Introduction
Spinal cord injury (SCI) often causes motor,
sensorial and autonomic nervous system dysfunction of the affected
segment (1). Normal defecation
processes are co-governed by the central nervous system (CNS) and
the autonomic nervous system [including the enteric nervous system
(ENS)] (2). When the spinal cord
is impaired, defecation is no longer under the control of the brain
(2). Over 1/3 of patients with SCI
have defecation dysfunction, which has a serious impact on their
quality of life (3,4). When SCI occurs above the conus
medullaris, the defecation reflex, which is controlled by the lower
defecation center at the sacral cord S2-4, is still intact; when
SCI occurs below the conus medullaris, both brain control and the
defecation reflex are lost (3,4). The
primary pathological manifestations of defecation dysfunction due
to SCI are decreased colonic motility and prolonged intestinal
transmission time, irrespective of trauma location (5,6).
The ENS governs gastrointestinal (GI) movements
using a variety of neurotransmitters. Serotonin (5-HT) is an
important neurotransmitter for modulating GI movement, and it is
also a signaling molecule for GI mucosa sensation feedback to the
nervous center (7). 5-HT augments
mucosa secretion and promotes the proliferation of the GI pacemaker
cells, known as interstitial cells of Cajal (ICC) (7–10).
The modulation of GI functions by 5-HT is exerted
directly on the smooth muscle, promoting its contraction (7). 5-HT3 receptor (5-HT3R) and 5-HT4R are
important 5-HT excitatory receptors and are widely present in the
GI tract (11,12). 5-HT3R is an ion channel linked
receptor that promotes GI movement by transmitting fast excitatory
postsynaptic potentials at the serotonergic neurons (13). It does this by augmenting the
secretion and release of neurotransmitters (including
acetylcholine, substance P and dopamine) (14), and by modulating ICC activities via
the regulation of extracellular Ca2+ concentration
(15). Additionally, 5-HT3R also
mediates the transmission of enteric sensory information to the
CNS, thus triggering the GI reflex (13,15–17).
The major enteric depot of 5-HT is found in mucosal
enterochromaffin cells, which are sensory transducers that use 5-HT
to activate both the intrinsic (via 5-HT1PR and 5-HT4R) and
extrinsic (via 5-HT3R) primary afferent nerves (18). Moreover, 5-HT3R enhances 5-HT
secretion from the gut mucosa (17,19).
Five subtypes of 5-HT3R have been characterized, among which
5-HT3AR is a functional receptor with specific subtype features
(16,20). 5-HT4R is a G protein-coupled
receptor. At the myenteric plexus, 5-HT4R promotes neurotransmitter
release from the cholinergic system, thus improving GI smooth
muscle contraction. At the mucosal epithelium, 5-HT4R mediates the
transmission of sensory information and augments secretion
(21–23). Moreover, 5-HT4R can enhance the
pacing of ICCs (24). 5-HT3AR and
5-HT4R are mainly expressed in the myenteric plexus, mucous
membrane, submucosal nerves and, in small quantities, in the
muscularis (25). Moreover, they
are expressed at varying degrees in neurons, epithelial cells,
goblet cells, chromaffin cells, Cajal mesenchymal cells and smooth
muscle cells (26–28). 5-HT3R and 5-HT4R antagonists
decrease GI motility, whereas 5-HT3R and 5-HT4R agonists enhance GI
emptying (29–31). 5-HT, 5-HT3AR and 5-HT4R are also
present in the spinal cord (32–34).
Intestinal serotonergic neurons are mainly distributed in the
submucosal plexus and myenteric plexus (11,12).
Serotonergic neurons are also distributed in the dorsal horn of the
spinal cord, which is related to visceral sensations (32). In addition, there are 5-HT
immunopositive nerve fibers in the lateral horn of the spinal cord,
which is related to visceral movement (24,27).
Synchronous anomalies of 5-HT expression in the spinal cord and
colon are detected in some enteric motility disorders (35). Nevertheless, the changes in 5-HT,
5-HT3AR and 5-HT4R in the colon and spinal cord of rats with SCI
remain to be clarified.
Sacral nerve electrical stimulation (SNS) enhances
the defecation reflex by improving colonic motility and shortening
colon transit time, thus alleviating constipation. Therefore, SNS
could be a therapeutic option for defecation dysfunction caused by
diverse etiologies, including SCI (36–39).
Electrical stimulation on nerve S3 triggers an anterograde impulse
on the whole colon that accelerates defecation frequency and
quantity (40,41). A previous study suggested that the
mechanism of action of SNS on intestinal dysfunction is through the
modulation of spinal and/or supraspinal afferent inputs (42). Another study highlighted that the
mechanisms of SNS are multifactorial and complex, and involve
rectal sensory threshold, recto-anal inhibitory reflex, rectal
evacuation and anorectal autonomic function (43). Nonetheless, the neuromodulation
mechanism underlying SNS therapy for SCI remains to be
determined.
Based on the available data, it was hypothesized
that SNS can improve defecation function by increasing 5-HT,
5-HT3AR and 5-HT4R in the colon of rat models of SCI. Therefore,
this study aimed to establish a rat model of acute severe SCI
(thoracic segments) to assess the influence of SNS on 5-HT, 5-HT3AR
and 5-HT4R in the colon and sacral cord, and to explore the
potential mechanisms for SNS in promoting defecation reflex.
Materials and methods
Animals and grouping
A total of 70 healthy adult female Sprague-Dawley
rats (specific pathogen-free grade; age, 8 weeks; weight, 200±20 g)
were purchased from Shanghai SIPPR-Bk Lab Animal Co., Ltd. (license
no. SCXK: 2008-0016). The animals were housed at 23±2°C, with 12-h
light-dark cycles and 50% humidity. The animals had free access to
food and drink, and were adaptively housed for 1 week prior to
experiments. The animals were caged individually after surgery. The
experiments were approved by the Animal Care and Use Committee of
Xi'an Jiaotong University Health Science Center. All efforts were
made to minimize animal numbers and suffering.
A total of 20 rats, using a random number table,
were randomly assigned to the sham operation group, and the
remaining 50 rats underwent severe SCI modeling. During the
operation, four rats died, which was within the acceptable limits
of the ethical approval obtained for the present study, and two
rats were excluded due to modeling failure. Forty rats were
randomly selected among the ones with successful modeling, and
randomized to the SCI and SNS groups (20 rats/group). The remaining
four rats received the same nursing interventions (such as
anti-infection treatment and assisted urination) as the rats with
SCI. After the experiment was completed, the rats were sacrificed
humanly.
The first day of the experiment was modeling. SNS
intervention was performed for 14 days, from day 2 to day 15. On
day 16, the time to first black stools was recorded (fasting
started on day 15 and the animals had free access to water for 24
h). Dry weight of the fecal pellets was recorded. The animals were
sacrificed on day 17.
Rat model of severe SCI
Prior to the induction of the severe SCI model, the
rats were deeply anesthetized by the intraperitoneal injection of
10% chloral hydrate (300 mg/kg). Thermal support was provided and
the depth of anesthesia was monitored by the toe pinch method. The
skin region was disinfected with iodophor and a median incision was
made from T10 to T13. The spinous process and vertebral plates were
exposed after the dissection of the superficial fascia and the
removal of the T11-T12 vertebral plates. A bone window was
generated and the spinal cord was exposed. SCI was induced by
striking the exposed spinal cord using a 10 g weight falling from
60 mm with the New York University (NYU) Impactor device (W.M. Keck
Center for Collaborative Neuroscience Rutgers, State University of
New Jersey); this apparatus was used because it avoids the
occurrence of heavy bleeding (44,45).
Successful SCI modeling was determined according to the improved
Basso, Beattie, Bresnahan locomotor rating (BBB) score (45). In the sham operation group, the
spinal cord was exposed at T10-T13, but the NYU impactor was not
used. In all rats, the wound was sutured within 5 min and covered
with gentamicin ointment.
BBB score
The improved BBB score was determined to evaluate
hind limb motor function (45).
The rats were placed in an open space before modeling (day 1), 24 h
after modeling (day 2) and on day 16. The following parameters were
observed independently by two observers: Joint motions of the hip,
knee and ankle; hind limb weight-bearing condition; walking
capability and hind limb-forelimb coordination; trunk stability;
paw position; and tail movement. The scores were between 0 and 21
points, higher scores indicated increased improvement of the hind
limb motor function.
Post-operative nursing
From the day of modeling, all rats received
intraperitoneal injections of gentamicin for 16 days at 5,000 U/kg
once daily. The lower abdomen, perineum and hind limbs of the rats
were cleaned daily, and passive movements of the hind limbs were
performed daily. The Crede maneuver (46) was applied every 12 h to assist
voiding: The SCI rats were held upright and gentle pressure was
applied on the bulging bladder from top to bottom for assisting
urination. Before voiding, the bladder was palpated and urinary
retention was estimated based on the degree of bladder filling.
The passive movements were performed after
Crede-assisted urination. The operator fixed the rat in the prone
position with one hand and grasped the toes of one hind limb with
the other hand. Then, the rat was pulled rearward and outwardly at
a 45° angle with the spine, until the knee and ankle joints were in
complete extension. After this, the hind limb was pushed towards
the trunk in the opposite direction until the hip and knee joints
were completely flexed and the ankle was completely dorsally
flexed. Flexions were performed 60 times/min for 1 min. The same
procedure was performed for the other hind limb. Passive movement
of both hind limbs was performed once every 12 h. An observer
blinded to grouping observed fur appearance, autonomic activity,
food and water intake, defecation and urination and bodyweight
during the study.
SNS
At 24 h after successful modeling, the rats in the
SNS group were immobilized in the prone position. Needle electrodes
were placed on bilateral S3 neural foramen. The electrodes were
connected to a Myolito electrical stimulator (MTR+ Vertriebs GmbH).
The stimulation settings were: Pulse width of 0.2 msec; frequency
of 10 Hz; stimulation duration of 10 sec; intervals of 5 sec; and
current of 2–3 mA. The appropriate stimulation degree was indicated
by slight shivering of the tail, but without braying. Each session
of SNS lasted 15 min, with one session per day for 14 days.
Assessment of intestinal transmission
function
The first black feces were discharged at 8:00 a.m.
on the 15th day of the experiment. Then, 10 rats from each group
were randomly selected and fasted for 24 h, but with free access to
water. At 8:00 a.m. on the 16th day of the experiment, these rats
were given 2 ml of 100 g/l activated charcoal suspension by gavage.
The time interval between charcoal gavage and the first black feces
was recorded.
The dry weight of feces discharged within 24 h was
recorded. In the remaining 10 rats/group, on the 16th day of
experiment, feces discharged within 24 h (8:00 a.m.-8:00 a.m.) were
collected, dried and weighed.
Tissue sampling
On the 17th day of the experiment, the rats were
sacrificed by decapitation after intraperitoneal injection of 10%
chloral hydrate (400 mg/kg), followed by exposure of the spinal
cord at S2-4 (in accordance with the vertebral bodies L3-5). The
fresh spinal cord tissue of these segments was rapidly harvested
and split into two parts. The abdomen was cut open for the quick
dissection of ~1 cm of distal colon, which was cut open along the
longitudinal axis to rinse off colonic contents with physiological
saline, and split into two parts. One part of fresh spinal and
colon tissues were directly preserved in liquid nitrogen for ELISA,
reverse transcription-quantitative PCR (RT-qPCR) and western
blotting. The other parts of spinal cord and spread colon tissues
were fixed in 4% paraformaldehyde for hematoxylin-eosin (H&E)
staining and immunohistochemistry (IHC).
Hence, the samples of the colon and spinal tissues
(20 rats/group) were both divided into two parts. One part was
fixed with 4% paraformaldehyde, while the other part was stored in
liquid nitrogen. Among the 20 samples fixed with 4%
paraformaldehyde, five were randomly selected for H&E staining,
five were randomly selected for IHC and the other 10 were stored
for eventual future use. Among the 20 samples stored in liquid
nitrogen, 10 were used for ELISA, five were used for RT-qPCR, four
were used for western blotting, and the remaining one was stored
for eventual future use.
H&E staining
Colon tissues of 5 rats in each group were fixed in
4% paraformaldehyde for 24 h at room temperature (23±2°C), followed
by routine paraffin embedding, and sectioning at 4 µm. Tissue
slides were subjected to routine H&E staining, as follows.
Dewaxing was performed using xylene and a descending ethanol series
(100, 100, 95, 95, 85 and 75%) for 5 min at each step. The sections
were treated with hematoxylin for 5 min, 1% hydrochloric acid in
ethanol for 2 sec, tap water for 10 min, and eosin for 3 min. The
sections were dehydrated in an ascending alcohol gradient (75, 85,
95, 95, 100 and 100%) and xylene, 3 min each step. The sections
were sealed with neutral gum. All steps were performed at room
temperature (23±2°C). Pathological changes of colon tissues were
observed in six different fields and photographed using a DMLS32
optical light microscope (magnification ×200; Leica Microsystems
GmbH).
ELISA
Colon and spinal cord tissues of 10 rats in each
group were homogenized in PBS (weight:volume ratio of 1:9) and
supernatants were extracted by centrifugation at 5,000 × g for 5–10
min at room temperature (23±2°C). The standards (24, 12, 6, 3, 1.5
and 0.75 ng/ml) from the 5-HT ELISA kit (cat. no. 201710; Nanjing
Jiancheng Bioengineering Institute Co., Ltd.) and test samples (50
µl) were added into the wells. Subsequently, the samples were
incubated with horseradish peroxidase-labeled 5-HT antibody (100
µl) for 60 min at 37°C, as per the kit instructions. Optical
density values were measured at a wavelength of 450 nm.
IHC for 5-HT3AR and 5-HT4R
Colon and spinal cord tissues from five rats in each
group were fixed in 4% paraformaldehyde for >24 h at room
temperature (23±2°C), followed by routine paraffin embedding and
sectioning (5-µm thick sections). One slide with an intact tissue
section from each animal was selected for IHC. For dewaxing and
hydration, the sections were successively immersed in xylene,
xylene, anhydrous ethanol, 95% ethanol, 85% ethanol and 70% ethanol
for 5 min each, followed by rinsing with PBS three times for 3 min
each. Endogenous peroxidase activity was blocked using 3%
H2O2 for 10 min at room temperature, followed
by rinsing with PBS three times for 3 min each. The sections were
blocked with 5% goat serum (50–100 µl; cat. no. C0265, Beyotime
Institute of Biotechnology) for 20 min at room temperature
(23±2°C). The primary antibodies (all purchased from Abcam)
included anti-5-HT3AR (cat. no. ab13897; 1:100) and anti-5-HT4R
(cat. no. ab60359; 1:200). The sections were incubated with the
primary antibody solution (50–100 µl) overnight at 4°C, followed by
washing three times with PBS, 3 min each. The sections were
incubated with horseradish-peroxidase goat anti-rabbit IgG (H+L)
secondary antibody (cat. no. 111-035-003; 1:200; Jackson
ImmunoResearch Laboratories, Inc.) at 37°C for 1 h. Chromogen
detection was performed by incubating the sections with DAB
solution for 15 min at room temperature (23±2°C). The sections were
counterstained with hematoxylin for 10 min at room temperature
(23±2°C) and washed with distilled water. Subsequently, the
sections were immersed in 70, 85, 95 and 100% ethanol for 5 min
each, and twice in xylene for 10 min each. Neutral gum was added to
seal the slides. The sections were examined under a DMLS32 optical
light microscope (Leica Microsystems GmbH). The positive staining
was examined (magnification, ×40, followed by magnifications,
×100/x400). Six high-power fields (HPF; magnification, ×400) were
randomly selected on every slide. The JD801 image analysis system
(Nanjing Jiancheng Bioengineering Institute Co., Ltd.) was used to
measure the average optical density (AOD) of the positive stained
area. Image-Pro software (version 6.0.0.309; Media Cybernetics,
Inc.) was used to count immunopositive cells in six HPFs
(magnification, ×400; 0.1323 mm2) per animal.
RT-qPCR
Cryopreserved colon and spinal cord tissues from 5
rats in each group were used for RNA extraction with TRIpure
reagent (cat. no. 2702026AX; Beijing Aidlab Biotechnologies Co.,
Ltd.). Total RNA (2 µg) was reverse transcribed to cDNA using a
OneScript cDNA Synthesis kit (cat. no. G234; Applied Biological
Materials, Inc.), according to the manufacturer's instructions.
qPCR was performed using the EvaGreen Express 2X qPCR MasterMix-ROX
kit (cat. no. MasterMix-ER; Applied Biological Materials).
Fluorescence qPCR was carried out in a TL988-IV qPCR system
(Tianlong Science and Technology Co., Ltd.), in triplicate. The
primer pairs used were as follows: 5-HT3AR forward,
5′-GGCACCTGGTCCTAGACAGAA-3′ and reverse,
5′-GGTTTCCCATGGCTGAGCAGT-3′; 5-HT4R forward,
5′-CCGTTTCTCCTCATGGTGCT-3′ and reverse, 5′-AACATCTGGATCTGCTGGGC-3′;
and β-actin forward, 5′-TGGGTATGGAATCCTGTGGCA-3′ and reverse,
5′-TGTTGGCATAGAGGTCTTTACGG-3′. The reaction conditions were:
Initial denaturation at 95°C for 10 min; and 40 cycles of
amplification at 95°C for 5 sec and 60°C for 10 sec. The issolution
curves of the PCR products were analyzed. The Cq values were
determined using the MED-TL software (version IV; Xi'an Tianlong
Science and Technology Co., Ltd.). The relative mRNA expression
levels were calculated using the 2−ΔΔCq method (47) and normalized to the internal
reference gene β-actin.
Western blotting
Cryopreserved colon and spinal cord tissues were
used for western blotting. Proteins were purified with the Protein
Extraction kit (cat. no. KGP250; Nanjing KeyGen Biotech Co., Ltd.).
Total protein was quantified using a bicinchoninic acid assay and
170 µg protein/lane were separated via SDS-PAGE on 10% gels. The
high mass of protein loaded per lane was due to the low expression
levels of 5-HT3AR and 5-HT4R in the samples. The separated proteins
were subsequently transferred onto nitrocellulose membranes and
blocked for 1.5–2 h at room temperature with 5% skim milk powder.
The membranes were incubated overnight on a shaking plate at 4°C
with primary antibodies against: 5-HT3AR (1:500; cat. no. ab13897,
Abcam), 5-HT4R (1:1,000; cat. no. ab60359; Abcam) and GAPDH (cat.
no. E12-052; Nanjing EnoGene Biotech Co., Ltd.). After rinsing with
TBST, the membranes were incubated for 1–2 h at room temperature
with a goat anti-rabbit IgG-HRP secondary antibody (1:5,000; cat.
no. E1WP318; Nanjing EnoGene Biotech Co., Ltd.). Protein bands were
visualized using the ECL Chemiluminescence kit (cat. no. E1WP3132;
Nanjing EnoGene Biotech Co., Ltd.), prior to scanning in the BOX
chemiXR5 chemiluminescence imaging system (SynGene Europe). The
band densities of the target proteins (5-HT3AR/5-HT4R) were
measured using Gel-Pro Analyzer software (version 32; Meyer
Instruments) with GAPDH as the loading control. The ratio of target
protein vs. internal control was considered as the relative
expression level of the target proteins.
Statistical analysis
SPSS 16.0 (SPSS, Inc.) was used for statistical
analysis. Data were expressed as the mean ± SD. Analyses of normal
distribution and homogeneity of variance were first conducted. For
data with normal distribution, comparisons among multiple groups
were carried out with one-way ANOVA, as appropriate, with the least
significant difference post hoc test (for data with homogeneity of
variance) or the post hoc Dunnett's T3 test (for data with
heterogeneity of variance). For the BBB score, the Wilcoxon
non-parametric test with Bonferroni's correction was used (same
experimental group, different time points). For comparisons between
unmatched data (same time point, different experimental group), the
Mann-Whitney U-test with Bonferroni's correction was used to
analyze the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Severe SCI model establishment
The NYU impactor device was used to establish the
rat model of severe thoracic SCI (44). In the sham group, hind limb
movement was not affected by the operation, whereas in the SCI and
SNS groups, hind limbs presented flaccid paralysis and complete
loss of motor function at the early stage after modeling. Within 2
weeks after SCI modeling, the hip and ankle joints of hind limbs
presented extensive motion in the SCI and SNS groups (data not
shown).
On the 17th day of the experiment, the rats in the
sham group showed bright fur, autonomic activity, food and water
intake, defecation and urination, and stable body weight (even with
some gain). In the SCI group, the rats showed fatigue, irritation
and aggressiveness, loose and dim fur, emaciation, decreased
autonomic activity, decreased food intake, decreased defecation,
urinary retention, hematuria and urinary incontinence (in some
rats), as well as muscular atrophy of various degrees in the hind
limbs. In the SNS group, the rats showed an improved state and fur
condition compared with the SCI group. They showed emaciation,
decreased autonomic activity, decreased food intake, decreased
defecation (although improved compared with the SCI group) and
urinary retention (although milder than in the SCI group; data not
shown as it was not an endpoint in the present study).
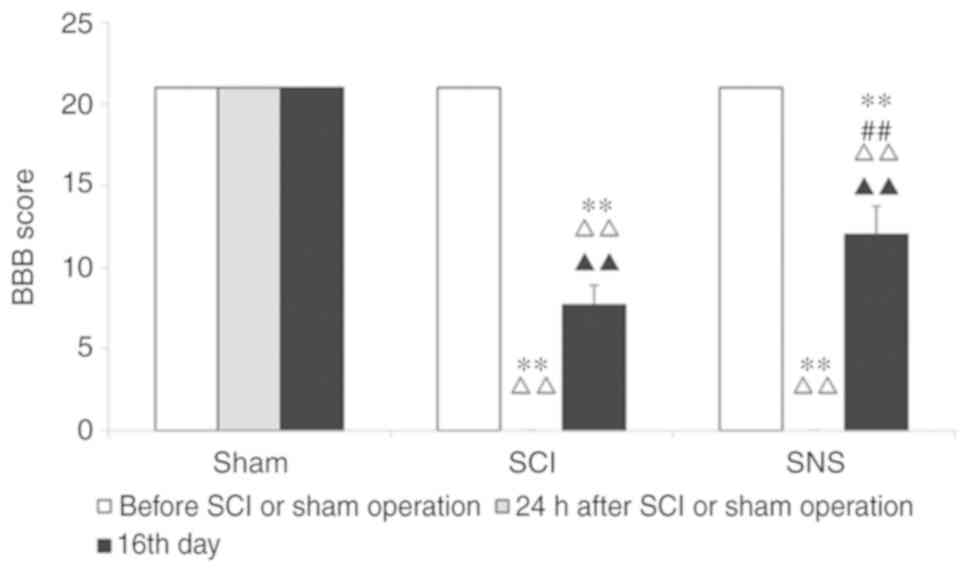
Before modeling, the BBB scores were 21 points for
all rats. At 24 h after modeling and compared to the sham group,
the BBB scores of the SCI and SNS groups were 0 (both P<0.01).
On the 16th day, compared to the sham operation group, BBB scores
of the SCI and SNS groups were decreased significantly (both
P<0.01). Compared with the SCI group, the BBB score of the SNS
group was higher (P<0.01; Fig.
1).
SNS improves intestinal transmission
function
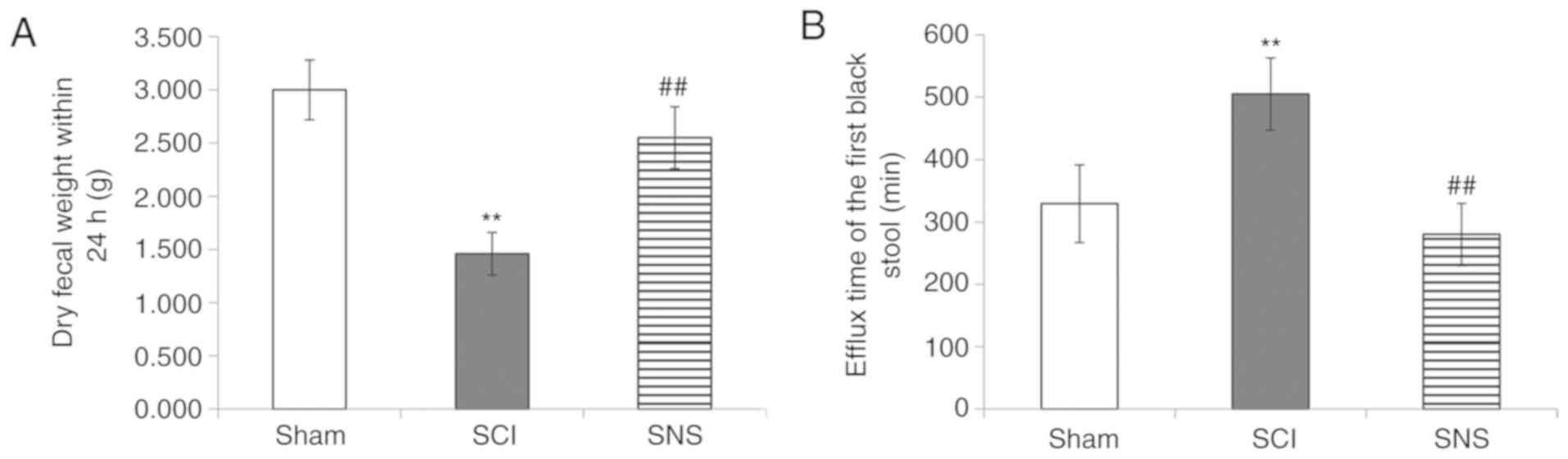
The effects of SCI and SNS on intestinal functions
were observed by the characterization of the fecal pellets and the
passage of black fecal pellets. On the 16th day, rats in the sham
group showed unobstructed defecation, with fecal pellets of a long
grainy shape, discharged as 2–3 consecutive pellets or a single
pellet with soft and humid texture. On the other hand, the rats in
the SCI group showed constipation, with fecal pellets showing a
small grainy shape, discharged as single pellet with hard and dry
texture, and the amount of defecation was significantly less than
that of the sham group (P<0.01; Fig. 2A). In the SNS group, rats showed
unobstructed defecation, with fecal pellets being slightly smaller
than in the sham group and with long grainy shape, discharged as
2–3 consecutive pellets or single pellet with slightly hard
texture, and the amount was larger than in the SCI group
(P<0.01).
In addition, the time interval between the operation
and subsequent first defecation was different among the three
groups (P<0.01). Compared with the sham group, the discharge of
the first black feces was significantly delayed in the SCI group
(P<0.01). Compared with the SCI group, the time interval was
significantly shortened in the SNS group (P<0.01; Fig. 2B).
Taken together, these results suggested that SCI
significantly impaired intestinal function. SNS could restore, at
least in part, intestinal function in SCI rats.
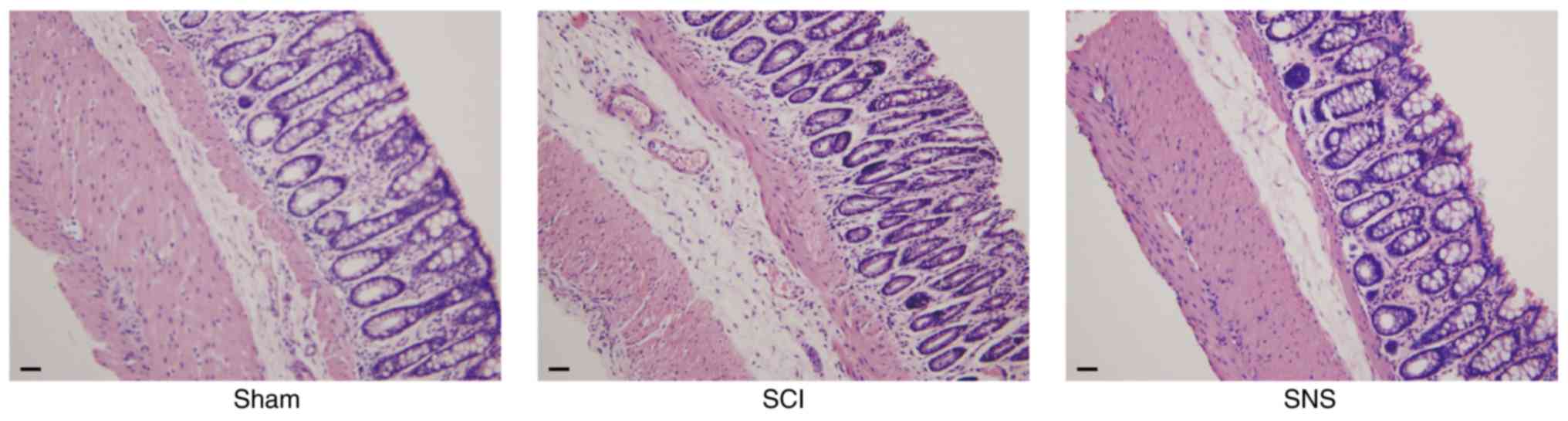
SNS improves the colon
histopathological features after SCI
The rats were sacrificed and colon histopathological
examination was carried out to determine the effects of SCI and SNS
on colon tissues. As shown in Fig.
3, compared with the sham group, the SCI group showed mucosal
erosion, lower number of glands, interstitial edema and prominent
atrophy of the muscular layer. The SNS group displayed mild atrophy
of the muscular layer and proper glands, mild interstitial edema
and with colon histology similar to that of the sham group. These
results suggested that SCI caused marked alterations to colon
histology, while SNS could alleviate those changes, at least in
part.
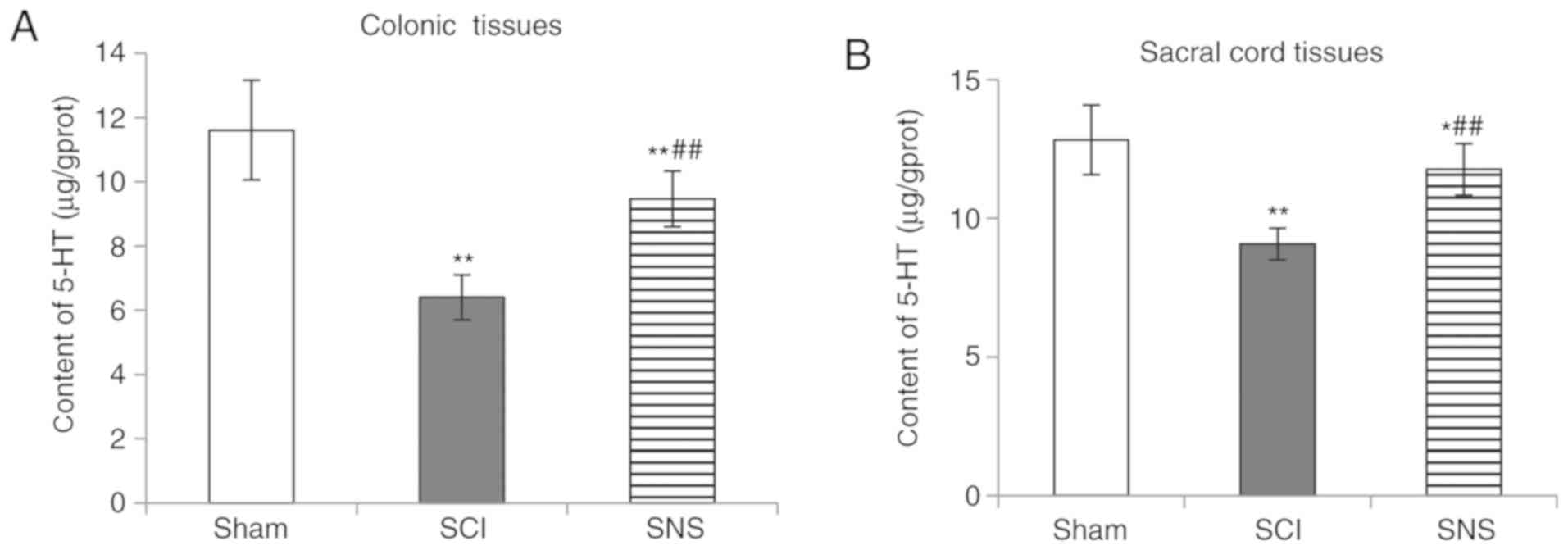
SNS increases 5-HT expression in the
colon and sacral cord of rats with SCI
The levels of 5-HT in the colon and sacral cord were
measured in rats with SCI, and those treated with SNS. As shown in
Fig. 4, compared with the sham
group, 5-HT expression levels in the colon and spinal cord tissues
were decreased in the SCI group (both P<0.01). Compared with the
SCI group, 5-HT expression levels were elevated in the SNS group
(both P<0.01). However, when compared to the sham group, 5-HT
content in the colon and spinal cord tissues was lower in the SNS
group (P<0.01 and P<0.05 respectively). These results
suggested that SNS could restore 5-HT expression after SCI.
SNS increases 5-HT3AR and 5-HT4R
protein levels
IHC was performed to determine the effects of SCI
and SNS on 5-HT3AR and 5-HT4R protein levels in the colonic
myenteric nerve plexus, colonic mucosa, sacral intermediolateral
nucleus and dorsal horn of sacral cord. As presented in Fig. 5A, positive staining for
5-HT3AR/5-HT4R was present in the colonic myenteric plexus
(cytoplasmic and nuclear staining). In the colonic myenteric
plexus, cells positive for 5-HT3AR and 5-HT4R were continuously and
densely distributed in the sham group; positive cells were
scattered (a few faintly stained cells) in the SCI group, but
densely distributed in the SNS group. In the SCI group, the AOD
values of 5-HT3AR and 5-HT4R staining were significantly lower in
the colonic myenteric plexus compared with the sham group
(P<0.01 and P<0.05, respectively). In the SNS group, the AOD
values of 5-HT3AR and 5-HT4R staining were elevated in the colonic
myenteric plexus vs. the SCI groups (P<0.01, P<0.05,
respectively; Fig. 5A). Compared
with the sham group, the numbers of 5-HT3AR positive cells in the
colonic myenteric nerve plexus of the SCI and SNS groups were
significantly lower (P<0.01). Compared with the sham group, the
numbers of 5-HT4R positive cells in the colonic myenteric nerve
plexus of the SCI group were significantly lower (P<0.01);
however, there was no significant difference in the SNS group.
Compared with the SCI group, the number of 5-HT3AR and 5-HT4R
positive cells in the SNS group was significantly higher
(P<0.01; Fig. 5A).
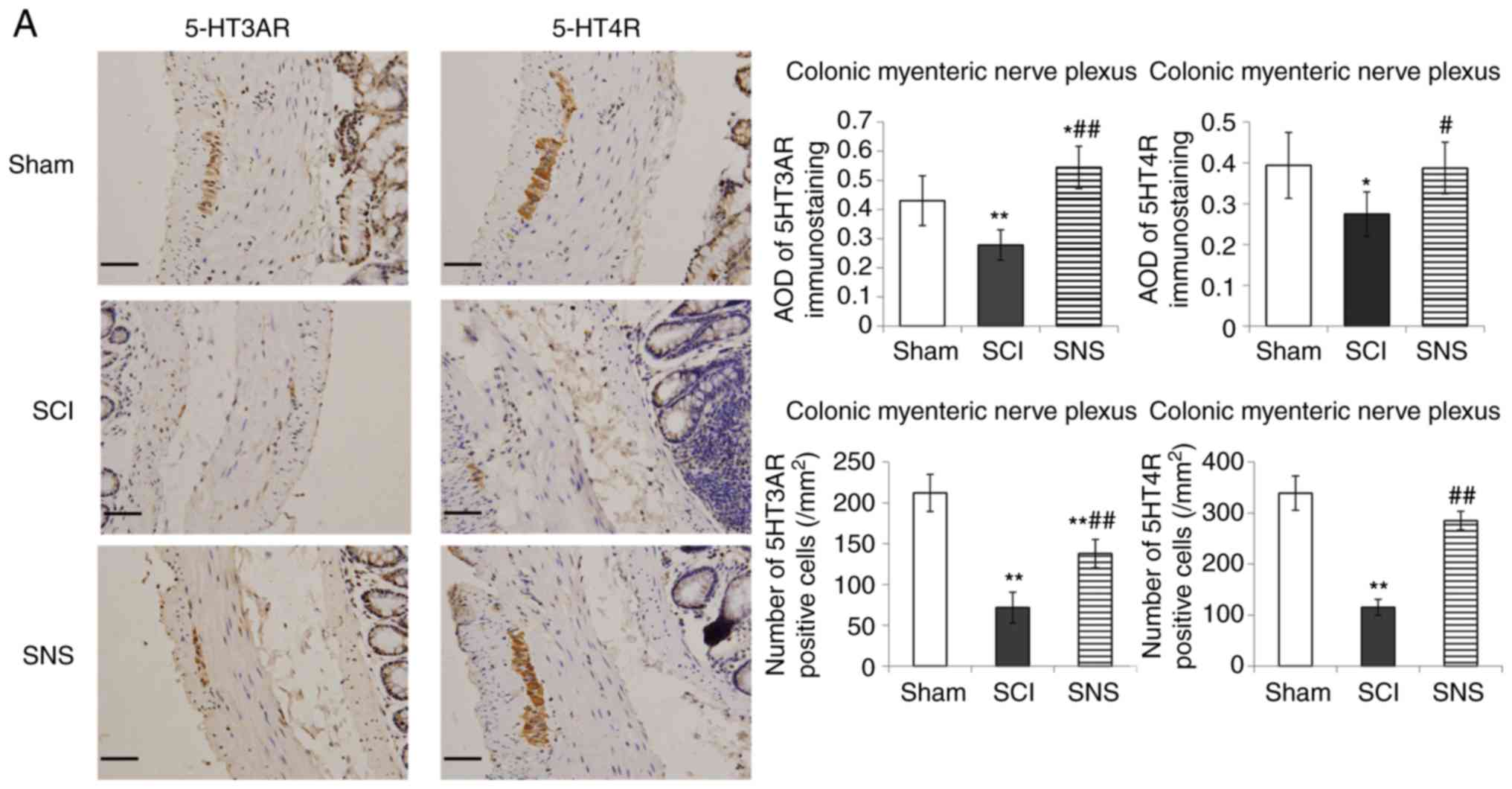 | Figure 5.Immunohistochemistry was performed to
determine the effects of SCI and SNS on 5-HT3AR and 5-HT4R protein
levels. Protein levels of 5-HT3AR and 5-HT4R in the (A) colonic
myenteric nerve plexus and (B) colonic mucosa (scale bar, 50 µm).
*P<0.05 vs. sham; **P<0.01 vs. sham; #P<0.05
vs. SCI; ##P<0.01 vs. SCI. SCI, spinal cord injury;
SNS, sacral nerve electrical stimulation; 5-HT, serotonin; R,
receptor; AOD, average optical density. Immunohistochemistry was
performed to determine the effects of SCI and SNS on 5-HT3AR and
5-HT4R protein levels. Effects of SCI and SNS on (C) 5-HT3AR and
(D) 5-HT4R protein levels in the sacral intermediolateral nucleus
and dorsal horn of the sacral cord (magnifications, ×100 and ×400;
n=5). *P<0.05 vs. sham; **P<0.01 vs. sham;
#P<0.05 vs. SCI; ##P<0.01 vs. SCI. SCI,
spinal cord injury; SNS, sacral nerve electrical stimulation; 5-HT,
serotonin; R, receptor; AOD, average optical density. |
In the colonic mucosa (Fig. 5B), cells positive for 5-HT3AR and
5-HT4R were densely distributed and strongly stained in the sham
group, while scattered and faintly stained in the SCI group, and
more densely distributed and moderately stained in the SNS group.
Compared with the sham group, the AOD values of 5-HT3AR and 5-HT4R
staining were significantly decreased in the colonic mucosa of the
SCI group (P<0.05). Compared with the SCI group, the AOD values
of 5-HT3AR and 5-HT4R staining in the colonic mucosa were
significantly elevated in the SNS group (P<0.01 and P<0.05,
respectively; Fig. 5B). Compared
with the sham group, the numbers of 5-HT3AR and 5-HT4R positive
cells in the colonic mucosa of the SCI group were significantly
lower (P<0.01); however, there was no significant difference in
the SNS group. Compared with the SCI group, the number of 5-HT3AR
and 5-HT4R positive cells in the SNS group was significantly higher
(P<0.01; Fig. 5B).
As shown in Fig. 5C and
D, in the sacral intermediolateral nucleus and the dorsal horn
of the sacral cord, 5-HT3AR and 5-HT4R positive cells were
moderately stained in the sham and SCI groups, while they were
strongly stained in the SNS group. There were no differences in the
AOD values and number of positive cells of 5-HT3AR and 5-HT4R
between the sham and SCI groups (all P>0.05). The AOD values of
5-HT3AR and 5-HT4R were higher in the SNS group compared with the
sham and SCI groups (all P<0.05), but there was no difference in
the number of positive cells (all P>0.05; Fig. 5C and D). Taken together, these
results suggested that SCI decreased 5-HT3AR and 5-HT4R protein
expression in the colon of rats, while SNS appeared to promote
5-HTR expression to above-sham levels.
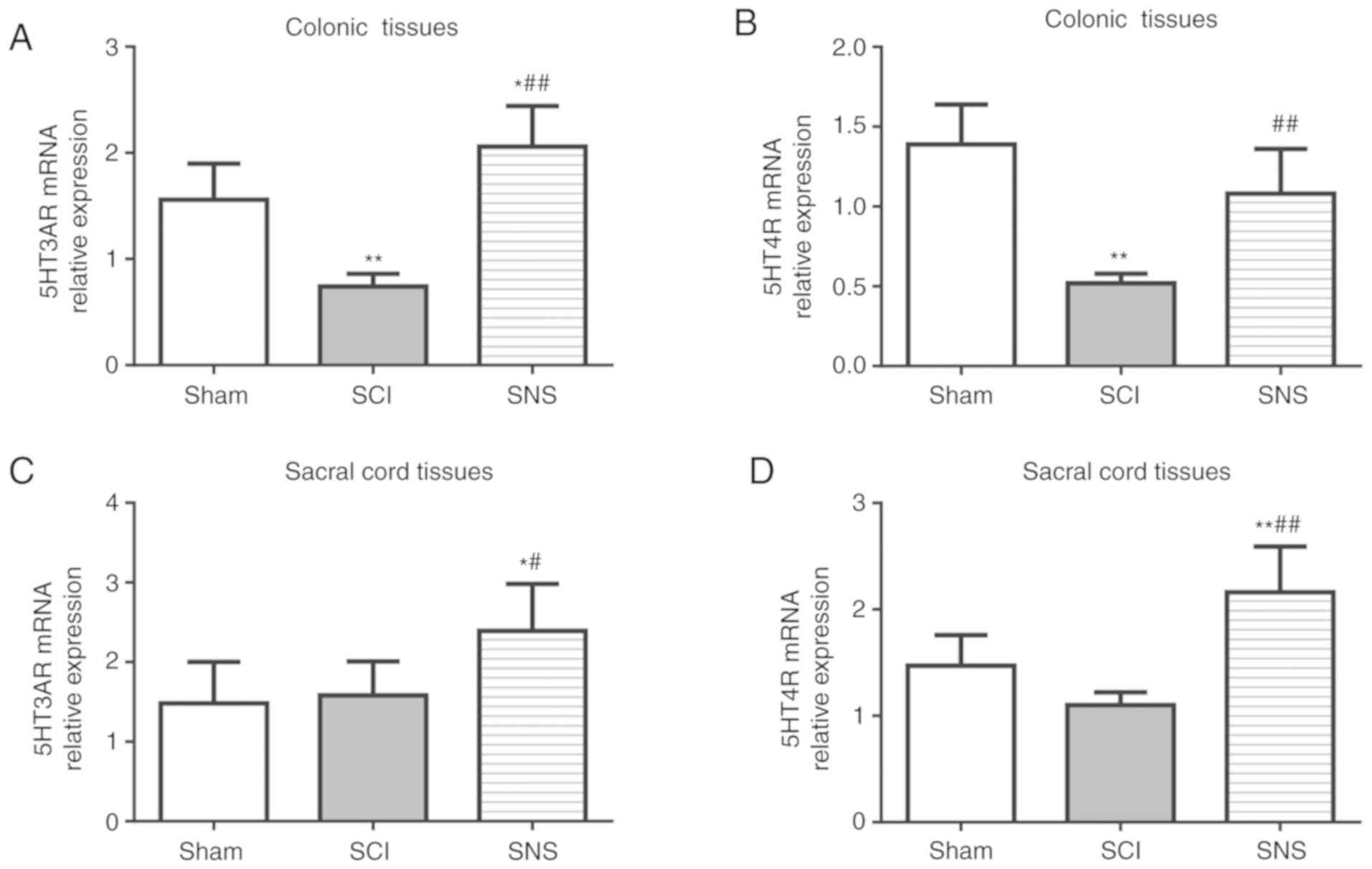
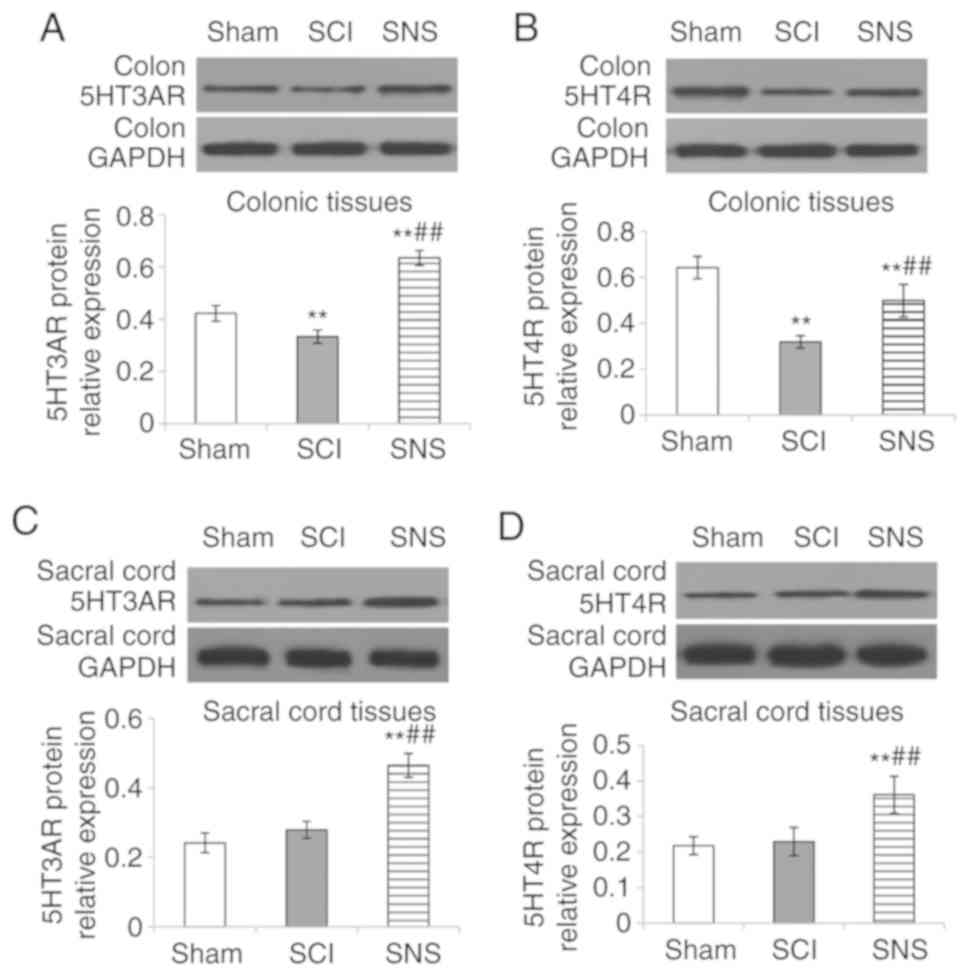
Validation of the effects of SNS on
5-HT3AR/5-HT4R gene and protein expression in the colon and sacral
spinal cord
In order to validate the IHC results, RT-qPCR and
western blotting were performed. Compared with the sham group, the
relative expression levels of 5-HT3AR and 5-HT4R mRNA and protein
were both downregulated in colon tissue (all P<0.05; Figs. 6A and B; 7A and B), but there was no significant
difference in the sacral cord (both P>0.05) in the SCI group
(Figs. 6C and D; 7C and D). In the SNS group, the relative
5-HT3AR mRNA (Fig. 6A) and protein
levels (Fig. 7A) were elevated
compared with the SCI group (all P<0.05) in the colon tissues.
The relative 5-HT3AR and 5-HT4R mRNA (Fig. 6C and D) and protein levels
(Fig. 7C and D) were both elevated
in sacral cord tissues compared with the SCI group (all P<0.05).
Compared with the SCI group, the relative expression levels of
5-HT3AR and 5-HT4R mRNA (Fig. 6)
and protein (Fig. 7) in the colon
and sacral cord tissues were elevated in the SNS group (all
P<0.05). Taken together, these results validated the IHC
results.
Discussion
Changes in 5-HT, 5-HT3AR and 5-HT4R expression in
the colon and spinal cord of rats with SCI remain to be clarified.
The neuromodulatory mechanism underlying SNS therapy for SCI also
remains to be determined. Therefore, the aim of the present study
was to establish a rat model of acute severe SCI (thoracic
segments) to assess the influence of SNS on 5-HT, 5-HT3AR and
5-HT4R in the colon and sacral cord. In SCI rats, SNS significantly
increased the amount of defecation, shortened the time to first
black feces, and improved the fecal texture and colon histology.
SNS elevated 5-HT contents in the colon and spinal cord tissues,
and enhanced 5-HT3AR and 5-HT4R protein expression and distribution
in the colonic myenteric plexus and mucosa, sacral
intermediolateral nucleus and dorsal horn. It also upregulated the
relative expression levels of 5-HT3AR/5-HT4R mRNA and protein in
the colon and spinal cord. Taken together, these results suggested
that SNS can elevate 5-HT3AR/5-HT4R expression in the sacral
defecation center and colon, and elevate colonic 5-HT contents,
thus improving defecation and accelerating recovery of the colonic
transmission function in acute SCI rats.
5-HT is mainly secreted by enterochromaffin cells;
>90% of 5-HT is present in the GI tract, while the remaining
portion is found in the CNS (7–10).
5-HT secreted in the gut upon sensation of pressure and chemical
stimulation exerts two types of effects; direct effect on the
smooth muscle and augmenting enteric motility, and activating the
intrinsic primary afferent neurons and modulating gut sensation,
motor function and secretion (7-10,13,15-17).
In the present study, SCI modeling led to a
significant decrease of 5-HT contents in the colon and sacral cord
of rats. It was hypothesized that histopathological changes in the
gut wall caused changes in the structure and function of
enterochromaffin cells that led to decreased secretion of 5-HT, and
thus decreased 5-HT contents in other locations such as the sacral
cord. Meanwhile, the downregulation of 5-HT3AR/5-HT4R was
associated with the impairment of the gut wall. The decrease in
5-HT contents and 5-HT3AR/5-HT4R expression in the colon not only
affected colonic excitability and lowered motility, but also
disabled feedback colonic sensation signaling to the nervous center
via the ascending afferent fibers of vagus and spinal nerves.
Thereby impeding, to a certain degree, the defecation reflex, and
increasing constipation. This mechanism of the involvement of 5-HT
and 5-HT3AR/5-HT4R in SCI has also been proposed in other studies
(48) and is supported by the
restoration of some colon function by intrathecal infusion of 5-HT
agonists (49). In addition,
impaired 5-HT axis is likely to affect enteric mucosal secretion
and the release of other neurotransmitters that further interfere
with the colonic transmission function. Moreover, according to Zhu
et al (50), SCI rats with
defecation dysfunction had decreased ICCs, degenerated colon
function that could be associated with decreased 5-HT contents, and
downregulated 5-HT3AR and 5-HT4R expression. Nevertheless, the
other neurotransmitters and factors secreted by the colonic mucosa
that could impact intestinal function remain to be determined in
detail.
SNS acts on the visceral sensory fibers of the
sacral nerve and sends excitatory impulses to the sacral cord,
thereby activating interneurons in the spinal cord, augmenting
afferent impulses of visceral sensation, and exciting the lower
center (41). Together, these
effects lead to efferent impulses via the visceral motor fibers and
increase the contraction of the lower part of the colon and rectum
through the pelvic nerve, thereby triggering defecation (43). There are a lack of studies
concerning the neurotransmitters and receptors involved in the
central and peripheral effects of SNS. Nevertheless, the effects of
SNS on improving colonic motility and shortening colon transit
time, thereby ameliorating constipation, are well known (36–39,41).
At the molecular level, after SNS, the mRNA and
protein levels of 5-HT3AR and 5-HT4R were upregulated in the spinal
cord at the S2-4 segments, where the sacral lower defecation center
is located (51). Since the
expression of 5-HT3AR and 5-HT4R in the spinal cord is positively
associated with visceral sensation (52), it is reasonable to assume that this
effect of SNS is at least one of the beneficial actions it has on
the colon. IHC staining showed that SNS increased the expression of
5-HT3AR and 5-HT4R proteins in the dorsal horn of spinal cord at
the S2-4 segments, implying that SNS generates excitatory visceral
sensation and conduction to the lower center, as supported by
previous studies (40,41). In addition, 5-HT3AR and 5-HT4R
proteins were upregulated in the intermediolateral nucleus at the
S2-4 segments, associated with the visceral motor and in colonic
myenteric plexus.
Based on previous studies and the known effects of
5-HT3AR and 5-HT4R on intestinal function (13,15–17),
it can be speculated that the effects of SNS are due to the
upregulation of 5-HT3AR and 5-HT4R. Nevertheless, the present study
was not designed to determine how SNS improved colonic histology
and function, and how it can upregulate 5-HT3AR and 5-HT4R
expression.
However, the present study does have limitations.
There was no control group of rats without SCI that were treated
with SNS. In addition, the present study was performed in the acute
phase of SCI and additional studies are necessary to confirm the
results in chronic SCI. Inflammation should also be examined in
future studies. As the low-level center for colonic motility is in
S2-S4, only the spinal samples of S2-S4 were obtained in this
study. Samples from the other levels were not obtained. Finally,
only 5-HT3AR and 5-HT4R were studied, it is likely that SNS affects
the neurons as a whole, rather than only specifically 5-HT3AR and
5-HT4R. In addition, 5-HT3AR and 5-HT4R were only examined at S2-4
and it is unknown whether they are changed at other levels.
Additional studies are necessary to examine these
issues. In particular, the studies of other neurotransmitters and
factors secreted by the colonic mucosa could shed additional light
on the matter. Period circadian protein 2 (Per2) is known to be
involved in the colonic circadian rhythm and electroacupuncture has
been shown to affect Per2 expression in rats with SCI (53). Per2 should be studied in relation
to 5-HT in SCI models. Nitric oxide and oxidative stress are also
involved in the effect of electroacupuncture on intestinal function
in SCI (54). The aim of the
present study was to investigate whether SNS could up-regulate 5-HT
and 5-HT3AR/5-HT4R to improve the recovery of fecal discharge
functions in rat models of SCI. Future studies should examine the
mechanisms responsible for SNS upregulating 5-HT and
5-HT3AR/5-HT4R. Taken together, these studies and the present one
indicate that intestinal function in SCI is a complex process.
Additional and comprehensive studies are necessary to unravel the
exact mechanisms.
To conclude, SNS increases 5-HT3AR/5-HT4R expression
in the sacral defecation center, increases 5-HT content and
5-HT3AR/5-HT4R expression in the colon, improves the defecation
reflex, and promotes the recovery of the colonic transmission
function in rats with SCI.
Acknowledgements
Not applicable.
Funding
The study was supported by grants from China
Postdoctoral Science Foundation (grant no. 2016M602847) and the
Natural Science Foundation of Shaanxi Province (grant no.
20168291).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JC carried out the studies, participated in
collecting data and drafted the manuscript. JY participated in the
analysis and interpretation of data. YY, JG, and WZ acquired the
data. BX performed the animal experiments and acquired the
behavioral data. HL and DH participated in the analysis and
interpretation of the data, and drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
We certify that all applicable institutional and
governmental regulations concerning the ethical use of animals were
followed during the course of this research. The experiments were
approved by the Animal Care and Use Committee of Xi'an Jiaotong
University Health Science Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NYU
|
New York University
|
|
SCI
|
spinal cord injury
|
|
CNS
|
central nervous system
|
|
IHC
|
immunohistochemistry
|
|
AOD
|
average optical density
|
|
5-HT
|
serotonin
|
|
5-HTR
|
serotonin receptor
|
References
|
1
|
DeVivo MJ and Vogel LC: Epidemiology of
spinal cord injury in children and adolescents. J Spinal Cord Med.
27 (Suppl 1):S4–S10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lynch AC, Antony A, Dobbs BR and Frizelle
FA: Bowel dysfunction following spinal cord injury. Spinal Cord.
39:193–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CW, Huang CC, Yang YH, Chen SC, Weng
MC and Huang MH: Relationship between neurogenic bowel dysfunction
and health-related quality of life in persons with spinal cord
injury. J Rehabil Med. 41:35–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piatt JA, Nagata S, Zahl M, Li J and
Rosenbluth JP: Problematic secondary health conditions among adults
with spinal cord injury and its impact on social participation and
daily life. J Spinal Cord Med. 39:693–698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brading AF and Ramalingam T: Mechanisms
controlling normal defecation and the potential effects of spinal
cord injury. Prog Brain Res. 152:345–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krassioukov A, Eng JJ, Claxton G,
Sakakibara BM and Shum S: Neurogenic bowel management after spinal
cord injury: A systematic review of the evidence. Spinal Cord.
48:718–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grundy D: 5-HT system in the gut: Roles in
the regulation of visceral sensitivity and motor functions. Eur Rev
Med Pharmacol Sci. 12 (Suppl 1):S63–S67. 2008.
|
|
8
|
Linan-Rico A, Ochoa-Cortes F, Beyder A,
Soghomonyan S, Zuleta-Alarcon A, Coppola V and Christofi FL:
Mechanosensory signaling in enterochromaffin cells and 5-HT
release: Potential implications for gut inflammation. Front
Neurosci. 10:5642016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wouters MM, Gibbons SJ, Roeder JL, Distad
M, Ou Y, Strege PR, Szurszewski JH and Farrugia G: Exogenous
serotonin regulates proliferation of interstitial cells of Cajal in
mouse jejunum through 5-HT2B receptors. Gastroenterology.
133:897–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camilleri M: Serotonergic modulation of
visceral sensation: Lower gut. Gut. 51 (Suppl 1):i81–i86. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sveshnikov DS, Torshin VI, Smirnov VM,
Kuchuk AV and Myasnikov IL: The significance of different
5-HT-receptors in regulation of gastrointestinal motility. Patol
Fiziol Eksp Ter. 45–51. 2014.(In Russian). PubMed/NCBI
|
|
12
|
Yu Y, Chen JH, Li H, Yang Z, Du X, Hong L,
Liao H, Jiang L, Shi J, Zhao L, et al: Involvement of 5-HT3 and
5-HT4 receptors in colonic motor patterns in rats.
Neurogastroenterol Motil. 27:914–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Machu TK: Therapeutics of 5-HT3 receptor
antagonists: Current uses and future directions. Pharmacol Ther.
130:338–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faerber L, Drechsler S, Ladenburger S,
Gschaidmeier H and Fischer W: The neuronal 5-HT3 receptor network
after 20 years of research-evolving concepts in management of pain
and inflammation. Eur J Pharmacol. 560:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu HN, Ohya S, Nishizawa Y, Sawamura K,
Iino S, Syed MM, Goto K, Imaizumi Y and Nakayama S: Serotonin
augments gut pacemaker activity via 5-HT3 receptors. PLoS One.
6:e249282011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozcan CU, Yilmaz O, Gurer DE, Ayhan S,
Taneli C and Genc A: Evaluation of the relation between
interstitial cells of cajal (CD117) and serotonin receptor (5HT-3A)
with postfundoplication dysphagia. Int J Surg. 13:137–141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Talley NJ: Review article:
5-hydroxytryptamine agonists and antagonists in the modulation of
gastrointestinal motility and sensation: Clinical implications.
Aliment Pharmacol Ther. 6:273–289. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gershon MD: Review article: Roles played
by 5-hydroxytryptamine in the physiology of the bowel. Aliment
Pharmacol Ther. 13 (Suppl 2):S15–S30. 1999. View Article : Google Scholar
|
|
19
|
Bhattarai Y, Schmidt BA, Linden DR, Larson
ED, Grover M, Beyder A, Farrugia G and Kashyap PC: Human-derived
gut microbiota modulates colonic secretion in mice by regulating
5-HT3 receptor expression via acetate production. Am J Physiol
Gastrointest Liver Physiol. 313:G80–G87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yaakob N, Malone DT, Exintaris B and
Irving HR: Heterogeneity amongst 5-HT(3) receptor subunits: Is this
significant? Curr Mol Med. 11:57–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L
and Yi-Xuan L: Effect of the 5-HT4 receptor and serotonin
transporter on visceral hypersensitivity in rats. Braz J Med Biol
Res. 45:948–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mader R, Kocher T, Haier J, Wieczorek G,
Pfannkuche HJ and Ito M: Investigation of serotonin type 4 receptor
expression in human and non-human primate gastrointestinal samples.
Eur J Gastroenterol Hepatol. 18:945–950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gilet M, Eutamene H, Han H, Kim HW and
Bueno L: Influence of a new 5-HT4 receptor partial agonist,
YKP10811, on visceral hypersensitivity in rats triggered by stress
and inflammation. Neurogastroenterol Motil. 26:1761–1770. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Geddis MS, Wen Y, Setlik W and
Gershon MD: Expression and function of 5-HT4 receptors in the mouse
enteric nervous system. Am J Physiol Gastrointest Liver Physiol.
289:G1148–G1163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emerit MB, Baranowski C, Diaz J, Martinez
A, Areias J, Alterio J, Masson J, Boué-Grabot E and Darmon M: A new
mechanism of receptor targeting by interaction between two classes
of ligand-gated ion channels. J Neurosci. 36:1456–1470. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glatzle J, Sternini C, Robin C, Zittel TT,
Wong H, Reeve JR Jr and Raybould HE: Expression of 5-HT3 receptors
in the rat gastrointestinal tract. Gastroenterology. 123:217–226.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu MT, Rayport S, Jiang Y, Murphy DL and
Gershon MD: Expression and function of 5-HT3 receptors in the
enteric neurons of mice lacking the serotonin transporter. Am J
Physiol Gastrointest Liver Physiol. 283:G1398–G1411. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freeman SL, Glatzle J, Robin CS, Valdellon
M, Sternini C, Sharp JW and Raybould HE: Ligand-induced 5-HT3
receptor internalization in enteric neurons in rat ileum.
Gastroenterology. 131:97–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morita H, Mochiki E, Takahashi N, Kawamura
K, Watanabe A, Sutou T, Ogawa A, Yanai M, Ogata K, Fujii T, et al:
Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on
gastrointestinal motor activity in dogs. World J Gastroenterol.
19:6604–6612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JH, Zhang Q, Yu Y, Li K, Liao H,
Jiang L, Hong L, Du X, Hu X, Chen S, et al: Neurogenic and myogenic
properties of pan-colonic motor patterns and their spatiotemporal
organization in rats. PLoS One. 8:e604742013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong EJ, Chung SY, Hong HN, Oh SW and Sim
JY: The novel, potent and highly selective 5-HT4 receptor agonist
YH12852 significantly improves both upper and lower
gastrointestinal motility. Br J Pharmacol. 175:485–500. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nardone R, Höller Y, Thomschewski A,
Höller P, Lochner P, Golaszewski S, Brigo F and Trinka E:
Serotonergic transmission after spinal cord injury. J Neural Transm
(Vienna). 122:279–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koyama Y, Kondo M and Shimada S: Building
a 5-HT3A receptor expression map in the mouse brain. Sci Rep.
7:428842017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou J, Li H, Liu XY, Wang YY, Li YQ and
Wu SX: Expression of 5-HT2, 4, 5 receptor subtype mRNAs in rat
spinal dorsal and ventral horns of different segments. J Fourth Mil
Med Univ. 25:1345–1348. 2004.
|
|
35
|
Sun J, Wu X, Meng Y, Cheng J, Ning H, Peng
Y, Pei L and Zhang W: Electro-acupuncture decreases 5-HT, CGRP and
increases NPY in the brain-gut axis in two rat models of
Diarrhea-predominant irritable bowel syndrome (D-IBS). BMC
Complement Altern Med. 15:3402015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iqbal F, Thomas GP, Tan E, Askari A,
Dastur JK, Nicholls J and Vaizey CJ: Transcutaneous sacral
electrical stimulation for chronic functional constipation. Dis
Colon Rectum. 59:132–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fassov J, Brock C, Lundby L, Drewes AM,
Gregersen H, Buntzen S, Laurberg S and Krogh K: Sacral nerve
stimulation changes rectal sensitivity and biomechanical properties
in patients with irritable bowel syndrome. Neurogastroenterol
Motil. 26:1597–1604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dinning PG, Hunt L, Patton V, Zhang T,
Szczesniak M, Gebski V, Jones M, Stewart P, Lubowski DZ and Cook
IJ: Treatment efficacy of sacral nerve stimulation in slow transit
constipation: A two-phase, double-blind randomized controlled
crossover study. Am J Gastroenterol. 110:733–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Worsøe J, Fynne L, Laurberg S, Krogh K and
Rijkhoff NJ: Acute effect of electrical stimulation of the dorsal
genital nerve on rectal capacity in patients with spinal cord
injury. Spinal Cord. 50:462–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Worsøe J, Rasmussen M, Christensen P and
Krogh K: Neurostimulation for neurogenic bowel dysfunction.
Gastroenterol Res Pract. 2013:5632942013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elkelini MS, Pravdivyi I and Hassouna MM:
Mechanism of action of sacral nerve stimulation using a transdermal
amplitude-modulated signal in a spinal cord injury rodent model.
Can Urol Assoc J. 6:227–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gourcerol G, Vitton V, Leroi AM, Michot F,
Abysique A and Bouvier M: How sacral nerve stimulation works in
patients with faecal incontinence. Colorectal Dis. 13:e203–e211.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abdel-Halim M: Studies of the Mechanisms
of Sacral Nerve Stimulation for Faecal Incontinence: Investigations
of Anorectal and Pelvic Floor Physiology and Function. University
College London. (Division of Surgery and Interventional Science).
2012.
|
|
44
|
Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI
and Chopp M: Progesterone is neuroprotective after acute
experimental spinal cord trauma in rats. Spine (Phila Pa 1976).
24:2134–2138. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barbalias GA, Klauber GT and Blaivas JG:
Critical evaluation of the Crede maneuver: A urodynamic study of
207 patients. J Urol. 130:720–723. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Awad RA: Neurogenic bowel dysfunction in
patients with spinal cord injury, myelomeningocele, multiple
sclerosis and Parkinson's disease. World J Gastroenterol.
17:5035–5048. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Guertin PA: New pharmacological approaches
against chronic bowel and bladder problems in paralytics. World J
Crit Care Med. 5:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu Y, Yang Y, Guo J, Zhang W, Zhu Z, Xie
B, Yu J and Cheng J: Abdominal manual therapy repairs interstitial
cells of cajal and increases colonic c-Kit expression when treating
bowel dysfunction after spinal cord injury. Biomed Res Int.
2017:14923272017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shafik A: Recto-colic reflex: Role in the
defecation mechanism. Int Surg. 81:292–294. 1996.PubMed/NCBI
|
|
52
|
Zhao JM, Li L, Chen L, Shi Y, Li YW, Shang
HX, Wu LY, Weng ZJ, Bao CH and Wu HG: Comparison of the analgesic
effects between electro-acupuncture and moxibustion with visceral
hypersensitivity rats in irritable bowel syndrome. World J
Gastroenterol. 23:2928–2939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng J, Wang X, Guo J, Yang Y, Zhang W,
Xie B, Zhu Z, Lu Y and Zhu Y: Effects of electroacupuncture on the
daily rhythmicity of intestinal movement and circadian rhythmicity
of colonic Per2 expression in rats with spinal cord injury. Biomed
Res Int. 2016:98602812016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Guo J, Zhu Y, Yang Y, Wang X, Chen B,
Zhang W, Xie B, Zhu Z, Yue Y and Cheng J: Electroacupuncture at
Zusanli (ST36) ameliorates colonic neuronal nitric oxide synthase
upregulation in rats with neurogenic bowel dysfunction following
spinal cord injury. Spinal Cord. 54:1139–1144. 2016. View Article : Google Scholar : PubMed/NCBI
|