Introduction
Atherosclerosis (AS) is a chronic inflammatory
disease that often occurs in the cardiovascular system. Important
causes of AS include vascular endothelial cell injury, smooth
muscle cell proliferation, monocyte or macrophage absorption of
lipids and inflammatory mediator release (1–4). It
has also been confirmed that the formation of AS is closely related
to excess cholesterol and cholesterol esters in foam cells. The
major sources of foam cells include macrophages, endothelial cells
and vascular smooth muscle cells (VSMCs) (5,6).
Among these sources, SMCs can migrate into the vascular intima to
form foam cells, thereby phagocytizing lipids. A large number of
accumulated foam cells can form lipid streaks and cholesterol
crystals, which eventually developed into atherosclerotic plaques
under the influence of various factors (7). MicroRNAs (miRNAs) are a class of
endogenous, non-coding RNAs of approximately 22 nucleotides in
length that participate in numerous biological processes in
vivo by regulating the expression of eukaryotic genes and the
activity of important signaling pathways (8–10).
Accumulating data have indicated that miRNAs are widely involved in
the regulation of AS. Brennan et al (11), reported that the let-7 miRNA family
exerted a protective effect on diabetes-associated AS via the
inhibition of VSMC inflammatory responses, which may provide a
possible therapeutic target for diabetes-associated AS. Research by
Qun et al (12), showed
that reduced miRNA-27b expression promoted plaque stability in AS
by regulating CCL20/CCR6 axis activity by targeting
N(α)-acetyltransferase 15, NatA auxiliary subunit. de Ronde et
al (13), also demonstrated
that individuals who smoked had higher miR-124-3p expression than
non-smokers, and this elevated miR-124-3p expression was associated
with a higher risk of advanced AS and subclinical AS. In their
paper, miR-124-3p was also shown to alter the phenotype of
monocytes, thereby increasing the risk of AS in smokers.
miR-133b is a specific miRNA that plays an important
role in neuron and embryonic heart development, adipocyte
differentiation and tumorigenesis (14–17).
Previous studies have confirmed that miR-133b is involved in the
pathogenesis of a number of diseases, including various cancers
(18), Parkinson's disease
(19), chronic Chagas disease
(20), atrial dilatation (21), cerebral ischemia (22) and AS (23). It has also been demonstrated that
miR-133b acts as a tumor suppressor by targeting matrix
metallopeptidase 9 (24), fascin
actin-bundling protein 1 (25),
peroxisome proliferator activated receptor γ (26), homeobox A9 (27) and epidermal growth factor receptor
(28). An miRNA profiling showed
that miR-133b-3p is upregulated in diabetic AS in rats (29). In addition, Zheng et al
(23) demonstrated that the
downregulation of miR-133b inhibits the immune responses of
macrophages, and attenuates the vulnerable plaque formation and
vascular remodeling in AS mice via the mastermind-like
transcriptional coactivator 1-mediated Notch-signaling pathway.
However, the specific regulatory role of miR-133b in AS and its
underlying mechanisms are not known.
The Notch pathway is involved in many physiological
processes, such as embryo development, tumorigenesis, immunological
function, and cardiac and vascular development (30,31).
Importantly, Notch signaling is involved in the occurrence,
progression and prognosis of AS (32). Liu et al (33), reported that the Notch pathway is
activated in AS plaques, resulting in endothelial inflammation and
senescence. Davis-Knowlton et al (34), showed that the Notch signaling
pathway is activated in the progression of AS, and plays a
receptor-specific regulatory role in smooth muscle cells. However,
it remains unclear whether the regulatory effect of miR-133b in AS
is related to the Notch signaling pathway.
In the present study, the specific regulatory role
of miR-133b in AS and its related-mechanism involving the Notch
signaling pathway were analyzed. These results may provide a new
experimental basis for the treatment of AS.
Materials and methods
Establishment of mouse AS model
A total of 60 healthy male apolipoprotein E-/- mice
(6 weeks old, weighing 18–22 g) were purchased from the College of
Pharmaceutical Sciences, Peking University. Among them, 20 mice
(the Sham group) were randomly selected and fed with a normal diet,
while the remaining 40 mice (the AS group) were fed with a high-fat
diet (21% fat and 0.5% cholesterol). Mice were maintained in a
controlled environment, at 23–25°C and 50–60% humidity, with a 12-h
light/dark cycle and free access to food and water. All the mouse
feeds were provided by Keao Xieli Experimental Animal Feed Co.,
Ltd.
Measurement of serum total cholesterol
(TC), triglyceride (TG), high density lipoprotein cholesterol
(HDL-C) and low density lipoprotein cholesterol (LDL-C)
After 13 weeks of feeding, all mice were starved for
12 h and anesthetized by intraperitoneal injection of diazepam (5
mg/kg) and ketamine (50 mg/kg) to collect cardiac venous blood. The
collected blood was centrifuged at 1,800 × g for 15 min at 4°C to
obtain serum. TC, TG, HDL-C and LDL-C levels in serum were detected
using OLYMPUS AU2700 automatic blood lipid analyzer (Olympus
Corporation).
Haematoxylin and eosin (H&E)
staining
At the 13th week, mice in the Sham group (n=10) and
the AS group (n=10) were randomly selected and anesthetized by
intraperitoneal injection of diazepam (5 mg/kg) and ketamine (50
mg/kg), and then sacrificed by cervical dislocation. After
immersion into 75% ethanol for 5 min, the mice were subjected to
thoracotomy to obtain the thoracic aorta tissues. Pre-cooled PBS
was used to remove residual blood from the thoracic aorta. Excess
extra vascular tissues were removed. Subsequently, the thoracic
aorta was fixed in 4% paraformaldehyde for 24 h at 25°C, embedded
in paraffin and sliced into 4-µm sections for H&E staining.
Briefly, these sections were de-waxed three times with toluene for
5 min each time, then dehydrated with gradient alcohol and washed
with pure water. The sections were stained with hematoxylin for 2
min and with eosin for 2 min, both at 25°C. After being dehydrated
by gradient alcohol and xylene, all sections were sealed with
neutral resin and observed under a light microscope (magnification,
×200).
Transfection in vivo
The miRNA delivery method used in this study was
performed in accordance with previous protocols (35–37).
This method may be used in the evaluation of the regulatory effects
of miR-133b overexpression on AS. A total of 20 mice randomly
selected from the AS group were divided into two groups, which were
named the miR-133b mimic + AS group and the miR-133b negative
control (NC) + AS group. According to the instructions of
EntransterTM-in vivo reagent (Engreen Biosystem Co., Ltd.),
the mice in both groups were intravenously injected with 10 mg/kg
miR-133b mimic (forward, 5′-UUUGGUCCCCUUCAACCAGCUA-3′ and reverse,
5′-GCUGGUUGAAGGGGACCAAAUU-3′) and miR-133b NC (forward, 5′-
UUCUCCGAACGUGUCACGUTT-3′ and reverse, 5′- ACGUGACACGUUCGGAGAATT-3′)
via the tail vein, respectively. The injection was administered
every week and lasted for 4 weeks. Before injection, the tail vein
was wiped with 75% alcohol so it was fully congested and expanded.
miR-133b mimic and miR-133b NC were synthesized by Shanghai Jima
Pharmaceutical Technology Co., Ltd. The subsequent experiments were
performed following 4 weeks of transfection.
Detection of inflammatory factors
Thoracic aorta tissues were homogenated. The levels
of tumor necrosis factor (TNF)-α (cat. no. KRC3011C) and monocyte
chemoattractant protein (MCP)-1 (cat. no. BMS6005TEN) in the
tissues were detected using ELISA kits (Thermo Fisher Scientific,
Inc.), according to the manufacturers' protocols.
Vascular reconstruction parameters and
Collagen/Vascular Area Ratio (CA/CVA) determination
The thoracic aorta tissues of mice in each group
were obtained 3 days after transfection according to the
acquisition method described above. Thoracic aorta tissues were
fixed in 4% paraformaldehyde at 25°C for 24 h. After being embedded
in paraffin, these vascular tissues were cut into 4-µm thick
sections and stained with Masson staining for 10 min at 25°C, then
washed with 0.2% aqueous acetic acid for 1 min and soaked with 1%
phosphotungstic acid for 4 min (Sigma-Aldrich; Merck KGaA). Before
staining with a bright green dye solution (Sigma-Aldrich; Merck
KGaA) for 30 sec at 25°C, all the sections were washed again with
0.2% aqueous acetic acid for 1 min. Gradient alcohol and xylene
were then used to dehydrate all sections. Lastly, these sections
were sealed with neutral gum and observed under a light microscope
(magnification, ×200). Intima thickness (IT), medial thickness (MT)
and collagen area (CA) were detected using Image-Pro Plus 5.1
Pathology Image Analysis System (Media Cybernetics, Inc.). The
ratio of CA/CVA (%) was calculated using the following equation:
(CA/CAV) × 100.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA in thoracic aorta tissues was collected
using TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. A total of 300 ng RNA was
synthesized into cDNA templates with the PrimeScript RT reagent kit
(Takara Bio, Inc.) at 42°C for 45 min. These cDNA templates were
amplified through RT-qPCR with a SYBR® Green kit (Takara
Bio, Inc.) on an ABI PRISM 7500 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used: 95°C for 3 min; and 40 cycles
of 95°C for 15 sec and 60°C for 30 sec. U6 was set as the internal
control for miRNAs, and GAPDH served as the internal control for
other genes. Primer sequences involved in this study were as
follows: miR-133b forward, 5′-TTTGGTCCCCTTCAACCAGCTA-3′ and
reverse, 5′-GTGCAGGGTCCGAGGT-3′; Notch1 forward,
5′-TCGCCGCAAGAGGCTTGAGATGCT-3′ and reverse,
5′-TCCGCTGCAGCACAGGCTTCA-3′; Notch3 forward,
5′-TCGCAACTGCCGAAGCGACA-3′ and reverse,
5′-GCGCAGGGCAGTATGGGGTTTT-3′; Jaggedl forward,
5′-CAGCGCACGCCGACAAAAC-3′ and reverse, 5′-TATGGCGGCACGCACTGTCT-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; GAPDH forward,
5′-CCACCCATGGCAAATTCCATGGCA-3′ and reverse,
5′-TCTAGACCGCAGGTCAGGTCCACC-3′. The relative expression of genes
was determined by the 2-ΔΔCq method (38).
Western blot analysis
The thoracic aorta tissues were ground in liquid
nitrogen and lysed with RIPA lysis buffer (cat. no. P0013C;
Beyotime Institute of Biotechnology). Total protein was quantified
by the BCA method and transferred to a PVDF membrane after
separation by 10% SDS-PAGE (30 µg/lane). Subsequently, the membrane
was blocked with 5% skimmed milk at 4°C for 1 h. Primary antibodies
used in this research were rabbit anti-Notch1 (cat. no. ab194123;
Abcam; 1:5,000), anti-Notch3 (cat. no. ab23426; Abcam; 1:5,000) and
anti-Jagged1 antibodies (cat. no. ab7771; Abcam; 1:5,000). The PVDF
membrane was incubated with primary antibodies at 4°C for 12 h.
Then, the membrane was washed with TBS with 0.1% Tween 20 (TBST)
for 15 min and incubated with a horseradish peroxidase-labeled goat
anti-rabbit IgG secondary antibody (cat. no. ab6721; Abcam;
1:5,000) for 2 h at 25°C. The membrane was then washed with TBST
for 15 min. Enhanced Chemiluminescence system (GE Healthcare) was
used to visualize proteins. Lab Works 4.5 software (UVP, LLC) was
selected to analyze the integrated optical density. β-actin was
used as an internal reference.
Cell culture and treatment
Mouse VSMCs (CRL-2797) were purchased from the
American Type Culture Collection. Cells were cultured with DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS and 1%
penicillin/streptomycin. VSMCs were treated with different
concentrations of oxidized low-density lipoprotein (ox-LDL; 0, 25,
50 and 75 µg/ml) for 24 h, or with 50 µg/ml ox-LDL for different
times (0, 12, 24 and 48 h), both at 25°C. VSMCs without treatment
were the control. Subsequently, VSMCs were transfected with 100 nM
miR-133b mimic (Control + mimic) and 100 nM miR-133b NC (Control +
NC) for 24 h. ox-LDL induced-VSMCs (24 h, 50 µg/ml) were
transfected with 100 nM miR-133b mimic (ox-LDL + mimic) and 100 nM
miR-133b NC (ox-LDL + NC) for 24 h. Cell transfection was performed
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), following the manufacturer's protocols.
The subsequent experiments were performed at 24 h
post-transfection.
CCK-8 assay
Cell proliferation was detected by a CCK-8 Assay Kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Simply, cells were seeded into a 96-well
plate and 50 µl CCK-8 solution was added into each well. After 3 h
of incubation, the optical density (OD) at 450 nm was detected by a
microplate reader (Molecular Devices).
Wound healing assay
Cell migration was detected by the wound healing
test. Briefly, cells were seeded into a 6-well plate and cultured
until 90% confluence, and then cultured in FBS-free DMEM overnight.
A sterile pipette tip was used to draw a vertical line on the cell
surface. Cells were subsequently cultured in FBS-free DMEM for 24
h, with the scratch width measured at 0 and 24 h. Cells were
observed under a light microscope (magnification, ×100). The
migration rate (%) was calculated as: (1 - scratch width at 24 h/
scratch width at 0 h) × 100.
Statistical analysis
Experimental data were expressed as mean ± SD. SPSS
19.0 (IBM Corp.) was used for data processing. Two-tailed Student's
t-test was used to compare the differences between two groups and
one-way ANOVA was selected to compare the differences among
multiple groups, followed by a Tukey's post hoc test. P<0.05 was
considered to be statistically significant and all experiments were
performed three times.
Results
Successful establishment of AS mouse
model
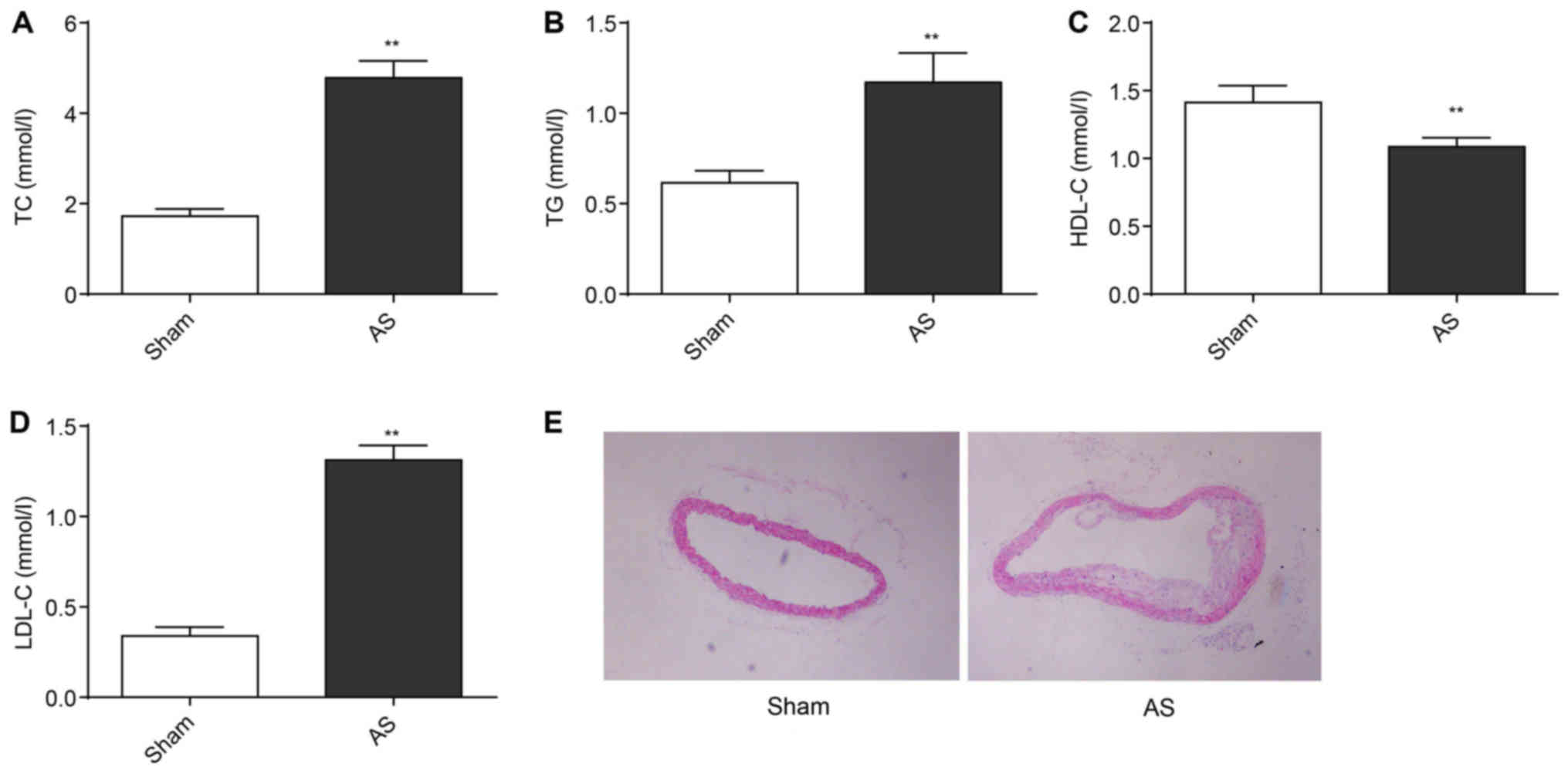
Serum TC, TG and LDL-C levels of mice in the AS
group were significantly higher than those in the Sham group
(P<0.01) at the 13th week. By contrast, the serum level of HDL-C
in the AS group was found to be significantly lower than the Sham
group (P<0.01; Fig. 1A-D). The
thoracic aorta vessels of mice in each group were stained with
H&E stain (Fig. 1E). In the
Sham group, thoracic aorta vessels had smooth intima, intact
endothelial cells and uniform blood vessel thickness. Smooth muscle
cell proliferation and inflammatory cell infiltration were not
observed. However, typical AS plaques protruding to the vascular
lumen was found in the thoracic aorta vessels of the AS group. The
subendothelial space was widened, and migration of smooth muscle
cells and foam cells were observed. Mesangial smooth muscle cells
showed obvious hyperplasia. There were a large number of newly
added smooth muscle cells near the outer membrane. Inflammatory
cell infiltration was easily detected. This showed the successful
construction of mice AS models.
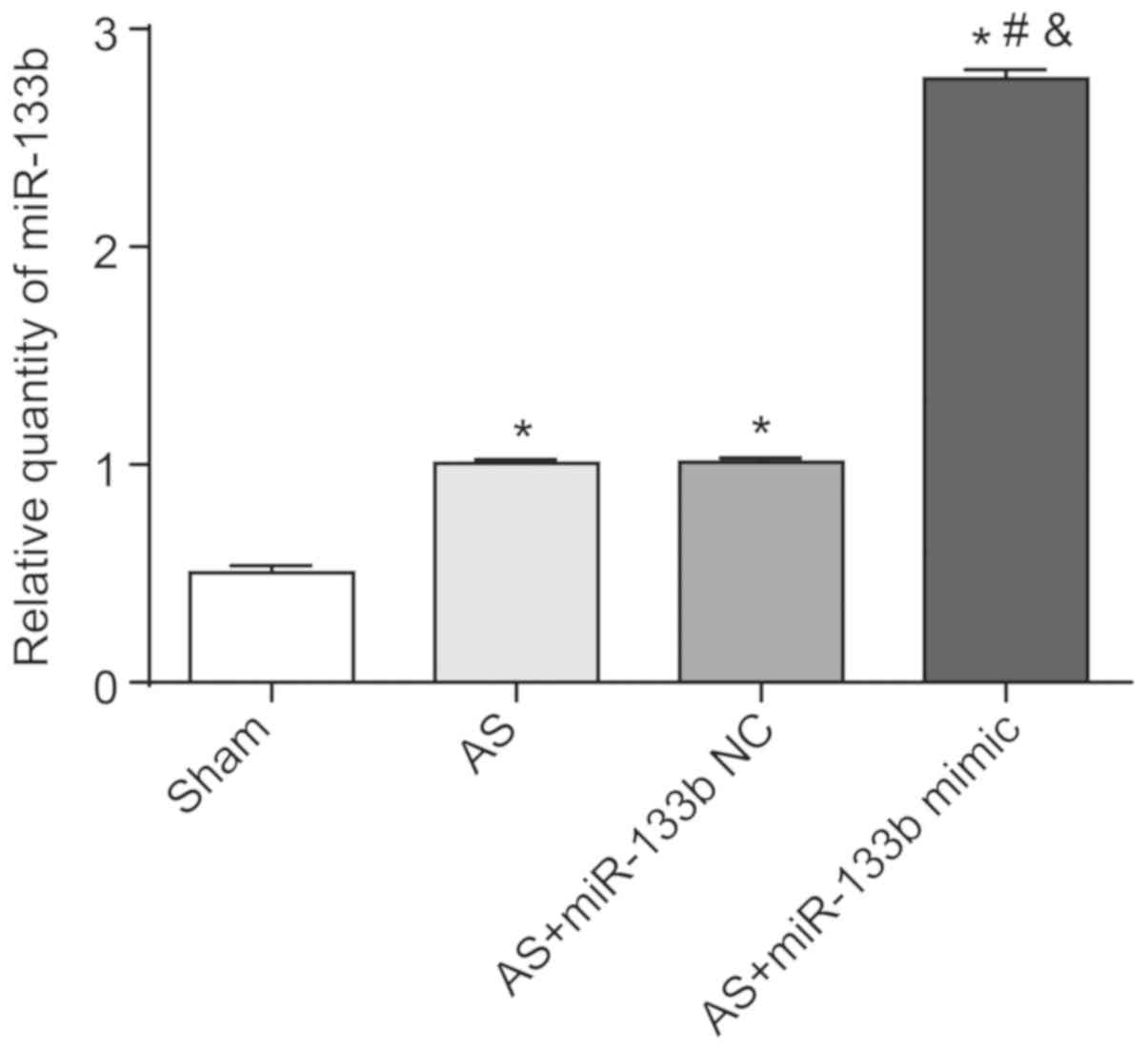
Successful transfection in mice
After 3 days of transfection in vivo, the
expression of miR-133b in the mouse thoracic aorta was determined
by RT-qPCR. As shown in Fig. 2,
the expression of relative miR-133b in the thoracic aorta of the AS
group, the AS + miR-133b NC group and the AS + miR-133b mimic group
were all significantly higher than that of the Sham group
(P<0.05). In addition, the relative expression of miR-133b in
thoracic aorta of the AS + miR-133b mimic group was significantly
higher than that of the AS group and the AS + miR-133b NC group
(P<0.05). No significant difference in relative miR-133b
expression was found in the thoracic aorta between the AS group and
the AS + miR-133b NC group. Therefore, miR-133b expression in the
thoracic aorta was successfully upregulated by transfection in
vivo.
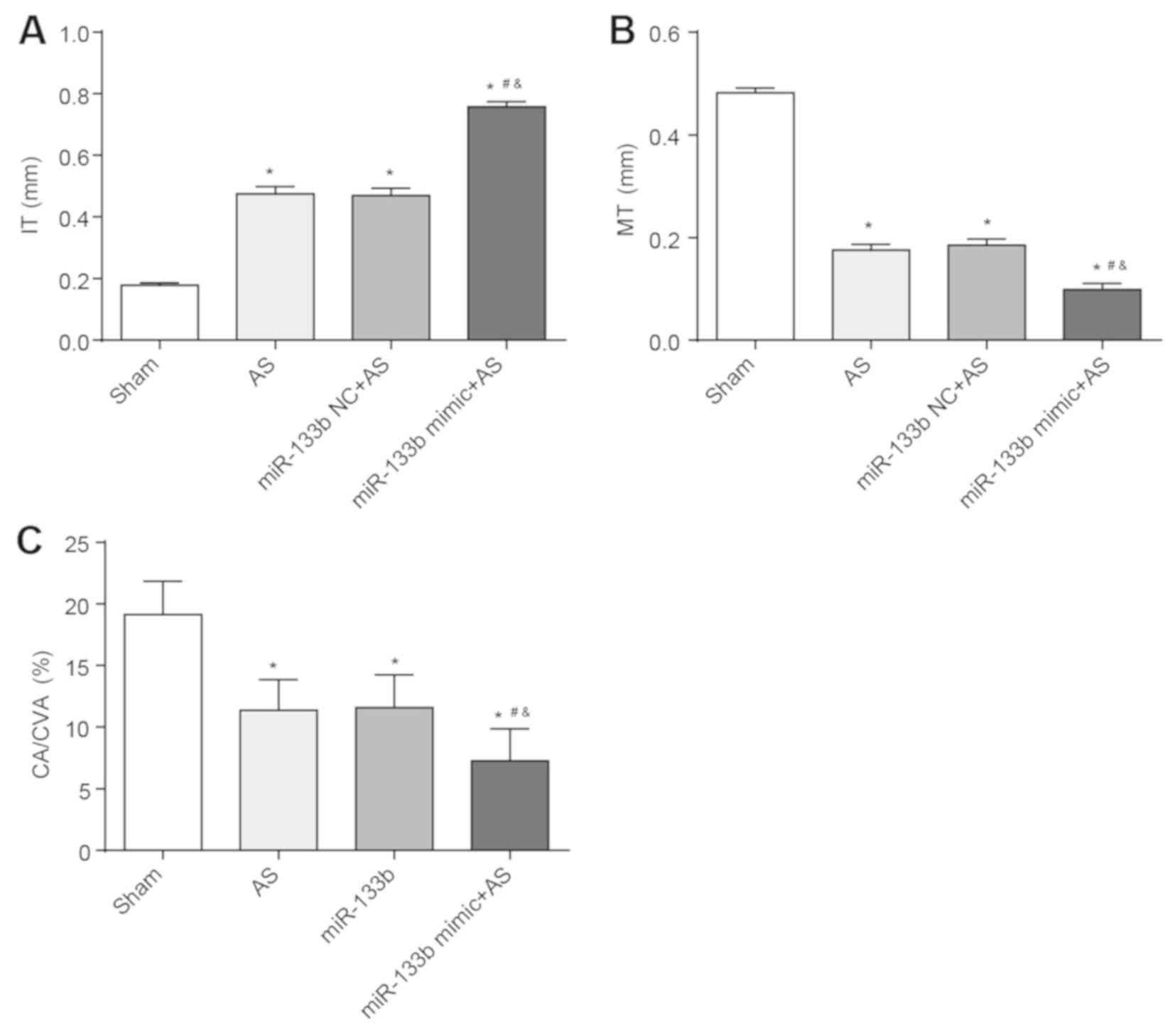
Overexpression of miR-133b aggravates
AS
Compared with the Sham group, IT increased in the
thoracic aorta of the AS group, the AS + miR-133b NC group and the
AS + miR-133b mimic group, whereas MT and the ratio of CA/CVA
decreased in the thoracic aorta of these groups (P<0.05).
Furthermore, these same results were also found in the thoracic
aorta of the AS + miR-133b mimic group when compared to the AS
group and the AS + miR-133b NC group (P<0.05). There was no
obvious difference in IT, MT and the ratio of CA/CVA between the AS
group and the AS + miR-133b NC group (Fig. 3A-C). All these results indicated
that overexpression of miR-133b aggravated AS.
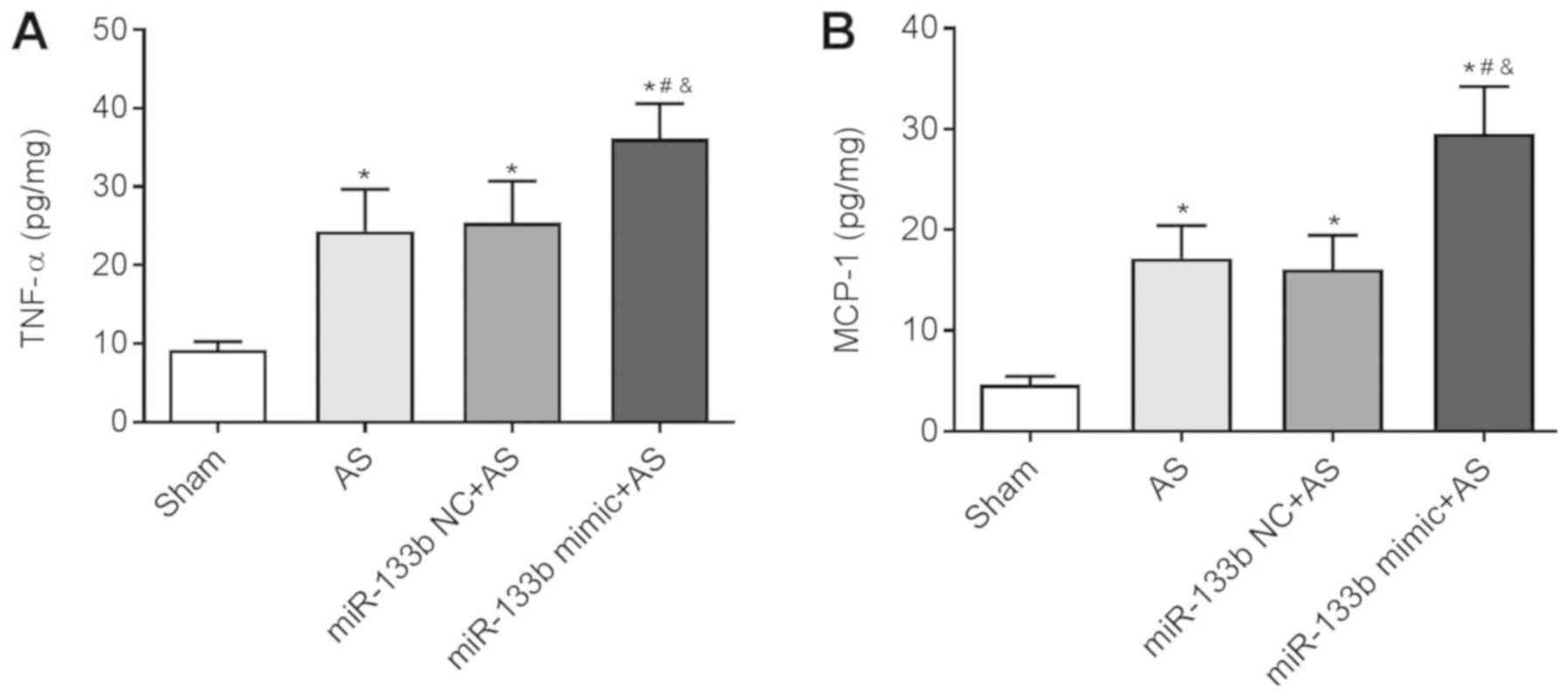
Overexpression of miR-133b increases
inflammatory factor levels
TNF-α and MCP-1 are two important inflammatory
factors (39). In the present
study levels of TNF-α and MCP-1 were significantly upregulated in
thoracic aorta tissues in mice of the AS group, the AS + miR-133b
NC group and the AS + miR-133b mimic group when compared with the
Sham group (P<0.05). This was also detected in thoracic aorta
tissues of the AS + miR-133b mimic group when compared with the AS
group and the AS + miR-133b NC group (P<0.05; Fig. 4A and B). Overexpression of miR-133b
increased the inflammatory factor levels of AS mice, thereby
worsening AS.
Overexpression of miR-133b aggravates
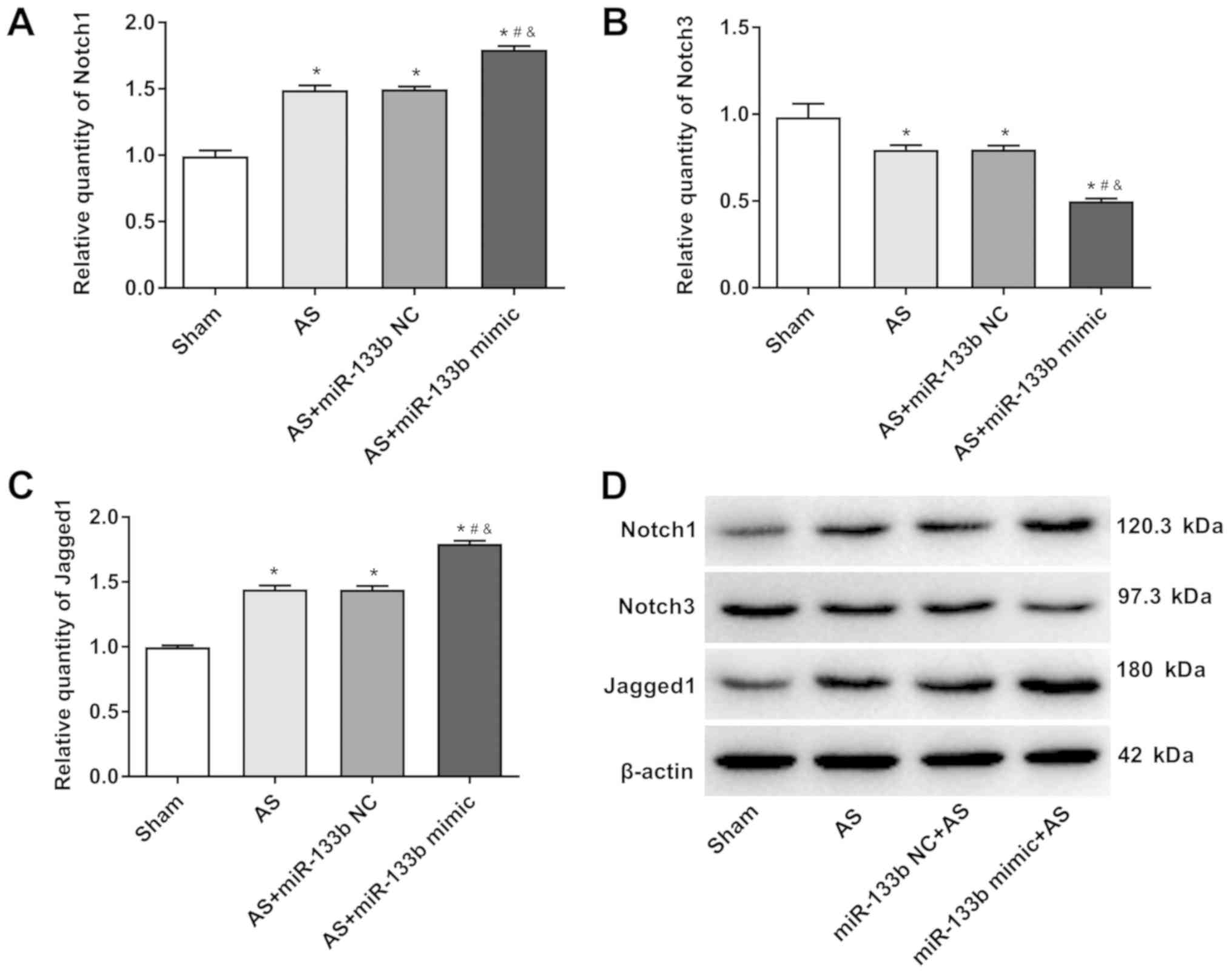
AS by activating the Notch signaling pathway
The Notch signaling pathway plays an important
regulatory role in a variety of diseases (40). Therefore, the effects of miR-133b
on the Notch signaling pathway in this paper were studied. It was
found that the relative expression of Notch1 and Jagged1 in the AS
+ miR-133b group was significantly higher than that in the AS group
and AS + miR-133b NC group when compared with the Sham group
(P<0.05). In addition, the relative expression of the Notch1 and
Jagged1 in the thoracic aorta in the AS + miR-133b mimic group was
significantly upregulated compared to the AS group and the AS +
miR-133b NC group (P<0.05). However, the Notch3 relative
expression level, was much lower in the AS + miR-133b mimic group
than that in the AS group and the AS + miR-133b NC group
(P<0.05; Fig. 5A-D). These
results suggested that overexpression of miR-133b may aggravate AS
by activating the Notch signaling pathway.
Overexpression of miR-133b promotes
cell proliferation and migration, and activates Notch signaling
pathway in ox-LDL-induced VSMCs
An in vitro AS model was induced in VSMCs by
the treatment of ox-LDL. As shown in Fig. 6A and B, ox-LDL treatment
significantly increased the expression of miR-133b in a dose- and
time-dependent manner (P<0.05). The 24 h ox-LDL treatment at a
concentration of 50 µg/ml was used for subsequent experiments. The
CCK-8 and wound healing assay showed that the OD450
value and the migration rate of ox-LDL-induced VSMCs were
significantly higher than those in the control group (P<0.01;
Fig. 6D and E). Western blotting
showed that the expression of Notch1 and Jagged1 significantly
increased, and the expression of Notch3 significantly decreased in
ox-LDL-induced VSMCs (P<0.01; Fig.
6F). To identify the regulatory role of miR-133b in AS,
miR-133b was overexpressed in VSMCs by the transfection of miR-133b
mimics. RT-qPCR showed that the expression of miR-133b was
significantly increased in miR-133b mimics-transfected VSMCs and
ox-LDL-induced VSMCs (P<0.01; Fig.
6C). The transfection of miR-133b mimics significantly
increased the OD450 value and migration rate,
upregulated Notch1 and Jagged1, and downregulated Notch3 in
ox-LDL-induced VSMCs (P<0.01; Fig.
6D-F). These results indicated that the miR-133b overexpression
promoted the proliferation and migration of ox-LDL-induced VSMCs,
and activated the Notch signaling pathway.
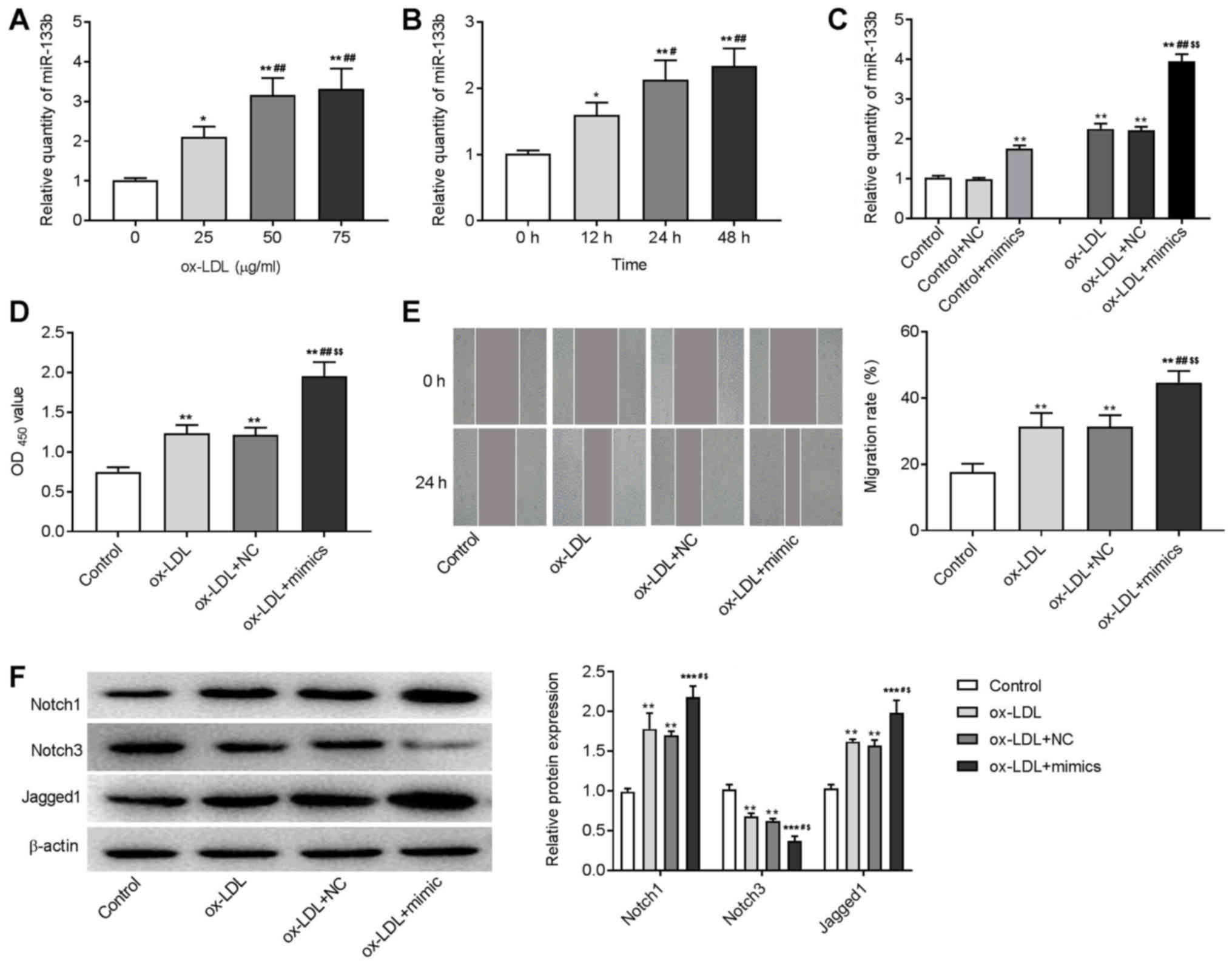 | Figure 6.Overexpression of miR-133b promoted
cell proliferation and migration, and activated Notch signaling
pathway in ox-LDL-induced VSMCs. Expression of miR-133b in VSMCs
treated with ox-LDL for (A) different concentrations and (B) times
was detected by RT-qPCR; *P<0.05, **P<0.01 vs. 0 µg/ml or 0
h; #P<0.05, ##P<0.01 vs. 25 µg/ml or 12
h. (C) Expression of miR-133b in VSMCs/ox-LDL-induced VSMCs
transfected with miR-133b mimic/NC was detected by RT-qPCR. (D)
Proliferation of VSMCs was detected by CCK-8 assay (OD 450). (E)
Migration of VSMCs was detected by wound healing assay
(magnification, ×400). (F) Protein expression of Notch1, Notch3 and
Jagged1 was detected by western blotting. **P<0.01,
***P<0.001 vs. Control group; #P<0.05,
##P<0.01 vs. ox-LDL group; $P<0.05,
$$P<0.01 vs. ox-LDL + NC group. miR, microRNA;
ox-LDL, oxidized low density lipoprotein; VSMCs, vascular smooth
muscle cells; RT-qPCR, reverse transcription-quantitative PCR; NC,
negative control; OD, optical density. |
Discussion
AS is the core pathological cause of acute cerebral
infarction, which can lead to carotid stenosis and reduce blood
supply to the brain (41). When
stenosis is continuously increased or the important cerebral artery
is blocked due to detached plaques, acute cerebral blood supply
blockage and ischemic hypoxia injury of the nervous system is more
likely to occur, thereby posing a great threat to the health and
life of patients (42,43). Early diagnosis and effective
treatment are important strategies to improve the prognosis of
patients with AS. miR-133b has been reported to be involved in the
development of a variety of tumors, such as gastric cancer, ovarian
cancer and non-small cell lung cancer (15,24,26,44,45).
However, the regulatory effect of miR-133b on AS development and
related action mechanism remains unclear. In the present study,
mice AS models were successfully constructed, and miR-133b was
shown to aggravate AS by activating the Notch signaling
pathway.
AS is a chronic inflammation and immune response to
the injury and stimulation of the arterial wall (46). In the early stages of AS formation,
monocytes that accumulate at the injury site pass through the
vascular endothelium, thereby activating and differentiating into
macrophages (47–50). The foam cells formed by the
lipidation of macrophages are the initiating factors of AS
(51). VSMCs, which are abundant
in the vascular media, migrate into the intima of vascular and
phagocytose the lipid to form muscle-derived foam cells, and
eventually form fibrous plaques (52,53).
In the present study, it was observed that overexpression of
miR-133b induced increased IT and decreased MT, which may be caused
by the migration of a large number of VSMCs into the intima. In
addition, the ratio of CA/CVA decreased as a result of miR-133b
overexpression. This is important because collagen is the main
component of vascular remodeling (54), it has been shown that if collagen
is reduced the original vascular lumen cannot be maintained
(55,56). Thus leading to the loss of the
compensatory mechanism where the vascular wall can expand,
therefore leading to luminal stenosis and aggravated AS (57–59).
Inflammatory responses are also important factors that trigger and
exacerbate AS (60). TNF-α and
MCP-1 are two important pro-inflammatory factors (61). TNF-α, which is mainly secreted by
activated monocytes/macrophages, is a cytokine with multiple
functions and an important mediator of inflammatory responses
(62). Tay et al (63), found that B2 lymphocytes promote
cell death and inflammation by increasing TNF-α production, which
is a key mechanism for promoting atherogenesis. Voloshyna et
al (64), also reported that
TNF-α could result in an atherogenic state by decreasing the
expression of ATP binding cassette transporters A1 as a
pro-inflammatory atherogenic cytokine. Gandhirajan et al
(65), discovered that TNF-α could
induce proliferation and migration of human VSMCs. In the present
study it was found that overexpression of miR-133b increased TNF-α
level, which might further aggravate AS by promoting the
proliferation and migration of VSMCs. MCP-1 has been considered as
an important factor in the formation of AS, and some scholars have
proposed to detect the degree of AS and predict the risk of
long-term cerebral infarction by detecting the level of MCP-1
(66). MCP-1 is capable of
chemotaxis of monocyte macrophages. It can induce the production of
various pro-inflammatory factors, promote the expression of
adhesion molecules and accelerate the formation of foam cells
(67). In the present research,
elevated MCP-1 levels induced by overexpression of miR-133b
exacerbated the development of AS. Notch signaling pathway has been
shown to have an important role in regulating the survival,
migration and phenotype of VSMCs (68). Beaten and Lilly (69) demonstrated that the Notch signaling
pathway was involved in the vascular development by influencing
angiogenesis, vessel patterning, arterial/venous specification and
VSMC differentiation. Sweeney et al (70), demonstrated that the apoptosis of
rat VSMCs significantly increased after the Notch signaling pathway
was inhibited using RPMS-1 or brefeldin A. Previously, researchers
revealed that in AS, the Notch signaling pathway was activated in
aged human endothelial cells, and several important inflammatory
factors were significantly upregulated in endothelial cells when
the Notch signaling pathway was activated (71). Thus, the Notch signaling pathway
was proposed to be a potential target for the treatment of AS. In
the present study it was discovered that the Notch signaling
pathway was activated by the upregulation of miR-133b, which
further led worsened AS. These findings indicated that miR-133b is
involved in the development of AS, which is consistent with
previous studies (23,29). However, the specific role of
miR-133b in AS is contrary to that in tumors, where it has been
shown to have a tumor inhibiting effect (24–28).
This conflict may attribute to the different functions of miR-133b
in different diseases.
This study exhibited some limitations. First, the
upstream and downstream genes that regulate miR-133b were not
investigated in relation to AS progression, so they are still
unclear. Second, the regulatory role of the Notch pathway in AS was
not evaluated. Third, the regulatory relationship between miR-133b
and other important signaling pathways, such as PI3K and MAPK
signaling were not evaluated in the AS model. Fourth, the effects
of miR-133b downregulation in AS was not analyzed. Lastly, the
effect of statin therapy on miR-133b expression in AS was not
analyzed. Further studies exploring these mechanisms are
needed.
To conclude, miR-133b was upregulated in the mouse
model of AS and ox-LDL-induced VSMCs. The overexpression of
miR-133b promoted vascular remodeling and inflammation in AS mice,
and the proliferation and migration of ox-LDL-induced VSMCs in
vitro. The regulatory role of miR-133b in AS was closely
associated with the activation of the Notch signaling pathway. It
is hypothesized that miR-133b downregulation may inhibit vascular
remodeling and inflammation, as well as the proliferation and
migration of VSMCs by blocking the Notch signaling pathway. Thus,
miR-133b may serve as a potential therapeutic target for the
treatment of AS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL conceived and designed the study; BH and SZ
acquired and analyzed the data; TL and BH drafted the manuscript;
and TL revised the manuscript for critically important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was conducted after obtaining the
approval of Weifang Hospital of Traditional Chinese Medicine's
Ethical Committee. Animal experiments were approved by the Animal
Experimental Ethics Committee of our hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Jiang C, Ma X and Wang J:
Notoginsenoside Fc attenuates high glucose-induced vascular
endothelial cell injury via upregulation of PPAR-γ in diabetic
Sprague-Dawley rats. Vascul Pharmacol. 109:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baumer Y, McCurdy S, Alcala M, Mehta N,
Lee BH, Ginsberg MH and Boisvert WA: CD98 regulates vascular smooth
muscle cell proliferation in atherosclerosis. Atherosclerosis.
256:105–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassan MO: The role of circulating
endotoxaemia as a proinflammatory mediator of atherosclerosis in
chronic kidney disease patients. BMJ. 288:283–284. 2016.
|
|
4
|
Groh L, Keating ST, Joosten LAB, Netea MG
and Riksen NP: Monocyte and macrophage immunometabolism in
atherosclerosis. Semin Immunopathol. 40:203–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chistiakov DA, Melnichenko AA, Myasoedova
VA, Grechko AV and Orekhov AN: Mechanisms of foam cell formation in
atherosclerosis. J Mol Med (Berl). 95:1153–1165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lao KH, Zeng L and Xu Q: Endothelial and
smooth muscle cell transformation in atherosclerosis. Curr Opin
Lipidol. 26:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Francis GA, Allahverdian S, Cheroudi AC,
Abraham T and McManus BM: Response to letter regarding article,
“contribution of intimal smooth muscle cells to cholesterol
accumulation and macrophage-like cells in human atherosclerosis”.
Circulation. 131:e252015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Y, Ma XY, Yang YB, Ren HT, Sun XH
and Wang LR: Identification and characterization of microRNAs and
their target genes from Nile tilapia (Oreochromis niloticus). Z
Natforsch C J Biosci. 71:215–223. 2016.
|
|
9
|
Xu X, Wang X, Fu B, Meng L and Lang B:
Differentially expressed genes and microRNAs in bladder carcinoma
cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp
Pathol. 8:12678–12687. 2015.PubMed/NCBI
|
|
10
|
Gao ZG, Chen QJ, Shao M, Qian YZ, Zhang
LF, Zhang YB and Xiong QX: Preliminary identification of key
miRNAs, signaling pathways, and genes associated with
Hirschsprung's disease by analysis of tissue microRNA expression
profiles. World J Pediatr. 13:489–495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennan E, Wang B, McClelland A, Mohan M,
Marai M, Beuscart O, Derouiche S, Gray S, Pickering R, Tikellis C,
et al: Protective effect of let-7 miRNA family in regulating
inflammation in diabetes-associated atherosclerosis. Diabetes.
66:2266–2277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qun L, Wenda X, Weihong S, Jianyang M, Wei
C, Fangzhou L, Zhenyao X and Pingjin G: miRNA-27b modulates
endothelial cell angiogenesis by directly targeting Naa15 in
atherogenesis. Atherosclerosis. 254:184–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Ronde MWJ, Kok MGM, Moerland PD, Van
den Bossche J, Neele AE, Halliani A, van der Made I, de Winther
MPJ, Meijers JCM, Creemers EE, et al: High miR-124-3p expression
identifies smoking individuals susceptible to atherosclerosis.
Atherosclerosis. 263:377–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim J, Inoue K, Ishii J, Vanti WB, Voronov
SV, Murchison E, Hannon G and Abeliovich A: A MicroRNA feedback
circuit in midbrain dopamine neurons. Science. 317:1220–1224. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez-Simon FM, Zhang XX, Loh HH, Law
P-Y and Rodriguez RE: Morphine regulates dopaminergic neuron
differentiation via miR-133b. Mol Pharmacol. 78:935–942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin H, Pasut A, Soleimani VD, Bentzinger
CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld
F, et al: MicroRNA-133 controls brown adipose determination in
skeletal muscle satellite cells by targeting Prdm16. Cell Metab.
17:210–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Wan X, Chen H, Yang S, Liu Y, Mo W,
Meng D, Du W, Huang Y, Wu H, et al: Identification of miR-133b and
RB1CC1 as independent predictors for biochemical recurrence and
potential therapeutic targets for prostate cancer. Clin Cancer Res.
20:2312–2325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li D, Xia L, Chen M, Lin C, Wu H, Zhang Y,
Pan S and Li X: miR-133b, a particular member of myomiRs, coming
into playing its unique pathological role in human cancer.
Oncotarget. 8:50193–50208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Mena L, Coto E, Cardo LF, Díaz M,
Blázquez M, Ribacoba R, Salvador C, Pastor P, Samaranch L, Moris G,
et al: Analysis of the Micro-RNA-133 and PITX3 genes in Parkinson's
disease. Am J Med Genet B Neuropsychiatr Genet. 153B:1234–1239.
2010.PubMed/NCBI
|
|
20
|
Ferreira LRP, Frade AF, Santos RHB,
Teixeira PC, Baron MA, Navarro IC, Benvenuti LA, Fiorelli AI,
Bocchi EA, Stolf NA, et al: MicroRNAs miR-1, miR-133a, miR-133b,
miR-208a and miR-208b are dysregulated in Chronic Chagas disease
Cardiomyopathy. Int J Cardiol. 175:409–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masè M, Grasso M, Avogaro L, Nicolussi
Giacomaz M, D'Amato E, Tessarolo F, Graffigna A, Denti MA and
Ravelli F: Upregulation of miR-133b and miR-328 in patients with
atrial dilatation: Implications for stretch-induced atrial
fibrillation. Front Physiol. 10:11332019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang B, Jiang X-C, Zhang T-Y, Hu YL,
Tabata Y, Chen Z, Pluchino S and Gao JQ: Peptide modified
mesenchymal stem cells as targeting delivery system transfected
with miR-133b for the treatment of cerebral ischemia. Int J Pharm.
531:90–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng CG, Chen BY, Sun RH, Mou XZ, Han F,
Li Q, Huang HJ, Liu JQ and Tu YX: miR-133b Downregulation Reduces
Vulnerable Plaque Formation in Mice with AS through Inhibiting
Macrophage Immune Responses. Mol Ther Nucleic Acids. 16:745–757.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhen Y, Liu J, Huang Y, Wang Y, Li W and
Wu J: miR-133b inhibits cell growth, migration, and invasion by
targeting MMP9 in non-small cell lung cancer. Oncol Res.
25:1109–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo L, Bai H, Zou D, Hong T, Liu J, Huang
J, He P, Zhou Q and He J: The role of microRNA-133b and its target
gene FSCN1 in gastric cancer. J Exp Clin Cancer Res. 33:992014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng Y, Jia B, Wang Y and Wan S: miR-133b
acts as a tumor suppressor and negatively regulates ATP citrate
lyase via PPARγ in gastric cancer. Oncol Rep. 38:3220–3226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Bu J, Liu X, Wang W, Mai W, Lv B,
Zou J, Mo X, Li X, Wang J, et al: miR-133b suppresses metastasis by
targeting HOXA9 in human colorectal cancer. Oncotarget.
8:63935–63948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, Wu D, Tao J, Qu P, Zhou Z and Hou
J: MicroRNA-133 inhibits cell proliferation, migration and invasion
by targeting epidermal growth factor receptor and its downstream
effector proteins in bladder cancer. Scand J Urol. 47:423–432.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Xiao L, Li J, Sun P, Shang L, Zhang
J, Zhao Q, Ouyang Y, Li L and Gong K: MicroRNA profiling of
diabetic atherosclerosis in a rat model. Eur J Med Res. 23:552018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fung E, Tang SM, Canner JP, Morishige K,
Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC and Aikawa
M: Delta-like 4 induces notch signaling in macrophages:
Implications for inflammation. Circulation. 115:2948–2956. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quillard T, Devallière J, Coupel S and
Charreau B: Inflammation dysregulates Notch signaling in
endothelial cells: Implication of Notch2 and Notch4 to endothelial
dysfunction. Biochem Pharmacol. 80:2032–2041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu ZJ, Tan Y, Beecham GW, Seo DM, Tian R,
Li Y, Vazquez-Padron RI, Pericak-Vance M, Vance JM,
Goldschmidt-Clermont PJ, et al: Notch activation induces
endothelial cell senescence and pro-inflammatory response:
Implication of Notch signaling in atherosclerosis. Atherosclerosis.
225:296–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Tan Y, Tian R, Li Y, Beecham GW,
Seo DM, Vazquez-Padron RI, Pericak-Vance MA, Vance JM,
Goldschmidt-Clermont PJ, et al: Notch Signaling Is A Potential
Novel Target In Atherosclerosis. J Surg Res. 165:3262011.
View Article : Google Scholar
|
|
34
|
Davis-Knowlton J, Turner JE, Turner A,
Damian-Loring S, Hagler N, Henderson T, Emery IF, Bond K, Duarte
CW, Vary CPH, et al: Characterization of smooth muscle cells from
human atherosclerotic lesions and their responses to Notch
signaling. Lab Invest. 99:290–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z, Wang Z, Wang T, Yuan J, Wang X and
Zhang Z: Inhibition of miR-34a-5p protected myocardial ischemia
reperfusion injury-induced apoptosis and reactive oxygen species
accumulation through regulation of Notch Receptor 1 signaling. Rev
Cardiovasc Med. 20:187–197. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin D, Cui B, Ma J and Ren J: MiR-183-5p
protects rat hearts against myocardial ischemia/reperfusion injury
through targeting VDAC1. Biofactors. 46:83–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ou M, Zhang C, Chen J, Zhao S, Cui S and
Tu J: Overexpression of microRNA-340-5p inhibits pulmonary arterial
hypertension induced by acute pulmonary embolism by down-regulating
the expression of inflammatory factors interleukin-1β and
interleukin-6. Available at SSRN 3365060. 2019. View Article : Google Scholar
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2− ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kasiewicz LN and Whitehead KA: Silencing
TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1
in an in vitro co-culture model of diabetic foot ulcers. Acta
Biomater. 32:120–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Talora C, Campese AF, Bellavia D, Felli
MP, Vacca A, Gulino A and Screpanti I: Notch signaling and
diseases: An evolutionary journey from a simple beginning to
complex outcomes. Biochim Biophys Acta. 1782:489–497. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Geng YR, Zhang HL, Dong Y, Liu GY, Xie J
and Wang H: Relationship between intercellular adhesion molecule-1
and cerebral infarction. Progress in Modern Biomedicine.
22:4373–4375. 2010.(In Chinese).
|
|
42
|
Xu R, Yin X, Xu W, Jin L, Lu M and Wang Y:
Assessment of carotid plaque neovascularization by
contrast-enhanced ultrasound and high sensitivity C-reactive
protein test in patients with acute cerebral infarction: A
comparative study. Neurol Sci. 37:1107–1112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Liu T, Huang P, Zhao H, Zhang R,
Ma B, Chen K, Huang F, Zhou X, Cui C, et al: A novel Golgi protein
(GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor
efficacy in hepatocellular carcinoma. Oncotarget. 6:13564–13578.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang L, Hou J, Cui XH, Suo LN and Lv YW:
MiR-133b regulates the expression of CTGF in epithelial-mesenchymal
transition of ovarian cancer. Eur Rev Med Pharmacol Sci.
21:5602–5609. 2017.PubMed/NCBI
|
|
45
|
Trajkovski M, Ahmed K, Esau CC and Stoffel
M: MyomiR-133 regulates brown fat differentiation through Prdm16.
Nat Cell Biol. 14:1330–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dumitriu IE and Kaski JC: The role of
lymphocytes in the pathogenesis of atherosclerosis: Focus on CD4+ T
cell subsets. Inflammatory Response in Cardiovascular Surgery.
Gabriel EA and Gabriel SA: Springer; London: pp. 9–14. 2013,
View Article : Google Scholar
|
|
47
|
Qin M, Luo Y, Meng XB, Wang M, Wang HW,
Song SY, Ye JX, Pan RL, Yao F, Wu P, et al: Myricitrin attenuates
endothelial cell apoptosis to prevent atherosclerosis: An insight
into PI3K/Akt activation and STAT3 signaling pathways. Vascul
Pharmacol. 70:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chhour P, Naha PC, O'Neill SM, Litt HI,
Reilly MP, Ferrari VA and Cormode DP: Labeling monocytes with gold
nanoparticles to track their recruitment in atherosclerosis with
computed tomography. Biomaterials. 87:93–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fuhrman B, Koren L, Volkova N, Keidar S,
Hayek T and Aviram M: Atorvastatin therapy in hypercholesterolemic
patients suppresses cellular uptake of oxidized-LDL by
differentiating monocytes. Atherosclerosis. 164:179–185. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He B, Zhou L, Shen LH, Ha LH, Pu J, Shao
Q, Wang L and Zeng JZ: RXR agonists inhibit PMA-induced
differentiation of monocytic THP-1 cells into macrophages.
Circulation. 118:S2772008.
|
|
51
|
Wang YS, Hsi E, Cheng HY, Hsu SH, Liao YC
and Juo SH: Let-7g suppresses both canonical and non-canonical
NF-κB pathways in macrophages leading to anti-atherosclerosis.
Oncotarget. 8:101026–101041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ping S, Li Y, Liu S, Zhang Z, Wang J, Zhou
Y, Liu K, Huang J, Chen D, Wang J, et al: Simultaneous increases in
proliferation and apoptosis of vascular smooth muscle cells
accelerate diabetic mouse venous atherosclerosis. PLoS One.
10:e01413752015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee GL, Wu JY, Tsai CS, Lin CY, Tsai YT,
Lin CS, Wang YF, Yet SF, Hsu YJ and Kuo CC: TLR4-activated
MAPK-IL-6 axis regulates vascular smooth muscle cell function. Int
J Mol Sci. 17:172016. View Article : Google Scholar
|
|
54
|
Liang Y, Gao H, Wang J, Wang Q, Zhao S,
Zhang J and Qiu J: Alleviative effect of grape seed
proanthocyanidin extract on small artery vascular remodeling in
spontaneous hypertensive rats via inhibition of collagen
hyperplasia. Mol Med Rep. 15:2643–2652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang L, Yaling H, Chenghui Y and Xiaoxiang
T: ASSA14-03-19 The change of cellular repressor of E1A-stimulated
genes during vascular remodelling in a mouse model of arterial
injury. Heart. 101 (Suppl 1):A14–A15. 2015. View Article : Google Scholar
|
|
56
|
Heijnen BF, Pelkmans LP, Danser AH,
Garrelds IM, Mullins JJ, De Mey JG, Struijker-Boudier HA and
Janssen BJ: Cardiac remodeling during and after renin-angiotensin
system stimulation in Cyp1a1-Ren2 transgenic rats. J Renin
Angiotensin Aldosterone Syst. 15:69–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bodle JD, Feldmann E, Swartz RH, Rumboldt
Z, Brown T and Turan TN: High-resolution magnetic resonance
imaging: An emerging tool for evaluating intracranial arterial
disease. Stroke. 44:287–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lim TT, Liang DH, Botas J, Schroeder JS,
Oesterle SN and Yeung AC: Role of compensatory enlargement and
shrinkage in transplant coronary artery disease. Serial
intravascular ultrasound study. Circulation. 95:855–859. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hecht HS, Achenbach S, Kondo T and Narula
J: High-Risk Plaque Features on Coronary CT Angiography. JACC
Cardiovasc Imaging. 8:1336–1339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Madrigal-Matute J, López-Franco O,
Blanco-Colio LM, Muñoz-García B, Ramos-Mozo P, Ortega L, Egido J
and Martín-Ventura JL: Heat shock protein 90 inhibitors attenuate
inflammatory responses in atherosclerosis. Cardiovasc Res.
86:330–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang M, Deng C, Wu D, Zhong Z, Lv X, Huang
Z, Lian N, Liu K and Zhang Q: The role of mononuclear cell tissue
factor and inflammatory cytokines in patients with chronic
thromboembolic pulmonary hypertension. J Thromb Thrombolysis.
42:38–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Torisu H, Ono M, Kiryu H, Furue M, Ohmoto
Y, Nakayama J, Nishioka Y, Sone S and Kuwano M: Macrophage
infiltration correlates with tumor stage and angiogenesis in human
malignant melanoma: Possible involvement of TNFalpha and IL-1α. Int
J Cancer. 85:182–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tay C, Liu YH, Hosseini H, Kanellakis P,
Cao A, Peter K, Tipping P, Bobik A, Toh BH and Kyaw T: B
cell-specific depletion of TNFα inhibits atherosclerosis
development and plaque vulnerability to rupture by reducing cell
death and inflammation. Cardiovasc Res. 111:385–397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Voloshyna I, Seshadri S, Anwar K,
Littlefield MJ, Belilos E, Carsons SE and Reiss AB: Infliximab
reverses suppression of cholesterol efflux proteins by TNF-α: A
possible mechanism for modulation of atherogenesis. BioMed Res Int.
2014:3126472014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gandhirajan RK, Staib PA, Minke K, Gehrke
I, Plickert G, Schlösser A, Schmitt EK, Hallek M and Kreuzer KA:
Small molecule inhibitors of Wnt/β-catenin/lef-1 signaling induces
apoptosis in chronic lymphocytic leukemia cells in vitro and in
vivo. Neoplasia. 12:326–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Z, Ma N, Zheng Y and Zhang L:
Association of serum immunoglobulin-G to Porphyromonas gingivalis
with acute cerebral infarction in the Chinese population. J Indian
Soc Periodontol. 19:628–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
He X, Li DR, Cui C and Wen LJ: Clinical
significance of serum MCP-1 and VE-cadherin levels in patients with
acute cerebral infarction. Eur Rev Med Pharmacol Sci. 21:804–808.
2017.PubMed/NCBI
|
|
68
|
Baeten JT and Lilly B: Differential
Regulation of NOTCH2 and NOTCH3 Contribute to Their Unique
Functions in Vascular Smooth Muscle Cells. J Biol Chem.
290:16226–16237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Baeten JT and Lilly B: Notch Signaling in
Vascular Smooth Muscle Cells. Adv Pharmacol. 78:351–382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sweeney C, Morrow D, Birney YA, Coyle S,
Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM and Cahill
PA: Notch 1 and 3 receptor signaling modulates vascular smooth
muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk
dependent pathway. FASEB J. 18:1421–1423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhao-Jun L, Yurong T, Beecham GW, et al:
Notch activation induces endothelial cell senescence and
pro-inflammatory response: Implication of Notch signaling in
atherosclerosis. J Vasc Surg. 53:81S–82S. 2015.
|