD34, CD45, and CD11b (44,45).
Thus, despite the three groups of mBM-MSCs exhibiting differential
p53 levels, no noticeable difference was observed in their
morphology and surface antigens presentation.
Previous studies in primary murine cells reported
that p53 accumulation and stabilization could promote increased
apoptosis and induce cell cycle arrest (46,47).
The present study demonstrated, through growth curve assays and DNA
cell cycle analysis, that p53+/− and p53−/−
mBM-MSCs possessed a higher proliferative potential compared with
p53+/+ mBM-MSCs, and that the absence, or partial
absence, of p53 corresponded with an increased S phase rate within
the cell cycle compared with basal p53 levels. These results
revealed that the differential expression of p53 influenced the
cell cycle distribution and proliferation of MSCs, which is
consistent with a previous study (18). The presence and correct functioning
of p53 is essential to avoid the spontaneous transformation of
MSCs; with the long-term absence of p53 promoting genomic
instability in MSCs, and eventually tumorigenesis (23). Results from the present study
indicated that the p53 status did not affect tumorigenic ability,
and no malignant transformation arising from mBM-MSCs with distinct
p53 status was evident over the three-month period in vivo,
indicating the maintenance of genomic stability of the three
mBM-MSC groups across the time period. Extended time-points and
cell passages may be required to observe the transformation process
in vivo.
MSCs are multipotent and differentiate into diverse
cell types; however, the role of p53 in the regulation of this
differentiation process is relatively unknown. He et al
(22) reported that mBM-MSCs
deficient in p53 exhibit enhanced osteogenic differentiation
properties, but similar adipogenic differentiation properties
compared with mBM-MSCs with wild-type p53. Boregowda et al
(24) demonstrated that the loss
of p53 strongly skews MSCs towards an osteogenic fate at the
expense of adipogenesis, due to the depletion of mitochondrial ROS,
which was induced in a closed low-oxygen (5%) cultured environment.
The present study revealed that p53+/− and
p53−/− mBM-MSCs have significantly increased adipogenic
and osteogenic differentiation ability compared with
p53+/+ mBM-MSCs, which agrees with a previous study by
Armesilla-Diaz et al (23).
Thus, the differentiation processes of MSCs may be controlled by
several factors, including p53, and the discrepancies of its role
in adipogenic differentiation will require further
investigation.
p53 can inhibit the proliferation and migration of
cancer cells. As demonstrated in the current study, a gradual
increase in the proliferative and migratory ability was observed
during the culture of p53+/− and p53−/−
mBM-MSCs compared with p53+/+ mBM-MSCs. These results
may be partly due to an increased S phase rate and an augmented
proliferation rate compared with p53+/+ cells. It has
been previously reported that the deregulation of p53 pathway
components is implicated in cancer cell stemness, invasion,
migration and proliferation; for example, the loss of p53 leads to
an increased expression of stemness markers, such as Sox2, SALL4
and Oct4, in breast tumors (48).
The loss of function of wild-type p53 has also been associated with
the promotion of bone metastasis in prostate cancer, partially
through the increased expression of stem-like markers in cancer
cells (49). Moreover, exosomes
from p53−/− mBM-MSC cells demonstrate increased
expression of stemness-related genes, such as Oct4, Nanog and Sox2
compared with p53+/+ mBM-MSC cells (41). Therefore, the present study
investigated these stemness-related proteins to determine the
relationship between p53 and stemness. Similar to previous studies
(41,46,47),
the expression levels of stemness-related proteins, such as LIN28B,
Sox2, c-Myc, SALL4 and Oct4 were increased in p53+/− and
p53−/− mBM-MSCs, suggesting a positive role of p53
inactivation in stemness maintenance.
Increasing evidence indicates that many miRNAs are
closely associated with p53, and p53 has been demonstrated to
promote the maturation and expression of miRNAs (50–52),
in addition to affecting protein expression and exerting different
biological effects through its relevant miRNAs (22,49,53).
miRNAs associated with tumor proliferation and metastasis are
differentially expressed between gastric cancer (GC) tissue-derived
MSCs and adjacent non-cancerous tissue-derived MSCs (54). Moreover, miR-3152 is involved in
the therapeutic response to neoadjuvant radio-chemotherapy
resistance observed in squamous cell carcinoma of esophagus (ESCC),
which suggested that miR-3152 could act as a predictive biomarker
for pre-therapeutic patient selection (28). miR-377 drives malignant
characteristics and acts as a prognostic biomarker in multiple
different cancers (29–32), whereas miR-221 is involved in
osteosarcoma and GC progression (33,34).
miR-155 has previously been observed to regulate melanoma cell
growth by acting as a tumor suppressor (35), and it is overexpressed in
hematological malignancies and solid tumors (36). The differential regulation of
miR-1288 was discovered to be related to the cancer location and
the pathological staging in colorectal cancers (37). In addition, the overexpression of
miR-1288 serves a crucial role in the pathogenesis of ESCC, with
its modulation demonstrating potential therapeutic value in
patients with ESCC (38). Serum
miR-4669 was expressed at lower levels in colon cancer compared to
colon controls, which may facilitate the diagnosis of colon cancer
(39). To determine whether the
above miRNAs were regulated by p53, the differential expression of
these miRNAs in p53+/+, p53+/− and
p53−/− mBM-MSCs was investigated. The results revealed
that miR-3152 and miR-377 were highly expressed in
p53+/− and p53−/− mBM-MSCs, which is
consistent with their expression levels in other types of cancer
(28–32) and suggested that they served an
oncogenic role following p53 inactivation. Conversely, miR-221,
miR-155 and miR-4669 expression levels were observed to be
decreased in p53+/− and p53−/− mBM-MSCs,
which based on previous studies (32–39),
suggested that they may serve a tumor suppressive role in MSCs. In
fact, p53 has been found to serve an important role in cell
proliferation and metastasis by acting through cancer-related
miRNAs (28–39,53,54).
Data from the present study revealed that the
protein levels of UBR2, RNF31 and MMP19 were significantly elevated
in p53+/− and p53−/− mBM-MSCs compared with
p53+/+. Similarly, it was previously reported that UBR2
was highly expressed in p53−/− mBM-MSC cells and
exosomes, which served an important role in GC progression
(41). RNF31 has been reported to
control important oncogenic pathways, such as p53, in breast cancer
(42). The increased expression of
MMP19 was associated with the progression of cutaneous melanoma and
may augment melanoma growth through promoting the invasion of tumor
cells (43). Thus, these results
suggested a relationship between p53 and UBR2, RNF31 and MMP19
proteins, which were determined to be novel proteins in the p53
signaling pathway and suggested that they may have an oncogenic
role in MSCs.
MSCs are known to secrete proteins, including
growth factors, inflammatory cytokines and chemokines to regulate
their biology in an autocrine or paracrine manner in accordance to
the surrounding microenvironment (55). TNF-α is an important factor in the
tumor microenvironment; it assists leukemia cells in immune
evasion, survival and resistance to chemotherapy (56). The BM microenvironment in patients
with multiple myeloma (MM) exhibit elevated concentrations of
IP-10, which might not only be a diagnostic tool, but also a
predictive biomarker for patients with MM (57). In the present study, it was
demonstrated that p53+/− and p53−/− mBM-MSCs
secreted higher levels of TNF-α and IP-10, which could subsequently
promote their proliferation, according to previous studies
(56,57).
Compared to p53+/+ MSCs, which expressed
the highest levels of p53 protein, the in vitro
p53+/− MSCs, rather than the complete absence of p53
(p53−/− MSCs), were sufficient to induce changes in the
biological functions observed in this study, such as cell
proliferation, migration, differentiation and the cell cycle.
Compared with p53+/− cells demonstrating an intermediate
level of p53 expression, p53−/− cells presented
inconsistent differences in the different biological functions
investigated. These observations suggested that distinct thresholds
of p53 protein expression levels were responsible for its various
functions during the progression from p53 knockdown to
inactivation. It is therefore necessary to determine whether
additional mechanisms are contributing as well to drive these
biological characteristics.
In conclusion, the present study compared the
differential characteristics of p53+/+,
p53+/− and p53−/− mBM-MSCs, and discovered
that they exhibited and shared typical MSC characteristics, but
also had some differences, such as the rate of proliferation,
differentiation, colony formation, migration and the expression of
stemness-related proteins, tumor-associated miRNAs and proteins, as
well as inflammatory cytokines. The partial or total absence of p53
may positively regulate the biological function of MSCs through its
relevant miRNAs and proteins. Moreover, the autocrine or paracrine
secretion of growth factors, inflammatory cytokines and chemokines
may provide the mechanism by which MSCs perform these roles.
However, the present study only briefly investigated the functions
of different p53 statuses in cells, and the relationship between
p53 and the molecules found to be differentially expressed was only
inferred; future studies are required to investigate the casual
relationship and the regulation between p53 and these molecules. In
addition, further research is required to investigate how p53
regulates the function of MSCs through these genes and proteins.
Overall, this study may provide new evidence for the biological
regulatory role of p53 in BM-MSCs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural
Science Foundation of China (grant no. 81702078), The Natural
Science Foundation of Jiangsu Province (grant no. BK20170356), The
Natural Science Fund for Colleges and Universities of Jiangsu
Province (grant no. 17KJB320016), The Suzhou Science and Technology
Project (grant no. SYS201728), The Postgraduate Research &
Practice Innovation Program of Jiangsu Province (grant no.
KYCX18_2535) and The Young Stuff Pre-research Fund Project of The
Second Affiliated Hospital of Soochow University (grant no.
SDFEYQN1718).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
HY and HD conceived and designed the experiments.
BW, LW, JM, HW, LX and YR performed the experiments. HY analyzed
the data and wrote the manuscript. All authors read, reviewed and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
in accordance with the Guide for the Care and Use of Laboratory
Animals and approved by the Animal Use Ethics Committee of Jiangsu
University (Zhejiang, China; 2012258).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frausin S, Viventi S, Verga Falzacappa L,
Quattromani MJ, Leanza G, Tommasini A and Valencic E: Wharton's
jelly derived mesenchymal stromal cells: Biological properties,
induction of neuronal phenotype and current applications in
neurodegeneration research. Acta Histochem. 117:329–338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iser IC, Bracco PA, Gonçalves CE, Zanin
RF, Nardi NB, Lenz G, Battastini AM and Wink MR: Mesenchymal stem
cells from different murine tissues have differential capacity to
metabolize extracellular nucleotides. J Cell Biochem.
115:1673–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung J, Choi JH, Lee Y, Park JW, Oh IH,
Hwang SG, Kim KS and Kim GJ: Human placenta-derived mesenchymal
stem cells promote hepatic regeneration in CCl4-injured rat liver
model via increased autophagic mechanism. Stem Cells. 31:1584–1596.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sargent A and Miller RH: MSC therapeutics
in chronic inflammation. Curr Stem Cell Rep. 2:168–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang F, Wang M, Yang T, Cai J, Zhang Q,
Sun Z, Wu X, Zhang X, Zhu W, Qian H and Xu W: Gastric
cancer-derived MSC-secreted PDGF-DD promotes gastric cancer
progression. J Cancer Res Clin Oncol. 140:1835–1848. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Homma A, Nakamura K, Matsuura K, Mizusawa
J, Onimaru R, Fukuda H and Fujii M: Dose-finding and efficacy
confirmation trial of superselective intra-arterial infusion of
cisplatin and concomitant radiotherapy for patients with locally
advanced maxillary sinus cancer (JCOG1212, RADPLAT-MSC). Jpn J Clin
Oncol. 45:119–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuiffo BG, Campagne A, Bell GW, Lembo A,
Orso F, Lien EC, Bhasin MK, Raimo M, Hanson SE, Marusyk A, et al:
MSC-regulated microRNAs converge on the transcription factor FOXP2
and promote breast cancer metastasis. Cell Stem Cell. 15:762–774.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
da Costa Gonçalves F, Grings M, Nunes NS,
Pinto FO, Garcez TN, Visioli F, Leipnitz G and Paz AH: Antioxidant
properties of mesenchymal stem cells against oxidative stress in a
murine model of colitis. Biotechnol Lett. 39:613–622. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ansboro S, Roelofs AJ and De Bari C:
Mesenchymal stem cells for the management of rheumatoid arthritis:
Immune modulation, repair or both? Curr Opin Rheumatol. 29:201–207.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nair BC, Krishnan SR, Sareddy GR, Mann M,
Xu B, Natarajan M, Hasty P, Brann D, Tekmal RR and Vadlamudi RK:
Proline, glutamic acid and leucine-rich protein-1 is essential for
optimal p53-mediated DNA damage response. Cell Death Differ.
21:1409–1418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Y, Du L, Lin L and Wang Y:
Tumour-associated mesenchymal stem/stromal cells: Emerging
therapeutic targets. Nat Rev Drug Discov. 16:35–52. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu B, Zhu J, Liu S, Wang L, Fan Q, Hao Y,
Fan C and Tang TT: Mesenchymal stem cells promote osteosarcoma cell
survival and drug resistance through activation of STAT3.
Oncotarget. 7:48296–48308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu M, Wang M, Yang F, Tian Y, Cai J, Yang
H, Fu H, Mao F, Zhu W, Qian H and Xu W: miR-155-5p inhibition
promotes the transition of bone marrow mesenchymal stem cells to
gastric cancer tissue derived MSC-like cells via NF-κB p65
activation. Oncotarget. 7:16567–16580. 2016.PubMed/NCBI
|
|
15
|
O'Malley G, Heijltjes M, Houston AM, Rani
S, Ritter T, Egan LJ and Ryan AE: Mesenchymal stromal cells (MSCs)
and colorectal cancer: A troublesome twosome for the anti-tumour
immune response? Oncotarget. 7:60752–60774. 2016.PubMed/NCBI
|
|
16
|
Zhang M, Gao CE, Li WH, Yang Y, Chang L,
Dong J, Ren YX and Chen D: Microarray based analysis of gene
regulation by mesenchymal stem cells in breast cancer. Oncol Lett.
13:2770–2776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li N, Xie C and Lu NH: p53, a potential
predictor of Helicobacter pylori infection-associated gastric
carcinogenesis? Oncotarget. 7:66276–66286. 2016.PubMed/NCBI
|
|
18
|
Berkers CR, Maddocks OD, Cheung EC, Mor I
and Vousden KH: Metabolic regulation by p53 family members. Cell
Metab. 18:617–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quintas-Cardama A, Hu C, Qutub A, Qiu YH,
Zhang X, Post SM, Zhang N, Coombes K and Kornblau SM: p53 pathway
dysfunction is highly prevalent in acute myeloid leukemia
independent of TP53 mutational status. Leukemia. 31:1296–1305.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XJ, L Jeffrey Medeiros, Bueso-Ramos
CE, Tang G, Wang S, Oki Y, Desai P, Khoury JD, Miranda RN, Tang Z,
et al: P53 expression correlates with poorer survival and augments
the negative prognostic effect of MYC rearrangement, expression or
concurrent MYC/BCL2 expression in diffuse large B-cell lymphoma.
Mod Pathol. 30:194–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tataria M, Quarto N, Longaker MT and
Sylvester KG: Absence of the p53 tumor suppressor gene promotes
osteogenesis in mesenchymal stem cells. J Pediatr Surg. 41:624–632.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Y, de Castro LF, Shin MH, Dubois W,
Yang HH, Jiang S, Mishra PJ, Ren L, Gou H, Lal A, et al: p53 loss
increases the osteogenic differentiation of bone marrow stromal
cells. Stem Cells. 33:1304–1319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armesilla-Diaz A, Elvira G and Silva A:
p53 regulates the proliferation, differentiation and spontaneous
transformation of mesenchymal stem cells. Exp Cell Res.
315:3598–3610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boregowda SV, Krishnappa V, Strivelli J,
Haga CL, Booker CN and Phinney DG: Basal p53 expression is
indispensable for mesenchymal stem cell integrity. Cell Death
Differ. 25:677–690. 2018. View Article : Google Scholar :
|
|
25
|
Huang Y, Yu P, Li W, Ren G, Roberts AI,
Cao W, Zhang X, Su J, Chen X, Chen Q, et al: p53 regulates
mesenchymal stem cell-mediated tumor suppression in a tumor
microenvironment through immune modulation. Oncogene. 33:3830–3838.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patocs A, Zhang L, Xu Y, Weber F, Caldes
T, Mutter GL, Platzer P and Eng C: Breast-cancer stromal cells with
TP53 mutations and nodal metastases. N Engl J Med. 357:2543–2551.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slotta-Huspenina J, Drecoll E, Feith M,
Habermehl D, Combs S, Weichert W, Bettstetter M, Becker K and
Langer R: MicroRNA expression profiling for the prediction of
resistance to neoadjuvant radiochemotherapy in squamous cell
carcinoma of the esophagus. J Transl Med. 16:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu H, Liu HY, Liu WJ, Shi YL and Bao D:
miR-377-5p inhibits lung cancer cell proliferation, invasion, and
cell cycle progression by targeting AKT1 signaling. J Cell Biochem.
Nov 28–2018.doi: 10.1002/jcb.28091 (Epub ahead of print).
|
|
30
|
Wang CQ, Chen L, Dong CL, Song Y, Shen ZP,
Shen WM and Wu XD: MiR-377 suppresses cell proliferation and
metastasis in gastric cancer via repressing the expression of
VEGFA. Eur Rev Med Pharmacol Sci. 21:5101–5111. 2017.PubMed/NCBI
|
|
31
|
EI Baroudi M, Machiels JP and Schmitz S:
Expression of SESN1, UHRF1BP1, and miR-377-3p as prognostic markers
in mutated TP53 squamous cell carcinoma of the head and neck.
Cancer Biol Ther. 18:775–782. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu WY, Yang Z, Sun Q, Yang X, Hu Y, Xie
H, Gao HJ, Guo LM, Yi JY, Liu M and Tang H: miR-377-3p drives
malignancy characteristics via upregulating GSK-3β expression and
activating NF-κB pathway in hCRC cells. J Cell Biochem.
119:2124–2134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu XH, Zhao ZX, Dai J, Geng DC and Xu YZ:
MicroRNA-221 regulates osteosarcoma cell proliferation, apoptosis,
migration, and invasion by targeting CDKN1B/p27. J Cell Biochem.
120:4665–4674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng Y, Bai F, You Y, Bai F, Wu C, Xin R,
Li X and Nie Y: Dysregulated microRNA expression profiles in
gastric cancer cells with high peritoneal metastatic potential. Exp
Ther Med. 16:4602–4608. 2018.PubMed/NCBI
|
|
35
|
DiSano JA, Huffnagle I, Gowda R,
Spiegelman VS, Robertson GP and Pameijer CR: Loss of miR-155
upregulates WEE1 in metastatic melanoma. Melanoma Res. 29:216–219.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Witten LW, Cheng CJ and Slack FJ: miR-155
drives oncogenesis by promoting and cooperating with mutations in
the c-Kit oncogene. Oncogene. 38:2151–2161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gopalan V, Pillai S, Ebrahimi F,
Salajegheh A, Lam TC, Le TK, Langsford N, Ho YH, Smith RA and Lam
AK: Regulation of microRNA-1288 in colorectal cancer: Altered
expression and its clinicopathological significance. Mol Carcinog.
53 (Suppl 1):E36–E44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gopalan V, Islam F, Pillai S, Tang JC,
Tong DK, Law S, Chan KW and Lam AK: Overexpression of microRNA-1288
in oesophageal squamous cell carcinoma. Exp Cell Res. 348:146–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang YN, Chen ZH and Chen WC: Novel
circulating microRNAs expression profile in colon cancer: A pilot
study. Eur J Med Res. 22:512017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang
H, Fu H, Yan Y, Zhang X, Wang M, et al: HucMSC Exosome-delivered
14-3-3ζ orchestrates self-control of the Wnt response via
modulation of YAP during cutaneous regeneration. Stem Cells.
34:2485–2500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mao J, Liang Z, Zhang B, Yang H, Li X, Fu
H, Zhang X, Yan Y, Xu W and Qian H: UBR2 Enriched in p53 deficient
mouse bone marrow mesenchymal stem Cell-Exosome promoted gastric
cancer progression via Wnt/β-catenin pathway. Stem Cells.
35:2267–2279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu J, Zhuang T, Yang H, Li X, Liu H and
Wang H: Atypical ubiquitin ligase RNF31: The nuclear factor
modulator in breast cancer progression. BMC Cancer. 16:5382016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Müller M, Beck IM, Gadesmann J, Karschuk
N, Paschen A, Proksch E, Djonov V, Reiss K and Sedlacek R: MMP19 is
upregulated during melanoma progression and increases invasion of
melanoma cells. Mod Pathol. 23:511–521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sineh Sepehr K, Razavi A, Saeidi M,
Mossahebi-Mohammadi M, Abdollahpour-Alitappeh M and Hashemi SM:
Development of a novel explant culture method for the isolation of
mesenchymal stem cells from human breast tumor. J Immunoassay
Immunochem. 39:207–217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang R, Zhou Y, Tan S, Zhou G, Aagaard L,
Xie L, Bünger C, Bolund L and Luo Y: Mesenchymal stem cells derived
from human induced pluripotent stem cells retain adequate
osteogenicity and chondrogenicity but less adipogenicity. Stem Cell
Res Ther. 6:1442015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li XF, Xu BZ and Wang SZ: Aspirin inhibits
the proliferation and migration of gastric cancer cells in
p53-knockout mice. Oncol Lett. 12:3183–3186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Donehower LA and Lozano G: 20 years
studying p53 functions in genetically engineered mice. Nat Rev
Cancer. 9:831–841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Dube C, Gibert M Jr, Cruickshanks
N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, et al:
The p53 pathway in Glioblastoma. Cancers (Basel). 10(pii):
E2972018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang
S, Zou X and Peng X: Wild-type p53 suppresses the
epithelial-mesenchymal transition and stemness in PC-3 prostate
cancer cells by modulating miR-145. Int J Oncol. 42:1473–1481.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tarasov V, Jung P, Verdoodt B, Lodygin D,
Epanchintsev A, Menssen A, Meister G and Hermeking H: Differential
regulation of microRNAs by p53 revealed by massively parallel
sequencing: MiR-34a is a p53 target that induces apoptosis and
G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cortez MA, Ivan C, Valdecanas D, Wang X,
Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, et al:
PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst. 108(pii):
djv3032015.PubMed/NCBI
|
|
52
|
Sachdeva M, Zhu S, Wu F, Wu H, Walia V,
Kumar S, Elble R, Watabe K and Mo YY: p53 represses c-Myc through
induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA.
106:3207–3212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang CJ, Chao CH, Xia W, Yang JY, Xiong
Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al: p53 regulates
epithelial-mesenchymal transition and stem cell properties through
modulating miRNAs. Nat Cell Biol. 13:317–323. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chan JK and Lam PY: Human mesenchymal stem
cells and their paracrine factors for the treatment of brain
tumors. Cancer Gene Ther. 20:539–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou X, Li Z and Zhou J: Tumor necrosis
factor α in the onset and progression of leukemia. Exp Hematol.
45:17–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cao Y, Luetkens T, Kobold S, Hildebrandt
Y, Gordic M, Lajmi N, Meyer S, Bartels K, Zander AR, Bokemeyer C,
et al: The cytokine/chemokine pattern in the bone marrow
environment of multiple myeloma patients. Exp Hematol. 38:860–867.
2010. View Article : Google Scholar : PubMed/NCBI
|