Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide due to the increase in cigarette smoking (1). Among the different types of lung
cancer, non-small cell lung cancer (NSCLC) has the highest
mortality, at ~85% (2). Lung
cancer tumor cell populations have a heterogeneous structure
consisting of cancer cells and a small numbers of cancer stem cells
(CSCs) (3). A CSC originates from
a mutated normal stem cell or a differentiated progenitor cell that
has accumulated various types of damage (3). Several studies have suggested that
identification of CSCs will enable a definitive treatment of the
tumor (4–6).
The fluorescence-activated cell sorting (FACS)
method can be used to identify and differentiate lung CSCs (LCSCs)
(7). LCSCs can be isolated using
specific surface markers such as CD44 and CD133. Using this method,
stem cells can be separated by molecules capable of irradiation at
certain wavelengths by binding to antibodies specific to these
surface markers (8).
Sirtuins are enzymes that act as nicotinamide
adenine dinucleotide (NAD+)-dependent deacetylases
(9). The mammalian sirtuin family
consists of seven homologs, SIRT1-7, classified by NAD+
binding and catalytic domain (9).
Sirtuin 1 (SIRT1), the most investigated of these variants, has
been reported to act as both a tumor promoter and a tumor
suppressor (10). In the present
study, the effects of SIRT1 activators (resveratrol and SRT1720)
and inhibitors (tenovin-6 and sirtinol) on SIRT1 and p53 expression
in CD44+/CD133+-enriched cells isolated from
the A549 cell line (NSCLC) were investigated to explore potential
new treatment approaches for lung cancer.
In the present study, cell sorting was performed to
enrich the cancer stem cells. Subsequently, proliferation and
cytotoxicity experiments were used to determine the optimum SIRT1
activator-inhibitor concentrations to use to treat the cells. Flow
cytometric analysis was used to analyze the apoptotic rate of cells
treated with the SIRT1 activators and inhibitors, and quantitative
(q)PCR and western blotting analysis was used to analyze the
expression levels of SIRT1 and p53 mRNA and protein,
respectively.
Materials and methods
Cell cultures and reagents
The cell culture media used in this study were
obtained from Biological Industries USA, Inc. Allophycocyanin
(APC)-labeled CD44 (cat. no. 130-098-110) and phycoerythrin
(PE)-labeled CD133/2 (cat. no. 130-098-046) antibodies were
obtained from Miltenyi Biotec GmbH. Cell sorting consumables were
procured from BD Biosciences. Antibodies for western blotting were
purchased from Cell Signaling Technology, Inc. SIRT1 activators and
inhibitors were obtained from Santa Cruz Biotechnology, Inc., and
stock solutions were prepared for each substance (Table SI). The A549 cell line was grown
in F-12 Nutrient Mixture (Ham's) medium supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin under standard culture conditions.
Cancer stem cell sorting
CD44+/CD133+ cells were sorted
using a BD FACSAria™ device (BD Biosciences). A549 cells were
incubated with 1% trypsin-EDTA for 5 min at 37°C and then collected
and centrifuged at room temperature for 10 min at 300 × g to obtain
a cell pellet. The cell pellet was washed with PBS containing 2%
FBS and 2 mM EDTA, centrifuged at room temperature for 10 min at
300 × g and then incubated in 100 µl PBS buffer with 10 µl each of
CD44 and CD133 antibodies (1:11) in the dark for 10 min at 4°C.
After incubation, PBS was added, the suspension was centrifuged at
room temperature for 10 min at 300 × g, and the supernatant was
discarded. The pellet was resuspended in 3 ml PBS and the APC- and
PE-labeled CD44+CD1 33+ cells were sorted
using the BD FACSAria™ device. The gating strategy used for the
separation of APC- and PE-marked CD44+ and
CD133+ cells is demonstrated in Fig. S1.
Cell proliferation and cytotoxicity
assays
The xCELLigence® Real-Time Cell Analyzer
(Roche Applied Science) was used for cell proliferation and
cytotoxicity analyses. After seeding
CD44+/CD133+-enriched A549 cells into an
E-Plate at a density of 10,000 cells/well, the impedance values
were measured for 72 h to obtain proliferation curves. For
cytotoxicity assays, cells were seeded into the E-Plates at the
same density and incubated for 24 h at 37°C. After this incubation,
SIRT1 activators (resveratrol and SRT1720) and inhibitors
(tenovin-6 and sirtinol) were applied, and the impedance values
were measured for 72 h. Each concentration was run in triplicate.
Data analysis was performed using the software included in the
xCELLigence device, and the IC50 of each active
substance was obtained.
Apoptosis analyses
Annexin V/PI staining was performed to evaluate the
apoptotic effects of the active substances. Cells were seeded onto
12-well plates at a density of 2×105 cells/well and
incubated for 24 h at 37°C to allow adhesion. After this
incubation, the active ingredients were applied at the
IC50 dose. After 72 h, the cells were released by
trypsinization, incubated with Annexin V/PI for 1 min at room
temperature, and then examined in the BD Accuri™ C6 flow cytometry
device and analyzed via BD Accuri™ C6 software (version 227.4; BD
Biosciences).
Quantitative (q)PCR analysis
After treating the
CD44+/CD133+- enriched A549 cells with
IC50 dose for 72 h, the cells were harvested, and RNA
was obtained using the MagNA Pure LC RNA Isolation kit (Roche
Applied Science). cDNA synthesis was performed using the iScript™
cDNA Synthesis kit (Bio-Rad Laboratories, Inc.). The following
reverse transcription temperature protocol was used: 30 min at
42°C, 85°C for 5 min and then maintained at 4°C. SIRT1 and p53 gene
expression levels were analyzed using Hs01009006-SIRT1 and
Hs01034249-TP53 TaqMan™ primers and TaqMan® FAM-MGB dye
probes (Applied Biosystems; Thermo Fisher Scientific, Inc.). GADPH
(Hs03929097-GAPDH) was used as a housekeeping gene. For qPCR, the
reaction mixture was prepared according to Table SII. The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
10 min; followed by 40 cycles of denaturation at 95°C for 10 sec
and extension at 60°C for 60 sec; prior to cooling to 40°C for 30
sec. The relative expression levels were calculated using the
2−ΔΔCq method (11) and
presented as fold change.
Western blot analysis
Western blot analyses of proteins collected from
cells treated with IC50 doses of the active substances
for 72 h and the control cells were performed using SIRT1, p53 and
β-actin antibodies. Proteins were obtained using Complete Lysis-M
Buffer (Roche Applied Science) and quantified using Bradford
solution (Fermentas; Thermo Fisher Scientific, Inc.) and the
Pierce™ Bovine Serum Albumin Standard Pre-Diluted set (Fermentas;
Thermo Fisher Scientific, Inc.). For each sample, 23.8 µg of
protein was run on 8% SDS-PAGE gel and transferred to a PVDF
membrane using the iBlot system (Invitrogen; Thermo Fisher
Scientific, Inc.; Table SIII).
The membranes were blocked with 5 ml blocking buffer (Western
Breeze® Chromogenic kit; Invitrogen; Thermo Fisher
Scientific, Inc.) for 45 min at room temperature. The membranes
were washed with 10 ml antibody washing solution diluted in 150 ml
distilled water three times for 5 min. The membranes were
subsequently incubated with the anti-SIRT1 (1.5:1,000; cat. no:
9475T; Cell Signaling Technology, Inc.), anti-p53 (1.5:1,000; cat.
no: 2527T; Cell Signaling Technology, Inc.) and anti-β-actin
(1.5:1,000; cat. no. 8457S; Cell Signaling Technology, Inc.)
primary antibodies at room temperature for 90 min. Following the
primary antibody incubation, the membranes were washed three times
with a washing solution and incubated with the ready to use
secondary antibody solution containing alkaline
phosphatase-conjugated secondary antibody (WesternBreeze™
Chromogenic kit; cat. no: WB7105; Invitrogen; Thermo Fisher
Scientific, Inc.) for 45 min at room temperature. The membranes
were washed three times with washing solution. Western Blot
Chromogenic Detection kit (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to visualize the protein bands and the expression
levels were analyzed using ImageJ software version 1.8 (National
Institutes of Health).
Statistical analysis
All experiments were performed at least in
triplicate. The results are expressed as the mean ± SD. Data were
analyzed by ANOVA followed by post-hoc Tukey's multiple comparisons
test. In the case of P<0.05, the difference was considered
statistically significant. GraphPad Prism 8.0 software (GraphPad
software, Inc.) was used for analyses.
Results
Cell proliferation and cytotoxicity
test
For the cell proliferation analysis, the
proliferation curve and the slope values of
CD44+/CD133+-enriched A549 cells were
determined to be 9×103, 18×103 and
36×103 cells/well, respectively, and 12×103
cells/well was calculated as the optimum cell density (Figs. S2 and S3). The IC50 values for each
agent were then determined in cells treated for a total of 72 h
(Table SIV).
When calculating the IC50 values at 24,
48 and 72 h for each agent, the IC50 values at 72 h were
preferred for apoptosis and protein expression experiments, while
48 h values were preferred for mRNA expression experiments. The
IC50 values of the
CD44+/CD133+-enriched A549 cell line treated
with resveratrol, sirtinol, tenovin-6 and SRT1720 for 48 h were
196, 37, 13.9 and 6.57 µM, respectively. The IC50 values
obtained with the treatment with resveratrol, sirtinol, tenovin-6
and SRT1720 for 72 h were 605, 35.8, 15.3 and 6.64 µM,
respectively. The cytotoxicity curves and the IC50 plots
of the A549-CSC line treated with the active agents are shown in
Figs. S4–S11. When the effects of the agents on
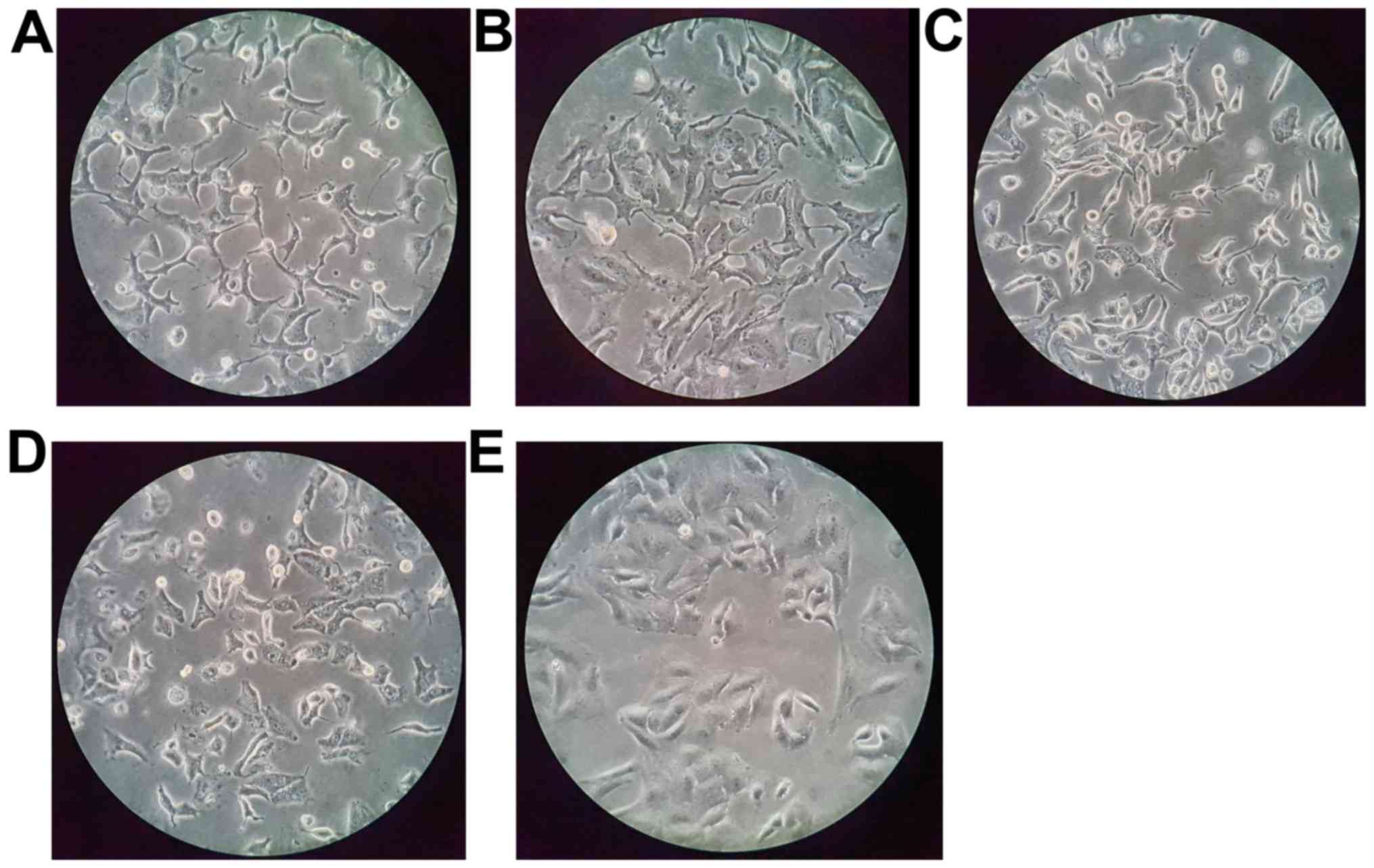
cell morphology were examined, the most significant change was
observed in the cells treated with sirtinol (Fig. 1); the cell membrane surface was
rough and furcation had occurred.
Apoptosis assay
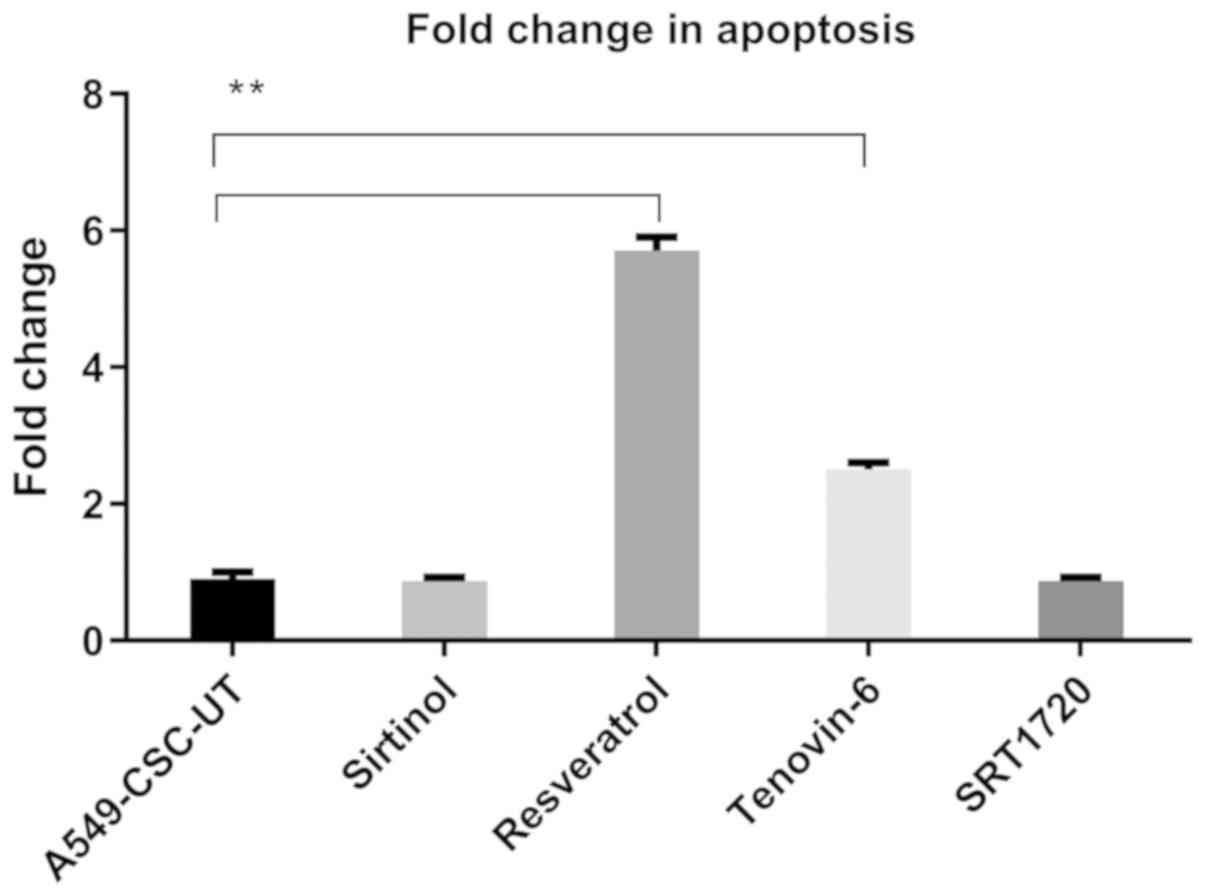
As shown in Fig. 2,
there was no significant difference in the apoptosis rates in the
CD44+/CD133+-enriched A549 cells treated with
sirtinol and SRT1720 compared with the control group, whereas
treatment with resveratrol and tenovin-6 significantly increased
the apoptosis rate by 5.7- and 2.5-fold, respectively.
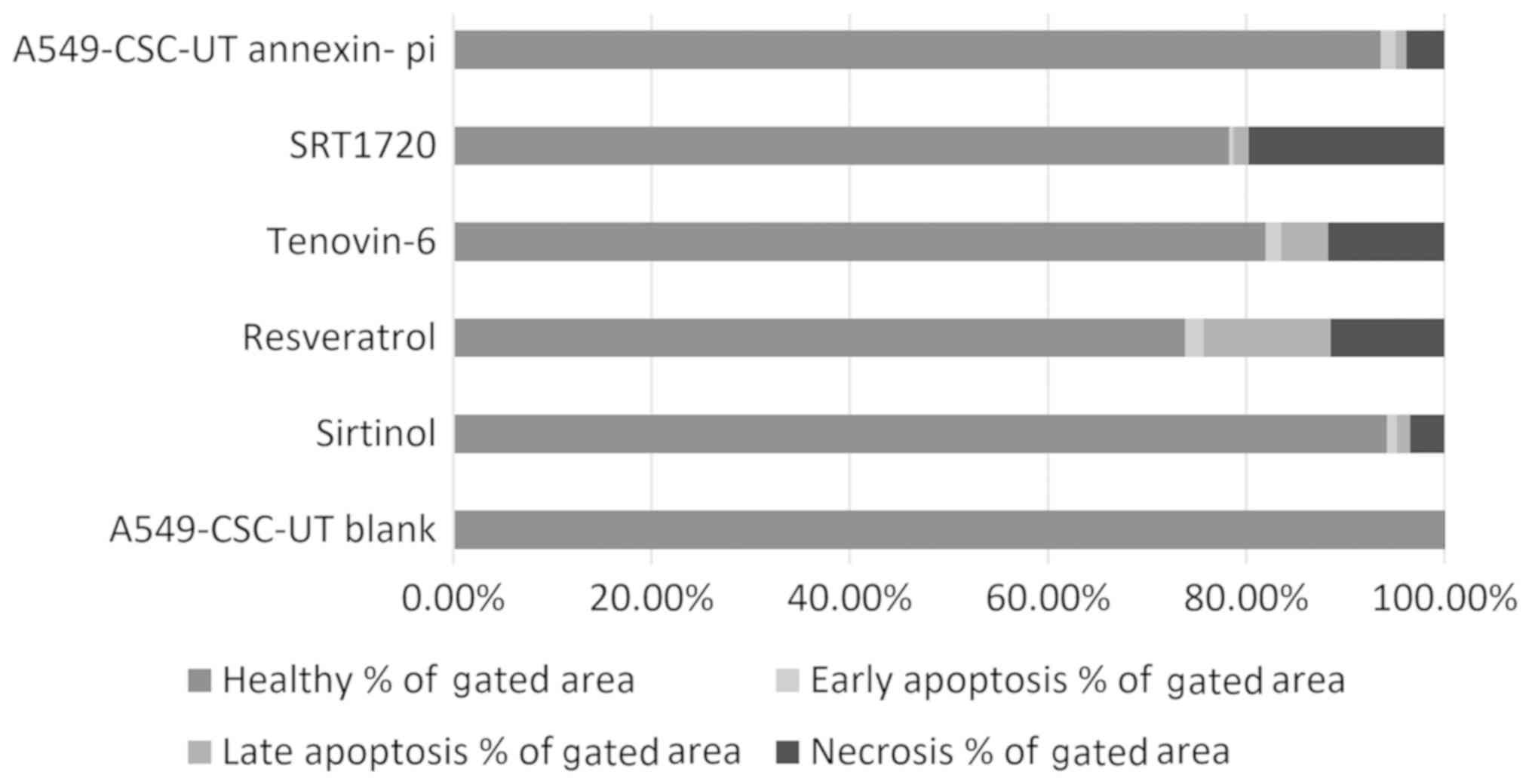
As shown in Table I
and Fig. 3, the rate of necrosis
was 19.75% with SRT1720 treatment and ~11% in cells treated with
tenovin-6 and resveratrol. Resveratrol was the strongest inducer of
apoptosis (14.64%).
 | Table I.A549 cell apoptosis rates with
Annexin/PI treatment of cancer stem cells as determined by flow
cytometry. |
Table I.
A549 cell apoptosis rates with
Annexin/PI treatment of cancer stem cells as determined by flow
cytometry.
|
| Healthy | Early
apoptosis | Late apoptosis | Necrosis |
|---|
|
|
|
|
|
|
|---|
| Treatment | % of gated area
(%) | % of all (%) | % of gated area
(%) | % of all (%) | % of gated area
(%) | % of all (%) | % of gated area
(%) | % of all (%) |
|---|
| A5469-CSCs-UT
blank | 99.99 | 61.82 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Siritinol | 94.20 | 49.51 | 1.03 | 0.54 | 1.32 | 0.69 | 3.45 | 1.82 |
| Resveratrol | 73.87 | 6.88 | 1.85 | 0.17 | 12.79 | 1.19 | 11.49 | 1.07 |
| Tenovin-6 | 81.95 | 0.77 | 1.62 | 0.02 | 4.72 | 0.04 | 11.71 | 0.11 |
| SRT1720 | 78.25 | 16.87 | 0.49 | 0.11 | 1.50 | 0.32 | 19.75 | 4.26 |
| A549-CSCs-UT
Annexin-PI | 93.60 | 67.73 | 1.49 | 1.08 | 1.09 | 0.79 | 3.82 | 2.76 |
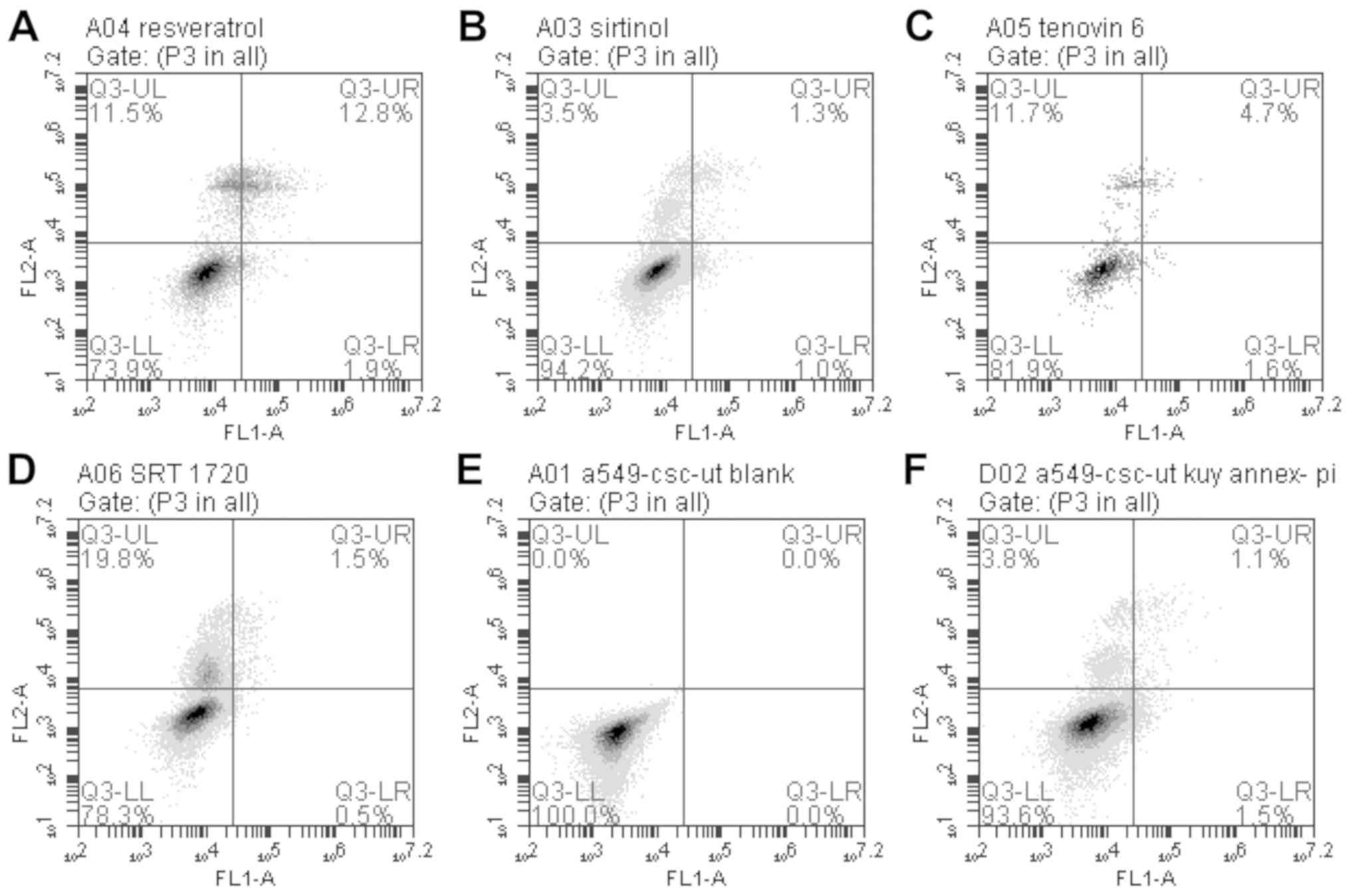
In the flow cytometric analysis, gates were drawn to
create four sections. According to these four sections, the lower
right quadrant represents early apoptosis (Q3-LR), the upper right
quadrant represents late apoptosis (Q3-UR), the lower left quadrant
represents the normal state (Q3-LL), and the upper left quadrant
represents necrosis (Q3-UL; Fig.
4).
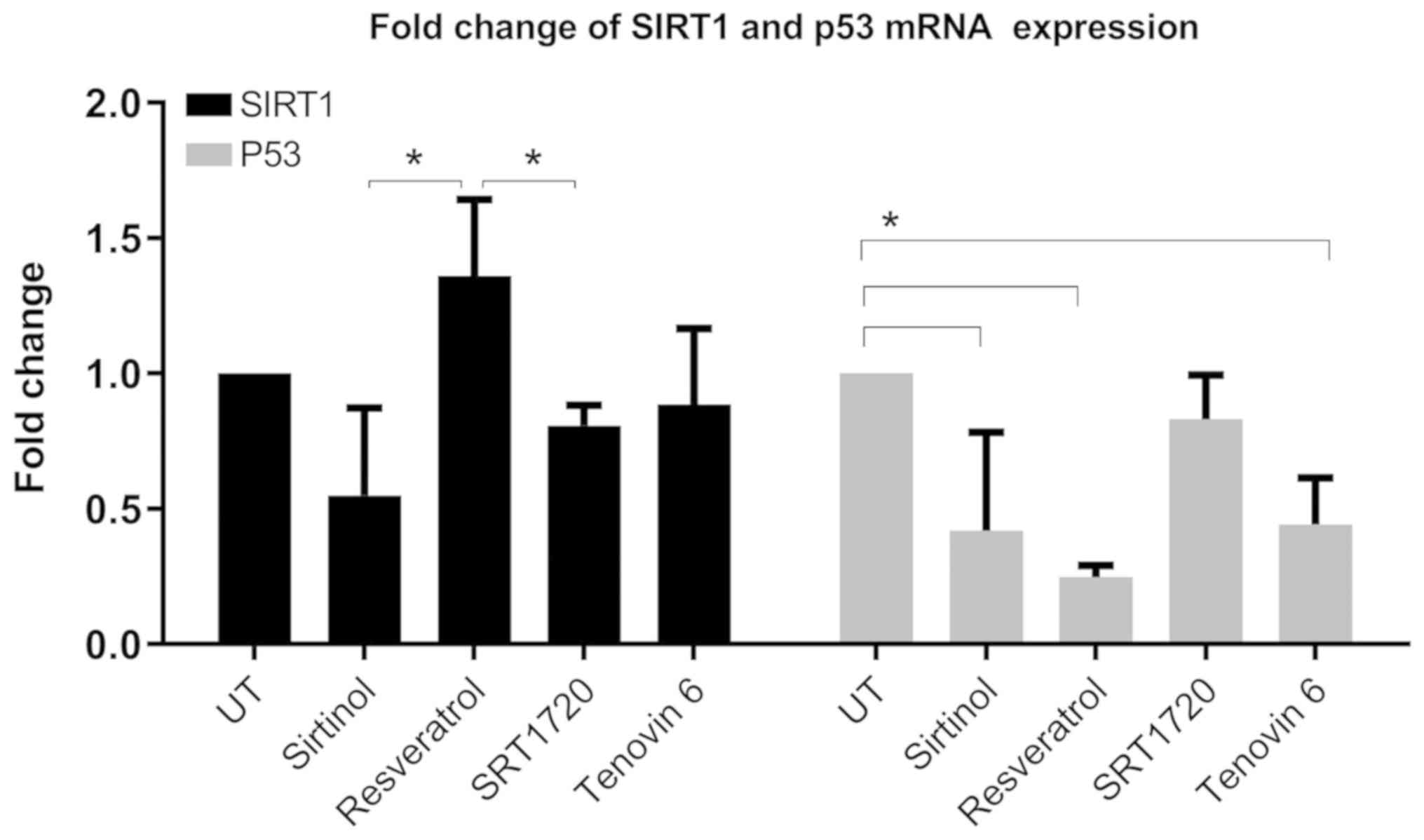
Analysis of mRNA expression
Analysis of p53 mRNA expression at 48 h revealed
that sirtinol, resveratrol and tenovin-6 treatments caused
significant differences in p53 expression (Fig. 5). SIRT1 expression decreased
significantly after sirtinol and SRT1720 compared to the
resveratrol treatment group. The most significant difference was a
4-fold decrease in p53 expression in resveratrol-treated cells.
Bradford protein quantification and
western blot analysis
Changes in the protein expression of SIRT1 and p53
in CSCs treated for 72 h were evaluated by western blotting
(Fig. S12). When the SIRT1 and
p53 protein expression levels of CSCs treated for 72 h with SIRT1
inhibitory agents were examined, it was observed that SIRT1
expression decreased by 79% after sirtinol treatment and by 78%
after treatment with tenovin-6. After treatment with resveratrol,
SIRT1 expression decreased by 58% and p53 expression increased by
37%, whereas treatment with SRT1720 decreased the SIRT1 expression
by 31% and increased the p53 expression by 25% (Fig. S13).
Discussion
To the best of our knowledge, this is the first
study to investigate the effects of SIRT1 activators and inhibitors
on the mRNA and protein expression of sirtuin 1 (SIRT1) and p53 in
CD44+/CD133+-enriched A549 non-small cell
lung cancer (NSCLC) cells. Different studies on NSCLC cells have
demonstrated that subpopulations with high expression of CD44 and
CD133 surface markers exhibit stem cell characteristics (12). In a study on NSCLC, Leung et
al (13) screened the
expression profiles of five stem cell markers (CD34, CD44, CD133,
BMI1 proto-oncogene, polycomb ring finger and octamer-binding
transcription factor 4) in 10 lung cancer cell lines by flow
cytometry and reported that cells rich in CD44 may have a role in
spheroid and tumor formation in vitro. In another study,
Tirino et al (14)
investigated the role of CD133 in the identification and
characterization of NSCLC tumor-initiating cells and found that 72%
of NSCLC samples contained CD133+ cells. In another
in vivo study, CD133+-rich cells isolated from
non-obese diabetic mice with severe combined immunodeficiency were
found to be more tumorigenic (14). Based on this information, a
CD44+/CD133+ cell subpopulation was obtained
from the A549 NSCLC cell line by the FACS method using APC- and
PE-labeled antibodies to CD44 and CD133 surface markers.
Resveratrol, a natural polyphenolic phytoalexin, has
been reported to have an antitumor effect by inducing cell
apoptosis via the activation of SIRT1 in human chondrosarcoma
(15). In addition, according to a
study by Wang et al (16)
resveratrol exhibited an inhibitory effect on proliferation, while
inducing apoptosis of NSCLCs. Similarly, the present findings
demonstrated that resveratrol had the maximum antitumor effect
among all agents on the cell subpopulation, which had cancer stem
cell (CSC) features, by increasing the rate of apoptosis by
5.7-fold after 72 h of treatment. However, this effect was observed
to occur with a 4.1-fold increased p53 expression and a 0.2-fold
decreased SIRT1 expression in the CD44+- and
CD133+-enriched A549 cells. Although most of the studies
have reported that resveratrol is an activator of SIRT1, the class
III NAD-dependent histone/protein deacetylase (17–19),
some studies have reported that the interactions between
resveratrol, SIRT1 and apoptosis, in fact, are more complex
(20–22). Frazzi et al (20) found that resveratrol exerted an
apoptosis-promoting effect by decreasing the activity of SIRT1 in
Hodgkin's lymphoma cells, which is consistent with the present
study. Furthermore, although the IC50 dose of
resveratrol in A549 cells enriched with CSCs was calculated at 173
µM (Table SIV), this value for
normal A549 cells was determined to be 98 µM by Wang et al
(23). Altogether, the possible
reasons for all these findings could be that resveratrol may have a
different pathway from SIRT1 by affecting apoptosis due to the
impact of CSCs.
Yuan et al (24) performed cell cycle analysis and
found that resveratrol downregulated the expression levels of
cyclin D1, cyclin-dependent kinase (CDK) 4 and CDK6, but it
upregulated the expression levels of the CDK inhibitors p21 and
p27, and induced cell cycle arrest in the G0/G1 phase. Similarly,
the increased expression of the tumor-suppressor protein p53
observed in the present study suggests that cancer cells could be
more easily forced into apoptosis. In fact, resveratrol produced a
5.7-fold increase in the rate of apoptosis as measured using the
Annexin method. On the other hand, a 1.37-fold increase in p53
protein expression was measured by western blotting. Yuan et
al (24) reported high levels
of p53 expression in the A549 cell line. This demonstrates the
difficulty of increasing p53 expression in CSCs. However, the
significant suppressive effect of resveratrol on the expression of
SIRT1, hypothesized to be a tumor promoter, makes it a significant
anti-tumorigenic agent.
Regarding the other SIRT1 activator, SRT1720, some
researchers have demonstrated that it has SIRT1-independent effects
and also indirectly activates SIRT1 (21). Therefore, the cancer stem-like A549
cells treated with SRT1720 experienced a higher necrosis rate than
apoptosis rate, which was 19.75%. Similar to the present result,
Lahusen and Deng (25) found that
SRT1720 induced necrosis by increasing lysosomal membrane
permeabilization in breast cancer cells.
In the present study, the SIRT1 inhibitors sirtinol
and tenovin-6 decreased the SIRT1 protein expression by 79 and 78%
and decreased the SIRT1 mRNA expression by 1.8- and 1.1-fold,
respectively, compared to those in the control group. These results
suggest that sirtinol and tenovin-6 block another intermediate step
in which SIRT1 protein expression does not suppress the level of
mRNA expression effectively. p53 mRNA expression decreased by 2.4-
and 2.3-fold upon treatment with sirtinol and tenovin-6,
respectively, whereas, sirtinol decreased the rate of apoptosis by
0.1-fold, and tenovin-6 increased the apoptosis rate by 2.5-fold.
When protein expressions of SIRT1 and p53 were evaluated using
western blotting, it was found that resveratrol, sirtinol and
tenovin-6 significantly decreased SIRT1 protein expression, whereas
there was no significant change in p53 expression.
Inhibition of SIRT1 with the activation of p53 is
associated with the pro-apoptotic effects of both tenovin-6 and
sirtinol in several tumors; therefore, in the present study it was
expected that these agents would increase the expression of
p53-dependent proteins, resulting in apoptosis (26–28).
On the other hand, any significant changes in p53 mRNA or protein
expression were not observed after treatment with these agents, and
there were also no marked changes in apoptosis. However, the
present results, in particular for tenovin-6, might be compatible
with the literature when looking at a study conducted by MacCallum
et al (29). These authors
observed that although tenovin-6 did not induce cellular apoptosis
or p53 mRNA expression activity, it increased apoptosis in acute
lymphoblastic leukemia cells (29).
Due to the budget limitation in the current study,
only the A549 cell line in the NSCLC family was used, and
acetylated-p53 could not be evaluated in the western blot assay.
Although this study did not show acetylated-p53 in Fig. S12, it should be considered that
the total p53 also contains acetylated-p53 regulated by SIRT1
(30).
Overall, although the current literature describes
the relationship between cancer, SIRT1 and SIRT1
activators/inhibitors, the available data are still controversial
and far from being definitive, in particular for CSCs. The results
of this study showed that resveratrol is an important agent for
inducing apoptosis in a CD44+/CD133+-enriched
A549 cell line.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by the Scientific
Research Projects Coordination Unit of Ege University (grant no.
15-TIP-061).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZE conceived and coordinated the study, and wrote
the manuscript; CE maintained the cells and performed the western
blotting and qPCR experiments; GO designed and supervised the cell
sorting experiments; VBC analyzed the data and revised the figures;
and ZD designed and performed the experiments, created the figures
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SIRT1
|
sirtuin 1
|
|
NSCLC
|
non-small cell lung cancer
|
|
CSCs
|
cancer stem cells
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
LCSCs
|
lung cancer stem cells
|
|
NAD
|
nicotinamide adenine dinucleotide
|
|
CD
|
cluster of differentiation
|
|
FBS
|
fetal bovine serum
|
|
CDK
|
cyclin-dependent kinase
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rich JN: Cancer stem cells: Understanding
tumor hierarchy and heterogeneity. Medicine (Baltimore). 95 (1
Suppl 1):S2–S7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leeman KT, Fillmore CM and Kim CF: Lung
stem and progenitor cells in tissue homeostasis and disease. Curr
Top Dev Biol. 107:207–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salama R, Tang J, Gadgeel SM, Ahmad A and
Sarkar FH: Lung cancer stem cells: Current progress and future
perspectives. J Stem Cell Res Ther S7. 0072012.
|
|
6
|
Luo J, Zhou X and Yakisich JS: Stemness
and plasticity of lung cancer cells: Paving the road for better
therapy. Onco Targets Ther. 7:1129–1134. 2014.PubMed/NCBI
|
|
7
|
Wang Y, Jiang M, Du C, Yu Y, Liu Y, Li M
and Luo F: Utilization of lung cancer cell lines for the study of
lung cancer stem cells. Oncol Lett. 15:6791–6798. 2018.PubMed/NCBI
|
|
8
|
Halim NHA, Zakaria N, Satar NA and Yahaya
BH: Isolation and characterization of cancer stem cells of the
non-small-cell lung cancer (A549) cell line. Methods Mol Biol.
1516:371–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haigis MC and Sinclair DA: Mammalian
sirtuins: Biological insights and disease relevance. Annu Rev
Pathol. 5:253–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mei Z, Zhang X, Yi J, Huang J, He J and
Tao Y: Sirtuins in metabolism, DNA repair and cancer. J Exp Clin
Cancer Res. 35:1822016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang HZ, Lin XG, Hua P, Wang M, Ao X,
Xiong LH, Wu C and Guo JJ: The study of the tumor stem cell
properties of CD133+CD44+ cells in the human lung adenocarcinoma
cell line A549. Cell Mol Biol (Noisy-le-grand). 56
(Suppl):OL1350–OL1358. 2010.PubMed/NCBI
|
|
13
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tirino V, Camerlingo R, Franco R, Malanga
D, La Rocca A, Viglietto G, Rocco G and Pirozzi G: The role of
CD133 in the identification and characterisation of
tumour-initiating cells in non-small-cell lung cancer. Eur J
Cardiothorac Surg. 36:446–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao SC, Chen YJ, Huang KH, Kuo KL, Yang
TH, Huang KY, Wang CC, Tang CH, Yang RS and Liu SH: Induction of
sirtuin-1 signaling by resveratrol induces human chondrosarcoma
cell apoptosis and exhibits antitumor activity. Sci Rep.
7:31802017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Li J, Cao N, Li Z, Han J and Li L:
Resveratrol, an activator of SIRT1, induces protective autophagy in
non-small-cell lung cancer via inhibiting Akt/mTOR and activating
p38-MAPK. Onco Targets Ther. 11:7777–7786. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Wan F, You W, Tan X, Liu G, Jin
Q, Wei C, Liu X, Zhao H, Liu Y and Zhang C: Comparison of apoptosis
between bovine subcutaneous and intramuscular adipocytes by
resveratrol via SIRT1. Anim Biotechnol. 1–9. 2019. View Article : Google Scholar
|
|
18
|
Yun JM, Chien A, Jialal I and Devaraj S:
Resveratrol up-regulates SIRT1 and inhibits cellular oxidative
stress in the diabetic milieu: Mechanistic insights. J Nutr
Biochem. 23:699–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chai R, Fu H, Zheng Z, Liu T, Ji S and Li
G: Resveratrol inhibits proliferation and migration through SIRT1
mediated post-translational modification of PI3K/AKT signaling in
hepatocellular carcinoma cells. Mol Med Rep. 16:8037–8044. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frazzi R, Valli R, Tamagnini I, Casali B,
Latruffe N and Merli F: Resveratrol-mediated apoptosis of hodgkin
lymphoma cells involves SIRT1 inhibition and FOXO3a
hyperacetylation. Int J Cancer. 132:1013–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pacholec M, Bleasdale JE, Chrunyk B,
Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis
P, Pabst B, et al: SRT1720, SRT2183, SRT1460, and resveratrol are
not direct activators of SIRT1. J Biol Chem. 285:8340–8351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi X, Ye Q, Li D, Peng Q, Wang Z, Wu X,
Zhang Y, Zhang Q and Jiang F: Inhibition of nucleolar stress
response by Sirt1: A potential mechanism of acetylation-independent
regulation of p53 accumulation. Aging Cell. 18:e129002019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Wang D and Zhao Y: Effect and
mechanism of resveratrol on the apoptosis of lung adenocarcinoma
cell line A549. Cell Biochem Biophys. 73:527–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan L, Zhang Y, Xia J, Liu B, Zhang Q,
Liu J, Luo L, Peng Z, Song Z and Zhu R: Resveratrol induces cell
cycle arrest via a p53-independent pathway in A549 cells. Mol Med
Rep. 11:2459–2464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lahusen TJ and Deng CX: SRT1720 induces
lysosomal-dependent cell death of breast cancer cells. Mol Cancer
Ther. 14:183–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirai S, Endo S, Saito R, Hirose M, Ueno
T, Suzuki H, Yamato K, Abei M and Hyodo I: Antitumor effects of a
sirtuin inhibitor, tenovin-6, against gastric cancer cells via
death receptor 5 up-regulation. PLoS One. 9:e1028312014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ueno T, Endo S, Saito R, Hirose M, Hirai
S, Suzuki H, Yamato K and Hyodo I: The sirtuin inhibitor tenovin-6
upregulates death receptor 5 and enhances cytotoxic effects of
5-fluorouracil and oxaliplatin in colon cancer cells. Oncol Res.
21:155–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Kim TH, Ahn MY, Lee J, Jung JH,
Choi WS, Lee BM, Yoon KS, Yoon S and Kim HS: Sirtinol, a class III
HDAC inhibitor, induces apoptotic and autophagic cell death in
MCF-7 human breast cancer cells. Int J Oncol. 41:1101–1109. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
MacCallum SF, Groves MJ, James J, Murray
K, Appleyard V, Prescott AR, Drbal AA, Nicolaou A, Cunningham J,
Haydock S, et al: Dysregulation of autophagy in chronic lymphocytic
leukemia with the small-molecule sirtuin inhibitor tenovin-6. Sci
Rep. 3:12752013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Solomon JM, Pasupuleti R, Xu L, McDonagh
T, Curtis R, DiStefano PS and Huber LJ: Inhibition of SIRT1
catalytic activity increases p53 acetylation but does not alter
cell survival following DNA damage. Mol Cell Biol. 26:28–38. 2006.
View Article : Google Scholar : PubMed/NCBI
|