Introduction
Neural stem/progenitor cells (NSPCs) remain in the
mammalian central nervous system (CNS) throughout life, where they
have the ability to self-renew and differentiate into multiple
different types of cell (1). In
the postnatal brain, the vast majority of NSPCs are spatially
restricted to two specific brain regions: The subventricular zone
of the lateral ventricles and the subgranular zone in the dentate
gyrus of the hippocampus (2).
Previous studies have indicated that postnatal NSPCs may be
activated in response to pathophysiological stimuli, such as
cerebral hemorrhage, traumatic brain injury and stroke, and that
they may participate in CNS damage repair and functional recovery
(3–5). Therefore, NSPC replacement therapy
may aid in the development of new treatment modalities for diseases
of the CNS. Nonetheless, this technique is restricted by the number
of NSPCs and newly generated neurons in the brain, thus it is
currently difficult to use NSPCs for clinical therapy (6). Therefore, there remains an urgent
requirement to identify the regulatory functions of NSPCs that
permit their positive enrichment in the CNS.
Numerous factors, including intracellular signal
molecules, the extracellular niche and microRNAs (miRNAs/miRs) have
been discovered to be involved in regulating the proliferation,
differentiation and maintenance of NSPCs (7–9). For
example, as a novel regulatory factor, miR-29a demonstrated huge
potential over the control of cell behavior not only in cancer
cells, but also in stem/progenitor cells (10,11).
More importantly, our previous study revealed that the
overexpression of miR-29 promoted the proliferation of cultured rat
NSPCs (12). These findings
indicated that miR-29a may be an important indicator of regulatory
factors in NSPC development. However, to the best of our knowledge,
very little is known about the function of miR-29a in NSPC
differentiation, let alone its intracellular signaling
mechanisms.
The present study aimed to investigate the effect of
miR-29a on the differentiation of NSPCs. The finding of the study
suggested that the overexpression of miR-29a may promote neural
differentiation, whilst having little influence on astrocyte
differentiation, potentially through regulating the zinc-finger
transcription factor Kruppel-like factor 4 (KLF4). This research
may offer novel insights into the onset of neurodevelopment and
provide a potential therapeutic target for the treatment of
diseases of the CNS.
Materials and methods
Rat NSPC culture
Rat NSPCs were prepared from E14.5 Sprague-Dawley
rat embryos, which were obtained from pregnant Sprague-Dawley rats
(certificate no. 22-9601018; Experimental Animal Center of Xi'an
Jiantong University Health Science Center) as previously described
with minor modifications (13,14).
A total of 5 pregnant Sprague-Dawley rats (age, 12 weeks; weight,
386–422 g) were used. All rats were maintained at 23±2°C on a 12-h
light/dark cycle with free access to standard rat food and water.
NSPCs were incubated in a humidified atmosphere of 5%
CO2 and 95% air at 37°C, and cultured in serum-free
complete medium, consisting of DMEM/F12 (1:1; Thermo Fisher
Scientific, Inc.), 1% N-2 supplement (cat. no. 17502048; Thermo
Fisher Scientific, Inc.), 2% B-27 supplement (cat. no. 17504044;
Thermo Fisher Scientific, Inc.), 20 ng/ml epidermal growth factor
(EGF; Thermo Fisher Scientific, Inc.) and 10 ng/ml basic fibroblast
growth factor (bFGF; Thermo Fisher Scientific, Inc.) The
differentiation medium was the same as the complete medium but did
not contain bFGF and EGF. After 3–5 days of culture, suspended
neurospheres of 80–200-µm in diameter were observed in the medium
using a binocular compound microscope (cat. no. CX200; Olympus
Corporation) (magnification, ×10). The neurospheres were
subsequently dissociated using TrypLE (Invitrogen; Thermo Fisher
Scientific, Inc.) into single NSPCs, which were used for follow-up
studies. For single-cell adhesive culture, single NSPCs in the
differentiation medium were allowed to attach onto poly-D-lysine
(PDL)-coated coverslips at 37°C. All experimental protocols were
approved by the Animal Care and Use Regulation of Xi'an Jiaotong
University Health Science Center. All efforts were made to minimize
the suffering of the animals and to keep the numbers of animals
used to a minimum.
Cell transfection
NSPCs were plated in PDL-coated 24-well or 6-well
plates and transfected with 50 nM miRNA or small interfering RNA
(siRNA) for 6 h at 37°C, then cells were cultured in normal
differentiation medium for 3 days prior to use in subsequent
experiments. The cells plated into the 24-well plates were used for
immunofluorescence staining, whereas the cells plated into the
6-well plates were used for the reverse transcription-quantitative
(RT-q)PCR and western blotting experiments. Transient transfections
were performed using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, and equivalent transfection medium was
used as a control.
The miR-29a mimic-negative control (NC), miR-29a
mimic, anti-miR-29a-NC, anti-miR-29a, miR-200c, miR-200c-NC, siRNA
targeting KLF4 (siKLF4) and non-specific siRNA (siNC) were
purchased from Shanghai GenePharma Co., Ltd. All siRNA was
pre-labeled by the supplier with fluorescent dye (fluorescein
amidite, FAM). The target sequences were as follows: miR-29a mimic,
5′-ACUGAUUUCUUUUGGUGUUCAG-3′; miR-29a mimic-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′; anti-miR-29a,
5′-ACTGATTTCAAATGGTGCT-3′; anti-miR-29a-NC,
5′-UUGUACUACACAAAAGUACUG-3′; miR-200c, 5′-CGUCUUACCCAGCAGUGUUUG-3′;
miR-200c-NC, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′; siKLF4,
5′-UCCAAAGAAGAAGGAUCUCUU −3′; and siNC,
5′-CGTACGCGGAATACTTCGATT-3′. Transfection efficiency was examined
using a fluorescence inverted microscope (magnification, ×10)
(DMI3000B; Leica Microsystems, Inc). Knockdown of KLF4 expression
levels was further evaluated via western blotting.
Dual-luciferase reporter assay
The dual-luciferase reporter assay was performed as
previously described (15).
Briefly, the sequence of the KLF4 3′ untranslated region (UTR) was
cloned into the pSICHECK2 vector (Promega Corporation), and the
following KLF4 primer: Forward, 5′-GCCTCGAGATCCCAGACAGTGGATAT-3′
and reverse, 5′-GCGCGGCCGCTTCAGATAAAATATTAT-3′. Cells were
co-transfected with plasmid for 6 h at 37°C in the presence of
Lipofectamine 2000, according to the manufacturer's instructions
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h
incubation, the media was removed and the cells were lysed in a
1.5-ml Eppendorf tube using a Dual-Luciferase Reporter assay system
(Promega Corporation), according to the manufacturer's protocol.
Fluorescence was detected using a microtiter plate reader (BioTek
Instruments, Inc). Relative luciferase activity was determined by
normalizing the Firefly luciferase activity (Flu value) to the
Renilla luciferase activity (Rlu value). Each experiment was
performed in triplicate. The Flu/Rlu value was calculated to obtain
a mean of three independent experiments.
RT-qPCR
RT-qPCR was performed as previously described with
minor modifications (12). Total
RNA was isolated from NSPCs using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Total RNA (1 µg) was reverse transcribed
into cDNA using Megaplexä Primer Pools (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and the TaqManä MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). All RT reactions were carried out in triplicate in a
Mastercycler ep gradient S cycler (Eppendorf) at 16°C for 30 min,
42°C for 30 min and 85°C for 5 min. qPCR was subsequently performed
using single tube TaqMan® MicroRNA assays and
TaqMan® 2X Universal PCR Master mix, No
AmpErase® UNG (cat. no. 4324018; Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturers'
protocols, in 10 µl reactions. All the reactions were performed in
triplicate in an iQ5 Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc.). To analyze the expression levels of KLF4 and
GAPDH, total RNA (1 µg) was reverse transcribed into cDNA using a
RevertAid First Strand cDNA synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) supplemented with a mix of OligodT and random
primers. qPCR was subsequently performed using a GoTaq qPCR Master
mix (Roche Diagnostics GmbH). The primer pairs used for the qPCR,
synthesized by Takara Biotechnology Co., Ltd., were as follows:
miR-29a forward, 5′-AGTGAATGAGGCCTTCGAGA-3′ and reverse,
5′-GCATCTGAGTCGCCACTGTA-3′; miR-200c forward,
5′-GGTTGCCCACTGGAAGAACACAAT-3′ and reverse,
5′-TAGACAATCCCAAGGCCAAGGTCTG-3′; KLF4 forward,
5′-GAAATTCGCCCGCTCCGATGA-3′ and reverse,
5′-CTGTGTGTTTGCGGTAGTGCC-3′; U6 forward, 5′-GCATCTGAGTCGCCACTGTA-3′
and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; and GAPDH forward,
5′-CATCACTGCCACCCAGAAGACTG-3′ and reverse,
5′-ATGCCAGTGAGCTTCCCGTTCAG-3′. The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
5 min; followed by 40 cycles at 95°C for 10 sec and 60°C for 1 min.
Expression levels were analyzed using the 2−ΔΔCq method
(16).
Immunofluorescence
The immunofluorescence procedure was conducted
according to a previous study (17). Briefly, following transfection and
incubating for 3 days, NSPCs plated onto PDL-coated coverslips were
fixed using 4% paraformaldehyde for 30 min at room temperature and
washed twice with PBS. The cells were subsequently permeabilized
with 0.3% Triton X-100 (diluted with PBS) and blocked with 10%
normal donkey serum (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. The cells were incubated with the following primary
antibodies diluted in PBS which contained 5% normal donkey serum
overnight at 4°C: Anti-nestin (1:200; cat. no. MAB353; EMD
Millipore), anti-neuron-specific class III β-tubulin (Tuj1; 1:200;
cat. no. ab78078; Abcam), anti-glial fibrillary acidic protein
(GFAP; 1:200; cat. no. ab7260; Abcam). Following the primary
antibody incubation, the cells were washed with PBS and incubated
with anti-mouse IgG ReadyProbesä secondary antibody, Alexa Fluor
488 (1:500; cat. no. R37114; Invitrogen; Thermo Fisher Scientific,
Inc.) or anti-rabbit IgG ReadyProbes™ secondary antibody, Alexa
Fluor 594 (1:500; cat. no. R37119; Invitrogen; Thermo Fisher
Scientific, Inc.) for 2 h at room temperature. The nuclei were
counterstained with 1 µg/ml DAPI at room temperature for 10 min.
Fluorescence images were observed and counted using a BX51
fluorescence microscope equipped with a DP70 digital camera
(magnification, ×400; Olympus Corporation) in 5 randomly selected
fields of view. For semi-quantification, Image-Pro Plus 5.1
software (Media Cybernectics, Inc.) was used. The percentage of
labeled cells was calculated and normalized by DAPI nuclei
staining. A total of three independently prepared NSPC cultures
were used for each assay. The experiments were performed in
triplicate and repeated independently ≥3 times.
Western blotting
At each end of the experiment, following
transfection and incubating for 3 days, total protein was extracted
by using RIPA lysis buffer (Pierce; Thermo Fisher Scientific, Inc.)
complemented with a Protease Inhibitor Cocktail (Roche Diagnostics
GmbH). Total protein was quantified using a Bradford assay
bicinchoninic acid assay (Pierce; Thermo Fisher Scientific, Inc.)
and 20–40 µg protein/lane (20 µg protein was used for GFAP and
β-Actin, 40 µg protein was used for remaining test) was subjected
to 12% SDS-PAGE. The separated proteins were subsequently
transferred onto PVDF membranes and blocked with 5% non-fat dry
milk at room temperature for 2 h. The membranes were incubated with
the following primary antibodies: Anti-Tuj1 (1:800), anti-KLF4
(1:1,000), anti-GFAP (1:1,000) and anti-β-actin (1:5,000; cat. no.
A1978; Sigma-Aldrich; Merck KGaA) overnight at 4°C. Following the
primary antibody incubation, the membranes were incubated at room
temperature for 2 h. with a horseradish peroxidase-conjugated
anti-rabbit IgG secondary antibody (1:10,000; cat. no. AP307P;
Sigma-Aldrich; Merck KGaA). Protein bands were visualized using an
enhanced chemiluminescent substrate (Pierce; Thermo Fisher
Scientific, Inc.), exposure to a Fuji X-ray film (Fujian Gutian
Yuanhang Medical Co., Ltd.) and a G:Box gel imaging system (Syngene
Europe, CHEMI–XT16). The expression levels were semi-quantified
using ImageJ 3.5 software (National Institutes of Health).
Statistical analysis
All statistical analyses were performed using SPSS
version 12.0 software (SPSS, Inc.) and the data are presented as
the mean ± SD from ≥3 independent in vitro experiments.
Statistical differences between groups were analyzed using a
one-way ANOVA, followed by Tukey's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
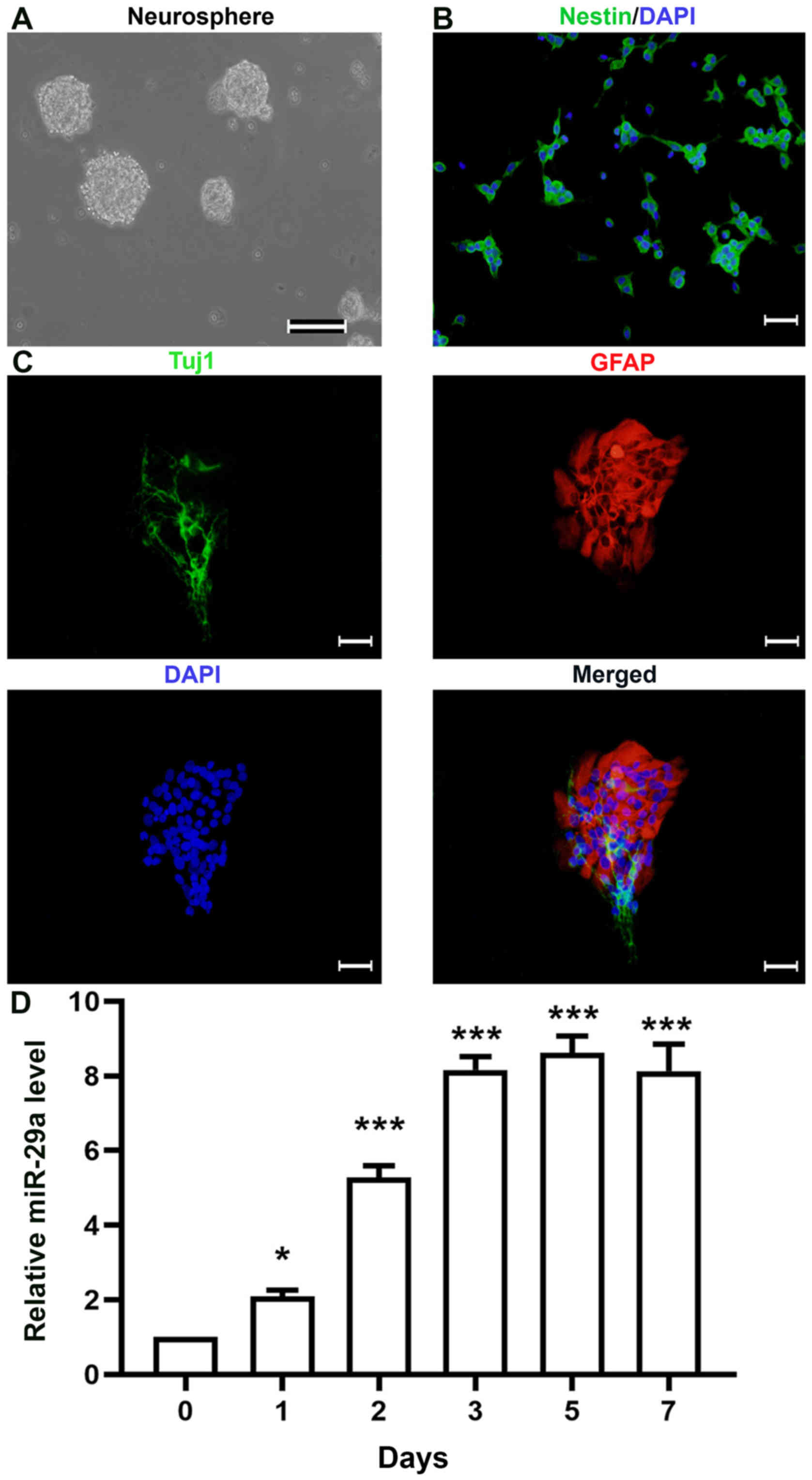
miR-29a is overexpressed during NSPC
differentiation
Following the culture of primary rat NSPCs for 3
days, ~100-µm diameter neurospheres were observed in the medium
(Fig. 1A). Subsequently, the
neurospheres were dissociated into single cells and plated onto
PDL-coated coverslips for immunofluorescence analysis (Fig. 1B); ≥95% of cells were discovered to
express nestin, a specific marker of NSPCs (data not shown)
(18). To observe the
differentiation of NSPCs, single cells were plated onto PDL-coated
coverslips and cultured in normal differentiation medium. Both
Tuj1-(a marker of immature neurons) and GFAP-(a marker of
astrocytes) positive cells were observed following
immunofluorescence staining (Fig.
1C). To determine the expression levels of miR-29a during NSPC
differentiation, the expression levels of miR-29a were analyzed
using RT-qPCR analysis. The results revealed that the expression
levels of miR-29a increased in a time-dependent manner (Fig. 1D), indicating that the expression
levels of miR-29a may increase during NSPC differentiation. There
were little differences observed between the miR-29a expression
levels in NSPCs at days 3, 5 and 7, thus cells cultured in normal
differentiation medium for 3 days was used in subsequent
experiments.
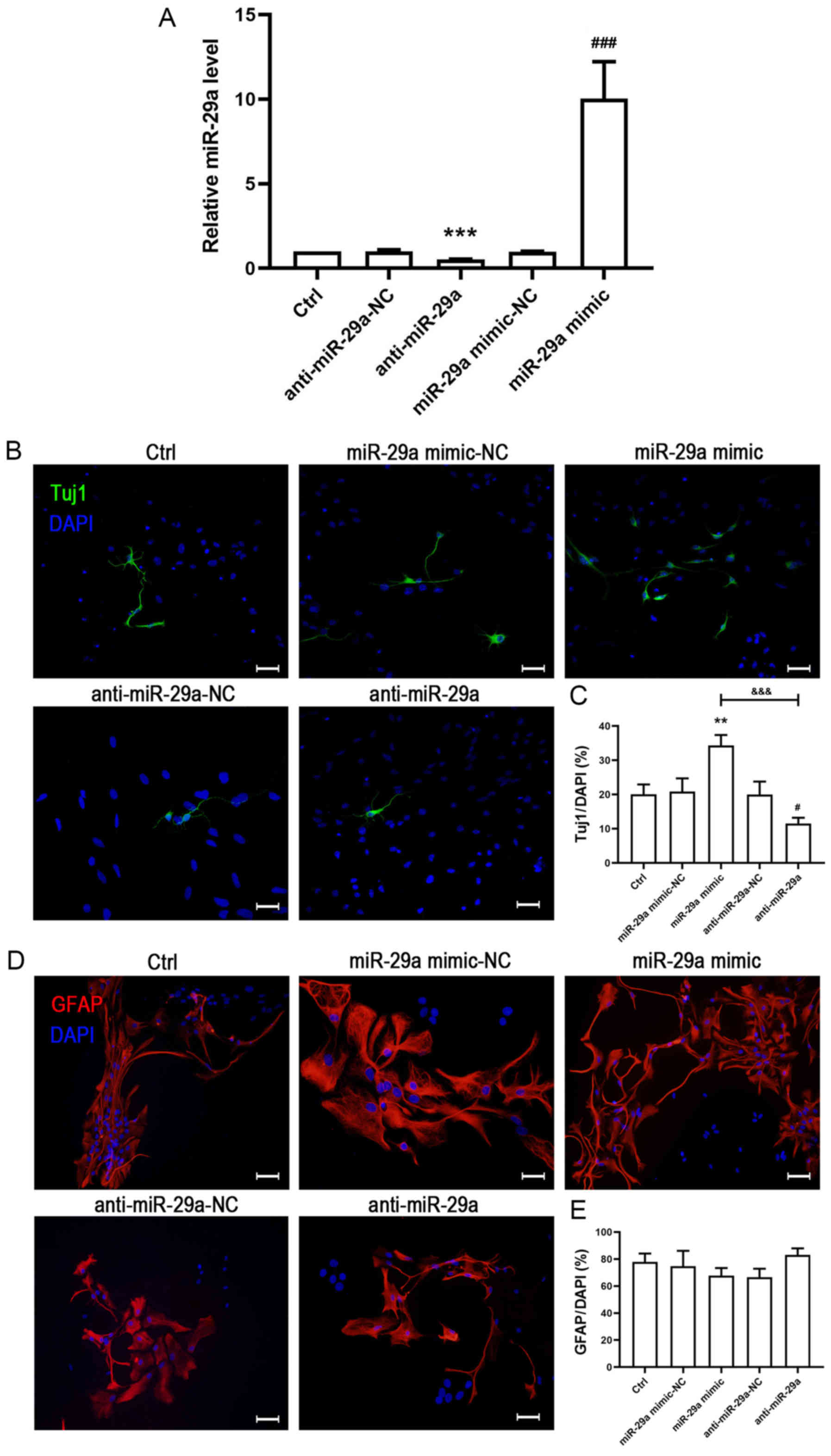
Effects of miR-29a on NSPC
differentiation
To investigate the effects of miR-29a on NSPC
differentiation in cultured rat NSPCs, cells were transfected with
miR-NC, miR-29a mimic, anti-miR-29a or anti-miR-29a-NC. Following
transfection, the cells were cultured in normal differentiation
medium for 3 days and the expression levels of miR-29a were
detected using RT-qPCR analysis. The results indicated that
compared with the control (Ctrl) group, both the NCs exerted no
effect on the expression levels of miR-29a (Fig. 2A). Notably, following the
transfection with the miR-29a mimic, the expression levels of
miR-29a were significantly increased compared with the miR-NC
group, whereas the anti-miR-29a group demonstrated the opposite
trend compared with the anti-miR-29a-NC group (Fig. 2A). These findings indicated that
both the miR-29a mimic and anti-miR-29a had a significant effect on
the miR-29a expression levels in NSPCs. Following the transfection,
Tuj1 and GFAP immunofluorescence staining was performed to
determine the impact of miR-29a on NSPC differentiation. Tuj1 and
GFAP immunofluorescence staining observed that there were no
significant differences in the expression of Tuj1 or GFAP between
the Ctrl and miR-29a mimic-NC or anti-miR-29a-NC groups (Fig. 2B-E), which suggested that the
transfection did not impact the differentiation of NSPCs.
Furthermore, compared with the miR-29a mimic-NC, the overexpression
of miR-29a significantly increased the number of Tuj1-positive
cells (Fig. 2B and C). In
contrast, the number of Tuj1-positive cells was decreased by
inhibiting the expression of miR-29a compared with the anti-miR-29a
NC (Fig. 2B and C). Notably,
despite the increased or decreased expression levels of miR-29a
achieved by the miR-29a mimic or anti-miR-29a, respectively, the
number of GFAP-positive cells was not altered across the groups
(Fig. 2D and E).
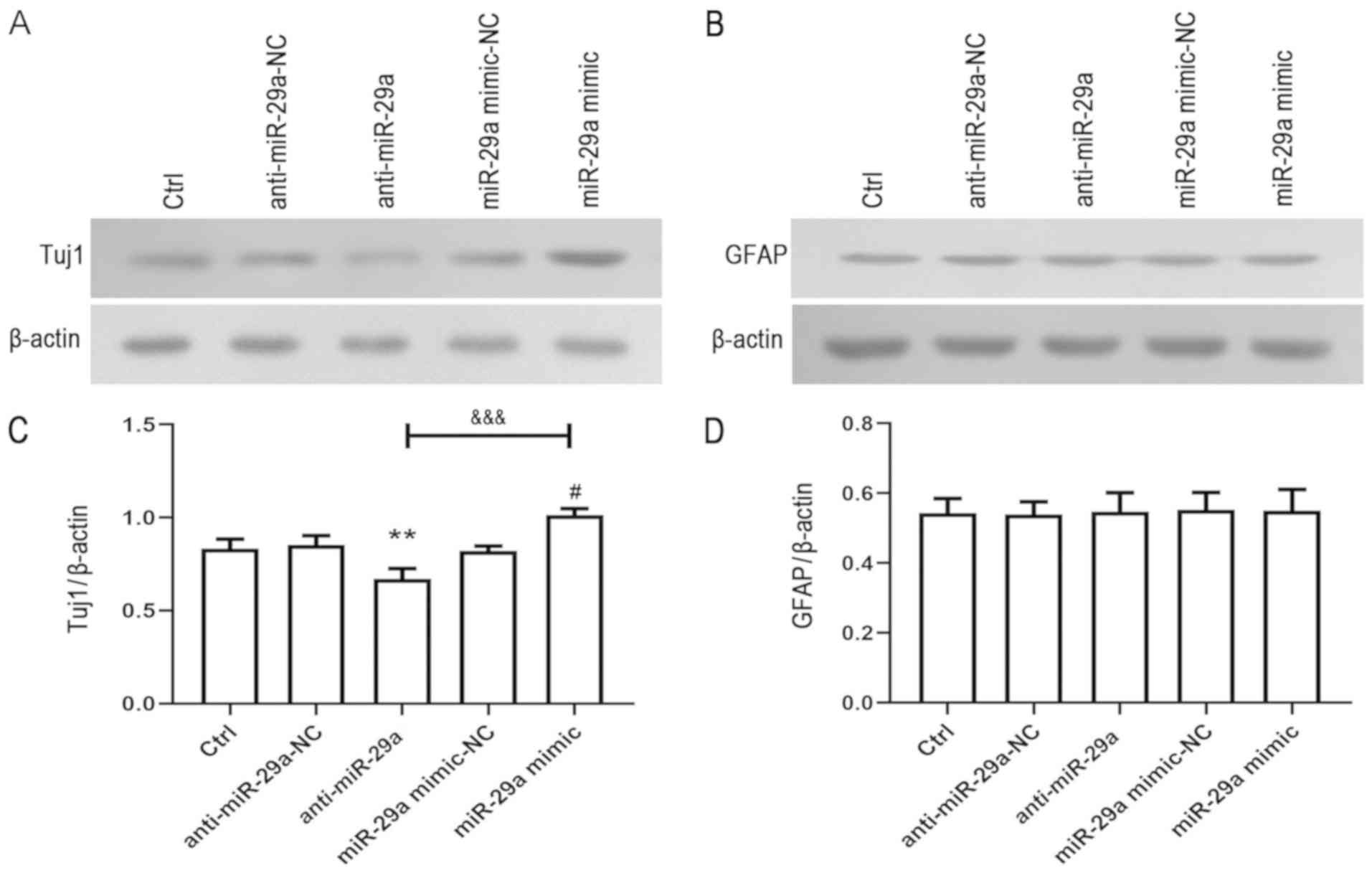
To further investigate the effects of miR-29a on
NSPC differentiation, the expression levels of Tuj1 and GFAP were
detected using western blotting. Similar to the immunofluorescence
staining results, the overexpression of miR-29a increased the
expression levels of Tuj1 compared with the miR-29a mimic-NC,
whereas the downregulation of miR-29a expression levels
demonstrated the opposite trend compared with the anti-miR-29a-NC
(Fig. 3A and C). Notably, no
significant differences were observed in the expression levels of
GFAP across all groups following the overexpression or
downregulation of miR-29a (Fig. 3B and
D). These results indicated that miR-29a may promote neural
differentiation, while having no impact on neuroglial
differentiation.
miR-29a modulates the expression
levels of KLF4 following NSPC differentiation
KLF4, as a member of the KLFs, has been reported to
be involved in a number of cellular processes, including
proliferation, differentiation and survival (19,20).
Thus, the expression levels of KLF4 during NSPC differentiation
were determined using RT-qPCR. The results demonstrated that the
expression levels of KLF4 were downregulated in a time-dependent
manner (Fig. 4A). To determine
whether KLF4 was a direct target gene of miR-29a, the possible
targets of miR-18a were predicted using TargetScan 6.2 (http://www.targetscan.org/vert_61/) and miRDB
databases (http://mirdb.org/). The results showed
that the KLF4 3′UTR containing the predicted miR-29a-binding site
was co-transfected into NSPCs to analyze the relative luciferase
activity in the presence of the miR-29a mimic, anti-miR-29a, and
negative control miRNA. The predicted binding site for miR-29a in
the KLF4 3′UTR is presented in Fig.
4B. As KLF4 was found to be targeted by miR-200c (21), exogenous miR-200c was used as a
control to repress the KLF4 3′UTR and a dual-luciferase reporter
assay and western blotting were performed. Dual-luciferase reporter
assay demonstrated that miR-29a mimic significantly decreased
luciferase activity, whereas anti-miR-29a had the opposite effect
(Fig. 4C). Furthermore,
overexpression of miR-29a significantly downregulated the
expression levels of KLF4 compared with the miR-29a mimic-NC group
(Fig. 4D and E). Conversely,
significantly upregulated expression levels of KLF4 were observed
in the anti-miR-29a group compared with the anti-miR-29a-NC group
(Fig. 4D and E). To determine
whether KLF4 regulated the effects of miR-29a on differentiation,
the expression level of KLF4 was knocked down using target siRNA.
The siRNA were effectively transfected into NSPC cultures and
significantly reduced the expression levels of KLF4 (Fig. 4F-H). Subsequently, KLF4 expression
levels were knocked down in the anti-miR-29a group; the inhibition
of KLF4 expression levels reversed the effects of anti-miR-29a on
the expression levels of Tuj1 (Fig. 4I
and J). These data indicated that miR-29a may modulate the
expression levels of KLF4.
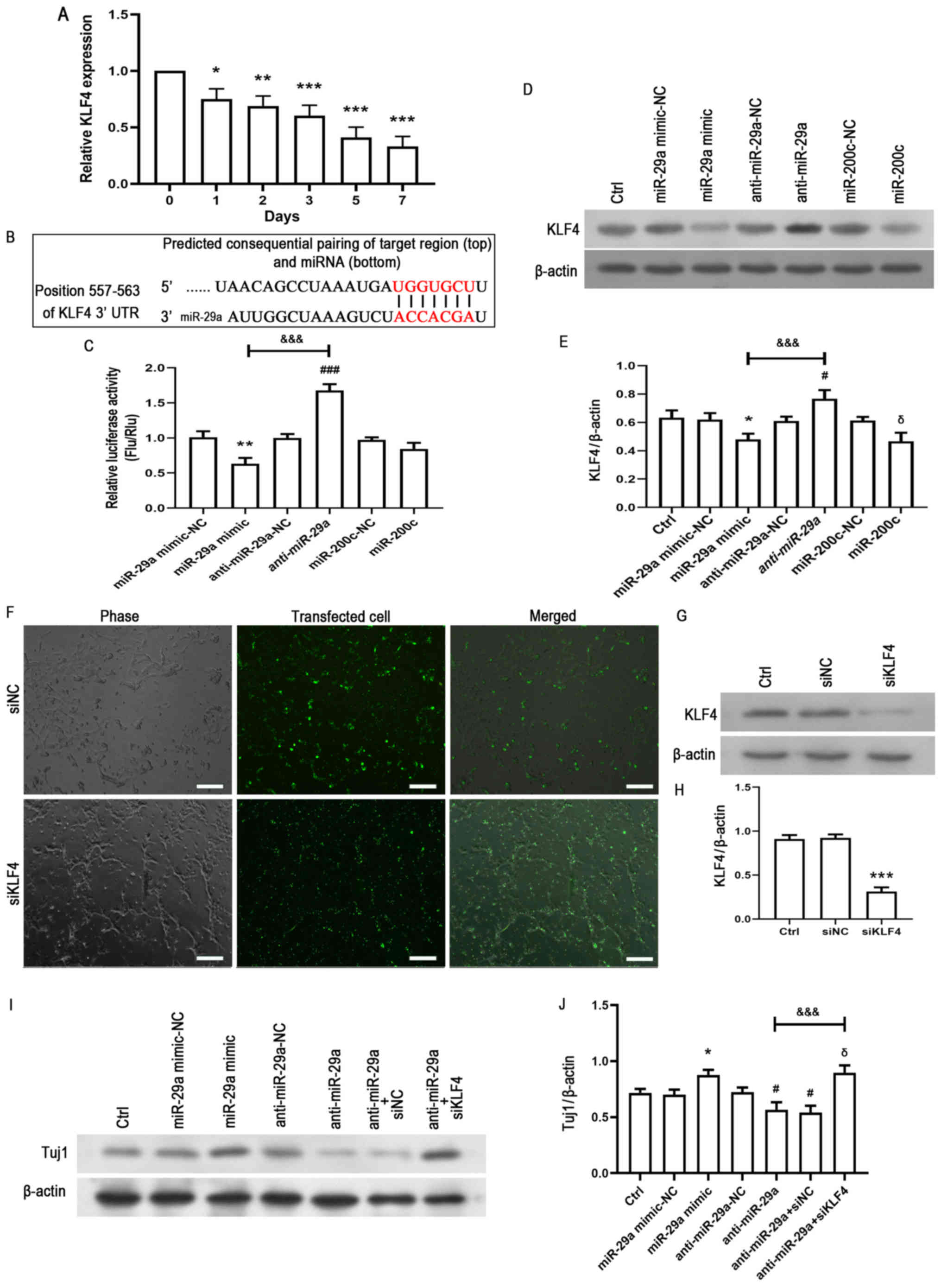 | Figure 4.miR-29a regulates the expression
levels of KLF4. (A) NSPCs were cultured in normal differentiation
medium and the expression levels of KLF4 were analyzed using
reverse transcription-quantitative PCR at different time points.
*P<0.05, **P<0.01, ***P<0.001 vs. control (0 d). (B)
Potential binding region of miR-29a on the KLF4 3′UTR was predicted
using TargetScan. (C) Dual-luciferase activity was performed to
determine the relative luciferase activity of NSPCs co-transfected
with the KLF4 3′UTR and the miR-29a mimic-NC, miR-29a mimic,
anti-miR-29a, anti-miR-29a-NC, miR-200c-NC or miR-200c. **P<0.01
vs. miR-29a mimic-NC. ###P<0.05 vs. anti-miR-29a-NC.
&&&P<0.001 vs. miR-29a mimic. (D) Single
NSPCs were transfected with miR-29a mimic-NC, miR-29a mimic,
anti-miR-29a, anti-miR-29a-NC, miR-200c (positive control) or
miR-200c-NC and cultured in the differentiation medium for 3 days.
Western blotting was used to analyze the expression levels of KLF4.
(E) Semi-quantification of the protein expression levels presented
in part (D). The data are presented as the mean ± SD of three
independent experiments. *P<0.05 vs. miR-29a mimic-NC.
#P<0.05 vs. anti-miR-29a-NC. δP<0.05
vs. miR-200c-NC. &&&P<0.001 vs. miR-29a
mimic. (F) NSPCs were transfected for 6 h with FAM-labeled
non-specific siNC or siKLF4 using Lipofectamine® 2000.
An equal volume of medium was added to the control group. On the
second day, >90% of cells were observed to be transfected
(green). Scale bar, 100 µm (G) Western blotting analysis and (H)
semi-quantification demonstrated that siKLF4 reduced the expression
levels of KLF4. ***P<0.001 vs. the siNC group. (I) Following the
transfection of NSPCs with miR-29a mimic-NC, miR-29a mimic,
anti-miR-29a, anti-miR-29a-NC or anti-miR-29a + siKLF4, NSPCs were
cultured in the differentiation medium for 3 days and Tuj1
expression levels were detected using western blotting. (J)
Semi-quantification of the protein expression levels presented in
part (I). The data are presented as the mean ± SD of three
independent experiments. *P<0.05 vs. miR-29a mimic-NC.
#P<0.05 vs. anti-miR-29a-NC. δP<0.05
vs. anti-miR-29a-NC+siNC. &&&P<0.001 vs.
anti-miR-29a. KLF4, Kruppel-like factor 4; miR/miRNA, microRNA;
UTR, untranslated region; NC, negative control; NSPCs, neural
stem/progenitor cells; Flu/Rlu, Firefly luciferase activity/Renilla
luciferase activity; Ctrl, control; Tuj1, neuron-specific class III
β-tubulin; si, small interfering RNA. |
Discussion
Previous research indicated that miR-29a had been
discovered to serve an important role in numerous biological
processes through regulating its target genes; it has been reported
to impact proliferation, apoptosis, invasion and oncogenesis
(10,22). Increasing evidence has suggested
that miR-29a may also be involved in regulating different types of
stem cells, including embryonic (11), mesenchymal (23) and hematopoietic stem cells
(24). The present study indicated
that the overexpression of miR-29a may promote neural
differentiation, as the expression levels of Tuj1 were found to be
increased. The induction and differentiation of NSPCs into
functional neurons have been identified as important steps in the
treatment of CNS disease (25).
Therefore, the results of the present study suggested that miR-29a
may be a novel therapeutic target to treat disorders of the
CNS.
The current study demonstrated that miR-29a promoted
neural differentiation in cultured rat NSPCs; however, miR-29a was
not found to have a role in neuroglial differentiation. It was
hypothesized that the excessive percentage of GFAP-positive cells
may offer a possible alternative explanation for these findings;
the percentage of GFAP-positive cells remained relatively high
following the transfection of the miR-29a mimic, leading to little
impact on the statistical results.
Accumulating evidence has reported that the miR-29
family target the expression of particular proteins that are
significantly involved in disease pathogenesis; for example,
miR-29a was reported to be a candidate biomarker for Alzheimer's
disease (AD) and Parkinson's disease (PD), due to the abnormal
expression levels of miR-29a identified in both AD and PD (26,27).
Furthermore, the miR-29b cluster was observed to be decreased in
patients with AD, where it participated in regulating the
expression levels of β-secretase 1, which subsequently promoted the
overproduction of amyloid β (28).
miR-29c was also reported to be involved in the regulation of
cerebral ischemia-induced cell death, which depended on its ability
to influence the expression levels of RE1-silencing transcription
factor and DNA (cytosine-5)-methyltransferase 3A (29). The present study also indicated
that miR-29a may promote neuronal differentiation in cultured rat
NSPCs, which provided novel insights into the role of miR-29a in
neurological disorders and its potential as a therapeutic
strategy.
As a zinc finger-containing transcription factor,
KLF4 has been found to be expressed in numerous types of mammalian
cells, where it regulates diverse cellular processes, such as
proliferation, differentiation and maintaining stemness (30–32).
For example, in a previous study, following the transfection of the
four genes, Oct4, Sox2, KLF4 and c-Myc, somatic cells were
reprogrammed and differentiated into induced pluripotent stem cells
(33,34). The current study demonstrated that
miR-29a downregulated the expression levels of KLF4, which were
detected using RT-qPCR and western blotting. These findings
suggested that miR-29a may influence neural differentiation by
regulating KLF4, which provided a novel insight into the potential
function of KLF4 in NSPCs. However, it should be noted that the
overexpression of miR-29a may reside in the potential lack of
faultless control, which manifests as an increased risk of cancer
or differentiation into unpredicted cell phenotype. Therefore,
additional research is required to determine the precise mechanism
by which miR-29a regulates the neural differentiation of NSPCs.
The present study demonstrated that miR-29a promotes
neuronal differentiation in cultured rat NSPCs via regulation of
KLF4 factor. The present study also provides a novel insight into
potential treatment strategies for CNS damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81000030) and the
Fundamental Research Funds for the Central Universities (grant no.
xzy012019104).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG, HQ, TZ and YH designed the experiments; YH
supervised the research; YG, HQ and ZL performed the majority of
the experiments; YG drafted the manuscript; and HQ and YH revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Animal Care and Use Regulation of Xi'an Jiaotong University Health
Science Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
bFGF
|
basic fibroblast growth factor
|
|
CNS
|
central nervous system
|
|
EGF
|
epidermal growth factor
|
|
KLF4
|
Kruppel-like factor 4
|
|
NSPCs
|
neural stem/progenitor cells
|
|
PD
|
Parkinson's disease
|
|
PDL
|
poly-D-lysine
|
References
|
1
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: Significant answers and significant questions.
Neuron. 70:687–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sierra A, Encinas JM and Maletic-Savatic
M: Adult human neurogenesis: From microscopy to magnetic resonance
imaging. Front Neurosci. 5:472011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abati E, Bresolin N, Comi GP and Corti S:
Preconditioning and cellular engineering to increase the survival
of transplanted neural stem cells for motor neuron disease therapy.
Mol Neurobiol. 56:3356–3367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellenchi GC, Volpicelli F, Piscopo V,
Perrone-Capano C and di Porzio U: Adult neural stem cells: An
endogenous tool to repair brain injury? J Neurochem. 124:159–167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Encinas JM and Fitzsimons CP: Gene
regulation in adult neural stem cells. Current challenges and
possible applications. Adv Drug Deliv Rev. 120:118–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imayoshi I and Kageyama R: The role of
Notch signaling in adult neurogenesis. Mol Neurobiol. 44:7–12.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ming GL and Song H: Adult neurogenesis in
the mammalian central nervous system. Annu Rev Neurosci.
28:223–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meza-Sosa KF, Pedraza-Alva G and
Pérez-Martínez L: microRNAs: Key triggers of neuronal cell fate.
Front Cell Neurosci. 8:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alizadeh M, Safarzadeh A, Beyranvand F,
Ahmadpour F, Hajiasgharzadeh K, Baghbanzadeh A and Baradaran B: The
potential role of miR-29 in health and cancer diagnosis, prognosis,
and therapy. J Cell Physiol. 234:19280–19297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajendran G, Dutta D, Hong J, Paul A, Saha
B, Mahato B, Ray S, Home P, Ganguly A, Weiss ML and Paul S:
Inhibition of protein kinase C signaling maintains rat embryonic
stem cell pluripotency. J Biol Chem. 288:24351–24362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Qiao H, Lu Z and Hou Y: miR29
promotes the proliferation of cultured rat neural stem/progenitor
cells via the PTEN/AKT signaling pathway. Mol Med Rep.
20:2111–2118. 2019.PubMed/NCBI
|
|
13
|
Zhang Z, Zheng X and Liu Y, Luan Y, Wang
L, Zhao L, Zhang J, Tian Y, Lu H, Chen X and Liu Y: Activation of
metabotropic glutamate receptor 4 regulates proliferation and
neural differentiation in neural stem/progenitor cells of the rat
subventricular zone and increases phosphatase and tensin homolog
protein expression. J Neurochem. Feb 12–2020.doi:
10.1111/jnc.14984. Online ahead of print. View Article : Google Scholar
|
|
14
|
Chen X, Tian Y, Yao L, Zhang J and Liu Y:
Hypoxia stimulates proliferation of rat neural stem cells with
influence on the expression of cyclin D1 and c-Jun N-terminal
protein kinase signaling pathway in vitro. Neuroscience.
165:705–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jia L, Chopp M, Wang L, Lu X, Zhang Y,
Szalad A and Zhang ZG: MiR-34a regulates axonal growth of dorsal
root ganglia neurons by targeting FOXP2 and VAT1 in postnatal and
adult mouse. Mol Neurobiol. 55:9089–9099. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Z, Hu F, Liu Y, Ma B, Chen X, Zhu K,
Shi Y, Wei T, Xing Y, Gao Y, et al: Activation of type 5
metabotropic glutamate receptor promotes the proliferation of rat
retinal progenitor cell via activation of the PI-3-K and MAPK
signaling pathways. Neuroscience. 322:138–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki S, Namiki J, Shibata S, Mastuzaki Y
and Okano H: The neural stem/progenitor cell marker nestin is
expressed in proliferative endothelial cells, but not in mature
vasculature. J Histochem Cytochem. 58:721–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi K and Yamanaka S: A decade of
transcription factor-mediated reprogramming to pluripotency. Nat
Rev Mol Cell Biol. 17:183–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Black AR, Black JD and Azizkhan-Clifford
J: Sp1 and krüppel-like factor family of transcription factors in
cell growth regulation and cancer. J Cell Physiol. 188:143–160.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Rooij E and Olson EN: MicroRNA
therapeutics for cardiovascular disease: Opportunities and
obstacles. Nat Rev Drug Discov. 11:860–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin M, Wu Y, Wang J, Ye W, Wang L, Yin P,
Liu W, Pan C and Hua X: MicroRNA-29 facilitates transplantation of
bone marrow-derived mesenchymal stem cells to alleviate pelvic
floor dysfunction by repressing elastin. Stem Cell Res Ther.
7:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Connell RM: Endogenous miR-29a regulates
HSC function in mammals. Blood. 125:2180–2181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barkho BZ and Zhao X: Adult neural stem
cells: Response to stroke injury and potential for therapeutic
applications. Curr Stem Cell Res Ther. 6:327–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller M, Jäkel L, Bruinsma IB, Claassen
JA, Kuiperij HB and Verbeek MM: MicroRNA-29a is a candidate
biomarker for Alzheimer's disease in cell-free cerebrospinal fluid.
Mol Neurobiol. 53:2894–2899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai X, Tang Y, Yu M, Wu L, Liu F, Ni J,
Wang Z, Wang J, Fei J, Wang W, et al: Downregulation of blood serum
microRNA 29 family in patients with Parkinson's disease. Sci Rep.
7:54112017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hébert SS, Horré K, Nicolaï L,
Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S,
Delacourte A and De Strooper B: Loss of microRNA cluster
miR-29a/b-1 in sporadic Alzheimer's disease correlates with
increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA.
105:6415–6420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pandi G, Nakka VP, Dharap A, Roopra A and
Vemuganti R: MicroRNA miR-29c down-regulation leading to
de-repression of its target DNA methyltransferase 3a promotes
ischemic brain damage. PLoS One. 8:e580392013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rowland BD and Peeper DS: KLF4, p21 and
context-dependent opposing forces in cancer. Nat Rev Cancer.
6:11–23. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghaleb AM and Yang VW: Krüppel-like factor
4 (KLF4): What we currently know. Gene. 611:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simmen RCM, Pabona JMP, Velarde MC,
Simmons C, Rahal O and Simmen FA: The emerging role of Krüppel-like
factors in endocrine-responsive cancers of female reproductive
tissues. J Endocrinol. 204:223–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tamanini S, Comi GP and Corti S: In vivo
transient and partial cell reprogramming to pluripotency as a
therapeutic tool for neurodegenerative diseases. Mol Neurobiol.
55:6850–6862. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vangapandu H and Ai W: Krüppel like factor
4 (KLF4): A transcription factor with diverse context-dependent
functions. Gene Ther Mol Biol 13a. 194–204. 2009.
|