Introduction
The human oral cavity is estimated to harbor nearly
700 bacterial species (1). Some
oral bacteria form biofilms called dental plaque on tooth surfaces.
Dental plaque accumulation is responsible for dental caries or
periodontal diseases. Oral biofilm formation is initiated by early
colonizing bacteria such as Streptococcus and
Actinomyces that adhere to the pellicle layer on tooth
surfaces (2).
Fusobacterium nucleatum (FN) is a
Gram-negative oral anaerobe that plays an important role in
subgingival plaque maturation by bridging early and late colonizers
(2,3). FN colonizes the oral cavity of
patients with periodontal diseases but is also found in healthy
subjects (4). Since the number of
FN increases as periodontal disease progresses, this anaerobe is
recognized as a periodontal pathogen (4). Inflammation evoked by
lipopolysaccharide of FN is reported to result in chronic
periodontitis and alveolar bone absorption (5–7). In
addition, FN produces methyl mercaptan and hydrogen sulfate that
are responsible for oral malodor (8,9). The
FadA adhesion protein of FN was recently shown to activate
Wnt/β-catenin signaling by binding to E-cadherin, which induces
colon epithelial proliferation and colon cancer (10). Thus, FN has attracted attention as
an etiologic agent not only for periodontal diseases but also for
other systemic diseases (11).
Chronic inflammation due to periodontitis is
implicated in various systemic conditions such as those associated
with diabetes mellitus, cardiovascular diseases, pulmonary
diseases, obesity, low-weight birth and preterm birth (12). Oral hygiene is an effective
prophylactic measure for various systemic diseases. Accordingly,
antimicrobial chemicals such as cetylpyridinium chloride (CPC),
chlorhexidine gluconate and isopropyl methylphenol are used as
additives in commercially available oral hygiene care products such
as toothpaste and mouth rinse solution. However, some of these
chemicals are associated with cytotoxicity or side effects
(13,14).
Natural products such as herbal plant extracts can
be used as alternative oral care reagents that have fewer adverse
effects (15). Essential oil from
Matricaria chamomilla is known to possess antimicrobial
activity and oral rinses containing this oil reduced oropharynx
colonization by Staphylococcus aureus and Streptococcus
pneumoniae in intensive care unit patients (16). Another study reported that the
medicinal plant, Baccharis dracunculifolia, reduced dental
plaque in healthy individuals (17), and a combination of herbal
essential oils promoted chlorhexidine gluconate-mediated inhibition
of biofilm formation by Streptococcus mutans or
Lactobacillus plantarum (18).
Nigella sativa L. (Ranunculaceae),
commonly known as black cumin (BC), is an annual flowering plant
native to South and Southeast Asia. N. sativa has a long
history as a medicinal plant for treatment of various ailments such
as metabolic syndrome as well as gastrointestinal, neuronal,
respiratory, urinary, reproductive disorders (19). BC seeds have been shown to possess
anti-diabetic, anti-cancer, anti-inflammatory, immunomodulatory,
antioxidant, antimicrobial, analgesic, spasmolytic, bronchodilatory
and hepatoprotective properties (19,20).
Most biological activities of BC are related to its essential oil
components. Thymoquinone (TQ) is a major (30-48%) component of BC
essential oil and contributes to its therapeutic effects (19,21).
In a rat model of periodontitis, Ozdemir et al (22) showed that gastric feeding of TQ (10
mg/kg, daily for 11 days) significantly reduced gingival
inflammation and alveolar bone loss. Based on these findings, TQ is
expected to have anti-bacterial and anti-inflammatory properties
that could prevent periodontal diseases. However, the effect of TQ
on FN-associated biofilms remains to be elucidated.
In this study, we evaluated the inhibitory effect of
TQ on FN-associated biofilm formation and discuss the therapeutic
potential of TQ for periodontal disease.
Materials and methods
Bacterial stains and culture
conditions
The bacterial strains used in this study were
Fusobacterium nucleatum ATCC25586 (FN), Actinomyces
naeslundii X600 (AN) and Streptococcus mitis ATCC 903
(SM). These bacterial strains were cultured anaerobically in BHIS
broth using the AnaeroPack System (Mitsubishi Gas Chemical Company,
Inc.). BHIS broth is Brain Heart Infusion medium (Eiken Chemical
Co.) supplemented with 5 µg/ml hemin (Sigma-Aldrich), 0.1%
L-cysteine hydrochloride monohydrate (Sigma-Aldrich), 0.5% yeast
extract (Becton Dickinson and Company) and 0.375% D-glucose
(Nacalai Tesque).
Preparation of biofilm containing
FN
Frozen stocks (−80°C) of FN, AN or SM were streaked
onto BHIS agar plates and incubated anaerobically for 48–72 h.
Single colonies of each strain were used to inoculate 3 ml of BHIS
broth that was then incubated at 37°C for 24 h in an anaerobic
chamber conditioned with 80% N2, 10% CO2, 10%
H2. After the incubation, the optical density of each
culture at 590 nm (OD590) was adjusted to 0.1 with BHIS
broth. Subsequently, FN culture was mixed with equal amounts of
cultured AN and/or SM, and 1 ml of the culture mixture was
dispensed into 24-well polystyrene tissue culture plates.
Gram-staining was performed with the culture used for biofilm
preparation to exclude contamination by other bacteria. Biofilms
formed by 4% paraformaldehyde (PFA)-fixed FN and/or AN were also
examined. For fixation, FN and/or AN cells collected from overnight
culture were suspended in 4% PFA in phosphate-buffered saline (PBS,
pH 7.4) and incubated 30 min at room temperature. After the fixed
cells were washed with PBS three times, the cells were resuspended
in BHIS to 0.1 OD590 and transferred to multi-titer
wells. Dual species biofilms was prepared on the bottom of wells in
24-well plates following anaerobic incubation at 37°C for 24 h. To
prepare biofilms containing FN alone, 1 ml of FN culture
(OD590 adjusted to 0.1) was applied to the wells and
incubated as described above. Scanning electron microscopy was used
to assess the prevalence of FN in biofilms. To prepare the
biofilms, sterilized plastic discs (Sensi-Disc, 13.5 mm, Sumitomo
Bakelite, Co., Ltd.) were inserted into wells before applying
bacterial mixtures. Biofilms that formed on the discs were fixed
with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2). After
fixation, the biofilms were dehydrated in a graded ethanol series
and dried in a Hitachi PCP-2 critical point drying apparatus. The
discs were coated with platinum/palladium in a Hitachi E-102
sputter coater and examined with a JEOL JCM-6000 scanning electron
microscope.
Chemicals
Test reagents used in this study were thymol (TM,
>98%), TQ (>99%), isopropyl methylphenol (IPMP, >99%) and
CPC (>99%). TQ, IPMP and CPC were purchased from Sigma-Aldrich
Co.). TM was obtained from Kanto Chemical Co., Inc. BC was prepared
from 500 g of BC seed as described previously (23), and 3.6 g of the oil was obtained
(recovery rate, 0.72%).
Inhibitory effect of TM or TQ on
FN-containing biofilm formation
Equal volumes of FN and AN cultures adjusted to
OD590 0.1 were mixed and dispensed into 24-well
polystyrene tissue culture plates. Before incubation, TM or TQ was
added to the culture at a final concentration of 0.1%. After
anaerobic incubation at 37°C for 24 h, the amount of biofilm mass
formed on the bottom of the wells was assessed using crystal violet
(Wako Pure Chemical) staining. After removing the test media, the
biofilms were gently washed three times with 1 ml saline and then
stained with 0.4 ml crystal violet solution (0.01%) at room
temperature for 1 h. Excess crystal violet solution was removed and
the stained biofilm was washed with 1 ml saline before eluting the
remaining dye with 1 ml acetic acid (33%, Wako Pure Chemical) and
gentle agitation for 30 min at room temperature. The eluent was
transferred to a 96-well plate for measurement at OD550
or OD600 to compare the biofilm mass.
Effect of TQ pretreatment on FN/AN
biofilm formation
After anaerobic culture of FN and AN in BHIS at 37°C
overnight, the cells were collected by centrifugation (6,000 g, 5
min, 4 C), suspended in PBS (pH 7.4) containing 0.05% TQ and
incubated for 30 min at room temperature in the dark. The cells
were again collected by centrifugation and washed three times with
PBS before resuspension in BHIS to OD590 to 0.1. FN/AN
biofilms were prepared using prefixed and unfixed cell
combinations. Biofilm mass was compared by crystal violet staining
as described above.
Cleansing effect on FN-containing
biofilms
Mixed FN and AN biofilms were washed by gentle
agitation with 0.5 ml 0.01 or 0.05% BC, TM, TQ, IPMP or CPC at 37°C
for 30 min. After removing the test reagents, crystal violet
staining was performed to compare the biofilm mass that remained
after treatment as described above. Confocal laser scanning
microscopy (CLSM) was also used to evaluate biofilm thickness and
bacterial viability after treatment. The remaining biofilm was
stained using a LIVE/DEAD® Biofilm Viability kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Stained biofilms were observed by CLSM (model LSM700;
Carl Zeiss) at 400× magnification and a 488 nm emission wavelength
(15% laser power). Signal/noise ratio, digital offset and digital
gain settings were optimized using untreated biofilm and biofilm
treated with 70% ethanol. The thickness of the remaining biofilm
was calculated using ZEN imaging software (ZEN 2012). The live/dead
ratio of bacteria in the biofilm was estimated from output data for
the arithmetic average luminance using ZEN imaging software.
Anti-inflammatory effect of TM and
TQ
The human monocytic leukemia cell line THP-1
(JCRB0112.1) was cultured in RPMI1640 medium (Wako Pure Chemical)
containing 10% fetal bovine serum, 100 U/ml penicillin and 100
µg/ml streptomycin (Pen Strep; Gibco; Thermo Fisher Scientific,
Inc.) at 37°C and 5% CO2. After seeding in 24-well
plates at an initial cell density of 5×105 cells/well,
the THP-1 cells were treated with a 5–50 µM TM or TQ for 1 h at
37°C under 5% CO2. Subsequently, FN suspension
(OD590=1.0) in PBS was added to the media at 10% (v/v)
and incubated for 3 h. The culture supernatants were collected by
centrifugation at 600 × g for 2 min at 4°C. TNF-α secreted from
THP-1 cells after stimulation by FN was quantified with a Human
TNF-α ELISA Ready Set-Go® assay (Thermo Fisher
Scientific, Inc.).
Toxicity of TM and TQ toward THP-1
cells
THP-1 cells were treated with TM and TQ as described
above. Cell viability was assessed by adding 8 µg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Dojindo Laboratories) to the media. Zinc dibutyldithiocarbamate
(ZDBC, 1 µg/ml, Wako Pure Chemical Corporation) was used as a
positive control.
Statistical analysis
Data are expressed as mean ± standard deviation.
Statistical analyses were performed with StatFlex ver. 6.0 (Artech
Co., Ltd.) wherein analysis of variance (ANOVA) was used to compare
the means of all groups, followed by Tukey's test (for all
combinations) or Dunnett's test (for comparison to control).
Statistically significant differences of the live/dead cell ratio
in biofilms were assessed by Chi-square test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Preparation of FN-containing
biofilm
To examine the effect of BC and its components on
FN-containing biofilm, we first prepared single species biofilms
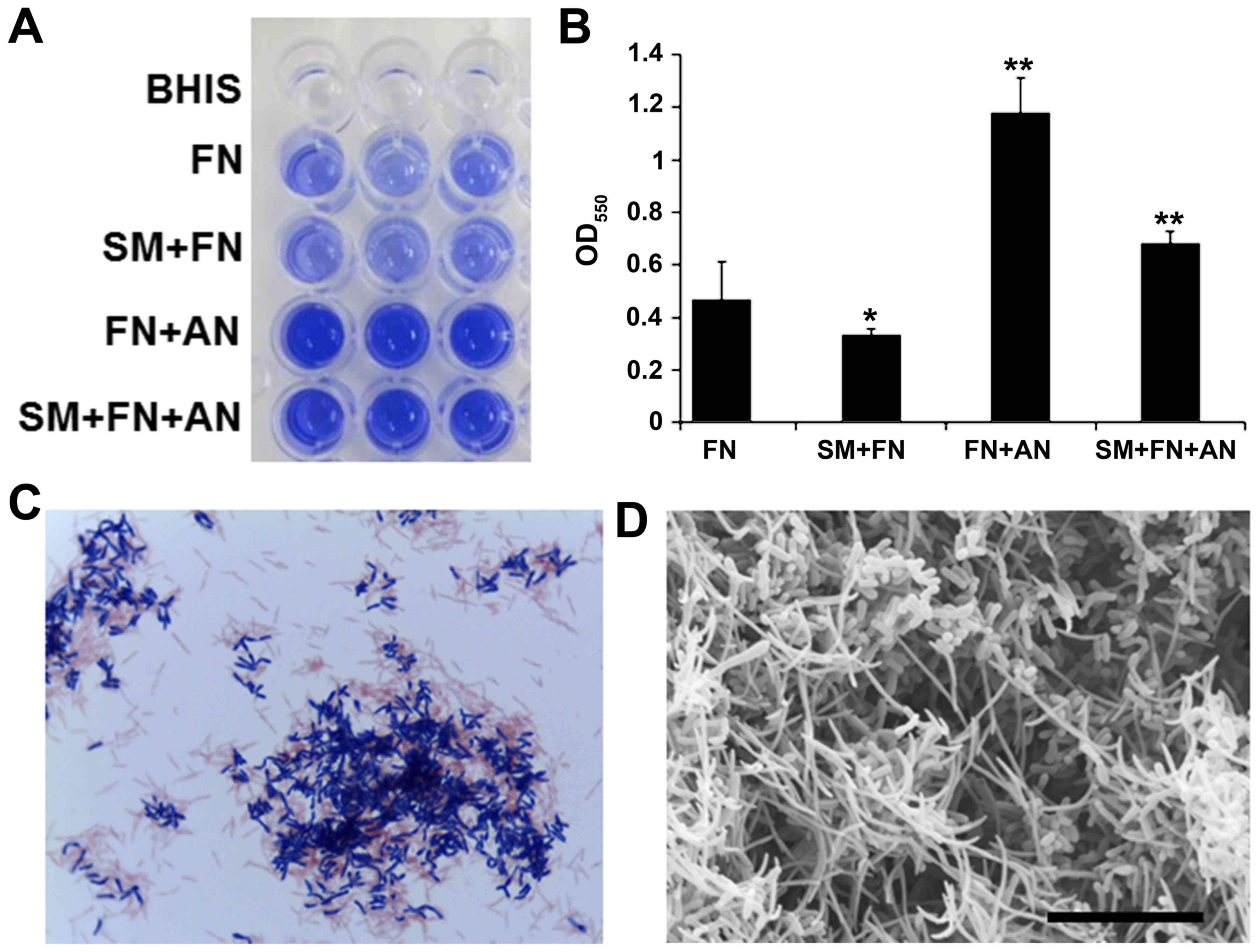
containing only FN in microtiter wells. Although FN grew well, it
formed thin and fragile biofilms (Fig.
1A). To increase the biofilm stability, FN was co-cultivated
with early colonizers in dental plaque, SM and/or AN.
Co-cultivation of FN with AN resulted in thick and firm biofilms
whereas SM and FN co-cultivation inhibited biofilm formation as
revealed by crystal violet staining (Fig. 1A and B). Gram-staining of the dual
species biofilm showed co-agglutination of FN (Gram-negative rods)
and AN (Gram-positive rods) (Fig.
1C). Scanning electron microscopy also showed that this dual
species biofilm contained substantial amounts of needle-shaped FN
cells (Fig. 1D). These dual
species FN/AN biofilms were used in subsequent experiments.
Inhibitory effect of BC components on
FN-containing biofilm
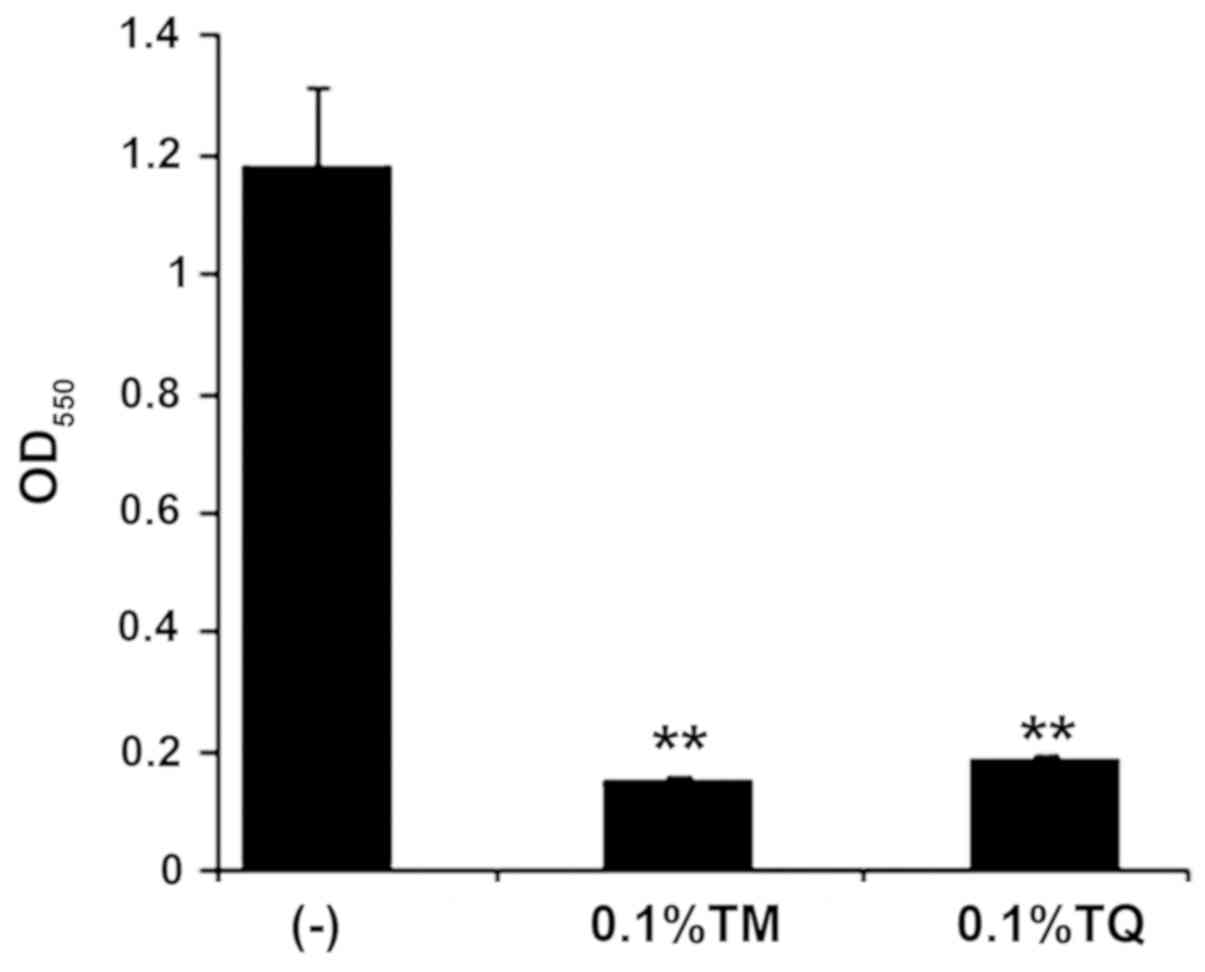
We examined whether the BC components TM and TQ
inhibited FN-associated biofilm formation. Upon addition of either
0.1% TM or TQ, dual species biofilm formation of FN and AN was
significantly inhibited as indicated by crystal violet staining
that showed decreased biofilm mass (Fig. 2). This effect was likely due to
growth inhibition since FN and AN co-cultures were only slightly
turbid after anaerobic incubation for 24 h in the presence of 0.1%
TM or TQ (data not shown).
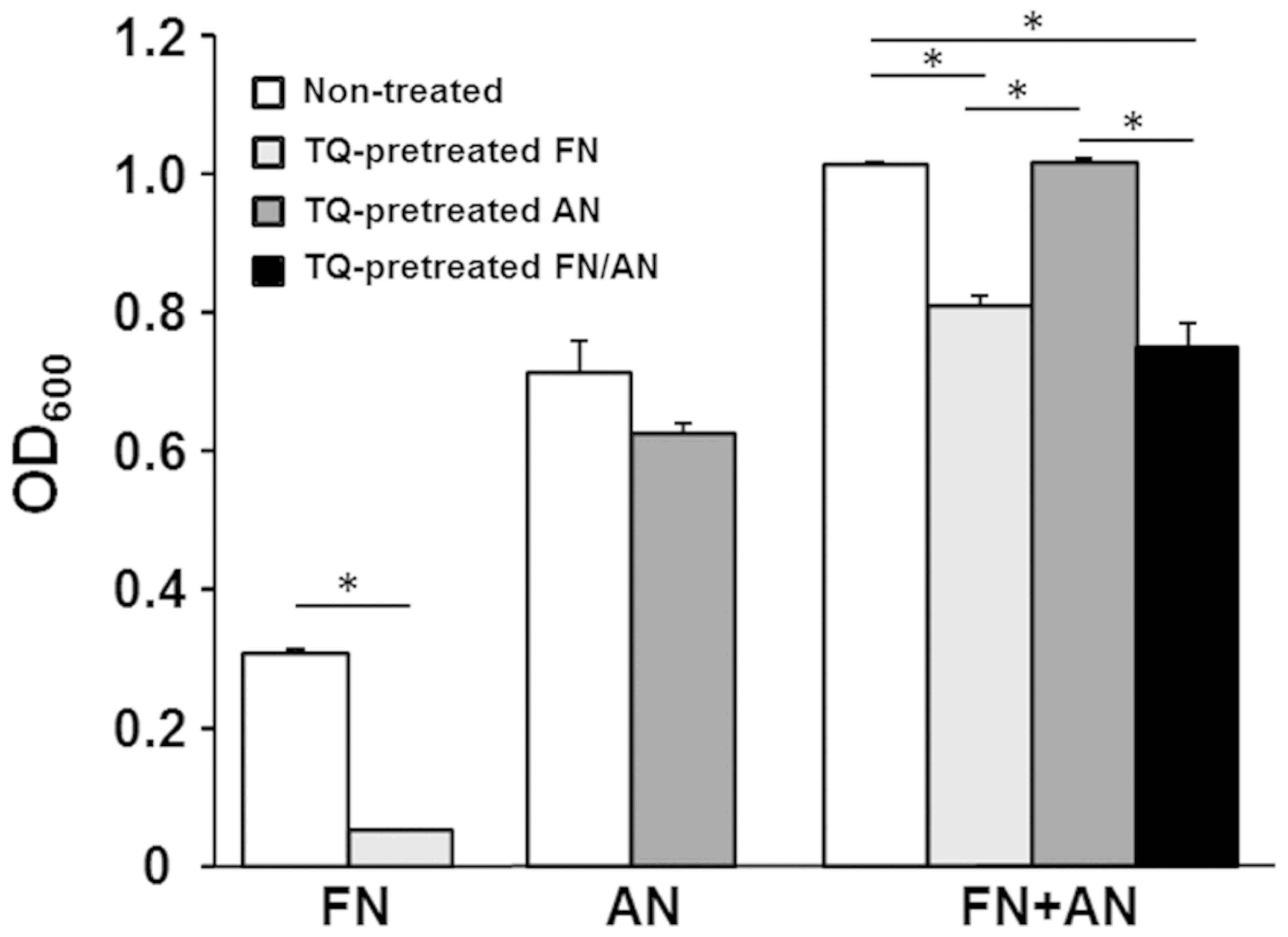
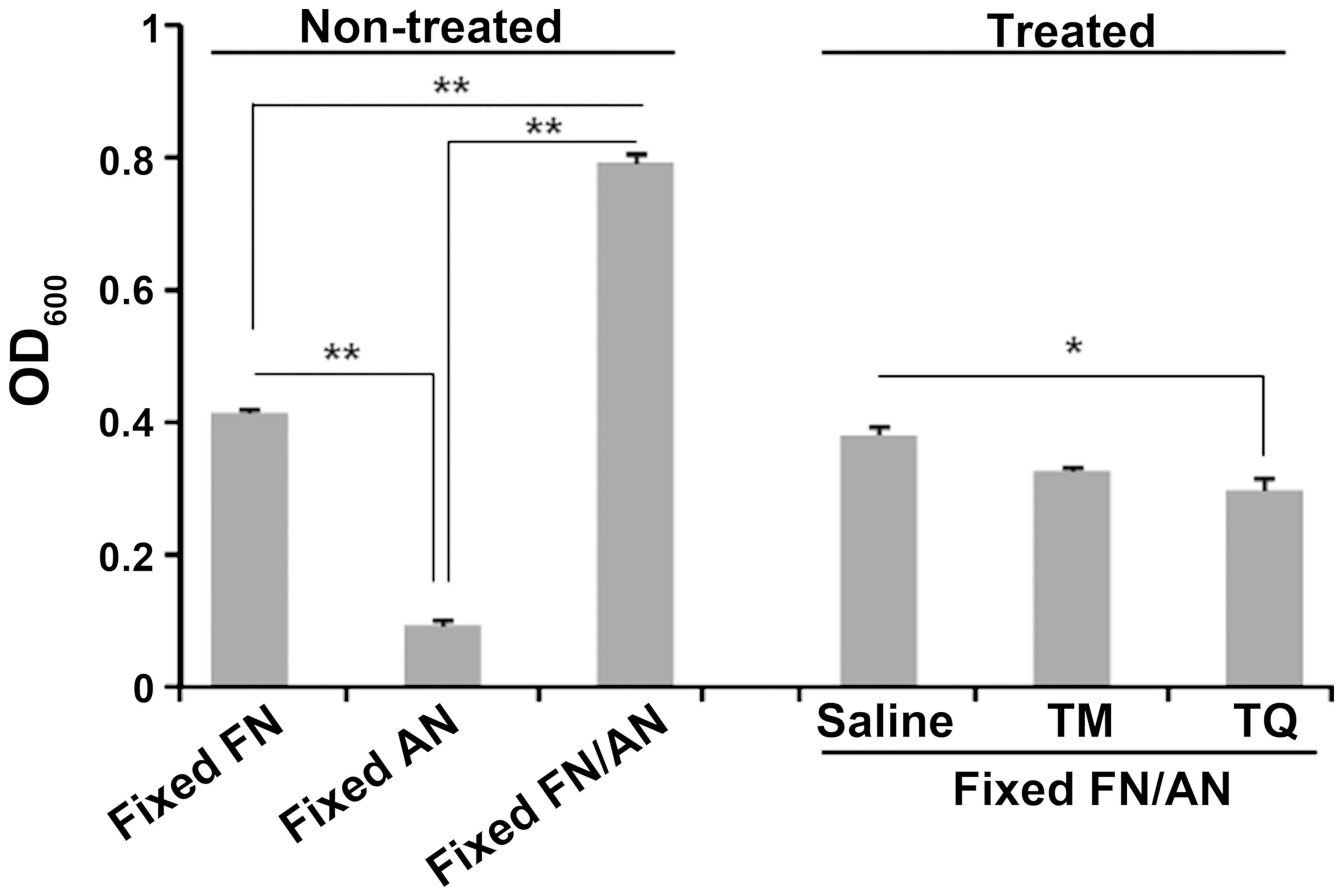
Effect of TQ pretreatment on FN/AN
biofilm formation
Biofilms were prepared with untreated or TQ-treated
FN and AN (Fig. 3). FN pretreated
with 0.01% TQ showed significantly decreased biofilm formation
whereas AN pretreated with TQ formed biofilms with efficiencies
similar to those seen for untreated AN. For dual species biofilms,
biofilm mass was significantly reduced when FN was pretreated with
TQ. These results indicate that FN is more sensitive to TQ than
AN.
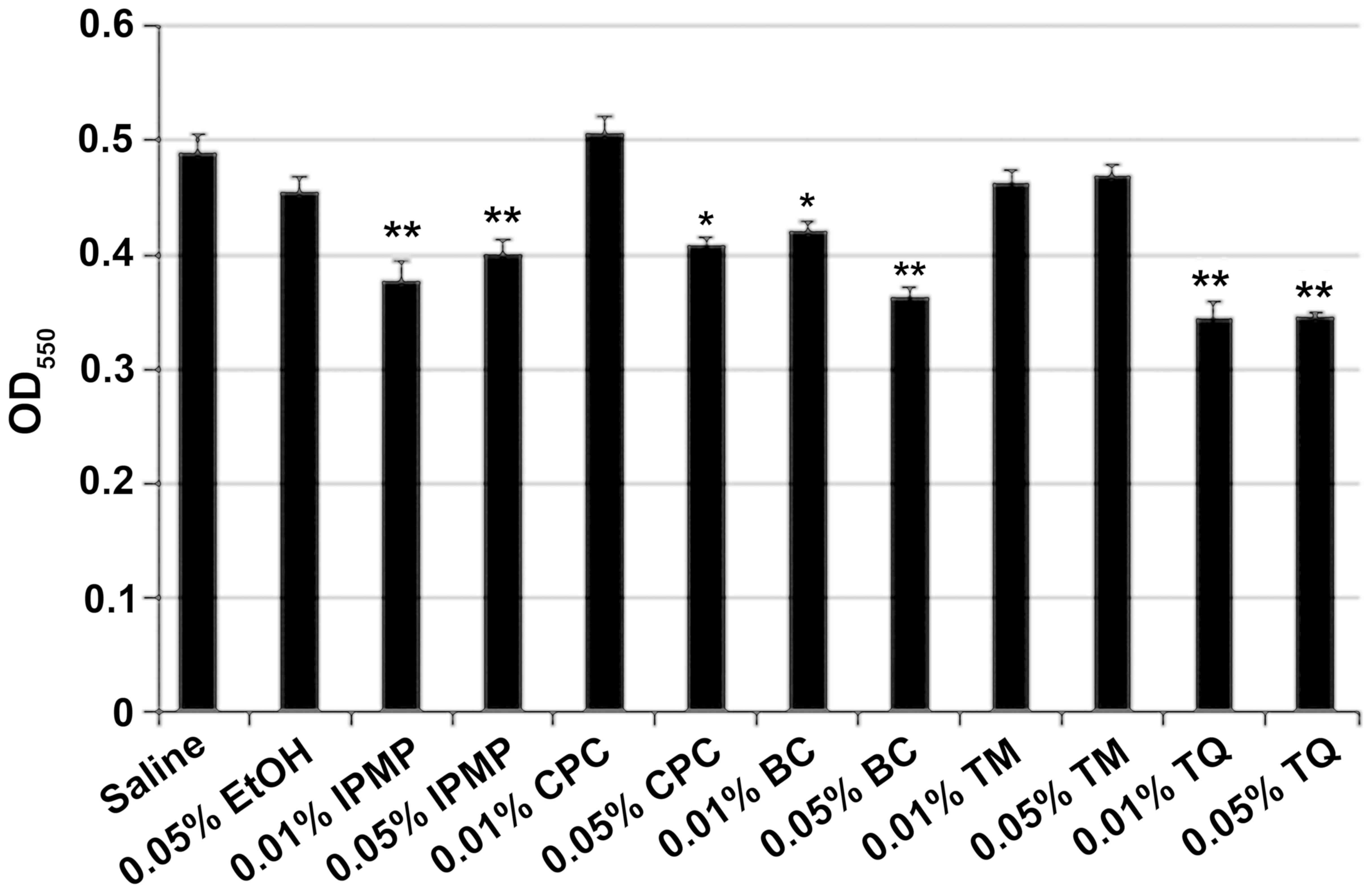
Cleansing effect of BC components on
FN-containing biofilm
After co-cultivation of FN and AN, the dual species
biofilms were washed with 0.01% or 0.05% BC, TM, TQ, IPMP or CPC.
Ethyl alcohol (0.05%) was also tested as an antimicrobial agent.
IPMP, BC, and TQ all significantly reduced biofilm mass (Fig. 4). CLSM analysis was also conducted
to examine the biofilm structure after treatment with the test
reagents (Fig. 5). Nearly 80% of
bacteria were viable in untreated biofilms, but upon treatment with
TQ, IPMP and CPC the percentage of dead cells in the biofilms
significantly increased (Fig. 5A and
B). Among these agents, CPC had the highest bactericidal
activity with over 70% of bacteria inactivated in the biofilm. All
of the test reagents reduced the biofilm thickness (Fig. 5C). Of these, TQ and IPMP were the
most effective for dislodging biofilms and decreased the average
biofilm thickness to nearly half that achieved with saline.
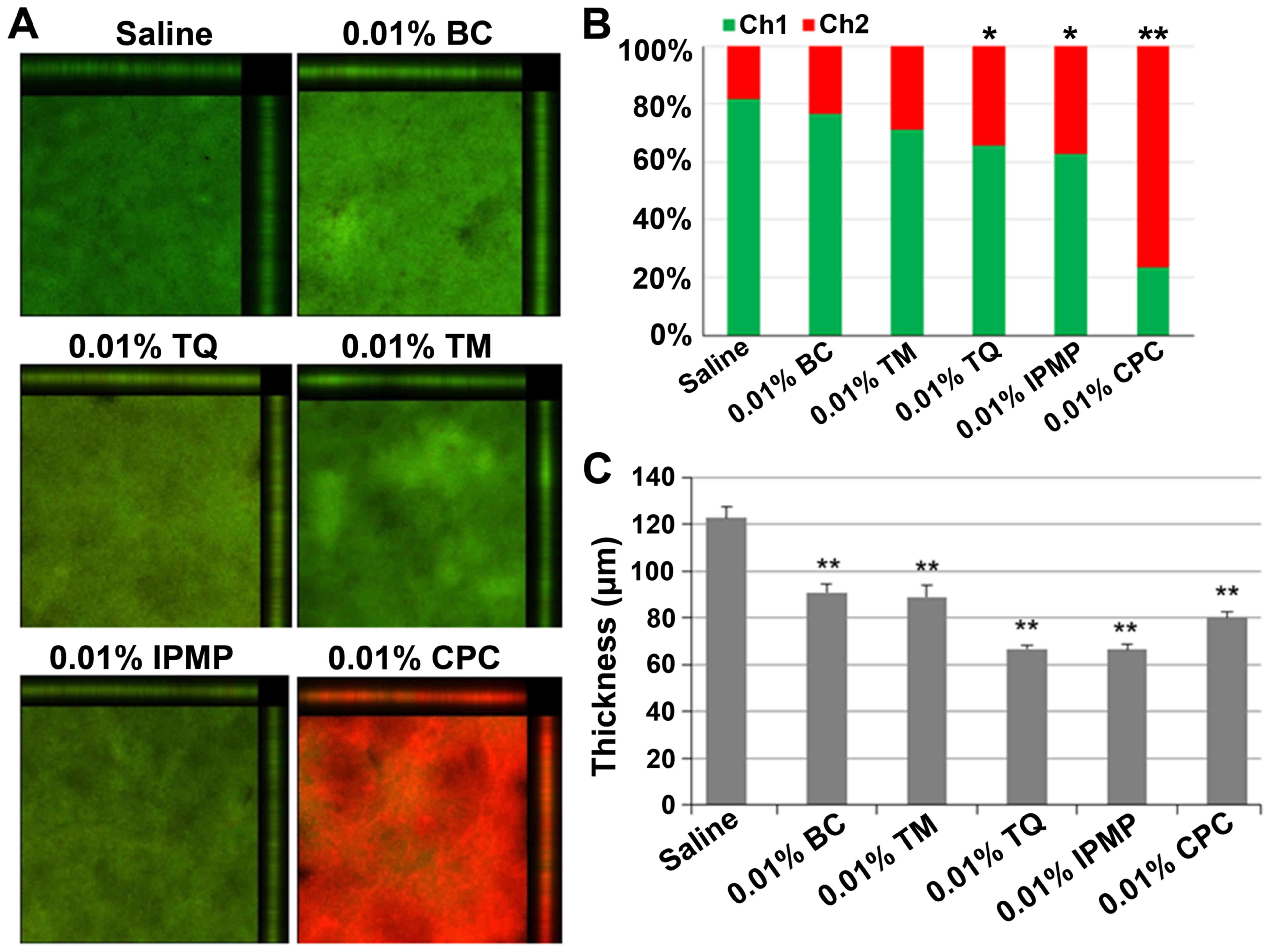 | Figure 5.Confocal laser scanning microscopy
analysis of dual species biofilms of FN and AN after treatment with
indicated test reagents. (A) Merged images of biofilms stained with
SYTO-9 (green) and propidium iodide (PI, red), which indicate live
and dead cells, respectively. (B) Arithmetic average luminance of
SYTO-9 (Ch1) and PI (Ch2) in the tested biofilm. Difference was
analyzed by Chi-squared test. *P<0.05 or **P<0.01 vs. Saline.
(C) Average thickness of biofilm retained after treatment. Data are
expressed as mean ± standard deviation from three repeats.
Differences were analyzed by ANOVA followed by Dunnett's test.
**P<0.01 vs. Saline. FN, Fusobacterium nucleatum; AN,
Actinomyces naeslundii; TM, thymol; TQ, thymoquinone; IPMP,
isopropyl methylphenol; BC, black cumin; CPC, cetylpyridinium
chloride. |
Effect of TQ on surface interaction
between FN and AN
We performed a biofilm assay using prefixed FN and
AN cells to evaluate the effect of TQ on surface interactions
between FN and AN. Interestingly, co-incubation of FN and AN
increased cell attachment to microtiter wells even after fixation
with 4% PFA (Fig. 6). Washing with
0.01% TQ, but not TM, significantly reduced the number of attached
cells compared to that seen with saline.
Effect of TM and TQ on proinflammaotry
response of THP-1 cells to FN
We examined the anti-inflammatory activity of TM and
TQ in the human monocytic cell line THP-1. Since FN is well known
to have proinflammatory potential, we used FN sonicate as immune
sensitizer to assess anti-inflammatory effect of TM or TQ. Before
stimulation with FN, THP-1 cells were treated with 5–50 µM TM or
TQ. The pretreated THP-1 cells were then exposed to FN, and the
amount of TNF-α secreted from the cells was quantified by ELISA. TM
or TQ itself did not induce TNF-α production (data not shown).
Pretreatment with TM or TQ significantly decreased the amount of
TNF-α secretion from THP-1 cells in response to FN (Fig. 7A). However, the anti-inflammatory
activity of TQ was higher than that of TM, and the inhibitory
effect on TNF-α secretion by TQ was dose-dependent. Pretreatment of
cells with 50 µM TQ prior to FN exposure decreased TNF-α levels to
one-eighth that seen for untreated cells.
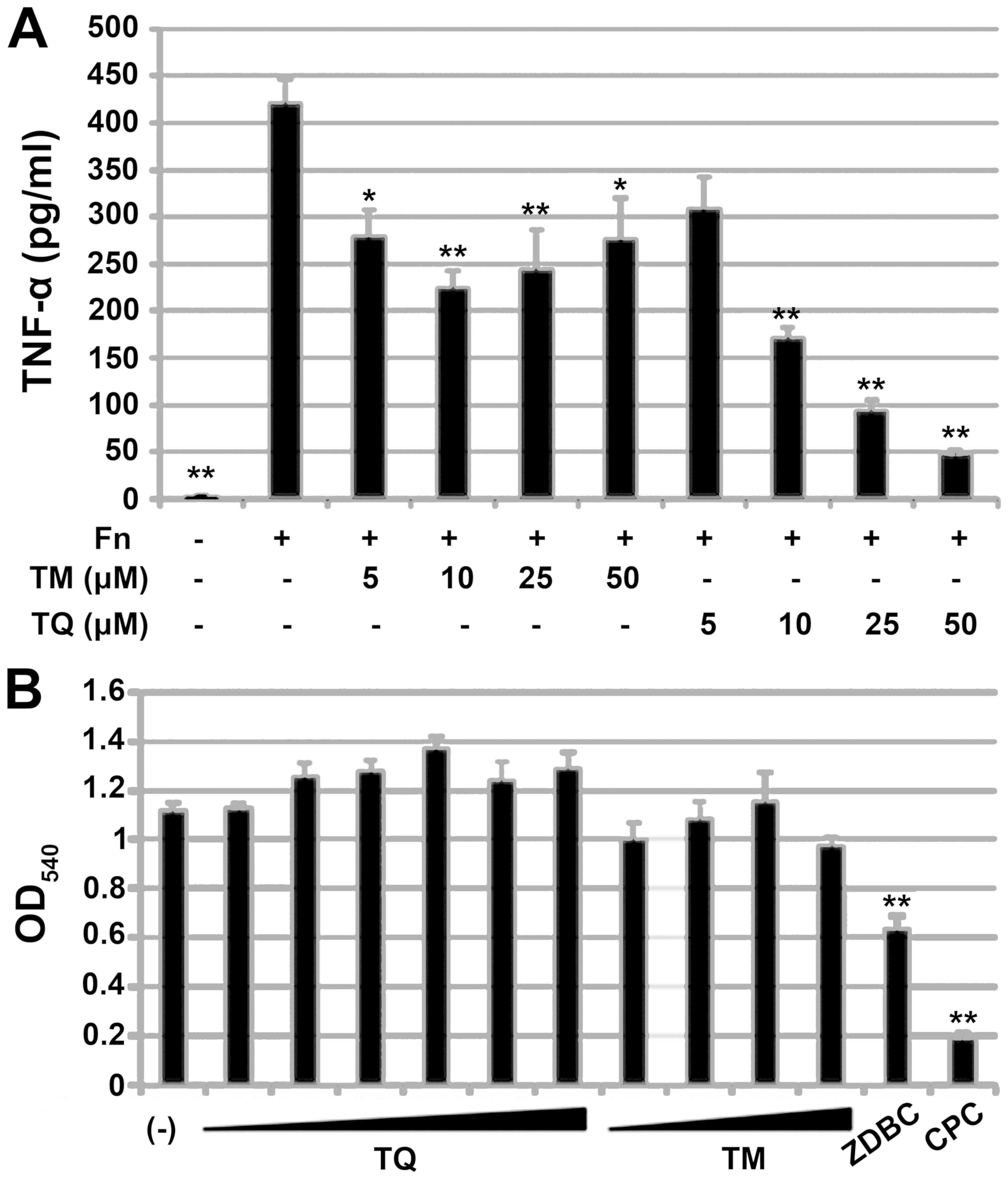 | Figure 7.Effect of TQ and TM on FN-mediated
proinflammatory response in the human monocytic cell line THP-1.
(A) TNF-α secreted from THP-1 after stimulation of FN strain
ATCC25586 (Fn). THP-1 pretreatment before Fn-stimulation is
indicated below the panel. Data are expressed as mean ± standard
deviation from three repeats. Differences were analyzed by ANOVA
followed by Dunnett's test. Significant difference from
FN-stimulation alone is shown by *P<0.05 or **P<0.01. (B) MTT
assay of THP-1 with or without TQ treatment (1, 2.5, 5, 10, 25 or
50 µM), TM (5, 10, 25 or 50 µM), 1 mg/ml ZDBC or 50 µM CPC under
FN-stimulation. Data are expressed as mean ± standard deviation of
three repeats. Differences were analyzed by ANOVA followed by
Dunnett's test. Significant differences from FN-stimulation alone
(indicated by -) are indicated by **P<0.01. TQ, thymoquinone;
TM, thymol; FN, Fusobacterium nucleatum; TNF, tumor necrosis
factor; CPC, cetylpyridinium chloride; ZDBC, zinc
dibutyldithiocarbamate. |
We performed a MTT assay to determine whether the
inhibitory effects of TM and TQ on TNF-α secretion were cytotoxic
effects on THP-1 cells. TM and TQ did not affect THP-1 cell
viability whereas the cytotoxic compound ZDBC did show a toxic
effect, as did 50 µM CPC (Fig.
7B).
Discussion
Although apparent virulence factors such as
exotoxins have not yet been identified in FN, the anaerobe is
considered to be a periodontal pathogen based on its crucial role
in the development of dental plaque that involves bridging diverse
types of oral bacteria during biofilm formation (3). The abundance of FN in the oral
microbiome is also known to increase in individuals with
periodontitis (24,25). Eradication of FN, which is expected
to reduce subgingival biofilm mass and inflammatory responses to
this anaerobe, is one therapeutic strategy for periodontitis.
Compounds having both antimicrobial and anti-inflammatory activity
are ideal therapeutics for periodontal diseases. TQ, a main
component of BC essential oil, exhibits antimicrobial activity as
well as anti-inflammatory effects (26–29).
In this study we examined the potential of this herbal quinone as a
therapeutic to treat FN-associated periodontitis. To the best of
our knowledge, this is the first report demonstrating that TQ shows
suppressive effects on FN-associated biofilm and inflammation.
Although we attempted to prepare FN biofilm in
multi-titer plates to examine the antibiofilm effect of TQ, FN did
not form stable biofilm. We hypothesized that FN requires
cooperation from other members of the oral microbiota to establish
intimate adherence to material surfaces. To test this hypothesis,
we co-cultivated FN with well-known early dental colonizers, AN or
SM. Co-cultivation of FN and AN significantly increased biofilm
mass, and microscopic analysis of biofilm suspensions showed
co-aggregation of these species. Multispecies biofilms having FN/AN
as the core components could serve as an in vitro
subgingival plaque model. Here, increases in FN/AN biofilm mass
appeared to be mainly attributable to FN since inactivation of FN
with TQ pretreatment abolished this increase (Fig. 3).
Microbial biofilm typically contains extracellular
polysaccharides (3), but SEM
examination of FN/AN-mixed biofilms showed no mucous matrices
within co-aggregates. Although aggregation factors that might
mediate FN/AN binding await elucidation, these interactions likely
involve physicochemical interactions of surface molecules, since
FN/AN co-aggregation was observed even after fixation with PFA.
Interestingly, co-cultivation of FN and SM reduced biofilm mass
over that seen for cultures containing FN alone. In addition, SM
inhibited FN/AN biofilm formation. This result suggests that some
resident Streptococci in the oral environment could have
inhibitory effects on FN-associated biofilms by competing with
binding receptors. Use of broad-spectrum antimicrobials for oral
hygiene care can compromise such indigenous anti-FN biofilm
activity of oral microbiome. Natural compounds such as herbal
extracts have mild and diverse pharmacological actions, and thus
are becoming attractive targets to develop oral hygiene care
products that have lower toxicity toward both beneficial components
of the oral microbiota as well as to human cells (15).
The BC essential oil components TM and TQ
significantly inhibited formation of FN/AN dual species biofilms.
This effect was due to growth inhibition of both species because
the optical density of the culture was not increased during
incubation. The bactericidal effect of TM or TQ appeared to be weak
as evidenced by CLSM results showing that after treatment with
0.01% TM or TQ over 70% of the bacteria within the biofilms
survived whereas treatment with 0.01% CPC killed over 70% of the
bacteria. Previous reports indicated that TQ has more pronounced
antimicrobial activity against Gram-positive bacteria than
Gram-negative bacteria (26,27).
However, in this study FN seemed to be more sensitive to TQ than AN
since biofilm mass was reduced with TQ pretreatment of FN but not
AN (Fig. 3). Thus, oral hygiene
care using BC oil and its components might inhibit dental plaque
formation by affecting a relatively narrow spectrum of bacteria and
minimizing damage to the healthy oral microbiome.
The most notable finding in this study was that TQ
reduced the mass of established FN/AN biofilms. This cleansing
effect was equivalent to that for IPMP and greater than that seen
for CPC. A similar trend was observed by CLSM, in which the
thickness of FN/AN biofilms was decreased by treatment with TQ or
IPMP to a greater degree than that seen for TM and CPC. TM and CPC
treatment resulted in a significant reduction in FN/AN biofilm
thickness, but did not decrease biofilm mass as evaluated by
crystal violet staining (Figs. 4
and 5). These results might
indicate that TQ and IPMP compromise FN/AN aggregation whereas TM
and CPC condense biofilm structures.
The biofilm assay using prefixed FN and AN (Fig. 6) indicated that the cleansing
effect of TQ on FN/AN biofilms likely involves interference with
cell surface interactions. Since TQ is hydrophobic and localizes to
the membrane fraction of BC seeds, TQ may be able to penetrate the
outer membrane of bacteria to affect membrane integrity, which in
turn can compromise FN/AN binding. In fact, TQ is reported to
induce membrane damage in pathogenic fungi (30).
Subgingival inflammation is one of the pathologies
of chronic periodontitis. Prolonged inflammation results in
gingival tissue damage and alveolar bone destruction. FN
lipopolysaccharides are known to induce extensive inflammatory
responses mediated through TLR2 and TLR4 signaling (5). TQ is reported to repress inflammatory
responses via diverse actions including inhibition of
5-lipoxygenase and leukotriene C4 synthase (26), inducible nitric oxide synthase
(31), suppression of NF-κB
signaling pathways (32) and
enhanced production of antioxidants such as catalase or glutathione
(33,34). Consistent with these findings, here
we showed that TQ repressed TNF-α production from a human monocytic
cell line in response to FN (Fig.
7). This effect was not due to TQ cytotoxicity to THP-1 cells
since cell viability was not affected at TQ concentrations of up to
50 uM, indicating that TQ does not compromise immunological
clearance of oral pathogens. Surprisingly, CPC, which is widely
used as an active component of oral care products, showed greater
cytotoxicity to THP-1 cells than did ZDBC. Meanwhile, with an
estimated LD50 of around 2.4 g/kg (range 1.52–3.77), TQ
is considered to have a low level of toxicity (19,35)
and only two cases of contact dermatitis have been reported in
clinical studies involving BC oil (36,37).
A limitation of this study is that all experiments
were performed in vitro. Dental plaque is more complex and
diverse among individuals and under in vivo conditions.
Although a suppressive effect of TQ on alveolar bone loss and
dental plaque accumulation has been demonstrated in a rat
periodontitis model (22) and
clinical study (38),
respectively, these studies did not monitor microbiome alterations
in saliva or dental plaque. Animal and clinical studies that focus
on FN, a keystone periodontal pathogen, will provide new insights
into the mechanisms for prophylactic and therapeutic effects of TQ
on periodontal disease.
In conclusion, the results of this study showed that
TQ effectively removes established FN/AN biofilms in vitro
by compromising FN binding to other dental plaque colonizers. TQ
effectively inactivated FN and suppressed inflammatory response to
this periodontal pathogen. Based on the crucial role of FN in
dental plaque maturation, agents that target FN, such as TQ, are
candidates for safe and effective active ingredients in oral
hygiene care products.
Acknowledgements
The authors would like to thank Dr. Hidenobu Senpuku
(National Institute of infectious Diseases in Japan) for the gift
of Actinomyces neaslandii strain X600.
Funding
This work was supported by JSPS KAKENHI (grant no.
18K17028).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AT, MI, KS and TK designed the current study. AT,
HNI, HYag and ME performed experiments to evaluate the effect of TQ
on FN-containing biofilm. TN performed statistical analyses. HYam
performed electron microscopic analyses. MI, KS and TK wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dewhirst FE, Chen T, Izard J, Paster BJ,
Tanner AC, Yu WH, Lakshmanan A and Wade WG: The human oral
microbiome. J Bacteriol. 192:5002–5017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kolenbrander PE and London J: Adhere
today, here tomorrow: Oral bacterial adherence. J Bacteriol.
175:3247–3252. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rickard AH, Gilbert P, High NJ,
Kolenbrander PE and Handley PS: Bacterial coaggregation: An
integral process in the development of multi-species biofilms.
Trends Microbiol. 11:94–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han YW: Fusobacterium nucleatum: A
commensal-turned pathogen. Curr Opin Microbiol. 23:141–147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Y, Shu R, Li CL and Zhang MZ:
Gram-negative periodontal bacteria induce the activation of
Toll-like receptors 2 and 4, and cytokine production in human
periodontal ligament cells. J Periodontol. 81:1488–1496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
To TT, Gümüş P, Nizam N, Buduneli N and
Darveau RP: Subgingival plaque in periodontal health antagonizes at
Toll-like receptor 4 and inhibits E-selectin expression on
endothelial cells. Infect Immun. 84:120–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong CY, Lin SK, Kok SH, Cheng SJ, Lee MS,
Wang TM, Chen CS, Lin LD and Wang JS: The role of
lipopolysaccharide in infectious bone resorption of periapical
lesion. J Oral Pathol Med. 33:162–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suwabe K, Yoshida Y, Nagano K and
Yoshimura F: Identification of an L-methionine γ-lyase involved in
the production of hydrogen sulfide from L-cysteine in
Fusobacterium nucleatum subsp. nucleatum ATCC 25586.
Microbiology. 157:2992–3000. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakano M, Shin K, Wakabayashi H, Yamauchi
K, Abe F and Hironaka S: Inactivating effects of the
lactoperoxidase system on bacterial lyases involved in oral
malodour production. J Med Microbiol. 64:1244–1252. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G
and Han YW: Fusobacterium nucleatum promotes colorectal
carcinogenesis by modulating E-cadherin/β-catenin signaling via its
FadA adhesin. Cell Host Microbe. 14:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen-Vercoe E, Strauss J and Chadee K:
Fusobacterium nucleatum: An emerging gut pathogen? Gut
Microbes. 2:294–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seymour GJ, Ford PJ, Cullinan MP, Leishman
S and Yamazaki K: Relationship between periodontal infections and
systemic disease. Clin Microbiol Infect. 13 (Suppl 4):S3–S10. 2007.
View Article : Google Scholar
|
|
13
|
Imai H, Kita F, Ikesugi S, Abe M, Sogabe
S, Nishimura-Danjobara Y, Miura H and Oyama Y: Cetylpyridinium
chloride at sublethal levels increases the susceptibility of rat
thymic lymphocytes to oxidative stress. Chemosphere. 170:118–123.
2010. View Article : Google Scholar
|
|
14
|
Hidalgo E and Dominguez C: Mechanisms
underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro.
15:271–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandra Shekar BR, Nagarajappa R, Suma S
and Thakur R: Herbal extracts in oral health care-A review of the
current scenario and its future needs. Pharmacogn Rev. 9:87–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Darvishi Khezri H, Haidari Gorji MA, Morad
A and Gorji H: Comparison of the antibacterial effects of matrica
& Persica™ and chlorhexidine gluconate mouthwashes in
mechanically ventilated ICU patients: A double blind randomized
clinical trial. Rev Chilena Infectol. 30:361–373. 2013.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pedrazzi V, Leite MF, Tavares RC, Sato S,
do Nascimento GC and Issa JP: Herbal mouthwash containing extracts
of Baccharis dracunculifolia as agent for the control of
biofilm: Clinical evaluation in humans. ScientificWorldJournal.
2015:7126832015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filoche SK, Soma K and Sissons CH:
Antimicrobial effects of essential oils in combination with
chlorhexidine digluconate. Oral Microbiol Immunol. 20:221–225.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali BH and Blunden G: Pharmacological and
toxicological properties of Nigella sativa. Phytother Res.
17:299–305. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gholamnezhad Z, Havakhah S and Boskabady
MH: Preclinical and clinical effects of Nigella sativa and
its constituent, thymoquinone: A review. J Ethnopharmacol.
190:372–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kokoska L, Havlik J, Valterova I, Sovova
H, Sajfrtova M and Jankovska I: Comparison of chemical composition
and antibacterial activity of Nigella sativa seed essential
oils obtained by different extraction methods. J Food Prot.
71:2475–2480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozdemir H, Kara MI, Erciyas K, Ozer H and
Ay S: Preventive effects of thymoquinone in a rat periodontitis
model: A morphometric and histopathological study. J Periodontal
Res. 47:74–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakasugi T, Murakawa T, Shibuya K and
Morimoto M: Deodorizing substance in black cumin (Nigella
sativa L.) seed oil. J Ole Sci. 66:877–882. 2017. View Article : Google Scholar
|
|
24
|
Saygun I, Nizam N, Keskiner I, Bal V,
Kubar A, Acikel C, Serdar M and Slots J: Salivary infectious agents
and periodontal disease status. J Periodontal Res. 46:235–239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou X, Liu X, Li J, Aprecio RM, Zhang W
and Li Y: Real-time PCR quantification of six periodontal pathogens
in saliva samples from healthy young adults. Clin Oral Investig.
19:937–946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaieb K, Kouidhi B, Jrah H, Mahdouani K
and Bakhrouf A: Antibacterial activity of thymoquinone, an active
principle of Nigella sativa and its potency to prevent
bacterial biofilm formation. BMC Complement Altern Med. 11:292011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kouidhi B, Zmantar T, Jrah H, Souiden Y,
Chaieb K, Mahdouani K and Bakhrouf A: Antibacterial and
resistance-modifying activities of thymoquinone against oral
pathogens. Ann Clin Microbiol Antimicrob. 10:292011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mansour M and Tornhamre S: Inhibition of
5-lipoxygenase and leukotriene C4 synthase in human blood cells by
thymoquinone. J Enzyme Inhib Med Chem. 19:431–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shokri H: A review on the inhibitory
potential of Nigella sativa against pathogenic and toxigenic
fungi. Avicenna J Phytomed. 6:21–33. 2016.PubMed/NCBI
|
|
31
|
El-Mahmoudy A, Matsuyama H, Borgan MA,
Shimizu Y, El-Sayed MG, Minamoto N and Takewaki T: Thymoquinone
suppresses expression of inducible nitric oxide synthase in rat
macrophages. Int Immunopharmacol. 2:1603–1611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sethi G, Ahn KS and Aggarwal BB: Targeting
nuclear factor-kappa B activation pathway by thymoquinone: Role in
suppression of antiapoptotic gene products and enhancement of
apoptosis. Mol Cancer Res. 6:1059–1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohamed A, Shoker A, Bendjelloul F, Mare
A, Alzrigh M, Benghuzzi H and Desin T: Improvement of experimental
allergic encephalomyelitis (EAE) by thymoquinone; an oxidative
stress inhibitor. Biomed Sci Instrum. 39:440–445. 2003.PubMed/NCBI
|
|
34
|
Sayed-Ahmed MM and Nagi MN: Thymoquinone
supplementation prevents the development of gentamicin-induced
acute renal toxicity in rats. Clin Exp Pharmacol Physiol.
34:399–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Badary OA, Al-Shabanah OA, Nagi MN,
Al-Bekairi AM and Elmazar MMA: Acute and subchronic toxicity of
thymoquinone in mice. Drug Develop Res. 44:56–61. 1998. View Article : Google Scholar
|
|
36
|
Steinmann A, Scatze M, Agathos M and Brett
R: Allergic contact dermatitis from black cumin (Nigella
sativa) oil after topical use. Contact Dermatitis. 36:268–269.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zedlitz S, Kaufmann R and Bochncke WH:
Allergic contact dermatitis from black cumin (Nigella
sativa) oil-containing ointment. Contact Dermatitis.
46:1882002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kapil H, Suresh DK, Bathia SC and Arora
KS: Assessment of clinical efficacy of locally delivered 0.2%
thymoquinone gel in the treatment of periodontitis. Saudi Dent J.
30:348–354. 2018. View Article : Google Scholar : PubMed/NCBI
|