Introduction
Diabetes mellitus (DM) is a metabolic disease that
is primarily characterized by hyperglycemia (1). Diabetic neuropathic pain (DNP) is one
of the intractable complications of DM and ~30% of patients with
diabetes suffer from DNP (2),
which can result in peripheral nerve dysfunctions, such as nerve
degeneration, allodynia, hyperalgesia and insensitivity (3). Thermal hyperalgesia and mechanical
allodynia are the most common forms of DNP (4). Compared with thermal hyperalgesia,
diabetic mechanical allodynia (DMA) severely affects the quality of
life of patients with diabetes (5). To a certain extent, thermal
hyperalgesia can be avoided by reducing contact with heat sources;
however, mechanical stimulation is ubiquitous and can cause
mechanical allodynia in patients when they are performing everyday
tasks, such as getting dressed (5). The molecular mechanisms underlying
DMA development and maintenance are not completely understood;
therefore, the clinical prevention and treatment of DMA is a
challenge.
Resveratrol (3,4′,5,-trihydroxystilbene; RES) is a
natural polyphenolic phytoalexin with a number of beneficial
abilities, such as neuroprotective, antitumorigenic, antioxidative
stress and anti-inflammatory effects (6–8). RES
promotes nerve regeneration, and improves the pathological and
behavioral outcomes of various peripheral and central nerve
injuries, including sciatic nerve crush (9), nerve root avulsion (10), chronic constriction of dorsal root
ganglion (DRG) (11), traumatic
brain (12) and spinal cord
(13) injuries. RES can also
alleviate pain by decreasing inflammatory responses via inhibition
of proinflammatory cytokines and induction of anti-inflammatory
cytokine release in the nerves or local tissue (14). In addition, the analgesic effect of
RES on trigeminal neuralgia involves suppression of glial
activation (15). It has been
reported that RES alone or in combination with insulin or 4-amino
1,8 naphthalimide (4-ANI) can attenuate thermal hyperalgesia from
the 4–8th week following the administration of streptozocin (STZ)
in rats (16–18). However, whether RES influences
mechanical allodynia within the 4 weeks following the injection of
STZ is still not completely understood.
ATP is a major signaling molecule in cell
communication and a major neurotransmitter in the nervous system
(19). The purinergic receptor
P2X3 (P2X3R) can be activated by ATP release following peripheral
tissue damage. P2X3R is primarily localized on small nociceptive
sensory neurons in the DRG, trigeminal ganglia and nodose ganglia,
and serves an important role in nociceptive transmission (20). The expression and function of P2X3R
are markedly enhanced in DRG neurons in diabetic model rats, and
hindpaw pain hypersensitivity is attenuated by the injection of a
P2X3R antagonist (21). Recently,
it was reported that RES can alleviate neuropathic pain in a model
of partial sciatic nerve ligation (pSNL) by suppressing increases
in P2X3R and ERK phosphorylation in the DRG (22). Furthermore, it has been reported
that protein expression levels of P2X3R in DRG neurons and spinal
dorsal horn (SDH) fibers are increased from the 2nd-4th week in a
rat model of STZ-induced DM (23).
However, whether the analgesic role of RES is associated with P2X3R
expression in DMA is not completely understood.
Therefore, the present study investigated the
effects of RES on DMA-related behaviors in rats. Moreover, to
investigate the analgesic role and possible mechanisms underlying
RES, alterations to the expression of P2X3R in the DRG and SDH
following ResED50 treatment were assessed.
Materials and methods
Animal preparation
Male Sprague-Dawley rats (weight, 180–220 g; age,
6–7 weeks) were supplied by the Experimental Animal Center of Xi'an
Jiao Tong University. All rats were housed in an environment with a
controlled temperature (22-25°C) and humidity (50-65%) with 12-h
light/dark cycles, and free access to food and water. Rats were
allowed to adapt to the environment for 7 days prior to subsequent
experiments. The present study was approved by the Animal Care and
Ethical Committee at Xi'an Medical University and all animal
experiments were performed according to the Guidelines of the
International Association for the Study of Pain (24). All efforts were made to minimize
animal suffering and to follow the 3Rs (reduction, refinement and
replacement).
Preparation of the rat model of DMA:
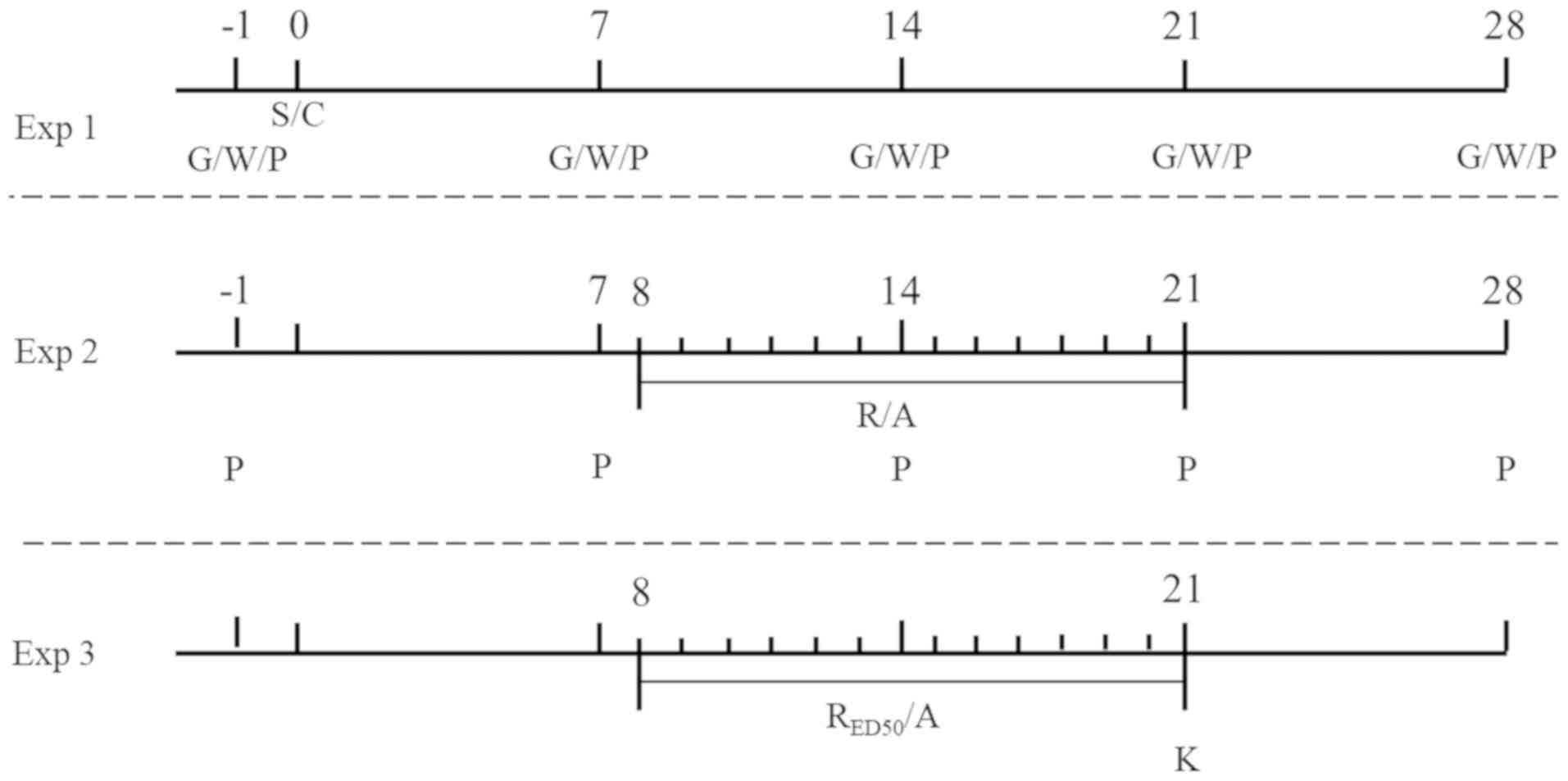
Experiment (Exp) 1
The preparation of the rat model of DMA is presented
in Fig. 1. Initially, a rat model
of DM was established. STZ (Sigma-Aldrich; Merck KGaA) was diluted
using citrate buffer (v:v=1:1.32; pH 4.5; cat. no. C1949; Tokyo
Chemical Industry UK Ltd.). The rats were randomly divided into two
groups: i) The DM group received an intraperitoneal injection of 60
mg/kg STZ; and ii) the vehicle group received an equal volume of
citrate buffer.
On 1 day prior to and 7, 14, 21 and 28 days post-STZ
injection, blood glucose, body weight and paw withdrawal threshold
(PWT) were measured. Rats with a non-fasting blood glucose
concentration >16.7 mmol/l (300 mg/dl) at 72 h after the STZ
injection were considered as DM model rats. Body weight was also
considered as an additional index for successful establishment of
the rat model of DM. Compared with the vehicle group, the increased
body weight in DM model rats was slow, and there was a significant
difference between two groups (P<0.01). Mechanical allodynia was
detected using flexile von Frey filaments to select for DMA model
rats among DM model rats, as previously described (25). Briefly, the rats were individually
placed in Plexiglas® boxes for ~20 min to allow them to
acclimate to the testing environment prior to the experiment.
Subsequently, von Frey filaments (0.6–15 g; Stoelting Co.) were
pressed perpendicularly on the plantar surface of the hind paw and
were bent with sufficient force for ~5 sec. Each test was repeated
at least 5 times at 3-min intervals. The PWT was recorded as the
minimal value resulting in at least 3 incidences of brisk
withdrawal or paw flinching. PWT tests were performed on 1 day
prior to and 7, 14, 21 and 28 days post-STZ administration to
estimate DMA-related pain behaviors. Rats displaying an obvious
decline in PWT on day 7 post-STZ injection were regarded as DMA
model rats. All behavioral experiments were blinded and were
performed between 09:00-18:00. Based on the results of PWT on the
7th day after STZ injection, a total of 99 DMA model rats were
randomly divided into groups: i) Exp 1 (n=15); ii) Exp 2 (n=60);
and iii) Exp 3 (n=24). A further 27 vehicle rats were used in the
present study (15 in Exp 1 and 12 in Exp 3). A total of 220 rats
were used in the present study, among which 126 (DMA 99 and vehicle
27) were used as effective experimental rats for the Exp1-3 and
including 94 rats that were not successfully prepared as DMA model
rats by STZ administration.
Evaluation of the analgesic effect of
RES and calculation of the RESED50
The aim of Exp 2 was to evaluate the repetitive
intragastric administration of various doses of RES on DMA-related
behaviors and to calculate the RESED50 (Fig. 1). RES (Sigma-Aldrich; Merck KGaA)
was diluted using alcohol-saline (AS; v:v=1:5). DMA model rats were
randomly divided into the following four groups (n=15 per group):
i) DMA + AS; ii) DMA + RES (25 mg/kg); iii) DMA + RES (100 mg/kg);
and iv) DMA + Res (400 mg/kg). DMA + AS group rats received the
same volume of AS and were regarded as the control groups. The
doses of RES selected for the present study were based on a
previous report (15). All groups
received AS/RES for 14 consecutive days from day 8–21 post-STZ
injection.
PWTs were measured weekly until one week post-RES/AS
withdrawal. The area under the time-course curve (AUC) values were
used to evaluate the overall effect of RES, according to previous
studies (26–30). The goodness of fit was presented as
the followings: Degrees of freedom=56; R2=0.8025;
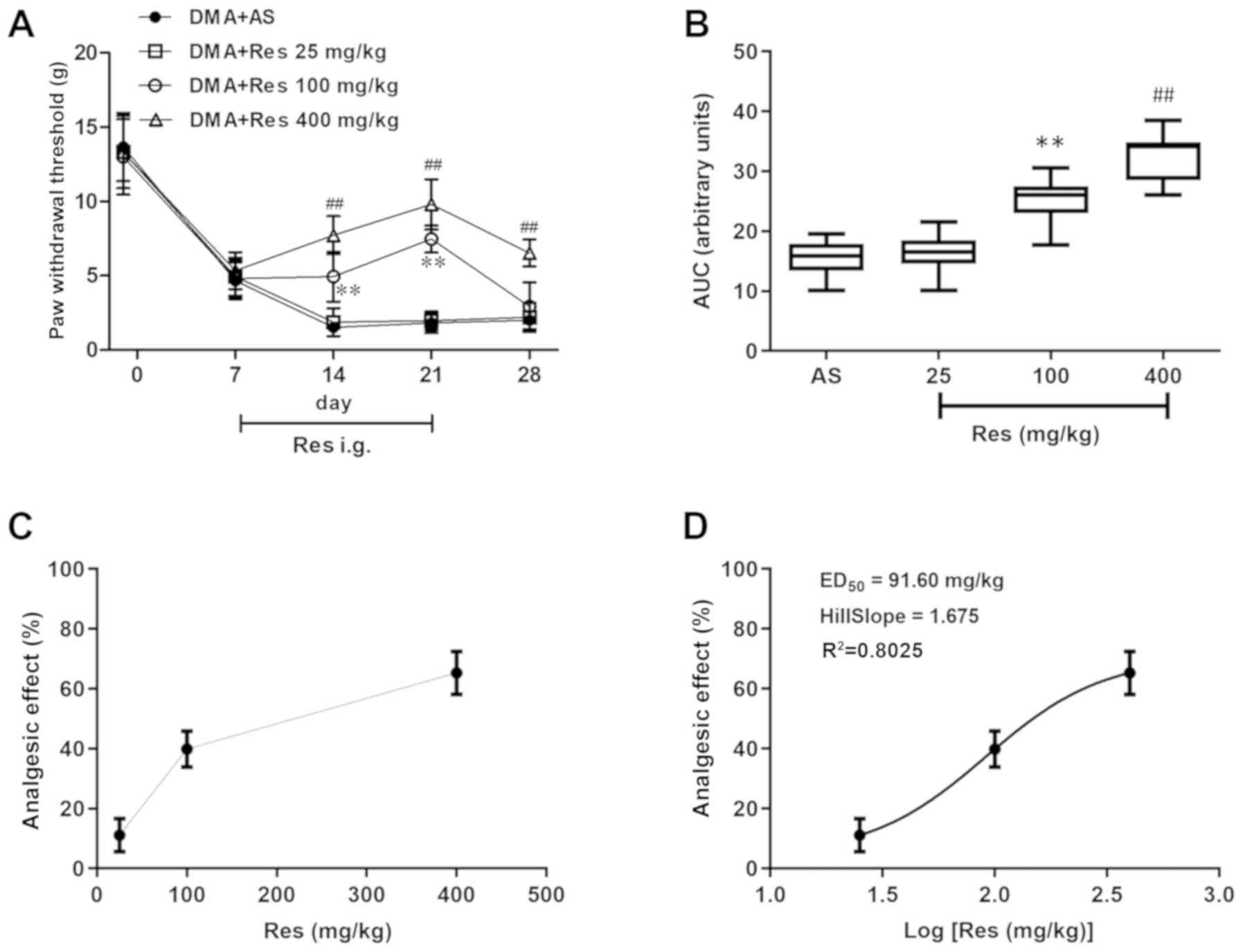
absolute sum of squares=11454; and Sy.x=60. The ED50 was
calculated from the best fitting curve. The doses of
intragastrically administered RES were transformed into the
logarithm dose and the non-line fit was created to establish the
dose-effect curve. Based on the dose-effect curve,
RESED50 (ED50=median effective dose) was calculated
according to the following formula:
Y=Emin+(Emax-Emin)/[1+10^((LogED50-X) × HillSlope)]. Best-fit
values in the formula were: Emin=4.359%, Emax=70.460%,
HillSlope=1.675, Y=50%, Best-fit X=2.170.
Assessment of alterations in the
expression of P2X3R after the administration of
RESED50
The effect of RESED50 on P2X3R protein
expression in the DRG and SDH was assessed (Exp 3; Fig. 1). The 24 DMA rats and 12 vehicle
rats were equally used for immunofluorescence staining and western
blotting, respectively. Then for each approach, rats were randomly
divided into three groups as follows (n=6 per group): i) The
vehicle + AS group, which consisted of vehicle rats receiving AS;
ii) the DMA + AS group, which consisted of DMA model rats receiving
AS; and iii) the DMA + RESED50 group, which consisted of
DMA model rats receiving RESED50. Rats received
AS/RESED50 for 14 consecutive days from day 8–21
post-STZ injection.
Immunofluorescence staining
Following 14 days of continuous administration of
AS/RESED50, rats were anaesthetized by the
intraperitoneal injection of sodium pentobarbital (65 mg/kg).
Subsequently, rats were transcardially perfused with 100 ml 0.9%
normal saline, followed by 500 ml PBS (pH 7.2; 0.1 mol/l)
containing 4% paraformaldehyde. The L4-5 spinal segments
and the corresponding DRGs were harvested and post-fixed in 4%
paraformaldehyde for 2–4 h at 4°C. Subsequently, the tissues were
cryoprotected overnight at 4°C in 0.1 mol/l PBS containing 30%
sucrose. Frozen transverse sections of spinal cord (SC) (thickness,
30 µm) and horizontal sections of DRG (thickness, 10 µm) were cut
using a CM1800 cryostat (Leica Microsystems GmbH). The sections
were immunofluorescently stained to detect P2X3R expression.
Briefly, tissue sections were rinsed with 0.01 mol/l PBS (pH 7.2)
and blocked with 10% FBS (cat. no. F8687; Sigma-Aldrich; Merck
KGaA) in PBS (0.01 mol/l) for 1 h at room temperature.
Subsequently, the sections were incubated with a rabbit anti-P2X3R
antibody (cat. no. ab10269; 1:1,000; Abcam) at 4°C overnight.
Following primary incubation, the sections were incubated with a
biotinylated donkey anti-rabbit IgG (cat. no. AP182F; 1:500; EMD
Millipore) secondary antibody at room temperature for 6–8 h. The
sections were also incubated with FITC-labeled avidin D (cat. no.
A-2001; 1:1,000; Vector Laboratories, Inc.) with DAPI (cat. no.
D9542; 1:1,000; Sigma-Aldrich; Merck KGaA) at room temperature for
2 h. DAPI staining was performed to count the number of DRG neurons
(data not shown). Between each step, the sections were rinsed with
PBS (0.01 mol/l). The incubation medium used for the primary and
second antibodies was PBS (0.01 mol/l; pH 7.4) supplemented with 2%
normal donkey serum (cat. no. ZB-2301; OriGene Technologies, Inc.),
0.3% Triton X-100 (pH 7.4), 0.25% λ-carrageen (cat. no. C1013;
Sigma-Aldrich; Merck KGaA) and 0.05% sodium azide. The incubation
medium used for FITC-avidin was PBS (0.01 mol/l; pH 7.4)
supplemented with 0.3% Triton X-100. Stained sections were observed
using a FV1000 confocal laser scanning microscope (Olympus
Corporation; magnification, ×100). The optical density of
P2X3R-immunoreactivity in DRG or spinal dorsal horn (SDH) was
measured by ImageJ (v1.46r; National Institutes of Health) and the
percentage of P2X3R positive cells over total neurons were
calculated.
Western blotting
Rats were anesthetized by the intraperitoneal
injection of sodium pentobarbital, and the L4-5 segments
of SDH and bilateral DRGs were immediately harvested on ice, as
previously described (31). The
protein was extracted from SDHs and DRGs using the lysate
(RIPA:PMSF=100:1; RAPI; cat. no. P0013; Beyotime Institute of
Biotechnology; PMSF; cat. no. ST506; Beyotime Institute of
Biotechnology). The protein concentration of samples was measured
by the bicinchoninic acid method using the albumin standard (cat.
no. 23209; Thermo Fisher Scientific, Inc.). Briefly, after heating
at 100°C for 5 min, 30 µg of protein were separated via SDS-PAGE on
a 10% gel and transferred onto PVDF membranes (EMD Millipore). The
membranes were blocked with TBS containing 0.02% Tween (TBST) and
5% non-fat dry milk for 1 h at room temperature. Subsequently, the
membranes were incubated overnight at 4°C with the following
primary antibodies: Anti-P2X3R (rabbit; cat. no. ab10269; 1:1,000;
Abcam) or anti-β-actin (mouse; cat. no. A228; 1:5,000;
Sigma-Aldrich; Merck KGaA). Following primary incubation, the
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit (cat. no. AP307P; 1:5,000; EMD Millipore) or anti-mouse
(cat. no. AP124P; 1:5,000; EMD Millipore) secondary antibodies for
1 h at room temperature. Between each step, the membranes were
rinsed three times with TBST for 10 min each. Proteins were
visualized with the ECL kit (cat. no. 32132; Thermo Fisher
Scientific, Inc.). Protein expression levels were quantified using
the ChemiDoc™ MP Imaging System (Bio-Rad Laboratories, Inc.) with
β-actin as the loading control.
Statistical analysis
Data are presented as the mean ± SEM. Researchers
were blinded to the behavioral test and reagents used. The data of
Figs. 2 and 3A were analyzed by repeated measures
ANOVA, and Fig. 4G-I were analyzed
using one-way ANOVA followed by Bonferroni's post hoc test,
respectively. Statistical analyses were performed using GraphPad
Prism software (version 5.01; GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
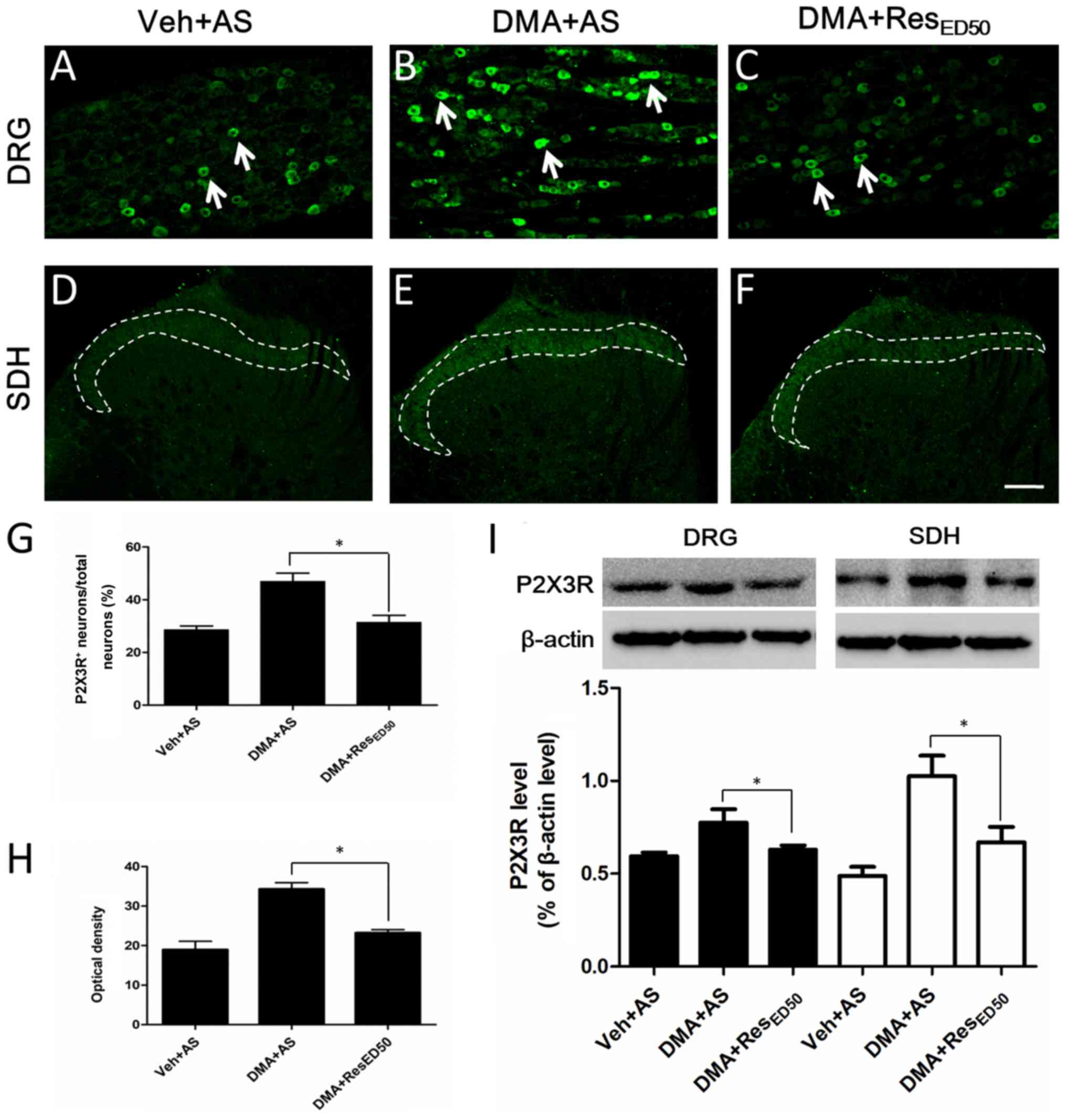 | Figure 4.Alterations to P2X3R expression in
the DRG and SDH. Representative images of P2X3R staining in the DRG
of the (A) Veh + AS, (B) DMA + AS and (C) DMA + RESED50
groups. Arrows indicate typical P2X3R-IR (scale bar=100 µm).
Representative images of P2X3R staining in the SDH of the (D) Veh +
AS, (E) DMA + AS and (F) DMA + RESED50 groups. Dotted lines
indicate typical P2X3R-IR (scale bar=100 µm). (G) Quantification of
the percentage of P2X3R-positive neurons/total neurons in the DRG.
(H) Optical density analysis of P2X3R staining in the SDH. (I)
P2X3R protein expression levels were measured by western blotting
in the DRG and SDH. *P<0.05, as indicated. P2X3R, purinergic
receptor P2X3; DRG, dorsal root ganglion; SDH, spinal dorsal horn;
Veh, vehicle; AS, alcohol-saline; DMA, diabetic mechanical
allodynia; RES, resveratrol; IR, immunoreactivity. |
Results
Characterization of DMA model
rats
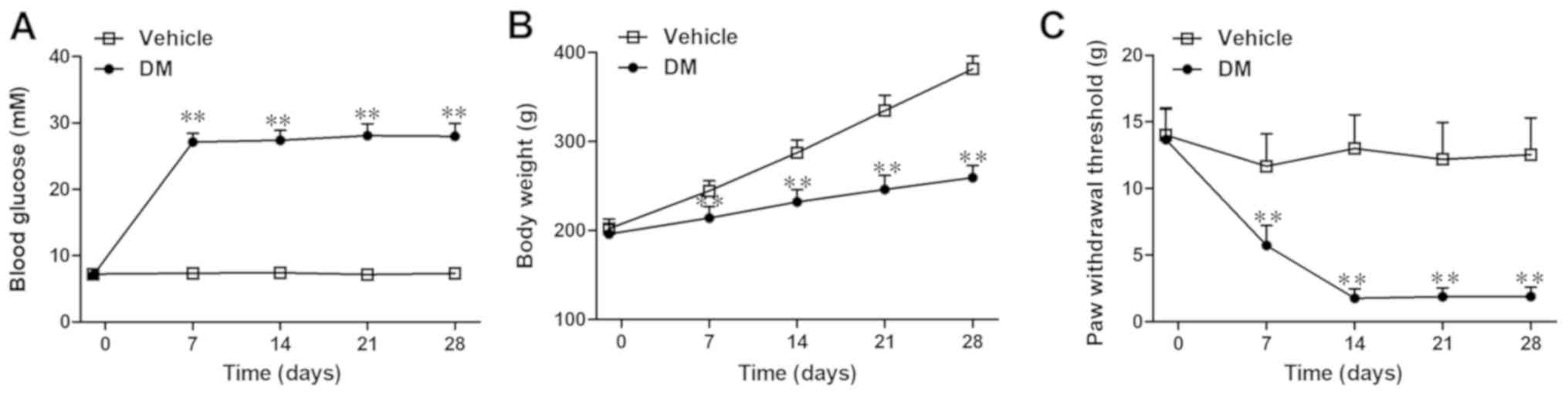
Rats in the vehicle group maintained normal blood
glucose levels, whereas rats in the DM group developed
hyperglycemia during the 4 weeks following the STZ injection (day
7: 27.1±1.3 vs. 7.35±0.6 mmol/l; P<0.01; Fig. 2A). Increased body weight was
significantly impaired in the DM group compared with the vehicle
group (day 7: 214.0±13.0 vs. 245.0±11.3 g; P<0.01; Fig. 2B). Furthermore, rats in the DM
group displayed a significant decrease in PWT on day 7 post-STZ
injection compared with the vehicle group, which indicated that the
DMA had been successfully induced (day 7: 5.7±1.5 vs. 11.7±2.4 g;
P<0.01; Fig. 2C). Moreover, the
DM group rats displayed evident mechanical allodynia from day 7–28
post-STZ injection, with a peak on day 14 compared with the vehicle
group (Fig. 2C).
RES alleviates STZ-induced diabetic
mechanical allodynia in rats
The analgesic effects of RES on DMA-related
behaviors were investigated. Compared with the DMA + AS group, both
the DMA + Res (400 mg/kg) and DMA + Res (100 mg/kg) groups
significantly increased the PWT, with the maximal pain-relieving
effect observed on day 21 post-STZ injection [DMA + Res (400
mg/kg), 9.8±1.7; DMA + Res (100 mg/kg), 7.5±0.9; DMA + AS, 1.8±0.7;
P<0.01; Fig. 3A]. After RES
administration was terminated on day 22 post-STZ injection, the
enhanced PWT was decreased by day 28 post-STZ injection to a level
similar to the sensitized level of DMA + AS group; however, the PWT
of the DMA + RES (400 mg/kg) group was still significantly
increased compared with the DMA + AS group. Subsequently, as
indicated by the AUC values of PWT, 100 and 400 mg/kg RES displayed
a significant analgesic effect on DMA compared with the DMA + AS
group (Fig. 3B). Furthermore, the
effects of various doses of RES on the PWT were assessed using the
dose or log(dose) vs. response curve, and RESED50 was
calculated as 91.60 mg/kg (Fig. 3C and
D).
RESED50 downregulates P2X3R
expression in DMA model rats
A previous study has indicated that the protein
expression of P2X3R is significantly increased during the
development of DMA, particularly on day 21 post-DMA induction
(23). Therefore, whether P2X3R
was associated with the analgesic effects of RES on DMA-related
behaviors was investigated. Compared with the Veh + AS group, the
expression of P2X3R was notably increased in the DMA + AS group,
particularly in the middle- and small-sized neurons in the DRG (as
indicated by arrows in Fig. 4B)
and in the fibers of laminae I and II in the SDH (as indicated by
dotted lines in Fig. 4E). P2X3R
expression was markedly decreased in the DRG and SDH in the DMA +
RESED50 group compared with the DMA + AS group (Fig. 4C and F). The percentage of
P2X3R-positive neurons/total neurons was significantly decreased in
the DMA + RESED50 group compared with the DMA + AS
group, decreasing from 47 to 31% (P<0.05; Fig. 4G). Similar alterations to P2X3R
expression levels were observed in the SDH (Fig. 4H), whereby the optical density of
P2X3R-immunoreactive fibers in the DMA + RESED50 group
was significantly lower compared with the DMA + AS group. The
results indicated that inhibition of P2X3R overexpression may be
the mechanism underlying RES-mediated alleviation of DMA-related
behaviors.
To further investigate the aforementioned
observations, P2X3R protein expression was examined by western
blotting. Similarly, in both the DRG and SDH, P2X3R expression was
significantly decreased in DMA tissues following RESED50
treatment compared with the DMA + AS group (P<0.05; Fig. 4I).
Discussion
In the present study, the effects and possible
mechanisms underlying RES in STZ-induced DMA model rats were
assessed using behavioral pharmacological methods, as well as
morphological and molecular biological analyses. The results
indicated that: i) The intragastric administration of RES
effectively alleviated DMA-associated pain behaviors; ii) the
ED50 of RES was 91.6 mg/kg for analgesic effects on
DMA-related behaviors; and iii) in the DRG neurons and SDH
terminals, the protein expression level of P2X3R was significantly
decreased after RESED50 administration for 14 continuous
days. The results suggested that RES effectively relieved
STZ-induced DMA in rats and that downregulation of P2X3R expression
may be a possible mechanism underlying RES-mediated effects.
RES displays anti-inflammatory, antioxidative,
antitumorigenic and neuroprotective effects (32–34).
It has also been reported that RES attenuates mechanical allodynia
and thermal hyperalgesia in rats with chronic constriction injury
by enhancing interleukin (IL)-4 receptor-mediated anti-inflammatory
responses in the spinal cord (35). A previous study demonstrated that
mechanical allodynia was present within the 2 weeks following
STZ-induced diabetes and persisted for a further 7 weeks (36). It has also been reported that the
daily oral administration of RES from week 4 post-STZ injection
mitigates thermal hyperalgesia in diabetic model mice (16). In the present study, the
intragastric administration of RES effectively alleviated
DMA-associated pain behaviors in a dose-dependent manner from week
2–4 post-STZ injection. Collectively, the results suggested that
RES displayed an analgesic effect against mechanical allodynia in
DM model rats.
DNP-associated hyperglycemia can lead to DRG
dysfunction, and may affect some ion channels or receptors in
neurons (37). Transient receptor
potential vanilloid 1 and voltage-gated sodium channel
NaV1.7 expression are significantly increased in DRG
neurons of DNP model rats (31,38).
It has been reported that increased P2X3R or P2X7R expression in
the DRG in an animal model of neuropathic pain is induced by pSNL
or chronic constriction injury, respectively. RES treatment
decreases the expression of P2X3R or P2X7R, which indicates that
RES alleviates pain behavior in rats with neuropathic pain by
inhibiting the upregulated expression of P2X receptors (11,22).
In a previous study, P2X3R protein expression levels increased in
the DRG and SDH with the development of DMA (23). In the present study, the results
suggested that repetitive administration of RES downregulated P2X3R
overexpression in DRG neurons and SDH terminals in DMA model rats.
The results indicated that RES may ameliorate DMA by attenuating
increased P2X3R expression in DMA model rats.
However, only the preliminary mechanism underlying
RES-mediated alleviation of STZ-induced diabetic mechanical
allodynia was investigated in the present study. According to a
previous study, P2X3 receptor can participate in mechanical and
thermal pain in pSNL model rats via the ERK signaling pathway
(22). Some proinflammatory
cytokines, such as tumor necrosis factor-α and IL-1β, also activate
signaling pathways in peripheral sensory neurons, leading to
downstream activation/sensitization of P2X3 (39). In addition, RES can inhibit
microglia activation via the AMP-activated protein kinase signaling
pathway, thereby reducing morphine tolerance (40). Therefore, future studies should
investigate the association between RES and the ERK signaling
pathways and proinflammatory cytokines.
In conclusion, the present study suggested that RES
displayed an analgesic effect against STZ-induced DMA and the
underlying mechanism may involve downregulation of P2X3R
expression. Therefore, RES may serve as a potential therapeutic
target for DMA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31600951 and
81873740), the Natural Science Basic Research Plan in Shaanxi
Province of China (grant nos. 2017JQ8048, 2018JM7094 and
2018JQ8003), the Research Plan of Xi'an Medical University (grant
nos. 2017PT28 and 2018PT11) and The Youth Innovation Team of
Shaanxi University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, LL and XG conceived and designed the study. YC,
YL and JN performed the majority of the experiments. LL, YM, XW and
ZQ analyzed the data and conducted some of the experiments. YC and
XG contributed reagents, materials and analysis tools. YC and LL
drafted the manuscript. XCG revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Ethical Committee at Xi'an Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association, . (2)
Classification and diagnosis of diabetes. Diabetes Care. 38
(Suppl):S8–S16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guastella V and Mick G: Strategies for the
diagnosis and treatment of neuropathic pain secondary to diabetic
peripheral sensory polyneuropathy. Diabetes Metab. 35:12–19.
2009.
|
|
3
|
Rajchgot T, Thomas SC, Wang JC, Ahmadi M,
Balood M, Crosson T, Dias JP, Couture R, Claing A and Talbot S:
Neurons and Microglia; A sickly-sweet duo in diabetic pain
neuropathy. Front Neurosci. 13:252019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scholz J, Rathmell JP, David WS, Chad DA,
Broderick AC, Perros SG, Shin NS, Wells JL, Davis JB, DiMaggio CJ,
et al: A standardized clinical evaluation of phenotypic diversity
in diabetic polyneuropathy. Pain. 157:2297–2308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dubin AE and Patapoutian A: Nociceptors:
The sensors of the pain pathway. J Clin Invest. 120:3760–3772.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castro OW, Upadhya D, Kodali M and Shetty
AK: Resveratrol for easing status epilepticus induced brain injury,
inflammation, epileptogenesis, and cognitive and memory
dysfunction-are we there yet? Front Neurol. 8:6032017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daverey A and Agrawal SK: Pre and post
treatment with curcumin and resveratrol protects astrocytes after
oxidative stress. Brain Res. 1692:45–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lancon A, Frazzi R and Latruffe N:
Anti-oxidant, anti-inflammatory and anti-angiogenic properties of
resveratrol in ocular diseases. Molecules. 21:3042016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding Z, Cao J, Shen Y, Zou Y, Yang X, Zhou
W, Guo Q and Huang C: Resveratrol promotes nerve regeneration via
activation of p300 acetyltransferase-mediated VEGF signaling in a
rat model of sciatic nerve crush injury. Front Neurosci.
12:3412018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oda H, Ohta S, Ikeguchi R, Noguchi T,
Kaizawa Y, Yurie H, Takeuchi H, Mitsuzawa S and Matsuda S:
Pretreatment of nerve grafts with resveratrol improves axonal
regeneration following replantation surgery for nerve root avulsion
injury in rats. Restor Neurol Neurosci. 36:647–658. 2018.PubMed/NCBI
|
|
11
|
Xie J, Liu S, Wu B, Li G, Rao S, Zou L, Yi
Z, Zhang C, Jia T, Zhao S, et al: The protective effect of
resveratrol in the transmission of neuropathic pain mediated by the
P2X7 receptor in the dorsal root ganglia. Neurochem Int.
103:24–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou P, Liu X, Li G and Wang Y: Resveratrol
pretreatment attenuates traumatic brain injury in rats by
suppressing NLRP3 inflammasome activation via SIRT1. Mol Med Rep.
17:3212–3217. 2018.PubMed/NCBI
|
|
13
|
Zhao H, Chen S, Gao K, Zhou Z, Wang C,
Shen Z, Guo Y, Li Z, Wan Z, Liu C and Mei X: Resveratrol protects
against spinal cord injury by activating autophagy and inhibiting
apoptosis mediated by the SIRT1/AMPK signaling pathway.
Neuroscience. 348:241–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao L, Ding Q, Gao C and Sun X:
Resveratrol attenuates neuropathic pain through balancing
pro-inflammatory and anti-inflammatory cytokines release in mice.
Int Immunopharmacol. 34:165–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang YJ, Hu L, Xia YP, Jiang CY, Miao C,
Yang CQ, Yuan M and Wang L: Resveratrol suppresses glial activation
and alleviates trigeminal neuralgia via activation of AMPK. J
Neuroinflammation. 13:842016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma S, Kulkarni SK and Chopra K: Effect
of resveratrol, a polyphenolic phytoalexin, on thermal hyperalgesia
in a mouse model of diabetic neuropathic pain. Fundam Clin
Pharmacol. 21:89–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma S, Chopra K and Kulkarni SK: Effect
of insulin and its combination with resveratrol or curcumin in
attenuation of diabetic neuropathic pain: participation of nitric
oxide and TNF-alpha. Phytother Res. 21:278–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma SS, Kumar A, Arora M and Kaundal
RK: Neuroprotective potential of combination of resveratrol and
4-amino 1,8 naphthalimide in experimental diabetic neuropathy:
Focus on functional, sensorimotor and biochemical changes. Free
Radic Res. 43:400–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edwards FA and Gibb AJ: ATP-a fast
neurotransmitter. FEBS Lett. 325:86–89. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bernier LP, Ase AR and Séguéla P: P2X
receptor channels in chronic pain pathways. Br J Pharmacol.
175:2219–2230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HH, Hu J, Zhou YL, Qin X, Song ZY,
Yang PP, Hu S, Jiang X and Xu GY: Promoted interaction of nuclear
factor-κB with demethylated purinergic P2X3 receptor gene
contributes to neuropathic pain in rats with diabetes. Diabetes.
64:4272–4284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo J, Wang C, Niu X, Zhou F, Li H and Gao
W: Effects of resveratrol in the signaling of neuropathic pain
involving P2X3 in the dorsal root ganglion of rats. Acta Neurol
Belg. Apr 19–2019.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Cui YY, Wu HH, Wa L, Shi J and Li YQ:
Spatio-temporal expression of P2X3 receptor in rats with diabetic
mechanical allodynia. Acta Anatomica Sinica. 45:540–544. 2014.
|
|
24
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HH, Yin JB, Zhang T, Cui YY, Dong YL,
Chen GZ and Wang W: Inhibiting spinal neuron-astrocytic activation
correlates with synergistic analgesia of dexmedetomidine and
ropivacaine. PLoS One. 9:e923742014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin JB, Zhou KC, Wu HH, Hu W, Ding T,
Zhang T, Wang LY, Kou JP, Kaye AD and Wang W: Analgesic effects of
Danggui-Shaoyao-San on various ‘Phenotypes’ of nociception and
inflammation in a formalin pain model. Mol Neurobiol. 53:6835–6848.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tallarida RJ: Drug synergism: Its
detection and applications. J Pharmacol Exp Ther. 298:865–872.
2001.PubMed/NCBI
|
|
29
|
Ke HH, Wu HH, Zhu Q, Zhang Y, Xiao JR, Su
XZ, Zheng WJ, Cai YP, Wu XZ, Wang YT and Chen GZ: Neuromuscular
effect of dexmedetomidine on sevoflurane: An open-label,
dose-escalation clinical trial. Minerva Anestesiol. 83:790–797.
2017.PubMed/NCBI
|
|
30
|
Bai L, Wang W, Dong YL, Wang W, Huang J,
Wang XY, Wang LY, Li YQ and Wu SX: Attenuation of mouse somatic and
emotional inflammatory pain by hydralazine through scavenging
acrolein and inhibiting neuronal activation. Pain Physician.
15:311–326. 2012.PubMed/NCBI
|
|
31
|
Cui YY, Xu H, Wu HH, Qi J, Shi J and Li
YQ: Spatio-temporal expression and functional involvement of
transient receptor potential vanilloid 1 in diabetic mechanical
allodynia in rats. PLoS One. 9:e1020522014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimazu Y, Shibuya E, Takehana S,
Sekiguchi K, Oshima K, Kamata H, Karibe H and Takeda M: Local
administration of resveratrol inhibits excitability of nociceptive
wide-dynamic range neurons in rat trigeminal spinal nucleus
caudalis. Brain Res Bull. 124:262–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takehana S, Sekiguchi K, Inoue M, Kubota
Y, Ito Y, Yui K, Shimazu Y and Takeda M: Systemic administration of
resveratrol suppress the nociceptive neuronal activity of spinal
trigeminal nucleus caudalis in rats. Brain Res Bull. 120:117–122.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu M, Cheng Z, Ding Z, Wang Y, Guo Q and
Huang C: Resveratrol enhances IL-4 receptor-mediated
anti-inflammatory effects in spinal cord and attenuates neuropathic
pain following sciatic nerve injury. Mol Pain.
14:17448069187675492018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zou L, Gong Y, Liu S and Liang S: Natural
compounds acting at P2 receptors alleviate peripheral neuropathy.
Brain Res Bull. 151:125–131. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schreiber AK, Nones CF, Reis RC, Chichorro
JG and Cunha JM: Diabetic neuropathic pain: Physiopathology and
treatment. World J Diabetes. 6:432–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McDonnell A, Collins S, Ali Z, Iavarone L,
Surujbally R, Kirby S and Butt RP: Efficacy of the Nav1.7 blocker
PF-05089771 in a randomised, placebo-controlled, double-blind
clinical study in subjects with painful diabetic peripheral
neuropathy. Pain. 159:1465–1476. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schiavuzzo JG, Teixeira JM, Melo B, da
Silva dos Santos DF, Jorge CO, Oliveira-Fusaro MC and Parada CA:
Muscle hyperalgesia induced by peripheral P2X3 receptors is
modulated by inflammatory mediators. Neuroscience. 285:24–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han Y, Jiang C, Tang J, Wang C, Wu P,
Zhang G, Liu W, Jamangulova N, Wu X and Song X: Resveratrol reduces
morphine tolerance by inhibiting microglial activation via AMPK
signalling. Eur J Pain. 18:1458–1470. 2014. View Article : Google Scholar : PubMed/NCBI
|