Introduction
It is well known that sepsis is a complex systemic
disorder. Sepsis is defined as a dysregulated immunological host
response to infection, usually caused by invading microorganisms
and their products (1). As the
most severe form of infection, sepsis can lead to multiple organ
dysfunction syndrome and tissue damage (2,3). It
is estimated that 40–60% of cases of multi-organ dysfunction are
associated with sepsis, including cardiorenal syndrome (CRS)
(4). Sepsis-associated CRS is
classified as type 5 CRS, which is characterized by a strong
systemic inflammatory reaction that can result in simultaneous
heart and kidney failure (5).
Cardiac dysfunction during sepsis mainly presents as reduced
cardiac contractility, impaired ventricular response to fluid
therapy and progressive ventricular dilatation (4,6). The
mortality rate in patients with myocardial dysfunction may reach up
to 70% (7). However, the molecular
mechanisms of myocardial dysfunction in sepsis have yet to be fully
elucidated.
Klotho is a type of single-pass transmembrane
protein that is commonly expressed in renal tubes (8). The Klotho gene family includes three
subtypes: α-, β- and γ-Klotho. Among them, α-Klotho is the main
type, the protective activity of which is crucial for the proper
function of numerous organs (9).
In addition, it has been confirmed that Klotho has antioxidative
activity in acute kidney injury and cardiovascular disease
(9,10). In the present study, it was found
that Klotho expression was decreased in mice with
lipopolysaccharide (LPS)-induced sepsis. Klotho treatment could
reverse the myocardial damage of CRS in sepsis. The data from the
present study further demonstrated that indoxyl sulfate decreased
the Klotho level and activated the reactive oxygen species
(ROS)-mitogen-activated protein kinase signaling pathway in
LPS-induced sepsis mice.
Materials and methods
Animal experiments
A total of 80 male wild-type C57BL/ 6 mice (aged 6–8
weeks, weight 20–24 g) were purchased from the Experimental Animal
Center of Zhejiang Academy of Medical Sciences. All were housed at
a specific pathogen-free laboratory (temperature, 20–24°C;
humidity, 50–70%; free access to food and water; 12-h light/dark
cycles). The animals were randomly divided into control and model
groups [LPS group, LPS + Klotho group, LPS + activated charcoal
(AST-120) group and indoxyl sulfate (IS) group]. There were 5 mice
per group. The control group received no treatment. The LPS group
was established through the intraperitoneal injection of LPS
(Sigma-Aldrich; Merck KGaA) at doses of 5, 10 and 20 mg/kg. The LPS
+ AST-120 group, after LPS injection, received charcoal oral
absorbent, 8% AST-120 in powder diet for 3 weeks. The IS group was
established through intraperitoneal injection for 4 weeks. The
final concentrations of IS were 5, 10 and 20 µM, with the mice
blood volume being 8.3% × weight. Pentobarbital sodium was
administered through intraperitoneal injection at a dose of 50
mg/kg for anesthesia. The health and behavior of the mice were
observed each day. The humane endpoints in the present study were
followed to the greatest extent possible to avoid mice mortality,
severe pain or suffering during the experiments. In addition, the
mice were euthanized when exhibiting the follow symptoms: Weight
loss of 15–20% of the original weight or no continuous weight gain
during the growth period, displaying cachexia and persistent muscle
wasting, loss of appetite [appetite loss for 24 h or poor appetite
(<50% of normal amount) for 3 days] or weakness (inability to
eat and drink, inability to stand for 24 h or extreme reluctance to
stand). There was no accidental mortality in each group during the
experiment. Finally, all the mice were sacrificed with euthanasia
by cervical dislocation. Mice exhibiting no breathing or heartbeat
were deemed to have succumbed in the present study. All animal
experiments conformed to the guidelines established by the Ministry
of Health (China) and were approved by the Animal Care and Welfare
Committee of Zhejiang Hospital.
Hematoxylin and eosin (HE)
staining
Heart and kidney tissues were fixed with 4% formalin
at room temperature for 24 h, embedded in paraffin and sectioned
into 4-mm thick sections. Subsequently, the tissue sections were
deparaffinized in xylene and rehydrated in a series of graded
alcohol solutions. Subsequently, the tissues sections were stained
with hematoxylin-eosin (cat. no. C0105; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Stained
sections were observed under a light microscope (magnifcation,
×200).
Reverse transcription-quantitative
(RT-q) PCR
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from kidney and
cardiomyocyte tissue samples (50–100 mg) according to the
manufacturer's instructions. Total RNA (1 µg) was reversed into
cDNA with a PrimeScript RT reagent kit (Takara Biotechnology Co.,
Ltd.). Prior to being reversed into cDNA, RNA samples were treated
with DNase I recombinant and RNasin inhibitor. The primers used
were: Atrial natriuretic peptide (ANP; sense:
5′-CGTATACAGTGCGGTGTCCA-3′, antisense: 5′-GGTTGACTTCCCCAGTCCAG-3′),
brain natriuretic peptide (BNP; sense:
5′-GCTGCTGGAGCTGATAAGAGAA-3′, antisense:
5′-CGATCCGGTCTATCTTGTGCC-3′) and GAPDH (sense:
5′-AGGAGCGAGACCCCACTAACA-3′, antisense:
5′-AGGGGGGCTAAGCAGTTGGT-3′). Each experiment was repeated three
times. qPCR was performed using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.). The PCR program used for the thermocycler
(Bio-Rad Laboratories, Inc.) was 95°C for 5 min, followed by 39
cycles at 95°C for 15 sec, 58°C for 30 sec and 72°C for 30 sec. The
relative changes in gene expression were normalized to GAPDH and
analyzed using the 2−ΔΔCq analysis method (11).
ELISA
Serum Klotho level was measured using a commercial
ELISA kit (cat. no. AB15504; Sigma-Aldrich; Merck KGaA) based on
the biotin double antibody sandwich technology. All serum samples
were run in duplicate according to the manufacturer's protocol. The
Klotho level was calculated as the average of duplicate
samples.
Western blotting
For western blotting, protein samples were extracted
from lysed tissues using RIPA (Beyotime Institute of Biotechnology)
and the levels were detected with a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Thereafter, protein
samples with the same quality (30 µg) were subjected to 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to a nitrocellulose membrane (Merck KGaA) using a wet method. The
membranes were blocked with 5% skim milk powder for 2 h at room
temperature. Subsequently, the membranes were incubated at 4°C for
overnight with primary antibodies targeted against: Klotho
(1:1,000; cat. no. ab15504; Abcam), p38MAPK (1:1,000; cat. no.
8690; Cell Signaling Technology, Inc.), phosphorylated-p38MAPK
(1:1000; cat. no. 5140; Cell Signaling Technology, Inc.) and GAPDH
(1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.).
Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody
(1:100,000; cat. no. 70-GAR007; MultiSciences Biotech Co., Ltd.)
for 1 h at room temperature. Protein bands were visualized using an
enhanced chemiluminescence method (EMD Millipore) with the Odyssey
imaging system (LI-COR Biosciences). Protein expression was
quantified using Image Pro Plus software (version 6.0; Media
Cybernetics, Inc.).
Flow cytometry for apoptosis and ROS
detection
For cell apoptosis analysis, H9c2 cells (ATCC) were
seeded (2×105 cells/well) and treated with IS (5, 10 and
20 µM) for 24 and 48 h. Floating and attached cells were all
harvested. Cell apoptosis was assessed using flow cytometry by
labeling with the Annexin V-fluorescein isothiocyanate and
propidium iodide apoptosis detection kit (MultiSciences Biotech
Co., Ltd.). For ROS analysis, heart tissue and H9c2 cells were
first incubated with dichlorodihydrofluorescein diacetate (H2DCFDA)
at 37°C for 20 min. The levels of intracellular
H2O2 were measured using H2DCFDA fluorescent
dye (Abcam). Cell apoptosis and ROS levels were determined using a
BD Accuri C6 flow cytometer (BD Biosciences) and analyzed using
FlowJo software (version 10; FlowJo LLC).
Cell viability assay
Cell viability was examined using the Cell Counting
Kit-8 (ccK8) assay (Beyotime institute of Biotechnology). Briefly,
cells were trypsinized and seeded (2×103 cells/well)
into 96-well plates. After culturing with IS for 48 h at 37°C, 10
µl CCK-8 reagent was added to each well and incubated at 37°C for 3
h. Subsequently, the absorbance of each well was measured at a
wavelength of 450 nm using a Multiskan MK3 spectrophotometer
(Thermo Fisher Scientific, Inc.).
Equipment
The FACScan cytometer was purchased from BD
Biosciences, the centrifuge was purchased from Eppendorf, the
microscope was obtained from Olympus Corporation, the NanoDrop1000
spectrophotometer was purchased from Thermo Fisher Scientific, as
was the cell incubator, and the protein electrophoresis transfer
device was purchased from Bio-Rad Laboratories, Inc.
Statistical analysis
All data are presented as mean ± standard error of
mean. For comparisons between two groups of normally distributed
data, the Student's t-test was used. For multiple comparisons of
normally distributed data, one-way analysis of variance was used
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
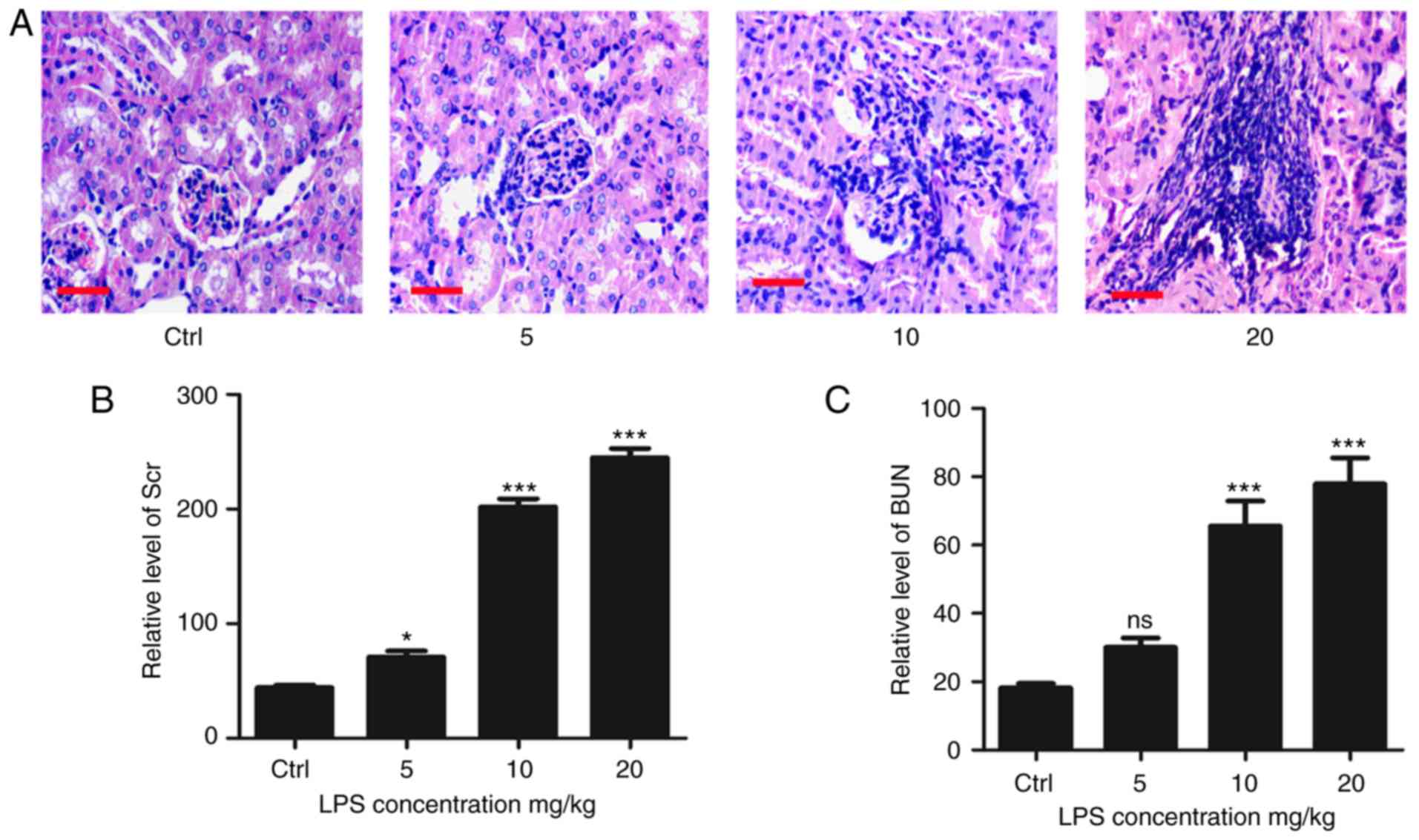
LPS-induced sepsis can lead to CRS in
mice
CRS is a common complication of sepsis. To study the
pathogenesis and pathological features of sepsis-related CRS, an
LPS-induced sepsis mice model (LPS concentrations: 5, 10 and 20
mg/kg) was established. The data from the present study
demonstrated that LPS injection can successfully induce sepsis in
mice. In addition, it was found that 10 mg/kg was the optimal LPS
concentration. Thereafter, the heart and kidney functions of mice
were evaluated. HE staining of renal tissue revealed obviously
edematous glomerular and tubular epithelium cells, as well as a
large number of inflammatory cell infiltrates in LPS-induced sepsis
mice. By contrast, the structure of the glomerulus and tubules was
clear and normal in control mice (Fig.
1A). The levels of serum creatinine and urea nitrogen were
significantly higher in LPS-induced sepsis mice (Fig. 1B and C).
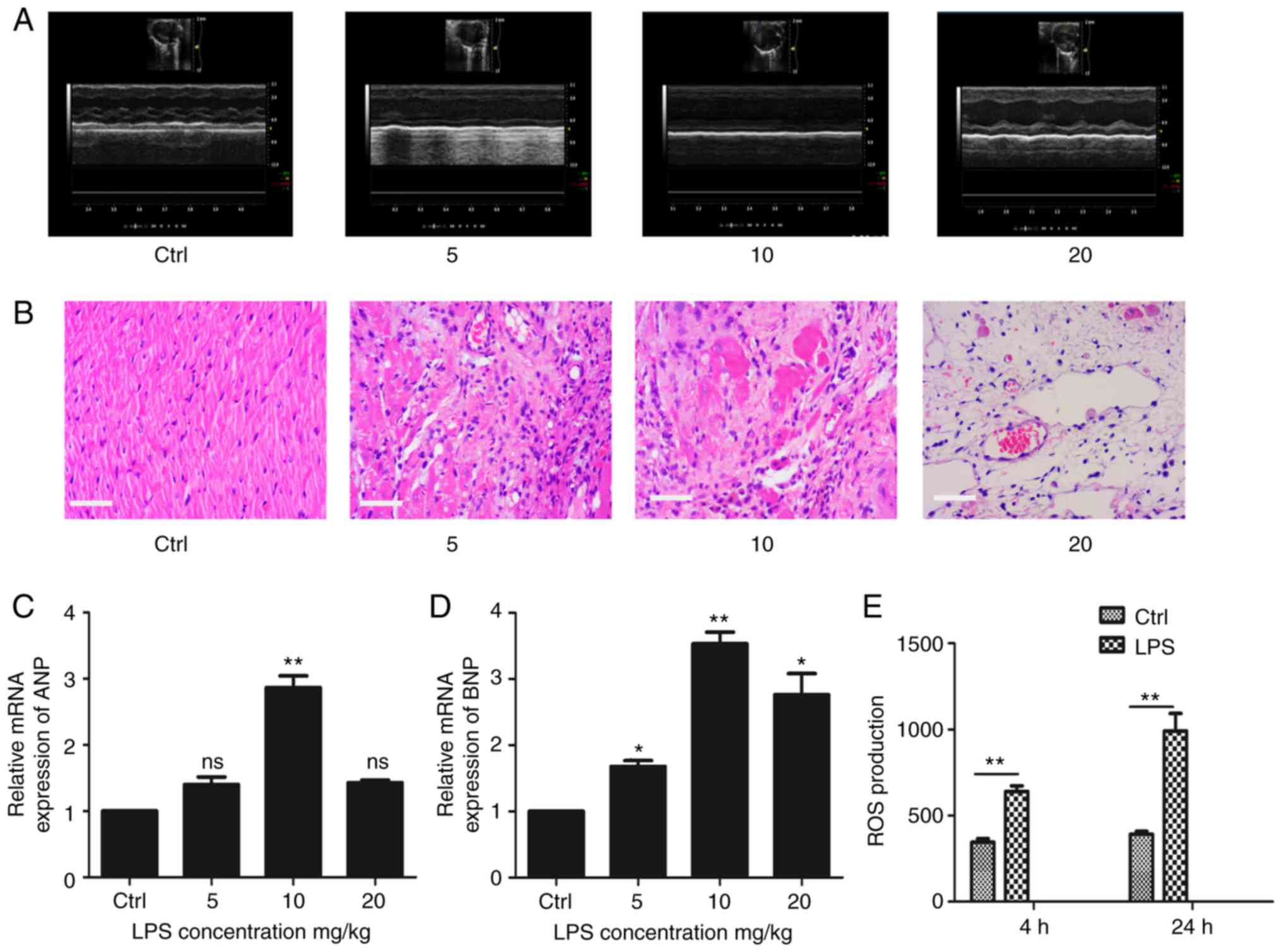
Cardiac dysfunction is another clinical feature of
sepsis. Cardiac echocardiography revealed that LPS-induced sepsis
mice had left ventricular systolic dysfunction and decreased
cardiac contractility (Fig. 2A).
Evaluation of the myocardial tissue morphology demonstrated that
the myocardial gap of the LPS-induced sepsis group was broader than
that of the control group, cardiac cells had severe edema,
myocardial hyperemia was evident, adherent inflammatory cells were
increased and some myocardial tissues demonstrated myocardial
degeneration and dissolution (Fig.
2B). The expression of myocardial injury markers such as ANP
and BNP were detected and, as shown in Fig. 2C and D, the expression of ANP and
BNP was upregulated in the LPS-induced sepsis group. In addition,
the ROS production of cardiomyocytes was also increased in
LPS-induced sepsis mice (Fig.
2E).
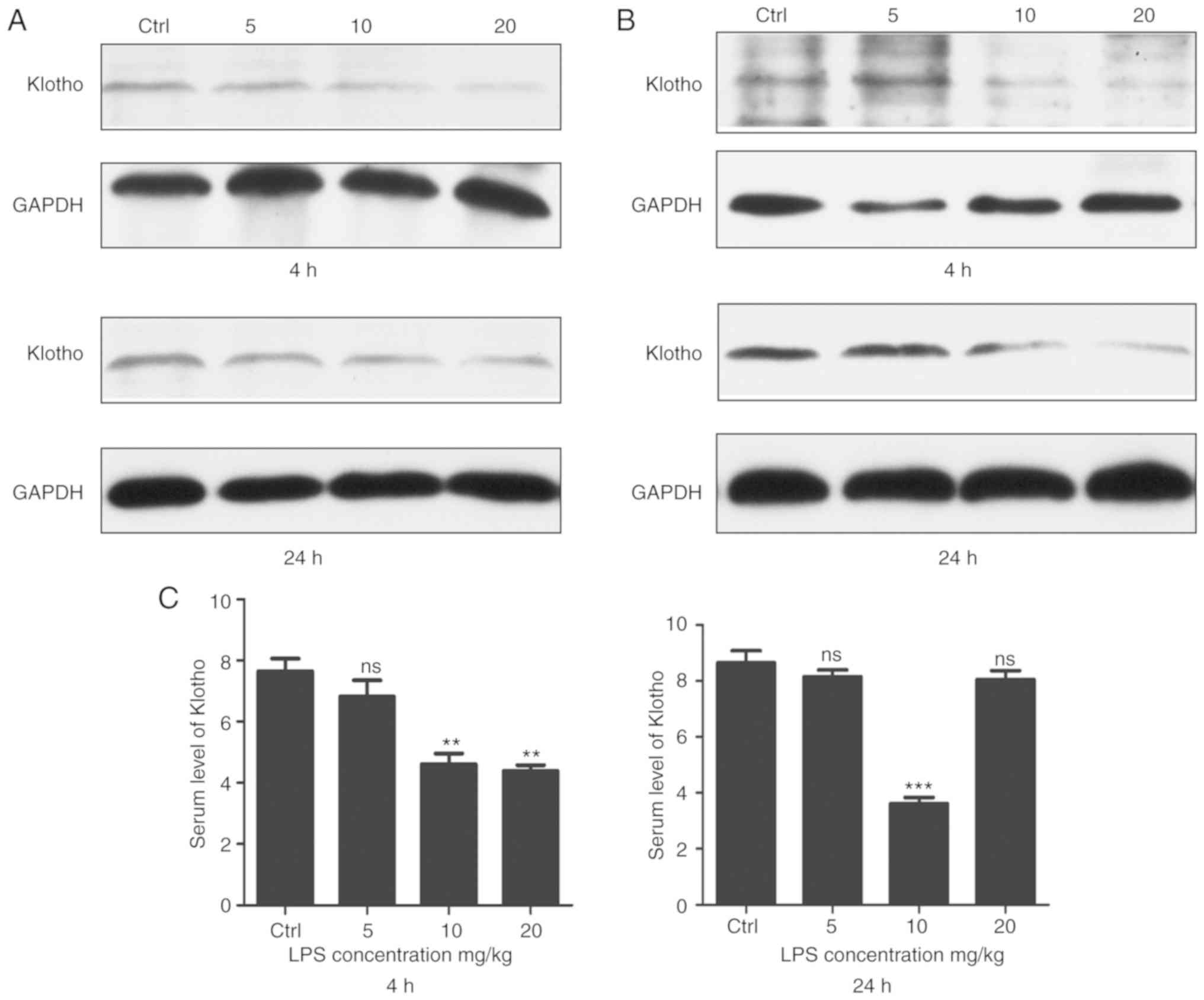
Klotho protein is significantly
decreased in LPS-induced sepsis mice
It has been reported that Klotho protein plays an
important role in numerous organs, such as the kidney and heart
(12,9). To ascertain whether Klotho protein
can affect the development of LPS-induced sepsis, the level of
Klotho was detected in LPS-induced sepsis mice. As shown in
Fig. 3A, the Klotho protein level
of kidney tissue was significantly decreased in the LPS-induced
sepsis group. The data further revealed that the expression of
Klotho protein was also downregulated in myocardial tissues and
that the downregulation of Klotho protein was dependent on the LPS
concentration (Fig. 3B). Serum
samples were collected for ELISA, which indicated that the level of
Klotho protein was decreased compared with that in the control
group (Fig. 3C).
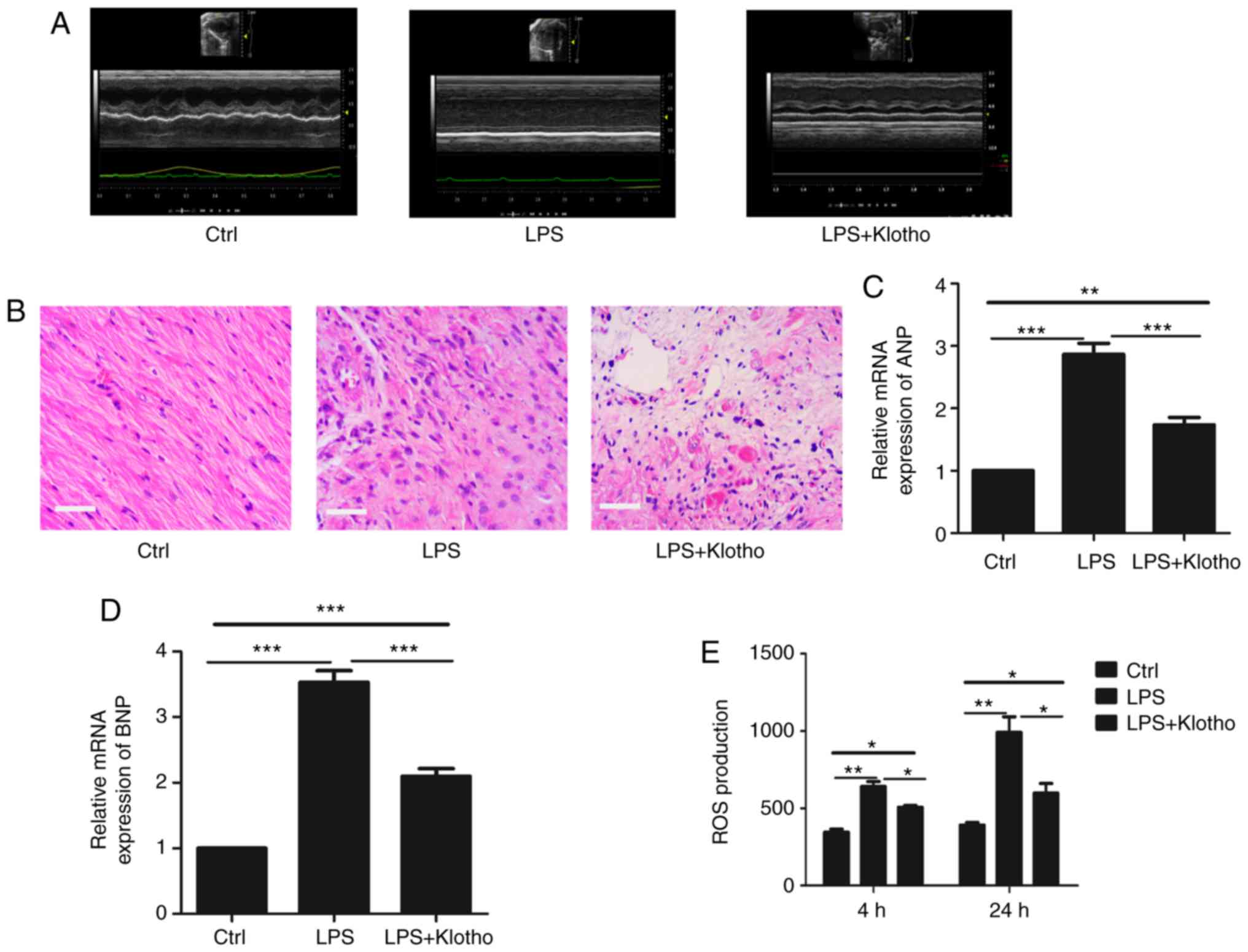
Klotho protein treatment reverses
myocardial injury in LPS-induced sepsis mice
To further investigate the function of Klotho in the
myocardial damage of CRS in sepsis, a Klotho protein intervention
experiment was next performed. After LPS injection at a dose of 10
mg/kg for 24 h, the mice were treated with Klotho protein (0.02
mg/kg) through intraperitoneal injection for 4 days. The results
indicated that the myocardial function of LPS-induced sepsis mice
was improved after Klotho protein injection (Fig. 4A and B). Similarly, the expression
levels of ANP and BNP were downregulated compared with the
LPS-induced sepsis group (Fig. 4C and
D). In addition, the ROS production of cardiomyocytes was also
decreased compared with that in the LPS-induced sepsis group
(Fig. 4E). These results confirmed
that Klotho had an important role in the myocardial damage of CRS
in sepsis.
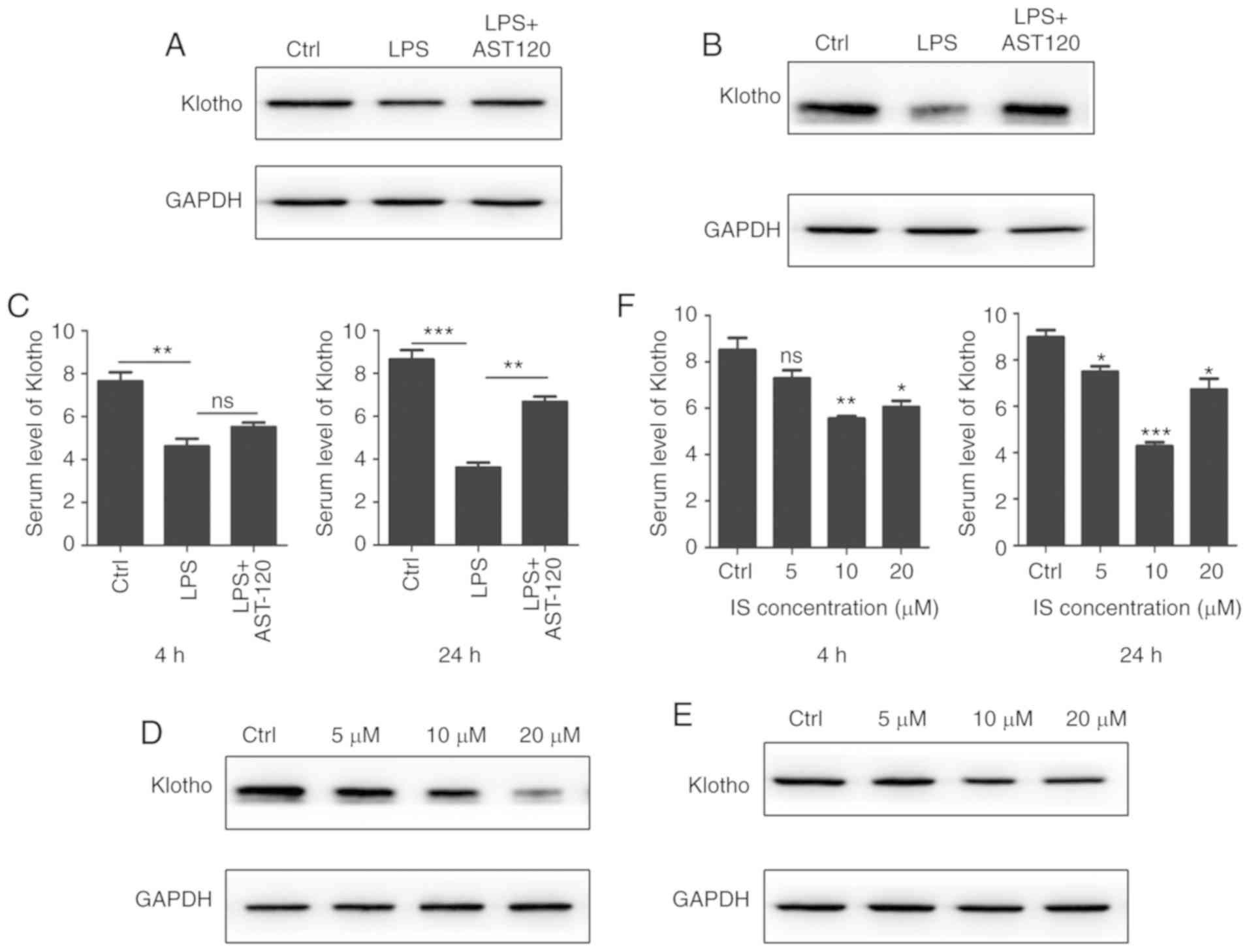
Klotho downregulation is associated
with redundant IS
As Klotho is essential in CRS, the cause of Klotho
downregulation required investigation. It is known that uremic
toxins often accumulate in sepsis. IS is a well-known protein-bound
uremic toxin that is correlated with sepsis (13). AST-120 (8%) was administered to
LPS-induced sepsis mice in order to remove the excess IS in serum.
Notably, western blot analysis demonstrated that the expression of
Klotho was improved in kidney and heart tissues (Fig. 5A and B). As shown by ELISA, the
serum level of Klotho protein was also increased (Fig. 5C). IS injection was then
administered to mice in order to simulate a similar septic model
and it was found that Klotho protein was decreased in kidney and
heart tissues (Fig. 5D and E). The
ELISA data demonstrated that the serum level of Klotho protein was
also decreased after IS injection (Fig. 5F). These results indicated that IS
decreased the expression of Klotho protein in LPS-induced
sepsis.
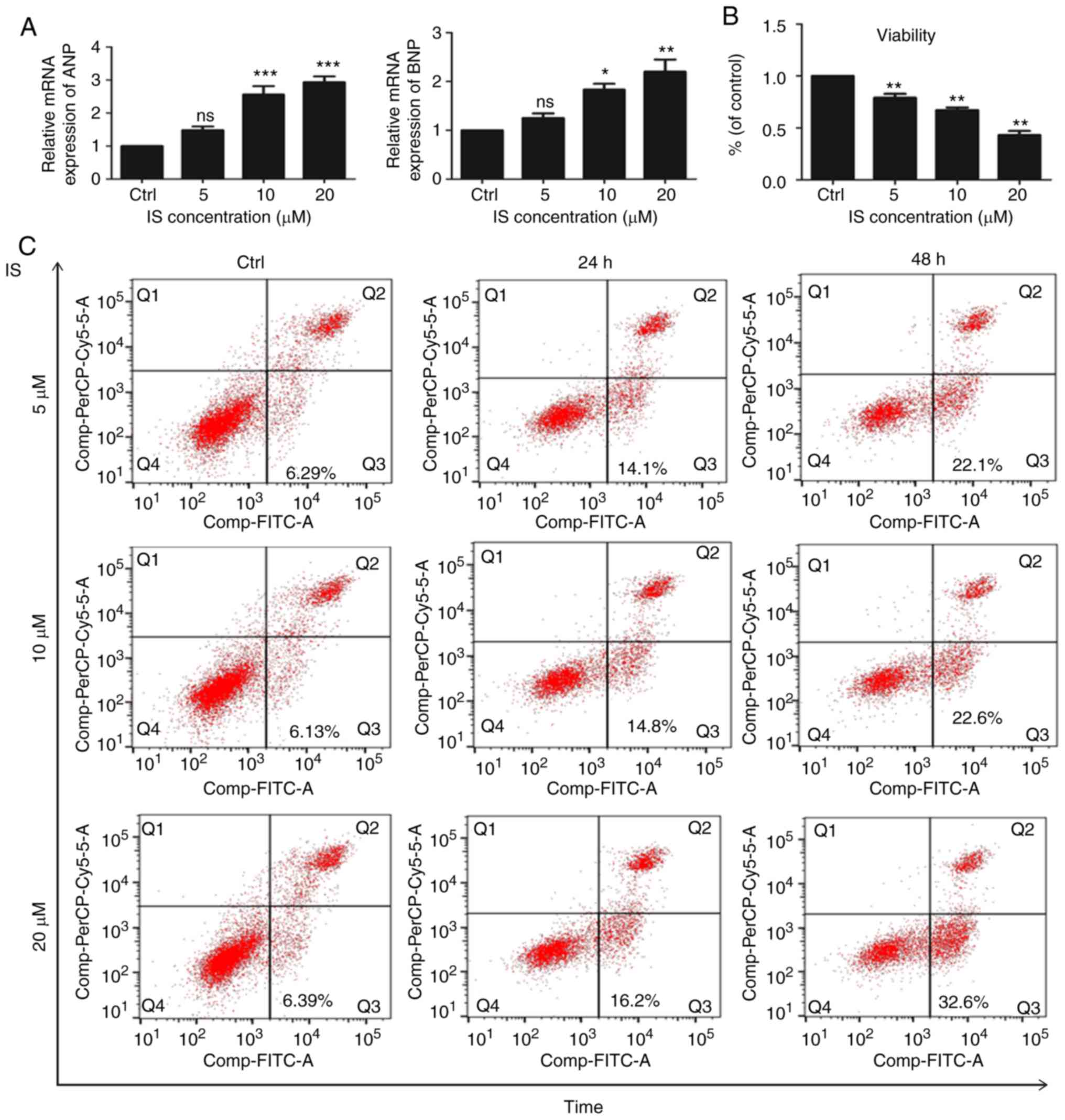
Klotho downregulation by IS activates
the ROS/p38 mitogen-activated protein kinase (MAPK) signaling
pathway in LPS-induced sepsis
To investigate the downstream effectors of Klotho in
the myocardial damage of CRS in sepsis, H9c2 cardiomyocyte cells
were treated with different IS concentrations to imitate Klotho
downregulation. As shown in Fig.
6A, the expression levels of ANP and BNP were upregulated in
the IS group. Fig. 6B shows that
cell proliferation was decreased after IS treatment. In addition,
the proportion of apoptotic cells was increased in the IS group
(Fig. 6C). The production of ROS
was also increased (Fig. 6D). As
the ROS/MAPK signaling pathway plays an important role in CRS
(14,15), the level of MAPK was then detected.
As shown in Fig. 6E, the
phosphorylated p38 MAPK level was increased in IS group. Exogenous
Klotho was added to the IS-treated H9c2 cells and it was found that
the expression of ANP and BNP had a certain degree of reversal
compared with the IS group (Fig.
6F). The expression of p38 MAPK was also decreased after Klotho
treatment (Fig. 6G). These results
indicated that decreased Klotho expression may cause the activation
of the ROS/p38 MAPK signaling pathway, leading to myocardial
damage.
Discussion
Myocardial injury caused by CRS is a common
complication in sepsis patients and has a high mortality rate.
Recent studies have indicated that myocardial depression in sepsis
is caused by a number of factors (16). For example, indocyanine green-001
can attenuate endotoxemia-induced cardiac depression through the
downregulation of the Wnt/β-catenin signaling pathway (17). In addition, it has been reported
that microRNA(miR)-135a is upregulated in sepsis-induced myocardial
depression (18) and a further
study confirmed that miR-135a aggravated sepsis-induced myocardial
dysfunction by regulating the p38 MAPK/nuclear factor-κB signaling
pathway (18). In addition, high
serum levels of soluble triggering receptor expressed on myeloid
cells-1 have been confirmed to be negatively correlated with left
ventricular ejection fraction in septic patients (19). However, the molecular mechanism of
this myocardial injury has yet to be fully elucidated. The results
of the present study confirmed that Klotho protein is a key
molecule in myocardial disorders in sepsis patients. Klotho is a
protein that is correlated with the suppression of several aging
phenotypes (20–22). It has been reported that Klotho can
affect phosphate and glucose metabolism, and has antioxidant,
adipogenic and other actions (23). In addition, previous studies have
demonstrated that Klotho protein has protective activity against
cardiovascular disease and heart injury (9,24). A
study confirmed that adults with higher plasma Klotho have a lower
risk of developing coronary artery disease (25). In addition, Klotho protein can
inhibit canonical transient receptor potential 6 channels to reject
pathological heart alterations (26). However, whether Klotho has a
protective function against the myocardial damage of CRS in sepsis
has yet to be elucidated. The present study found that Klotho
protein was downregulated in the heart and kidney tissues of septic
mice and that the serum level of Klotho was also significantly
decreased. In addition, the myocardial function of LPS-induced
septic mice was significantly improved after treatment with Klotho
protein. These results suggested that Klotho played an important
role in sepsis-associated myocardial injury. Therefore, this raises
the question of what role the downregulation of Klotho protein
expression plays in sepsis-associated myocardial injury. The data
from the present study demonstrated that IS negatively regulated
the expression of Klotho. It has been reported that IS can induce
cell apoptosis through oxidative stress and the MAPK signaling
pathway (27). In addition, a
previous study confirmed that the protective function of Klotho
protein in cardiovascular disease mainly depends on its
antioxidative and antiapoptotic activity (9). The data from the present study
confirmed that decreased Klotho induced the activation of the
ROS/p38 MAPK signaling pathway, leading to cardiomyocyte apoptosis
and finally resulting in myocardial damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Nature
Science Fund (grant no. LY16H150005), the Medical Scientific
Research Foundation of Zhejiang Province (grant no. WKJ-ZJ-1401)
and Zhejiang Provincial Program for the Cultivation of High-level
Innovative Health talents.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FY initiated and designed the present study. FY and
YF performed the experiments and interpreted the results. FY, YF
and JY conducted all of the experiments. JC designed the
experiments and revised the manuscript. All authors read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Animal Care and Welfare Committee of Zhejiang Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krivan S, Kapelouzou A, Vagios S,
Tsilimigras D, Katsimpoulas M, Moris D, Aravanis CV, Demesticha TD,
Schizas D, Mavroidis M, et al: Increased expression of Toll-like
receptors 2, 3, 4 and 7 mRNA in the kidney and intestine of a
septic mouse model. Sci Rep. 9:40102019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aird WC: The role of the endothelium in
severe sepsis and multiple organ dysfunction syndrome. Blood.
101:3765–3777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Virzi GM, Clementi A, Brocca A and Ronco
C: Endotoxin effects on cardiac and renal functions and cardiorenal
syndromes. Blood Purif. 44:314–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kotecha A, Vallabhajosyula S, Coville HH
and Kashani K: Cardiorenal syndrome in sepsis: A narrative review.
J Crit Care. 43:122–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Virzi GM, Clementi A, Brocca A, de Cal M,
Marcante S and Ronco C: Cardiorenal syndrome type 5 in sepsis: Role
of endotoxin in cell death pathways and inflammation. Kidney Blood
Press Res. 41:1008–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clementi A, Virzi GM, Brocca A and Ronco
C: The role of endotoxin in the setting of cardiorenal syndrome
type 5. Cardiorenal Med. 7:276–283. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blanco J, Muriel-Bombín A, Sagredo V,
Taboada F, Gandía F, Tamayo L, Collado J, García-Labattut A,
Carriedo D, Valledor M, et al: Incidence, organ dysfunction and
mortality in severe sepsis: A Spanish multicentre study. Critic
care. 12:R1582008. View
Article : Google Scholar
|
|
8
|
Abolghasemi M, Yousefi T, Maniati M and
Qujeq D: The interplay of Klotho with signaling pathway and
microRNAs in cancers. J Cell Biochem. 120:14306–14317. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olejnik A, Franczak A, Krzywonos-Zawadzka
A, Kaluzna-Oleksy M and Bil-Lula I: The biological role of klotho
protein in the development of cardiovascular diseases. Biomed Res
Int. 2018:51719452018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oh HJ, Oh H, Nam BY, You JS, Ryu DR, Kang
SW and Chung YE: The protective effect of klotho against
contrast-associated acute kidney injury via the anti-oxidative
effect. Am J Physiol Renal Physiol. 317:F881–F889. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu MC, Shi M, Gillings N, Flores B,
Takahashi M, Kuro-O M and Moe OW: Recombinant α-Klotho may be
prophylactic and therapeutic for acute to chronic kidney disease
progression and uremic cardiomyopathy. Kidney Int. 91:1104–1114.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Veldeman L, Vanmassenhove J, Van Biesen W,
Massy ZA, Liabeuf S, Glorieux G and Vanholder R: Evolution of
protein-bound uremic toxins indoxyl sulphate and p-cresyl sulphate
in acute kidney injury. Int Urol Nephrol. 51:293–302. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu D, Li M, Tian Y, Liu J and Shang J:
Luteolin inhibits ROS-activated MAPK pathway in myocardial
ischemia/reperfusion injury. Life Sci. 122:15–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Zhang Y, Zhong S, Gao F, Chen Y, Li
W, Zheng F and Shi G: N-n-butyl haloperidol iodide protects against
hypoxia/reoxygenation injury in cardiac microvascular endothelial
cells by regulating the ROS/MAPK/Egr-1 pathway. Front Pharmacol.
7:5202016.PubMed/NCBI
|
|
16
|
Zanotti-Cavazzoni SL and Hollenberg SM:
Cardiac dysfunction in severe sepsis and septic shock. Curr Opin
Crit Care. 15:392–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yousif NG, Hadi NR and Hassan AM:
Indocyanine green-001 (ICG-001) attenuates Wnt/β-catenin-induces
myocardial injury following sepsis. J Pharmacol Pharmacother.
8:14–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng G, Pan M, Jin W, Jin G and Huang Y:
MicroRNA-135a is up-regulated and aggravates myocardial depression
in sepsis via regulating p38 MAPK/NF-κB pathway. Int
Immunopharmacol. 45:6–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Zhang E, Hu Y, Liu Y and Chen B:
High Serum sTREM-1 correlates with myocardial dysfunction and
predicts prognosis in septic patients. Am J Med Sci. 351:555–562.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reish NJ, Maltare A, McKeown AS, Laszczyk
AM, Kraft TW, Gross AK and King GD: The age-regulating protein
klotho is vital to sustain retinal function. Invest Ophthalmol Vis
Sci. 54:6675–6685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Y and Sun Z: Molecular basis of klotho:
From gene to function in aging. Endocr Rev. 36:174–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flotyńska J, Uruska A, Araszkiewicz A and
Zozulińska-Ziółkiewicz D: Klotho protein function among patients
with type 1 diabetes. Endokrynol Pol. 69:696–704. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Y, Zhuang X, Huang Z, Zou J, Yang D,
Hu X, Du Z, Wang L and Liao X: Klotho protects the heart from
hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated
inflammation both in vitro and in vivo. Biochim Biophys Acta Mol
Basis Dis. 1864:238–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Semba RD, Cappola AR, Sun K, Bandinelli S,
Dalal M, Crasto C, Guralnik JM and Ferrucci L: Plasma klotho and
cardiovascular disease in adults. J Am Geriatrics Soc.
59:1596–1601. 2011. View Article : Google Scholar
|
|
26
|
Xie J, Cha SK, An SW, Kuro OM, Birnbaumer
L and Huang CL: Cardioprotection by Klotho through downregulation
of TRPC6 channels in the mouse heart. Nat Commun. 3:12382012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin YT, Wu PH, Tsai YC, Hsu YL, Wang HY,
Kuo MC, Kuo PL and Hwang SJ: Indoxyl sulfate induces apoptosis
through oxidative stress and mitogen-activated protein kinase
signaling pathway inhibition in human astrocytes. J Clin Med.
8(pii): E1912019. View Article : Google Scholar : PubMed/NCBI
|