Introduction
Gastric cancer (GC) displays the highest morbidity
and mortality among all malignant tumors in the majority of Asian
countries, especially in China, where it ranks second among the
most common types of cancer (1).
With the development of medical technology, surgery is the only
radical treatment strategy available for GC. Although the 5-year
survival rate of patients with stage I and II GC is >70%, the
rate of early diagnosis is low and the majority of patients are
diagnosed during the advanced stages of the disease, when surgery
is no longer an optimal treatment strategy (2,3).
Patients with advanced GC have a poor prognosis, with a median
overall survival of 10–12 months (4). At present, there are a number of
issues regarding the early detection of GC; therefore, it is
important to explore the pathogenesis of GC to provide a
theoretical basis for the development of effective diagnostic and
therapeutic strategies for GC.
Long non-coding RNAs (lncRNAs) are transcripts that
have no protein-coding potential and are >200 nucleotides in
length, but display important regulatory roles in tissue physiology
and disease processes (5). lncRNAs
can be used as potential biomarkers for the diagnosis and prognosis
of various types of cancer, but can also enhance the specificity
and sensitivity of existing biomarkers (6). The lncRNA nicotinamide nucleotide
transhydrogenase-antisense RNA1 (NNT-AS1), located at 5p12 with 3
exons, is a newly identified lncRNA (7). NNT-AS1 has been reported to be
involved in the progression of lung, ovarian, breast and gastric
cancer, as well as osteosarcoma (8–13).
It has also been reported that NNT-AS1 expression was increased in
GC tissues and cell lines (14);
however, the molecular mechanism underlying NNT-AS1 in GC is not
completely understood and requires further investigation.
MicroRNAs (miRNAs) are a class of non-coding RNAs
that are ~21 nucleotides in length and are involved in a series of
processes associated with human physiology and pathology (15,16).
As stable biomarkers in tumors and circulation, miRNAs serve as an
attractive option for the development of tumor detection, diagnosis
and prognosis assessment strategies (17,18).
Moreover, it has been reported that miR-142-5p is associated with
the progression of a variety of different types of cancer (19–22).
Furthermore, Yan et al (22) reported that miR-142-5p knockdown
could promote tumor metastasis in GC; however, the molecular
mechanism underlying miR-142-5p in GC has not been previously
reported.
Sex-determining region Y-related high mobility group
box 4 (SOX4), a member of the sex-determining region Ybox family,
is associated with a number of different types of cancer, including
GC (23–26). However, the regulatory mechanism
between NNT-AS1 and SOX4 in GC has not been previously
reported.
The present study demonstrated that NNT AS1 serves a
cancerogenic role in GC. NNT AS1 knockdown curbed GC progression
via regulating the miR-142-5p/SOX4 axis, providing a potential
target for GC treatment.
Materials and methods
Patient samples
A total of 25 paired GC tissues and adjacent
non-cancerous tissues were collected from patients with GC who
underwent surgery resection at The First People's Hospital of
Guangyuan. Participants in the present study had not received
chemotherapy, radiation or other anticancer drugs before surgery.
The patients included 14 males and 11 females (age, 35–72 years).
The histology and pathology of the fresh tissue samples were
examined by at least two pathologists. Adjacent non-cancerous
tissues were sampled ≥3 cm away from tumor margin. The samples were
collected from August 2014 to August 2017. All samples were
instantly frozen in liquid nitrogen and stored at −80°C until
further analysis. The present study was approved by the Ethics
Committee of The First People's Hospital of Guangyuan. Written
informed consent was obtained from all participants.
Cell culture and transfection
The human GC AGS and HGC-27 cell lines and the human
gastric mucosal epithelial GES-1 cell line were acquired from BeNa
Culture Collection (Beijing Beina Chunglian Biotechnology Research
Institute). Cells were cultured in RPMI-1640 medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2.
For transfection, short hairpin (sh)RNAs targeting
NNT-AS1 (sh-NNT-AS1: 5′-GCAATGATGGGCCACTAAATTG-3′) or SOX4
(sh-SOX4: 5′-AGCAAACCGCACGCCAAGCTCATCCTGGC-3′), and a shRNA
negative control (sh-NC) were synthesized by Shanghai GenePharma
Co., Ltd. miR-142-5p mimic (miR-142-5p:
5′-CAUAAAGUAGAAAGGAGCACUACU-3′), miR-142-5p inhibitor
(anti-miR-142-5p: 5′-AGTAGTGCTCCTTTCTACTTTATG-3′) and their
corresponding negative controls (miR-NC or anti-miR-NC) were
purchased from Shanghai GenePharma Co., Ltd. The sequences of
NNT-AS1 and SOX4 were cloned into the pcDNA 3.1 vector (Invitrogen;
Thermo Fisher Scientific, Inc.) to establish NNT-AS1 and SOX4
overexpression vectors, respectively. The pcDNA 3.1 vectors (pcDNA)
were used as a negative control for overexpression vectors. AGS and
HGC-27 cells were transfected with sh-NC (500 ng/µl), sh-NNT-AS1
(500 ng/µl), sh-SOX4 (500 ng/µl), miR-142-5p (40 nM),
anti-miR-142-5p miR-NC (40 nM), anti-miR-NC (40 nM), pcDNA (15 nM),
NNT-AS1 (15 nM) or SOX4 (15 nM) using Lipofectamine®
2000 reagent (Thermo Fisher Scientific, Inc.) when cell confluence
reached 80%. According to the manufacturer's protocol. The
subsequent experiments were performed 48 h post-transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from GC tissues and cells
using TRlzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using the RT Reagent kit (Takara
Biotechnology Co., Ltd.) or the miRNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.). Subsequently, qPCR was performed
using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.). The
following primer pairs were used for qPCR: NNT-AS1 forward,
5′-AGTTCCACCAAGTTTCTTCA-3′ and reverse, 5′-AGGTTTTGCCAGCATAGAC-3′;
SOX4 forward, 5′-GTGAGCGAGATCTCGGG-3′ and reverse,
5′-CAGGTTGGAGATGCTGGACTC-3′; GAPDH forward,
5′-CACATCGCTCAGACACCATG-3′ and reverse, 5′-TGACGGTGCCATTGGAATTT-3′;
U6: Forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; and miR-142-5p: Forward,
5′-AACTCCAGCTGGTCCTTAG-3′ and reverse, 5′-TCTTGAACCCTCATCCTGT-3′.
The amplification parameters were as follows: Denaturation at 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 30
sec, annealing at 60°C for 30 sec and extension at 72°C for 1 min.
mRNA and miRNA expression levels were quantified using the
2−ΔΔCq method (27) and
normalized to the internal reference genes GAPDH and U6,
respectively.
Western blotting
Total protein was isolated from GC tissues and cells
using RIPA buffer (Beyotime Institute of Biotechnology) with a
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Total
protein was quantified via a BCA Protein Aassay kit (Beyotime
Institute of Biotechnology). Total protein (30 µg) was separated
via 10% SDS-PAGE and transferred onto PVDF membranes. Subsequently
the membranes were blocked with 5% non-fat milk in PBS for 2 h at
room temperature. The membranes were incubated at 4°C overnight
with the following primary antibodies: Anti-SOX4 (1:1,000; cat. no.
sc-518016; Santa Cruz Biotechnology, Inc.), anti-E-cadherin (1:500;
cat. no. sc-21791 Santa Cruz Biotechnology, Inc.), anti-GAPDH
(1:2,000; cat. no. sc-32233; Santa Cruz Biotechnology, Inc.),
anti-β-catenin (1:200; cat. no. ab16051; Abcam), anti-c-Myc (1:200;
cat. no. ab168727; Abcam) and anti-Bcl-2 (1:100; cat. no. ab185002;
Abcam). Following primary incubation, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-mouse (1:2,000;
cat. no. sc-2354; Santa Cruz Biotechnology, Inc.) or anti-rabbit
(1:2,000; cat. no. ab97051; Abcam) antibodies for 1 h at room
temperature. Protein bands were visualized using Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore). Protein expression
levels were semi-quantified using ImageJ software (version 1.50;
National Institutes of Health) with GAPDH as the loading
control.
MTT assay
Cell proliferation was detected using the MTT assay
(Promega Corporation), according to the manufacturer's
instructions. AGS and HGC-27 cells were seeded (5×103
cells/well) into 96-well plates and cultured for 24, 48 or 72 h at
37°C. Subsequently, AGS and HGC-27 cells were transfected.
Following transfection, 20 µl MTT (5 mg/ml) was added to each well
and incubated for 4 h at 37°C. The purple formazan crystals were
dissolved using 200 µl DMSO. The optical density of each well was
measured at a wavelength of 490 nm using a microplate reader.
Migration and invasion assays
The migration and invasion of AGS and HGC-27 cells
were determined using Transwell plates (pore size, 8 mm; BD
Biosciences). Transfected AGS and HGC-27 cells were seeded
(1×105 cells/well) into the upper chambers of Transwell
plates with serum-free RPMI-1640 medium. RPMI-1640 medium
containing 10% FBS was plated into the lower chambers of Transwell
plates. Following incubation for 48 h in humidified air at 37°C
with 5% CO2, cells on the lower surface of the Transwell
membrane were stained using 0.1% crystal violet at room temperature
for 10 min. Stained cells were observed using an inverted
microscope (magnification, ×100). For the invasion assay, the upper
chambers of Transwell plates were precoated with
Matrigel® (BD Biosciences): Transwell plates were
covered with cold Matrigel, and then kept at 37°C for 30 min.
Flow cytometry assay
Following transfection, AGS and HGC-27 cell
apoptosis was detected using the FITC Annexin V Apoptosis Detection
kit I (BD Biosciences). Briefly, transfected AGS and HGC-27 cells
were collected and re-suspended in 500 µl binding buffer.
Subsequently, cells were incubated with 5 µl Annexin V-FITC
specific antibodies and 5 µl propidium iodide for 15 min in the
dark at room temperature. Apoptotic cells were detected by flow
cytometry using a FACScan® flow cytometer (BD
Biosciences). The data were analyzed with the CellQuest software
(version 5.1; BD Biosciences).
Dual-luciferase reporter assay
The binding sites between miR-142-5p and NNT-AS1 or
SOX4 were predicted by starBase (version 2.0) (starbase.sysu.edu.cn/starbase2). The
wild-type (WT) and mutant (MUT) sequences of NNT-AS1 and the
3′-untranslated region (UTR) of SOX4 were synthesized and inserted
downstream of pGL3-control luciferase reporter vectors (Promega
Corporation) to construct the following luciferase reporter
vectors: WT-NNT-AS1, MUT-NNT-AS1, SOX4 3′UTR-WT and SOX4 3′UTR-MUT.
AGS and HGC-27 cells were co-transfected with the luciferase
reporter vectors and miR-142-5p or miR-NC using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
when cell growth reached 70% and cultured for 48 h at 37°C.
Lipofectamine® 2000 reagent was used according to the
manufacturer's protocol. Luciferase activities were detected using
the Dual-Luciferase Reporter assay kit (Promega Corporation). The
luciferase intensities were determined by normalizing the firefly
luminescence to Renilla luminescence.
Statistical analysis
The experiments were repeated at least three times.
Statistical analyses were performed using GraphPad Prism (version
6.00; GraphPad Software, Inc.) and SPSS (version 13.0; SPSS, Inc.)
software. Spearman's correlation analysis was used to analyze the
correlation between NNT-AS1 and SOX4 or miR-142-5p in GC tissues.
Differences between two groups were analyzed using unpaired
Student's t-test. The differences between GC tissues and adjacent
non-cancerous tissues were analyzed using paired Student's t-test.
Differences among multiple groups were analyzed using one-way ANOVA
followed by Sidak's post hoc test. Data are presented as the mean ±
SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
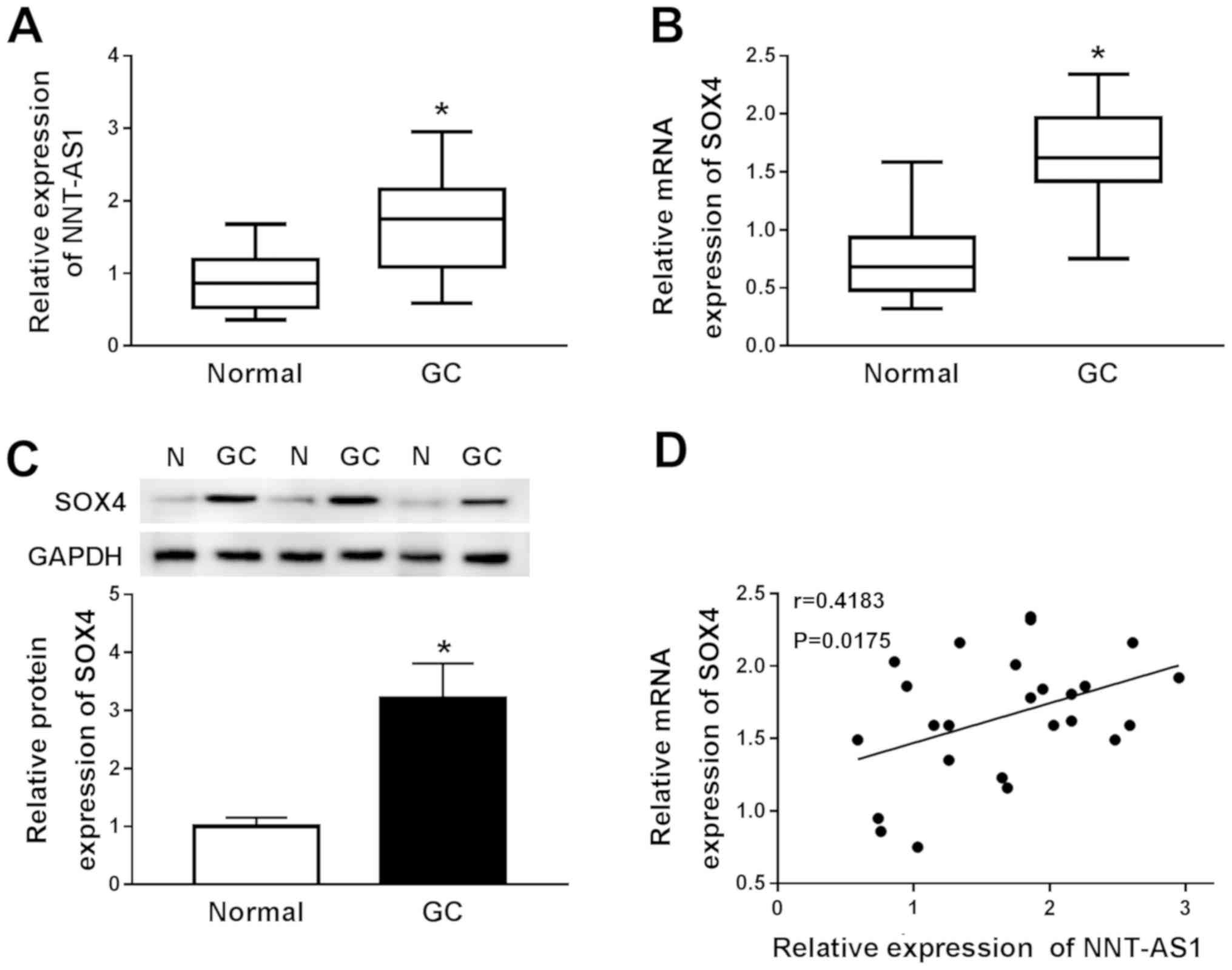
NNT-AS1 and SOX4 are upregulated in GC
tissues
RT-qPCR was performed to measure the expression
levels of NNT-AS1 and SOX4 in 25 paired GC and normal adjacent
tissues to investigate the role of NNT-AS1 and SOX4 in GC. NNT-AS1
and SOX4 expression levels were significantly increased in GC
tissues compared with adjacent normal tissues (Fig. 1A and B). Subsequently, the protein
expression levels of SOX4 in GC and adjacent normal tissues were
determined by western blotting. Compared with adjacent normal
tissues, SOX4 protein expression levels were significantly
increased in GC tissues (Fig. 1C).
The relationship between NNT-AS1 and SOX4 was further investigated
using Spearman's rank test. The results indicated that the
expression levels of NNT-AS1 and SOX4 in GC tissues were positively
correlated (Fig. 1D).
Collectively, the results indicated that NNT-AS1 and SOX4 may serve
an oncogenic role in GC.
NNT-AS1 knockdown inhibits GC cell
proliferation, migration and invasion, and promotes GC cell
apoptosis
To investigate the role of NNT-AS1 during GC
progression, the expression levels of NNT-AS1 in AGS and HGC-27
cells were assessed. NNT-AS1 expression was significantly increased
in AGS and HGC-27 cells compared with GES-1 cells (Fig. 2A). Based on the aforementioned
finding, the possible role of NNT-AS1 in GC cells was further
investigated using loss-of-function experiments. NNT-AS1 expression
was significantly reduced in the sh-NNT-AS1 group compared with the
sh-NC group in AGS and HGC-27 cells (Fig. 2B). Subsequently, the MTT assay
indicated that NNT-AS1 knockdown significantly reduced the
proliferation of AGS and HGC-27 cells compared with the sh-NC group
(Fig. 2C and D). The flow
cytometry results indicated that NNT-AS1 knockdown significantly
increased AGS and HGC-27 cell apoptosis compared with the sh-NC
group (Fig. 2E). The migration and
invasion of AGS and HGC-27 cells was then determined using
Transwell assays. The results indicated that the migration and
invasion of AGS and HGC-27 cells were significantly decreased in
the sh-NNT-AS1 group compared with the sh-NC group (Fig. 2F and G). Overall, the results
suggested that NNT-AS1 knockdown inhibited GC cell proliferation,
migration and invasion, and induced GC cell apoptosis.
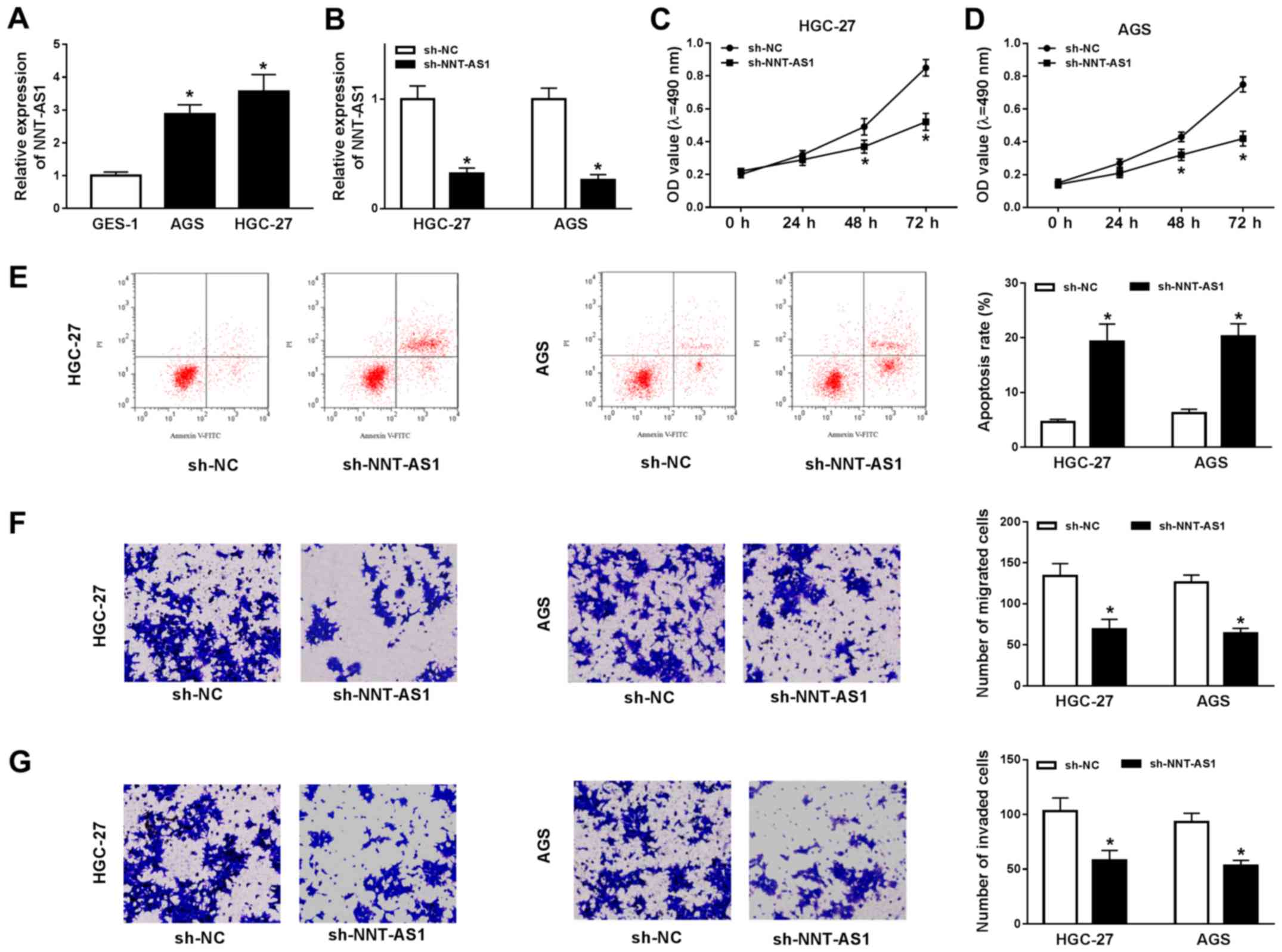 | Figure 2.NNT-AS1 knockdown alters GC cell
proliferation, migration, invasion and apoptosis. AGS and HGC-27
cells were transfected with sh-NNT-AS1 or sh-NC. (A) Expression of
NNT-AS1 in GES-1, AGS and HGC-27 cells. (B) Transfection efficiency
of NNT-AS1 in AGS and HGC-27 cells. The MTT assay was performed to
determine (C) HGC-27 and (D) AGS cell proliferation. (E) Flow
cytometry was performed to detect AGS and HGC-27 cell apoptosis.
Transwell assays were performed to assess AGS and HGC-27 cell (F)
migration and (G) invasion. The cells were observed using an
inverted microscope (magnification, ×100). *P<0.05 vs. sh-NC.
NNT-AS1, nicotinamide nucleotide transhydrogenase-antisense RNA1;
GC, gastric cancer; sh, short hairpin RNA; NC, negative control;
PI, propidium iodide; OD, optical density. |
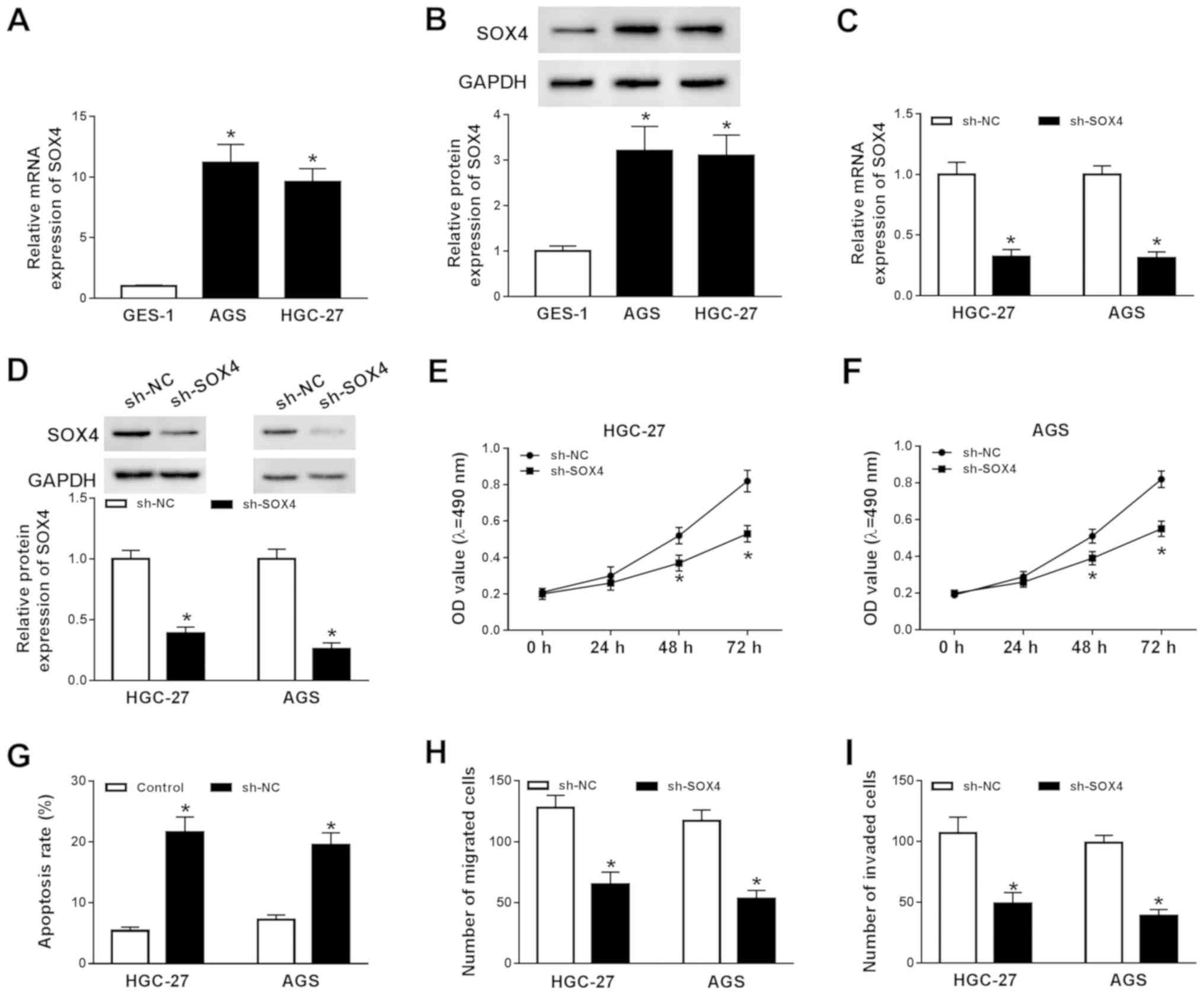
SOX4 knockdown decreases GC cell
proliferation, migration and invasion, and induces GC cell
apoptosis
To explore the function of SOX4 during GC
progression, the mRNA and protein expression levels of SOX4 in
GES-1, AGS and HGC-27 cells were determined by RT-qPCR and western
blotting, respectively. The mRNA and protein expression levels of
SOX4 were significantly increased in AGS and HGC-27 cells compared
with GES-1 cells (Fig. 3A and B).
Subsequently, AGS and HGC-227 cells were transfected with sh-SOX4
or sh-NC to knockdown SOX4 expression. Compared with the sh-NC
group, the mRNA and protein expression levels of SOX4 were
significantly decreased in the sh-SOX4 group (Fig. 3C and D). Subsequently, the effects
of SOX4 knockdown on AGS and HGC-27 cell proliferation, migration,
invasion and apoptosis were investigated. The MTT assay indicated
that SOX4 knockdown significantly decreased AGS and HGC-27 cell
proliferation compared with the sh-NC group (Fig. 3E and F). The flow cytometry results
suggested that SOX4 knockdown significantly increased AGS and
HGC-27 cell apoptosis compared with the sh-NC group (Fig. 3G). Moreover, the results of the
Transwell assays indicated that SOX4 knockdown significantly
reduced AGS and HGC-27 cell migration and invasion (Fig. 3H and I). In summary, the results
indicated that SOX4 knockdown inhibited GC cell proliferation,
migration and invasion, and induced GC cell apoptosis.
SOX4 overexpression reverses NNT-AS1
knockdown- mediated effects on GC cell proliferation, apoptosis,
migration and invasion
To further investigate the relationship between
NNT-AS1 and SOX4 during GC progression, the expression levels of
NNT-AS1 in AGS and HGC-27 cells transfected with pcDNA-NNT-AS1 or
pcDNA were determined. NNT-AS1 expression was significantly
upregulated in AGS and HGC-27 cells transfected with pcDNA-NNT-AS1
compared with the pcDNA group (Fig.
S1). The mRNA and protein expression levels of SOX4 in AGS and
HGC-27 cells transfected with sh-NNT-AS1, sh-NC, pcDNA or NNT-AS1
were determined by RT-qPCR and western blotting, respectively.
NNT-AS1 knockdown significantly decreased the mRNA and protein
expression levels of SOX4 in AGS and HGC-27 cells, whereas NNT-AS1
overexpression significantly increased the mRNA and protein
expression levels of SOX4 in AGS and HGC-27 cells, compared with
the corresponding control groups (Fig.
4A-D). Moreover, SOX4 mRNA and protein expression levels were
upregulated in AGS and HGC-27 cells transfected with SOX4 compared
with the pcDNA group (Fig. S2).
Furthermore, the effects of SOX4 overexpression on AGS and HGC-27
cell proliferation, migration, invasion and apoptosis following
NNT-AS1 knockdown were investigated. NNT-AS1 knockdown-mediated
inhibition of AGS and HGC-27 cell proliferation was reversed by
SOX4 overexpression (Fig. 4E and
F). Furthermore, SOX4 overexpression reversed NNT-AS1
knockdown-mediated AGS and HGC-27 cell apoptosis induction
(Fig. 4G and H). The results of
the Transwell assays also indicated that SOX4 overexpression
reversed NNT-AS1 knockdown-mediated inhibition of AGS and HGC-27
cell migration and invasion (Fig.
4I-L). Collectively, the results suggested that SOX4
overexpression reversed NNT-AS1 knockdown-mediated effects on GC
cell proliferation, apoptosis, migration and invasion.
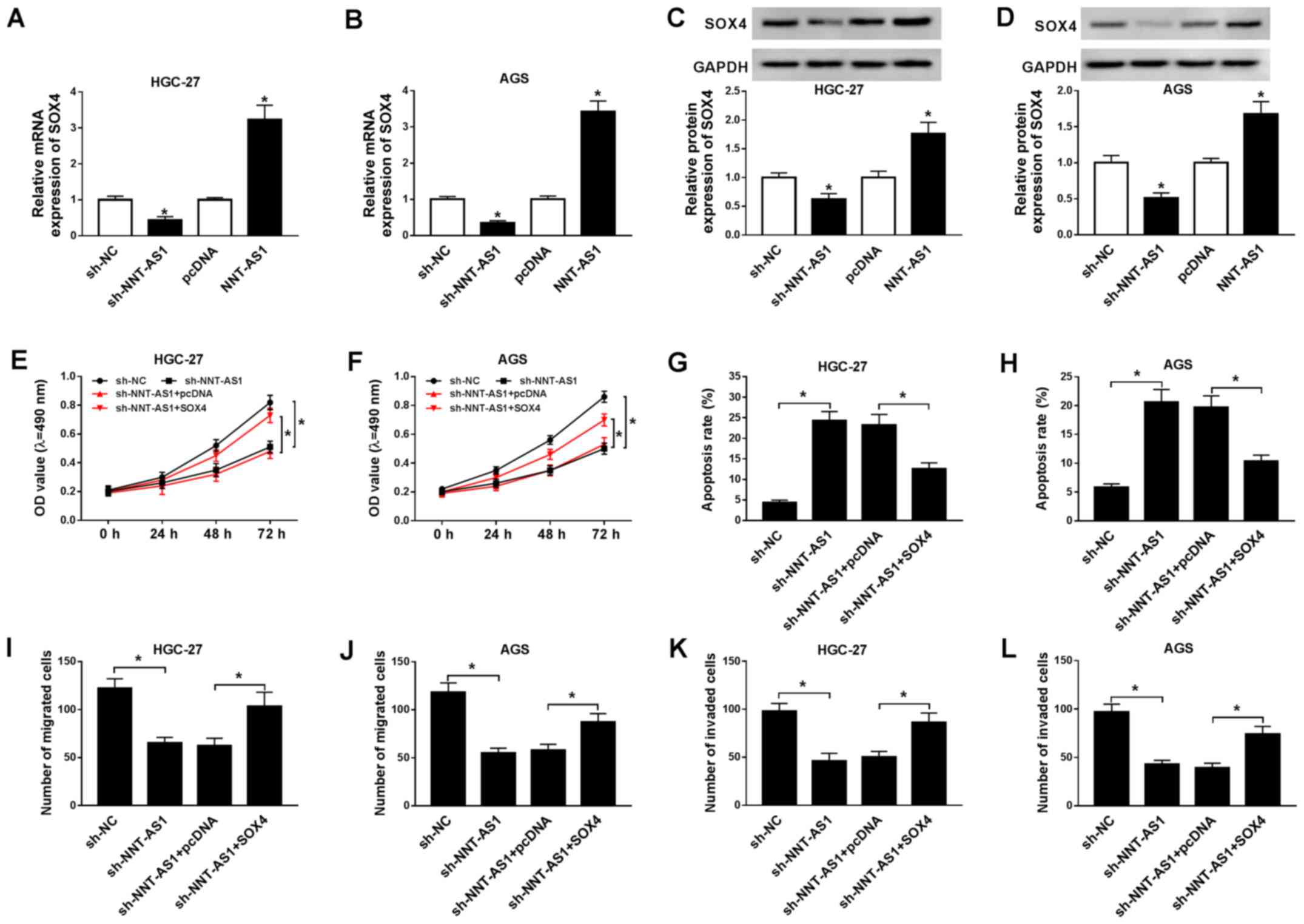 | Figure 4.SOX4 overexpression reverses NNT-AS1
knockdown-mediated effects on GC cell proliferation, apoptosis,
migration and invasion. (A-D) AGS and HGC-27 cells were transfected
with sh-NNT-AS1, sh-NC, pcDNA, or NNT-AS1. The mRNA expression
levels of SOX4 in (A) HGC-27 and (B) AGS cells. Protein expression
levels of SOX4 in (C) HGC-27 and (D) AGS cells. *P<0.05 vs.
sh-NC or pcDNA. (E-L) AGS and HGC-27 cells were transfected with
sh-NNT-AS1, sh-NC, sh-NNT-AS1 + pcDNA or sh-NNT-AS1 + SOX4. Effect
of NNT-AS1 knockdown and SOX4 overexpression on (E) HGC-27 and (F)
AGS cell proliferation. Effect of NNT-AS1 knockdown and SOX4
overexpression on (G) HGC-27 and (H) AGS cell apoptosis. Effect of
NNT-AS1 knockdown and SOX4 overexpression on (I) HGC-27 and (J) AGS
cell migration. Effect of NNT-AS1 knockdown and SOX4 overexpression
on (K) HGC-27 and (L) AGS cell invasion. *P<0.05 vs. sh-NC or
sh-NNT-AS1 + SOX4. SOX4, sex-determining region Y-related high
mobility group box 4; NNT-AS1, nicotinamide nucleotide
transhydrogenase-antisense RNA1; GC, gastric cancer; sh, short
hairpin RNA; NC, negative control; OD, optical density. |
NNT-AS1 regulates SOX4 expression by
sponging miR-142-5p in GC cells
It has been reported that miR-142-5p is associated
with the recurrence and poor prognosis of GC (22). The expression of miR-142-5p in GC
tissues and cells was determined using RT-qPCR. Compared with the
adjacent normal tissues, miR-142-5p expression was significantly
reduced in GC tissues (Fig. 5A).
Similarly, miR-142-5p expression was significantly decreased in GC
cells compared with GES-1 cells (Fig.
5B). Moreover, a negative correlation between NNT-AS1 and
miR-142-5p expression was identified in GC tissues (Fig. 5C). Additionally, miR-142-5p
expression was significantly increased in AGS and HGC-27 cells
transfected with miR-142-5p compared with the control group, and
significantly decreased in AGS and HGC-27 cells transfected with
anti-miR-142-5p, compared with the corresponding control cells
(Fig. S3). Subsequently, AGS and
HGC-27 cells were transfected with miR-142-5p or miR-NC to explore
the effects of miR-142-5p overexpression on GC cell proliferation,
migration, invasion and apoptosis. The MTT assay indicated that
miR-142-5p overexpression significantly reduced AGS and HGC-27 cell
proliferation compared with the miR-NC group (Fig. 5D and E). Moreover, AGS and HGC-27
cell apoptosis was significantly increased by miR-142-5p
overexpression compared with the miR-NC group (Fig. 5F). The Transwell assays indicated
that miR-142-5p overexpression significantly inhibited AGS and
HGC-27 cell migration and invasion compared with the miR-NC group
(Fig. 5G and H). It has been
reported that lncRNAs can act as sponges for miRNAs to modulate
miRNA expression (28); therefore,
the binding sites between miR-142-5p and NNT-AS1 or SOX4 were
predicted using starBase. miR-142-5p displayed binding sites for
NNT-AS1 and SOX4 (Fig. 5I).
Furthermore, the dual-luciferase reporter assay indicated that the
luciferase activities of the WT-NNT-AS1 and SOX4 3′UTR-WT reporter
vectors were significantly reduced in the miR-142-5p overexpression
group compared with the miR-NC group in AGS and HGC-27 cells. By
contrast, the luciferase activities of the MUT-NNT-AS1 and SOX4
3′UTR-MUT reporter vectors were not significantly altered by
miR-142-5p overexpression in AGS and HGC-27 cells (Fig. 5J-M). In addition, the protein
expression levels of SOX4 in the miR-142-5p-overexpression group
were significantly decreased compared with the miR-NC group in AGS
and HGC-27 cells. MiR-142-5p overexpression-induced effects on SOX4
expression were reversed by NNT-AS1 overexpression (Fig. 5N and O). Collectively, the results
indicated that NNT-AS1 regulated SOX4 expression via miR-142-5p in
GC cells.
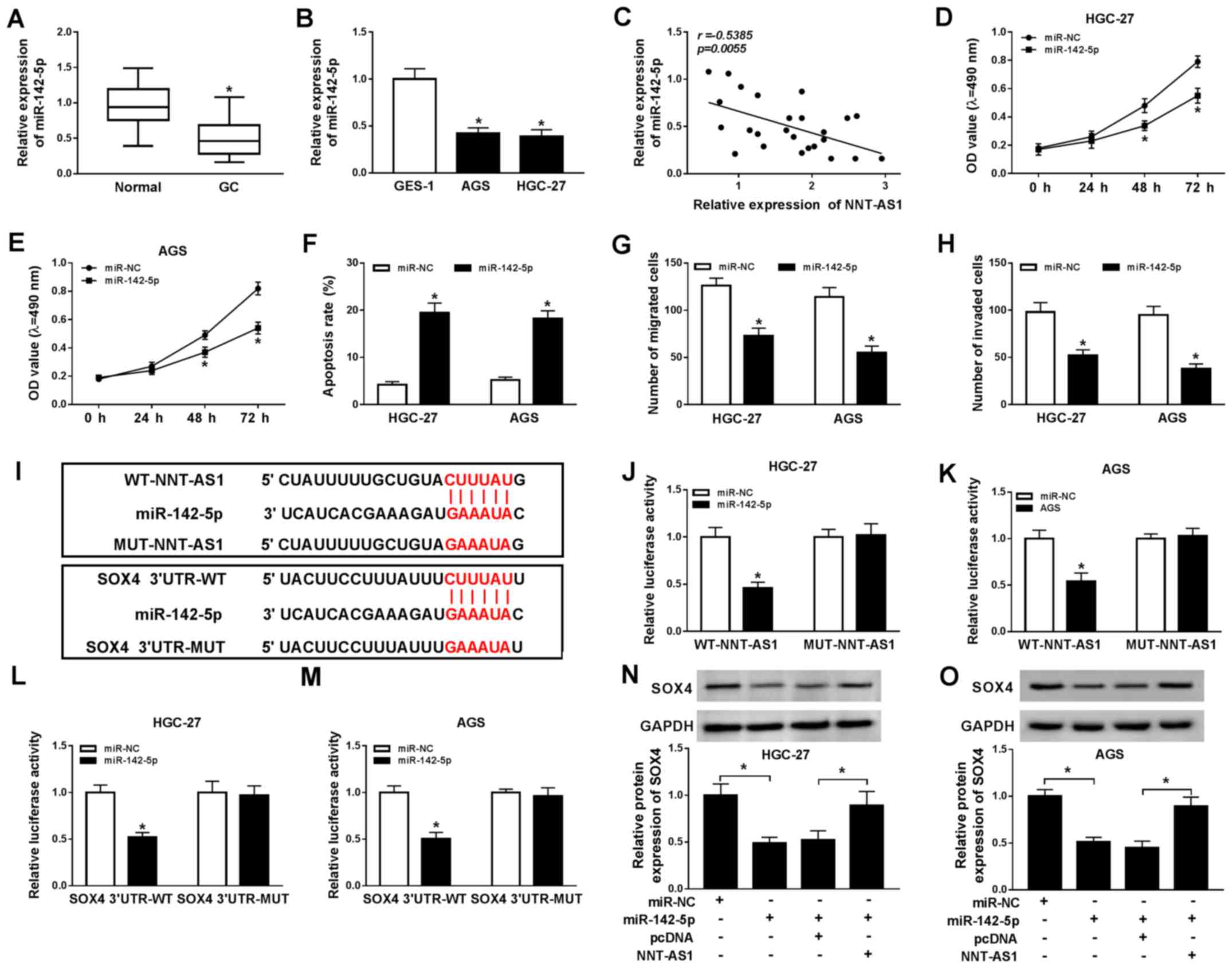 | Figure 5.NNT-AS1 regulates SOX4 expression via
miR-142-5p. miR-142-5p expression levels in GC (A) tissues and (B)
cells. (C) The relationship between NNT-AS1 and miR-142-5p
expression in GC tissues. Effect of miR-143-5p overexpression on
(D) HGC-27 and (E) AGS cell proliferation. Effect of miR-143-5p
overexpression on HGC-27 and AGS cell (F) apoptosis, (G) migration
and (H) invasion. (I) The binding sites between miR-142-5p and
NNT-AS1 or SOX4 were predicted using starBase. The luciferase
activities of WT-NNT-AS1, MUT-NNT-AS1 in (J) HGC-27 and (K) AGS
cells. The luciferase activities of SOX4 3′UTR-WT and SOX4
3′UTR-MUT in (L) HGC-27 and (M) AGS cells. Protein expression
levels of SOX4 in (N) HGC-27 and (O) AGS cells. *P<0.05 vs.
corresponding control. NNT-AS1, nicotinamide nucleotide
transhydrogenase-antisense RNA1; SOX4, sex-determining region
Y-related high mobility group box 4; miR, microRNA; GC, gastric
cancer; WT, wild-type; MUT, mutant; 3′UTR, 3′-untranslated region;
NC, negative control; OD, optical density. |
NNT-AS1 knockdown blocks the
Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis
To investigate whether the Wnt/β-catenin signaling
was associated with the regulatory mechanism underlying NNT-AS1
during GC, the expression levels of the Wnt/β-catenin signaling
pathway-associated proteins, β-catenin, c-Myc, Bcl-2 and
E-cadherin, were assessed by western blotting. NNT-AS1 knockdown
reduced the protein expression levels of β-catenin, c-Myc and
Bcl-2, but enhanced the protein expression level of E-cadherin
compared with the sh-NC group in AGS and HGC-27 cells (Fig. 6A and B). However, miR-142-5p
knockdown and SOX4 overexpression reversed NNT-AS1
knockdown-mediated effects on the protein expression levels of
β-catenin, c-Myc, Bcl-2 and E-cadherin in AGS and HGC-27 cells
(Fig. 6A and B). The results
suggested NNT-AS1 knockdown blocked the Wnt/β-catenin signaling
pathway via the miR-142-5p/SOX4 axis.
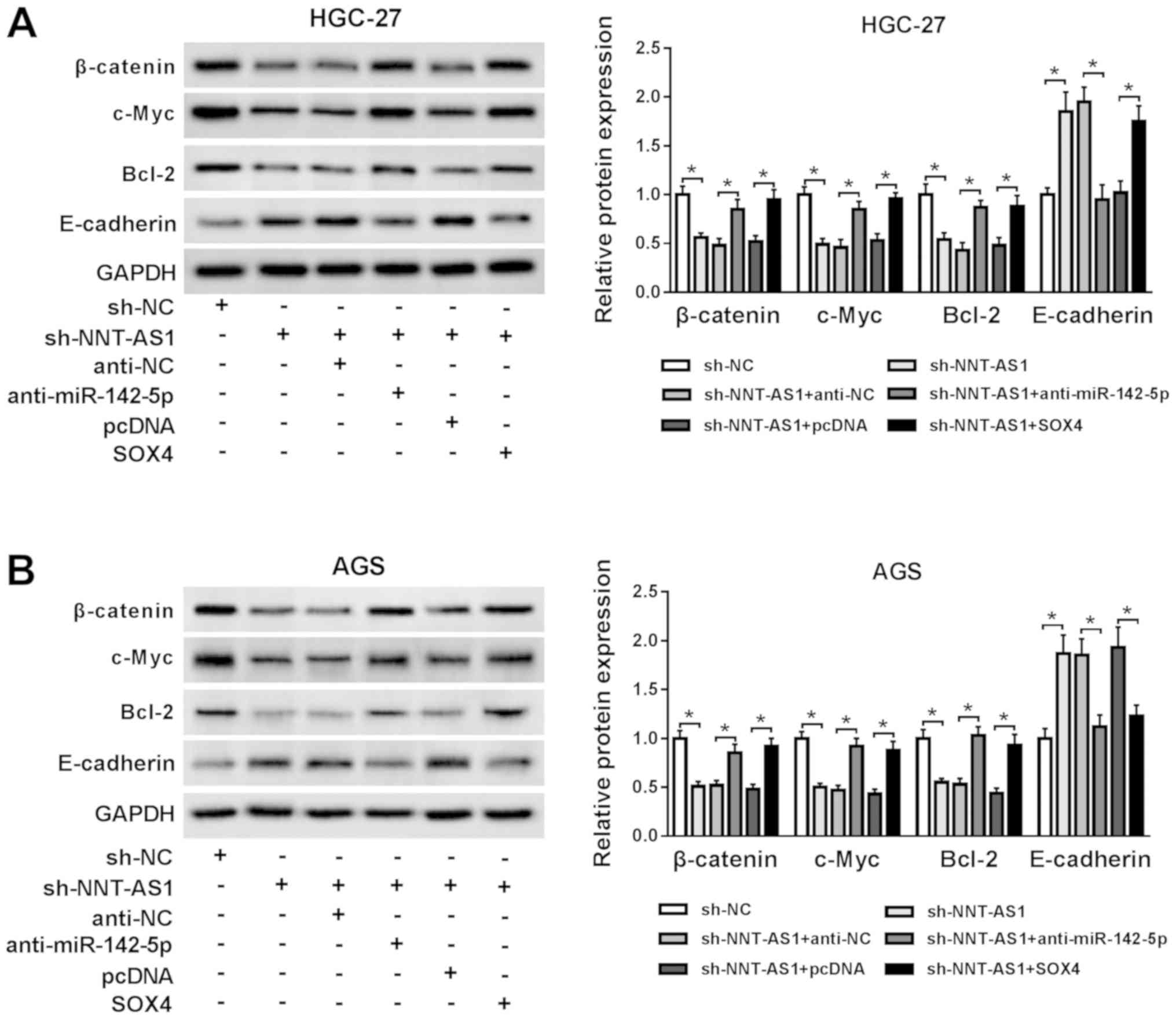 | Figure 6.NNT-AS1 knockdown blocks the
Wnt/β-catenin signaling pathway via the miR-142-5p/SOX4 axis.
Protein expression levels of β-catenin, c-Myc, Bcl-2 and E-cadherin
in (A) HGC-27 and (B) AGS cells transfected with sh-NC, sh-NNT-AS1,
sh-NNT-AS1 + anti-NC, sh-NNT-AS1 + anti-miR-142-5p, sh-NNT-AS1 +
pcDNA or sh-NNT-AS1 + SOX4. *P<0.05, as indicated. NNT-AS1,
nicotinamide nucleotide transhydrogenase-antisense RNA1; miR,
microRNA; SOX4, sex-determining region Y-related high mobility
group box 4; sh, short hairpin RNA; NC, negative control. |
Discussion
GC is a malignant tumor with high morbidity and
mortality, which seriously threatens human health and life
worldwide (29). Recent studies
have indicated that lncRNA dysregulation participates in a number
of different types of human cancers, including GC (14,30,31);
therefore, identifying the regulatory mechanisms underlying lncRNAs
is important for the diagnosis and treatment of GC.
It has been reported that NNT-AS1 dysregulation
displays vital roles during the progression of various types of
cancer. For example, Hua et al (32) reported that NNT-AS1 overexpression
promoted cervical cancer cell proliferation and invasion. Another
study reported that NNT-AS1 overexpression was related to
osteosarcoma progression and poor prognosis (12). Chen et al (14) also revealed that NNT-AS1 expression
was notably elevated in GC tissues and cells. In the present study,
NNT-AS1 expression levels were upregulated in GC tissues and cells
compared with normal tissues and cells. Moreover, NNT-AS1 knockdown
inhibited GC cell proliferation, migration and invasion, and
promoted GC cell apoptosis. The study of Chen et al
(14) also demonstrated that
NNT-AS1 knockdown decreased GC cell proliferation and invasion, and
induced cell-cycle progression arrest at
G0/G1 phase in GC cells. The results of the
present study were therefore consistent with the aforementioned
studies, which indicated that NNT-AS1 may serve a carcinogenic role
during GC.
lncRNAs bind to miRNAs as a competitive endogenous
RNA to regulate the expression of miRNA target genes (28). Li et al (9) reported that NNT-AS1 accelerated
breast cancer cell proliferation and metastasis by upregulating
zinc finger E-box binding homeobox 1 (ZEB1) expression via sponging
miR-142-3p (9). Increasing
evidence has suggested that abnormal miRNA expression displays
essential roles in tumorigenesis (33,34).
In hepatocellular cancer cells, miR-142-5p enhances cell apoptosis
and suppresses cell proliferation (35). Moreover, enhanced miR-142-5p
expression induced cell apoptosis and inhibited cell proliferation
in osteosarcoma (36). In the
present study, the results indicated that miR-142-5p acted as a
target for NNT-AS1. Moreover, miR-142-5p expression was decreased
in GC tissues and cells compared with normal tissues and cells, and
the expression of miR-142-5p in GC tissues was negatively
correlated with NNT-AS1. In addition, miR-142-5p overexpression
induced cell apoptosis and decreased cell proliferation, migration
and invasion. A previous study demonstrated that miR-142-5p
inhibition enhanced GC cell migration (22); therefore, it was hypothesized that
miR-142-5p may display an anticancer role during GC. By contrast,
miR-142-5p promotes tumor growth in renal cell and colorectal
cancer, which might be related to tissue specificity (20,37).
Subsequently, the results of the present study indicated that
miR-142-5p directly targeted SOX4 in GC cells. Moreover, SOX4
expression was upregulated in GC tissues and cells compared with
normal tissues and cells, and SOX4 knockdown promoted GC cell
apoptosis and decreased GC cell proliferation, migration, and
invasion. A previous study reported that SOX4 overexpression
reversed miR-138-mediated inhibition of GC cell proliferation in
vitro and in vivo (38). Zhang et al (39) demonstrated that SOX4 knockdown
decreased GC cell migration and invasion, indicating that SOX4
served as a carcinogen during GC. In addition, the results of the
present study suggested that NNT-AS1 regulated SOX4 expression via
miR-142-5p in GC cells. Furthermore, SOX4 overexpression reversed
NNT-AS1 knockdown-mediated effect on GC cell proliferation,
apoptosis, migration and invasion. Therefore, the present study
indicated that NNT-AS1 exerted its effects via the miR-142-5p/SOX4
axis in GC.
It has been reported that aberrant activation of the
Wnt/β-catenin signaling pathway is associated with the occurrence
and development of various types of cancer, such as colorectal and
prostate cancer (30,31,40).
Β-catenin is a component of the Wnt signaling cascade that
accumulates in the cytoplasm and transfers to the nucleus to
activate downstream target genes, including c-Myc (41). E-cadherin is a calcium-dependent
cell-cell adhesion molecule that displays vital roles in epithelial
cell behavior, tissue formation and cancer inhibition (42). Bcl-2 is an apoptosis suppressor
gene that reduces cadherin-mediated expression of cell adhesion,
leading to unregulated cell proliferation and tumorigenesis
(43). Previous studies have
demonstrated that activation of the Wnt/β-catenin signaling pathway
promoted GC progression (44,45).
In the present study, NNT-AS1 knockdown blocked the Wnt/β-catenin
signaling pathway via the miR-142-5p/SOX4 axis in GC cells. The
results indicated that the Wnt/β-catenin signaling pathway was
involved in the development of GC, which was consistent with the
results of previous studies.
In summary, NNT-AS1 and SOX4 expression levels were
upregulated in GC tissues and cells compared with normal tissues
and cells. NNT-AS1 knockdown and SOX4 knockdown inhibited GC cell
proliferation, migration and invasion, and promoted GC cell
apoptosis. NNT-AS1 regulated SOX4 expression via sponging
miR-142-5p. SOX4 overexpression reversed NNT-AS1 knockdown-mediated
effects on GC cell proliferation, migration, invasion and
apoptosis. Moreover, NNT-AS1 knockdown inhibited the Wnt/β-catenin
signaling pathway via the miR-142-5p/SOX4 axis. Collectively, the
results indicated that NNT-AS1 knockdown inhibited GC cell
proliferation, migration and invasion, and promoted GC cell
apoptosis by modulating the miR-142-5p/SOX4/Wnt/β-catenin signaling
pathway. Therefore, NNT-AS1 may serve as a potential biomarker and
target for the diagnosis, prognosis assessment and treatment of
GC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and KZ conceived and designed the study. YH
performed the experiments. KZ and YH analyzed the data and wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isobe Y, Nashimoto A, Akazawa K, Oda I,
Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y and
Kaminishi M: Gastric cancer treatment in Japan: 2008 annual report
of the JGCA nationwide registry. Gastric Cancer. 14:301–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasechnikov V, Chukov S, Fedorov E,
Kikuste I and Leja M: Gastric cancer: Prevention, screening and
early diagnosis. World J Gastroenterol. 20:13842–13862. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Digklia A and Wagner AD: Advanced gastric
cancer: Current treatment landscape and future perspectives. World
J Gastroenterol. 22:2403–2414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chandra Gupta S and Nandan Tripathi Y:
Potential of long non-coding RNAs in cancer patients: From
biomarkers to therapeutic targets. Int J Cancer. 140:1955–1967.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Yang L, Hu X, Jiang Y, Hu Y, Liu
Z, Liu J, Wen T, Ma Y, An G and Feng G: Upregulated NNT-AS1, a long
noncoding RNA, contributes to proliferation and migration of
colorectal cancer cells in vitro and in vivo. Oncotarget.
8:3441–3453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li C, Zhang S, Qiu T, Wang Y, Ricketts DM
and Qi C: Upregulation of long non-coding RNA NNT-AS1 promotes
osteosarcoma progression by inhibiting the tumor suppressive
miR-320a. Cancer Biol Ther. 20:413–422. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Lv M, Song Z, Lou Z, Wang R and
Zhuang M: Long non-coding RNA NNT-AS1 affects progression of breast
cancer through miR-142-3p/ZEB1 axis. Biomed Pharmacother.
103:939–946. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen Q and Jiang Y: LncRNA NNT-AS1
promotes the proliferation, and invasion of lung cancer cells via
regulating miR-129-5p expression. Biomed Pharmacother. 105:176–181.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Ren M, Li Y, Hu J, Lu G, Ma W, Guo
D, Lu X and He S: Long noncoding RNA NNT-AS1 promotes gastric
cancer proliferation and invasion by regulating microRNA-363
expression. J Cell Biochem. 120:5704–5712. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye H, Lin J, Yao X, Li Y, Lin X and Lu H:
Overexpression of long non-coding RNA NNT-AS1 correlates with tumor
progression and poor prognosis in osteosarcoma. Cell Physiol
Biochem. 45:1904–1914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Shi J and Xu Y: Long non-coding
RNA NNT-AS1 contributes to cell proliferation, metastasis and
apoptosis in human ovarian cancer. Oncol Lett. 15:9264–9270.
2018.PubMed/NCBI
|
|
14
|
Chen B, Zhao Q, Guan L, Lv H, Bie L, Huang
J and Chen XB: Long non-coding RNA NNT-AS1 sponges miR-424/E2F1 to
promote the tumorigenesis and cell cycle progression of gastric
cancer. J Cell Mol Med. 22:4751–4759. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhaskaran M and Mohan M: MicroRNAs:
History, biogenesis, and their evolving role in animal development
and disease. Vet Pathol. 51:759–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu A, Hou C, Chen H, Zong X and Zong P:
Genetics and epigenetics of glioblastoma: Applications and overall
incidence of IDH1 mutation. Front Oncol. 6:162016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Chen W, Jin Y, Xue R, Su J, Mu Z, Li
J and Jiang S: miR-142-5p enhances cisplatin-induced apoptosis in
ovarian cancer cells by targeting multiple anti-apoptotic genes.
Biochem Pharmacol. 161:98–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Xiao Z, Ai F, Liu F, Chen X, Cao K,
Ren W, Zhang X, Shu P and Zhang D: miR-142-5p promotes development
of colorectal cancer through targeting SDHB and facilitating
generation of aerobic glycolysis. Biomed Pharmacother.
92:1119–1127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Liu Z, Fang X and Yang H:
miR-142-5p suppresses tumorigenesis by targeting PIK3CA in
non-small cell lung cancer. Cell Physiol Biochem. 43:2505–2515.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan J, Yang B, Lin S, Xing R and Lu Y:
Downregulation of miR-142-5p promotes tumor metastasis through
directly regulating CYR61 expression in gastric cancer. Gastric
Cancer. 22:302–313. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu B, Zhang H, Wang Z, Zhang F, Wei H and
Li L: lncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance
in non-small-cell lung cancer cell line by targeting SOX4. Cancer
Biol Ther. 18:974–983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee H, Goodarzi H, Tavazoie SF and Alarcón
CR: TMEM2 is a SOX4-regulated gene that mediates metastatic
migration and invasion in breast cancer. Cancer Res. 76:4994–5005.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sandbothe M, Buurman R, Reich N, Greiwe L,
Vajen B, Gürlevik E, Schäffer V, Eilers M, Kühnel F, Vaquero A, et
al: The microRNA-449 family inhibits GF-β-mediated liver cancer
cell migration by targeting SOX4. J Hepatol. 66:1012–1021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun R, Jiang B, Qi H, Zhang X, Yang J,
Duan J, Li Y and Li G: SOX4 contributes to the progression of
cervical cancer and the resistance to the chemotherapeutic drug
through ABCG2. Cell Death Dis. 6:e19902015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Wang Y and Wang H: Use of
immunotherapy in the treatment of gastric cancer. Oncol Lett.
18:5681–5690. 2019.PubMed/NCBI
|
|
30
|
Fu X, Zhu X, Qin F, Zhang Y, Lin J, Ding
Y, Yang Z, Shang Y, Wang L, Zhang Q and Gao Q: Linc00210 drives
Wnt/β-catenin signaling activation and liver tumor progression
through CTNNBIP1-dependent manner. Mol Cancer. 17:732018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo C and Wang X, Chen LP, Li M, Li M, Hu
YH, Ding WH and Wang X: Long non-coding RNA MALAT1 regulates
ovarian cancer cell proliferation, migration and apoptosis through
Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci.
22:3703–3712. 2018.PubMed/NCBI
|
|
32
|
Hua F, Liu S, Zhu L, Ma N, Jiang S and
Yang J: Highly expressed long non-coding RNA NNT-AS1 promotes cell
proliferation and invasion through Wnt/β-catenin signaling pathway
in cervical cancer. Biomed Pharmacother. 92:1128–1134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deris Zayeri Z, Tahmasebi Birgani M,
Mohammadi Asl J, Kashipazha D and Hajjari M: A novel infram
deletion in MSH6 gene in glioma: Conversation on MSH6 mutations in
brain tumors. J Cell Physiol. 234:11092–11102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lou K, Chen N, Li Z, Zhang B, Wang X, Chen
Y, Xu H, Wang D and Wang H: MicroRNA-142-5p overexpression inhibits
cell growth and induces apoptosis by regulating FOXO in
hepatocellular carcinoma cells. Oncol Res. 25:65–73. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng D, Li J, Zhang L and Hu L:
miR-142-5p suppresses proliferation and promotes apoptosis of human
osteosarcoma cell line, HOS, by targeting PLA2G16 through the
ERK1/2 signaling pathway. Oncol Lett. 17:1363–1371. 2019.PubMed/NCBI
|
|
37
|
Liu L, Liu S, Duan Q, Chen L, Wu T, Qian
H, Yang S, Xin D, He Z and Guo Y: MicroRNA-142-5p promotes cell
growth and migration in renal cell carcinoma by targeting BTG3. Am
J Transl Res. 9:2394–2402. 2017.PubMed/NCBI
|
|
38
|
Pang L, Li B, Zheng B, Niu L and Ge L:
miR-138 inhibits gastric cancer growth by suppressing SOX4. Oncol
Rep. 38:1295–1302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang M, Huang S and Long D: miR-381
inhibits migration and invasion in human gastric carcinoma through
downregulatedting SOX4. Oncol Lett. 14:3760–3766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shiina H, Igawa M, Shigeno K, Terashima M,
Deguchi M, Yamanaka M, Ribeiro-Filho L, Kane CJ and Dahiya R:
Beta-catenin mutations correlate with over expression of C-myc and
cyclin D1 Genes in bladder cancer. J Urol. 168:2220–2226. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Backer J, Wong AS, Schwanke EL,
Stewart BG and Pasdar M: Bcl-2 expression decreases
cadherin-mediated cell-cell adhesion. J Cell Sci. 116:3687–3700.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu F, Li J, Guo N, Wang XH and Liao YQ:
miRNA-27a promotes the proliferation and invasion of human gastric
cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling
pathway. Am J Cancer Res. 7:405–416. 2017.PubMed/NCBI
|
|
45
|
Shan Y, Ying R, Jia Z, Kong W, Wu Y, Zheng
S and Jin H: LINC00052 promotes gastric cancer cell proliferation
and metastasis via activating the Wnt/β-catenin signaling pathway.
Oncol Res. 25:1589–1599. 2017. View Article : Google Scholar : PubMed/NCBI
|