Introduction
Gestational diabetes mellitus (GDM) refers to
glucose intolerance with first onset and diagnosis during
pregnancy, and is the most common perinatal complication (1). The global prevalence of GDM is
expected to increase annually, particularly in Asia (2), possibly due to the observed increase
in maternal age and obesity in this continent (3). GDM usually resolves after childbirth,
but it is associated with an increased risk of prenatal, perinatal
and postnatal adverse events (4).
If blood glucose is poorly controlled, GDM may induce
hyperglycemia, which affects both the mother and fetus (4). The short-term adverse consequences of
hyperglycemia include infection, pre-eclampsia and hypertension for
the mother, and birth trauma due to macrosomia for the fetus
(5). GDM also has long-term health
effects (6). For the mother, the
risk of GDM recurrence is increased by 35–50% in subsequent
pregnancies, and 26–70% of pregnant women with GDM develop type 2
diabetes mellitus within 10–15 years following delivery (5). For the children of mothers with GDM,
the risk of developing obesity and type 2 diabetes mellitus
increase throughout their lifespan (7), and those born with macrosomia are at
an increased risk of cardiovascular disease and leukemia in the
future (4,8). However, even if the control of blood
glucose level of pregnant women with GDM is satisfactory, the
pregnancy outcome may not significantly improved (9). The specific reasons and underlying
mechanism remain elusive.
The mother and fetus are connected by the placenta.
The placenta is an appendage of the fetus that has major endocrine
and transport functions (10). It
serves a key role in the growth and development of the fetus, and
can synthesize numerous hormones, cytokines and transporters
(11). Aquaporin 3 (AQP3) is a
subtype of the AQP family, whose functions include solute transport
and signal transduction (12).
AQP3 is also expressed in the placenta and may transport water and
glycerol to the fetal circulation. It may also serve an important
role in fetal growth and development, and its expression level may
be affected by the maternal environment. Hydramnios is a common
complication of pregnancy in women with GDM (13). AQP participates in the regulation
of amniotic fluid balance, and the AQP level in the placenta is
positively correlated with amniotic fluid volume in pregnant women
with abnormal glucose metabolism (14). However, for the majority of
pregnant women with GDM with a normal amniotic fluid index (AFI),
it is unclear whether the expression of placental AQP3 gene is
altered, and whether the AQP3 level is associated with AFI. In
addition to abnormal glucose metabolism, pregnant women with GDM
usually have dysregulated lipid metabolism (15). The AQP3 protein also transports
glycerol, which is involved in lipid metabolism (16). However, there are few reports on
whether the expression of AQP3 in the placenta is altered, and
whether this is associated with GDM.
Adiponectin (APN) is an adipokine secreted by
adipose tissue and has insulin-sensitizing, anti-atherosclerotic
and anti-inflammatory properties (17). In some cases, APN can also reduce
body weight, which is associated with maintaining body metabolism
and energy balance (18).
Decreases in APN levels serve an important role in
obesity-associated diseases, including insulin resistance/diabetes
and cardiovascular diseases (19).
APN in the umbilical cord blood also serves a key role in
regulating fetal growth, development and fat reserves, and is an
important index for predicting the pregnancy outcome (20). For pregnant women with GDM, even if
the blood glucose is well controlled, the serum APN level is
downregulated, and the insulin resistance is increased compared
with that of pregnant women with normal glucose tolerance (NGT)
(21). However, there are few
reports on how APN changes in the umbilical cord blood.
In the present study, the expression of AQP3 in the
placenta was detected by immunohistochemistry, reverse
transcription-quantitative PCR (RT-qPCR) and western blotting, and
the APN level in the umbilical artery blood was determined by
ELISA. The associations of AQP3 in the placenta and APN in the
umbilical artery blood with GDM and pregnancy outcome were further
discussed. The aim was to provide a reference for perinatal health
care.
Materials and methods
Patients
The present study was conducted at the North China
University of Science and Technology Affiliated Hospital, between
November 2017 and October 2018, and performed in accordance with
the ethical guidelines of the Declaration of Helsinki for
experiments involving human subjects. The present study was
approved by the ethics committee of the North China University of
Science and Technology Affiliated Hospital. Informed consent was
obtained from all patients. A total of 60 pregnant women with GDM
and 60 pregnant women with NGT were recruited. The inclusion
criteria for pregnant women with GDM were as follows: A diagnosis
of GDM according to oral glucose tolerance test (22) or by documented clinical diagnosis
in the medical records; aged 20–40 years; single pregnancy; simple
control of blood glucose through diet and exercise; no recent
history of taking drugs affecting blood lipid levels; normal
pregnancy screenings, such as Down's screening and four-dimensional
color Doppler ultrasound; complete clinical data; and the provision
of informed consent. The exclusion criteria were: Unsatisfactory
blood glucose control; and patients with pregnancy complications
such as thyroid disease, pregnancy-induced hypertension, or severe
heart, liver, kidney and other diseases. The criteria for
satisfactory blood glucose control during pregnancy were as
follows: Blood glucose should be 5.3–6.7 mmol/l prior and
subsequent to meals; blood glucose at night should not be <3.3
mmol/l; and glycosylated hemoglobin during pregnancy should not be
<5.5% (23). The inclusion
criteria for the patients with NGT were as: Aged 20–40 years;
single pregnancy; no recent history of taking drugs affecting blood
lipid levels; normal pregnancy screenings, such as Down's screening
and four-dimensional color Doppler ultrasound; complete clinical
data; and provision of informed consent. The exclusion criteria
were: Diagnosis of pre-pregnancy diabetes (including types 1 and
2); control of blood glucose using drugs; and patients with
pregnancy complications such as thyroid disease, pregnancy-induced
hypertension, and severe heart, liver, kidney and other
diseases.
There were four pregnancy outcomes in the present
study: i) Macrosomia, defined as fetal weight ≥4,000 g; ii) fetal
distress, defined as type III electronic fetal heart rate
monitoring graphics, meconium contamination of the amniotic fluid,
or abnormal fetal movement; iii) neonatal asphyxia, defined as (a)
5 min Apgar score <7 points and no effective breathing
established; (b) umbilical artery blood gas pH <7.15; (c)
exclusion of other causes of low Apgar score; (d) the presence of
prenatal high-risk factors that may lead to asphyxia (a, b and c
were the necessary conditions, d was the reference condition); and
iv) cesarean section, defined as an obstetric surgical procedure
for abnormal delivery, indicating scarred uterus, cephalopelvic
disproportion or fetal distress. Any cases of macrosomia, fetal
distress, neonatal asphyxia or cesarean section were considered as
abnormal outcomes; otherwise, the outcomes were considered as
normal.
Blood, tissue samples and biochemical
analyses
Placental tissue collection
Immediately following delivery, 3 placental tissue
samples ~1 cm3 were removed from the central
non-calcified area of the placenta; after washing with normal
saline, 1 of the samples was fixed with 10% neutral formalin at
room temperature for 1 day for immunohistochemical study, and the
remaining 2 samples were placed in a freezing tube and stored in a
freezer at −80°C for western blotting and RT-qPCR analyses.
Umbilical artery blood collection
Within 1 min after delivery, 3 ml umbilical artery
blood was extracted by syringe and injected into a
coagulation-promoting tube; serum was then separated by
centrifugation (1,000 × g at 4°C for 15 min), sealed and stored in
the refrigerator at −80°C for detection of APN.
Biochemical analyses
Routine blood tests, including pre-delivery fasting
plasma glucose (FPG), glycosylated hemoglobin (HbA1c) and serum
triglyceride (TG) were completed by the Laboratory Department of
North China University of Science and Technology Affiliated
Hospital. Clinical examination data, including AFI and neonatal
weight, were obtained in the Department of Obstetrics and
Gynecology. The APN level in the umbilical artery serum was
detected using commercial ELISA kits (cat. no. 20180906451;
Shanghai YL Biotech Co., Ltd.) according to the manufacturer's
protocol.
Immunohistochemistry analysis
Formalin-fixed (at 4°C for 24 h) placental tissue
samples were dehydrated through graded alcohols (50, 60, 80, 90, 95
and 100% alcohol), embedded in paraffin, and cut into sections
(3×3×3 mm). After routinely dewaxing and hydration (100, 95, 85 and
75% alcohol), antigen retrieval was performed in EDTA antigen
retrieval solution (Shanghai Guangrui Biotechnology Co., Ltd.;
http://www.shgrsw.com/.). Subsequently,
endogenous peroxidase activity was blocked by incubating the slides
with 3% H2O2 for 15 min at room temperature.
The sections were incubated with rabbit anti-human AQP3 antibody
(cat. no. AF5222, Affinity BioReagents, Inc.; 1:200) at 4°C
overnight and 37°C for 40 min, and followed by incubation with goat
anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary
antibodies (cat. no. ZB-2301; 1:20; OriGene Technologies, Inc.) at
37°C for 30 min. The color developing was performed with DAB color
developer solution (OriGene Technologies, Inc.) and the sections
were stained with hematoxylin at room temperature for 30 sec.
Immunohistochemical staining images (magnification, ×400) were
obtained by a Micro Publisher 5.0 microscope (Roper Industries).
Image-Pro-Plus 6.0 software (Media Cybernetics, Inc.) was used to
analyze the results of immunohistochemistry and calculate the
integral optical density. A positive result for the AQP3 protein
staining was the presence of brown granules in the cell membrane
and cytoplasm.
Western blotting
Total cytoplasmic protein was extracted from
placental tissue using RIPA lysis buffer (BestBio Co., Ltd.;
http://www.bestbio.com.cn/) and
quantified using a BCA Protein Assay kit (Shanghai Guangrui
Biotechnology Co., Ltd.). Protein (20 µl/lane) was denatured and
resolved by 8% SDS-PAGE (Shanghai Huyu Biotechnology Co., Ltd.;
http://www.shhymall.com/.), and transferred at
240 mA for 2 h onto a PVDF membrane (Bio-Rad Laboratories, Inc.).
Non-specific regions of the PVDF membrane were blocked by
incubating the membrane with 5% skimmed milk at room temperature
for 2 h. Then, the PVDF membrane was probed with primary rabbit
anti-human AQP3 antibody (cat. no. sc518001; Santa Cruz
Biotechnology Inc.; 1:1,000) or rabbit anti-human β-actin antibody
(cat. no. sc58675; Santa Cruz Biotechnology Inc.; 1:5,000)
overnight at 4°C, followed by incubation with goat anti-rabbit IgG
HRP-conjugated secondary antibodies (cat. no. 111-035-003; Jackson
ImmunoResearch Laboratories, Inc.; 1:2,000) for 2 h at room
temperature. Finally, detection of protein signals was performed
using an ECL Chemiluminescence kit (GrBio), and observed by Image
Lab5.0 software (Bio-Rad Laboratories, Inc.). To obtain the
relative expression of AQP3, the AQP3 signal was normalized to the
β-actin signal.
RT-qPCR
Total RNA was extracted form placental tissue using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reversely transcribed to cDNA by the M5 First Strand
cDNA Synthesis kit (Mei5bio; Beijing Jumei Biotechnology Co., Ltd.)
according to the manufacturer's protocol. Then, qPCR was performed
by the SYBR Green Realtime PCR Mix kit (Mei5bio; Beijing Jumei
Biotechnology Co., Ltd.). β-actin was selected as an internal
reference. The following primers were used: AQP3 forward:
5′-GAAGTCAGGTCATAAGTT-3′ and reverse: 5′-GAAGTCAGGTCATAAGTT-3′;
β-actin forward: 5′-GAAGTCAGGTCATAAGTT-3′ and reverse:
5′-ACTCTTCCAGCCTTCCTT-3′. The PCR conditions were as follows:
Denaturation of the DNA at 95°C for 10 min, followed by denaturing
at 95°C for 15 sec, annealing at 65°C for 15 sec, and extension at
72°C for 60 sec for 40 cycles. The value of 2−ΔΔCq
(ΔCq=Cq value of APQ3 - Cq value of β-actin; ΔΔCq=Cq value of GDM
group - Cq value of NGT group) was used as the relative expression
of AQP3 mRNA and calculated according to a previous report
(24).
Statistical analysis
Statistical analyses were performed using SPSS 23.0
statistical software (IBM Corp.). A Mann-Whitney U test was used to
analyze the immunohistochemistry results. Quantitative data are
presented as mean ± standard deviation, and the results between two
groups were compared with Student's t-test. Classified count data
were analyzed using the χ2 test. The associations of
AQP3 and APN with GDM and pregnancy outcome were analyzed by
logistic regression analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical data analysis
As demonstrated in Table I, there were no significant
differences in age, pre-pregnancy body mass index, gestational
week, number of pregnancies, number of births, pre-delivery FPG,
HbA1c and AFI between the GDM and NGT groups (P>0.05). The
gestational weight gain, TG and neonatal weight in the GDM group
were increased compared with those in the NGT group, and the
differences were statistically significant (P<0.05).
 | Table I.Clinical data analysis results of the
two groups. |
Table I.
Clinical data analysis results of the
two groups.
|
Characteristics | GDM (n=60) | NGT (n=60) | P-value |
|---|
| Age, years | 30.57±4.83 | 29.85±4.07 | 0.38 |
| BMI prior to
pregnancy | 23.23±4.34 | 22.22±3.88 | 0.18 |
| Gestational week,
weeks | 39.04±0.88 | 39.11±0.90 | 0.64 |
| Number of
pregnancies, n | 2.75±2.00 | 2.17±1.42 | 0.07 |
| Number of births,
n | 0.63±0.71 | 0.43±0.50 | 0.08 |
| Gestational weight
gain, kg | 17.60±4.97 | 14.66±4.60 | 0.00 |
| FPG, mmol/l | 4.50±0.64 | 4.35±0.60 | 0.18 |
| HbA1c, % | 5.04±0.41 | 4.90±0.44 | 0.08 |
| TG, mmol/l | 3.85±1.64 | 2.93±1.15 | 0.00 |
| AFI, cm | 6.71±1.67 | 6.83±2.04 | 0.88 |
| Neonatal weight,
g |
3,619.17±384.78 |
3,434.00±493.37 | 0.02 |
As shown in Table
II, the pregnancy outcomes of cesarean section, macrosomia,
fetal distress and neonatal asphyxia were compared between the GDM
and NGT groups. The incidence of macrosomia in the GDM group was
significantly increased compared with that in the NGT group
(P<0.05), but there was no significant difference in the
incidence of cesarean section, fetal distress and neonatal
asphyxia. This demonstrated that good control of blood glucose
levels in pregnant women with GDM may not singularly improve
pregnancy outcomes.
 | Table II.Comparison of pregnancy outcomes
between the two groups |
Table II.
Comparison of pregnancy outcomes
between the two groups
|
| n |
|
|
|---|
|
|
|
|
|
|---|
| Outcome | Total | GDM | NGT | χ2 | P-value |
|---|
| Cesarean
section | 63 | 34 | 29 | 0.84 | 0.36 |
| Macrosomia | 15 | 12 | 3 | 4.88 | 0.03 |
| Fetal distress | 7 | 4 | 3 | 0.00 | 1.00 |
| Neonatal
asphyxia | 3 | 2 | 1 | 0.00 | 1.00 |
AQP3 and APN levels
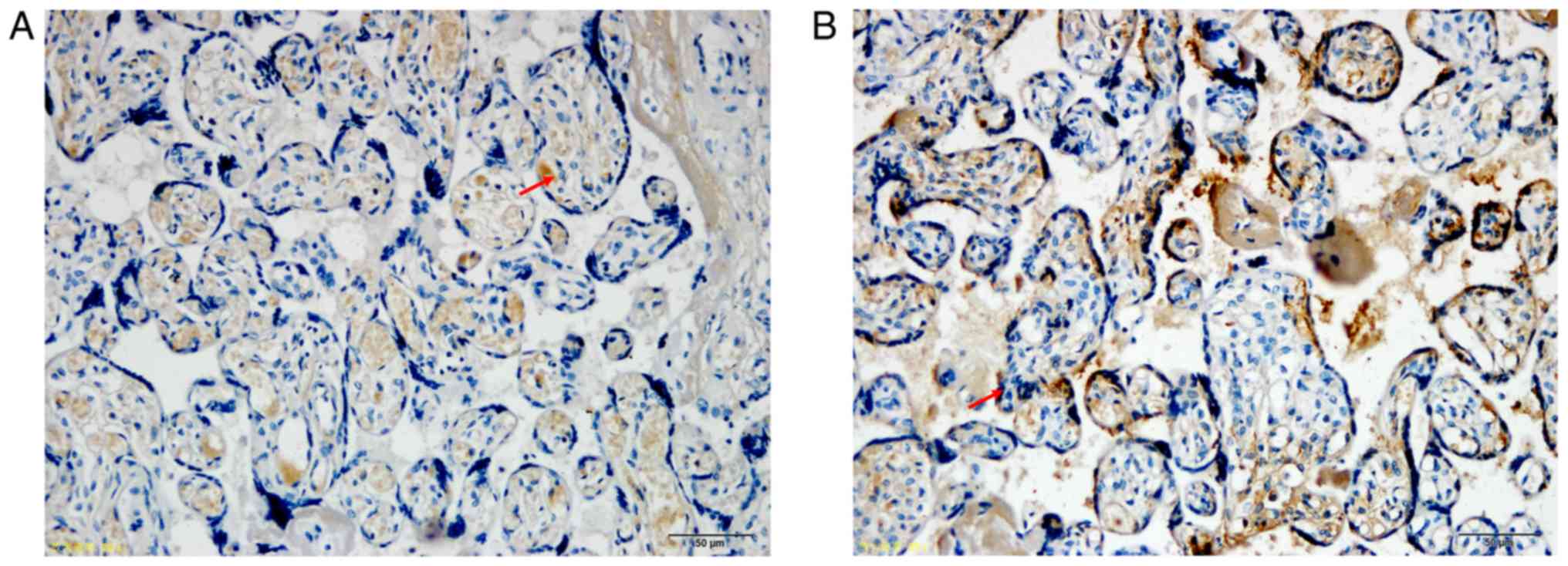
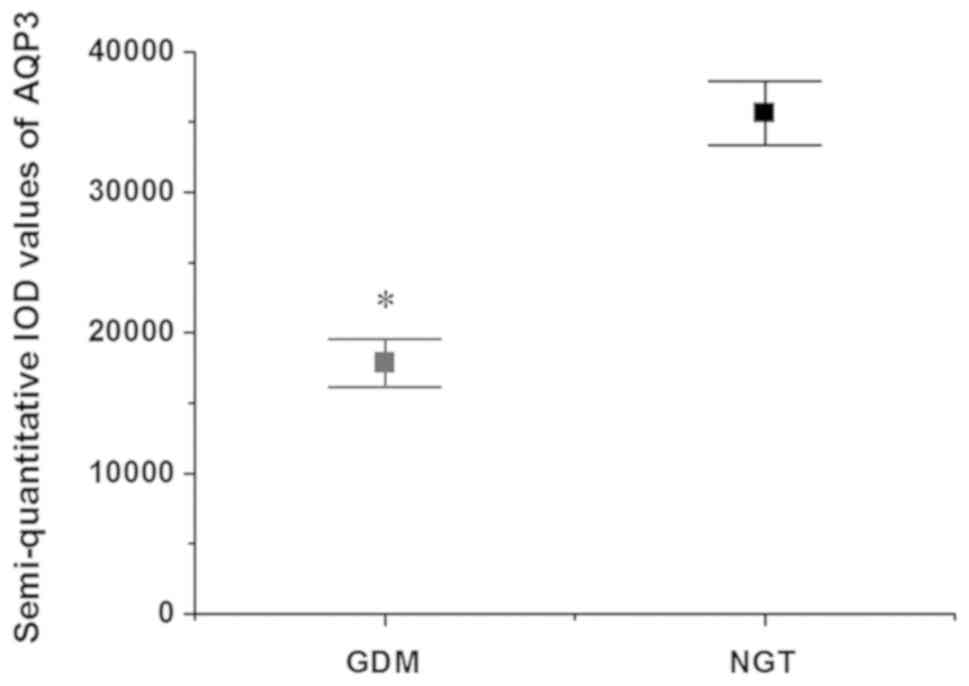
As shown in Fig. 1,
the AQP3 protein, which was expressed in the cytoplasm and membrane
of placental trophoblasts, was detected as brown and yellow
granules, and its expression was weakly positive in the GDM group
and positive in the NGT group. According to the semi-quantitative
results (Fig. 2), the difference
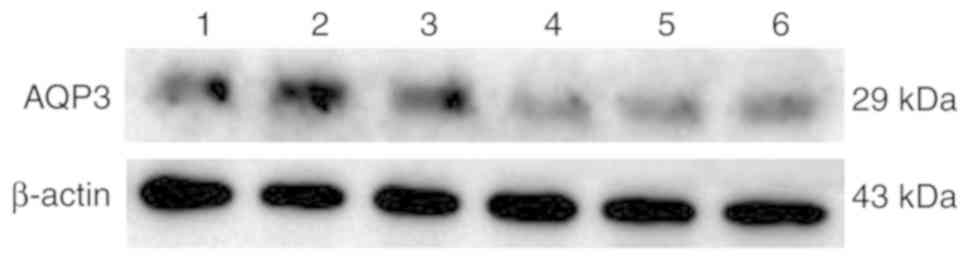
was statistically significant (P<0.05). According to the western
blotting results (Fig. 3), the
expression of the AQP3 protein in placental tissues of the GDM
group was also significantly decreased compared with that in the
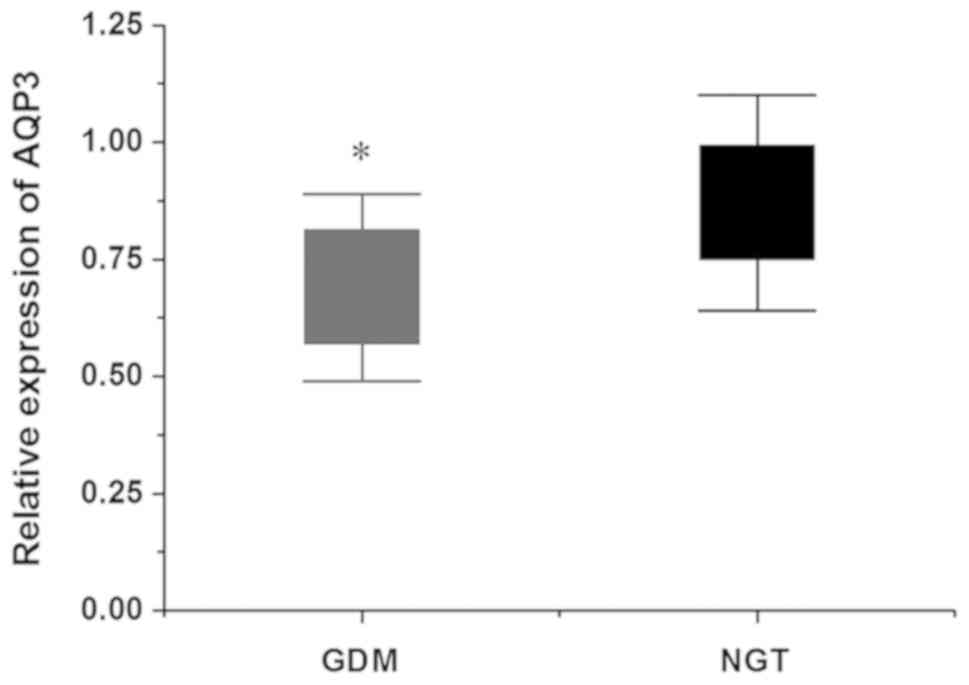
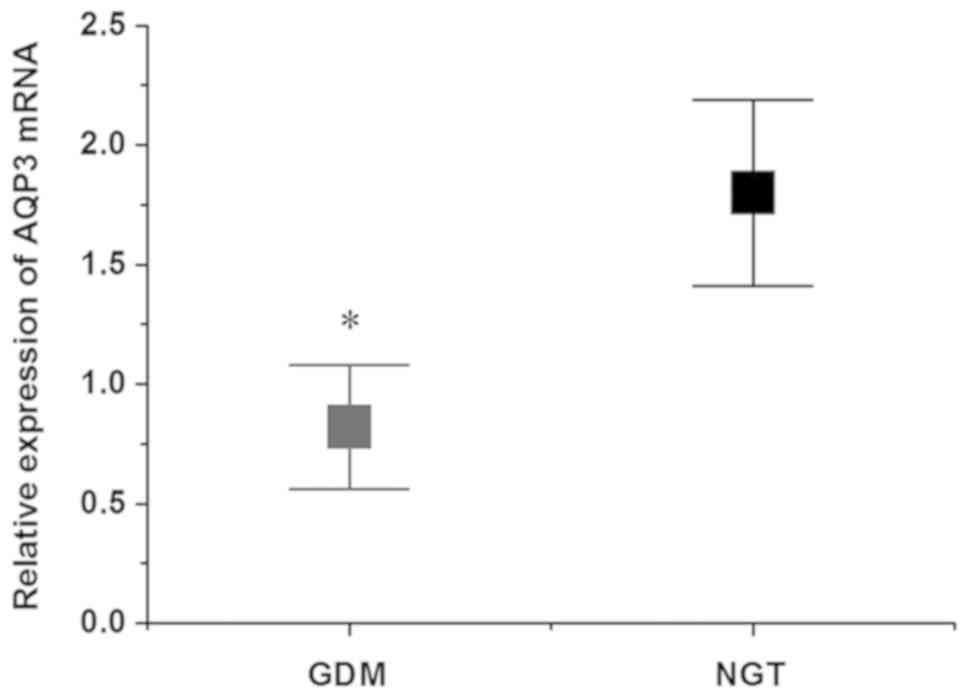
NGT group (Fig. 4). The RT-qPCR
results demonstrated that the relative expression of AQP3 mRNA in
placental tissues of the GDM group was decreased compared with that
of the NGT group, and the difference was statistically significant
(P<0.05; Fig. 5). The results
of immunohistochemistry, western blotting and RT-qPCR were
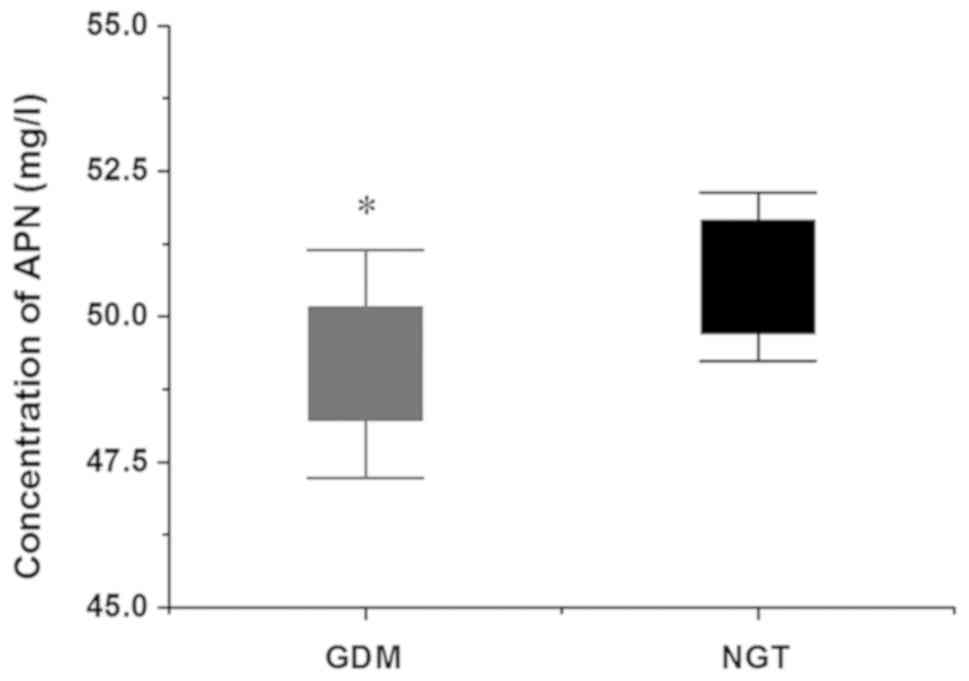
consistent. The APN level in the umbilical artery serum was
detected by ELISA. As shown in Fig.
6, the APN level in the GDM group was decreased compared with
that in the NGT group, and the difference was statistically
significant (P<0.05).
Association analysis
As shown in Table
III, the mean relative expression levels of AQP3 in the
placenta (0.78) and the mean APN level in the umbilical artery
serum (49.93 mg/l) were used as the threshold between high and low
levels. Univariate logistic regression analysis revealed that the
expression levels of AQP3 and APN were associated with GDM. The
risk of GDM in pregnant women with low expression levels of AQP3
and APN were increased 5.29- and 2.98-fold compared with those in
the women with high levels of AQP3 and APN, respectively.
 | Table III.Univariate logistic regression
analysis of factors affecting the development of GDM in
patients. |
Table III.
Univariate logistic regression
analysis of factors affecting the development of GDM in
patients.
| A, AQP3 |
|---|
|
|---|
| Patient group | n | High level
(≥0.78a) | Low level
(<0.78) | χ2 | P-value | OR | 95% CI |
|---|
| GDM | 60 | 14 | 46 | 18.04 | 0.00 | 5.29 | 2.39–11.68 |
| NGT | 60 | 37 | 23 |
|
|
|
|
|
| B, APN,
mg/l |
|
| Patient
group | n | High level
(≥49.93) | Low level
(<49.93) |
χ2 | P-value | OR | 95% CI |
|---|
|
|---|
| GDM | 60 | 22 | 38 | 8.53 | 0.00 | 2.98 | 1.42–6.27 |
| NGT | 60 | 38 | 22 |
|
|
|
|
To investigate the risk factors of GDM, the
indicators that differed significantly between the GDM and NGT
groups, including AQP3, APN, TG, gestational weight gain and
neonatal weight, were selected as independent variables, and their
means were used as the threshold between high and low levels. As
shown in Table IV, multivariate
logistic regression analysis indicated that AQP3, APN and TG were
statistically significant different (P<0.05), and the odds
ratios were 5.00, 2.98 and 3.48, respectively. Their corresponding
95% confidence intervals were 2.13–11.75, 1.29–6.89 and 1.50–8.10,
respectively.
 | Table IV.Multivariate logistic regression
analysis of factors affecting the development of GDM in
patients. |
Table IV.
Multivariate logistic regression
analysis of factors affecting the development of GDM in
patients.
| Factor | B | SE |
Walsχ2 | P-value | OR | 95% CI |
|---|
| AQP3 | 1.61 | 0.44 | 13.64 | <0.01 | 5.00 | 2.13–11.75 |
| APN | 1.09 | 0.43 | 6.51 | 0.01 | 2.98 | 1.29–6.89 |
| TG | 1.25 | 0.43 | 8.41 | <0.01 | 3.48 | 1.50–8.10 |
As shown in Table
V, univariate logistic regression analysis demonstrated that
the AQP3 level was significantly associated with pregnancy outcome;
the risk of abnormal pregnancy outcome in the GDM and NGT groups
with low levels of AQP3 expression was increased 5.50- and
12.00-fold, respectively, compared with that in the respective
groups with high AQP3 expression. Similarly, the APN level also had
a significant effect on pregnancy outcomes; the risk of abnormal
pregnancy outcome in the GDM and NGT groups with low levels of APN
was increased 3.40- and 22.67-fold, respectively, compared with
that in the respective groups with high APN expression. The AQP3
and APN levels in the NGT group were more closely associated with
pregnancy outcome compared with those in the GDM group, possibly
due to other factors affecting pregnancy outcome in the GDM
group.
 | Table V.Univariate logistic regression
analysis of factors affecting pregnancy outcome. |
Table V.
Univariate logistic regression
analysis of factors affecting pregnancy outcome.
| A, AQP3 |
|---|
|
|---|
| Patient group | Pregnancy
outcome | n | High level
(≥0.78a) | Low level
(<0.78) | χ2 | P-value | OR | 95% CI |
|---|
| GDM | Normal | 24 | 2 | 22 | 3.73 | 0.04 | 5.50 | 1.11–27.37 |
|
| Abnormal | 36 | 12 | 24 |
|
|
|
|
| NGT | Normal | 20 | 5 | 15 | 17.06 | <0.01 | 12.00 | 3.35–42.93 |
|
| Abnormal | 40 | 32 | 8 |
|
|
|
|
|
|
|
| B, APN,
mg/l |
|
| Patient
group | Pregnancy
outcome | n | High level
(≥49.93) | Low level
(<49.93) |
χ2 | P-value | OR | 95% CI |
|
| GDM | Normal | 24 | 5 | 19 | 4.32 | 0.04 | 3.40 | 1.04–11.09 |
|
| Abnormal | 36 | 17 | 19 |
|
|
|
|
| NGT | Normal | 20 | 4 | 16 | 24.26 | 0.00 | 22.67 | 5.60–91.71 |
|
| Abnormal | 40 | 34 | 6 |
|
|
|
|
Multivariate logistic regression analysis was
conducted with abnormal pregnancy outcome as the dependent variable
and AQP3 and APN as independent variables. As shown in Table VI, the results indicated that both
the AQP3 and APN levels significantly affected pregnancy outcome.
The risk of abnormal pregnancy outcome in pregnant women with low
levels of AQP3 and APN expression was increased 4.64- and
5.41-fold, respectively, compared with that associated with high
levels of AQP3 and APN expression.
 | Table VI.Multivariate logistic regression
analysis of factors affecting pregnancy outcome. |
Table VI.
Multivariate logistic regression
analysis of factors affecting pregnancy outcome.
| Factor | B | SE |
Walsχ2 | P | OR | 95% CI |
|---|
| AQP3 | 1.53 | 0.51 | 9.24 | 0.00 | 4.64 | 1.72–12.46 |
| APN | 1.69 | 0.47 | 12.87 | 0.00 | 5.41 | 2.15–13.61 |
Discussion
GDM may be caused by a number of factors. For
example, in normal pregnancy, levels of insulin resistance may
approach those normally observed in patients with type 2 diabetes
mellitus and can be inhibited by excessive secretion of β cells.
However, if β cells are unable to secrete enough insulin, pregnant
women are more likely to develop GDM (25). The pathogenesis of GDM also
includes genetic factors, inflammatory factors, adipokines and
decreased expression of estrogen receptors (26,27).
Changes in lipid metabolism are a predictor of GDM. Excessive free
fatty acids can decrease the sensitivity of surrounding tissues to
insulin, cause insulin resistance, and subsequently lead to GDM
(28). Therefore, the blood lipid
levels of pregnant women with GDM are generally increased. As
maternal serum TG and free fatty acids are associated with fetal
blood lipids, fetal growth and fat quality, fatty acids and
glycerol may affect fetal growth and fat quality, which may lead to
abnormal pregnancy outcomes, including cesarean section,
macrosomia, fetal distress and neonatal asphyxia (29,30).
In the present study, there was no significant difference in FPG
and HbA1c between the GDM and NGT groups, suggesting that the blood
glucose in the GDM group was well-controlled. However, the blood TG
level of the GDM group was significantly increased compared with
that of the NGT group, and gestational weight gain and neonatal
weight values were both increased compared with those in the GDM
group. Therefore, although the blood glucose control was
satisfactory, the levels of insulin resistance and lipid metabolism
of the pregnant women with GDM did not improve, and the blood TG
level in the GDM group remained increased compared with the NTG
group. This result suggested that pregnant women with GDM cannot
alter their rates of lipid metabolism or weight gain by controlling
blood glucose alone. In addition, the incidence of macrosomia in
the GDM group was significantly increased compared with that in the
NGT group. This indicated that the pregnancy outcome of GDM was not
only associated with maternal blood glucose, but also with other
factors, such as blood TG. Although the incidence of macrosomia
increased in the GDM group, there was no significant difference in
the cesarean section rate between the two groups, which may be
associated with the larger proportion of uterine scarring in the
GDM group. The incidence of fetal distress and neonatal asphyxia
was not statistically significantly different, which may be
attributed to the small sample.
AQP is an integral membrane protein family that
includes 13 subtypes (12). The
AQP8 and AQP8 mRNA levels in GDM are associated with amniotic fluid
volume, and increase concomitantly with the increase in the
amniotic fluid volume (31,32).
In the present study, there was no significant difference in the
AFI values between the NGT and GDM groups with good blood glucose
control. However, the expression level of the AQP3 protein in the
placenta of the GDM group was significantly decreased compared with
that of the NGT group, indicating that the level of AQP in the
placenta of the GDM group with normal AFI and good blood sugar
control may still be affected. Therefore, the placental AQP level
is not associated with AFI, and blood glucose level exerts no
significant effect on AQP level.
Microarray global gene expression analysis revealed
that the GDM placenta has 66 differentially expressed genes,
involving not only AQPs, but also cell activation, immune response,
organ development and regulation of cell death (33). In the present study, the blood TG
level of the GDM group was significantly increased compared with
that of the NGT group, and the expression of AQP3 in the placenta
of the GDM group was significantly decreased compared with that in
the NGT group. The fetuses of pregnant women with GDM exhibited
macrosomia, with a high transfer rate of water and glycerol in the
placenta. As a negative feedback of fetal overgrowth, the AQP3
level in the GDM placenta was downregulated, which may decrease the
transport of maternal glycerol to the fetus through the placenta.
Accordingly, the reduction of glycerol transported to the fetus
increased the level of free glycerol in the maternal circulation,
which, in turn, increased the level of TG in the blood of pregnant
women with GDM. In addition, downregulation of AQP3 may result in
impaired intestinal barrier integrity by opening tight junction
complexes, suggesting that the effect of AQP3 may be more complex
than the simple facilitation of membrane permeability (34). Therefore, it may be inferred that
AQP3 serves an important role in glycerol diffusion and placental
lipid metabolism in GDM, and a regulatory role in fetal growth and
development.
APN, as an adipokine that regulates sugar and lipid
metabolism, is closely associated with GDM (35). As fetal growth and development are
affected by insulin and glucose metabolism, APN is also an
important factor in regulating fetal intrauterine development
(36), and the APN level in the
umbilical cord blood was identified to be negatively correlated
with neonatal weight (37). In the
present study, the APN level in the umbilical cord blood of the GDM
group was significantly decreased compared with that of the NGT
group, demonstrating that pregnant women with lower levels of APN
in the umbilical cord blood are at an increased risk of developing
GDM. The neonatal weight in the GDM group was significantly
increased compared with that in the NGT group, indicating that the
decrease in APN in the umbilical cord blood may be associated with
the increase of the fetal fat reserve in the uterus.
During pregnancy, APN can recognize its receptor,
induce protein kinase activation, increase the production of
intracellular cAMP through the protein kinase A pathway, and then
induce trophoblast differentiation through cAMP (38). As the transport, gating and
redistribution of AQP3 are regulated by phosphorylation, and the
phosphorylation of AQP3 is dependent on cAMP (39), an increase in intracellular cAMP
may promote the phosphorylation of AQP3, thereby increasing the
transport of water and glycerol through the cell membrane.
Therefore, APN in the maternal serum may act on placental
trophoblasts and increase the activity of AQP3 in placental tissues
through the cAMP-PKA pathway. In addition, APN receptor signaling
is transmitted by peroxisome proliferator activated receptor
(PPAR), and AQP3 is the target of PPAR (40). In the present study, the
downregulation of AQP3 expression in the GDM group decreased the
regulation of APN on glucose and fat metabolism. The levels of AQP3
in the placenta and of APN in the umbilical artery blood were
identified to be negatively association with GDM and pregnancy
outcomes: The decrease in AQP3 and APN levels increased the risks
of developing GDM and abnormal pregnancy outcomes. Unfortunately,
the evaluation model could not be established in the present study,
as there were numerous factors affecting pregnancy outcome, blood
lipids values and other indicators, and there were only a few
indicators examined. Thus, the authors plan to establish an
evaluation model and prediction model that could evaluate the
outcome of pregnancy in future studies.
In conclusion, the level of AQP3 expression in the
placenta was identified to be associated with GDM. The decrease in
AQP3 expression levels in the placenta increased the risk of
developing GDM. It was inferred that the expression of AQP3 in the
placenta was associated with lipid metabolism at the placental
interface in patients with GDM. The specific mechanism of AQP3
regulation of fetal growth and development requires further
investigation. The decrease in the APN level in the umbilical
artery blood was also identified to be associated with GDM and was
an important factor in regulating fetal intrauterine development.
In addition, low levels of AQP3 in the placenta and APN in the
umbilical cord blood increased the risk of abnormal pregnancy
outcomes, such as cesarean section, macrosomia, fetal distress and
neonatal asphyxia. Understanding these risk factors of GDM may be
helpful to identify high-risk pregnant women, formulate effective
preventive measures, and provide a basis for the effective
management of GDM. The limitations of the present study included a
lack of analysis of the maternal pregnancy outcome and
establishment of an evaluation model, which will be included in
future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ and YC designed the study. CZ, YL and JW
performed the experiments. CL analyzed the data. CZ and YC wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the North China University of Science and Technology
Affiliated Hospital. Informed consent was obtained from all
patients.
Patient consent for publication
All the individuals who participated in the study
provided written informed consent for the publication of any
associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gilbert L, Gross J, Lanzi S, Quansah DY,
Puder J and Horsch A: How diet, physical activity and psychosocial
well-being interact in women with gestational diabetes mellitus: An
integrative review. BMC Pregnancy Childbirth. 19:602019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee KW, Ching SM, Ramachandran V, Yee A,
Hoo FK, Chia YC, Wan Sulaiman WA, Suppiah S, Mohamed MH and Veettil
SK: Prevalence and risk factors of gestational diabetes mellitus in
Asia: A systematic review and meta-analysis. BMC Pregnancy
Childbirth. 18:4942018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laine MK, Kautiainen H, Gissler M, Raina
M, Aahos I, Järvinen K, Pennanen P and Eriksson JG: Gestational
diabetes in primiparous women-impact of age and adiposity: A
register-based cohort study. Acta Obstet Gynecol Scand. 97:187–194.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harrison AL, Shields N, Taylor NF and
Frawley HC: Exercise improves glycaemic control in women diagnosed
with gestational diabetes mellitus: A systematic review. J
Physiother. 62:188–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McIntyre HD, Catalano P, Zhang C, Desoye
G, Mathiesen ER and Damm P: Gestational diabetes mellitus. Nat Rev
Dis Primers. 5:472019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zamanfar D, Farhadi R and Shahbaznejad L:
Neonate of diabetic mother, pathogenesis and complications. J Clin
Excell. 2:90–103. 2014.(In Persian).
|
|
7
|
Scholtens DM, Kuang A, Lowe LP, Hamilton
J, Linder B, Lawrence JM, Lebenthal Y, McCance D, Nodzenski M,
Talbot O, et al: Hyperglycemia and adverse pregnancy outcome
follow-up study (hapo fus): Maternal gestational diabetes mellitus
and childhood glucose metabolism. Diabetes Care. 42:381–392. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walsh JM and McAuliffe FM: Prediction and
prevention of the macrosomic fetus. Eur J Obstet Gynecol Reprod
Biol. 162:125–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fantinelli S, Marchetti D, Verrocchio MC,
Franzago M, Fulcheri M and Vitacolonna E: Assessment of
psychological dimensions in telemedicine care for gestational
diabetes mellitus: A systematic review of qualitative and
quantitative studies. Front Psychol. 10:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burton GJ and Jauniaux E: What is the
placenta? Am J Obstet Gynecol. 213:S6.e1, S6–8. 2015. View Article : Google Scholar
|
|
11
|
Reijnders IF, Mulders AGMGJ and Koster
MPH: Placental development and function in women with a history of
placenta-related complications: A systematic review. Acta Obstet
Gynecol Scand. 97:248–257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marlar S, Jensen HH, Login FH and Nejsum
LN: Aquaporin-3 in Cancer. Int J Mol Sci. 18(pii): E21062017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frank Wolf M, Peleg D, Stahl-Rosenzweig T,
Kurzweil Y and Yogev Y: Isolated polyhydramnios in the third
trimester: Is a gestational diabetes evaluation of value? Gynecol
Endocrinol. 33:849–852. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beall MH, Wang S, Yang B, Chaudhri N,
Amidi F and Ross MG: Placental and membrane aquaporin water
channels: Correlation with amniotic fluid volume and composition.
Placenta. 28:421–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korkmazer E and Solak N: Correlation
between inflammatory markers and insulin resistance in pregnancy. J
Obstet Gynaecol. 35:142–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Li B, Zhang L, Chen L, Sun G, Zhang
Q, Wang J, Zhi X, Wang L, Xu Z and Xu H: The proliferation
impairment induced by AQP3 deficiency is the result of glycerol
uptake and metabolism inhibition in gastric cancer cells. Tumor
Biol. 37:9169–9179. 2016. View Article : Google Scholar
|
|
17
|
Kawwass JF, Summer R and Kallen CB: Direct
effects of leptin and adiponectin on peripheral reproductive
tissues: A critical review. Mol Hum Reprod. 21:617–632. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ott R, Stupin JH, Melchior K, Schellong K,
Ziska T, Dudenhausen JW, Henrich W, Rancourt RC and Plagemann A:
Alterations of adiponectin gene expression and DNA methylation in
adipose tissues and blood cells are associated with gestational
diabetes and neonatal outcome. Clin Epigenetics. 10:1312018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chakraborti CK: Role of adiponectin and
some other factors linking type 2 diabetes mellitus and obesity.
World J Diabetes. 6:1296–1308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santana MG, de Velasco PC, Oliveira ORC,
Santo RE, Spreafico F, Almeida LB, Sardinha FLC and
Tavares-do-Carmo MDG: Adiponectin, insulin and leptin levels in the
cord plasma of the neonates from adolescent and adult mothers and
their relationship with anthropometric parameters and fetal
sex-gender. J Perinatol. 38:489–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Q, Xiong R, Wang L, Cui J, Shi L, Liu Y
and Luo B: Luo, Associations of dietary habits, physical activity
and cognitive views with gestational diabetes mellitus among
Chinese women. Public Health Nutr. 17:1850–1857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng H, Zhu WW, Yang HX, Wei YM, Wang C,
Su RN, Hod M and Hadar E: Relationship between oral glucose
tolerance test characteristics and adverse pregnancy outcomes among
women with gestational diabetes mellitus. Chin Med J (Engl).
130:1012–1018. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Casey BM, Duryea EL, Abbassi-Ghanavati M,
Tudela CM, Shivvers SA, McIntire DD and Leveno KJ: Glyburide in
women with mild gestational diabetes. Obstet Gynecol. 126:303–309.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative CT method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Correa PJ, Vargas JF, Sen S and lllanes
SE: Prediction of gestational diabetes early in pregnancy:
Targeting the long-term complications. Gynecol Obstet Inves.
77:145–149. 2014. View Article : Google Scholar
|
|
26
|
Lowe LP, Metzger BE, Lowe WL Jr, Dyer AR,
McDade TW and McIntyre HD; HAPO Study Cooperative Research Group, :
Inflammatory mediators and glucose in pregnancy: Results from a
subset of the hyperglycemia and adverse pregnancy outcome (HAPO)
study. J Clin Endocr Metab. 95:5427–5434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fasshauer M, Blüher M and Stumvoll M:
Adipokines in gestational diabetes. Lancet Diabetes Endo.
2:488–499. 2014. View Article : Google Scholar
|
|
28
|
Lingying K and Huixia Y: Lipid metabolism
and transfer across placenta in women with gestational mellitus.
Chin J Obstet Gynecol Pediatr. 9:5–8. 2013.
|
|
29
|
Herrera E and Ortega-Senovilla H: Lipid
metabolism during pregnancy and its implications for fetal growth.
Curr Pharm Biotechno. 15:24–31. 2014. View Article : Google Scholar
|
|
30
|
Gorgal R, Gonçalves E, Barros M, Namora G,
Magalhães A, Rodrigues T and Montenegro N: Gestational diabetes
mellitus: A risk factor for non-elective cesarean section. J Obstet
Gynaecol Re. 38:154–159. 2012. View Article : Google Scholar
|
|
31
|
Zhu X, Jiang S, Hu Y, Zheng X, Zou S, Wang
Y and Zhu X: The expression of aquaporin 8 and aquaporin 9 in fetal
membranes and placenta in term pregnancies complicated by
idiopathic polyhydramnios. Early Hum Dev. 86:657–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang SS, Zhu XJ, Ding SD, Wang JJ, Jiang
LL, Jiang WX and Zhu XQ: Expression and localization of aquaporins
8 and 9 in term placenta with oligohydramnios. Reprod Sci.
19:1276–1284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Enquobahrie DA, Williams MA, Qiu C, Meller
M and Sorensen TK: Global placental gene expression in gestational
diabetes mellitus. Am J Obstet Gynecol. 200:206.e1–206.e13. 2009.
View Article : Google Scholar
|
|
34
|
Zhi X, Tao J, Li Z, Jiang B, Feng J, Yang
L, Xu H and Xu Z: MiR-874 promotes intestinal barrier dysfunction
through targeting AQP3 following intestinal ischemic injury. FEBS
Lett. 588:757–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang LT, Wu SL, Liao X, Ma SJ and Tan HZ:
Adiponectin gene polymorphisms and risk of gestational diabetes
mellitus: A meta-analysis. World J Clin Cases. 7:572–584. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thagaard IN, Hedley PL, Holm JC, Lange T,
Larsen T, Krebs L and Christiansen M: Leptin and adiponectin as
markers for preeclampsia in obese pregnant women, a cohort study.
Pregnancy Hypertens. 15:78–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altinova AE, Toruner F, Bozkurt N, Bukan
N, Karakoc A, Yetkin I, Ayvaz G, Cakir N and Arslan M: Circulating
concentrations of adiponectin and tumor necrosis factor-α in
gestational diabetes mellitus. Gynecol Endocrinol. 23:161–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benaitreau D, Dos Santos E, Leneveu MC, De
Mazancourt P, Pecquery R and Dieudonné MN: Adiponectin promotes
syncytialisation of BeWo cell line and primary trophoblast cells.
Reprod Biol Endocrinol. 8:128–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen Q, Wang J, Zhou Q, Shen Z, Luo H, Tao
X and Zhu X: Linking expression of aquaporin 3 to activation of JNK
pathway. Front Biosci. 22:258–267. 2017. View Article : Google Scholar
|
|
40
|
Tardelli M, Claudel T, Bruschi FV,
Moreno-Viedma V and Trauner M: Adiponectin regulates AQP3 via PPARα
in human hepatic stellate cells. Biochem Bioph Res Commun.
490:51–54. 2017. View Article : Google Scholar
|