Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer and is the leading cause of cancer-related
deaths worldwide (1,2). Although surgical resection combined
with post-surgery radio-chemotherapy has achieved great progress,
the median survival time for patients with HCC remains
unsatisfactory (3). It is
estimated that ~700,000 individuals succumb to HCC each year
globally (4). Therefore, further
investigations into the molecular basis of HCC are required to
explore innovative targets for its diagnosis and treatment.
Circular RNA (circRNA) is a newly discovered class
of endogenous noncoding RNAs that are characterized by a covalently
closed continuous loop (5). Due to
their special structure, circRNAs are highly evolutionarily
conserved and stable (6). Recent
studies have indicated that circRNAs may serve important roles in
driving cancer initiation and progression, and have the potential
to serve as biomarkers for predicting cancer progression (7,8).
Accumulating evidence has demonstrated that circRNAs regulate HCC
progression and serve as potential biomarkers for predicting cancer
prognosis (9–15). However, a considerable number of
circRNAs remain to be elucidated in HCC.
Insulin-like growth factor 2 (IGF2) is a
genomic imprinting gene involved in development and growth, which
is located on the short arm of chromosome 11 (16). This gene is a paternally imprinted
growth factor regulated by four promoters. During fetal stages, the
expression of IGF2 is monoallelically regulated from 3
promoters (P2, P3 and P4) in human liver and in adults, its
expression is regulated by both alleles of promoter P1 (17). Although IGF2 is highly
active during fetal development, it is much less active after birth
(18). However, studies have
suggested that IGF2 overexpression occurs in numerous types
of cancers and is associated with resistance to chemotherapy and a
worse prognosis (18,19). This might be partly explained by
the reactivation of IGF2 transcription from the
fetal-specific promoters or demethylation of its fetal promoter
(20,21). However, further studies are still
needed to elucidate the mechanisms of IGF2 overexpression in
HCC.
Previous studies have demonstrated that circRNAs may
function as competing endogenous RNAs (ceRNA) by sponging miRNAs to
regulate target gene expression (22–24).
In a previous study, Han et al (25) characterized the expression profile
of circRNAs in human HCC tissues and paired adjacent liver tissues.
They demonstrated that circMTO1 suppresses HCC progression by
acting as a sponge for oncogenic miR-9 to promote p21 expression.
In the present study, the sequencing data used in the study of Han
et al was downloaded from the Gene Expression Omnibus (GEO)
database and these data were re-analyzed using a series of
bioinformatics methods. A ceRNA network was constructed and
IGF2 was found to be involved in a ceRNA network of
hsa_circRNA_100084-hsa-miR-23a-5p-IGF2. The present study
further validated the ceRNA association in HCC tissues and liver
cancer cells.
Materials and methods
RNA sequencing data
The expression profile of lncRNAs in human HCC was
downloaded from the GEO database (26) (accession number: GSE97332), which
was deposited by Han et al (25). This dataset contained seven pairs
of HCC tumor tissues and matched non-tumor tissues and was based on
the Agilent-069978 Arraystar Human CircRNA microarray V1 platform.
Original expression data as well as the platform probes annotation
files were downloaded.
Identification of differentially
expressed (DE)-circRNAs in HCC
The original expression profiles of HCC and normal
tissues were analyzed using GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/), which is an
online tool for processing data using GEO queries and limma
packages (27) in R from the
Bioconductor project (28). The
raw data were preprocessed by background correction and
normalization by log2 transformation. The raw P-value was adjusted
by the Benjamini and Hochberg method to a false discovery rate
(FDR). The circRNAs with thresholds of FDR <0.05 and |logfold
change (FC)| >2 were considered as DE-circRNAs. Heatmap for the
top 10 upregulated circRNAs and the top 10 downregulated circRNAs
were constructed using pheatmap method in R package (http://finzi.psych.upenn.edu/R/library/pheatmap/html/pheatmap.html).
Construction of a circRNA-miRNA-mRNA
network
The top 10 upregulated circRNAs and top 10
downregulated circRNAs were selected for further analysis. Since
circRNA nomenclature differs among platforms, the present study
first mapped the probe sequences of the top 10 upregulated circRNAs
and top 10 downregulated circRNAs into circBase (http://www.circbase.org/cgi-bin/webBlat). The
associations with the highest matching score were selected. The
miRNAs related to the 20 DE-circRNAs were predicted by miRanda
v.3.3a (http://www.microrna.org/microrna/home.do) with the
criteria of Score ≥140 and Energy ≤-10 (29).
The HCC-associated miRNAs were searched on the
miR2Disease (www.mir2disease.org) database using ‘hepatocellular
carcinoma’ as key words. Then, the overlapping miRNAs of
HCC-associated miRNAs and the predicted miRNAs in the previous step
were noted for further study.
Prediction of miRNA target genes
The online tool miRWalk2.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/)
(30) was used for predicting the
target genes of miRNAs in 7 databases: miRWalk (http://mirwalk.umm.uni-heidelberg.de/),
miRanda (http://www.microrna.org/microrna/home.do), miRDB
(http://mirdb.org/), miRMap (https://mirmap.ezlab.org/), miRNAMap (http://mirnamap.mbc.nctu.edu.tw/), RNA22
(https://cm.jefferson.edu/rna22/) and
TargetScan (http://www.targetscan.org/vert_72/). The target genes
predicted by at least 6 databases were retained. To further filter
the target genes, miRanda was used to calculate the score and
Energy for the combination of miRNA-mRNA (3′-untranslated region).
The miRNA-target genes relationships with scores ≥140 and Energy
≤-10, were subjected to further analysis.
The circRNA-miRNA-mRNA network was constructed using
Cytoscape software (31) based on
the obtained circRNAs, miRNAs and mRNAs.
Clinical samples and cell culture
The present study was approved by the ethics
committee of the Lishui Municipal Central Hospital (Lishui, China)
and written informed consent was obtained from all patients
included in this study. A total of 37 pairs of HCC and adjacent
normal tissues without preoperative treatment were collected from
surgical resections in the hospital between March 2018 and August
2018 and stored at −80°C until use. HCC was diagnosed via
histological confirmation. The adjacent normal tissues were
collected 3 cm away from the HCC tissue edge. The clinical
characteristics of patients are presented in Table I.
 | Table I.Clinicopathological characteristics
of patients with HCC cancer in this study (n=37). |
Table I.
Clinicopathological characteristics
of patients with HCC cancer in this study (n=37).
| Characteristic | Patients, n
(%) |
|---|
| Age, years |
|
|
<60 | 16 (43.2%) |
|
≥60 | 21 (56.8%) |
| Sex |
|
|
Male | 23 (62.2%) |
|
Female | 14 (37.8%) |
| Serum AFP,
ng/ml |
|
|
Negative | 13 (35.1%) |
|
Positive | 24 (64.9%) |
| Smoking |
|
|
Negative | 18 (48.6%) |
|
Positive | 19 (51.4%) |
| Alcohol |
|
|
Negative | 27 (73%) |
|
Positive | 10 (27%) |
| Cirrhosis |
|
|
Present | 28 (75.7%) |
|
Absent | 9 (24.3%) |
| T stage |
|
|
T1-T2 | 15 (40.5%) |
|
T3-T4 | 22 (59.5%) |
| Regional lymph node
metastasis |
|
|
Yes | 16 (43.2%) |
| No | 21 (56.8%) |
| Distant
metastasis |
|
|
Yes | 11 (29.7%) |
| No | 26 (70.3%) |
| Tumor size |
|
| <5
cm | 26 (70.3%) |
| ≥5
cm | 11 (29.7%) |
Human liver cancer cell lines with a stepwise
metastatic potential, including MHCC97H with high metastatic
potential, and HepG2 (a hepatoblastoma cell line) (32) and Hep3B with very low invasiveness,
and the normal human hepatic stellate cell LX2 were purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences and iCell Bioscience Inc. All cells were authenticated via
STR profiling. Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) with 10%
fetal bovine serum (FBS, Invitrogen; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5%
CO2 at 37°C.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was extracted from tissue and cells
(1×106) with TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The quantity and concentration of total RNA were
determined by a NanoDrop 2000 instrument (Thermo Fisher Scientific,
Inc.). RT-qPCR was performed as described previously (33). Briefly, total RNA was reverse
transcribed to cDNA using a PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd.). qPCR was performed in a 96-well plate on
an ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with PowerUp SYBR-Green Master Mix (Thermo Fisher Scientific,
Inc.) as per the procedure provided by the manufacturer. For
detecting hsa-miR-23a-5p, a hsa-miR-23a-5p-specific stem-loop
primer (Guangzhou RiboBio Co., Ltd.) was used for reverse
transcription and RT-qPCR amplification was performed using the
Bulge-Loop miRNA RT-qPCR Starter kit (Guangzhou RiboBio Co., Ltd.).
The thermocycling conditions were 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 60 sec. GAPDH (for circRNA
and mRNA) or U6 (for miRNA) was used as reference control. Relative
expression level was calculated by the 2−ΔΔCq method
(34). Primer sequences are listed
in Table II.
 | Table II.Primer sequences used for
RT-qPCR. |
Table II.
Primer sequences used for
RT-qPCR.
| Gene | Sequence
(5′à3′) |
|---|
|
hsa_circRNA_100084 | F:
AGAATGCAGGTCCAACCA |
|
| R:
GAGACAGCGGGAGTGAAG |
|
hsa-miR-23a-5p | RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGCAAATCC |
|
| F:
GGGGTTCCTGGGGATG |
|
| R:
GTGCAGGGTCCGAGGT |
| IGF2 | F:
GTCGGCCCAAACCGAG |
|
| R:
CGAAGGCCAAGAAGGTGAGAA |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| GAPDH | F:
AGAAGGCTGGGGCTCATTTG |
|
| R:
AGGGGCCATCCACAGTCTTC |
Western blot analysis
The protein expression of IGF2 was determined by
western blotting. Briefly, total protein was extracted using RIPA
lysis buffer and protein concentration was determined by BCA assay
(Beyotime Institute of Biotechnology). Proteins (30 µg/lane) were
separated by SDS-PAGE on 12% gel and transferred onto a PVDF
membrane (Millipore). Following blocking with 5% skimmed milk for 1
h at room temperature (~25°C), the PVDF membrane was incubated with
primary antibodies against IGF2 (1:1,000, cat. no. ab9574; Abcam)
and GAPDH (1:1,000, cat. no. ab8245; Abcam)_at 4°C overnight.
Following washing with Tris-buffered saline containing 0.05%
Tween-20, horseradish peroxidase-conjugated secondary antibody IgG
(H&L, 1:4,000, cat. no. ab6728; Abcam) was added and incubated
at room temperature (~25°C) for 1 h. Protein bands were visualized
using the enhanced chemiluminescence ECL method (Bio-Rad
Laboratories, Inc.) and analyzed with ImageJ software v.1.6.0
(National Institutes of Health).
Cell transfection
Sh-hsa_circ_100084
(5′-AACCCGUUCUCCGAAUUCCUAdTdT-3′), hsa-miR-23a-5p mimics, the miRNA
negative control (NC), pcDNA3.1-IGF2, and pcDNA3.1-NC were obtained
from Guangzhou RiboBio Co., Ltd. These constructs were transfected
into HepG2 cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. Briefly, 5×105 cells were seeded in each
well of six-well plates. Sh-hsa_circ_100084 (1 µg/well),
hsa-miR-23a-5p mimics (50 pmol/well), pcDNA3.1-IGF2 (1 µg/well), or
NC (1 µg/well for Sh-NC and 50 pmol/well for miR-NC) and
Lipofectamine® 2000 were added in each well and
incubated for 24 h. Transfection efficiency was determined by
RT-qPCR after 24 h.
Cell proliferation, migration and
invasion assays
Cell proliferation was analyzed by a Cell Counting
Kit-8 (CCK-8, Beyotime Institute of Biotechnology) according to
manufacturer's instructions. Briefly, 1×104 cells/well
were plated on 96-well plates and incubated at 37°C in 5%
CO2 for 24 h. After 48 h of transfection, the cells were
incubated for another 24, 48 and 72 h. Then, 10 µl of CCK-8 was
added into each well and the absorbance at 450 nm was measured with
an MK3 microplate reader (Thermo Scientific, Inc.).
Cell migration and invasion assays
were performed using Transwell assays
For invasion assays, 1×105 cells were
suspended into 250 µl of serum-free DMEM with 0.1% bovine serum
albumin (Invitrogen; Thermo Fisher Scientific, Inc.) and seeded
into the upper chamber of a 24-well Transwell insert (pore size: 8
µm; BD Biosciences) which were precoated with Matrigel at 37°C for
30 min (BD Biosciences). The lower chamber was filled with DMEM
containing 2.5% FBS. For migration assays, 1×105
suspended cells were seeded into the upper chambers without a
Matrigel coating. After 48 h, the invaded or migrated cells were
fixed and stained with 0.5% crystal violet at room temperature
(~25°C) for 15 min and counted under a microscope (Olympus
Corporation). Five images were randomly captured for each
sample.
Luciferase reporter assay
HepG2 cells were seeded into 24-well plates and
co-transfected with hsa_circ_100084-wt, hsa_circ_100084-mut,
IGF2-wt, or IGF2-mut plasmids. Then, hsa-miR-23a-5p mimics or
negative controls (Guangzhou RiboBio Co., Ltd.) were transfected
into cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Luciferase assays were conducted 48 h
following transfection using a Dual Luciferase Reporter Assay
System (Promega Corporation). The firefly luciferase activity was
normalized to Renilla luciferase activity.
Immunohistochemistry (IHC)
The protein level of IGF2 in hepatic tissues was
examined by IHC. Briefly, biopsies were fixed in 4% buffered
formaldehyde and embedded in paraffin for 4 h. Then, 5-µm sections
were blocked with 5% BSA (cat. no. 810652; Sigma-Aldrich; Merck
KGaA) for 30 min at 37°C, and incubated with a primary antibody
against IGF2 (1:500; cat. no. ab9574, Abcam) overnight at 4°C,
followed by incubation with a biotinylated secondary anti-Rabbit
IgG antibody (1:500; cat. no. SA00004-2, ProteinTech Group, Inc.)
and peroxidase-labeled streptavidin at room temperature for 15 min.
Representative images at magnification, ×20 and ×40 were captured
under an inverted microscope (Leica Microsystems, Inc.).
Statistical analysis
All experiments were performed in triplicate and
data were analyzed by SPSS 20.0 (IBM Corp.). Comparisons between
two groups were analyzed by Student's t-test or χ2 test
when appropriate, while comparisons among multiple groups were
conducted using one-way ANOVA with LSD post hoc analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification of DE-circRNAs in HCC
samples
The circRNA expression profile of HCC deposited by
Han et al (25), was
downloaded from the GEO database and GEO2R was used to identify
DE-circRNAs in the HCC samples compared with the adjacent liver
tissues. According to the criteria of FDR <0.05 and |logFC|
>2, 147 DE-circRNAs were identified, including 50 downregulated
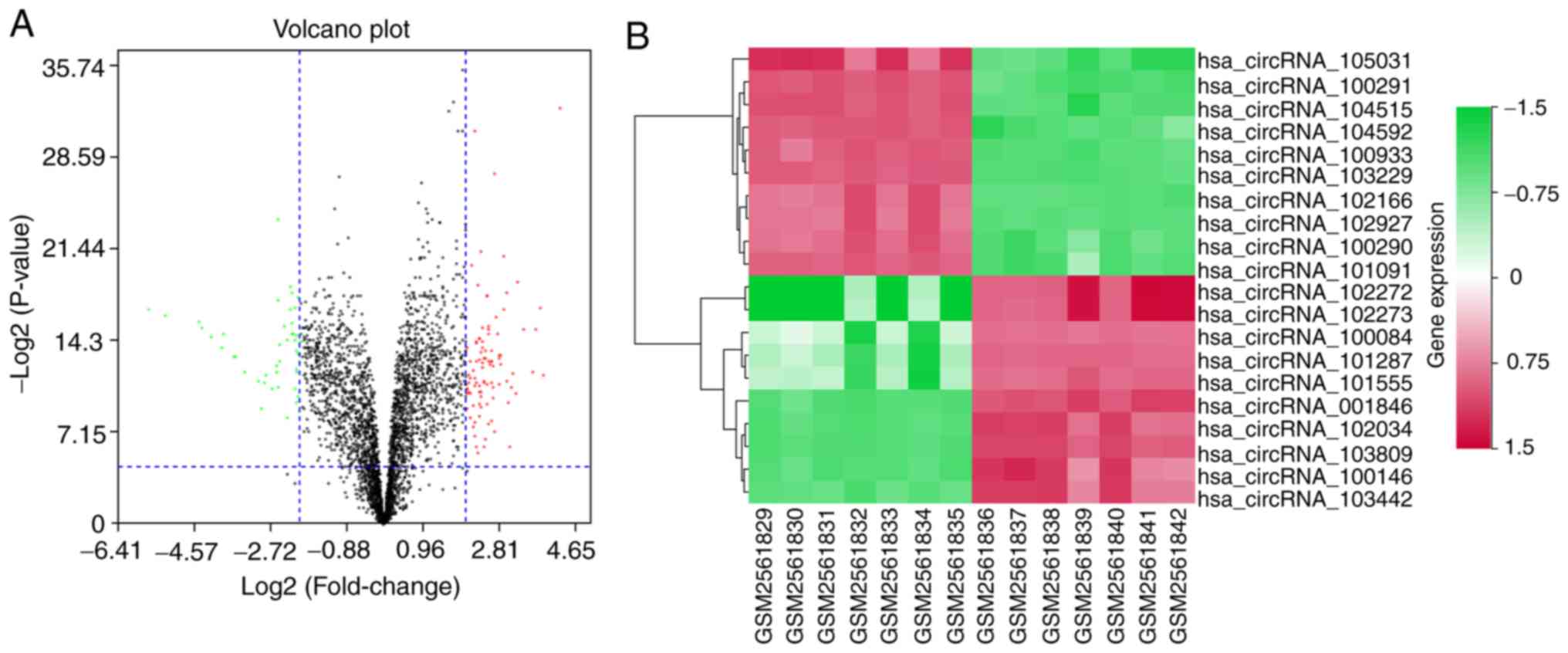
circRNAs (34.01%) and 97 upregulated circRNAs (65.99%; Fig. 1A). The top 10 upregulated circRNAs
and top 10 downregulated circRNAs are shown in Fig. 1B and listed in Table III.
 | Table III.Top 10 upregulated circRNAs and top
10 downregulated circRNAs between HCC and normal tissues. |
Table III.
Top 10 upregulated circRNAs and top
10 downregulated circRNAs between HCC and normal tissues.
| ID | P-value | FDR | logFC | Hsa_circRNAs |
|---|
| Downregulated
circRNAs |
| ASCRP005278 |
1.73×10−10 |
4.01×10−08 | −6.4099 |
hsa_circRNA_105031 |
| ASCRP004769 |
3.51×10−07 |
9.18×10−06 | −5.69472 |
hsa_circRNA_104515 |
| ASCRP000679 |
6.00×10−07 |
1.30×10−05 | −5.29939 |
hsa_circRNA_100291 |
| ASCRP004845 |
8.86×10−07 |
1.75×10−05 | −4.45749 |
hsa_circRNA_104592 |
| ASCRP003527 |
1.62×10−06 |
2.55×10−05 | −4.41726 |
hsa_circRNA_103229 |
| ASCRP001306 |
3.37×10−06 |
4.20×10−05 | −4.19757 |
hsa_circRNA_100933 |
| ASCRP000678 |
7.84×10−06 |
7.18×10−05 | −3.92181 |
hsa_circRNA_100290 |
| ASCRP001459 |
2.60×10−06 |
3.47×10−05 | −3.89008 |
hsa_circRNA_101091 |
| ASCRP003234 |
1.74×10−05 |
1.19×10−04 | −3.61817 |
hsa_circRNA_102927 |
| ASCRP002498 |
1.69×10−05 |
1.16×10−04 | −3.58558 |
hsa_circRNA_102166 |
| Upregulated
circRNAs |
| ASCRP000474 |
2.74×10−04 |
8.82×10−04 | 3.2099071 |
hsa_circRNA_100084 |
| ASCRP000343 |
3.35×10−08 |
2.04×10−06 | 3.2221957 |
hsa_circRNA_001846 |
| ASCRP003740 |
1.65×10−06 |
2.57×10−05 | 3.3864621 |
hsa_circRNA_103442 |
| ASCRP001647 |
5.29×10−05 |
2.64×10−04 | 3.610212 |
hsa_circRNA_101287 |
| ASCRP000535 |
1.71×10−06 |
2.62×10−05 | 3.6841615 |
hsa_circRNA_100146 |
| ASCRP002369 |
3.13×10−07 |
8.37×10−06 | 3.766187 |
hsa_circRNA_102034 |
| ASCRP001906 |
6.85×10−05 |
3.12×10−04 | 3.8742393 |
hsa_circRNA_101555 |
| ASCRP002600 |
5.00×10−15 |
1.74×10−11 | 4.0273536 |
hsa_circRNA_102272 |
| ASCRP002601 |
1.89×10−13 |
1.64×10−10 | 4.249804 |
hsa_circRNA_102273 |
| ASCRP004099 |
6.85×10−08 |
3.60×10−06 | 4.6531421 |
hsa_circRNA_103809 |
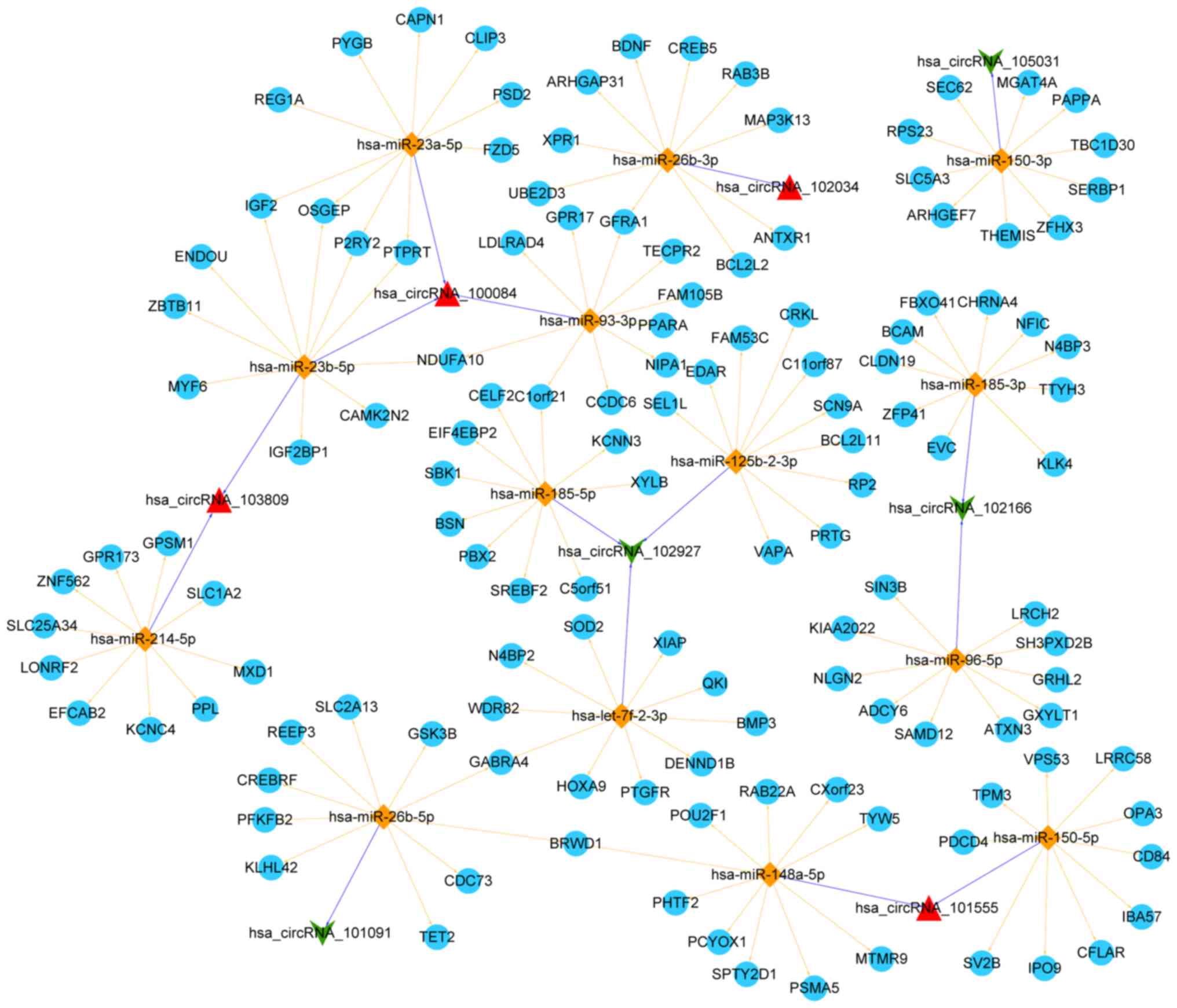
Construction of circRNA-miRNA-mRNA
network
By using miRanda to predict which miRNAs were
related to the top 20 DE circRNAs, 409 miRNAs were obtained at the
criteria of score ≥140 and Energy ≤-10. Following searching in miR2
Disease, 17 circRNA-miRNA relationships involving 16 miRNAs and 8
circRNAs were obtained. Additionally, a total of 1,669 target genes
associated with these miRNAs were predicted in 6 of 7 searched
databases and 923 target genes were further filtered. The top 10
target genes for each miRNA were used for constructing a ceRNA
network in Cytoscape. This ceRNA network involved 15 circRNA-miRNA
relationships and 140 miRNA-mRNA relationships (Fig. 2).
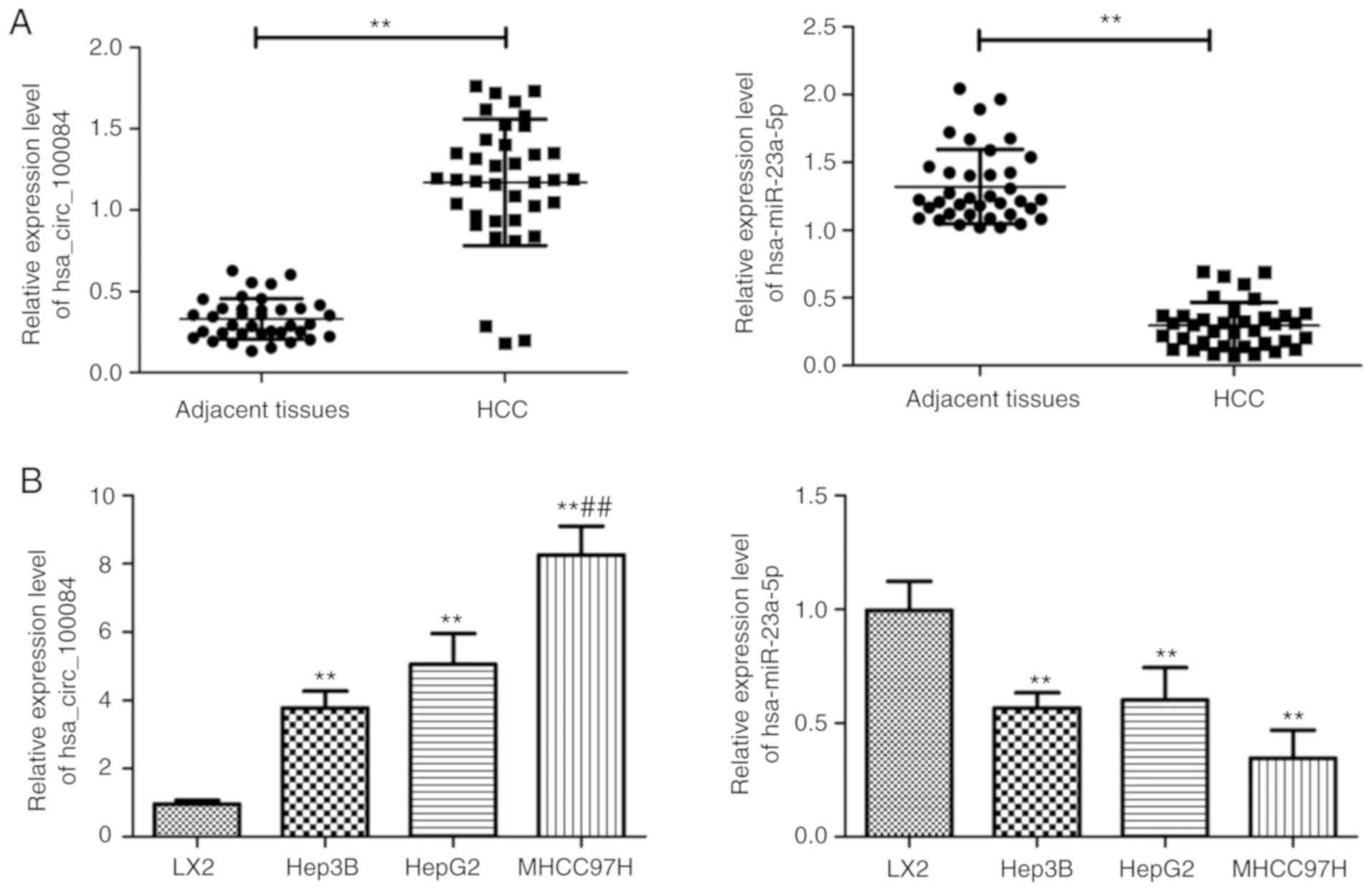
Differential expression of
hsa_circRNA_100084, hsa-miR-23a-5p, and IGF2 in HCC tissues and
cells
Findings showed that IGF2 was involved in the ceRNA
relationship of hsa_circRNA_100084-hsa-miR-23a-5p-IGF2. Therefore,
the expression level of hsa_circRNA_100084, hsa-miR-23a-5p, and
IGF2 in HCC tissues and liver cancer cells we validated by RT-qPCR,
IHC and western blotting. As shown in Fig. 3A, compared with levels in the
adjacent normal tissues, the relative expression levels of
hsa_circRNA_100084 in HCC tissues were significantly upregulated
(P<0.01), while the expression of hsa-miR-23a-5p was
significantly downregulated (P<0.01). Then, the expression
patterns of hsa_circRNA_100084 and hsa-miR-23a-5p were analyzed in
three liver cancer cell lines with different metastatic potential,
including MHCC97H, Hep3B and HepG2 cells. Consistently, the
expression of hsa_circRNA_100084 was significantly upregulated in
liver cancer cells (Hep3B and MHCC97H) and a hepatoblastoma cell
line (HepG2) (P<0.01), while the expression of hsa-miR-23a-5p
was significantly downregulated compared with levels in the human
normal hepatic cell line LX2 (P<0.01, Fig. 3B). Additionally, the mRNA and
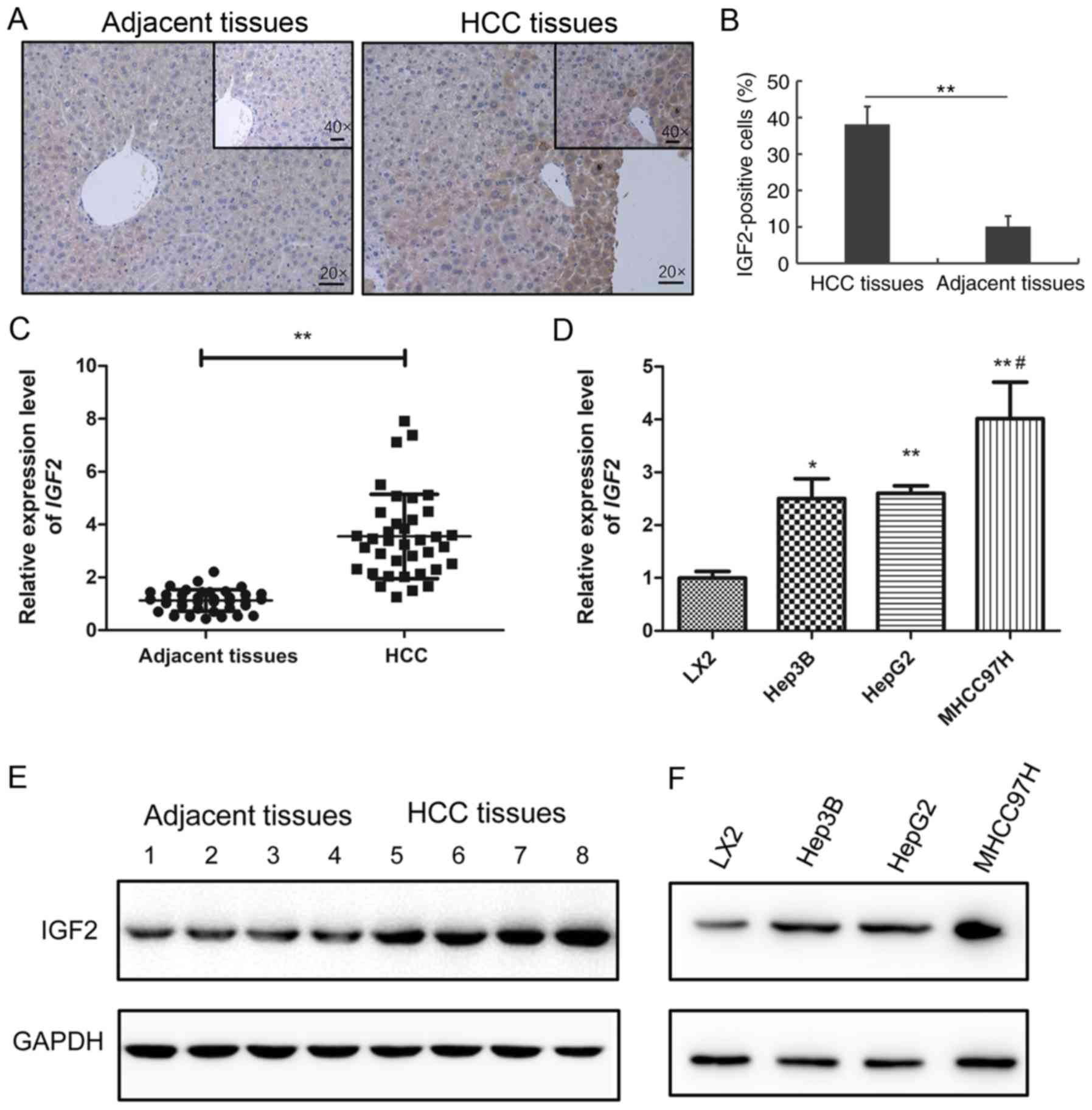
protein expression of IGF2 in HCC tissues and adjacent tissues were
tested by RT-qPCR, IHC and western blotting (Fig. 4). IHC results showed that the
number of IGF2-positive cells in HCC tissues was much higher
compared with adjacent normal tissues (Fig. 4A and B). qPCR and western blotting
further confirmed that IGF2 was upregulated in HCC tissues and
cells (Fig. 4C-E). Taken together,
these data suggested that hsa-miR-23a-5p, hsa_circRNA_100084 and
IGF2 might be involved in HCC progression and that there may
be competing relationships among them.
hsa_circRNA_100084 promotes IGF2
expression by acting as a sponge of hsa-miR-23a-5p in liver cancer
cells
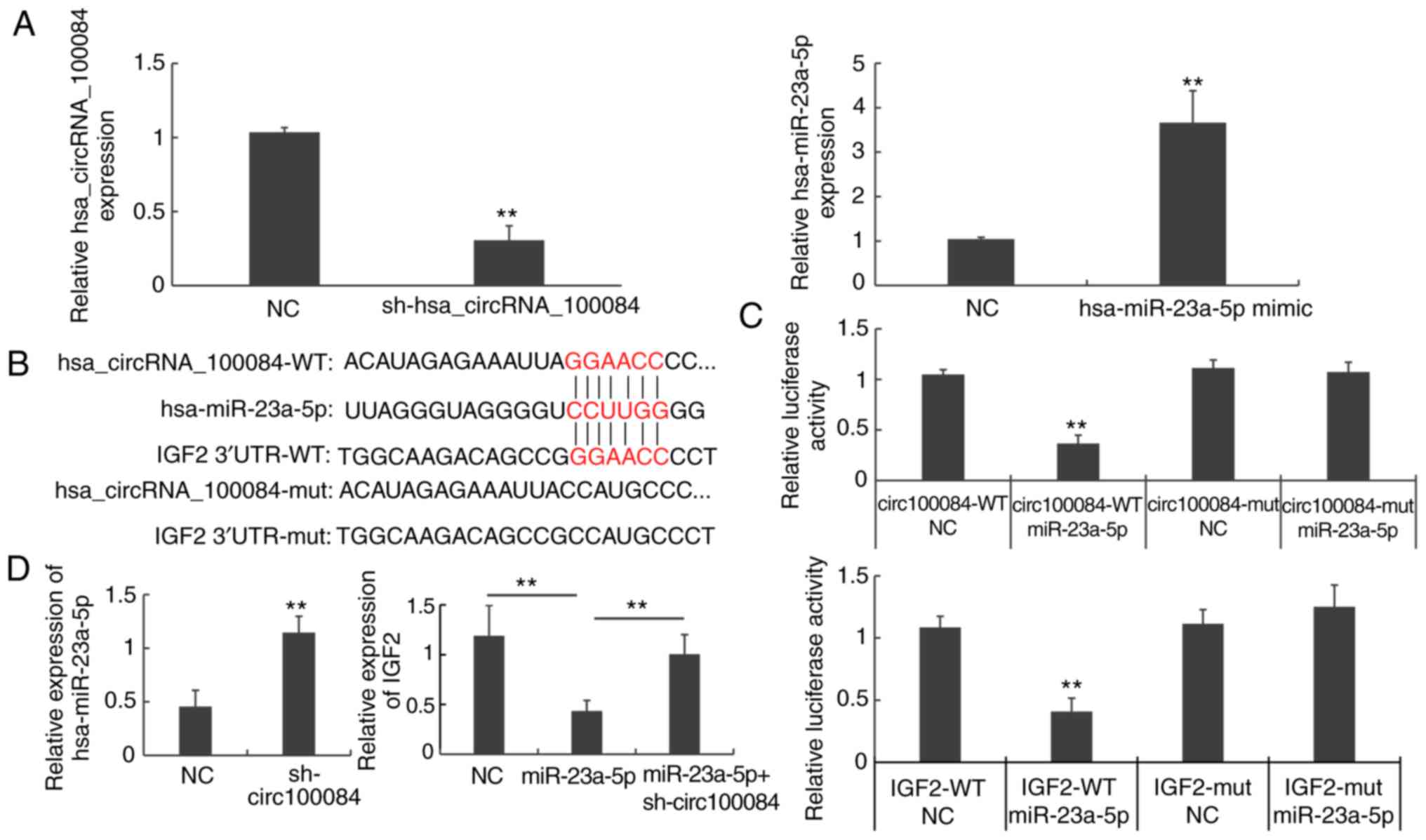
RT-qPCR results showed that expression of
hsa_circRNA_100084 was significantly decreased following the
transfection of sh-hsa_circRNA_100084, while hsa-miR-23a-5p
expression was significantly increased following the transfection
of hsa-miR-23a-5p mimics (P<0.01; Fig. 5A). Bioinformatics analysis
demonstrated that hsa_circRNA_100084 and IGF2 may bind to
hsa-miR-23a-5p (Fig. 5B).
Luciferase reporter assays showed that hsa-miR-23a-5p mimics could
regulate the luciferase activity of wild-type hsa_circRNA_100084
and IGF2 (P<0.01; Fig.
5C), rather than mutant hsa_circRNA_100084 and IGF2 (P>0.05;
Fig. 5C). To further investigate
the relationships among hsa_circRNA_100084, hsa-miR-23a-5p and
IGF2, HepG2 cells were transfected with
sh-hsa_circRNA_100084, hsa-miR-23a-5p, mimics or
sh-hsa_circRNA_100084 + hsa-miR-23a-5p mimics, respectively.
RT-qPCR results showed that sh-hsa_circRNA_100084 transfection
increased the levels of hsa-miR-23a-5p (P<0.01; Fig. 5D). Additionally, overexpression of
hsa-miR-23a-5p decreased the expression of IGF2 in HepG2
cells. However, sh-hsa_circRNA_100084 could also attenuate the
effect of hsa-miR-23a-5p overexpression on the expression of
IGF2 (P<0.01; Fig. 5D).
Taken together, the results of the present study demonstrated that
hsa_circRNA_100084 promoted the expression of IGF2 by acting
as a sponge of hsa-miR-23a-5p in liver cancer cells.
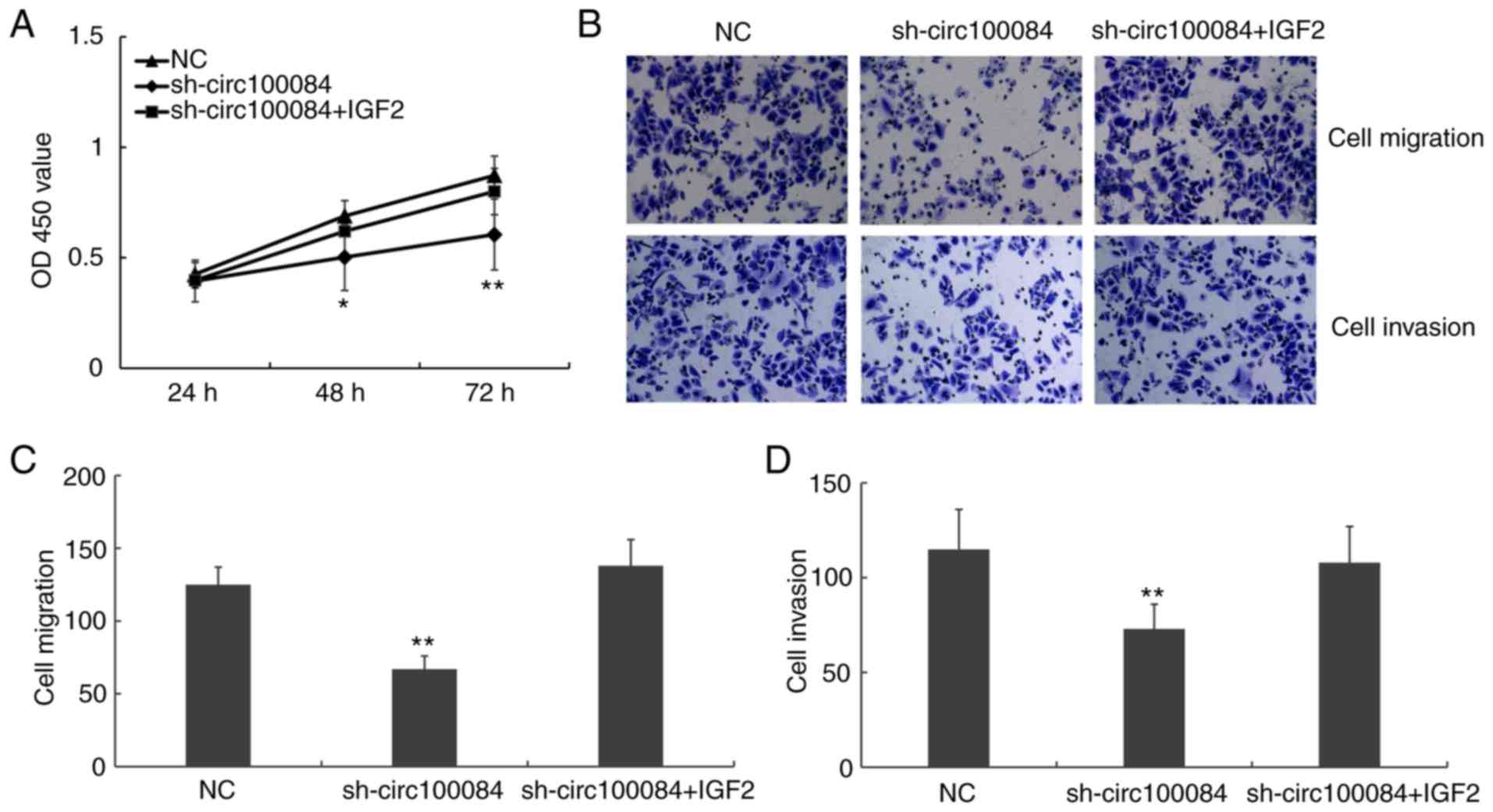
Hsa_circRNA_100084 promotes
proliferation, migration and invasion via regulating IGF2
CCK-8 assays and Transwell assays were performed to
investigate the role of hsa_circRNA_100084 on cell proliferation,
migration and invasion using HepG2 cells. The results demonstrated
that sh-hsa_circRNA_100084 inhibited the proliferation, migration
and invasion of liver cancer cells. However, transfection of
pcDNA3.1-IGF2 simultaneously could reverse the effects of
sh-hsa_circRNA_100084 (Fig. 6).
Taken together, the results suggested that hsa_circRNA_100084
promotes liver cancer cell proliferation, migration and invasion by
regulating IGF2 via acting as a sponge of
hsa-miR-23a-5p.
Discussion
HCC is regarded as the most malignant type of liver
cancer because of its high incidence rate and poor prognosis
(35). Therefore, it is necessary
to investigate the biological basis and identify novel targets for
HCC. Due to their special structure, circRNAs are evolutionarily
conserved and stable. Previous studies have demonstrated that
circRNAs are disease-, tissue- and stage-specifically expressed,
suggesting their particular roles in disease initiation and
development (36–38). The present study re-analyzed the
gene expression profile GSE97332 and identified 147 DE-circRNAs,
including 50 downregulated circRNAs (34.01%) and 97 upregulated
circRNAs (65.99%). Then, a ceRNA network was constructed for these
DE-circRNAs. In this ceRNA network, it was found that IGF2
was involved in a ceRNA relationship of
hsa_circRNA_100084-hsa-miR-23a-5p-IGF2. Further studies
demonstrated that hsa_circRNA_100084 promoted liver cancer cell
proliferation, migration and invasion by competitively binding
hsa-miR-23a-5p, leading to the upregulation of IGF2.
Elucidating the molecular mechanisms of HCC will be
important for the development of therapies to successfully treat
HCC. Several studies have demonstrated that IGF2 is upregulated in
a number of cancers, including HCC, and is associated with
resistance to chemotherapy and a worse prognosis (18,19,39).
Though loss of imprinting, loss of heterozygosity, or reactivation
of IGF2 transcription could partially explain the
upregulation of IGF2 in cancer, further studies are
necessary to explore these possibilities. The present study found
that IGF2 is involved in a ceRNA relationship of
hsa_circRNA_100084-hsa-miR-23a-5p- IGF2. Consistently, Zhen
et al (40) demonstrated
that circHMGCS1 promotes hepatoblastoma cell proliferation by
regulating IGF2.
The results of the present study demonstrated that
knocking down hsa_circRNA_100084 could significantly inhibit the
proliferation, migration and invasion of liver cancer cells,
suggesting that hsa_circRNA_100084 might have potential to be used
us a promising therapeutic strategy for HCC. By searching circBase,
hsa_circRNA_100084 was found to correspond to circEIF4G3, which is
located on chr1:21329205-21415706. However, the role of this
circRNA in cancer initiation and development has not been
investigated previously.
The mechanisms of circRNAs in cancer initiation and
progression have not been clearly elucidated. It has been reported
that circRNAs can regulate the expression of oncogenes or
tumor-suppressive genes in different patterns (41). The most reported pattern is the
ceRNA hypothesis. In this hypothesis, circRNAs have been proposed
to communicate with mRNAs by competing for binding to shared miRNA
targets (42). This hypothesis has
been confirmed in a number of studies. For example, circMTO1
suppresses HCC progression by acting as a sponge of miR-9 (25). circSMAD2 can inhibit the
epithelial-mesenchymal transition via targeting miR-629 in HCC
(43). circ_0067934 promotes tumor
metastasis and growth in HCC via the inhibition of miR-1324
(44). It was hypothesized that
hsa_circRNA_100084 might act as a miRNA sponge. Therefore, it was
predicted the miRNAs related with DE-circRNAs by bioinformatics
analysis. The combination of hsa-miR-23a-5p with
hsa_circRNA_100084, as well as IGF2, was validated by a
dual-luciferase reporter assay. As expected, hsa-miR-23a-5p could
diminish the fluorescence of the wildtype of hsa_circRNA_100084 and
IGF2, but not the mutated forms. In addition, overexpression
of hsa-miR-23a-5p could decrease the expression of IGF2 in
HepG2 cells. However, sh-hsa_circRNA_100084 at the same time could
attenuate the effect of hsa-miR-23a-5p overexpression on the
expression of IGF2.
However, the present study inevitably possess some
limitations. First, although a ceRNA relationship of
hsa_circRNA_100084-hsa-miR-23a-5p- IGF2 axis was identified
and their relationship confirmed by experiments, the expression
downstream of IGF-2, such as the insulin receptor substrate
1/PI3K/Akt axis and sarcomatoid hepatocellular carcinoma/growth
factor receptor-bound protein 2/Ras/mitogen-activated protein
kinase axis has not been examined. Besides, more direct evidence,
such as RNA immunoprecipitation analysis of the interaction of
circRNA_100084 and miR-23a-5p was not performed. Therefore, further
researches are still needed to make more validate conclusions.
In conclusion, the present study demonstrated that
hsa_circRNA_100084 is upregulated in HCC tissue compared with the
matched non-tumor liver tissues and may act as a ceRNA to increase
IGF2 expression by sponging hsa-miR-23a-5p, which
consequently contributes to HCC proliferation, migration and
invasion. The deregulated circRNAs in HCC will be the subject of
continuing investigation in further studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
JY and YHW designed the study. YL and JL analyzed
and interpreted the RNA sequencing data and patients' data. JY,
ZCY, YFZ and JFT performed the in vitro experiments, and JY
and YHW were major contributors in writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
the Lishui Municipal Central Hospital and written informed consent
was obtained from all patients included in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JI, Kim JK, Kim DY, Ahn SH, Park JY,
Kim SU, Kim BK, Han KH and Lee KS: Prognosis of hepatocellular
carcinoma patients with extrahepatic metastasis and the
controllability of intrahepatic lesions. Clin Exp Metastasis.
31:475–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nigro JM, Cho KR, Fearon ER, Kern SE,
Ruppert JM, Oliner JD, Kinzler KW and Vogelstein B: Scrambled
exons. Cell. 64:607–613. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen LL: The biogenesis and emerging roles
of circular RNAs. Nat Rev Mol Cell Biol. 17:205–211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: CircRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui S, Qian Z, Chen Y, Li L, Li P and Ding
H: Screening of up- and downregulation of circRNAs in HBV-related
hepatocellular carcinoma by microarray. Oncol Lett. 15:423–432.
2018.PubMed/NCBI
|
|
10
|
Kou P, Zhang C, Lin J and Wang H: Circular
RNA hsa_circ_0078602 may have potential as a prognostic biomarker
for patients with hepatocellular carcinoma. Oncol Lett.
17:2091–2098. 2019.PubMed/NCBI
|
|
11
|
Nakamura M, Chiba T, Kanayama K, Hiroaki
Kanzaki H, Saito T, Kusakabe Y and Kato N: Epigenetic dysregulation
in hepatocellular carcinoma: An up-to-date review. Hepatol Res.
49:3–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv Y, Wei W, Huang Z, Chen Z, Fang Y, Pan
L, Han X and Xu Z: Long non-coding RNA expression profile can
predict early recurrence in hepatocellular carcinoma after curative
resection. Hepatol Res. 48:1140–1148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Y, Zhang C, Zhang B, Yu H and Yu Q:
circRNA of AR-suppressed PABPC1 91 bp enhances the cytotoxicity of
natural killer cells against hepatocellular carcinoma via
upregulating UL16 binding protein 1. Oncol Lett. 17:388–397.
2019.PubMed/NCBI
|
|
14
|
Xie B, Zhao Z, Liu Q, Wang X, Ma Z and Li
H: CircRNA has_circ_0078710 acts as the sponge of microRNA-31
involved in hepatocellular carcinoma progression. Gene.
683:253–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang XY, Huang ZL, Zhang PB, Huang XY,
Huang J, Wang HC, Xu B, Zhou J and Tang ZY: CircRNA-100338 is
associated with mTOR signaling pathway and poor prognosis in
hepatocellular carcinoma. Front Oncol. 9:3922019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu CM, Lin PM, Lin HC, Lai CC, Yang CH,
Lin SF and Yang MY: Altered expression of imprinted genes in
squamous cell carcinoma of the head and neck. Anticancer Res.
36:2251–2258. 2016.PubMed/NCBI
|
|
17
|
Vu TH and Hoffman AR: Promoter-specific
imprinting of the human insulin-like growth factor-II gene. Nature.
371:714–717. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livingstone C: IGF2 and cancer. Endocr
Relat Cancer. 20:R321–R339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brouwer-Visser J and Huang GS: IGF2
signaling and regulation in cancer. Cytokine Growth Factor Rev.
26:371–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tovar V, Alsinet C, Villanueva A, Hoshida
Y, Chiang DY, Solé M, Thung S, Moyano S, Toffanin S, Mínguez B, et
al: IGF activation in a molecular subclass of hepatocellular
carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol.
52:550–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinez-Quetglas I, Pinyol R, Dauch D,
Torrecilla S, Tovar V, Moeini A, Alsinet C, Portela A,
Rodriguez-Carunchio L, Solé M, et al: IGF2 is up-regulated by
epigenetic mechanisms in hepatocellular carcinomas and is an
actionable oncogene product in experimental models.
Gastroenterology. 151:1192–1205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang C, Wu D, Gao L, Liu X, Jin Y, Wang D,
Wang T and Li X: Competing endogenous RNA networks in human cancer:
Hypothesis, validation, and perspectives. Oncotarget.
7:13479–13490. 2016.PubMed/NCBI
|
|
23
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du H and Chen Y: Competing endogenous RNA
networks in cervical cancer: Function, mechanism, and perspective.
J Drug Target. 27:1–47. 2018.PubMed/NCBI
|
|
25
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets--update. Nucleic Acids Res. 41(D1): D991–D995.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article32004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Turner DA: Miranda: A non-strict
functional language with polymorphic types. Proc. of a Conference
on Functional Programming Languages and Computer Architecture.
Jouannaud JP: Springer-Verlag; Berlin, Heidelberg: pp. 1–16. 1985,
View Article : Google Scholar
|
|
30
|
Jiang Q, Wang Y, Hao Y, Juan L, Teng M,
Zhang X, Li M, Wang G and Liu Y: miR2Disease: A manually curated
database for microRNA deregulation in human disease. Nucleic Acids
Res. 37:(Database). D98–D104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
33
|
Xu D, Yu J, Gao G, Lu G, Zhang Y and Ma P:
LncRNA DANCR functions as a competing endogenous RNA to regulate
RAB1A expression by sponging miR-634 in glioma. Biosci Rep.
38:BSR201716642018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu RX, Seto WK, Lai CL and Yuen MF:
Epidemiology of hepatocellular carcinoma in the Asia-Pacific
region. Gut Liver. 10:332–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zatkova A, Rouillard JM, Hartmann W, Lamb
BJ, Kuick R, Eckart M, von Schweinitz D, Koch A, Fonatsch C,
Pietsch T, et al: Amplification and overexpression of the IGF2
regulator PLAG1 in hepatoblastoma. Genes Chromosomes Cancer.
39:126–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhen N, Gu S, Ma J, Zhu J, Yin M, Xu M,
Wang J, Huang N, Cui Z, Bian Z, et al: CircHMGCS1 promotes
hepatoblastoma cell proliferation by regulating the IGF signaling
pathway and glutaminolysis. Theranostics. 9:900–919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Luo P, Jing W, Zhou H, Liang C
and Tu J: circSMAD2 inhibits the epithelial-mesenchymal transition
by targeting miR-629 in hepatocellular carcinoma. OncoTargets Ther.
11:2853–2863. 2018. View Article : Google Scholar
|
|
44
|
Zhu Q, Lu G, Luo Z, Gui F, Wu J, Zhang D
and Yong Ni Y: CircRNA circ_0067934 promotes tumor growth and
metastasis in hepatocellular carcinoma through regulation of
miR-1324/FZD5/Wnt/β-catenin axis. Biochem Biophys Res Commun.
497:626–632. 2018. View Article : Google Scholar : PubMed/NCBI
|