Introduction
Alzheimer's disease (AD) is a progressive,
age-related neurodegenerative disease that presents with
progressive intellectual deterioration involving memory, language
and judgement, ultimately leading to a total dependence on nursing
care for affected patients (1).
One of the major histopathological hallmarks of AD is cerebral
deposits of extracellular amyloid β (Aβ) peptides. Senile plaques,
composed of Aβ, are an important criterion for the verification of
AD (2). Aβ40 and
Aβ42, which are derived from amyloid precursor protein
(APP), affect the pathogenesis of AD because of their strong
aggregative ability and neurotoxicity (3). However, there is no effective therapy
for AD that can reverse or slow its progression.
Autophagy is one of the main mechanisms of
maintaining cellular homeostasis; it degrades and recycles old
proteins and organelles using cellular machinery (4). However, excessive autophagy may also
lead to autophagic neuron death and apoptosis (5). Previous studies have reported that
autophagy may be involved in AD pathogenesis (6). In addition, autophagy serves an
important role in clearing Aβ aggregation and preserving neuronal
function in AD (7). Another recent
study also indicated that Aβ1-42 can induce autophagic
cell death and autophagic vacuoles (8). Autophagosomes can be observed during
Aβ1-42-induced cytotoxicity in PC12 cells (8,9), and
it has been reported that autophagy exhibits protective effects by
reducing the deposition of Aβ and attenuating damage related to
memory and cognitive dysfunction in AD animal models (10). β-asarone, a chief constituent of
Acorus tatarinowii Schott, can easily pass through the blood
brain barrier and exhibits various neuroprotective effects against
neurodegenerative disease in vivo and in vitro models
(11–14). A study using SH-SY5Y cells
demonstrated that β-asarone prevents Aβ25-35-induced
inflammatory responses and autophagy through the downregulation of
Beclin-1 and light chain (LC)3B and the upregulation of Bcl-2
(15). In addition, it has been
identified that the upregulation of Beclin-1-dependent autophagy
protects against β-amyloid-induced cell injury in PC12 cells
(16). β-asarone also has
cerebrovascular protective effects in AD rats (17). However, its effect on AD remains to
be elucidated.
The present study evaluated the neuroprotective
effect of β-asarone in an APP/presenilin-1 (PS1) transgenic mouse
model of AD and identified its underlying mechanism. APP/PS1 mutant
transgenic mouse models are used to assess the pharmacodynamics of
potential amyloidosis-lowering and pro-cognitive compounds
(18). Control groups were treated
with the autophagy inhibitor 3-methyladenine (3-MA) or the
autophagy activator rapamycin. By monitoring autophagy, the present
study aimed to explore if β-asarone may be a potential therapeutic
agent for the prevention and treatment of AD by affecting
autophagy.
Materials and methods
Animals
A total of 60 APP/PS1 double transgenic mice (30
males and 30 females; weight, 20–25 g; age, 3 months), that is,
C57BL/6 mice co-expressing mutant human APPswe and mutant human PS1
gene lacking exon 9, PSl-ΔE9; and 10 wild-type littermates (5 males
and 5 females; weight, 20–25 g; age, 3 months) were purchased from
Nanjing Biomedical Research Institute of Nanjing University. The
animals were housed under standard conditions, at a temperature of
20–22°C and 40–60% humidity, with a 12-h light/dark cycle and free
access to water and food. All procedures were approved by the
Guangzhou University of Chinese Medicine Institutional Animal Care
and Use Committee (Guangzhou, China) (ethics no. 2014013).
Preparation of β-asarone
β-asarone was extracted from Acorus
tatarinowii Schott and then purified by freezing
crystallization, as reported previously (19). The obtained β-asarone was ≤99.55%
pure (17), as confirmed by the
China National Analytical Center using gas chromatography-mass
spectrometry, infrared spectroscopy and nuclear magnetic resonance
spectroscopy.
Experimental design
The normal control group comprised 10 wild-type
C57BL/6 mice. The APP/PS1 transgenic mice were randomly divided
into 6 groups (n=10 each): i) model group; ii) low-, iii) medium-
and iv) high-dose β-asarone groups, which were given β-asarone by
intragastric administration at doses of 10, 20 or 40 mg/kg body
weight, respectively; v) 3-MA treated group, which was given 3-MA
(cat. no. M9281; Sigma-Aldrich; Merck KGaA) by intraperitoneal
injection at a dose of 30 mg/kg body weight; and vi) the rapamycin
treated group, which was given rapamycin (cat. no. R0395;
Sigma-Aldrich; Merck KGaA) by intraperitoneal injection at a dose
of 1 mg/kg body weight. The treatments were continuously
administered to all mice one per day for 30 days. At the end of the
experiment, all mice were sacrificed as described below, for the
removal of hippocampal samples, which were subsequently stored at
−80°C for flow cytometry, ELISA, immunofluorescence staining,
immunohistochemistry, transmission electron microscopy (TEM),
reverse transcription-quantitative PCR (RT-qPCR) and western blot
analysis.
Immunohistochemical staining of senile
plaque formations
A total of 10 mice from each group were deeply
anesthetized with 10% chloral hydrate (350 mg/kg, intraperitoneal;
no signs of peritonitis were observed) and then transcranially
perfused with 0.9% normal saline (30 ml) until colorless liquid
flowed from the right atrial appendage. When the run-off became
clear, perfusion was performed with 4% paraformaldehyde (30 ml)
until the upper limbs became white and stiff. The hippocampus was
rapidly dissected from the brains and was placed on ice. Tissues
were fixed in 10% formalin at room temperature for 24 h and
embedded in paraffin. Paraffin-embedded tissues were subsequently
cut into serial 5-µm coronal sections using a microtome. Sections
were deparaffinized at 60°C for 1 h, washed in xylene twice for 10
min and rehydrated in a descending alcohol series. Following the
quenching of endogenous peroxidase activity for 10 min in PBS
containing 3% H2O2 at 37°C, sections were
heated in antigen retrieval solution (0.01 mol/l citrate buffer, pH
8.0) at 90°C for 10 min, cooled in water and immersed for 5 min in
PBS containing 0.3% Triton X-100 at 37°C. Sections were blocked
with 5% BSA (cat. no. 810652; Sigma-Aldrich; Merck KGaA) for 30 min
at 37°C and the sections were then incubated with
anti-Aβ42 antibodies (1:50; cat. no. ab10148; Abcam) for
1 h at 37°C, amplified with avidin biotin-peroxidase complex
labelling (1:100; cat. no. SV1022; Wuhan Boster Biological
Technology, Ltd.), developed with DAB (cat. no. AR1022; Wuhan
Boster Biological Technology, Ltd.) and imaged using a U-SPT light
microscope (magnification, ×200; Olympus Corporation). Data were
analyzed using ImageJ version 1.48 software (National Institutes of
Health). The average optical density (%) was calculated using the
following equation: (Integrated optical density/measurement area)
×100.
ELISA analysis of Aβ40 and
Aβ42 levels
The hippocampus was weighed and homogenized with
icecold normal saline (1 µl/3 mg) and then centrifuged at 3,000 × g
for 10 min at 4°C to obtain the supernatant. Aβ40 (cat.
no. A226FC) and Aβ42 (cat. no. A227FC) levels in the
supernatant were determined separately using ELISA kits, according
to the manufacturer's instructions (Elixir Canada Medicine Company
Ltd.).
RT-qPCR of APP and Beclin-1 mRNA
levels
Total RNA was extracted from hippocampus tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
concentration of RNA was determined using a NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed into cDNA using a Hifair® III 1st
Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) kit
[cat. no. 11141ES60; Yeasen Biotechnology (Shanghai) Co., Ltd.],
according to the manufacturer's protocol. Quantitative analysis of
the mRNA expression levels of APP and Beclin-1 was performed by
qPCR using a Hieff UNICON® Power qPCR SYBR Green Master
mix [no Rox; Yeasen Biotechnology (Shanghai) Co., Ltd.], according
to the manufacturer's protocol, 96-well optical reaction plates and
the CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc.). qPCR was performed using the following conditions: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing and extension at 60°C
for 30 sec. All experiments were performed four times. The
following primers were used: GAPDH, forward
5′-AGAAGGTGGTGAAGCAGGCATC-3′, reverse
5′-CGAAGGTGGAAGAGTGGGAGTTG-3′; APP, forward
5′-TGCAGCAGAACGGATATGAGAAT-3′, reverse
5′-GTCAAAAGCCGAGGGTGAGTAAA-3′; and Beclin-1, forward
5′-ATACTGTTCTGGGGGTTTGCG-3′, reverse 5′-GTCTCTCCTTTTTCCACCTCTTC-3′.
The primers used in the present study were selected from the PubMed
database and were synthesized by Shanghai Jierui Biological
Engineering Co., Ltd. Data were analyzed using the relative gene
expression (2−ΔΔCq) method (20) and normalized to GAPDH.
Flow cytometric analysis of Beclin-1,
LC3A/B and p62
Beclin-1, LC3A/B and p62 expression levels were
quantitatively determined using our previously established method
(21,22). Hippocampal tissues were prepared as
single cell suspensions, and then counted and adjusted to a density
of 1×106 cells/ml. The cells were blocked with a protein
block solution (2% BSA; cat. no. 810652; Merck KGaA) for 20 min and
fixed with 1% paraformaldehyde for 20 min, as directed in the
instructions for the IntraPrep permeabilization reagent (cat. no.
GAS003; Invitrogen; Thermo Fisher Scientific, Inc.). Next, the
cells were incubated with rabbit anti-Beclin-1 (1:100; cat. no.
sc-11427, Santa Cruz Biotechnology, Inc.), mouse anti-p62 (1:100;
cat. no. ab56416; Abcam) and rabbit anti-LC3A/B antibody (1:100;
cat. no. 4108; Cell Signaling Technology, Inc.) in the dark at room
temperature for 30 min. Subsequently the cells were incubated for 1
h at 37°C with the following secondary antibodies: Anti-rabbit
(1:500; cat. no. CW0114S; CoWin Biosciences) and anti-mouse
(1:1,000; cat. no. 4408S; Cell Signaling Technology, Inc.) and
washed twice with PBS. Finally, the labelled cells were fixed at
37°C overnight in 4% paraformaldehyde and prepared for flow
cytometric analysis. The control cells were incubated with
secondary antibody alone. Data were collected from 20,000 events
for every analysis. FACS data were collected using an ALTRA flow
cytometer (Beckman Coulter, Inc.) equipped with EXPOTM32 MultiCOMP
software.
Western blot analysis of Beclin-1,
LC3A/B, p62 and GAPDH
Total protein was extracted from the hippocampal
tissue using phenylmethanesulfonylfluoride lysis buffer
(Sigma-Aldrich; Merck KGaA). The lysates were incubated for 30 min
at 4°C and centrifuged at 13,000 × g for 15 min at 4°C. Total
protein was quantified using a bicinchoninic acid assay kit (Wuhan
Boster Biological Technology, Ltd.) and 100 µg protein/lane was
separated by 12% SDS-PAGE (cat. no. P0012A; Beyotime Institute of
Biotechnology). The separated proteins were subsequently
transferred onto nitrocellulose membranes (0.2 µm; Bio-Rad
Laboratories, Inc.). The membranes were blocked in 5% BSA (cat. no.
810652; Merck KGaA) for 1 h at 4°C. The membranes were washed and
incubated with antibodies against Beclin-1 (1:1,000; cat. no.
ab62557; Abcam), p62 (1:1,000; cat. no. ab56416; Abcam), GAPDH
(1:1,000; cat. no. ab8245; Abcam) and LC3A/B (1:1,000; cat. no.
4108; Cell Signaling Technology, Inc.) for 12 h at 4°C. Following
the primary antibody incubation, membranes were washed and
incubated with horseradish peroxidase-linked secondary antibodies
(1:2,000; cat nos. CW0103 and CW0102; CoWin Biosciences) for 1 h at
room temperature. GAPDH was used as an internal control. The
membranes were washed with TBS-20% Tween and visualized using an
ECL kit (Bio-Rad Laboratories, Inc.) and a ChemiDoc XRS™ imager
(Bio-Rad Laboratories, Inc.). Blots were repeated ≥3 times for each
condition. Expression levels were quantified using Image-Pro Plus
6.0 analysis software (Media Cybernetics, Inc.) and normalized to
GAPDH.
Immunofluorescence staining analysis
of Beclin-1, LC3A/B and p62
The fixed coronal sections (5-µm) of the
hippocampus, aforementioned, were incubated with PBS containing 3%
H2O2 at 37°C for 10 min to quench endogenous
peroxidase activity, heated in antigen retrieval solution (EDTA, pH
8.0) at 90°C for 10 min, chilled in water and then immersed for 5
min in PBS at 37°C. Sections were then incubated with rabbit
anti-Beclin-1 antibody (1:50; cat. no. ab62557; Abcam), rabbit
anti-LC3A/B antibody (1:50; cat. no. 4108; Abcam) and rabbit
anti-p62 antibody (1:50; cat. no. ab56416; Abcam) for 60 min at
37°C. Expression was then amplified with avidin biotin-peroxidase
complex labelling and visualized using an IX71 inverted
fluorescence microscope (magnification, ×400; Olympus Corporation).
Data analysis was performed using ImageJ version 1.48 software
(National Institutes of Health).
TEM
To further clarify the effect of β-asarone on
autophagy, TEM, the standard method for detecting autophagy, was
employed (16). Hippocampal tissue
from each group were fixed with 2.5% glutaraldehyde in 0.1 mol/l
PBS (pH 7.4) at room temperature for 90 min and post-fixed in 1%
osmium tetroxide for 30 min. Following washing with PBS, the cells
were progressively dehydrated in a 10% graded series of 50–100%
ethanol and propylene oxide and embedded in Epon 812 resin. The
blocks were cut into ultrathin sections (50–150 µm) with a UC7
vibratome (Leica Microsystems GmbH) and the sections were then both
stained with 3% saturated uranyl acetate and 3% lead citrate at
room temperature for 30 min. Sections were used to observe
dopaminergic neuron morphology under an H-7650 transmission
electron microscope (magnification, ×30,000; Hitachi, Ltd.), as
previously described (11).
Statistical analysis
All statistical analyses were performed with SPSS
version 13.0 statistical software (SPSS, Inc.). Data are expressed
as the mean ± SD, and significant differences among different
groups were determined by one-way ANOVA followed by Bonferroni's
post hoc test for multiple comparisons. Each experiment was
repeated ≥3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
β-asarone reduces the formation of
senile plaques in the hippocampus
Senile plaques composed of Aβ peptides are an
important criterion for verifying AD (2); inhibiting Aβ accumulation is a
potential strategy for preventing AD. The immunohistochemical
staining analysis presented in Fig.
1 demonstrated that the hippocampal tissues of mice in the
model group had more senile plaques compared with mice in the
normal control group (P<0.01). In addition, the number of senile
plaques in the hippocampus of the β-asarone-, 3-MA-and
rapamycin-treated groups was significantly reduced compared with
the model group (P<0.05 or P<0.01). Furthermore, the number
of senile plaques in the hippocampus was higher in the β-asarone-
and rapamycin-treated groups compared with the 3-MA-treated group
(P<0.01), but lower in the β-asarone- and 3-MA-treated groups
compared with the rapamycin-treated group (P<0.05 or
P<0.01).
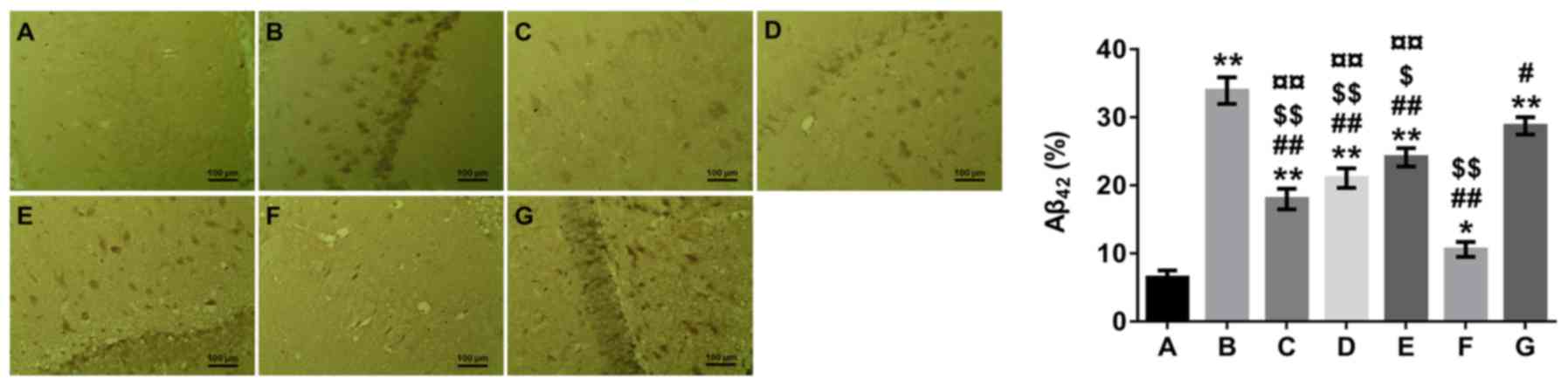 | Figure 1.Effects of β-asarone on the formation
of senile plaques in the hippocampus of Alzheimer's disease model
mice by Aβ42 immunohistochemical staining analysis. A,
Normal group; B, model group; C, low-dose β-asarone group; D,
medium-dose β-asarone group; E, high-dose β-asarone group; F,
3-MA-treated group; and G, rapamycin-treated group. Data are
presented as the mean ± SD of 3 mice. Scale bar, 100 µm. Data were
analyzed using a medical image analysis system. *P<0.05 and
**P<0.01 vs. normal group; #P<0.05 and
##P<0.01 vs. model group; $P<0.05 and
$$P<0.01 vs. rapamycin-treated group;
¤¤P<0.01 vs. 3-MA-treated group. 3-MA,
3-methyladenine. Aβ, amyloid β. |
β-asarone decreases hippocampal levels
of Aβ40 and Aβ42
The two main Aβ peptide isoforms produced from APP,
Aβ40 and Aβ42, serve an important role in the
pathogenesis of AD because of their strong aggregative ability and
neurotoxicity (2). ELISA analysis
revealed that hippocampal levels of Aβ40 and
Aβ42 were significantly elevated in the model group
compared with the normal control group (P<0.01; Table I). By contrast, a significant
decrease in Aβ40 and Aβ42 levels was observed
in the β-asarone-, 3-MA- and rapamycin-treated groups compared with
the model group (P<0.05 or P<0.01; Table I). A significant increase in
Aβ40 and Aβ42 levels was observed in the
β-asarone- and rapamycin-treated groups compared with the
3-MA-treated group (P<0.05 or P<0.01), whereas a significant
decrease was noted in the β-asarone-treated groups compared with
the rapamycin treated group (P<0.01).
 | Table I.Aβ40 and Aβ42
levels in the hippocampus determined by ELISA. |
Table I.
Aβ40 and Aβ42
levels in the hippocampus determined by ELISA.
| Groupa | Aβ40
(pg/g tissue weight) | Aβ42
(pg/g tissue weight) |
|---|
| Normal | 1.11±0.09 | 1.44±0.24 |
| Model |
9.18±0.38c |
9.86±0.19c |
| Low-dose
β-asarone |
4.87±0.40c,e,g,h |
4.46±0.30c,e,f,h |
| Medium-dose
β-asarone |
5.28±0.45c,e,g,h |
4.80±0.27c,e,g,h |
| High-dose
β-asarone |
5.69±0.40c,e,g,h |
5.60±0.24c,e,g,h |
| 3-MA |
2.81±0.12c,e |
3.37±0.20b,e |
| Rapamycin |
7.89±0.78c,d,g |
7.38±0.67c,d,g |
Effect of β-asarone on APP and
Beclin-1 mRNA expression levels in the hippocampus
β-asarone treatment also exhibited a notable effect
on APP and Beclin-1 mRNA expression levels in the hippocampus of
APP/PS1 transgenic mice (Table
II). RT-qPCR analysis revealed a significant increase in APP
and Beclin-1 mRNA expression levels in the model group compared
with the normal control group (P<0.01). In addition, a
significant decrease in APP and Beclin-1 mRNA levels was observed
in the β-asarone-, 3-MA- and rapamycin-treated groups compared with
the model group (P<0.05 or P<0.01; Table II). Furthermore, a significant
increase in APP and Beclin-1 mRNA levels was observed in the
β-asarone- and rapamycin-treated groups compared with the
3-MA-treated group (P<0.05 or P<0.01), but the opposite was
observed in the β-asarone-treated groups compared with the
rapamycin-treated group (P<0.01).
 | Table II.APP and Beclin-1 mRNA levels in the
hippocampus by reverse transcription-quantitative PCR. |
Table II.
APP and Beclin-1 mRNA levels in the
hippocampus by reverse transcription-quantitative PCR.
| Groupa | APP | Beclin-1 |
|---|
| Normal | 1.00±0.00 | 1.00±0.00 |
| Model |
4.21±0.11c |
3.81±0.09c |
| Low-dose
β-asarone |
1.42±0.04b,e,f,h |
1.39±0.03b,e,g,h |
| Medium-dose
β-asarone |
1.77±0.07c,e,g,h |
1.64±0.05b,e,g,h |
| High-dose
β-asarone |
2.06±0.08c,e,g,h |
1.86±0.06c,e,g,h |
| 3-MA |
1.25±0.05b,e |
1.15±0.03b,e |
| Rapamycin |
2.82±0.13c,e,g |
3.07±0.11c,d,g |
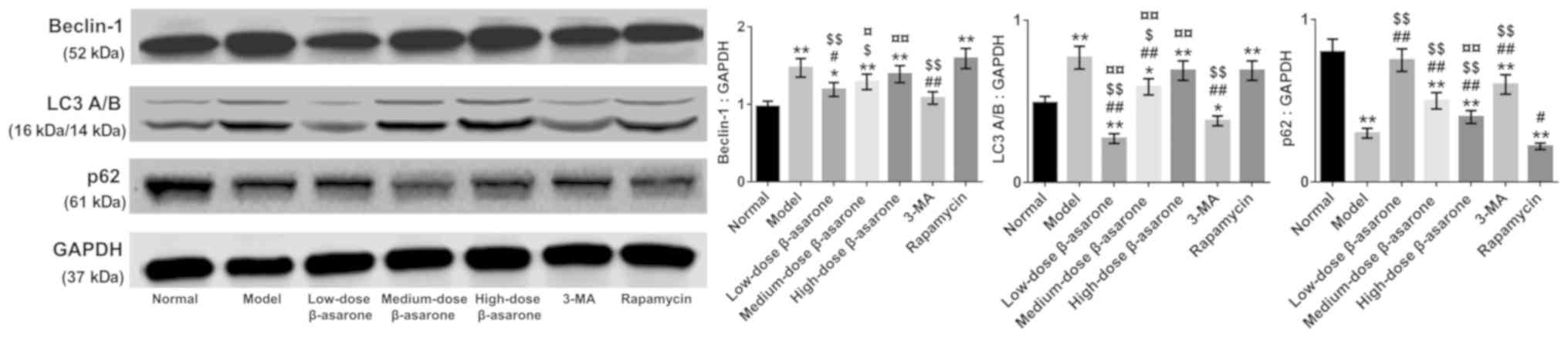
Effect of β-asarone on autophagy in
the hippocampus
The autophagy inhibitor-3-MA and autophagy
activator-rapamycin were used, and β-asarone-treated groups were
compared with 3-MA- and rapamycin-groups to determine whether
expressions of the autophagy markers Beclin-1 and LC3A/B were
altered. The results from Figs.
2–4 demonstrated a significant
decrease in the expression of Beclin-1 and LC3A/B accompanied by an
increased in the expression of p62 in the low-dose-β-asarone- and
3-MA-treated groups compared with the model group (P<0.05 or
P<0.01; Fig. 3; P<0.05 or
P<0.01; Figs. 2 and 4), but there was a decrease in p62
expression in the rapamycin-treated group compared with the model
group (P<0.05; Figs. 2 and
3; P<0.01; Fig. 4). In addition, Beclin-1 and LC3A/B
expression increased, and p62 expression decreased significantly in
the high-dose-β-asarone-and rapamycin-treated groups compared with
the 3-MA-treated group (P<0.01; Figs. 2–4). However, Beclin-1 and LC3A/B
expression was reduced and p62 expression was significantly
augmented in the low-dose- and medium-dose β-asarone-treated groups
compared with the rapamycin-treated group (P<0.05 or P<0.01;
Fig. 3; P<0.01; Figs. 2 and 4).
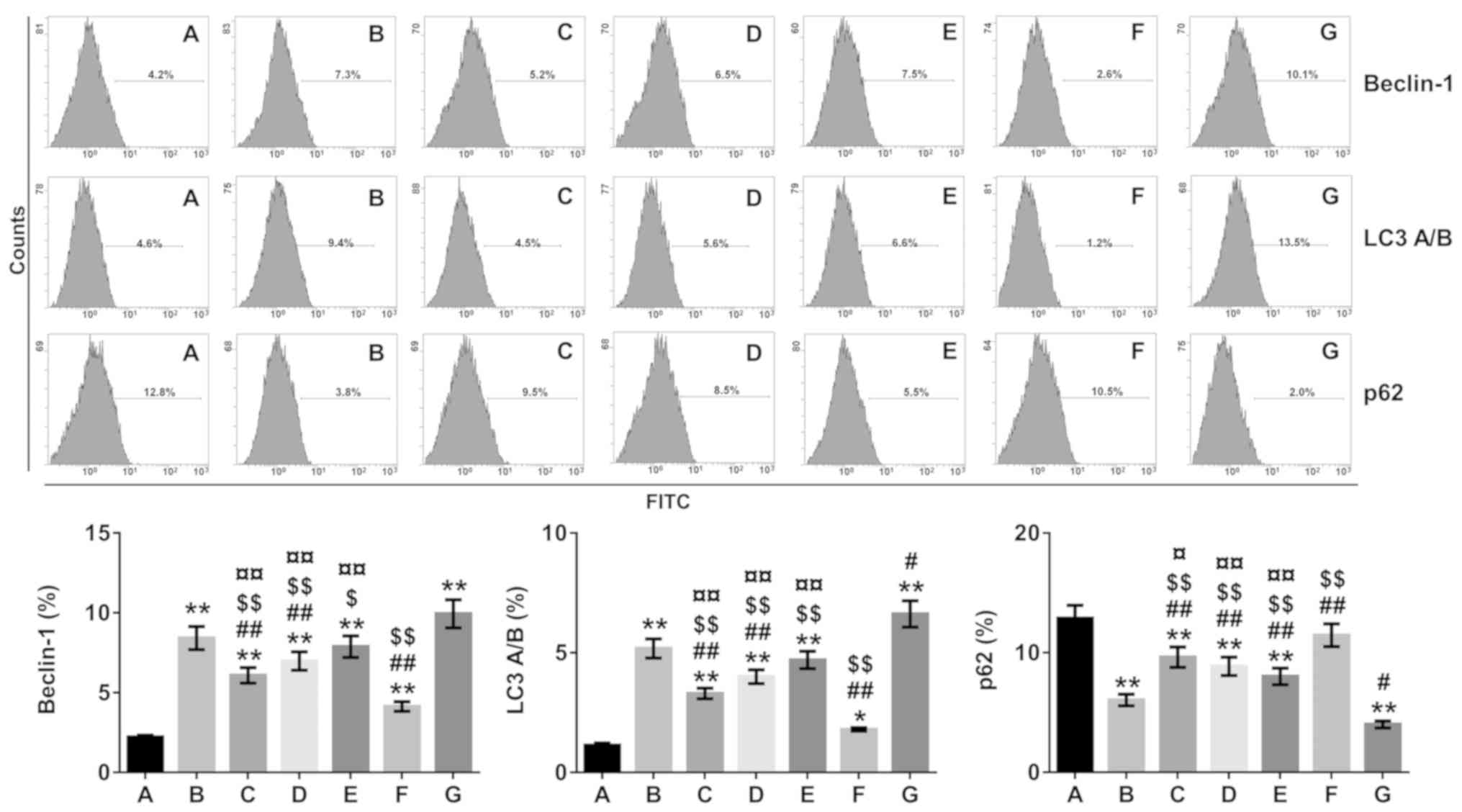 | Figure 2.Beclin-1, LC3A/B and p62 expression
in the hippocampus of Alzheimer's disease model mice determined by
flow cytometry. A, Normal group; B, model group; C, low-dose
β-asarone group; D, medium-dose β-asarone group; E, high-dose
β-asarone group; F, 3-MA-treated group; and G, the
rapamycin-treated group. Data are presented as the mean ± SD of 7
mice. *P<0.05 and **P<0.01 vs. normal group;
#P<0.05 and ##P<0.01 vs. model group;
$P<0.05 and $$P<0.01 vs.
rapamycin-treated group; ¤P<0.05 and
¤¤P<0.01 vs. 3-MA-treated group. 3-MA,
3-methyladenine; LC3, light chain 3. |
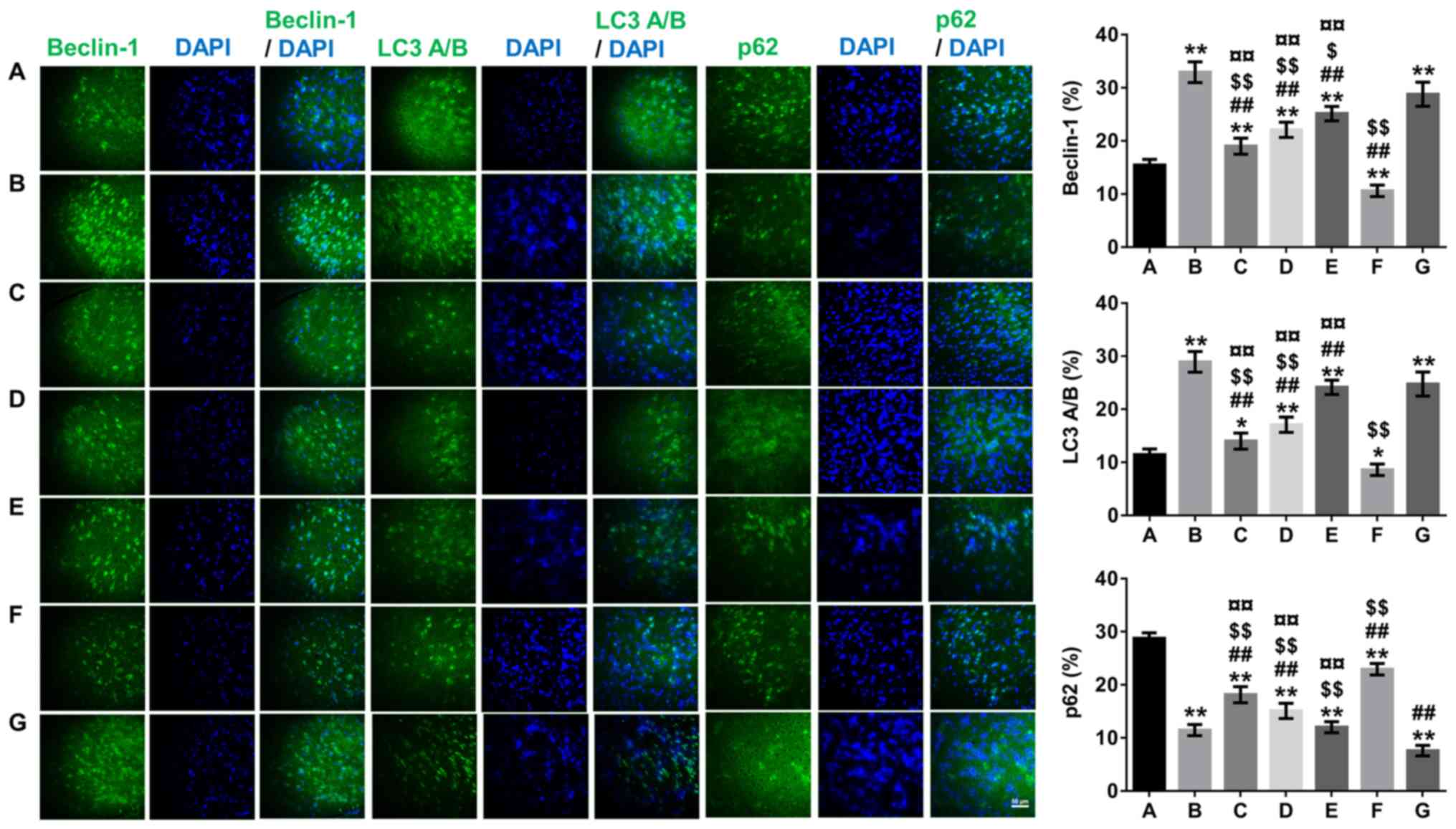 | Figure 4.Effects of β-asarone on
autophagy-related protein expression (Beclin-1, LC3 and p62) in the
hippocampus of Alzheimer's disease model mice by immunofluorescence
staining analyses. The samples detected were tissues rather than
cells and most of the expressions were normal. Images were captured
with an Olympus fluorescence microscope (not taken by confocal
microscopy due to experimental conditions) so the images presented
are not punctate; magnification, ×400. A, Normal group; B, model
group; C, low-dose β-asarone group; D, medium-dose β-asarone group;
E, high-dose β-asarone group; F, 3-MA-treated group; and G, the
rapamycin-treated group. Data are presented as the mean ± SD of 10
mice. *P<0.05 and **P<0.01 vs. normal group;
##P<0.01 vs. model group; $P<0.05 and
$$P<0.01 vs. rapamycin-treated group;
¤¤P<0.01 vs. 3-MA-treated group. 3-MA,
3-methyladenine; LC3, light chain 3. |
TEM was used to analyze the number of autophagosomes
(Fig. 5). The number of
autophagosomes in the model and rapamycin-treated groups were
increased compared with that in the normal control group
(P<0.01), whereas the number of autophagosomes in the 3-MA group
and in all β-asarone-treated groups was significantly lower
compared with the model group (P<0.01).
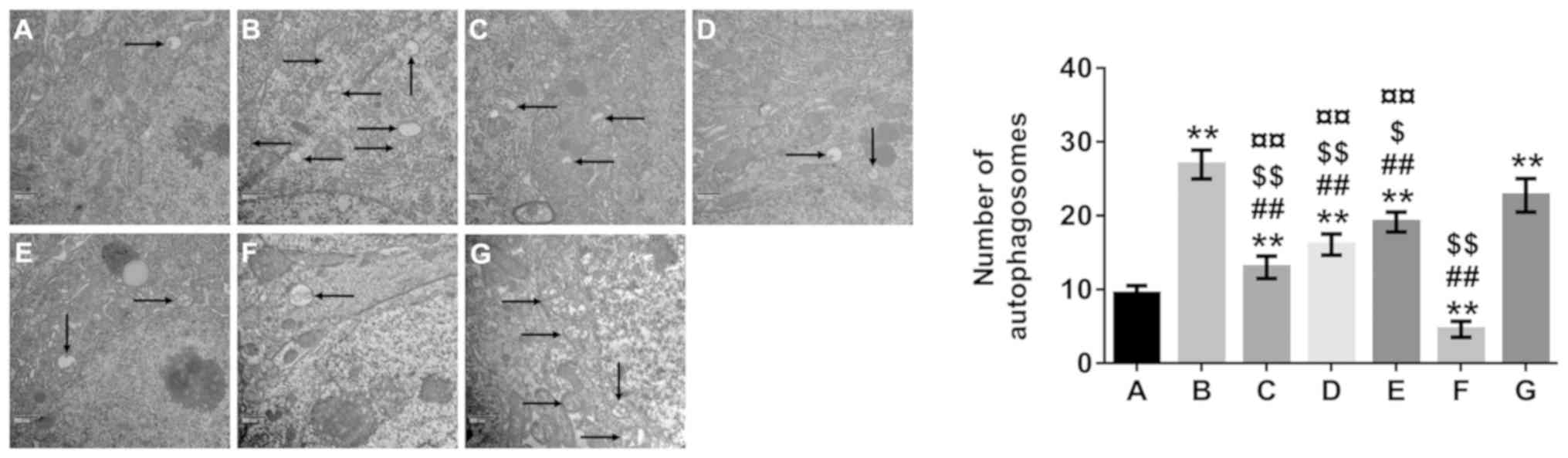 | Figure 5.Effect of β-asarone on autophagy in
Alzheimer's disease model mice. Transmission electron microscopy
(×30,000 magnification) demonstrated that autophagosome formation
was barely observed in the normal group. In addition, there were
more autophagosomes in the model- and rapamycin-treated groups
compared with the 3-MA and β-asarone treated groups. A, Normal
group; B, model group; C, low-dose β-asarone group; D, medium-dose
β-asarone group; E, high-dose β-asarone group; F, 3-MA-treated
group; and G, the rapamycin-treated group. Data are presented as
the mean ± SD of 3 mice. Black arrows indicate autophagosomes.
**P<0.01 vs. normal group; ##P<0.01 vs.
model group; $P<0.05 and $$P<0.01 vs.
rapamycin-treated group; ¤¤P<0.01 vs. 3-MA-treated
group. 3-MA, 3-methyladenine. |
Discussion
The pathological characteristics of AD include
senile plaques, intracellular neurofibrillary tangles, activated
microglia and astrocyte accumulation around Aβ plaques, and
degenerative neurons (23). Senile
plaques composed of Aβ peptides are an important criterion for
verifying AD (24).
Aβ40 and Aβ42, the two-main Aβ isoforms, are
produced from the APP by β-secretase and γ-secretase, respectively
(2). In addition to the formation
of Aβ oligomers and amyloid deposits, Aβ42 has been
linked to AD pathology (25).
Inhibiting Aβ accumulation in the brain may prove to be an
important therapeutic strategy for AD. In the present study, the
APP/PS1 transgenic mice in the model group exhibited increased
intracellular levels of Aβ40 and Aβ42 APP
mRNA, and a higher number of senile plaques compared with the
normal control group, which suggested increased Aβ production in
the hippocampus of these animals. β-asarone administration in
APP/PS1 transgenic mice decreased Aβ40, Aβ42
and APP levels and the number of senile plaques, suggesting that
β-asarone may have a protective effect. These results indicated
that β-asarone had a positive effect on reducing Aβ40,
Aβ42 peptide and APP mRNA levels in the senile plaques
of APP/PS1 transgenic mice.
Beclin-1, LC3A/B and p62 expression levels have been
used as specific markers of autophagy (26). Beclin-1, a key gene in autophagy
regulation that was first discovered in mammals, regulates the
localization of other autophagy-related proteins to autophagosomes
(27). A previous study revealed
that Beclin-1 deficiency increases APP and APP-like proteins,
accelerates Aβ accumulation and promotes neurodegeneration in mice,
implying that Beclin-1 is involved in the pathogenesis of AD
(28). The current study aimed to
investigate the effects of β-asarone on Aβ accumulation and to
determine whether APP mRNA reduction was associated with changes in
Beclin-1 levels in the hippocampus of APP/PS1 transgenic mice. In
the present study, it was demonstrated that β-asarone treatment led
to a reduction in APP and Beclin-1 mRNA levels; however, this
finding is not consistent with existing research. These results
suggested that the inhibition of APP mRNA expression by β-asarone
may be correlated with autophagy.
To further investigate the effects of β-asarone on
autophagy in APP/PS1 transgenic mice, Beclin-1, LC3A/B and p62
expression levels were detected in the hippocampus by flow
cytometry, western blotting and immunofluorescence staining
analyses. LC3 staining did not demonstrate punctate LC3A/B
fluorescence in our immunofluorescence staining method, and this
was consistent with the results of Bel et al (29). As an important process that
modulates the turnover of proteins, autophagy can serve a
protective role in many human diseases that are characterized by
toxic aggregation of misfolded proteins by eliminating the
aggregated proteins (5).
Aβ1-42 serves a vital role in the pathogenesis of AD
owing to its strong aggregative ability and neurotoxicity (2). Autophagy can protect against AD by
clearing Aβ deposition and preserving neuronal function (7). Furthermore, autophagic activity
increases when cells experience injury, nutritional deficiency,
growth factor deficits or high energy demand (4). However, excessive autophagy can also
lead to autophagic neuron death and apoptosis (5). Previous studies have reported that
almost all cells with increased Beclin-1 expression exhibit
punctate LC3B fluorescence (30),
and autophagy dysfunction or defects cause p62 upregulation
(31). Based on the evidence of
p62 partial restoration, a previous study has shown that
chloroquine is an autophagy inhibitor that further increases LC3A/B
expression in rats (32). This
indicated that there may be a negative correlation between p62 and
LC3A/B expression in autophagy.
In the present study, 3-MA was used as an inhibitor
of autophagy and rapamycin was used as an activator of autophagy
(33,34). It was demonstrated that that
Beclin-1 and LC3A/B expression levels in the model group were
higher compared with the normal control group. This result
suggested that the increased expression of these proteins in the
APP/PS1 transgenic mouse may activate autophagy in neuronal cells.
In addition, Beclin-1 and LC3A/B expression levels in all β-asarone
dose groups and the 3-MA-treated group were lower compared with
level in the model group. Together, these results indicated that
low-dose β-asarone may suppress autophagic activity by
downregulating Beclin-1 and LC3A/B expression. In addition, it also
revealed that p62 expression was higher in the 3-MA treated group,
near levels seen in the normal control group. By contrast, p62
expression was the lowest in the rapamycin treated group. These
results suggested that p62 overexpression may inhibit autophagy.
The expression of p62 was higher in all β-asarone dose groups and
in the 3-MA treated groups compared with the model group, which
indicated that β-asarone may inhibit autophagic activity by
upregulating p62 expression. Finally, there were fewer
autophagosomes in all β-asarone dose groups compared with the model
group. Together, these results suggested that β-asarone inhibited
autophagy by decreasing Beclin-1 and LC3A/B expression and
increasing p62 expression in the hippocampus of APP/PS1 transgenic
mice.
In conclusion, the present study demonstrated that
β-asarone could decrease Aβ40, Aβ42, APP,
Beclin-1 and LC3A/B levels and the number of senile plaques, and
enhance p62 expression. Collectively, these findings indicated that
β-asarone may protect against AD by clearing Aβ accumulation and
suppressing autophagic activity, and should be explored as a
potential therapeutic agent for AD treatment.
Acknowledgements
Not applicable.
Funding
The current study was supported by a Joint Research
Project of the Science and Technology Department of Guangdong
Province and the Guangdong Province Academy of Traditional Chinese
Medicine (grant no. 2012A032400006) and the cultivation of
excellent doctoral dissertations and special project funds of
Guangzhou University of Chinese Medicine (grant no.
A1-AFD018161Z01024). It was also supported by The Lingnan Normal
University-level talent project (grant no. ZL1801), The Natural
Science Foundation of Guangdong province of China (grant no.
2018A030307037), The Hainan Natural Science Foundation of China
(grant no. 20168266), The Program of Hainan Association for Science
and Technology Plans to Youth R&D Innovation (grant no.
HAST201635), The Scientific Research Cultivating Fund of Hainan
Medical University (grant no. HY2015-01), The National Natural
Science Foundation of China (grant nos. 81904104 and 31900297) and
The Administration of Traditional Chinese Medicine of Guangdong
Province, China (grant no. 20181114).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD and LH conceived the study, designed the
experiments, analyzed the data and prepared the manuscript. MD, LH
and XZ obtained samples for the present study. XZ, MD and LH
performed the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Guangzhou
University of Chinese Medicine Institutional Animal Care and Use
Committee and conformed to the National Institutes of Health Guide
for the Care and Use of Animals in Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Selkoe D, Mandelkow E and Holtzman D:
Deciphering Alzheimer disease. Cold Spring Harb Perspect Med.
2:a0114602012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gouras GK, Almeida CG and Takahashi RH:
Intraneuronal Abeta accumulation and origin of plaques in
Alzheimer's disease. Neurobiol Aging. 26:1235–1244. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitehouse IJ, Brown D, Baybutt H, Diack
AB, Kellett KA, Piccardo P, Manson JC and Hooper NM: Ablation of
prion protein in wild type human amyloid precursor protein (APP)
transgenic mice does not alter the proteolysis of APP, levels of
amyloid-β or pathologic phenotype. PLoS One. 11:e01591192016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oczypok EA, Oury TD and Chu CT: It's a
cell-eat-cell world: Autophagy and phagocytosis. Am J Pathol.
182:612–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han K, Kim J and Choi M: Autophagy
mediates phase transitions from cell death to life. Heliyon.
1:e000272015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salminen A, Kaarniranta K, Kauppinen A,
Ojala J, Haapasalo A, Soininen H and Hiltunen M: Impaired autophagy
and APP processing in Alzheimer's disease: The potential role of
Beclin 1 interactome. Prog Neurobiol. 106-107:33–54. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gali CC, Fanaee-Danesh E, Zandl-Lang M,
Albrecher NM, Tam-Amersdorfer C, Stracke A, Sachdev V, Reichmann F,
Sun Y, Avdili A, et al: Amyloid-beta impairs insulin signaling by
accelerating autophagy-lysosomal degradation of LRP-1 and IR-β in
blood-brain barrier endothelial cells in vitro and in 3XTg-AD mice.
Mol Cell Neurosci. 99:1033902019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lauritzen I, Pardossi-Piquard R, Bourgeois
A, Pagnotta S, Biferi MG, Barkats M, Lacor P, Klein W, Bauer C and
Checler F: Intraneuronal aggregation of the β-CTF fragment of APP
(C99) induces Aβ-independent lysosomal-autophagic pathology. Acta
Neuropathol. 132:257–276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hertel C, Terzi E, Hauser N, Jakob-Rotne
R, Seelig J and Kemp JA: Inhibition of the electrostatic
interaction between beta-amyloid peptide and membranes prevents
beta-amyloid-induced toxicity. Proc Natl Acad Sci USA.
94:9412–9416. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Liu C, Wang J, Li Q, Ping H, Gao
S and Wang P: MiR-299-5p regulates apoptosis through autophagy in
neurons and ameliorates cognitive capacity in APPswe/PS1dE9 mice.
Sci Rep. 6:245662016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang L, Deng M, He Y, Lu S, Ma R and Fang
Y: β-asarone and levodopa co-administration increase striatal
dopamine level in 6-hydroxydopamine induced rats by modulating
P-glycoprotein and tight junction proteins at the blood-brain
barrier and promoting levodopa into the brain. Clin Exp Pharmacol
Physiol. 43:634–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim HW, Kumar H, Kim BW, More SV, Kim IW,
Park JI, Park SY, Kim SK and Choi DK: β-Asarone
(cis-2,4,5-trimethoxy-1-allyl phenyl), attenuates pro-inflammatory
mediators by inhibiting NF-κB signaling and the JNK pathway in LPS
activated BV-2 microglia cells. Food Chem Toxicol. 72:265–272.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong H, Gao Z, Rong H, Jin M and Zhang X:
β-asarone reverses chronic unpredictable mild stress-induced
depression-like behavior and promotes hippocampal neurogenesis in
rats. Molecules. 19:5634–5649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang QS, Wang ZH, Zhang JL, Duan YL, Li
GF and Zheng DL: Beta-asarone protects against MPTP-induced
Parkinson's disease via regulating long non-coding RNA MALAT1 and
inhibiting α-synuclein protein expression. Biomed Pharmacother.
83:153–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang W and Teng J: β-asarone prevents
Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y
cells: Down expression Beclin-1, LC3B and up expression Bcl-2. Int
J Clin Exp Med. 8:20658–20663. 2015.PubMed/NCBI
|
|
16
|
Xue Z, Guo Y, Zhang S, Huang L, He Y, Fang
R and Fang Y: Beta-asarone attenuates amyloid beta-induced
autophagy via Akt/mTOR pathway in PC12 cells. Eur J Pharmacol.
741:195–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Fang YQ, Xue ZF, He YP, Fang RM and
Li L: Beta-asarone attenuates ischemia-reperfusion-induced
autophagy in rat brains via modulating JNK, p-JNK, Bcl-2 and Beclin
1. Eur J Pharmacol. 680:34–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsiao K, Chapman P, Nilsen S, Eckman C,
Harigaya Y, Younkin S, Yang F and Cole G: Correlative memory
deficits, Abeta elevation, and amyloid plaques in transgenic mice.
Science. 274:99–102. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L and Fang YQ: Analysis of the
distribution of β-asarone in rat hippocampus, brainstem, cortex and
cerebellum with gas chromatography-mass spectrometry (GC-MS). J Med
Plants Res. 5:1728–1734. 2011.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang LP, Deng MZ, He YP and Fang YQ:
β-asarone and levodopa co-administration protects against
6-hydroxydopamine-induced damage in parkinsonian rat mesencephalon
by regulating autophagy: Down-expression Beclin-1 and light chain
3B and up-expression P62. Clin Exp Pharmacol Physiol. 42:269–277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Y, Mo Z, Xue Z and Fang Y: Establish a
flow cytometric method for quantitative detection of Beclin-1
expression. Cytotechnology. 65:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonham LW, Desikan RS and Yokoyama JS;
Alzheimer's Disease Neuroimaging Initiative, : The relationship
between complement factor C3, APOE ε4, amyloid and tau in
Alzheimer's disease. Acta Neuropathol Commun. 4:652016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abad S, Ramon C, Pubill D, Camarasa J,
Camins A and Escubedo E: Adolescent exposure to MDMA induces
dopaminergic toxicity in substantia nigra and potentiates the
amyloid plaque deposition in the striatum of APPswe/PS1dE9 mice.
Biochim Biophys Acta. 1862:1815–1826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gautam V, D'Avanzo C, Berezovska O, Tanzi
RE and Kovacs DM: Synaptotagmins interact with APP and promote Aβ
generation. Mol Neurodegener. 10:312015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmitz KJ, Ademi C, Bertram S, Schmid KW
and Baba HA: Prognostic relevance of autophagy-related markers LC3,
p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients
with respect to KRAS mutational status. World J Surg Oncol.
14:1892016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pattingre S, Espert L, Biard-Piechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin 1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Brien CE and Wyss-Coray T: Sorting
through the roles of beclin 1 in microglia and neurodegeneration. J
Neuroimmune Pharmacol. 9:285–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bel S, Pendse M, Wang Y, Li Y, Ruhn KA,
Hassell B, Leal T, Winter SE, Xavier RJ and Hooper LV: Paneth cells
secrete lysozyme via secretory autophagy during bacterial infection
of the intestine. Science. 357:1047–1052. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi J, Jung W and Koo JS: Expression of
autophagy-related markers beclin-1, light chain 3A, light chain 3B
and p62 according to the molecular subtype of breast cancer.
Histopathology. 62:275–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang H, Zhu J, Li Y, Zhang L, Gu J, Xie
Q, Jin H, Che X, Li J, Huang C, et al: Upregulation of SQSTM1/p62
contributes to nickel-induced malignant transformation of human
bronchial epithelial cells. Autophagy. 12:1687–1703. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon I, Lee Y, Cosio-Lima LM, Cho JY and
Yeom DC: Effects of long-term resistance exercise training on
autophagy in rat skeletal muscle of chloroquine-induced sporadic
inclusion body myositis. J Exerc Nutrition Biochem. 19:225–234.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei X, Zhou Z, Li L, Gu J, Wang C, Xu F,
Dong Q and Zhou X: Intrathecal injection of 3-methyladenine reduces
neuronal damage and promotes functional recovery via autophagy
attenuation after spinal cord ischemia/reperfusion injury in rats.
Biol Pharm Bull. 39:665–673. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Liu F, Wang Y, Li D, Guo F, Xu L,
Zeng Z, Zhong X and Qian K: Rapamycin-induced autophagy sensitizes
A549 cells to radiation associated with DNA damage repair
inhibition. Thorac Cancer. 7:379–386. 2016. View Article : Google Scholar : PubMed/NCBI
|