Introduction
Bone development and maintenance are regulated by
highly dynamic remodelling processes that are influenced by
bone-degrading osteoclasts, bone-forming osteoblasts and
mechanical-sensing osteocytes (1).
Osteoclasts are the only cell type able to remove bone tissue, and
they are responsible for both physiological and pathological bone
resorption, which is indispensable during skeletal development and
maintenance (2). Osteoclasts are
multinucleated cells that are formed by the fusion of myeloid
hematopoietic precursors formed in the bone marrow (3). Osteoclast precursors are circulating
blood monocytes that are recruited to sites of bone remodelling
where they fuse into osteoclasts following specific stimuli
(4). Therefore, identifying agents
capable of suppressing osteoclast differentiation may facilitate
the development of a therapeutic strategy for the treatment of
pathological bone loss.
Bacteria and their by-products can induce the
release of pro-inflammatory cytokines, such as macrophage
chemoattractant protein-1 (MCP-1), tumour necrosis factor-α
(TNF-α), interleukins (ILs) and prostaglandin E2, which directly or
indirectly stimulate osteoclast differentiation (5,6). In
a previous study, Jiang et al (7) showed the relationship between
inflammatory cytokines and bone resorption. Macrophage-derived
chemokines induce macrophages and osteoclast precursor cells to
polarize and migrate to inflammatory tissues, whereas inflammatory
cytokines promote osteoclast differentiation and suppress
osteoblast formation.
Among various inflammatory cytokines, MCP-1 is one
of the most abundantly released and is responsible for macrophage
cell recruitment and activation during acute inflammation,
promoting multinuclear cells to fuse into osteoclasts (8,9).
Seven-amino acid truncated (7ND) protein is a mutant version of the
MCP-1 protein. The 7ND protein lacks seven amino acids between
position 2 and 8 of MCP-1, located in the N-terminus and functions
as a dominant negative inhibitor of MCP-1 (6,10).
In a previous study, Yao et al (6) showed that n7ND protein effectively
decreased MCP-1-induced migration of THP-1 macrophages in
vitro. In addition, n7ND protein injection or delivery from a
layer-by-layer coating platform significantly inhibited wear
particle-induced osteolysis in vivo (7,11).
However, the efficacy of 7ND on human TNF superfamily member 11
(TNFSF11)-induced osteoclast differentiation and lipopolysaccharide
(LPS)-induced osteolysis has yet to be demonstrated. Therefore, in
the present study, the effects of 7ND proteins on human peripheral
blood mononuclear cell (PBMC) proliferation and osteoclast
differentiation were investigated in vitro, and the ability
of 7ND to decrease LPS-induced bone erosion was examined in
vivo.
Materials and methods
Reagents
Recombinant human colony-stimulating factor 1
(rhCSF1), TNFSF11 (rhTNFSF11) and MCP-1 (rhMCP-1) were purchased
from PeproTech, Inc. α-minimal essential medium (α-MEM),
penicillin-streptomycin solution, PBS without calcium and
magnesium, FBS, DAPI staining solution and Rhodamine-conjugated
phalloidin were purchased from Gibco; Thermo Fisher Scientific,
Inc. The tartrate-resistant acid phosphatase (TRAP) staining kit,
Escherichia coli (E. coli) LPS, and haematoxylin and
eosin (H&E) were purchased from Sigma-Aldrich; Merck KGaA. Cell
Counting Kit-8 (CCK-8) reagent was obtained from Dojindo Molecular
Technologies, Inc. 7ND protein was purified as previously reported
(12).
PBMC isolation
Heparinized blood (100 ml) was collected from each
healthy volunteer (4 males and 5 females; 25–35 years of age;
recruitment date from September 2016 to January 2018) at the
Department of Operative Dentistry and Endodontics at the Affiliate
Stomatology Hospital of Sun Yat-sen University. To be included
volunteers had to be Chinese, healthy, ~30 years of age and
medication-free for one month. The patients provided written
informed consent. The study was approved by The Research Ethics
Committee of Guanghua School of Stomatology, Affiliated
Stomatological Hospital, Sun Yat-sen University. Blood was
collected in BD vacutainer cell preparation tubes (CPT; BD
Biosciences) with 0.100 M sodium citrate. Heparinized blood was
centrifuged at 1,500 × g and 0°C for 30 min, and the cell layer
above the Ficoll-Paque formed in the CPT was collected, resuspended
in 10 ml of α-MEM and recentrifuged at 195.65 × g and 37°C for 5
min. Then, the cells were cultured in 0.5 ml of α-MEM supplemented
with 10% FBS and 1% penicillin-streptomycin solution in a 24-well
plate at a density of 3×105 cells/well and incubated at
37°C in a humidified atmosphere with 5% CO2 for 1 day.
On the second day, the complete medium was replaced, floating cells
were discarded, and the adherent cells were identified as PBMCs
(12). PBMCs were cultured and
used for subsequent experiments.
Cell viability assay
PBMCs were incubated at 37°C in 96-well plates at a
density of 1×105 cells/well for 1 day, the complete
medium was replaced and increasing concentrations of 7ND protein
(0–100 ng/ml) were added. After 1, 2 and 3 days, CCK-8 reagent (10
µl) was added to each well followed by incubation at 37°C in the
dark for 3 h, according to the manufacturer's protocols. The
absorbance was measured at 450 nm using a microplate reader (BioTEK
Instruments, Inc.).
Osteoclast differentiation and
identification
PBMCs were plated in 24-well plates and supplemented
with rhCSF1 (25 ng/ml) and rhTNFSF11 (40 ng/ml) to induce
osteoclast differentiation. Groups were arranged as follows: i)
Untreated monocytes; ii) monocytes treated with rhCSF1 (25 ng/ml)
and rhTNFSF11 (40 ng/ml); iii) monocytes treated with rhCSF1 (25
ng/ml), rhTNFSF11 (40 ng/ml) and rhMCP-1 (25 ng/ml); and iv)
monocytes treated with rhCSF1 (25 ng/ml), rhTNFSF11 (40 ng/ml) and
7ND protein (25 ng/ml). The medium and all cytokines were replaced
every 3 days for 15 days, and osteoclasts were cultured as
previously described (13,14).
For TRAP staining, cells were fixed in 4%
paraformaldehyde for 20 min at room temperature (18-26°C) and then
stained with TRAP staining solution at 37°C for 1 h, according to
the manufacturer's protocol (Sigma-Aldrich; Merck KGaA). The images
were captured by a light microscope (Carl Zeiss AG) using
magnification, ×100. TRAP-positive cells with ≥3 nuclei were
considered multinucleated osteoclasts.
For actin cytoskeleton staining, cells were washed
with PBS three times, permeabilized with 0.2% Triton X-100 in PBS
for 10 min and stained with fluorescent phalloidin (1:200) diluted
in PBS for 20 min at room temperature (18-26°C). Nuclei
counterstaining was performed by DAPI (1:500) for 5 min at room
temperature (18-26°C), followed by fluorescence microscopy (Carl
Zeiss AG) using magnification, ×100. In total, four fields were
randomly selected, and the numbers of osteoclasts and filamentous
(F)-actin rings were counted by two independent investigators.
In vivo animal model of bone loss by
LPS
A total of 78 five-week-old male C57BL/6J mice
(16.3–3.3 g) were purchased from The Animal Resources Center of Sun
Yat-sen University and housed at a controlled temperature (22-24°C)
and humidity (55-60%) under a 12-h light/dark cycle with free
access to food and water. All protocols were reviewed and approved
by the Sun Yat-sen University Ethics Committee (approval nos.
IACUC-DB-17-1105 and IACUC-DD-17-1112).
To establish an LPS-induced osteolysis murine model,
the mice were randomly divided into three groups (n=20/group) and
injected with E. coli LPS or PBS subcutaneously, over the
calvaria. Groups were arranged as follows: i) Control group
injected with PBS; ii) mice injected with 5 mg/kg LPS; and iii)
mice injected with 25 mg/kg LPS. Animals were monitored every day
and the weight of each mouse was recorded on the last experimental
day. In total, five mice from each group were sacrificed on day 1,
3, 5 and 7, and all skull bones were dissected and soaked in 75%
ethanol at room temperature (18-26°C) for micro-computed tomography
(µ-CT) analysis.
To study the function of 7ND protein in vivo,
the mice were injected subcutaneously over the calvaria and
randomly divided into three groups (n=6 in each group): i) Negative
control group injected with PBS on day 1 and day 3; ii) Positive
control group injected with LPS (25 mg/kg) on day 1 and PBS on day
3; and iii) Experimental group injected with LPS (25 mg/kg) and 7ND
protein (3 µg in 100 µl PBS) on day 1 and 7ND protein (3 µg in 100
µl PBS) without LPS on day 3. The animals were sacrificed on day 6,
and their skull bones were dissected and soaked in 75% ethanol at
room temperature (18-26°C) for µ-CT analysis.
µ-CT imaging and analysis
All calvaria were scanned by a µ-CT scanner (µCT50;
SCANCO Medical AG). The scanning parameters were 8 W, 70 kV, 114
µA, 360° rotation and a pixel size of 20 µm. After scanning,
VGStudio Max software (version 1.2.1; Volume Graphics GmbH) was
used to reconstruct the three-dimensional (3D) images.
Subsequently, the 3D images were converted into graphic images by a
blinded observer. To study the murine LPS-induced osteolysis model,
the resorption areas of a region of interest in the frontal and
parietal bones were defined with Adobe Photoshop (version 12.0.3;
Adobe Systems, Inc.) by two independent investigators. ImageJ
software (version 6.0; National Institutes of Health) was used to
analyse the images of resorption areas and to determine the
percentage of the calvarial resorption area of each sample.
For quantitative analysis of the function of 7ND
protein with LPS-induced osteolysis, the software of µCT50 (SCANCO
Medical AG) was used to analyse 4 mm thick (200 slices) skull
sections from the midline suture of the skull in its centre to
calculate bone mineral density (BMD), bone volume (BV) and the bone
volume per tissue volume (BV/TV). The skulls were then fixed in 4%
paraformaldehyde at room temperature (18-26°C) for 1 day for
histological analysis.
Histological evaluation of
osteolysis
All skulls were fixed in 4% paraformaldehyde for 1
day at room temperature (18-26°C) and the soft tissue was removed.
The remaining calvaria bones were decalcified for 3 weeks at room
temperature (18-26°C) using 10% EDTA, pH 7.4, dehydrated and
embedded in paraffin. Serial sections of 5-µm thickness were cut by
a Leica microtome RM2255 (Leica Microsystems GmbH), including the
centre of the calvaria, the site of drug injection. For
histological examination, the slides were stained with H&E at
room temperature (18-26°C) and TRAP at 37°C for 1 h according to
the manufacturer's protocols. Images in six randomly selected
fields were captured by a light microscope (Carl Zeiss AG) at
magnification, ×100. TRAP-positive osteoclasts in the skull bones
were counted by two independent investigators.
Statistical analysis
Each experiment was performed >3 times. All
statistical analyses were performed using SPSS (version 20; IBM
Corp.). Data are presented as the mean ± SD. Data were evaluated
for a normal distribution using Shapiro-Wilk test. The data were
normally distributed, and the groups were compared using one-way
ANOVA followed by Bonferroni's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
7ND protein inhibits osteoclast
differentiation in PBMCs
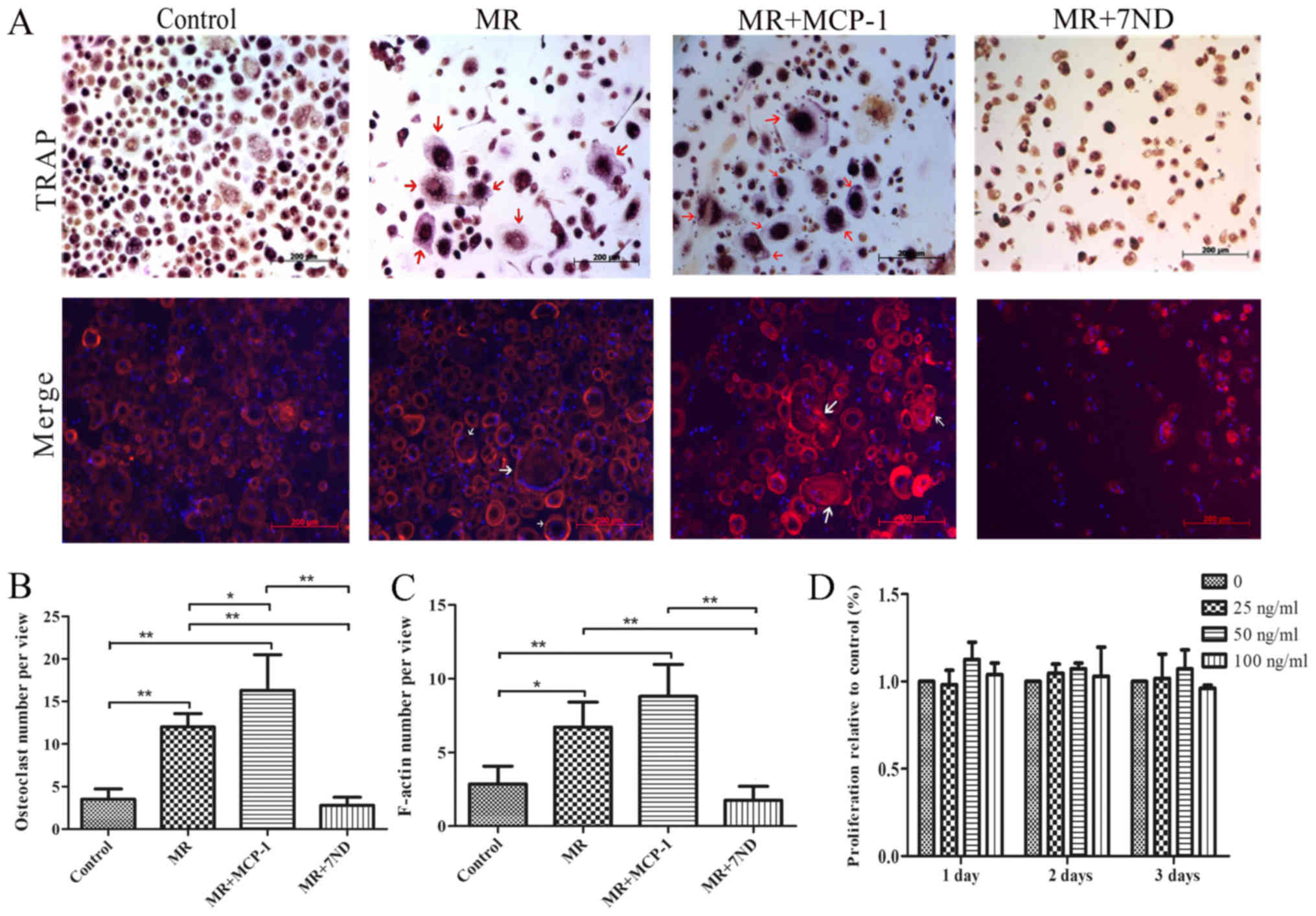
PBMCs were induced to differentiate into osteoclasts
in the positive control group by adding rhCSF1 and rhTNFSF11 to the
culture for 15 days (Fig. 1A). The
7ND protein (25 ng/ml) was added in osteoclast differentiation
media, and TRAP staining revealed that significantly fewer
osteoclasts formed in the presence of 7ND protein. In contrast,
significantly more osteoclasts formed in the presence of rhMCP-1
(Fig. 1A and B).
Immunofluorescence results suggested similar results, with
significantly decreased F-actin signal in osteoclasts in the
presence of the 7ND protein (Fig. 1A
and C). Compared with the differentiation media, a decreased
number of osteoclasts were observed in the negative control group
(Fig. 1A-C).
7ND protein does not affect PBMC
proliferation
A CCK-8 assay was performed to determine the
proliferation of PBMCs treated with 7ND protein. The results
indicated that treatment with 7ND protein at various concentrations
(0–100 ng/ml) for 1–3 days did not affect PBMC proliferation
(Fig. 1D).
Establishment of a mouse LPS-induced
bone loss model
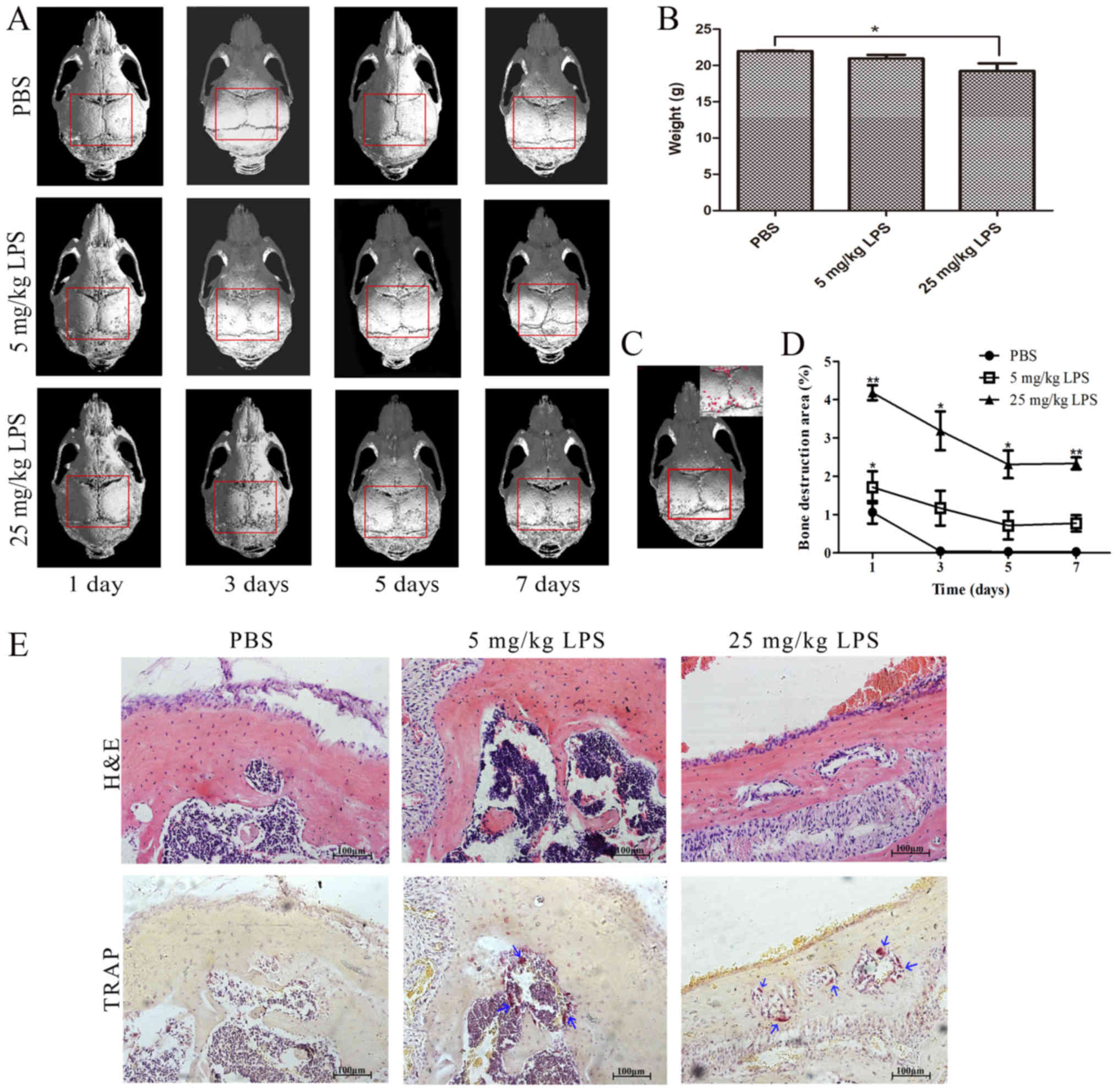
µ-CT imaging confirmed that LPS injection induced
osteolysis. Increasing concentrations of LPS were associated with
more defects in the entire calvarial bone (Fig. 2A). The defects were indicated by
the percentage of bone destruction area, and osteolysis induced by
25 mg/kg LPS was found to last longer compared with 5 mg/kg LPS
(Fig. 2A, C and D). The weights of
the mice indicated the health status of the mice (Fig. 2B). H&E and TRAP staining of
calvarial bones confirmed that LPS was able to induce osteoclast
differentiation (Fig. 2E).
Collectively, the percentage of the bone destruction area and the
weights of the mice showed that a high concentration (25 mg/kg) of
LPS induced osteolysis that was detected for >5 days, whereas a
low concentration (5 mg/kg) of LPS induced significant osteolysis
that was detected for 1 day.
µ-CT imaging and analysis indicates
that 7ND protein decreases osteolysis
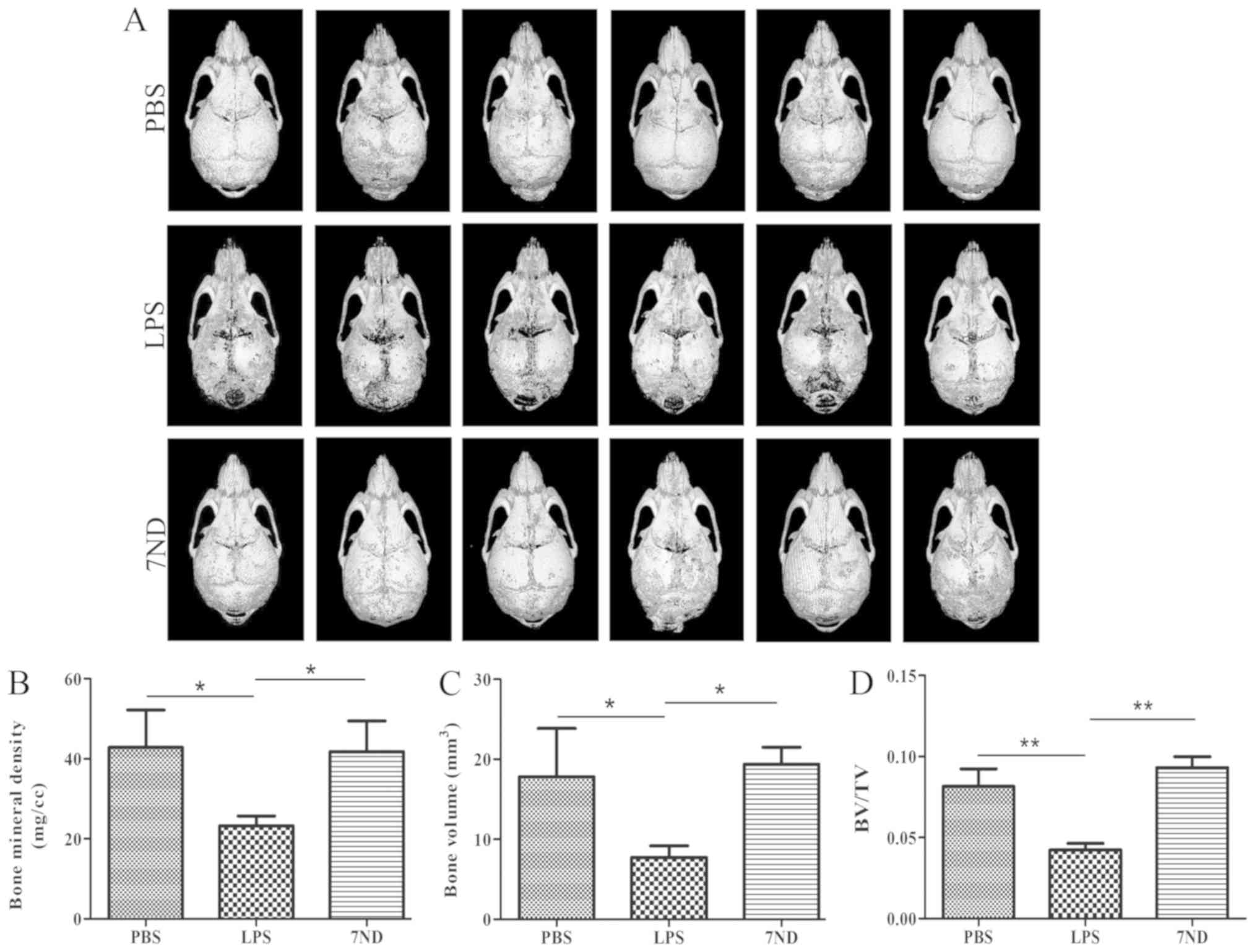
µ-CT imaging suggested that 7ND protein injection
reduced LPS-induced bone resorption. Bone defects decreased in the
entire calvarial bone following treatment with 7ND protein compared
with the LPS group (Fig. 3A). BMD,
BV and BV/TV were used to measure the degree of bone loss. After
treatment with 7ND, BMD, BV and BV/TV were significantly increased
compared in the LPS group (Fig.
3B-D). Importantly, treatment with 7ND did not present
significant differences compared with the PBS group (Fig. 3B-D), suggesting that 7ND protein
significantly inhibited osteolysis.
7ND protein decreases the number of
osteoclasts in the bone resorption lacunae
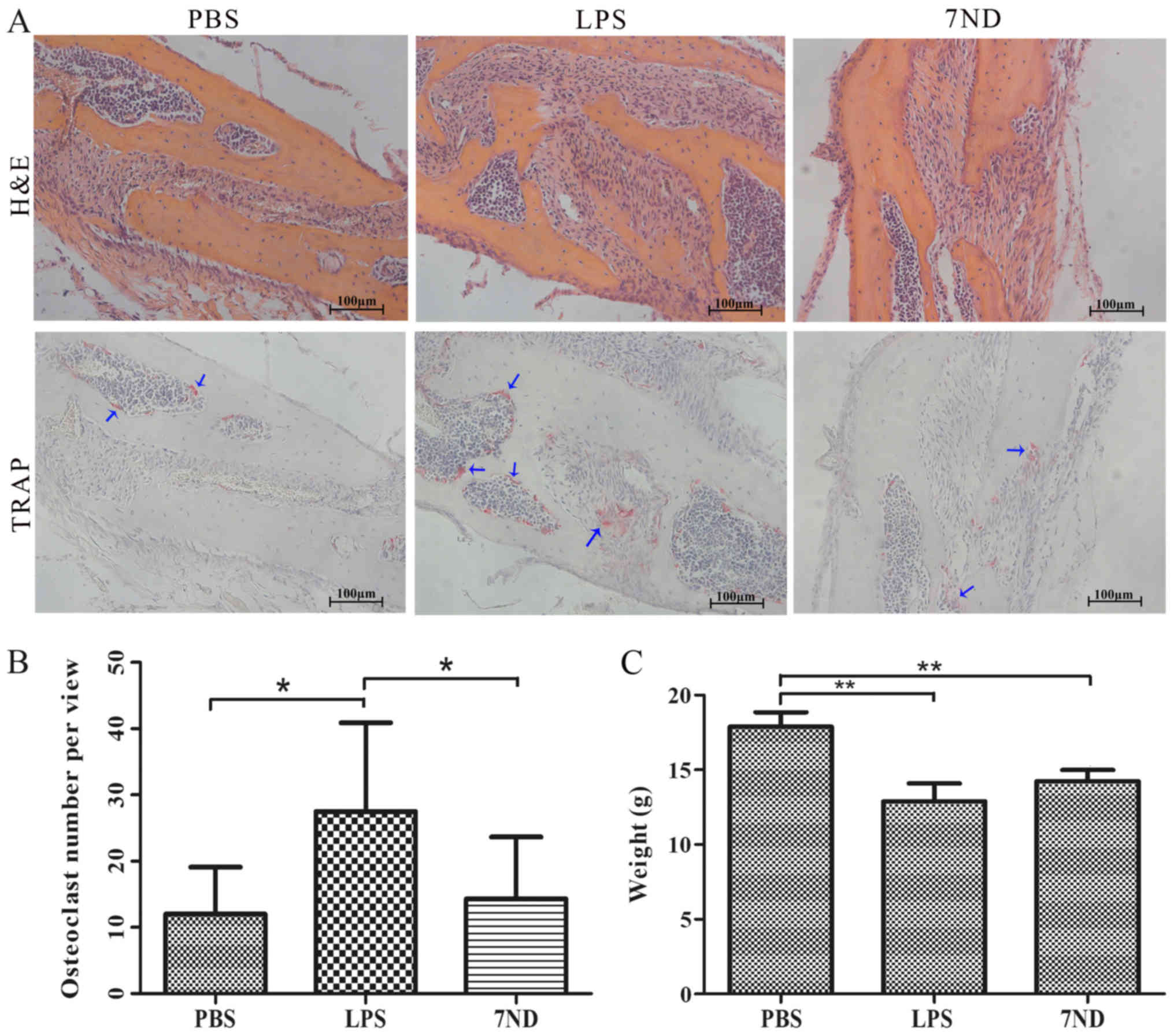
H&E and TRAP staining were used to visualize and
count osteoclasts accumulating in the resorption lacunae. The
results demonstrated that significantly more osteoclasts were
detected in the LPS group compared with the PBS and 7ND groups,
whereas the 7ND group showed no significant difference compared
with the PBS group (Fig. 4A and
B), in line with the µ-CT results. As mice in the LPS and 7ND
groups were injected with LPS, their weight was lower compared to
the PBS group where mice were not injected with LPS (Fig. 4C).
Discussion
7ND protein is a mutant form of MCP-1 without the
seven N-terminal amino acids at positions 2–8. 7ND binds to the
MCP-1 receptor on macrophages and functions as a dominant-negative
inhibitor of MCP-1 (6,10). MCP-1 plays a critical role in
chemotaxis and inflammation, activating cell mobilization and
upregulating a series of pro-inflammatory cytokines and chemokines
(15). However, Yao et al
(6) showed that 7ND protein
treatment decreased not only MCP-1-induced migration of THP-1 cells
in a dose-dependent manner, but also the THP-1 chemotactic effects
of conditioned media from Raw 264.7 murine macrophages exposed to
polymethylmethacrylate particles with and/or without LPS. In
addition, the 7ND protein is able to bind to the MCP-1 receptor
without inducing cell activation, and interference of the MCP-1-C-C
motif chemokine receptor 2 ligand-receptor axis was able to
decrease the release of inflammatory cytokines without adverse
effects (6,16).
The present results suggested that 7ND protein
efficiently inhibited PBMCs differentiation into osteoclasts
without influencing cell proliferation, in line with the results by
Kim et al (13,14). The present study has demonstrated
the significant effects of 7ND protein in vitro, which is
the first step for animal experiments in vivo. Previous
studies identified that 7ND protein exhibited promising results in
decreasing osteolysis. Morrison et al (17) found that 7ND blocked calmodulin 1
(CALM1), Jun proto-oncogene (JUN), AP-1 transcription factor
subunit and nuclear factor of activated T cells 2 (NFATC2)
induction in colony forming unit-granulocyte macrophages and
inhibited human osteoclast differentiation at the molecular level.
Yao et al (6) showed that
7ND protein decreased macrophage migration and the release of
inflammatory cytokines in a dose-dependent manner. Therefore, in
the present study, the effects of 7ND protein on osteoclast
differentiation were investigated, and F-actin ring formation and
TRAP expression were examined.
Various previous studies demonstrated that 7ND
protein treatment significantly decreased osteolysis induced by
orthopaedic implant wear particles (7,12,18).
Wear particles are by-products of all joint replacements and are
able to stimulate chronic inflammation, thus leading to osteolysis.
Wear particles increase the production of pro-inflammatory
chemokines, including MCP-1, TNF-α, ILs and granulocyte-monocyte
CSF (6). However, a study of the
effect of 7ND protein on bacterial osteolysis has yet to be
reported. It has been hypothesized that osteolysis induced by
chronic Gram-negative bacterial infection underlies bone diseases,
and it has been demonstrated that LPS is a major virulence factor
found in the Gram-negative bacterial cell wall (19). LPS was identified to induce
intracellular activation of mitogen-activated protein kinases 8 and
14, and nuclear factor κB in macrophages and monocytes, thus
promoting the release of pro-inflammatory cytokines that are
recognized as key pathogens of inflammatory osteolytic diseases,
such as osteomyelitis, septic arthritis, periodontitis and
infection of orthopaedic implants (20,21).
Among various mouse models of osteolysis, an inflammation-induced
bone loss model through LPS injection into the calvaria was used in
the present study. Compared with other animal models (22–25),
this model exhibits the advantage of accurate and reproducible
injection into the calvaria between the ears of mice (19,26,27).
In addition, the present model is not affected by interferences
caused by possible animal activity.
Inhibition of LPS-induced osteolysis is important to
prevent bone destruction in infective bone diseases. Assessing the
effects of novel agents on LPS-induced osteoclastogenesis should be
studied in vivo using murine models. However, various
studies established LPS-induced osteolysis models in multiple ways,
and no standard protocols were described to determine the injection
time in the murine model (22–27).
To solve these problems, the present study established an
LPS-induced bone loss mouse model. The present µ-CT imaging results
suggested that the percentage of the bone destruction area,
indicating osteolysis, induced by high concentrations (25 mg/kg) of
LPS lasted >5 days, whereas low concentrations (5 mg/kg) induced
osteolysis for 1 day. The present results suggested that 25 mg/kg
LPS is recommended for long-term experiments (5–7 days), whereas 5
mg/kg LPS is recommended for short-term experiments (1–3 days).
Based on the present findings, a long-term murine osteolysis model
following treatment with 25 mg/kg LPS was used to determine the
efficacy of local 7ND protein delivery in vivo.
Based on the inhibitory effects of 7ND protein on
osteoclast differentiation in vitro, further in vivo
experiments were performed. As assessed by µ-CT imaging, injection
of 7ND protein effectively mitigated LPS-induced bone loss. 7ND
treatment significantly reduced bone osteolysis and maintained
normal bone morphology. Quantitative analyses of µ-CT images
revealed that 7ND protein treatment increased the BMD, BV fraction
and BV/TV compared with PBS. Histological analyses indicated that
7ND protein effectively reduced the number of osteoclasts.
Importantly, µ-CT and histological analyses showed no significant
differences between local delivery of 7ND protein in the
LPS-induced-osteolysis model and PBS in normal mice, demonstrating
that 7ND protein significantly inhibited LPS-induced osteolysis.
Subcutaneous injection of 7ND protein may have an antiresorptive
effect on LPS-induced bone loss, and the molecular mechanism of 7ND
protein requires further investigation in vivo. Nabeshima
et al (18) showed that 7ND
protein coating decreased the number of infiltrating macrophages in
a continuous polyethylene particle infusion model. Therefore,
further investigation is required to clarify the mechanism of 7ND
protein on LPS-induced bone loss. Further in vivo study may
identify the direct effects of 7ND protein on osteoclast
differentiation and its indirect anti-inflammatory effects.
In the present study, 7ND protein efficiently
inhibited osteoclast differentiation in PBMCs in vitro and
significantly reduced LPS-induced osteolysis in vivo.
Morrison et al (17) showed
that colony forming unit-granulocyte macrophages increased by
1,000-fold the differentiation potential of MCP-1 within 24 h of
TNFSF11 treatment, and 7ND inhibited osteoclast differentiation by
suppressing the expression levels of CALM1, JUN and NFATC2.
Therefore, in the present study, it was hypothesized that the 7ND
protein inhibited human osteoclast formation at an early stage of
differentiation by interfering with the expression of
pro-inflammatory cytokines, thus inhibiting the signalling pathways
involved in inflammation-mediated stimulation. However, the
molecular mechanisms underlying 7ND protein function on the
inhibition of osteoclast differentiation requires further
investigation.
In conclusion, the present study suggested the
potential of the 7ND protein in inhibiting osteoclast
differentiation and reducing bone loss. The present results
suggested that the 7ND protein may represent a novel therapeutic
candidate to treat various osteoclast-associated bone diseases.
Acknowledgements
Not applicable.
Funding
The present work was supported by The Natural
Science Foundation of Guangdong Province (grant no. 2017A030313713)
and The National Natural Science Foundation of China (grant nos.
8150079 and 81870750).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
HJ and QG conceived the present study. WL, JQ, YL
and JL performed the experiments. WL and JQ designed the study,
performed the experiments, analysed the data and wrote the
manuscript. WL and JQ contributed equally. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by The Sun Yat-sen
University Ethics Committee (ethics certificate nos.
IACUC-DB-17-1105 and IACUC-DD-17-1112).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lenertz LY, Baughman CJ, Waldschmidt NV,
Thaler R and van Wijnen AJ: Control of bone development by P2X and
P2Y receptors expressed in mesenchymal and hematopoietic cells.
Gene. 570:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakashima T and Takayanagi H: New
regulation mechanisms of osteoclast differentiation. Ann N Y Acad
Sci. 1240:E13–E18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyce BF: Advances in the regulation of
osteoclasts and osteoclast functions. J Dent Res. 92:860–867. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muto A, Mizoguchi T, Udagawa N, Ito S,
Kawahara I, Abiko Y, Arai A, Harada S, Kobayashi Y, Nakamichi Y, et
al: Lineage-committed osteoclast precursors circulate in blood and
settle down into bone. J Bone Miner Res. 26:2978–2990. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko SY: Myricetin suppresses LPS-induced
MMP expression in human gingival fibroblasts and inhibits
osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW
264.7 cells. Arch Oral Biol. 57:1623–1632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao Z, Keeney M, Lin TH, Pajarinen J,
Barcay K, Waters H, Egashira K, Yang F and Goodman S: Mutant
monocyte chemoattractant protein 1 protein attenuates migration of
and inflammatory cytokine release by macrophages exposed to
orthopedic implant wear particles. J Biomed Mater Res A.
102:3291–3297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang X, Sato T, Yao Z, Keeney M,
Pajarinen J, Lin TH, Loi F, Egashira K, Goodman S and Yang F: Local
delivery of mutant CCL2 protein-reduced orthopaedic implant wear
particle-induced osteolysis and inflammation in vivo. J Orthop Res.
34:58–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Foo SS, Taylor A, Lulla A, Merits
A, Hueston L, Forwood MR, Walsh NC, Sims NA, Herrero LJ and
Mahalingam S: An inhibitor of monocyte chemotactic protein
synthesis, protects against bone loss induced by chikungunya virus
infection. J Virol. 89:581–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sierra-Filardi E, Nieto C, Domínguez-Soto
A, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven
J, Ardavín C, Rodríguez-Fernández JL, et al: CCL2 shapes macrophage
polarization by GM-CSF and M-CSF: Identification of
CCL2/CCR2-dependent gene expression profile. J Immunol.
192:3858–3867. 2015. View Article : Google Scholar
|
|
10
|
Zhang Y, Ernst CA and Rollins BJ: MCP-1:
Structure/activity analysis. Methods. 10:93–103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keeney M, Waters H, Barcay K, Jiang X, Yao
Z, Pajarinen J, Egashira K, Goodman SB and Yang F: Mutant MCP-1
protein delivery from layer-by-layer coatings on orthopedic
implants to modulate inflammatory response. Biomaterials.
34:10287–10295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo S, Zhou C, Zhang J, Chen M, Li H,
Zheng S and Quan J: Mutant monocyte chemoattractant protein-1
protein (7ND) inhibits osteoclast differentiation and reduces oral
squamous carcinoma cell bone invasion. Oncol Lett. 15:7760–7768.
2018.PubMed/NCBI
|
|
13
|
Kim MS, Day CJ, Selinger CI, Magno CL,
Stephens SR and Morrison NA: MCP-1-induced human osteoclast-like
cells are tartrate-resistant acid phosphatase, NFATc1, and
calcitonin receptor-positive but require receptor activator of
NFkappaB ligand for bone resorption. J Biol Chem. 281:1274–1285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MS, Day CJ and Morrison NA: MCP-1 is
induced by receptor activator of nuclear factor-{kappa}B ligand,
promotes human osteoclast fusion, and rescues granulocyte
macrophage colony-stimulating factor suppression of osteoclast
formation. J Biol Chem. 280:16163–16169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gibon E, Ma T, Ren PG, Fritton K, Biswal
S, Yao Z, Smith L and Goodman SB: Selective inhibition of the
MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of
macrophages in the presence of UHMWPE particles. J Orthop Res.
30:547–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y and Rollins BJ: A dominant
negative inhibitor indicates that monocyte chemoattractant protein
1 functions as a dimer. Mol Cell Biol. 15:4851–4855. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morrison NA, Day CJ and Nicholson GC:
Dominant negative MCP-1 blocks human osteoclast differentiation. J
Cell Biochem. 115:303–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nabeshima A, Pajarinen J, Lin TH, Jiang X,
Gibon E, Córdova LA, Loi F, Lu L, Jämsen E, Egashira K, et al:
Mutant CCL2 protein coating mitigates wear particle-induced bone
loss in a murine continuous polyethylene infusion model.
Biomaterials. 117:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Zhang C, Wang X, An B, Zhang P and
Zhu Z: Berberine inhibits lipopolysaccharide- and polyethylene
particle-induced mouse calvarial osteolysis in vivo. J Surg Res.
73:e47–e52. 2012. View Article : Google Scholar
|
|
20
|
Hou GQ, Guo C, Song GH, Fang N, Fan WJ,
Chen XD, Yuan L and Wang ZQ: Lipopolysaccharide (LPS) promotes
osteoclast differentiation and activation by enhancing the MAPK
pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med.
32:503–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robertson Remen KM, Lerner UH, Gustafsson
JÅ and Andersson G: Activation of the liver X receptor-β potently
inhibits osteoclastogenesis from lipopolysaccharide-exposed bone
marrow-derived macrophages. J Leukoc Biol. 93:71–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baek JM, Kim JY, Ahn SJ, Cheon YH, Yang M,
Oh J and Choi MK: Dendrobium moniliforme exerts inhibitory effects
on both receptor activator of nuclear factor kappa-B
ligand-Mediated osteoclast differentiation in vitro and
lipopolysaccharide-induced bone erosion in vivo. Molecules.
21:2952016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baek JM, Kim JY, Cheon YH, Park SH, Ahn
SJ, Yoon KH, Oh J and Lee MS: Aconitum pseudo-laeve var. erectum
inhibits receptor activator of nuclear factor kappa-B
ligand-induced osteoclastogenesis via the c-Fos/nuclear factor of
activated T-Cells, cytoplasmic 1 signaling pathway and prevents
lipopolysaccharide-induced bone loss in mice. Molecules.
19:11628–11644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baek JM, Kim JY, Jung Y, Moon SH, Choi MK,
Kim SH, Lee MS, Kim I and Oh J: Mollugin from rubea cordifolia
suppresses receptor activator of nuclear factor-κB ligand-induced
osteoclastogenesis and bone resorbing activity in vitro and
prevents lipopolysaccharide-induced bone loss in vivo.
Phytomedicine. 22:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JY, Baek JM, Ahn SJ, Cheon YH, Park
SH, Yang M, Choi MK and Oh J: Ethanolic extract of schizonepeta
tenuifolia attenuates osteoclast formation and activation in vitro
and protects against lipopolysaccharide-induced bone loss in vivo.
BMC Complement Altern Med. 16:3012016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Khansari A, Shapira L, Graves DT and
Amar S: Contribution of interleukin-11 and prostaglandin(s) in
lipopolysaccharide-induced bone resorption in vivo. Infect Immun.
70:3915–3922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Espirito Santo AI, Ersek A, Freidin A,
Feldmann M, Stoop AA and Horwood NJ: Selective inhibition of TNFR1
reduces osteoclast numbers and is differentiated from anti-TNF in a
LPS-driven model of inflammatory bone loss. Biochem Biophys Res
Commun. 464:1145–1150. 2015. View Article : Google Scholar : PubMed/NCBI
|