Introduction
Obstructive sleep apnea syndrome (OSAS) is a common
and complex disorder consisting of complete or partial upper airway
obstruction and sleep fragmentation, affecting >4% of the
world's general population and 30–45% of patients with obesity
(1–3). As a result of upper airway collapse,
OSAS causes repeated nocturnal hypoxia and alternate episodes of
normoxia, such chronic intermittent hypoxia (CIH) during sleep,
resembling the pathophysiologic mechanisms in ischemia/reperfusion
multi-organ injury, which can trigger cardiovascular morbidity,
lung injury and chronic kidney disease (4–7).
Moreover, previous studies have reported that CIH contributes to
the pathogenesis of non-alcoholic fatty liver disease and
exacerbates liver fibrogenesis (2–8).
However, the underlying mechanisms of hepatic fibrosis in patients
with OSAS are not fully understood.
Epidemiological studies and clinical investigations
have shown that OSAS is a type of chronic and mild systemic
inflammatory response disease (8,9). For
example, inflammatory factors such as interleukin (IL)-β, IL-6,
IL-18, tumor necrosis factor-α (TNF-α), interferon-γ and C-reactive
protein (CRP) can be detected at high levels in the blood of
patients with OSAS (10,11). Furthermore, Ryan and McNicholas
(11) revealed a significant
positive association between serum TNF-α levels and OSAS severity,
with higher serum levels of TNF-α in patients with OSAS compared
with non-OSAS subjects. In addition, Drager et al (12) reported that the expression of CRP
declined in adult patients with OSAS after effective treatment with
continuous positive airway pressure. Therefore, these findings
suggest that CIH may be associated with inflammation and may
contribute to the increased incidence of liver fibrogenesis in
patients with OSAS. However, although several studies have closely
linked CIH to systemic inflammation, the mechanism of this
association has not been fully established.
Recent studies have shown that inflammation is
associated with innate immune activation, including that involving
Toll-like receptors (TLRs) and its underlying signal pathway
(13,14). TLRs belong to a class of receptors
with well-known pattern recognition, and can accurately perceive
pathogens and bacterial-derived molecules (15). Moreover, activation of TLRs can
lead to inflammatory responses, thus increasing the production of
proinflammatory cytokines (16).
TLR4, a typical representative of TLRs, mediates both innate and
adaptive immune responses and plays an essential role in promoting
inflammation activation (14,17).
It has also been reported that TLR4 can trigger the main adaptor
protein, myeloid differentiation factor 88 (MyD88)-dependent
pathway, leading to rapid activation of the classical NF-κB and
mitogen-activated protein kinase (MAPK) signaling pathway, which
upregulates the transcription and translation of proinflammatory
genes, such as IL-1β, IL-6, IL-8, IL-18 and TNF-α (18,19).
Previous studies have revealed that CIH can increase mRNA and
protein expression levels of TLR4 in the heart and hypothalamus of
rats, and can induce myocardial remodeling and neuronal cell damage
in hippocampus, which may be involved in CIH-induced inflammation
(20,21). Furthermore, it has been shown that
TLR4 expression in the serum of patients with OSAS is significantly
increased compared with healthy individuals (22). However, the relationship between
abnormal expression of TLR4 and the severity of liver injury in
patients with OSAS has not been fully elucidated, and there is
limited information on the downstream changes of TLR signal
transduction after CIH exposure.
The aims of the present study were as follows: i) To
investigate whether CIH exposure can induce similar liver fibrosis
pathological changes in rats as in patients with OSAS; ii) to
examine whether CIH affects proinflammatory cytokine production in
the liver; and iii) to identify the underlying molecular mechanisms
and signaling pathways that may be involved in CIH-induced liver
fibrosis.
Materials and methods
Animals
A total of 24 adult male Sprague-Dawley rats (age, 9
weeks; weight, 200±10 g) were purchased from Shanghai Xipuer-Bikai
Laboratory Animal Co., Ltd. Rats were fed and housed in a standard
pathogen-free environment with a 12 h light/12 h dark cycle (7:00
a.m. lights on and 7:00 p.m. lights off automatically). The rats
were provided with special compound diet and sterilization water
ad libitum. Room temperature and humidity were controlled at
~23±2°C and 50±10%, respectively. All animal procedures were
approved by the Ethical Committee of Experimental Animals of Fujian
Medical University and The Second Affiliated Hospital of Fujian
Medical University. Animal experiments were carried out in
accordance with animal welfare requirements and the Guidelines for
the Care and Use of Laboratory Animals published by the P.R. China
Ministry of Health (January 25, 1998) (23).
CIH animal model construction and
experimental design
Rats were divided into the control (Con) group
(n=6), CIH group (n=6), CIH + TLR4 short hairpin (sh)RNA lentivirus
group (CIH-TLR4) (n=6) and CIH + TLR4 empty vector lentivirus group
(CIH-Vector) (n=6). Firstly, 10 µl (2×106 TU/µl) TLR4
shRNA lentivirus
(5′-GATCCGCACTCTTGATTGCAGTTTCATTCAAGAGATGAAACTGCAATCAGAGTGCTTTTTTG-3′),
scrambled TLR4 shRNA
(5′-GTAGCTAAATGATGAACAACATGGCCTTATATCTCCTAGTAATCTTCGTGGCTTCGTAGTGAT-3′)
and TLR4 shRNA empty vector lentivirus (Shanghai GenePharma Co.,
Ltd.) were separately injected into the rats of the CIH-TLR4 group,
Con group or CIH-Vector group, respectively, via the tail vein.
After 1 week of recovery, each rat was placed in a transparent
plastic container (length, 40 cm; width, 30 cm; height, 18 cm),
which was connected to a device for controlling O2
concentration and pressure (Puhe Biotechnology). For consistency
with the rat sleep cycle, the experimental time was set from 8:00
to 20:00 every day. Rats in the Con group were supplied with air,
whereas those in the CIH, CIH-TLR4 and CIH-Vector group were
subjected to intermittent hypoxia treatment, by injecting 95%
N2 for 20 sec to rapidly reduce O2
concentration to 7.4–7.8%. Container pressure was reduced to 600
mmHg. The set-up was held for 12 sec, and then injected with air
for 28 sec to recover O2 concentration to 21% and
achieve normal air pressure. The entire experiment lasted for 4
weeks.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
After CIH modeling, all rats were anesthetized
(sodium pentobarbitone, 40 mg/kg) and sacrificed by exsanguination.
A small piece of liver in each group was quickly cut and removed.
RNA was extracted using a RNeasy Mini kit (Qiagen GmbH) following
the manufacturer's protocol and stored at −80°C for subsequent use.
Total RNA was reverse transcribed into cDNA at 42°C for 30 min
using a QuantiTect Reverse Transcription kit (Qiagen GmbH),
according to the manufacturer's protocol. qPCR reactions were
performed using iTaq SYBR Green kits (Toyobo Life Science),
according to the manufacturer's protocol. All reactions were
carried out in duplicate and the cycles were run on a Bio-Rad CFX96
RT system (Bio-Rad Laboratories, Inc.). The following thermocycling
conditions were used: Initial denaturation at 95°C for 30 sec;
followed by 35 cycles of annellation at 58°C for 40 sec and
elongation at 72°C for 30 sec. The primers used were as follows:
IL-1β forward, 5′-AGGAGAGACAAGCAACGACAA-3′ and reverse,
5′-GTTTGGGATCCACACTCTCCA-3′; IL-8 forward,
5′-ATGGCTGCTGAACCAGTAGA-3′ and reverse, 5′-CTAGTCTTCGTTTTGAACAG-3′;
monocyte chemotactic-1 (MCP-1) forward, 5′-TCCACCACTATGCAGGTCTC-3′
and reverse, 5′-TGGACCCATTCCTTATTGGG-3′; TNF-α forward,
5′-AGAACTCCAGCCGGTGTCTGTG-3′ and reverse,
5′-GTGGCAAATCGGCTGACGGTGT-3′; intercellular adhesion molecule-1
(ICAM-1) forward, 5′-GGCGTCCATTTACACCTATTA-3′ and reverse,
5′-TTCCTTTTCTTCTCTTGCTTG-3′; vascular cell adhesion molecule-1
(VCAM-1) forward, 5′-AACTGCACGGTCCCTAAT-3′ and reverse,
5′-AGATGGTGGGTTCTTTCG-3′; and β-actin forward,
5′-AGCCATGTACGTAGCCATCC-3′ and reverse, 5′-ACCCTCATAGATGGGCACAG-3′.
Expression levels were quantified using the 2−ΔΔCq
method (24).
Tissue immunohistochemistry
After CIH modeling, all rats were anesthetized
(sodium pentobarbitone, 40 mg/kg) and sacrificed by exsanguination.
A small piece of liver in each group was quickly cut and fixed with
10% formalin for 48 h at 4°C. Samples were then embedded in
paraffin and cut into tissue sections (3-µm) using a tissue
microtome. The deparaffinized tissue sections were subjected to
hematoxylin and eosin (H&E) staining, both for 10 min at room
temperature, and observed under a light microscope (magnification,
×400) to obtain accurate pathological diagnosis.
Different sections in each group were stained via
standard Sirius red staining at room temperature for 30 min to
observe and determine reactive fibrosis, along with its
distribution as described previously (25). Liver tissues were blocked with 5%
BSA (Toyobo Life Science) for 1 h at room temperature followed by
incubation with IL-1β (Abcam; cat. no. ab9787), IL-8 (Santa Cruz
Biotechnology, Inc.; cat. no. sc-376750), MCP-1 (Abcam; cat. no.
ab25124), TNF-α (Abcam; cat. no. ab9755), ICAM-1 (Abcam; cat. no.
ab171123), VCAM-1 (Abcam; cat. no. ab78712), TLR4 (Abcam; cat. no.
ab95562) and NF-κB antibodies (Abcam; cat. no. ab16502) diluted at
1:100 in PBS with 5% BSA (Toyobo Life Science) at 4°C overnight.
After washing with PBS three times, all tissue sections were
incubated with horseradish peroxidase (HRP)-labeled secondary
antibodies (Abcam; cat. nos. ab7090 and ab97040) diluted at 1:200
at room temperature for 30 min to assess liver inflammation in
rats. The sections were then stained with 3′3-diaminobenzidine at
room temperature for 15 min and the immune reaction results were
observed by light microscopy (magnification, ×400). The statistical
results of these immunohistochemical images were analyzed by ImageJ
version 1.52t software (National Institutes of Health).
Western blot analysis
Total protein of each group was isolated from the
liver tissue by ultrasonic homogenization (20 kHZ; 15 sec; 4°C) in
pre-cooled cell lysis solution (Sangon Biotech Co., Ltd.)
containing protease and phosphatase inhibitors to inhibit protein
degradation (Sangon Biotech Co., Ltd.). Then, cell lysate products
were centrifuged at 10,000 × g for 30 min at 4°C in a refrigerated
centrifuge. Total protein was quantified using the bicinchoninic
acid assay method. Proteins (30 µg/µl/lane) were separated by 10%
SDS-PAGE in Tris-glycine-SDS buffer by vertical electrophoresis for
90 min. After separation via electrophoresis, proteins were
immediately transferred to prepared nitrocellulose (NC) membranes
using a trans-blot transfer system (Sangon Biotech Co., Ltd.). NC
membranes were blocked with 5% skim milk diluted by 2% PBS (Sangon
Biotech Co., Ltd.) for 60 min at room temperature and then
incubated with primary anti-TLR4 (Abcam; cat. no. ab95562;
1:1,000), anti-inhibitor of NF-κB (IκB; Abcam; cat. no. ab32518;
1:500), anti-MAPK-1 (Abcam; cat. no. ab32081; 1:500),
anti-phosphorylated (p)-MAPK-1 (Abcam; cat. no. ab223500; 1:500),
anti-NF-κB p65 (Abcam; cat. no. ab16502; 1:500) and anti-β-actin
(Abcam; cat. no. ab8226; 1:3,000) antibodies overnight at 4°C.
Then, the antibodies were diluted with 5% BSA (Sangon Biotech Co.,
Ltd.) in TBS-0.1% Tween-20. After washing with PBS three times, NC
membranes were incubated with 5% skim milk diluted HRP-conjugated
secondary antibodies (cat. nos. D110261 and D110273, 1:1,000;
Sangon Biotech Co., Ltd.) for 60 min at room temperature. The
membrane blots were then visualized using a chemiluminescence
instrument and enhanced chemiluminescence liquid kit (Pierce;
Thermo Fisher Scientific, Inc.). Optical density value of each blot
was determined using Image Lab 3.0 software (Bio-Rad Laboratories,
Inc.) for the chemiluminescence instrument and ImageJ 1.52t
software (National Institutes of Health).
Hepatic stellate cell (HSC)
culture
HSC lines were purchased from Sangon Biotech Co.,
Ltd. and cultured in DMEM (HyClone; GE Healthcare Life Sciences)
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences)
and 1% penicillin-streptomycin at 37°C in a cell incubator with 5%
CO2. HSCs (3×105/cm2) in the
hypoxia/reoxygenation (HR) group were seeded on a 6-well plate and
incubated at 37°C for 24 h. After treatment with or without 10 µM
MAPK inhibitor U0126 (MedChemExpress) at room temperature for 5
min, cells were placed in 94% N2, 5% CO2 and
1% O2 humidified culture incubator for 6 h at 37°C,
followed by reoxygenation with 5% CO2 and 95% air for 12
h until harvest. Cells in the Con group were cultured in a cell
incubator with 5% CO2 (normoxic conditions) and
harvested at the same time as the experimental group (18 h).
Statistical analysis
Data are presented as the mean ± SEM (n=6/group).
Data were analyzed using one-way ANOVA followed by Tukey's post hoc
test using SPSS 18.0 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
CIH induces liver fibrosis in
rats
To investigate the effects of CIH exposure in rats,
Sirius red staining was used to evaluate liver histological
structure. It was identified that 4 weeks of CIH exposure induced a
substantial amount of collagen fibers around the macrovesicular and
microvesicular structures, while collagen fibers were rarely
detected in the Con group (Fig. 1A and
B). When the gene expression of TLR4 was knocked down by TLR4
shRNA lentivirus, liver fibrosis was alleviated (Fig. 1B and D; CIH-TLR4 group vs. CIH
group). Furthermore, the results indicated that there was no
difference between the CIH-Vector group and CIH group (Fig. 1B and C). Therefore, it was
speculated that 4 weeks of CIH can induce liver fibrosis in rats
and this condition may be associated with TLR4 protein
expression.
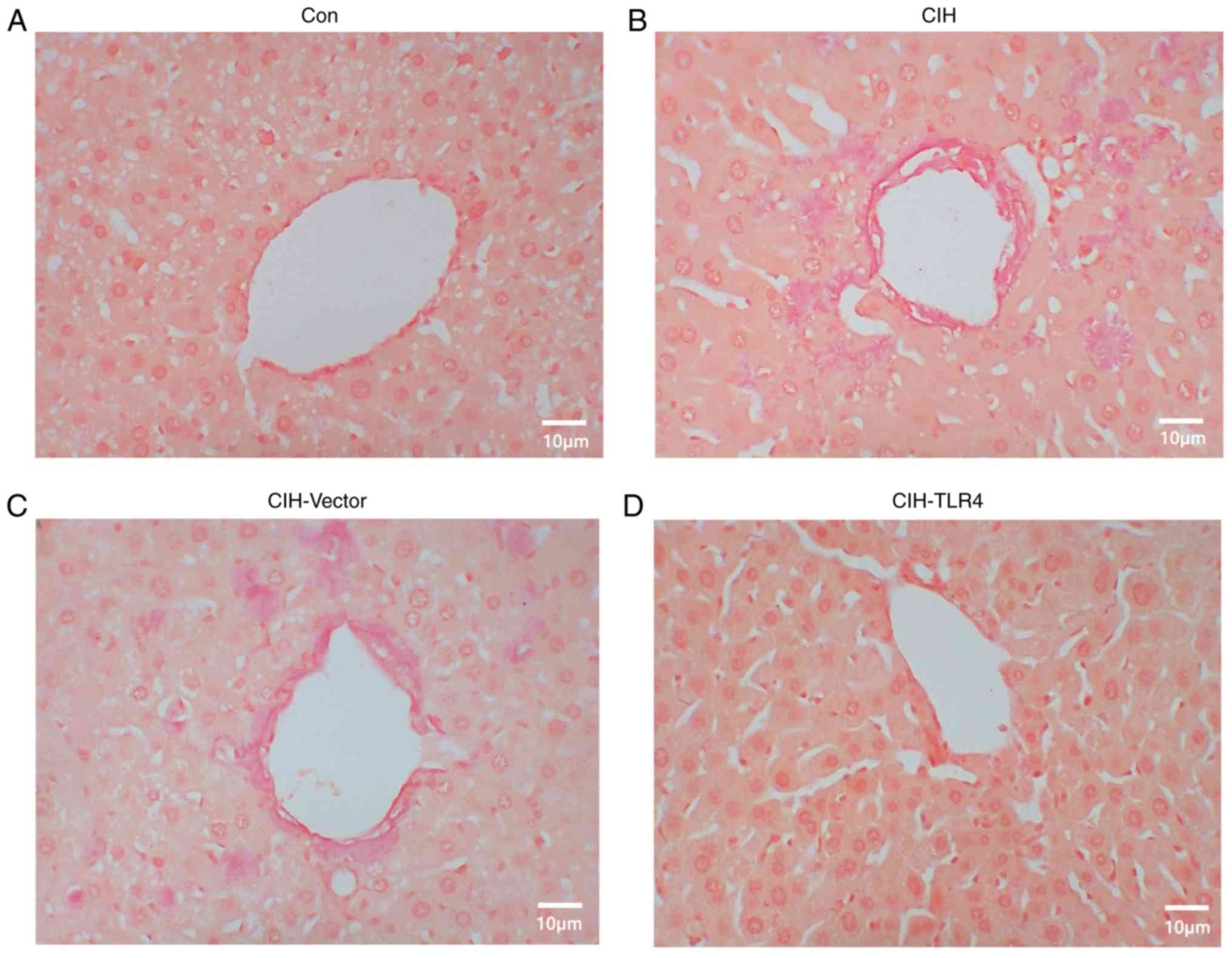 | Figure 1.CIH-induced liver fibrosis. After
exposing rats to CIH for 4 weeks, liver tissues were harvested and
subjected to Sirius red staining. (A) Con group, (B) CIH group, (C)
CIH-Vector and (D) CIH-TLR4 group. Representative images of
immunohistochemistry. Magnification, ×400. Con, control; CIH,
chronic intermittent hypoxia; CIH-Vector group, CIH + empty vector
lentivirus; CIH-TLR4 group, CIH + TLR4 shRNA lentivirus; shRNA,
short hairpin RNA; TLR4, Toll-like receptor 4. |
CIH induces liver inflammation in
rats
Previous studies have reported that systemic
inflammation is a primary cause of myocardium fibrosis (11). Therefore, the present study
examined whether CIH-induced liver fibrosis in rats was associated
with inflammation. RT-qPCR and immunohistochemistry were used to
detect the expression levels of inflammatory cytokines in the liver
of the model animals. RT-qPCR (Fig.
2), immunohistochemical (Fig.
3) and immunohistochemical image analysis (Fig. 4) demonstrated that, compared with
Con group, the livers of the CIH and CIH-Vector groups had
significantly higher expression levels of IL-1β, IL-8, MCP-1,
TNF-α, ICAM-1 and VCAM-1 (P<0.01 vs. Con group). Moreover, it
was found that these effects were reversed by TLR4 shRNA lentivirus
treatment (P<0.05 or P<0.01 vs. CIH group). However, no
significant differences were found between the CIH-Vector group and
CIH group. Thus, the results suggested that inflammation may play
an important role in CIH exposure-induced liver fibrosis.
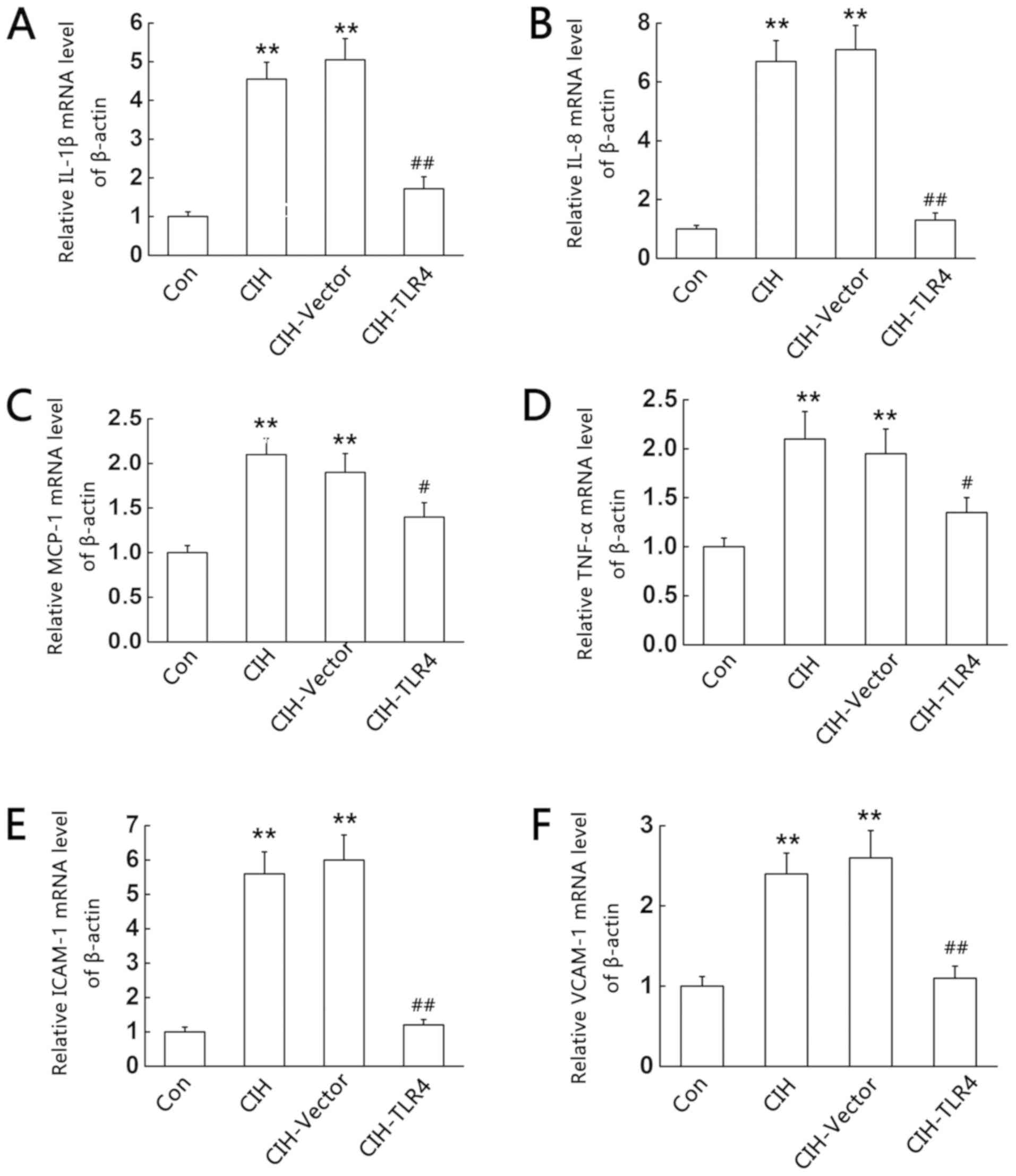 | Figure 2.CIH-induced liver mRNA expression
levels of IL-1β, IL-8, MCP-1, TNF-α, ICAM-1 and VCAM-1. After
exposing rats to CIH for 4 weeks, liver tissues were harvested and
subjected to reverse transcription-quantitative PCR. (A) IL-1β, (B)
IL-8, (C) MCP-1, (D) TNF-α, (E) ICAM-1 and (F) VCAM-1 expression
levels. **P<0.01 vs. control; #P<0.05,
##P<0.01 vs. CIH. Con, control; CIH, chronic
intermittent hypoxia; CIH-Vector group, CIH + empty vector
lentivirus; CIH-TLR4 group, CIH + TLR4 shRNA lentivirus; shRNA,
short hairpin RNA; TLR4, Toll-like receptor 4; IL, interleukin;
TNF, tumor necrosis factor; MCP-1, monocyte chemotactic-1; ICAM-1,
intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion
molecule-1. |
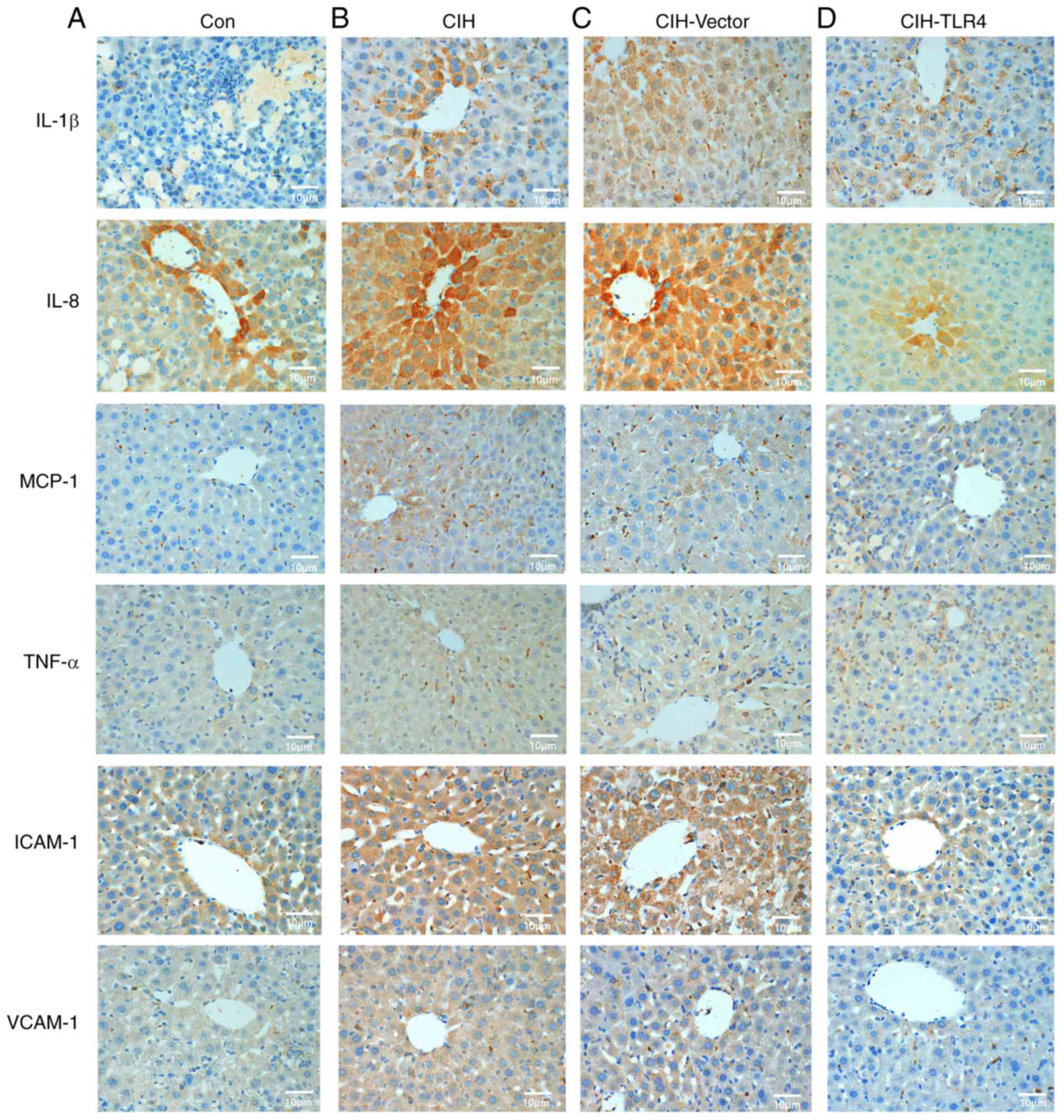 | Figure 3.CIH-induced liver inflammation. After
exposing rats to CIH for 4 weeks, liver tissues were harvested and
subjected to immunohistochemistry staining with antibodies of
IL-1β, IL-8, MCP-1, TNF-α, ICAM-1 and VCAM-1. (A) Con group, (B)
CIH group, (C) CIH-Vector group and (D) CIH-TLR4 group.
Representative images of immunohistochemistry assay. Magnification,
×400. Con, control; CIH, chronic intermittent hypoxia; CIH-Vector
group, CIH + empty vector lentivirus; CIH-TLR4 group, CIH + TLR4
shRNA lentivirus; shRNA, short hairpin RNA; TLR4, Toll-like
receptor 4; IL, interleukin; TNF, tumor necrosis factor; MCP-1,
monocyte chemotactic-1; ICAM-1, intercellular adhesion molecule-1;
VCAM-1, vascular cell adhesion molecule-1. |
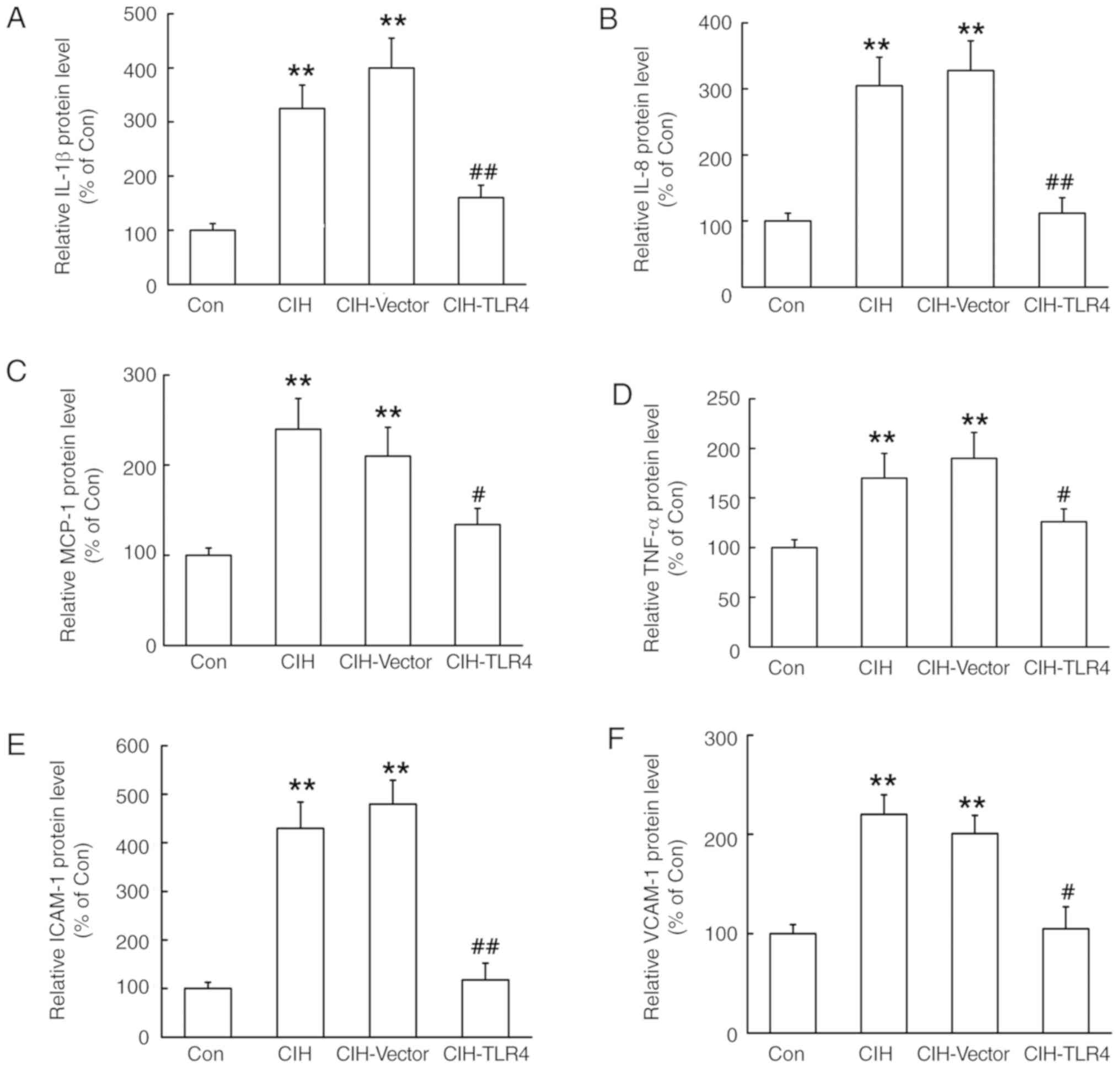 | Figure 4.Statistical results of
immunohistochemical images. After exposing rats to CIH for 4 weeks,
liver tissues were harvested and subjected to immunohistochemistry
staining with antibodies of IL-1β, IL-8, MCP-1, TNF-α, ICAM-1 and
VCAM-1. (A) IL-1β, (B) IL-8, (C) MCP-1, (D) TNF-α, (E) ICAM-1 and
(F) VCAM-1 expression levels. **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. CIH. Con,
control; CIH, chronic intermittent hypoxia; CIH-Vector group, CIH +
empty vector lentivirus; CIH-TLR4 group, CIH + TLR4 shRNA
lentivirus; shRNA, short hairpin RNA; TLR4, Toll-like receptor 4;
IL, interleukin; TNF, tumor necrosis factor; MCP-1, monocyte
chemotactic-1; ICAM-1, intercellular adhesion molecule-1; VCAM-1,
vascular cell adhesion molecule-1. |
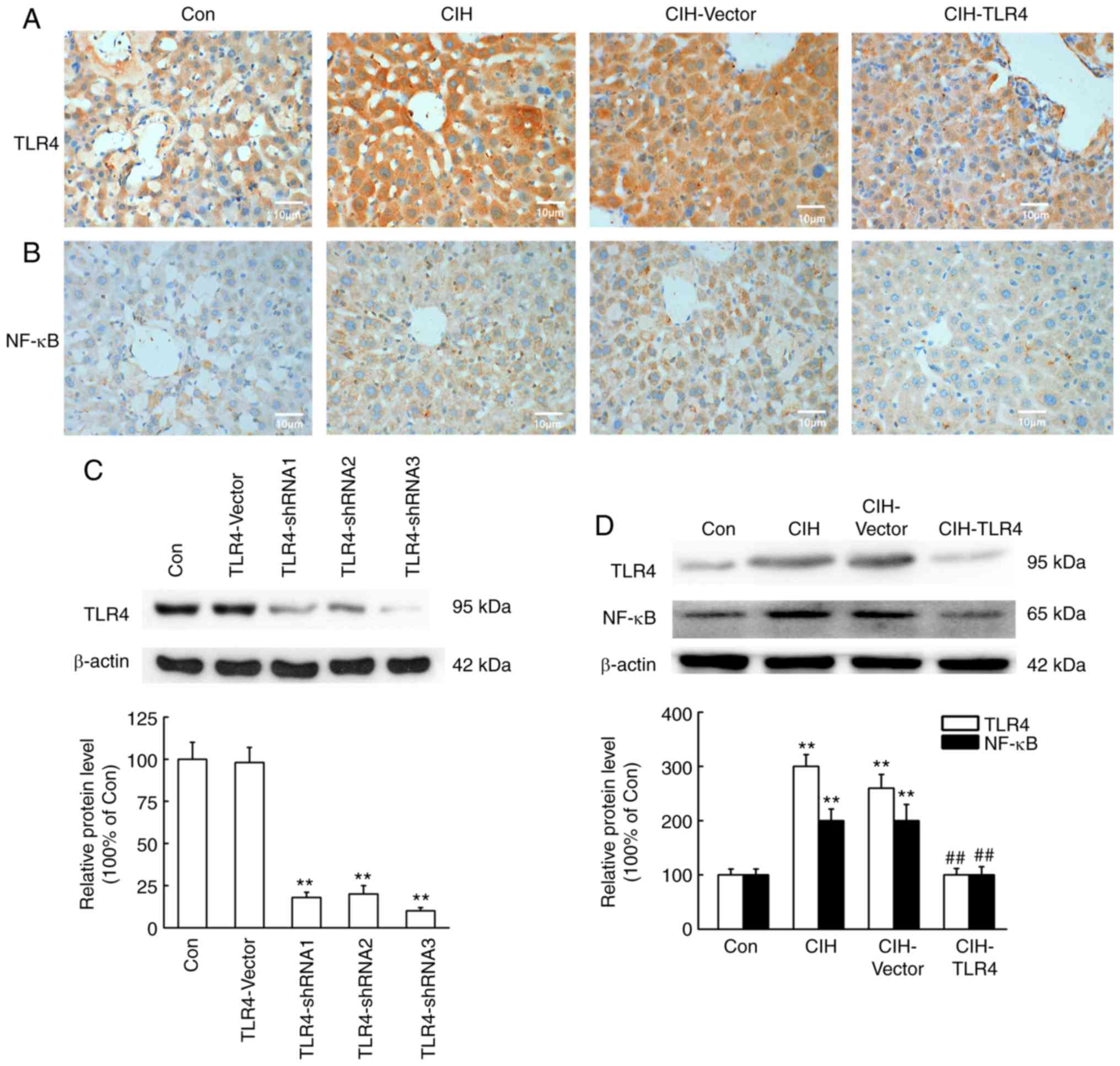
TLR4/NF-κB/MAPK signaling pathway is
involved in CIH-induced liver fibrosis
To determine whether the TLR4/NF-κB/MAPK signaling
pathway was involved in CIH-induced liver fibrosis, TLR4 and NF-κB
expression levels were measured by immunohistochemistry and western
blot analysis in rats. It was identified that TLR4 and NF-κB
expression levels were significantly increased in the CIH and
CIH-Vector groups (Fig. 5;
P<0.01 vs. Con group), while TLR4 shRNA lentivirus treatment
decreased the expression levels of these proteins (Fig. 5; P<0.01 vs. CIH group).
The induction of TLR4, NF-κB, IκB and p-MAPK-1 was
assessed by western blot analysis of HSCs. It was demonstrated that
the protein expression levels of TLR4, NF-κB, IκB and p-MAPK-1 in
the hypoxia/reoxygenation (HR) group were significantly higher
compared with the Con group (P<0.01; Fig. 6). Furthermore, these effects were
significantly reversed by application of U0126, a type of MAPK
inhibitor. Collectively, the results indicated the involvement of
the TLR4/NF-κB/MAPK signaling pathway in CIH-induced liver
fibrosis.
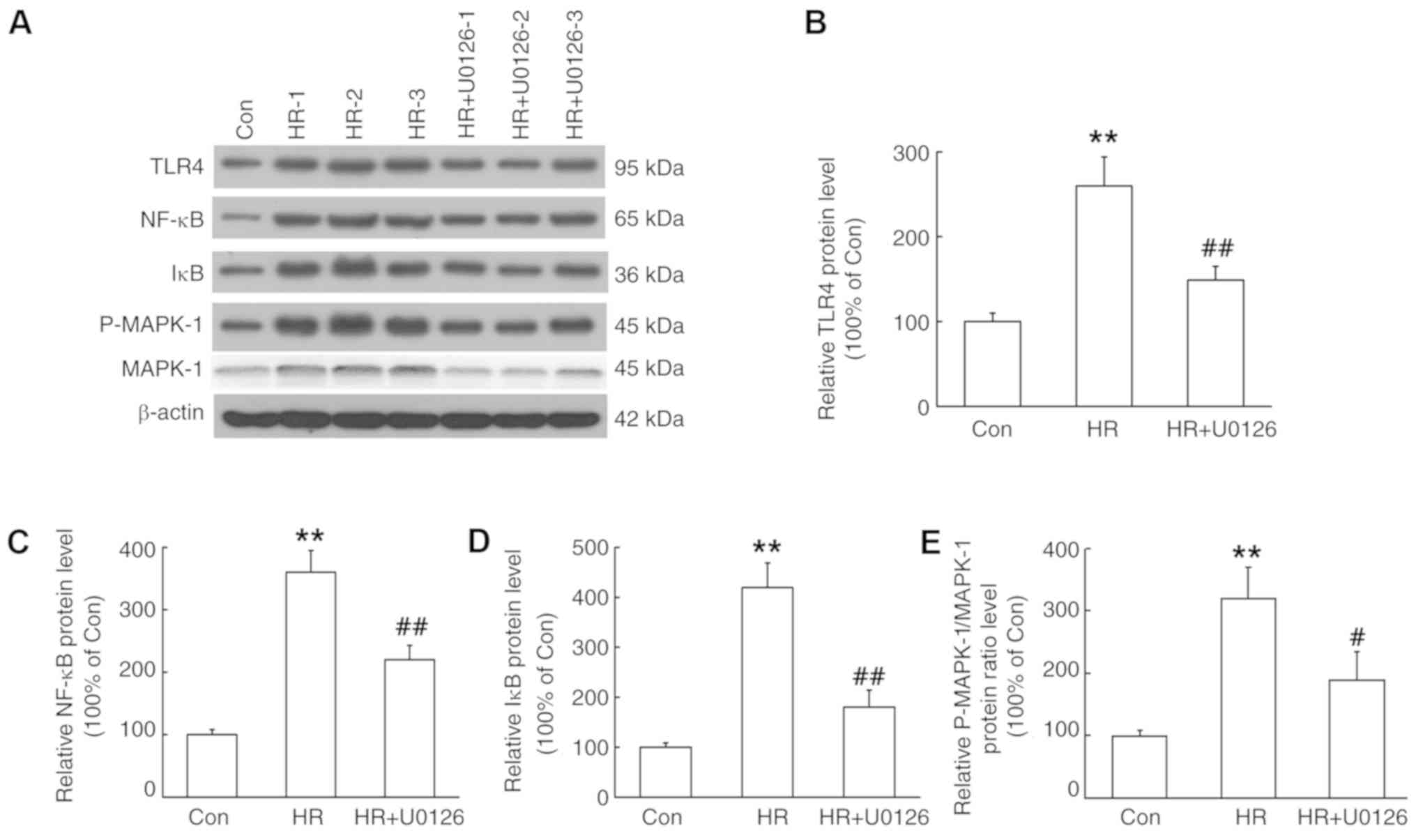 | Figure 6.Effects of HR and MAPK inhibitor
U0126 on the expression levels of TLR4, NF-κB, IκB and p-MAPK-1 in
HSCs. After treating hepatic stellate cells with or without MAPK
inhibitor U0126 (10 µM), cells were placed in a 94% N2,
5% CO2 and 1% O2 humidified culture incubator
for 6 h, followed by reoxygenation with 5% CO2 and 95%
air for 12 h. Protein expression was determined by western blot
analysis. (A) Representative protein bands. Protein expression
levels of (B) TLR4, (C) NF-κB, (D) IκB and (E) p-MAPK-1. Data are
presented as the mean ± SEM, n=6 in each group. **P<0.01 vs.
control; #P<0.05, ##P<0.01 vs. HR. Con,
control; p, phosphorylated; IκB, inhibitor of NF-κB; TLR4,
Toll-like receptor 4; MAPK, mitogen-activated protein kinase; HR,
hypoxia/reoxygenation. |
Discussion
Numerous animal models used in the study of hypoxia
have been developed over the past years, of which the most widely
used is the CIH model, which simulates the intermittent hypoxia
factor of OSAS (3,8). There are several different methods to
verify the successful establishment of the OSAS model, such as
monitoring the electroencephalogram, oronasal air flow, dynamic
blood oxygen for 2 h, mean blood oxygen saturation, minimum blood
oxygen saturation and sleep apnea index (6). In the present study, a CIH model was
established and used to study the underlying mechanisms of liver
fibrosis in OSAS. It was found that 4 weeks of CIH exposure induced
distinct hepatic fibrosis around the macrovesicular and
microvesicular structures. Moreover, it was demonstrated that
knockdown of TLR4 using TLR4 shRNA lentivirus resulted in
alleviated liver fibrosis. Immunohistochemical results also
identified that liver samples of the CIH and CIH-Vector groups
presented an inflammatory state with increased expression levels of
IL-1β, IL-8, MCP-1, TNF-α, ICAM-1 and VCAM-1, which could be
reversed by TLR4 shRNA lentivirus treatment. These results were
consistent with previous findings reporting that patients with OSAS
have increased systemic inflammation (2), which contributes to liver fibrosis
and may be alleviated by inhibiting TLR4 expression.
Previous studies have shown that OSAS is an
inflammatory state characterized by increases in the levels of
circulating biomarkers of inflammation (2,26).
While the inflammatory mechanism underlying OSAS has not been fully
elucidated, it has been observed that the increased inflammation is
partly mediated by HR (26,27).
Savransky et al (28)
revealed that CIH is a potent effective proinflammatory cytokine
that not only induces hyperglycemia and liver lipid peroxidation,
but also enhances the activity of NF-κB, which is the main
regulator of the inflammatory response. In addition, it has been
observed that patients with OSAS exhibit significantly increased
serum NF-κB activities (2).
Furthermore, serum levels of multiple NF-κB-dependent
proinflammatory cytokine and adhesion molecules, such as IL-1β,
IL-6, IL-8, TNF-α, MCP-1 and VCAM-1, are also elevated in patients
with OSAS (29,30). Moreover, Aron-Wisnewsky et
al (8) reported that CIH is
strongly associated with increased systemic inflammatory responses,
as well as with more serious fibrosis or inflammatory liver
injuries. The present results suggested that 4 weeks of CIH
exposure induced liver fibrosis in the CIH and CIH-Vector groups,
while the CIH-Vector group presented an inflammatory state with
increased expression of IL-1β, IL-8, MCP-1, TNF-α, ICAM-1 and
VCAM-1. NF-κB is considered to be an oxidant-sensitive
transcription factor, and activation of NF-κB promotes inflammation
and multiple tissue injury in response to CIH and in liver disease
conditions (31,32). Furthermore, the present results
indicated that 4 weeks of CIH exposure increased NF-κB and TLR4
expression levels in the liver. In addition, it was found that
protein expression levels of TLR4, NF-κB, IκB and p-MAPK-1 in the
HR group were significantly higher compared with the Con group, and
these effects could be reversed by application of the MAPK
inhibitor U0126. However, a limitation of the present study was
that p-NF-κB was not detected; p-NF-κB is the activated state of
NF-κB, thus it would be beneficial to detect the effects of CIH and
CIH-TLR4 on p-NF-κB.
CIH can induce an unbalanced production of large
amounts of reactive oxygen species (ROS) and endogenous antioxidant
defense mechanisms, which enhance oxidative stress (33,34).
ROS can also activate nuclear transcriptional factors, including
NF-κB and hypoxia-inducible factors (HIFs), which in turn promote
the production of inflammatory cytokines, such as IL-1β and IL-8,
as observed in a CIH model (35,36).
HIFs are the main transcriptional regulators of the hypoxia
response, and belong to a family of heterodimeric transcription
factors (37). Furthermore, in
almost all tissues and cells, HIFs act as the main regulator of the
body to maintain homeostasis in response to hypoxia (38). Activated HIFs can be rapidly
transferred to the nucleus, where they bind to hypoxia response
elements of the target gene promoter region to regulate gene
transcription (37,39). Previous studies have revealed that
activation of HIF1α plays a key role in downstream signaling
transduction of lipopolysaccharide (LPS) stimulation via the
pattern recognition receptor TLR4. For example, LPS can upregulate
HIF1α expression in rat liver, thus upregulating the expression of
aldolase, a type of HIF1α target gene (40,41).
In addition, it has been shown that the activity of LPS-induced
HIF1α mainly depends on NF-κB, the inflammatory master regulator of
a group of proteins, which is predominantly regulated by inhibiting
the transcriptional action of IκB (38,42).
Clinical and animal studies have reported that the
expression of TLR4 is closely related to activation of inflammatory
response in patients with OSAS (20,22).
Moreover, TLR4 is a typical pattern recognition receptor located
upstream of NF-κB, and is closely related to the activation of
inflammatory responses mainly via MyD88- and TIR-domain-containing
adapter-inducing interferon-β-dependent pathways (43). It has also been revealed that
activation of the TLR4/MyD88 signaling pathway in HR is positively
correlated with myocardial injury in animal models (44). Shimamoto et al (45) observed that myocardial ischemia
activates the TLR4/MyD88-dependent signal pathway and increases the
activation of NF-κB, which ultimately leads to the release of
innate cytokines in the heart. Furthermore, Takahashi et al
(46) showed that healthy mice and
TSK mouse fibrosis models exhibited notable fibrosis after
bleomycin treatment, while TLR4 knockout mice were protected from
fibrosis, thus indicating that TLR4 plays an important role in the
fiberization process. Moreover, non-functional TLR4 mutations or
TLR4 knockout can effectively protect mice against the development
of renal tissue dysfunction, inflammatory damage and fibrosis in a
model of chronic kidney injury (15,16).
It was found that similar injury also occurred in the rat liver in
the present study, and this condition could be significantly
alleviated by knocking down TLR4. Therefore, it was speculated that
TLR4 and NF-κB mediated CIH-induced inflammation and liver
fibrosis.
In conclusion, the preliminary results indicated
that CIH could induce liver fibrosis in rats, and this effect was
positively associated with inflammation and the TLR4/NF-κB/MAPK
signaling pathway. Furthermore, it was demonstrated that the
expression of TLR4 was associated with the pathogenesis of liver
fibrosis in CIH; thus, the development of a novel method that
inhibits TLR4 expression may be a viable strategy for clinical
prevention of liver fibrosis in patients with OSAS.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science
Foundation of Fujian Province (grant nos. 2018J0105, 2018Y0032 and
2016J01441) and Young and Middle-aged Backbone Key Research Project
of Fujian Province (grant no. 2016ZQN55).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZPL, XPY, YJZ and SYC conducted the experiments and
analyzed the data. HLL conceived and supervised the project,
contributed to the design of the experiments, discussed the data
and wrote the manuscript with contributions from ZPL, XPY, YJZ and
SYC. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Ethical
Committee of Experimental Animals of Fujian Medical University and
The Second Affiliated Hospital of Fujian Medical University. Animal
experiments were carried out in accordance with animal welfare
requirements and the Guidelines for the Care and Use of Laboratory
Animals published by the P.R. China Ministry of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Philip P, Bioulac S, Altena E, Morin CM,
Ghorayeb I, Coste O, Monteyrol PJ and Micoulaud-Franchi JA:
Specific insomnia symptoms and self-efficacy explain CPAP
compliance in a sample of OSAS patients. PLoS One. 13:e01953432018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paschetta E, Belci P, Alisi A, Liccardo D,
Cutrera R, Musso G and Nobili V: OSAS-related inflammatory
mechanisms of liver injury in nonalcoholic fatty liver disease.
Mediators Inflamm. 2015:8157212015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnold J, Sunilkumar M, Krishna V,
Yoganand SP, Kumar MS and Shanmugapriyan D: Obstructive sleep
apnea. J Pharm Bioallied Sci. 9 (Suppl 1):S26–S28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Halloran KD, Lewis P and McDonald F:
Sex, stress and sleep apnoea: Decreased susceptibility to upper
airway muscle dysfunction following intermittent hypoxia in
females. Respir Physiol Neurobiol. 245:76–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang NY, Ivanovska J, Tamir-Hostovsky L,
Belik J and Gauda EB: Chronic intermittent hypoxia in premature
infants: The link between low fat stores, adiponectin receptor
signaling and lung injury. Adv Exp Med Biol. 1071:151–157. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Q, Lin G, Huang J, Chen G, Huang X
and Lin Q: Expression profile of long non-coding RNAs in rat models
of OSA-induced cardiovascular disease: New insight into
pathogenesis. Sleep Breath. 23:795–804. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coelho NR, Dias CG, João Correia M, Grácio
P, Serpa J, Monteiro EC, Diogo LN and Pereira SA: Cysteine
oxidative dynamics underlies hypertension and kidney dysfunction
induced by chronic intermittent hypoxia. Adv Exp Med Biol.
1071:83–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aron-Wisnewsky J, Clement K and Pépin JL:
Nonalcoholic fatty liver disease and obstructive sleep apnea.
Metabolism. 65:1124–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sozer V, Kutnu M, Atahan E, Caliskaner
Ozturk B, Hysi E, Cabuk C, Musellim B, Simsek G and Uzun H: Changes
in inflammatory mediators as a result of intermittent hypoxia in
obstructive sleep apnea syndrome. Clin Respir J. 12:1615–1622.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu CX, Liu Y and Zhang JC: Chronic
intermittent hypoxia and hypertension: A review of systemic
inflammation and Chinese medicine. Chin J Integr Med. 19:394–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryan S and McNicholas WT: Inflammatory
cardiovascular risk markers in obstructive sleep apnoea syndrome.
Cardiovasc Hematol Agents Med Chem. 7:76–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drager LF, Tavoni TM, Silva VM, Santos RD,
Pedrosa RP, Bortolotto LA, Vinagre CG, Polotsky VY, Lorenzi-Filho G
and Maranhao RC: Obstructive sleep apnea and effects of continuous
positive airway pressure on triglyceride-rich lipoprotein
metabolism. J Lipid Res. 59:1027–1033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ropert C: How toll-like receptors reveal
monocyte plasticity: The cutting edge of antiinflammatory therapy.
Cell Mol Life Sci. 76:745–755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohan S and Gupta D: Crosstalk of
toll-like receptors signaling and Nrf2 pathway for regulation of
inflammation. Biomed Pharmacother. 108:1866–1878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chowdhury P, Sacks SH and Sheerin NS:
Toll-like receptors TLR2 and TLR4 initiate the innate immune
response of the renal tubular epithelium to bacterial products.
Clin Exp Immunol. 145:346–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
González-Guerrero C, Cannata-Ortiz P,
Guerri C, Egido J, Ortiz A and Ramos AM: TLR4-mediated inflammation
is a key pathogenic event leading to kidney damage and fibrosis in
cyclosporine nephrotoxicity. Arch Toxicol. 91:1925–1939. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukherjee S, Karmakar S and Babu SP: TLR2
and TLR4 mediated host immune responses in major infectious
diseases: A review. Braz J Infect Dis. 20:193–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang
C, Ge W, Wu J, Du P and Chen Y: Boosting mTOR-dependent autophagy
via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB
pathway quenches intestinal inflammation and oxidative stress
injury. EBioMedicine. 35:345–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu H and Li H: Prunetin inhibits
lipopolysaccharide-induced inflammatory cytokine production and
MUC5AC expression by inactivating the TLR4/MyD88 pathway in human
nasal epithelial cells. Biomed Pharmacother. 106:1469–1477. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan X, Deng Y, Guo X, Shang J, Zhu D and
Liu H: Atorvastatin attenuates myocardial remodeling induced by
chronic intermittent hypoxia in rats: Partly involvement of
TLR-4/MYD88 pathway. Biochem Biophys Res Commun. 446:292–297. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng Y, Yuan X, Guo XL, Zhu D, Pan YY and
Liu HG: Efficacy of atorvastatin on hippocampal neuronal damage
caused by chronic intermittent hypoxia: Involving TLR4 and its
downstream signaling pathway. Respir Physiol Neurobiol. 218:57–63.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Viciani E, Montagnani F, Tavarini S,
Tordini G, Maccari S, Morandi M, Faenzi E, Biagini C, Romano A,
Salerni L, et al: Paediatric obstructive sleep apnoea syndrome
(OSAS) is associated with tonsil colonisation by Streptococcus
pyogenes. Sci Rep. 6:206092016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Z, Shang H, Chen YQ, Pan LL, Bhatia M
and Sun J: Sulforaphane protects pancreatic acinar cell injury by
modulating Nrf2-Mediated oxidative stress and NLRP3 inflammatory
pathway. Oxid Med Cell Longev. 2016:78641502016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng X, Guo R, Dong M, Zheng J, Lin H and
Lu H: Contribution of TLR4 signaling in intermittent
hypoxia-mediated atherosclerosis progression. J Transl Med.
16:1062018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryan S, Taylor CT and McNicholas WT:
Selective activation of inflammatory pathways by intermittent
hypoxia in obstructive sleep apnea syndrome. Circulation.
112:2660–2667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye J, Liu H, Li Y, Liu X and Zhu JM:
Increased serum levels of C-reactive protein and matrix
metalloproteinase-9 in obstructive sleep apnea syndrome. Chin Med J
(Engl). 120:1482–1486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Savransky V, Bevans S, Nanayakkara A, Li
J, Smith PL, Torbenson MS and Polotsky VY: Chronic intermittent
hypoxia causes hepatitis in a mouse model of diet-induced fatty
liver. Am J Physiol Gastrointest Liver Physiol. 293:G871–G877.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Lima FF, Mazzotti DR, Tufik S and
Bittencourt L: The role inflammatory response genes in obstructive
sleep apnea syndrome: A review. Sleep Breath. 20:331–338. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamaki S, Yamauchi M, Fukuoka A, Makinodan
K, Koyama N, Tomoda K, Yoshikawa M and Kimura H: Production of
inflammatory mediators by monocytes in patients with obstructive
sleep apnea syndrome. Intern Med. 48:1255–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Wang Y, Xia T, Liu Y, Liu T, Shi X
and Li Y: Pathogenesis of abnormal hepatic lipid metabolism induced
by chronic intermittent hypoxia in rats and the therapeutic effect
of N-acetylcysteine. Med Sci Monit. 24:4583–4591. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song D, Fang G, Mao SZ, Ye X, Liu G, Gong
Y and Liu SF: Chronic intermittent hypoxia induces atherosclerosis
by NF-κB-dependent mechanisms. Biochim Biophys Acta.
1822:1650–1659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morgan BJ, Bates ML, Rio RD, Wang Z and
Dopp JM: Oxidative stress augments chemoreflex sensitivity in rats
exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol.
234:47–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lüneburg N, Siques P, Brito J, Arriaza K,
Pena E, Klose H, Leon-Velarde F and Böger RH: Long-Term chronic
intermittent hypobaric hypoxia in rats causes an imbalance in the
asymmetric dimethylarginine/nitric oxide pathway and ROS activity:
A possible synergistic mechanism for altitude pulmonary
hypertension? Pulm Med. 2016:65785782016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Anazi A, Parhar R, Saleh S, Al-Hijailan
R, Inglis A, Al-Jufan M, Bazzi M, Hashmi S, Conca W, Collison K and
Al-Mohanna F: Intracellular calcium and NF-κB regulate
hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in
human adipocytes. Life Sci. 212:275–284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang X, Chen GT, Wang YQ, Xian S, Zhang L,
Zhu SM, Pan F and Cheng YX: TLR4 promotes the expression of HIF-1α
by triggering reactive oxygen species in cervical cancer cells in
vitro-implications for therapeutic intervention. Mol Med Rep.
17:2229–2238. 2018.PubMed/NCBI
|
|
37
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ullah K, Rosendahl AH, Izzi V, Bergmann U,
Pihlajaniemi T, Mäki JM and Myllyharju J: Hypoxia-inducible factor
prolyl-4-hydroxylase-1 is a convergent point in the reciprocal
negative regulation of NF-κB and p53 signaling pathways. Sci Rep.
7:172202017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu D, He C, Wei J, Zhang Z, Luo Y, Tan H
and Ren C: PGK1 is a potential survival biomarker and invasion
promoter by regulating the HIF-1α-mediated epithelial-mesenchymal
transition process in breast cancer. Cell Physiol Biochem.
51:2434–2444. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shirasuna K, Shimamura N, Seno K, Ohtsu A,
Shiratsuki S, Ohkuchi A, Suzuki H, Matsubara S, Nagayama S, Iwata H
and Kuwayama T: Moderate hypoxia down-regulates interleukin-6
secretion and TLR4 expression in human Sw.71 placental cells. Cell
Physiol Biochem. 36:2149–2160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Menon D, Coll R, O'Neill LA and Board PG:
GSTO1-1 modulates metabolism in macrophages activated through the
LPS and TLR4 pathway. J Cell Sci. 128:1982–1990. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siegert I, Schodel J, Nairz M, Schatz V,
Dettmer K, Dick C, Kalucka J, Franke K, Ehrenschwender M, Schley G,
et al: Ferritin-mediated iron sequestration stabilizes
hypoxia-inducible factor-1α upon LPS activation in the presence of
ample oxygen. Cell Rep. 13:2048–2055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Funami K, Matsumoto M, Oshiumi H, Inagaki
F and Seya T: Functional interfaces between TICAM-2/TRAM and
TICAM-1/TRIF in TLR4 signaling. Biochem Soc Trans. 45:929–935.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu
C and Duan J: The protective effect of luteolin on myocardial
ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3
inflammasome pathway. Biomed Pharmacother. 91:1042–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shimamoto A, Chong AJ, Yada M, Shomura S,
Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring
DJ, et al: Inhibition of Toll-like receptor 4 with eritoran
attenuates myocardial ischemia-reperfusion injury. Circulation. 114
(1 Suppl):I270–I274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takahashi T, Asano Y, Ichimura Y, Toyama
T, Taniguchi T, Noda S, Akamata K, Tada Y, Sugaya M, Kadono T and
Sato S: Amelioration of tissue fibrosis by toll-like receptor 4
knockout in murine models of systemic sclerosis. Arthritis
Rheumatol. 67:254–265. 2015. View Article : Google Scholar : PubMed/NCBI
|