Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
metabolic disease that is a global public health concern. From 1989
to 2015, the incidence rate of NAFLD was 28.01–52.34 per 1,000
people for Asia and Israel (1). In
2018, the prevalence of NAFLD was ~25% of the world population
(2). The clinical process of NAFLD
includes simple steatosis, steatohepatitis, hepatic
fibrosis/cirrhosis and hepatocellular carcinoma (HCC) (3). The two-hit hypothesis of
non-alcoholic steatohepatitis (NASH) suggests that hepatic
steatosis (‘first hit’) is a prerequisite for subsequent events
(‘second hits’) that lead to hepatic injury (4). Free fatty acids (FFAs) are the
primary mediators of excessive lipid accumulation in the liver.
Notably, circulating FFA levels are significantly increased in
patients with NAFLD, and evidence indicates that plasma FFAs are
associated with disease severity (5). Excess FFA-induced hepatic injury
results from the limited capacity of hepatocytes to transform FFA
into triglycerides in lipid droplets (5). Excess lipid droplets in cells can
result in mitochondrial dysfunction and oxidative stress,
potentially contributing to hepatic inflammation and fibrosis in
NASH (5).
The initiation of cancer may be the result of
mutations in stem cells that interfere with differentiation.
Certain tumor cells share properties with stem cells, which
indicates that cancer stem cells (CSCs) may be responsible for
cancer initiation and progression. These distinctive properties of
CSCs are the capacities for self-renewal and cell proliferation
(spherogenesis), which are primary causes of cancer recurrence and
metastasis (6). The sonic hedgehog
(Shh) signaling pathway and stemness-connected transcription
factors (such as Sox2 and Oct4) are primarily responsible for CSC
proliferation (7,8). CSCs have been hypothesized to be
responsible for the carcinogenesis of HCC. An increasing number of
studies have demonstrated that growth factors, such as epidermal
growth factor (EGF) or fibroblast growth factor 2 (FGF2), can
stimulate cell proliferation of CSCs and sphere formation in a
three-dimensional culture system. Sphere formation is a practical
approach for enriching certain CSC subpopulations with self-renewal
properties and CSC marker expression levels (9,10).
Research has demonstrated that liver CSC
subpopulations can be isolated by certain cell surface markers,
specifically CD133 (11,12), CD90 (13–15),
CD44 (16), the epithelial cell
adhesion molecule (17) and CD13
(18). A previous study
demonstrated that undifferentiated multipotent neural stem cells
could be expanded via suspension using sphere assay (19). Sphere assays have been used to
study adult stem cells in numerous organs and tissues, including
the liver, nerves, prostate and mammary (9,20–22).
Furthermore, sphere formation assay is widely recognized for its
ability to enrich potential CSC subpopulations via stimulation of
EGF or FGF2 (9,23–25).
Our previous study reported that exposure of
hepatocytes to palmitate (PA) induced reactive oxygen species (ROS)
production, NFκB activation and inflammatory cytokine expression
levels in primary rat hepatocytes (PRHs) (26) and HepG2 cells (27). This increase in proinflammatory
cytokines induced by PA could serve a key role in hepatic stellate
cell activation and the development of hepatic fibrosis.
Furthermore, it was demonstrated that PA induced CSC sphere
formation in HepG2 cells but not in PRHs. Conversely, inhibition of
NFκB activity significantly limited HepG2 CSC sphere formation
(27). In the present study, it
was hypothesized that PA would induce CSC properties via
palmitoylation, as palmitoylation has been demonstrated to regulate
CSC markers, estrogen receptors and epidermal growth factor (EGF)
receptors (28). Liquid
chromatography-tandem mass spectrometry (LC/MS/MS) was used to
identify the palmitoylation of proteins in PA-treated HepG2 CSC
spheres. The present study demonstrated that numerous proteins were
palmitoylated in CSC spheres formed following PA treatment. After
treating the HepG2 cells with two palmitoylation inhibitors,
tunicamycin and 2-bromohexadecanoic acid, the PA-treated CSC
spheres were notably inhibited. Taken together, the results of the
present study verified the hypothesis that PA-induced
palmitoylation is key for HepG2 CSC sphere formation. Thus, the
inhibition of palmitoylation may be a chemopreventive strategy for
use in treating patients with NAFLD.
Materials and methods
Chemicals
PA (cat. no. 102553) and BSA were acquired from
Sigma-Aldrich (Merck KGaA). PA stock solution was prepared as
previously described (27). In
brief, 400 mM PA stock solution was prepared in DMSO. The 3% (w/v)
FFA-free BSA solution was prepared in DMEM and maintained at 55°C.
A 5 mM FFA/BSA solution was prepared by dissolving the 128.2 mg of
PA in 1 ml (500 mM) ethanol and heating at 70°C for 10 min. 10% BSA
solution was made in DMEM medium, filtered and incubated at 37°C
for 10 min. Subsequently, 10 ul of dissolved PA was added to 990 µl
of 10% BSA, and the solution was vortexed and incubated at 55°C for
30 min. The final PA concentration in BSA solution was 5 mM. The
FFA/BSA solution was diluted to the concentration used in the
experiments using DMEM. All solutions were freshly prepared before
use. The 1 mg/ml tunicamycin and 1 mM 2-bromohexadecanoic acid
(cat. nos. T7765 and 21604, respectively; both Sigma-Aldrich; Merck
KGaA) stock solutions were prepared in ethanol and stored at
−20°C.
Cell culture
The human liver cancer cell line HepG2 was obtained
from the Bioresource Collection and Research Center. Authentication
of the HepG2 cell line used in the present study was performed via
the short tandem repeat-PCR method. Cells were maintained as a
monolayer in culture medium (Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), 100
IU/ml penicillin and 100 µg/ml streptomycin. Cells were maintained
at 37°C in a humidified 5% CO2 incubator. During sphere
assays, cells were harvested and washed with PBS to remove serum.
Cells were then suspended in ultralow-attachment 6-well plates
(Corning, Inc.) at a density of 5,000 cells/well. Serum-free
DMEM/F12 culture medium was supplemented with 100 IU/ml penicillin,
100 µg/ml streptomycin, 20 ng/ml human recombinant EGF, 10 ng/ml
human recombinant FGF2, 2% B27 supplement and 1% N2
supplement (Thermo Fisher Scientific, Inc.). Mycoplasma testing was
performed for the cell lines used. The maintenance and culture of
HepG2 cells were performed as previously described (27). Cell morphology was observed and
recorded using a light microscope (magnification, ×40) and the most
representative image was selected.
Oil Red O staining
After HepG2 cells were exposed to 200 µM PA
(Sigma-Aldrich; Merck KGaA) for 12 h at 37°C, cells were stained
with Oil Red O to investigate the amount of fat accumulation in
cells. Briefly, cells were washed with PBS and fixed in 10% neutral
formalin at room temperature (RT) for 30 min. After two washes with
propylene glycol, 10% Oil Red O was added and cells were stained
for 7 min at RT before being washed with 85% propylene glycol.
Cells were rinsed in distilled water and counterstained with 10%
hematoxylin solution at RT for 8 min. A total of three images were
captured per well using a light microscope (magnification, ×40) and
the most representative image was selected.
CSC sphere formation assay
After cells were cultured under serum-free medium
supplemented with EGF and FGF2 for sphere formation, spheres were
collected via centrifugation at 700 × g at RT for 3 min.
Subsequently, cells were dissociated using trypsin-EDTA and
mechanically disrupted with a pipette to obtain single cells. The
sphere-derived single cells were then centrifuged at 700 × g at RT
for 3 min to remove the trypsin-EDTA and resuspended in serum-free
medium for cell counting using trypan-blue exclusion assay (Thermo
Fisher Scientific, Inc.) using a light microscope (magnification,
×100) and the replication of sphere assays. The spheres were
passaged every 7 days until they reached a diameter of 100 µm.
Mass spectrometry
PA-treated HepG2 CSCs were collected to extract
protein lysates using lysis buffer [1 ml ice-cold buffer (20 mM
Tris-Hcl, pH 7.4; 150 mM NaCl; 1% (v/v) Triton X-100; 0.5% (v/v)
Nonidet P40; 1 mM EDTA; 1 mM PMSF; 2 µg/ml Antipain C; 50 µg/ml
tosyl phenylalanyl chloromethyl ketone; 10 µg/ml Leupeptin; 1 mM
NaF; 1 mM NaVO3; 5 mM
Na4P2O7)]. A total of 10 µg of
protein lysates were further separated using 10% SDS-PAGE and
stained with 0.25% Coomassie brilliant blue R-250 solution (Bio-Rad
Laboratories, Inc.) at RT for 1 h. All subsequent procedures for
in-gel digestion and mass spectrometry were performed as previously
described (29).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way analysis of variance was performed to compare the
statistical differences in biochemical and molecular parameters.
The post hoc test performed was Tukey's multiple comparisons test.
PRISM software (version 6.0; GraphPad Software, Inc.) was used for
this purpose. A total of three independent experiments were
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
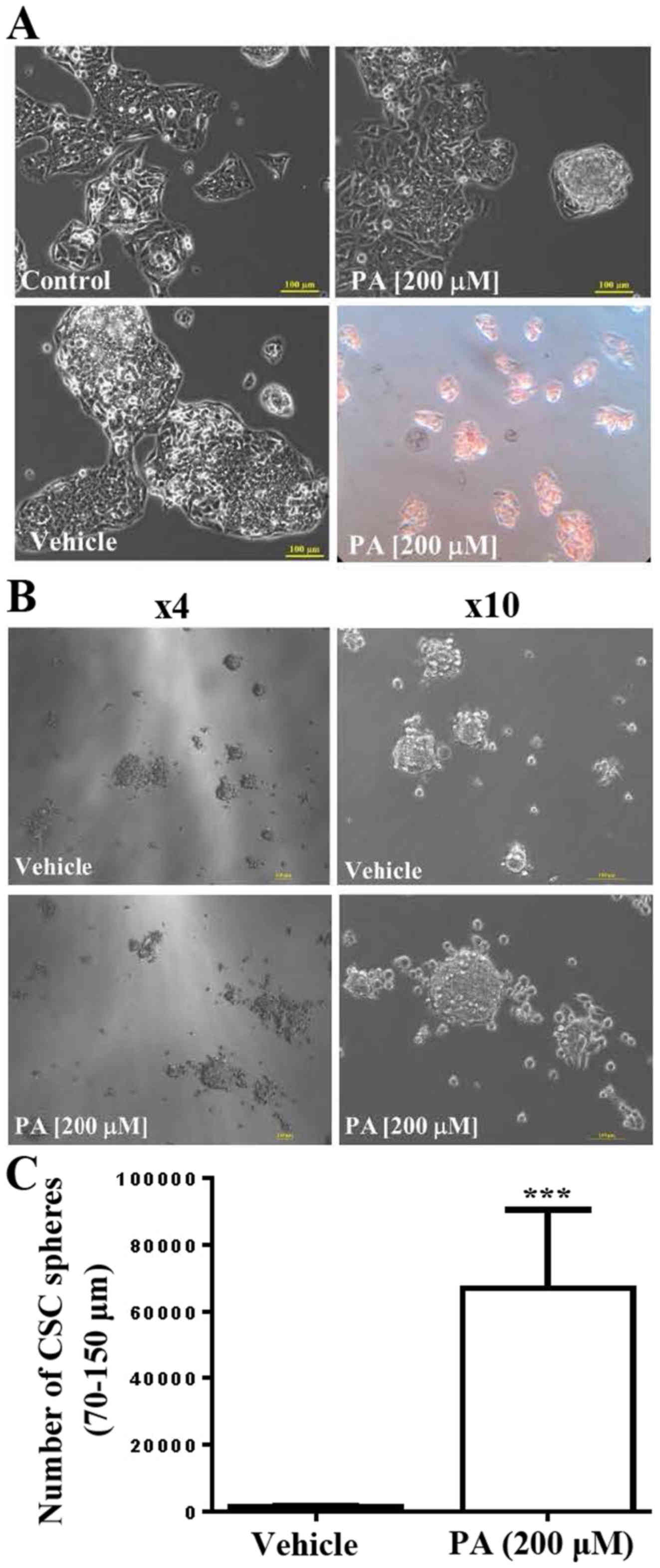
Effects of PA on HepG2 cells
The present study investigated the underlying
mechanisms of the effects of PA on human HepG2 cells. The results
indicated that, at a concentration of 200 µM, PA did not affect the
growth and survival rate of HepG2 cells but did induce
morphological changes in HepG2 cells in the 10% FBS culture medium
(Fig. 1A). The results of Oil Red
O staining indicated that lipid droplets accumulated inside HepG2
cells following PA treatment (Fig.
1A). Furthermore, the present study investigated the effects of
PA on the cancer sphere formation ability of HepG2 cells; it was
demonstrated that PA treatment significantly increased the cancer
sphere formation ability of HepG2 cells (n=3; P<0.001).
Representative images are presented in Fig. 1B and C.
LC/MS/MS analysis to identify sites of
PA-induced HepG2-CSC sphere palmitoylation on cell proteins
PA treatment has previously been shown to
significantly increase sphere formation of HepG2 cells (27). In the present study, it was
demonstrated that 200 µM PA significantly increased HepG2 CSC
sphere formation, particularly at the sphere size 75–150 µm
(Fig. 1). Subsequently, PA-treated
HepG2 CSC spheres were collected and total proteins were isolated
for LC/MS/MS analysis to investigate the effects of PA treatment on
the palmitoylation of HepG2-CSC spheres. It was noted that certain
proteins were palmitoylated, namely α-enolase, c-Myc
promoter-binding protein 1, epididymis luminal protein 114,
epithelial protein lost in neoplasm β variant (fragment),
LIM-domain and actin-binding protein 1, AHNAK, RNA-binding motif
protein, X chromosomes, plectin, spectrin α chain, non-erythrocytic
1 SFPQ protein (fragment), thyroid hormone receptor-associated
protein 3 and vinculin (Table
I).
 | Table I.Identification of novel types of
palmitoylation in palmitate-induced HepG2-CSC spheres using mass
spectrometry. |
Table I.
Identification of novel types of
palmitoylation in palmitate-induced HepG2-CSC spheres using mass
spectrometry.
| Protein name | Protein ID | Modified
peptidesa | Positiona | Calc. m/z | Obs. m/z | dM,
calc.-Obs.b | dM,
ppmc |
|---|
| α-enolase | P06733 | (−)IEEELGS#K | 413-420 | 381.568 | 381.576 | −0.008 | −20.959 |
| C-myc
promoter-binding protein 1 | E2DRY6 | (−)IEEELGS#K | 317-324 | 381.568 | 381.576 | −0.008 | −20.959 |
| Epididymis luminal
protein 114 | V9HWK2 |
(−)NLGPGMTK#MAK | 163-173 | 693.416 | 693.405 | 0.011 | 16.103 |
| Epithelial protein
lost in neoplasm β variant | Q53GG0 |
(−)RS#NTENLSQHFR | 54-65 | 432.495 | 432.501 | −0.005 | −12.570 |
| (Fragment) |
|
(−)KGWSM*SEQSEES#VGGR | 614-629 | 502.758 | 502.770 | −0.012 | −24.776 |
| LIM domain and
actin-binding protein 1 | Q9UHB6 |
(−)RS#NTENLSQHFR | 54-65 | 432.495 | 432.501 | −0.005 | −12.570 |
|
|
|
(−)KGWSM*SEQSEES#VGGR | 614-629 | 502.758 | 502.770 | −0.012 | −24.776 |
| AHNAK | Q09666 |
(−)M*KM*PTFS#TPGAK | 631-642 | 392.225 | 392.224 | 0.001 | 2.163 |
|
|
|
(−)IS#M*PDFDLNLK | 3,671-3,681 | 387.476 | 387.479 | −0.003 | −6.553 |
| RNA-binding motif
protein, X chromosome | P38159 |
(−)PSFES#GRRGPPPPPR | 87-101 | 624.700 | 624.707 | −0.007 | −11.174 |
|
|
|
(−)DYGHSS#SRDDYPSR | 245-258 | 470.735 | 470.746 | −0.012 | −24.794 |
|
|
|
(−)DYSDHPSGGSYRDS#YESYGNSR | 271-292 | 685.065 | 685.071 | −0.006 | −9.169 |
| Plectin | Q15149 |
(−)S#EFERLECLQR | 523-533 | 584.995 | 584.995 | 0.000 | −0.554 |
|
|
|
(−)AS#FEKAAAGKAELELELGR | 2,069-2,087 | 557.828 | 557.816 | 0.012 | 20.636 |
| Spectrin α chain,
non-erythrocytic 1 | Q13813 | (−)SCK#KFM*LFR | 1,090-1,098 | 380.480 | 380.477 | 0.003 | 6.954 |
| SFPQ protein
(Fragment) | Q9BSV4 |
(−)DMRMGGGGAMNM*GDPYGS#GGQK | 536-557 | 607.785 | 607.785 | 0.000 | −0.257 |
| Thyroid hormone
receptor-associated protein 3 | Q9Y2W1 | (−)SSSDRS#RR | 149-156 | 396.906 | 396.904 | 0.003 | 7.305 |
|
|
|
(−)SSSNHSRVESS#K | 162-173 | 514.954 | 514.955 | −0.001 | −1.561 |
|
|
|
(−)S#GKWEGLVYAPPGK | 468-481 | 432.509 | 432.501 | 0.008 | 18.812 |
| Vinculin | P18206 |
(−)NLGPGMTK#MAK | 163-173 | 693.416 | 693.405 | 0.011 | 16.103 |
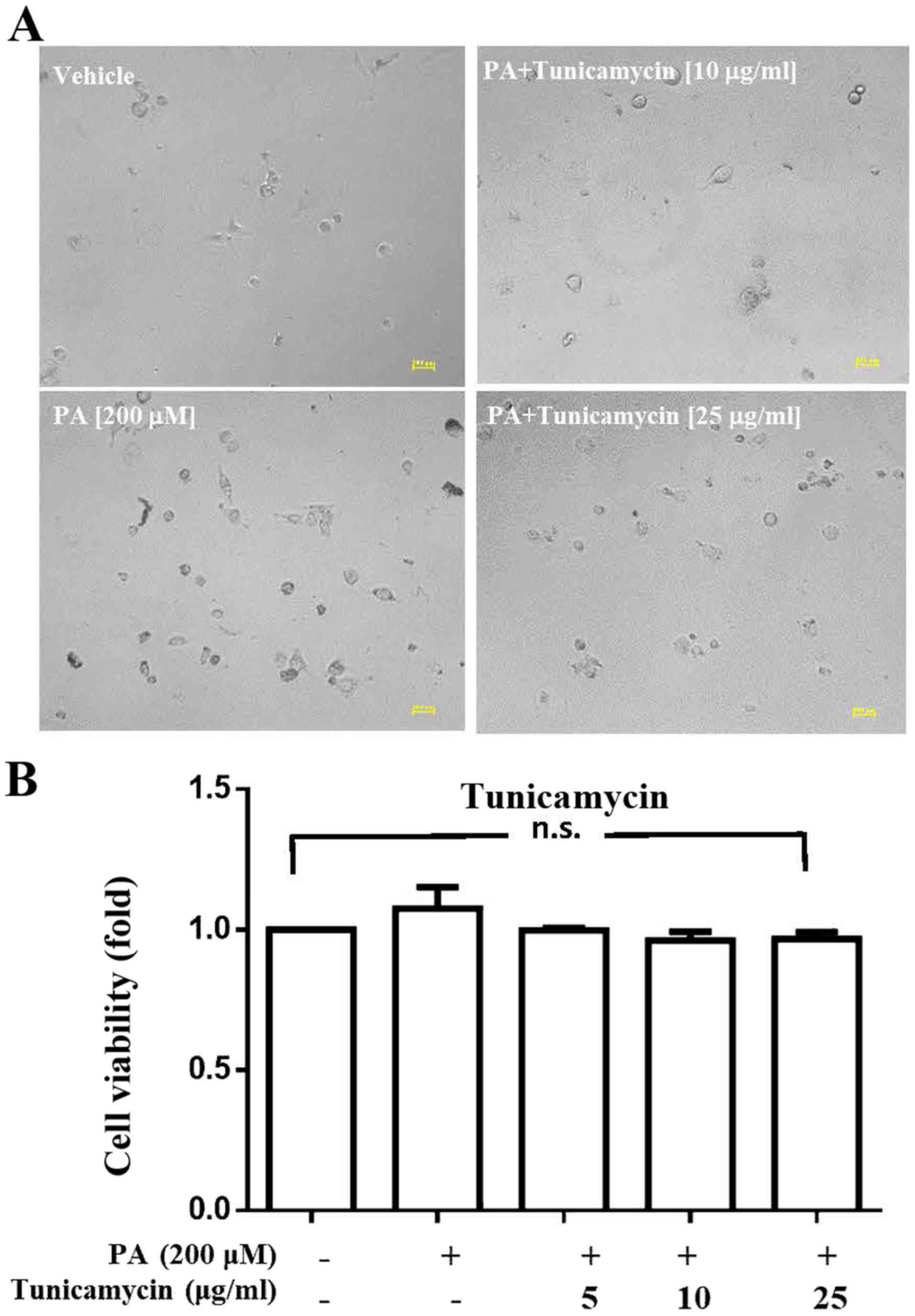
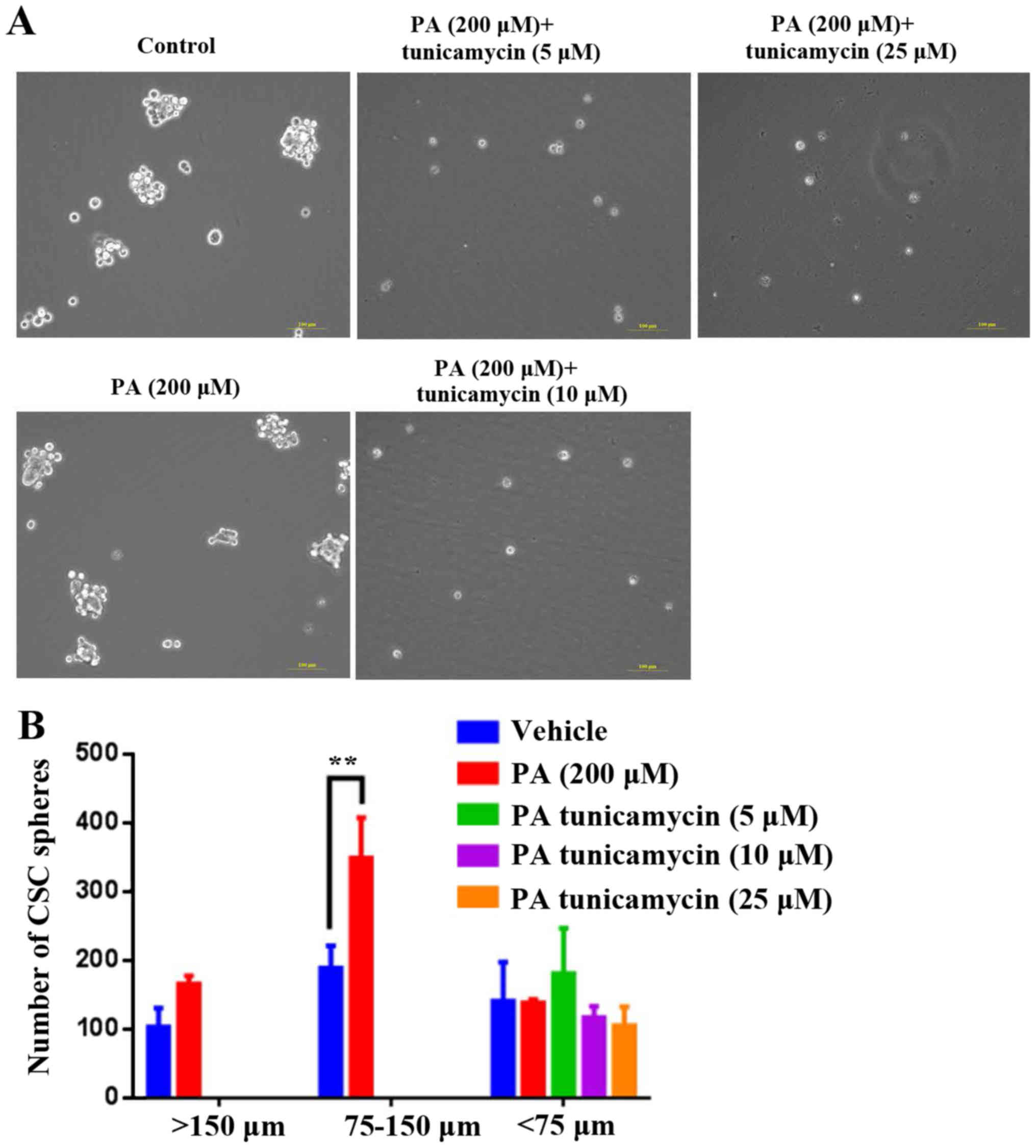
Analysis of the effects of tunicamycin
on viability and CSC sphere formation ability of HepG2 cells
After demonstrating that numerous proteins were
palmitoylated, the present study investigated whether inhibition of
palmitoylation alters cell viability and CSC sphere formation. The
results indicated that tunicamycin, a specific inhibitor of
palmitoylation, did not affect the viability of PA-treated HepG2
cells at concentrations of 5, 10 or 25 µg/ml (Fig. 2); however, at these concentrations,
tunicamycin notably decreased PA-induced HepG2-CSC sphere formation
(>150 µm and 75–150 µm; Fig.
3).
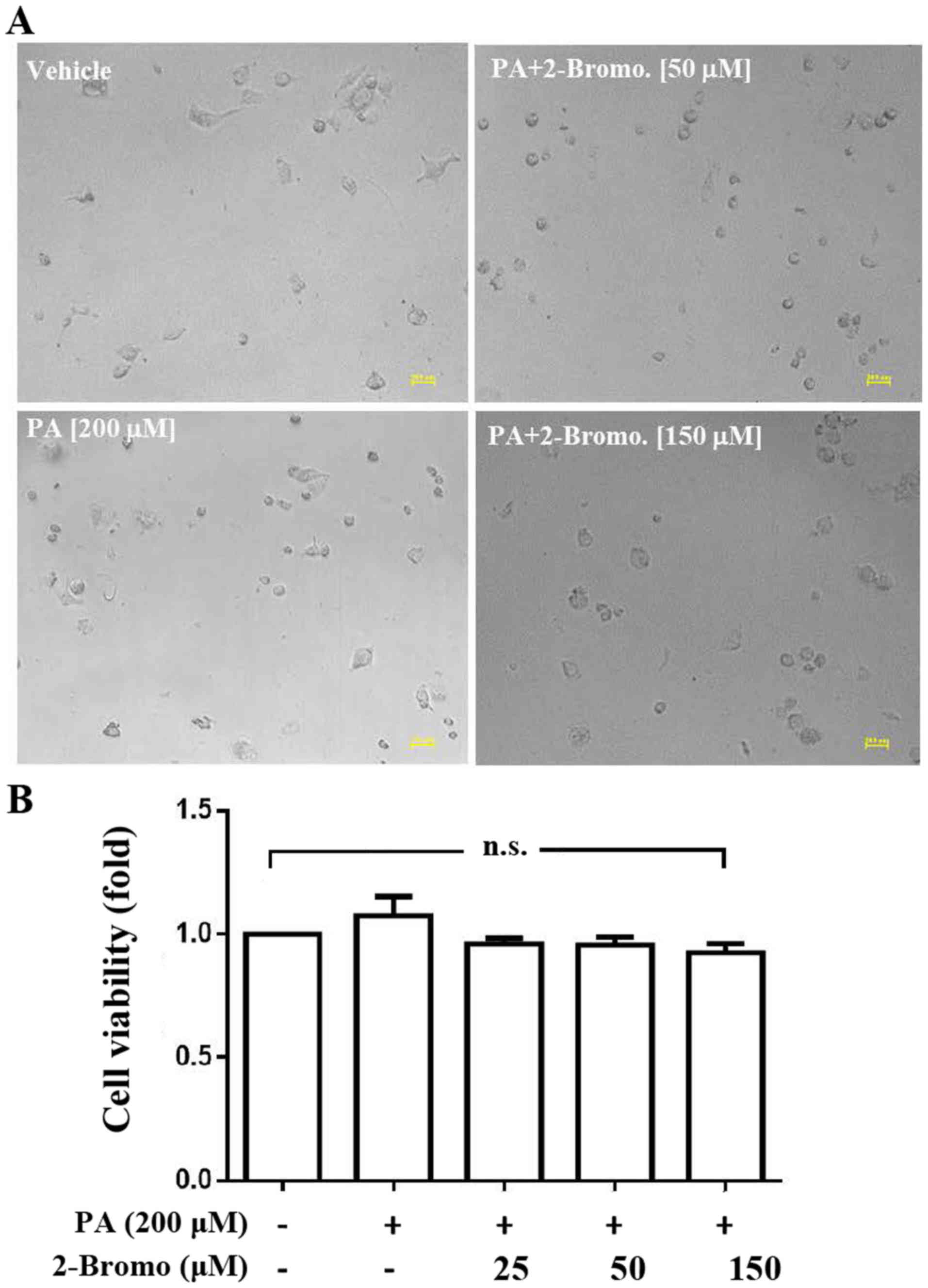
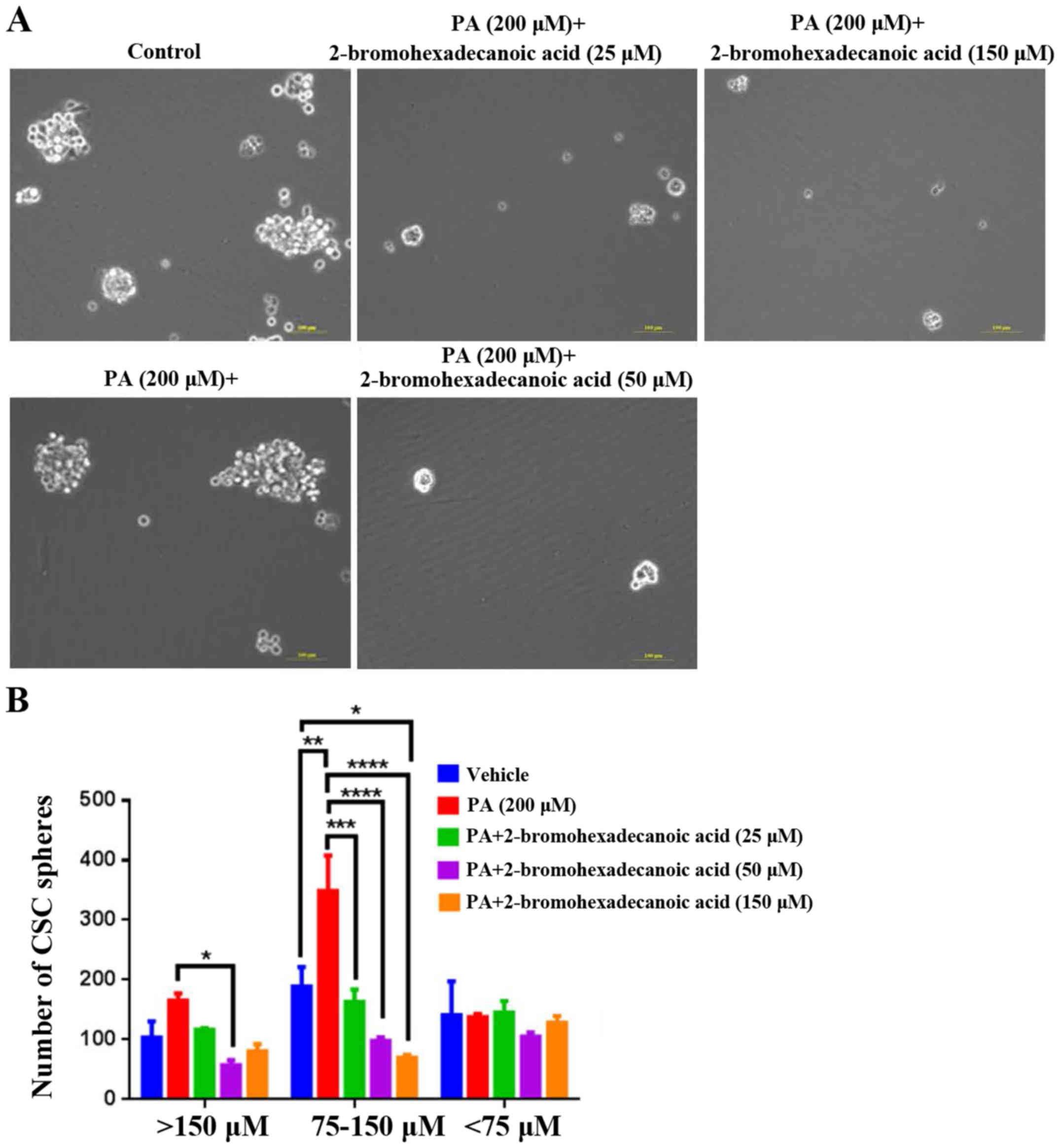
Analysis of the effects of
2-bromohexadecanoic acid on viability and CSC sphere formation
ability of HepG2 cells
The present study demonstrated that
2-bromohexadecanoic acid, another inhibitor of palmitoylation, did
not affect viability of PA-treated HepG2 cells at concentrations of
25, 50 and 150 µM (Fig. 4);
however, it significantly decreased PA-induced HepG2-CSC sphere
formation (75–150 µm) at these concentrations (Fig. 5).
Discussion
NAFLD is the most common type of liver disease
worldwide and is expected to become a primary cause of HCC in the
near future. Previous research has investigated the effects and
underlying mechanisms of saturated FFAs, such as PA, on liver
cells. Our previous study demonstrated that PA increased
intracellular hydrogen peroxide levels in PRHs and the human liver
cancer cell line HepG2, and induced the expression levels of
proinflammatory cytokines, such as TNF-α, IL6 and intercellular
adhesion molecule 1. Furthermore, PA activated hepatic stellate
cells and contributed to steatosis-associated hepatic fibrogenesis
(30). In addition, PA has been
reported to significantly increase CSC sphere formation in HepG2
cells but not in PRH (27).
Furthermore, PA (50, 100 and 200 µM) activated the expression
levels of pluripotent genes, including Sox2, Oct4 and Shh in a
dose-dependent manner, and 200 µM PA significantly increased the
sphere formation capacity of HepG2 cells (27). In the present study, 200 µM PA
significantly increased the sphere formation capacity of HepG2
cells. Notably, in a previous study, PA (10, 50, 100 and 200 µM)
dose-dependently induced production of ROS in primary hepatocytes
and HepG2 cells but did not affect the viability of primary
hepatocytes and HepG2 cells (30).
Furthermore, PA not only induced proinflammatory responses in
hepatocytes and HepG2 cells (30)
but also activated the CSC-like properties of HepG2 cells (27) to increase sphere formation.
However, long-term PA treatment may cause cells to become
senescent, as certain types of cell are prone to senescence rather
than apoptosis following high exogenous stress (31).
The primary saturated fatty acid in HepG2 cells is
PA, which undergoes post-translation palmitoylation. Research
indicates that palmitoylation modulates protein function at all
stages of protein processing (26). In addition, other saturated fatty
acids, such as myristic and stearic acid, and unsaturated fatty
acids, such as oleic and arachidonic acid, also undergo
post-translational modification. The present study used LC/MS/MS to
identify palmitoylation-modified proteins in PA-treated HepG2 CSC
spheres (Fig. 1); numerous
proteins were demonstrated to be palmitoylated.
Tunicamycin is a nucleoside antibiotic that can
inhibit protein palmitoylation. Tunicamycin has been reported to
inhibit palmitoylation of the endothelial isoform of nitric oxide
synthase, promote nitric oxide synthesis in aortic endothelial
cells and increase the influx of Ca2+ across the plasma
membrane (32). In the present
study, tunicamycin did not affect HepG2 cell viability in 10% FBS
culture medium but decreased PA-induced formation of HepG2 CSC
spheres in the sphere formation culture medium. Although
2-bromohexadecanoic acid (2-bromopalmitate) is used as a
non-selective inhibitor of lipid metabolism, it has been
demonstrated to be a general inhibitor of protein S-palmitoylation.
It has been hypothesized that 2-bromopalmitate is converted to
2-bromopalmitoyl-CoA in cells. Notably, both 2-bromopalmitate and
2-bromopalmitoyl-CoA interact with DHHC palmitoyl acyl
transferases, which catalyze protein S-palmitoylation. The present
study demonstrated that 2-bromohexadecanoic acid did not affect
HepG2 cell viability in the 10% FBS culture medium, but decreased
PA-induced formation of HepG2 CSC spheres in the sphere formation
culture medium. Neither 2-bromopalmitate nor tunicamycin affected
viability of HepG2 cells under serum-containing culture conditions.
However, they both significantly decreased the number of HepG2
spheres formed under serum-free culture conditions. One explanation
for this finding is that serum supplementation may activate cell
survival to eliminate ER stress when cells are treated with
2-bromopalmitate or tunicamycin. The present study investigated the
effects of PA and two inhibitors, 2-bromopalmitate and tunicamycin,
on palmitoylation. Although 2-bromopalmitate is traditionally used
as an inhibitor of PA, its effects extend beyond protein
palmitoylation. Tunicamycin can also function as an inhibitor of
protein N-glycosylation and induce cellular ER stress. Further
research is necessary to determine other mechanisms of
2-bromopalmitate and tunicamycin and to investigate their ability
to decrease PA-induced protein palmitoylation.
The effects of tunicamycin at concentrations of 5,
10 and 25 µg/ml, and 2-bromopalmitate at a concentration of 150 µM
observed in the present study are consistent with those reported in
the literature (6,7). Sobocińska et al (33) used 2-bromopalmitate (125 and 250
µM) to inhibit lipopolysaccharide-induced S-palmitoylation and
activation of PI4KIIβ; this activation generated
phosphatidylinositol-4-phosphate, which is involved in signaling
pathways controlling the production of proinflammatory cytokines.
Patterson and Skene (34)
demonstrated that tunicamycin (10 µg/ml) significantly inhibited
growth cone protein palmitoylation in intact neuronal cells. On the
other hand, the results of the present study demonstrated that PA
may induce protein palmitoylation and CSC properties via inducing
expression levels of carnitine O-palmitoyltransferase 1 and the CSC
marker CD44. Additionally, tunicamycin and 2-bromopalmitate
treatment notably suppressed the expression levels of carnitine
O-palmitoyltransferase 1 and CD44, respectively (Data S1),
indicating a novel mechanism of tunicamycin and 2-bromopalmitate in
the inhibition of palmitoylation.
In breast cancer, palmitoylation has been
hypothesized to control the function of commonly dysregulated
genes, including estrogen and EGF receptors, and CSC marker
proteins. Palmitoylation also regulates the formation of complexes,
such as integrins, at the plasma membrane (26). However, it is unclear whether
aberrant palmitoylation can contribute to tumor initiation and
growth in the liver. The results of the present study indicated
that PA-mediated palmitoylation may be a key factor for inducing
CSC activation in the fatty liver of patients with NAFLD. In
conclusion, the development of specific inhibitors for the
suppression of PA-mediated palmitoylation may present a promising
chemopreventive strategy to treat patients with NAFLD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Shin Kong Wu Ho
Su Memorial Hospital (grant no. SKH 8302-105-DR-06), the Ministry
of Science and Technology of Taiwan Government (grant nos.
107-2314-B-715-004-MY3, 103-2314-B-715-001-MY2,
104-2314-B-715-003-MY3, 105-2320-B-039-059-MY3 and
108-2320-B-039-013) and Mackay Medical College (grant nos. 1052B07,
1051B23, 1061B09, 1071B12 and 1081E03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LWC, YCH and CCL were involved in the
conceptualization, supervision and funding acquisition of the
present study. LWC, YCH was involved in project administration.
LWC, CLT, KCY, CCL and YCH were involved in the investigation,
methodology and analysis of the present study, and in the writing
of the manuscript. The final manuscript was read and approved by
LWC, CLT, KCY, CCL and YCH.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-Meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Younossi Z, Tacke F, Arrese M, Chander
Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George
J, Fan J and Vos MB: Global perspectives on non-alcoholic fatty
liver disease and non-alcoholic steatohepatitis. Hepatology.
69:2672–2682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angulo P and Lindor KD: Treatment of
non-alcoholic steatohepatitis. Best Pract Res Clin Gastroenterol.
16:797–810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malaguarnera M, Di Rosa M, Nicoletti F and
Malaguarnera L: Molecular mechanisms involved in NAFLD progression.
J Mol Med (Berl). 87:679–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leamy AK, Egnatchik RA and Young JD:
Molecular mechanisms and the role of saturated fatty acids in the
progression of non-alcoholic fatty liver disease. Prog Lipid Res.
52:165–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang P, Qiu J, Li B, Hong J, Lu C, Wang
L, Wang J, Hu Y, Jia W and Yuan Y: Role of Sox2 and Oct4 in
predicting survival of hepatocellular carcinoma patients after
hepatectomy. Clin Biochem. 44:582–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun C, Sun L, Li Y, Kang X, Zhang S and
Liu Y: Sox2 expression predicts poor survival of hepatocellular
carcinoma patients and it promotes liver cancer cell invasion by
activating Slug. Med Oncol. 30:5032013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin X, Li YW, Zhang BH, Ren ZG, Qiu SJ, Yi
Y and Fan J: Coexpression of stemness factors Oct4 and Nanog
predict liver resection. Ann Surg Oncol. 19:2877–2887. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma XL, Sun YF, Wang BL, Shen MN, Zhou Y,
Chen JW, Hu B, Gong ZJ, Zhang X, Cao Y, et al: Sphere-forming
culture enriches liver cancer stem cells and reveals Stearoyl-CoA
desaturase 1 as a potential therapeutic target. BMC Cancer.
19:7602019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stafman LL, Williams AP, Garner EF, Aye
JM, Stewart JE, Yoon KJ, Whelan K and Beierle EA: Targeting PIM
kinases affects maintenance of CD133 tumor cell population in
hepatoblastoma. Transl Oncol. 12:200–208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maehara O, Ohnishi S, Asano A, Suda G,
Natsuizaka M, Nakagawa K, Kobayashi M, Sakamoto N and Takeda H:
Metformin regulates the expression of CD133 through the AMPK-CEBPβ
pathway in hepatocellular carcinoma cell lines. Neoplasia.
21:545–556. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WC, Chang YS, Hsu HP, Yen MC, Huang
HL, Cho CY, Wang CY, Weng TY, Lai PT, Chen CS, et al: Therapeutics
targeting CD90-integrin-AMPK-CD133 signal axis in liver cancer.
Oncotarget. 6:42923–42937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He J, Liu Y, Zhu T, Zhu J, Dimeco F,
Vescovi AL, Heth JA, Muraszko KM, Fan X and Lubman DM: CD90 is
identified as a candidate marker for cancer stem cells in primary
high-grade gliomas using tissue microarrays. Mol Cell Proteomics.
11:M111.010744. 2012. View Article : Google Scholar
|
|
15
|
Zhang K, Che S, Pan C, Su Z, Zheng S, Yang
S, Zhang H, Li W, Wang W and Liu J: The SHH/Gli axis regulates
CD90-mediated liver cancer stem cell function by activating the
IL6/JAK2 pathway. J Cell Mol Med. 22:3679–3690. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei S, Liu K, He Q, Gao Y and Shen L: PES1
is regulated by CD44 in liver cancer stem cells via miR-105-5p.
FEBS Lett. 593:1777–1786. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamashita T, Honda M, Nakamoto Y, Baba M,
Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H, et al:
Discrete nature of EpCAM+ and CD90+ cancer stem cells in human
hepatocellular carcinoma. Hepatology. 57:1484–1497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christ B, Stock P and Dollinger MM: CD13:
Waving the flag for a novel cancer stem cell target. Hepatology.
53:1388–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki A, Oyama K, Fukao K, Nakauchi H and
Taniguchi H: Establishment of clonal colony-forming assay system
for pancreatic stem/progenitor cells. Cell Transplant. 11:451–453.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi X, Gipp J and Bushman W:
Anchorage-independent culture maintains prostate stem cells. Dev
Biol. 312:396–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gou S, Liu T, Wang C, Yin T, Li K, Yang M
and Zhou J: Establishment of clonal colony-forming assay for
propagation of pancreatic cancer cells with stem cell properties.
Pancreas. 34:429–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rappa G, Mercapide J, Anzanello F,
Prasmickaite L, Xi Y, Ju J, Fodstad O and Lorico A: Growth of
cancer cell lines under stem cell-like conditions has the potential
to unveil therapeutic targets. Exp Cell Res. 314:2110–2122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wobser H, Dorn C, Weiss TS, Amann T,
Bollheimer C, Büttner R, Schölmerich J and Hellerbrand C: Lipid
accumulation in hepatocytes induces fibrogenic activation of
hepatic stellate cells. Cell Res. 19:996–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chong LW, Chou RH, Liao CC, Lee TF, Lin Y,
Yang KC and Hsu YC: Saturated fatty acid induces cancer stem
cell-like properties in human hepatoma cells. Cell Mol Biol
(Noisy-le-Grand). 61:85–91. 2015.PubMed/NCBI
|
|
28
|
Anderson AM and Ragan MA: Palmitoylation:
A protein S-acylation with implications for breast cancer. NPJ
Breast Cancer. 2:160282016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang MH, Liao CC, Hung JH, Lai XT, Yen CH
and Chen YA: Utilizing proteomic approach to identify nuclear
translocation related serine kinase phosphorylation site of GNMT as
downstream effector for benzo[a]pyrene. J Food Drug Anal.
27:603–609. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chong LW, Hsu YC, Lee TF, Lin Y, Chiu YT,
Yang KC, Wu JC and Huang YT: Fluvastatin attenuates hepatic
steatosis-induced fibrogenesis in rats through inhibiting paracrine
effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol.
15:222015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alessio N, Del Gaudio S, Capasso S, Di
Bernardo G, Cappabianca S, Cipollaro M, Peluso G and Galderisi U:
Low dose radiation induced senescence of human mesenchymal stromal
cells and impaired the autophagy process. Oncotarget. 6:8155–8166.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buckley BJ and Whorton AR: Tunicamycin
increases intracellular calcium levels in bovine aortic endothelial
cells. Am J Physiol. 273:C1298–C1305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sobocińska J, Roszczenko-Jasińska P,
Zaręba-Kozioł M, Hromada-Judycka A, Matveichuk OV, Traczyk G,
Łukasiuk K and Kwiatkowska K: Lipopolysaccharide upregulates
palmitoylated enzymes of the phosphatidylinositol cycle: An insight
from proteomic studies. Mol Cell Proteomics. 17:233–254. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patterson SI and Skene JH: Novel
inhibitory action of tunicamycin homologues suggests a role for
dynamic protein fatty acylation in growth cone-mediated neurite
extension. J Cell Biol. 124:521–536. 1994. View Article : Google Scholar : PubMed/NCBI
|