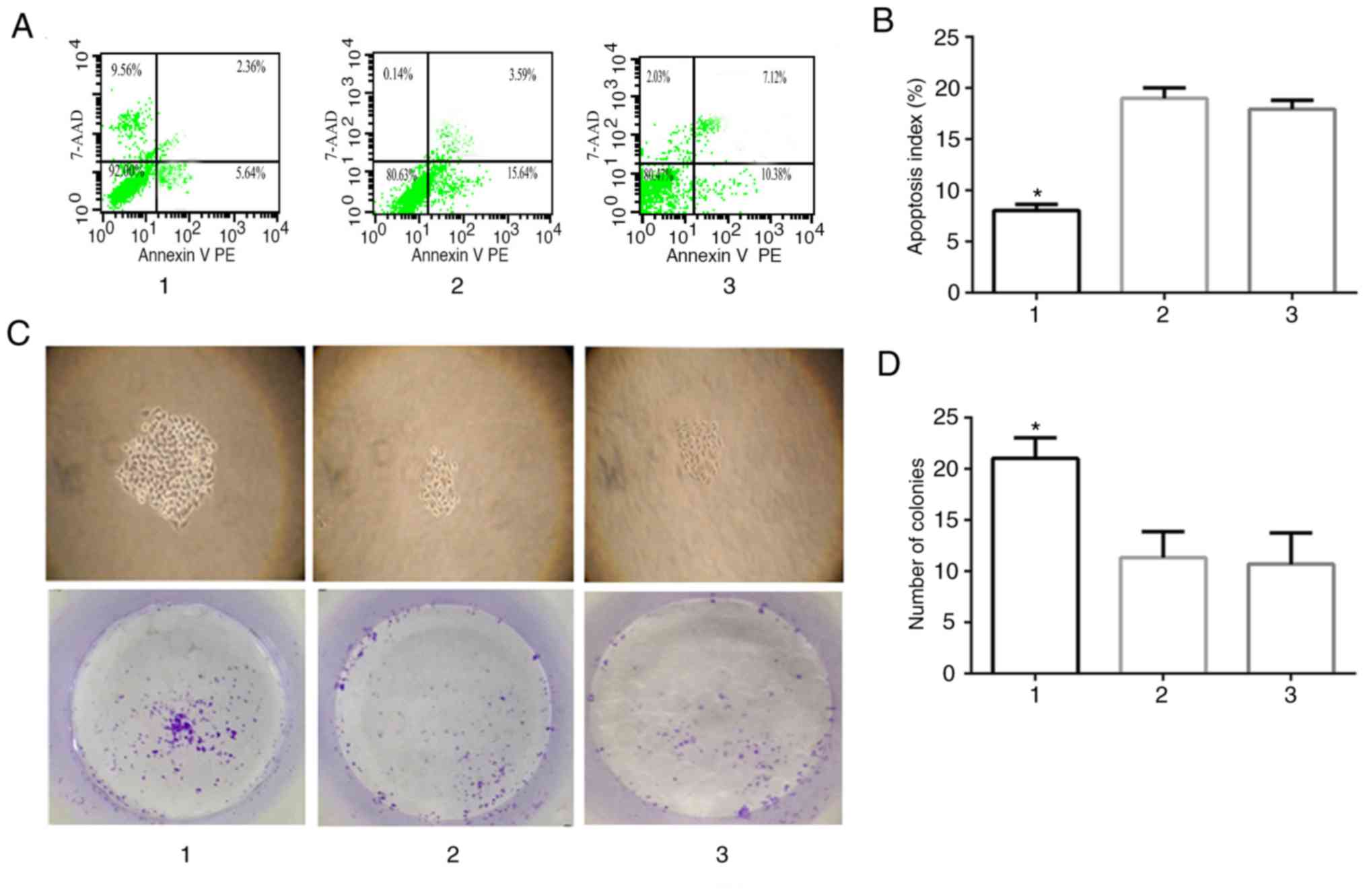
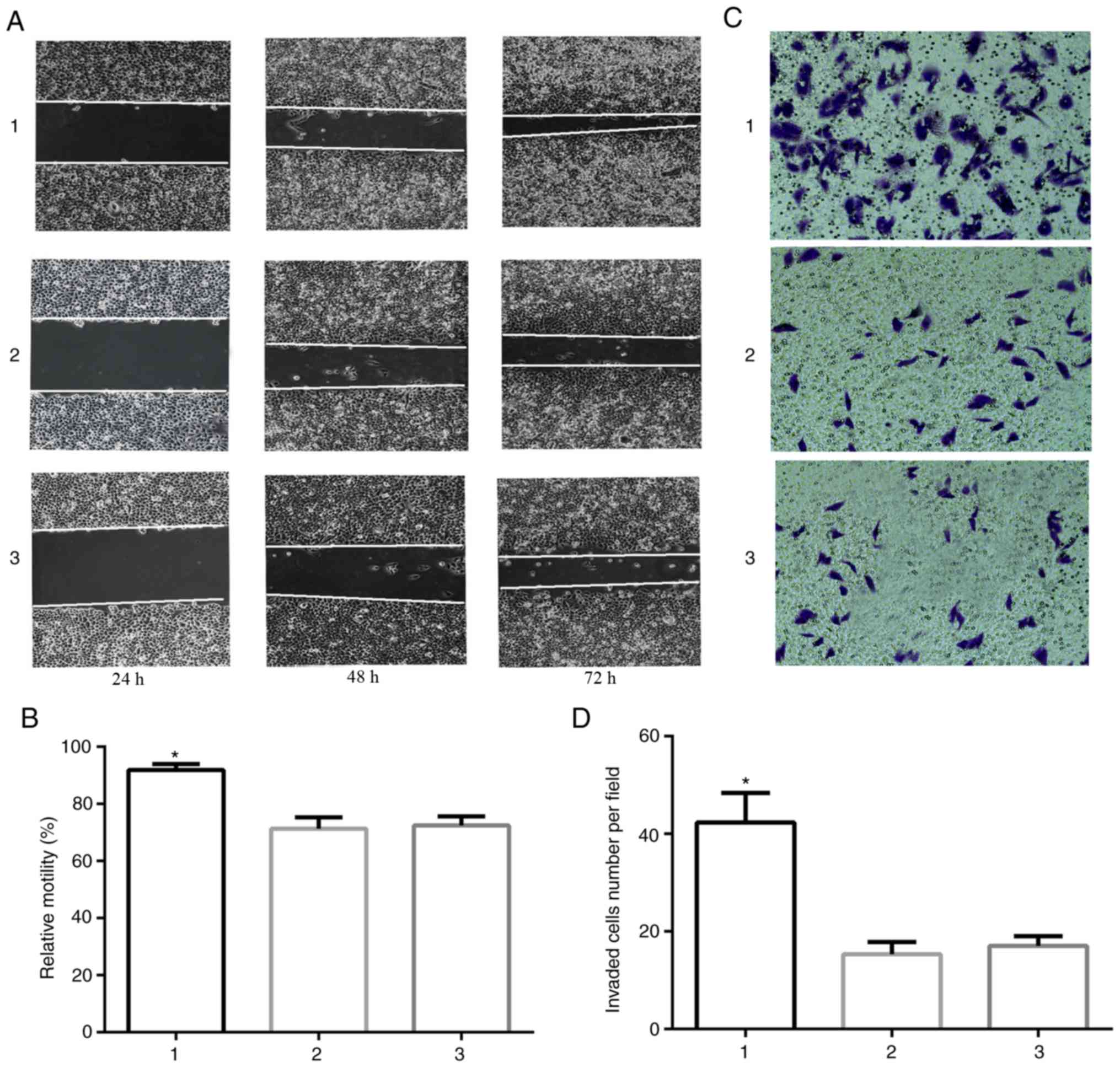
|
1
|
Emmings E, Mullany S, Chang Z, Landen CN
Jr, Linder S and Bazzaro M: Targeting mitochondria for treatment of
chemoresistant ovarian cancer. Int J Mol Sci. 20:2292019.
View Article : Google Scholar
|
|
2
|
Kongsema M, Wongkhieo S, Khongkow M, Lam
EW, Boonnoy P, Vongsangnak W and Wong Ekkabut J: Molecular
mechanism of Forkhead box M1 inhibition by thiostrepton in breast
cancer cells. Oncol Rep. 42:953–962. 2019.PubMed/NCBI
|
|
3
|
Zeng L, Liao Q, Zou Z, Wen Y, Wang J, Liu
C, He Q, Weng N, Zeng J, Tang H, et al: Long non-coding RNA
XLOC_006753 promotes the development of multidrug resistance in
gastric cancer cells through the PI3K/AKT/mTOR signaling pathway.
Cell Physiol Biochem. 51:1221–1236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen S, Wu J, Jiao K, Wu Q, Ma J, Chen D,
Kang J, Zhao G, Shi Y, Fan D, et al: MicroRNA-495-3p inhibits
multidrug resistance by modulating autophagy through GRP78/mTOR
axis in gastric cancer. Cell Death Dis. 9:10702018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozovska Z, Gabrisova V and Kucerova L:
Colon cancer: cancer stem cells markers, drug resistance and
treatment. Biomed Pharmacother. 68:911–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alamolhodaei NS, Tsatsakis AM, Ramezani M,
Hayes AW and Karimi G: Resveratrol as MDR reversion molecule in
breast cancer: An overview. Food Chem Toxicol. 103:223–232. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu T, Ma Y, Wang Z, Zhang W and Yang X:
Siva 1 inhibits cervical cancer progression and its clinical
prognosis significance. Cancer Manag Res. 12:303–311. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M,
Jacquot S and Schlossman SF: CD27, a member of the tumor necrosis
factor receptor family, induces apoptosis and binds to Siva, a
proapoptotic protein. Proc Natl Acad Sci USA. 94:6346–6351. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen JY, Yang LX and Huang ZF: The N
terminal 33 amino acid domain of Siva 1 is sufficient for nuclear
localization. Braz J Med Biol Res. 46:1021–1027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cottle DL, McGrath MJ, Wilding BR, Cowling
BS, Kane JM, D'Arcy CE, Holdsworth M, Hatzinisiriou I, Prescott M,
Brown S, et al: SLIMMER (FHL1B/KyoT3) interacts with the
proapoptotic protein Siva-1 (CD27BP) and delays skeletal myoblast
apoptosis. J Biol Chem. 284:26964–26977. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chu F, Borthakur A, Sun X, Barkinge J,
Gudi R, Hawkins S and Prasad KV: The Siva-1 putative amphipathic
helical region (SAH) is sufficient to bind to BCL-XL and sensitize
cells to UV radiation induced apoptosis. Apoptosis. 9:83–95. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balci-Peynircioglu B, Waite AL, Hu C,
Richards N, Staubach-Grosse A, Yilmaz E and Gumucio DL: Pyrin,
product of the MEFV locus, interacts with the proapoptotic protein,
Siva. J Cell Physiol. 216:595–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin FT, Lai YJ, Makarova N, Tigyi G and
Lin WC: The lysophosphatidic acid 2 receptor mediates
down-regulation of Siva-1 to promote cell survival. J Biol Chem.
282:37759–37769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rapisarda V, Ledda C, Matera S, Fago L,
Arrabito G, Falzone L, Marconi A, Libra M and Loreto C: Absence of
t(14;18) chromosome translocation in agricultural workers after
short term exposure to pesticides. Mol Med Rep. 15:3379–3382. 2107.
View Article : Google Scholar
|
|
15
|
Li T, Lv M, Chen X, Yu Y, Zang G and Tang
Z: Plumbagin inhibits proliferation and induces apoptosis of
hepatocellular carcinoma by downregulating the expression of SIVA.
Drug Des Devel Ther. 13:1289–1300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vachtenheim J Jr, Lischke R and
Vachtenheim J: Siva 1 emerges as a tissue specific oncogene beyond
its classic role of a proapoptotic gene. Onco Targets Ther.
11:6361–6367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Nostrand JL, Brisac A, Mello SS,
Jacobs SB, Luong R and Attardi LD: The p53 target gene SIVA enables
non-small cell lung cancer development. Cancer Discov. 5:622–635.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du W, Jiang P, Li N, Mei Y, Wang X, Wen L,
Yang X and Wu M: Suppression of p53 activity by Siva1. Cell Death
Differ. 6:1493–1504. 2009. View Article : Google Scholar
|
|
19
|
Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv
L, Liu J, Xu Y, Shen Y and Yang M: Noncoding RNAs in gastric
cancer: Implications for drug resistance. Mol Cancer. 19:622020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XT, Xie YB and Xiao Q: Lentivirus
mediated RNA interference targeting E2F 1 inhibits human gastric
cancer MGC 803 cell growth in vivo. Exp Mol Med. 43:638–645. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan LH, Wang XT, Yang J, Lian C, Kong FB,
Wei WY, Luo W, Xiao Q and Xie YB: Reversal of multidrug resistance
in gastric cancer cells by CDX2 downregulation. World J
Gastroenterol. 19:4155–4165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan LH, Wei WY, Cao WL, Zhang XS, Xie YB
and Xiao Q: Overexpression of CDX2 in gastric cancer cells promotes
the development of multidrug resistance. Am J Cancer Res.
5:321–332. 2014.PubMed/NCBI
|
|
23
|
Lorendeau D, Dury L, Genoux Bastide E,
Lecerf Schmidt F, Simões Pires C, Carrupt PA, Terreux R, Magnard S,
Di Pietro A, Boumendjel A, et al: Collateral sensitivity of
resistant MRP1 overexpressing cells to flavonoids and derivatives
through GSH efflux. Biochem Pharmacol. 90:235–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang ZZ, Yu WX, Zheng M, Liao XH, Wang
JC, Yang DY, Lu WX, Wang L, Zhang S, Liu HK, et al: PIN1 Inhibition
Sensitizes Chemotherapy in Gastric Cancer Cells by Targeting Stem
Cell-like Traits and Multiple Biomarkers. Mol Cancer Ther.
19:906–919. 2020.PubMed/NCBI
|
|
26
|
Peng L, Li Y, Wei S, Li X, Dang Y, Zhang W
and Zhang G: LAMA4 activated by Androgen receptor induces the
cisplatin resistance in gastric cancer. Biomed Pharmacother.
124:1096672020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barkinge JL, Gudi R, Sarah H, Chu F,
Borthakur A, Prabhakar BS and Prasad KV: The p53-induced Siva-1
plays a significant role in cisplatin-mediated apoptosis. J
Carcinog. 8:22009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okuno K, Yasutomi M, Nishimura N, Arakawa
T, Shiomi M, Hida J, Ueda K and Minami K: Gene expression analysis
in colorectal cancer using practical DNA array filter. Dis Colon
Rectum. 44:295–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Jiang P, Du W, Wu Z, Li C, Qiao M,
Yang X and Wu M: Siva1 suppresses epithelial-mesenchymal transition
and metastasis of tumor cells by inhibiting stathmin and
stabilizing microtubules. Proc Natl Acad Sci USA. 108:12851–12856.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei SH, Lin F, Wang X, Gao P and Zhang HZ:
Prognostic significance of stathmin expression in correlation with
metastasis and clinicopathological characteristics in human ovarian
carcinoma. Acta Histochem. 110:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Huang S, Jiang Y, Liu Y, Song T,
Li D and Yang L: Reactive astrocytes increase the expression of P
gp and Mrp1 via TNF α and NF κB signaling. Mol Med Rep.
17:1198–1204. 2017.PubMed/NCBI
|
|
32
|
Cui X, Shen D, Kong C, Zhang Z, Zeng Y,
Lin X and Liu X: NF-κB suppresses apoptosis and promotes bladder
cancer cell proliferation by upregulating survivin expression in
vitro and in vivo. Sci Rep. 7:407232017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen MY, Wang Y, Cui SY, Wu XL, Guo Y and
Xu RR: MicroRNA-125a regulates proliferation and apoptosis of acute
myeloid leukemia through targeting NF-κB pathway. Eur Rev Med
Pharmacol Sci. 23:3594–3601. 2019.PubMed/NCBI
|
|
34
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF κB is
essential for epithelial mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Investig. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu T, Wei R, Zhang Y, Chen W and Liu H:
Association between NF-κB expression and drug resistance of liver
cancer. Oncol Lett. 17:1030–1034. 2018.PubMed/NCBI
|
|
37
|
Tanaka T, Hozumi Y, Iino M and Goto K:
NAP1L1 regulates NF-κB signaling pathway acting on anti-apoptotic
Mcl-1 gene expression. Biochim Biophys Acta Mol Cell Res.
1864:1759–1768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei W, Cao W, Zhan Z, Yan L, Xie Y and
Xiao Q: miR-1284 suppresses gastric cancer progression by targeting
EIF4A1. OncoTargets Ther. 12:3965–3976. 2019. View Article : Google Scholar
|
|
39
|
Cao W, Wei W, Zhan Z, Xie D, Xie Y and
Xiao Q: Regulation of drug resistance and metastasis of gastric
cancer cells via the microRNA647 ANK2 axis. Int J Mol Med.
41:1958–1966. 2018.PubMed/NCBI
|
|
40
|
Strouse JJ, Ivnitski Steele I, Njus HM,
Foutz TD, Yao T, Weiner WS, Schroeder CE, Simpson DS, Maki BE, Li
K, et al: Selective Efflux Inhibition of ATP binding Cassette Sub
family G Member 2. Probe Reports from the NIH Molecular Libraries
Program (Internet) (Bethesda (MD)). National Center for
Biotechnology Information (US). Nov 30–2010.9:(updated Feb 25,
2013).
|
|
41
|
Bentires-Alj M, Barbu V, Fillet M, Chariot
A, Relic B, Jacobs N, Gielen J, Merville MP and Bours V: NF-kappaB
transcription factor induces drug resistance through MDR1
expression in cancer cells. Oncogene. 22:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan Z, Liang X, Zhan Y, Wang Z, Xu J, Qiu
Y, Wang J, Cao Y, Le VM, Ly HT, et al: Targeting CD133 reverses
drug resistance via the AKT/NF κB/MDR1 pathway in colorectal
cancer. Br J Cancer. 122:1342–1353. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang AK, Zhou ZW, Wei MQ, Liu JP and Zhou
SF: Modulators of multidrug resistance associated proteins in the
management of anticancer and antimicrobial drug resistance and the
treatment of inflammatory diseases. Curr Top Med Chem.
10:1732–1756. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sisodiya SM, Lin WR, Harding BN, Squier MV
and Thom M: Drug resistance in epilepsy: Expression of drug
resistance proteins in common causes of refractory epilepsy. Brain.
125:22–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han F, Liu G, Sun C and Wei J: Ailanthone
reverses multidrug resistance by inhibiting the P glycoprotein
mediated efflux in resistant K562/A02 cells. Cell Mol Biol (Noisy
le grand). 64:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|