Introduction
Obesity is currently considered to be a serious
global health problem due to its high prevalence (39%), according
to the World Health Organization 2016 survey (1). This pathological entity characterized
by an excess of body fat is measured by body mass index (2) Moreover, it is now well established
that maternal obesity plays an important role in early life
programming. Obesity in pregnant females often leads to gestational
diabetes, which results in the adaptation of the fetus to a
hyperglycemic, metabolically altered intrauterine environment
(3). However, during postnatal
life, such adaptation becomes detrimental to the individual because
it increases the susceptibility to excess body fat and
subsequently, an impaired metabolic state during adulthood
(4). In the first instance, a high
percentage of body fat leads to a chronic low-grade inflammatory
state caused by the dysregulation of adipokine synthesis and
macrophage infiltration within adipocytes events that precede the
development of other diseases, such as insulin resistance, diabetes
mellitus, cardiovascular complications (5–8) and
different types of cancer, such as lung, endometroid and breast
cancer (9–11).
In previous years, it has been demonstrated that
abnormal epigenetic regulation due to an adverse intrauterine
environment is a robust explanation for the increased risk of
developing metabolic diseases in postnatal life (12). Within this context, Castro et
al (13) described a strong
correlation between maternal blood concentrations of leptin and
adiponectin, and the fat percentage of offspring. Likewise, studies
have demonstrated that the administration of a high-fat diet to
adiponectin knockout (KO) females had an impact on the offspring's
body weight (14,15). Similarly, the administration of a
diet with 30% sugar has proven effective in inducing obesity in
experimental models, which imitates the amount of carbohydrates
present in the diet that most individuals have today (16–19).
Additionally, it has been reported that adipokines are not only
secreted from adipose tissues, there are also several adipokines,
like adiponectin and leptin that have been identified in
reproductive organs of different species, including the
hypothalamic-pituitary-gonadal axis (20,21).
Furthermore, several research groups have identified that maternal
metabolic disorders affect the expression of some adipokines at
both the gene and protein level in reproductive organs of the
offspring (9,22).
Due to its implications in different metabolic
disorders, lipocalin-2 (Lcn2) has been widely studied (23,24).
For example, circulating concentrations of Lcn2 are higher in women
with gestational diabetes and preeclampsia, which suggests that
Lcn2 may be of value as a possible marker of fetal programming in
humans (25). Other studies
conducted in humans and animal models indicated a sex specific
regulation of Lcn2 by estrogen (26–28);
ovariectomized mice treated with estrogen showed an increase in
Lcn2 gene expression levels in white adipose tissue, liver
and serum (29). This adipokine is
a member of the lipocalin superfamily, characterized by the
presence of three conserved motifs comprising a single
eight-stranded antiparallel β-barrel similar to a calyx that is
able to bind numerous ligands. These three specific features confer
a vast functional diversity and lipocalins are therefore involved
in a number of different processes, such as iron intake, cellular
apoptosis and inflammation (30,31).
In 2008, by means of a DNA microarray assay, the
present group group identified the Lcn2 gene within a
cluster of DNA sequences whose expression profiles were increased
in the perinatal murine ovary (32). Later, the current group also
identified Lcn2 and its receptor 24p3 (24p3R) mRNA and protein in
the gonads of Sprague Dawley rats, and found their expression to be
sexually dimorphic during the perinatal period (33). Based on these previous observations
and the fact that several studies have demonstrated that adipokine
synthesis can be altered by early life programming due to maternal
obesity, the expression levels of Lcn2 and 24p3R mRNA
and their respective protein profiles were analyzed in the ovaries
and testes of offspring of obese mothers in the present study. It
should also be taken into account that only a few studies have
addressed the expression of this adipokine in reproductive organs
and even though it is well established that 24p3R participates in
apoptosis and cellular iron intake (34), its expression in murine
reproductive organs has not been documented until recently
(33). Therefore, it is important
to further investigate the role of Lnc2 and its receptor in order
to establish the effect of obesity in gonadal development during
gestation.
Materials and methods
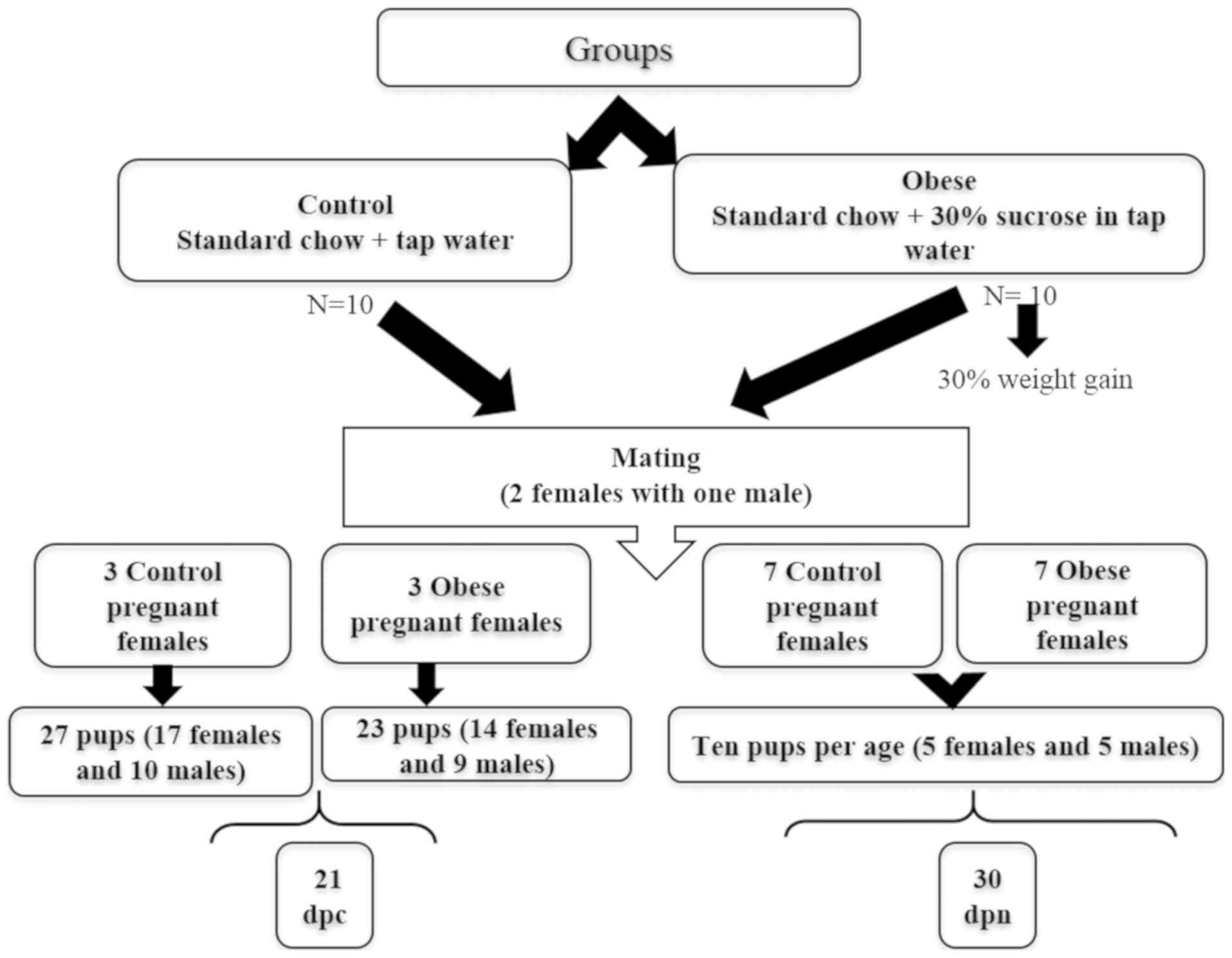
Animals
The animal experiments were conducted using
2-month-old Sprague Dawley rats obtained from an inbred colony at
the National Medical Center, Mexican Social Security Institute
(Mexico City, Mexico). A total of 20 female (200±10 g) and 10 male
rats (300±20 g) were housed under a controlled photoperiod (12-h
light/dark cycle, lights on at 7:00 h) and temperature (21±2°C).
Male rats had free access to rodent chow and tap water (5008
Formulab Diet; PMI Nutrition International). This diet is
formulated to supply a complete life-cycle nutrition in rat
breeding colonies, providing calories from 26.8% protein, 16.7% fat
and 56.4% carbohydrates. Littermate female rats were assigned
randomly to two nutritional groups (n=10) ~8 weeks before mating.
The first group was fed standard chow ad libitum and had
free access to tap water (control group); the second group received
standard chow ad libitum and had free access to water with
30% sucrose, prepared by adding 30% w/v of commercial brown sugar
(obese group) in order to induce obesity according to the model
employed in previous studies (16–19).
The experimental protocol was approved by the Research Ethics
Committees of the National Medical Center of the Mexican Social
Security Institute and the National Autonomous University of Mexico
(approval nos. R-2011-3604-2 and UNAM-003-2013, respectively), and
the study was conducted following the American Association for
Accreditation of Laboratory Care and National Institutes of Health
guidelines (35). All animal
procedures complied with government published recommendations for
the use of laboratory animals. In order to ensure a successful
pregnancy, female rats were nulliparous, and had an average body
weight of 200±10 g.
Although a daily report on dietary and liquid intake
of female dams was not included, these observations were made daily
during the experiment. The amount of water and food consumption was
not different between the control and the experimental groups. The
weight of the female dams from both groups was measured weekly.
Once the females of the experimental group reached a weight 30%
higher than that of control females and following what is described
elsewhere for the generation of a murine model of obesity (16–19),
both control and experimental female rats were mated with a
3-month-old male (2 females with 1 male). The next morning, males
were separated from the females and returned to their original
cages (2 per cage) in order to be employed in further breeding.
Females were examined for the presence of vaginal plugs and this
was considered the first day of gestation. At 21 days
postconception (dpc), three pregnant females of the control and
experimental groups were anesthetized with sodium pentobarbital (25
mg/kg, i.p.) until they were unconscious (36). To verify the state of
unconsciousness, which implies a reversible insensitivity to
external stimuli, a minimum pinch in the tail of the rat was made
to ensure the absence of pain in the presence of such stimulus.
Immediately after, rats were culled by cervical dislocation
following the National Institutes of Health guide for the care and
use of Laboratory Animals (NIH Publications no. 8023, revised 1978)
and the Mexican regulations for the use and care of laboratory
animals (policy no. NOM-062-ZOO-1999). Euthanasia after cervical
dislocation was confirmed by corroborating the separation of the
cervical vertebrae from the skull manually, also the cessation of
breathing, heartbeat, eye movements and the absence of response to
external stimuli were verified before performing an abdominal
incision to retrieve the fetuses from each mother. Upon retrieval,
27 fetuses (17 females and 10 males) from the control group, and 23
fetuses (14 females and 9 males) from the experimental group were
decapitated and their gonads were dissected and frozen on dry ice
for RNA isolation (Fig. 1).
For the remaining pregnant rats (7 in total for each
group, 1 per age), the day of birth was designated as postnatal day
0. In order to ensure adequate and standardized nutrition until
weaning, litter sizes were standardized to 10 pups per litter (5
females and 5 males in each group; Fig. 1), the remaining pups were culled by
decapitation. During lactation, the mothers were fed standard chow
ad libitum and either tap water (control group) or water
plus 30% sucrose (obese group) accordingly. The weight and glucose
blood levels of both litters were recorded at each time point
before sacrifice.
Upon collection, tissues were either frozen on dry
ice for RNA extraction or fixed for immunohistochemistry. A total
of 10 ovaries and 10 testicles were collected from Sprague Dawley
rats at 0, 2, 4, 6, 12, 20 and 30 days postnatal (dpn; 10 per
group) immediately after sacrifice. These specific time-points were
selected based on the authors' previous results (32,33).
RNA isolation
Total RNA was isolated using the RNeasy Mini kit
(Qiagen, Inc.), following the manufacturer's protocol and as
described previously (33).
Briefly, the tissue was homogenized in TRIzol reagent (Molecular
Research Center, Inc.), and the aqueous and organic phases were
separated by the addition of one volume of bromo-3-chloropropane
(Sigma-Aldrich; Merck KGaA), followed by centrifugation in a
MiniSpin® eppendorf centrifuge at 13, 800 × g for 15 min
at 4°C. Next, 350 µl of 70% ethanol (Sigma-Aldrich; Merck KGaA)
were added to all samples and each sample was applied to an RNeasy
mini-column. The columns were washed by centrifugation at 735 × g
for 2 min at room temperature with buffers containing guanidine and
ethanol. In order to elute the RNA, RNase-free water (30 µl) was
added directly onto the silica-gel membrane of the columns and each
column was centrifuged for 1 min at 13, 800 × g at room
temperature. The RNA was quantified in a SmartSpec Plus
spectrophotometer (Bio-Rad Laboratories, Inc.) by measuring
absorbance at 260 nm and was stored at −85°C until use. The quality
of each RNA sample was assessed on 2% formaldehyde denaturing
agarose gels.
Semi-quantitative reverse
transcription PCR
Total RNA from all samples was reverse transcribed
using the Superscript™ First-Strand Synthesis system (Invitrogen;
Thermo Fisher Scientific, Inc.), according to manufacturer's
protocol and as described previously (28). All reactions were carried out in a
total volume of 20 µl. First, 300 ng of total RNA, isolated from
the gonads collected at 21 dpc and 0, 2, 4, 6, 12, 20 and 30 dpn,
were annealed at 65°C for 5 min to 0.5 µg of oligo (dT)12-18 primer
(0.5 µg/µl) and 1 µl of a dNTP cocktail (10 mM). The annealed
RNA-primer samples were incubated for 1 h at 42°C with the
following components of the First-Strand Synthesis system
(Invitrogen; Thermo Fisher Scientific, Inc.): RT buffer (10X),
MgCl2 (25 mM), RNaseOUT (40 U/µl), and Superscript II
reverse transcriptase (50 U/µl). The reactions were terminated by
incubation at 70°C for 15 min, followed by incubation at 37°C for
20 min with 2 U of Escherichia coli RNase H (2 U/µl)
(Invitrogen; Thermo Fisher Scientific, Inc.).
The PCR amplification of the reverse-transcribed
products was carried out in a total volume of 20 µl, using 10 µl of
2X KAPA Taq ReadyMix (Kapa Biosystems; Roche Diagnostics) and 1 µl
of cDNA template annealed to 10 pmol of the Lcn2, 24p3R or
GAPDH specific primers (Table
I). The PCR conditions used were 5 min at 94°C, followed by 35
cycles of denaturation at 94°C for 30 sec, annealing for 30 sec at
60°C for Lcn2 and at 58°C for 24p3R and GAPDH,
and 1 min of extension at 72°C, with a 10 min final extension at
72°C. Each sample was amplified in triplicate in a Biometra
TProfessional thermocycler (Biometra Ltd, Jena Analytic). A total
of 20 µl of the PCR reactions were electrophoresed on 2% agarose
gels stained with ethidium bromide. The gels were scanned
electronically and the images were quantitated by densitometry
using image-analysis software (Kodak Molecular Imaging Software
4.0, Kodak Image Station 4000R; Carestream Health, Inc.). Relative
expression levels of Lcn2 and its receptor were obtained by
dividing the average number of pixels of the experimental
transcript (Lcn2 or 24p3R) by the average number of
pixels of the control transcript (GAPDH).
 | Table I.Primer sequences used for
semi-quantitative PCR. |
Table I.
Primer sequences used for
semi-quantitative PCR.
| Gene | Primer sequence
(5′→3′) | Length, bp |
|---|
| Lcn2 | F:
TCTCGATTCCGTCGGGTGGTGG | 592 |
|
| R:
CCTGGGTGTCCTGTGTCTG |
|
| 24p3R | F:
AGGACTGGGACTACAACGGA | 507 |
|
| R:
GTGCGGACTCCAGAAACAGA |
|
| GAPDH | F:
CAAGGTCATCCATGACAACTTTG | 496 |
|
| R:
GTCCACCACCCTGTTGCTGTAG |
|
Immunohistochemistry
In order to determine Lcn2 and 24p3R protein
signaling within gonads of the offspring of wild-type and obese
rats, 5-µm sections were obtained from formalin-fixed,
paraffin-embedded gonadal samples collected from four 30 dpn rats
(2 females and 2 males from each group) and mounted on glass slides
previously coated with poly-L-lysine. The sections were then
deparaffinized and rehydrated in a decreasing alcohol series (100,
90, 70 and 30%) until water. The sections were microwave heated in
high mode at 60°C with antigen retrieval solution (Vector
Laboratories, Inc.), rinsed in 1X phosphate buffered saline (PBS;
pH 7.4), incubated for 30 min with four drops of the background
sniper, which is an endogenous peroxidase-inactivation solution of
the Starr Trek Universal HRP detection system (Biocare Medical,
LLC), and subsequently blocked with 10% bovine serum albumin (BSA)
in 1X PBS for 30 min at room temperature. Tissues were then
incubated with either primary rat polyclonal antibody against Lcn2
or against 24p3R antibody (1:150; cat. no. ab41105 and ab124506;
Abcam), at 4°C overnight.
The sections were washed in PBS, incubated at room
temperature for 2 h with the Starr Trek Universal HRP detection
system (Biocare Medical, LLC), and washed with 1X PBS. The
peroxidase reaction was developed with diaminobenzidine and
H2O2, generating a brown precipitate.
Finally, the slides were counterstained with hematoxylin for 5 sec
at room temperature, dehydrated and mounted with synthetic resin.
The positive control consisted of sections of uterus collected from
the same wild-type female (sections of rat uterus were used
following immunohistochemistry protocol´s recommendation in which
it is suggested to be used as positive control, the latter because
it is well established that lipocalin 2 is highly expressed in
uterus). The negative control consisted of replacing the primary
antibody with BSA in PBS. The brown precipitate signal was analyzed
semi-quantitatively using the integrated optical density (IOD)
provided by the Image-Pro Plus software 7 (Media Cybernetics,
Inc.). A total of five sections of each of the three slides were
analyzed at high magnification (×40) under a light microscope.
Percentage was used to express the relative changes in the IODs of
the gonadal tissue of experimental rats compared with the IODs of
control rats.
Statistical analysis
Values from three experimental repeats are expressed
as the mean ± standard error. An unpaired two-tailed Student's
t-test was used for comparisons between the two groups. Two-way
ANOVA followed by Tukey's post hoc test was used to compare the
relative expression of cDNA between gonads of the offspring of
wild-type rats and offspring of obese mothers. Statistical analyses
were performed using GraphPad Prism version 7 for Windows
(GraphPad, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Taking into account that in murine species during
the perinatal and prepubertal periods, key molecular processes for
gonadal function take place and based on the fact that in 2008, our
group identified the expression of the gene that encodes for Lcn2
in the mouse ovary (32), first we
decided to analyze the expression levels of Lcn2 and its
corresponding receptor (24p3R) in female and male gonads from
wild-type Sprague Dawley rats collected at different time-points
(21 dpc, 0, 2, 4, 6, 12, 20 and 30 dpn), demonstrating that the
relative expression of both Lcn2 and 24p3R is
significantly different in female and male murine gonads at
perinatal and prepubertal stages of development (33). In the present study,
semi-quantitative PCR was used to assess possible changes in the
relative expression levels of Lcn2 and 24p3R mRNA in
the gonads of the offspring of obese rats, by comparing these
levels of expression with those observed in the gonads of offspring
of the control group (Figs.
2,3). Immunohistochemistry was
performed to determine if such changes were also present at the
protein level. A significant difference in the expression level of
this adipokine and its receptor was only found at two specific time
points (21 dpc and 30 dpn) (Figs.
4,5).
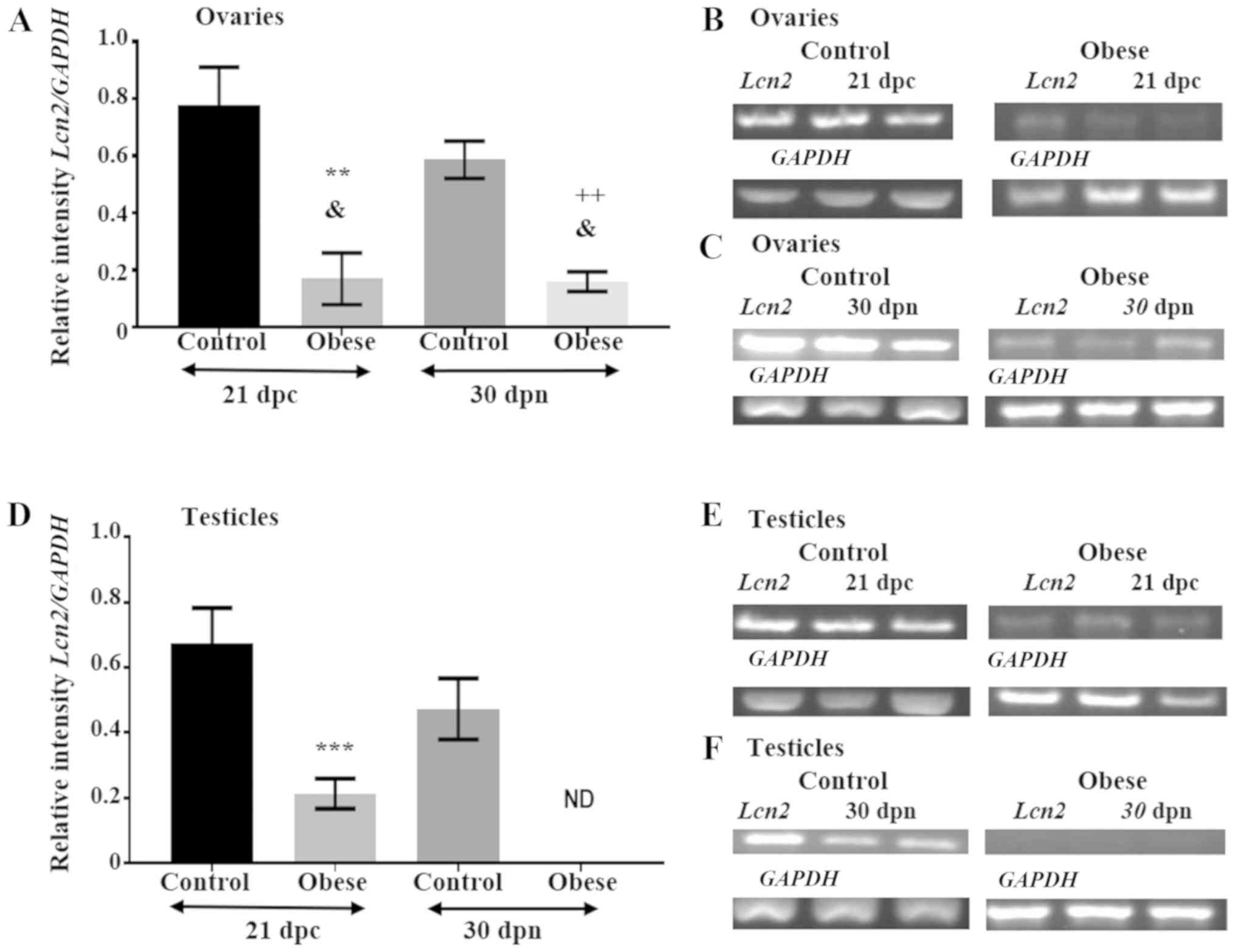 | Figure 2.Relative expression levels of Lcn2
mRNA are altered in the gonads of the offspring of obese rats. (A)
Graphic presentation of the densitometric changes detected in the
electrophoresis of PCR products amplified from the total RNA
isolated from the ovaries collected at 21 dpc and at 30 dpn. The
Y-axis indicates the expression levels of Lcn2 cDNA in the
ovaries of offspring of control and obese rats relative to the
expression levels of the GAPDH gene. The X-axis indicates
age in dpc and dpn. At 21 dpc and 30 dpn, mRNA expression of the
adipokine is abundant in the ovaries of the control group, while
Lcn2 mRNA expression is downregulated in the ovaries of the
experimental group. Values are expressed as the mean ± standard
error. (B) Representative image of the electrophoresis of PCR
products, Lcn2 is abundant in ovaries of the perinatal
offspring of the control group and downregulated in the ovaries of
the experimental group. The constitutively expressed GAPDH
gene was used as the normalizing unit. (C) Representative image of
the electrophoresis of PCR products, Lcn2 is abundant in
ovaries of the prepubertal offspring of the control group and
downregulated in the ovaries of the experimental group. The
constitutively expressed GAPDH gene was used as the
normalizing unit. (D) Graphic presentation of the densitometric
changes detected in the electrophoresis of PCR products amplified
from the total RNA isolated from the testicles collected at 21 dpc
and at 30 dpn. Y-axis indicates the expression levels of
Lcn2 cDNA in the testicles of offspring of control and obese
rats relative to the expression levels of the GAPDH gene.
The X-axis indicates age in dpc and dpn. At 21 dpc and 30 dpn, mRNA
expression levels of the adipokine are abundant in the testicles of
the control group, while Lcn2 mRNA expression is either down
regulated or absent in the testicles of the experimental group.
Values are expressed as the mean ± standard error. (E)
Representative image of the electrophoresis of PCR products,
Lcn2 is abundant in testicles of the perinatal offspring of
the control group and downregulated in the testicles of the
experimental group. The constitutively expressed GAPDH gene
was used as the normalizing unit. (F) Representative image of the
electrophoresis of PCR products, Lcn2 is abundant in
testicles of the prepubertal offspring of the control group and not
detected in the testicles of the experimental group. The
constitutively expressed GAPDH gene was used as the
normalizing unit. RNA was isolated from male and female gonads
collected at 21 dpc and 30 dpn from both groups. At 21 dpc, n=27
animals in control group (17 females and 10 males), and n=23
animals in the experimental group (14 females and 9 males). At 30
dpn, n=20 animals (5 females and 5 males per group). Tukey's test
was performed to compare all possible pair of means.
&P=0.002; **P<0.01, obese female group vs.
control female group at 21 dpc; ++P<0.01, obese
female group vs. control female group at 30 dpn; ***P<0.001,
obese male group vs. control male group at 21 dpc. Lcn2,
lipocalin-2; dpc, days postconception; dpn, days postnatal; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; ND, not detected. |
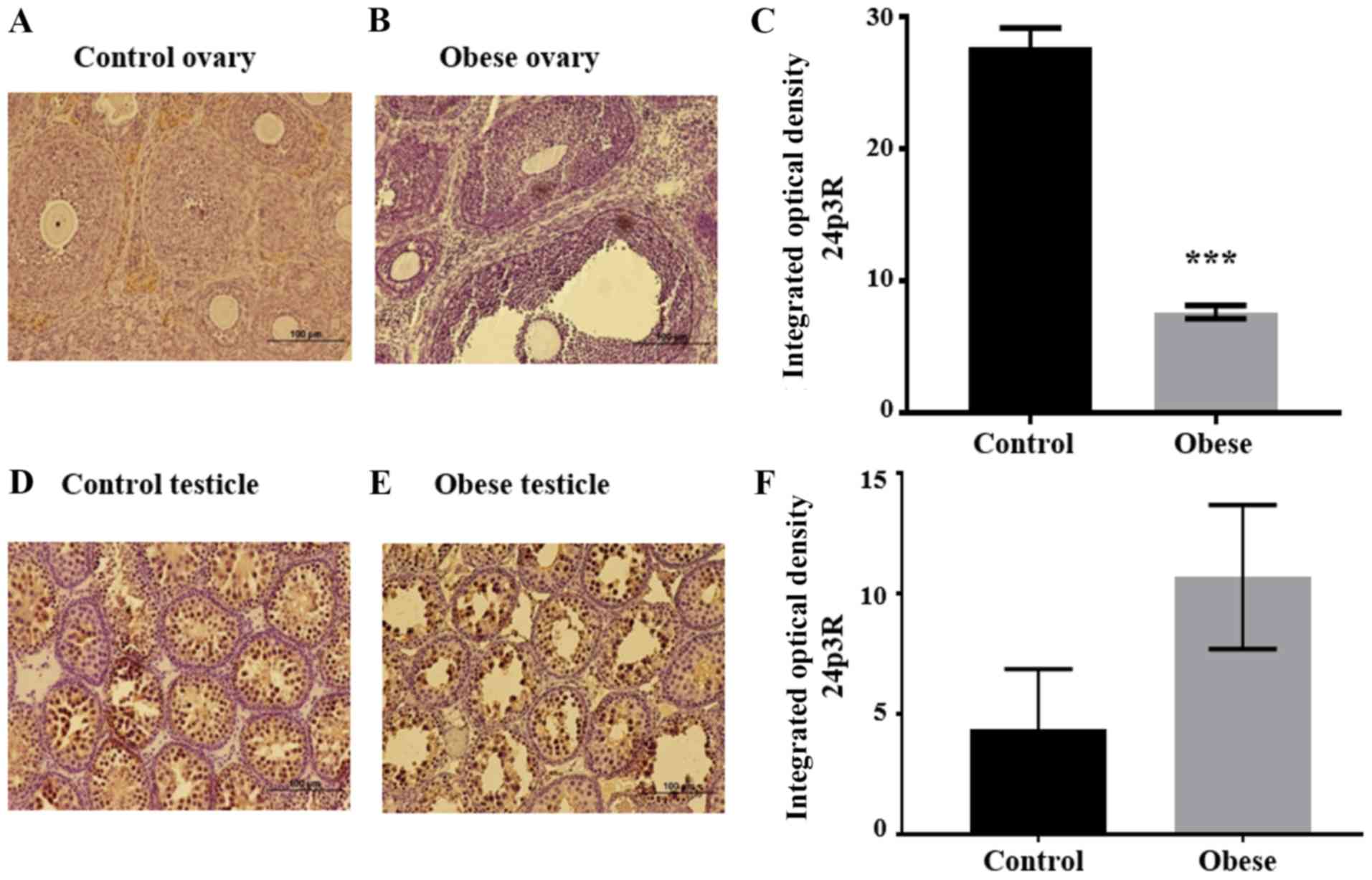 | Figure 5.24p3R expression levels in the gonads
of prepubertal offspring of control and obese rats. (A) The 24p3R
signal is strong in the stroma of ovary sections from the offspring
of wild-type rats, while the signal was barely visible in the
remaining follicular cells, including oocytes, granulosa, theca
cells and the corpus luteum. (B) In paraffin-embedded ovarian
sections from one ovary collected from the offspring of obese
mothers, overall intensity decreased notably. (C) Graph depicting
statistically significant differences in overall mean IOD values
between ovarian samples of the control and experimental groups. (D)
Immunoreactivity to 24p3R in a normal testicle, with staining
observed in Sertoli and germinal cells of different developmental
stages. (E) A stronger signal is observed in Sertoli and germinal
cells of the testicle from the offspring of the experimental group.
(F) Graph depicting higher mean IOD values in the testicular
structures of the offspring of obese mothers. The increase in the
IOD values was not statistically significant. Magnification, ×40;
scale bar, 100 µm. n=3 slides with serial sections from 2 ovaries
and 2 testicles (1 gonad per group, control or experimental), were
used in the assay. ***P<0.001 experimental group vs. control
group. 24p3R, 24p3 receptor; IOD, integrated optical density. |
Relative expression levels of Lcn2
mRNA changes in the gonads of the offspring of obese dams during
the perinatal period
During the perinatal period, the relative expression
level of Lcn2 mRNA was higher in the ovaries and testicles
of offspring in the control group (0.78±0.13 and 0.68±0.11,
respectively (Fig. 2A, B, D and
E). However, at the same age, Lcn2 mRNA expression in
the obese group decreased by >50% in the ovaries (0.17±0.09) and
in the testicles (0.21±0.05; Fig. 2A,
B, D and E).
Relative expression of Lcn2 mRNA
decreases significantly in the gonads of 30 dpn offspring of obese
dams
At 30 dpn, in the offspring of wild-type rats,
expression of Lcn2 was abundant in the ovaries (0.59±0.06)
and in the testicles (0.47±0.09; Fig.
2A, C, D and F), while in the ovaries of the experimental
group, the relative expression level of Lcn2 decreased to
0.16±0.03 (Fig. 2A and C).
Notably, the mRNA expression level of Lcn2 was null in the
male gonads (Fig. 2D and F).
Statistical analysis revealed a significant difference in the
expression levels of Lcn2 between control and experimental
groups at the two specific time points in ovaries (P<0.01) and
testicles (P<0.001) (Fig. 2A and
D).
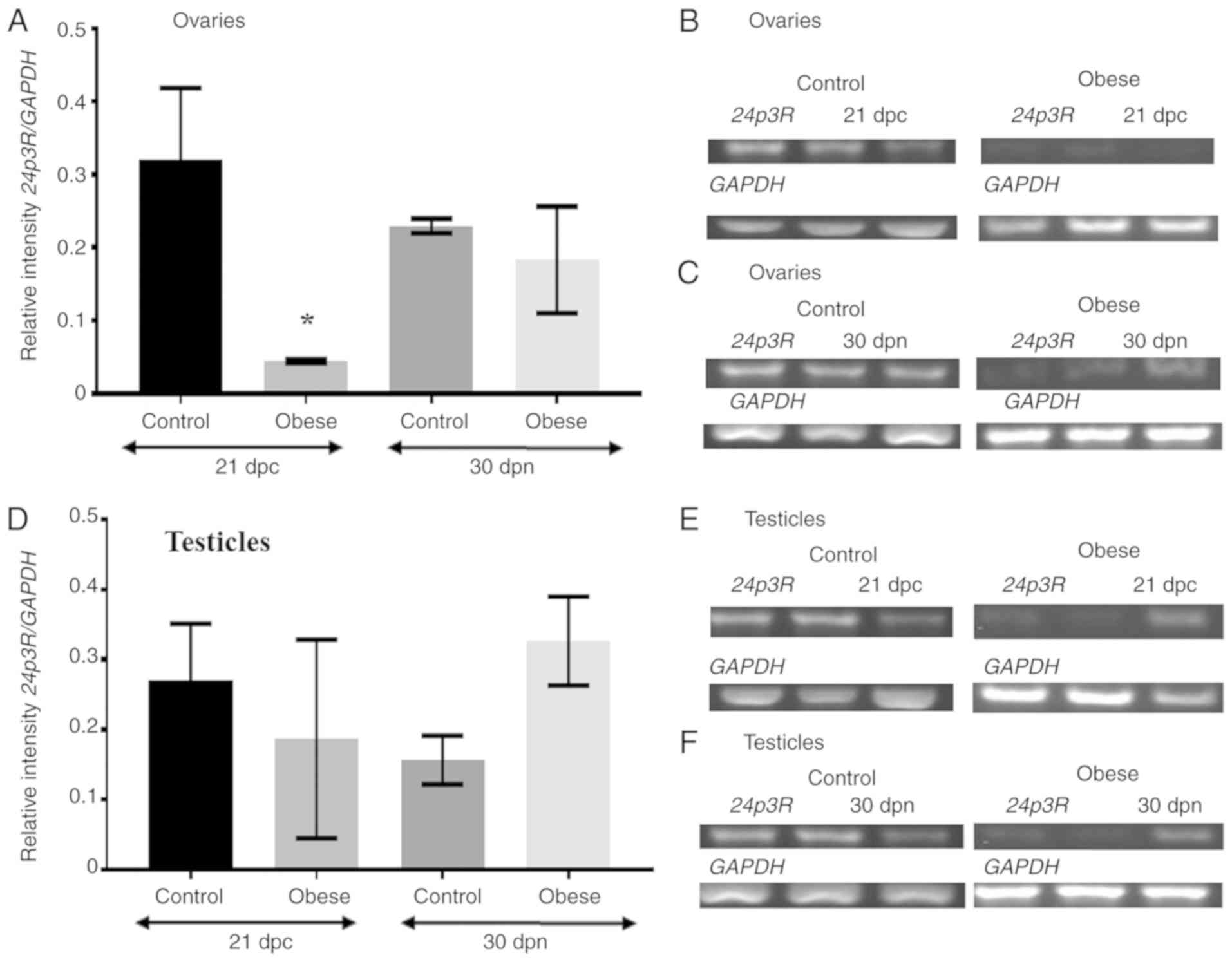
Relative expression level of 24p3R
mRNA is not modified in the gonads of the offspring of obese
dams
The mRNA expression level of 24p3R was also
analyzed. Electrophoresis of the PCR products revealed a
significant change in the relative expression level of this
receptor in perinatal ovarian samples obtained from the offspring
of obese mothers. As shown in Fig. 3A
and B 24p3R mRNA expression levels were downregulated in
the experimental group (0.04±0.001) compared with the control group
(0.32±0.09 (P<0.05). As shown in Fig. 3D and E, the change in the relative
expression level of 24p3R in the perinatal testicles of the
experimental group was not significant in the experimental group
(0.19±0.14) compared with the control group (0.27±0.08).
At 30 dpn, the relative expression of 24p3R
in the ovaries of the experimental group did not change (0.18±0.07)
compared with the control group (0.23±0.01), as shown in Fig. 3A and C. Conversely, at 30 dpn, the
relative expression of 24p3R increased slightly in the
testicles of pups of obese mothers (0.33±0.06) compared with the
control group (0.16±0.03). These changes were not statistically
significant (Fig. 3D and F).
As a positive control, a fragment (496 bp) of the
ubiquitous GAPDH gene obtained from the same cDNA samples
was also amplified. The signal intensity obtained from the PCRs of
GAPDH was also used to normalize the signal intensity
generated by the amplification of the experimental genes
(Lcn2 and 24p3R).
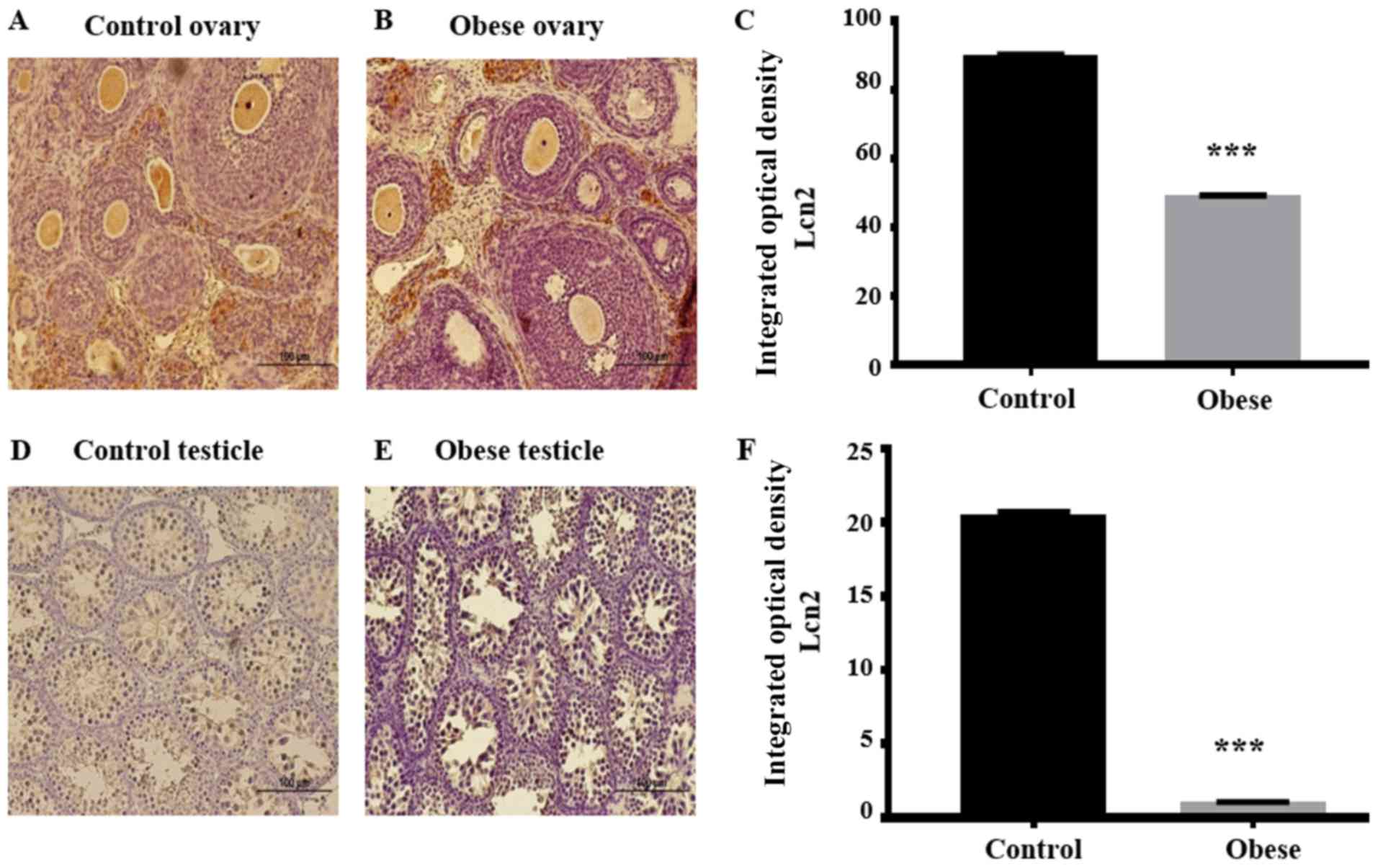
Immunohistochemistry analysis of the
Lcn2 and 24p3R proteins
Immunohistochemistry was performed to determine
possible changes in protein synthesis or cellular localization of
Lcn2 and 24p3R within the gonads of 30 dpn offspring of obese rats
(Figs. 4 and 5) compared with the findings observed
previously in the gonads of 30 dpn offspring of wild-type rats
(33). In paraffin-embedded
ovarian sections from offspring of the control group, Lcn2
immunostaining was strong in the oocytes, zona pellucida, antrum,
corpus luteum and stroma of developed follicles. A signal of minor
intensity was also present in the theca and granulosa cells of
primary and growing follicles (Fig.
4A).
The Lcn2 signal in ovarian sections of the offspring
of obese rats was less intense in all follicular structures, except
for the corpus luteum where the signal was slightly increased
compared to the same cellular structure in the ovaries of control
rat offspring (Fig. 4B). The IOD
generated by each follicular structure was quantitated by employing
the IOD tool of the Image-Pro Plus software. Differences between
the sum of all IODs of the follicular structures of the control
group (90±0.4) and the corresponding IODs of the respective
follicular structures of the experimental group (49.34±0.2) were
statistically significant, with the IOD being lower in the latter
group (P<0.001) (Fig. 4C).
Regarding the 24p3R, intense staining was observed
in the ovarian stroma of wild-type offspring, while the signal was
barely visible in oocytes, granulosa and theca cells (Fig. 5A). The overall intensity decreased
markedly in ovaries of the offspring of obese rats (Fig. 5B). IOD decreased in the
experimental group (7.67±0.5) compared with the control group
(27.69±1.5), this was statistically significant (P<0.001)
(Fig. 5C).
In testicles of wild-type offspring, Lcn2 was only
detected in Sertoli and Leydig cells. Neither germinal nor myoid
cells presented immunopositivity for this protein (Fig. 4D). On the other hand, in the
experimental group, this adipokine was only detected in Sertoli
cells (Fig. 4E). Overall, the Lcn2
IOD was higher in the testicles of wild-type offspring (20.73±0.2)
compared with the IOD observed in Sertoli cells of testicles of the
experimental group (1.08±0.01), this was statistically significant
(P<0.001) (Fig. 4F). The 24p3R
signal was detected in Sertoli and germinal cells at different
developmental stages (Fig. 5D and
E) and, contrary to what was expected, the IOD was higher in
the two testicular structures of the experimental group (10.7±1.0)
compared with the same structures of the control group (4.36±1.5).
However, the difference between groups was not statistically
significant (Fig. 5F).
Discussion
At present, obesity is considered a serious global
health problem to which a considerable number of human and economic
resources are allocated. It is well established that a state of
low-grade chronic inflammation is involved in obesity, affecting
not only individuals but also their offspring through fetal
programming (37). Furthermore, it
has been demonstrated that there is an association between
imbalanced concentrations of anti-inflammatory and pro-inflammatory
adipokines, and the development of the chronic inflammatory state,
which affects different organs, including those comprising the
hypothalamus-pituitary-gonadal axis (9).
The present study describes the changes in mRNA and
protein expression patterns of the adipokine Lcn2 and its
corresponding receptor (24p3R), in the gonads of the offspring of
obese mothers during perinatal and prepubertal development. Even
though the expression profiles of the two proteins were analyzed at
different time points starting from hours before birth to 30 dpn, a
significant change in these expression profiles was only observed
at 21 dpc and 30 dpn compared with those observed in the gonads of
the offspring of control dams. The modification in the expression
profile of both the adipokine and the receptor at these specific
time points coincides with mechanisms that are essential for
gonadal development, such as the onset of folliculogenesis and
spermatogenesis in perinatal murine gonads or the beginning of the
gonadotropin-dependent cascade in the prepubertal stage (38–40).
Although different studies have associated an
increase in serum Lcn2 concentrations with obesity and related
cardiometabolic diseases (41,42),
in the present study, this adipokine was downregulated in the ovary
both at 21 dpc and 30 dpn and was downregulated to a greater extent
in the testicle, where the expression level of this adipokine was
non-existent at 30 dpn. Rees and Hay (43) observed that Lcn2 mRNA
expression level was decreased in the fetal liver of offspring
exposed to a maternal low-protein soy oil diet compared with the
relative expression observed in the fetal liver of offspring
exposed to a high-protein diet. Nevertheless, the same was not
observed when the diet was prepared with corn oil, which led the
authors to suggest that a difference in fatty acid composition
between the oil and corn diets rather than protein content could be
driving the changes in Lcn2 mRNA expression level in the
fetal liver. Moreover, these changes in the expression level of
this adipokine persisted until adulthood, suggesting that Lcn2
plays an essential role in fetal programming of hepatic metabolism.
In the same manner, in the current study, the relative expression
of Lcn2 decreased in the gonads of fetal and prepubertal
offspring from obese dams fed with a high sucrose diet. This type
of diet was used because it has been demonstrated that its
administration leads to the development of maternal insulin
resistance, which elicits perinatal insulinemia, considered to be
one of the causes of genetic programming (44). It has been observed that in order
to survive an abnormal intrauterine environment, the offspring of
obese mothers develop an adaptive response to such challenges via
changes in epigenetic regulation (45). Therefore, the downregulation in
Lcn2 expression observed in the present study could also be
a result of fetal programming.
It is now commonly known that an adverse fetal
environment often leads to genetic reprogramming through DNA
methylation (12). Houde et
al (3) demonstrated that
maternal hyperglycemia causes promoter hypermethylation of the
leptin and adiponectin genes. Thus, DNA silencing could be a
plausible explanation for the downregulation of Lcn2 in the
gonads of offspring of obese mothers. Further DNA methylation
studies are needed to confirm this hypothesis.
On the other hand, Law et al (46) demonstrated that Lcn2-KO mice showed
improved systemic energy homeostasis and insulin sensitivity under
both basal and obesogenic conditions. From 11 weeks of age onwards,
Lcn2-KO mice had lower fasting glucose and serum insulin levels
than their wild-type counterparts. This improved metabolic
condition is in line with the current findings observed in 30 dpn
offspring of obese rats, which had normal serum glucose levels
despite a considerable increase in body weight (data not shown). At
21 dpc, the offspring of these obese dams presented normal serum
glucose levels and exhibited no weight alterations. Therefore, the
present results indicated that maternal obesity is associated with
molecular alterations in the fetal gonads, which are the result of
genetic programming.
Jungheim et al (22) demonstrated that ovarian follicular
function is severely affected in the presence of excess circulating
glucose concentrations, including granulosa cell apoptosis,
abnormal follicular development and delayed maturation. In the
present study, the immunohistochemical results showed that, in the
female gonads of 30 dpn obese offspring, exposure to a high-glucose
diet until weaning led to a considerable number of atretic
follicles characterized by an irregular shape and a reduced
granulosa cell layer.
A specific role of Lcn2 in the regulation of cell
differentiation has been demonstrated in spermatogenesis, in which
spermatogonia drive the expression levels of Lcn2 in Sertoli cells
via activation of the NF-κB pathway (47). This pathway is also involved in
apoptotic processes in pancreatic β-cells, where the endoplasmic
reticulum stress-unfolded protein response is activated in order to
ameliorate the effects of a metabolically adverse maternal
environment (48). In the present
study, the testicular seminiferous tubules of obese offspring were
narrower and the number of gametic cells was reduced. However,
these results should be interpreted with caution as they may be the
result of dysregulation in the cell differentiation signaling
pathway, in which Lcn2 could be involved via NF-κB in the gonads,
triggering activation of the apoptotic process. Lcn2 also
participates in the apoptotic pathway by binding to the 24p3R in
order to internalize iron captured by the adipokine, thereby
increasing intracellular iron levels that induce the mitochondrial
pro-apoptotic cascade (49).
In 21 dpc ovaries of offspring of obese mothers, the
expression of 24p3R mRNA and protein were downregulated,
while at 30 dpn, the expression level profile was similar to that
observed in female gonads of the offspring of wild-type rats. In
the fetal male gonads, the relative expression of this receptor was
similar between the control and experimental groups; surprisingly,
at the prepubertal stage, mRNA and protein abundance increased in
the testes of the offspring of obese mothers. The reason for this
difference in the expression pattern of 24p3R between ovaries and
testicles is not clear. A recent study performed by Chella Krishnan
et al (50) demonstrates
that Lcn2 in conjunction with megalin, not the 24p3R, exerts its
metabolic function in liver and adipose tissue of mice in a
sexually dimorphic pattern. The latter may account for the
differences between female and male gonads regarding the expression
of both Lcn2 and 24p3R. As for 24p3R, the insignificant change
during the fetal stage and the slight increase in its expression
profile within the prepubertal testis, suggested that megalin could
be the receptor participating in Lcn2 signaling within the male
gonad. Perhaps different regulation mechanisms take place in each
gonad. Further experiments are necessary in order to elucidate this
difference.
Finally, immunohistochemistry studies indicated that
Lcn2 and the 24p3R are expressed in both germinal and somatic
cells, which is consistent with the localization of other
adipokines in gonads (20,51). Even though the present study found
that both the mRNA and the protein profiles of Lcn2 and 24p3R are
modified in the gonads of the offspring of obese mothers, it is
limited since reverse transcription-quantitative PCR was not
performed, therefore such modifications may not be that accurate,
also it does not experimentally demonstrate the cause of this
alteration or the subsequent consequences of a disturbed Lcn2/24p3R
signaling pathway. It is now well established that this adipokine
signals via its receptors in order to regulate gonadal cell
differentiation. Therefore, assessing the effect that a change in
the expression profile of either one might have in the fertility
and/or the hormonal milieu of the offspring's gonads is essential.
This is why in the first instance, the participation of this
adipokine and its corresponding receptor in gonadal development,
including cell differentiation, gametic cell maturation and
steroidogenesis, needs to be determined.
Acknowledgements
The authors would like to thank Mrs Noemí Castillo
(Cardiovascular and Metabolic Diseases Research Unit, National
Medical Center, Mexican Social Security Institute, Mexico City,
Mexico) for her assistance with the histological procedures.
Funding
The present study was supported in part by the
Mexican Social Security Institute (grant no.
FIS/IMSS/PROT/014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EdlC participated in the conception and design of
the study, performed most of the semi-quantitative and
immunohistochemistry experiments, participated in the analysis and
interpretation of the data, and prepared the manuscript. LMA
participated in the data analysis and revision of the manuscript.
LD, RLB, and EC collected the biological samples and performed the
remaining semi-quantitative reverse transcription PCR and
immunohistochemistry experiments. MCRM participated in the analysis
and interpretation of the data. JPM participated in the design of
the study and prepared and revised the manuscript for its
intellectual content. The final version of the manuscript was read
and approved by all authors and each author believes that the
manuscript represents honest work.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Research Committees of both the National Medical Center and the
National Autonomous University of México, México City, and was
performed in accordance with the American Association for
Accreditation of Laboratory Care and National Institutes of Health
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO), . Global
Health Observatory (GHO) data. Overweight and obesity. WHO.
(Geneva). 2016.https://www.who.int/gho/ncd/risk_factors/overnweight/en
|
|
2
|
Apovian CM: Obesity: Definition,
comorbidities, causes and burden. Am J Manag Care. 22 (7
Suppl):S176–S185. 2016.PubMed/NCBI
|
|
3
|
Houde AA, Hivert MF and Bouchard L: Fetal
epigenetic programming of adipokines. Adipocyte. 2:41–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O´Reilly JR and Reynolds RM: The risk of
maternal obesity to the long-term health of the offspring. Clin
Endocrinol (Oxf). 78:9–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wellen KE and Hotamisligil GS:
Inflammation, stress, and diabetes. J Clin Invest. 115:1111–1119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gencer M, Gazi E, Hacivelioğlu S,
Binnetoğlu E, Bartçu A, Türkön H, Temiz A, Altun B, Vural A,
Cevizci S, et al: The relationship between subclinical
cardiovascular disease and lipocalin-2 levels in women with PCOS.
Eur J Obstet Gynecol Reprod Biol. 181:99–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kolb R, Sutterwala FS and Zhang W: Obesity
and cancer: Inflammation bridges the two. Curr Opin Pharmacol.
29:77–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsatsanis C, Dermitzaki E, Avgoustinaki P,
Malliaraki N, Mytaras V and Margioris AN: The impact of adipose
tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG)
axis. Hormones (Athens). 14:549–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balistreri CR, Caruso C and Candore G: The
Role of adipose tissue and adipokines in obesity-related
inflammatory diseases. Mediators Inflamm. 2010:8020782010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lillycrop KA and Burdge GC: Epigenetic
changes in early life and future risk of obesity. Int J Obes
(Lond). 35:72–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Castro NP, Euclydes VV, Simoes FA,
Vaz-de-Lima LR, De Brito CA, Luzia LA, Devakumar D and Rondó PH:
The relationship between maternal plasma leptin and adiponectin
concentrations and newborn adiposity. Nutrients. 9:E1822017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao L, Yoo HS, Madon A, Kinney B, Hay WW
Jr and Shao J: Adiponectin enhances mouse fetal fat deposition.
Diabetes. 61:3199–3207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao L, Wattez JS, Lee S, Guo Z, Schaak J,
Hay WW Jr, Zita MM, Parast M and Shao J: Knockout maternal
adiponectin increases fetal growth in mice: Potential role for
trophoblast IGFBP-1. Diabetologia. 59:2417–2425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson RJ, Segal MS, Sautin Y, Nakagawa
T, Feig DI, Kang DH, Gersch MS, Benner S and Sánchez-Lozada LG:
Potential role of sugar (fructose) in the epidemic of hypertension,
obesity and the metabolic syndrome, diabetes, kidney disease, and
cardiovascular disease. Am J Clin Nutr. 86:899–906. 2007.PubMed/NCBI
|
|
17
|
Shapiro A, Mu W, Roncal C, Cheng KY,
Johnson RJ and Scarpace PJ: Fructose-induced leptin resistance
exacerbates weight gain in response to subsequent high-fat feeding.
Am J Physiol Regul Integr Comp Physiol. 295:R1370–R1375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beck B, Richy S, Archer ZA and Mercer JG:
Ingestion of carbohydrate-rich supplements during gestation
programs insulin and leptin resistance but not body weight gain in
adult rat offspring. Front Physiol. 3:2242012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perez-Capistran T, Chávez-Negrete A,
Palomares M, Damasio-Santana L, Ramírez A, Pérez MI, Villatoro
Martínez A and Manuel-Apolinar L: Relationship of receptors of
adipokines with hypertension and obesity. Murine model. Rev Med
Inst Mex Seguro Soc. 55 (Suppl 4):S358–S364. 2017.PubMed/NCBI
|
|
20
|
Dobrzyn K, Smolinska N, Kiezun M, Szeszko
K, Rytelewska E, Kisielewska K, Gudelska M and Kaminski T:
Adiponectin: A new regulator of female reproductive system. Int J
Endocrinol. 2018:79650742018. View Article : Google Scholar
|
|
21
|
Kawwass JF, Summer R and Kallen CB: Direct
effects of leptin and adiponectin on peripheral reproductive
tissues: A critical review. Mol Hum Reprod. 21:617–632. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jungheim ES, Macones GA, Odem RR,
Patterson BW, Lanzendorf SE, Ratts VS and Moley KH: Associations
between free fatty acids, cumulus oocyte complex morphology and
ovarian function during in vitro fertilization. Fertil Steril.
95:1970–1974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Lam KS, Kraegen EW, Sweeney G,
Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL and Xu A:
Lipocalin-2 is an inflammatory marker closely associated with
obesity, insulin resistance, and hyperglycemia in humans. Clin
Chem. 53:34–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thraikill KM, Moreau CS, Cockrell GE, Jo
CH, Bunn RC, Morales-Pozzo AE, Lumpkin CK and Fowlkes JL: Disease
and gender-specific dysregulation of NGAL and MMP-9 in type 1
diabetes mellitus. Endocrine. 37:336–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Anna R, Baviera G, Corrado F, Giordano
D, Recupero S and Di BA: First trimester serum neutrophil
gelatinase-associated lipocalin in gestational diabetes. Diabet
Med. 26:1293–1295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fried SK and Greenberg AS: Lipocalin2: A
‘sexy’ adipokine that regulates 17β estradiol and obesity.
Endocrinology. 153:1582–1584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Drew BG, Hamidi H, Zhou Z, Villanueva CJ,
Krum SA, Calkin AC, Parks BW, Ribas V, Kalajian NY, Phun J, et al:
Estrogen receptor (ER) α-regulated lipocalin 2 expression in
adipose tissue links obesity with breast cancer progression. J Biol
Chem. 290:5566–5581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu YF, Li MY, Yan YP, Wei W, Li B, Pan
HY, Yang ZM and Liang XH: ERα- dependent stimulation of Lcn2 in
uterine epithelium during mouse early pregnancy. Reproduction.
159:493–501. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JH, Meyers MS, Khuder SS, Abdallah SL,
Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C and
Mauvais-Jarvis F: Tissue-selective estrogen complexes with
bazedoxifene prevent metabolic dysfunction in female mice. Mol
Metab. 3:177–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flower DR: The lipocalin protein family:
Structure and function. Biochem J. 318:1–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kjeldsen L, Cowland JB and Borregaard N:
Human neutrophil gelatinase-associated lipocalin and homologous
proteins in rat and mouse. Biochim Biophys Acta. 1482:272–283.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De la Chesnaye E, Kerr B, Paredes A,
Merchant-Larios H, Méndez JP and Ojeda S: Fbxw15/Fbxo12J is an F
Box protein-encoding gene selectively expressed in oocytes of the
murine ovary. Biol Reprod. 78:714–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De la Chesnaye E, Manuel-Apolinar L,
Damasio L, Olivares A, Palomino MA, Santos I and Méndez JP:
Expression profiling of lipocalin-2 and 24p3 receptor in murine
gonads at different developmental stages. Exp Ther Med. 16:213–221.
2018.PubMed/NCBI
|
|
34
|
Devireddy LR, Gazin C, Zhu X and Green MR:
A cell-surface receptor for lipocalin 24p3 selectively mediates
apoptosis and iron uptake. Cell. 123:1293–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gettayacamin M and Retnam L: AAALAC
international standards and accreditation process. Toxicol Res.
33:183–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Loza-Medrano SS, Baiza-Gutman LA,
Manuel-Apolinar L, García-Macedo R, Damasio-Santana L, Martínez-Mar
OA, Sánchez-Becerra MC, Cruz-López M, Ibáñez-Hernández MA and
Díaz-Flores M: High fructose-containing drinking water-induced
steatohepatitis in rats is prevented by the nicotinamide-mediated
modulation of redox homeostasis and NADPH-producing enzymes. Mol
Biol Rep. 47:337–351. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chandrasekaran S and Neal-Perry G:
Long-term consequences of obesity on female fertility and the
health of the offspring. Curr Opin Obstet Gynecol. 29:180–187.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cohen PE and Pollard JW: Regulation of
meiotic recombination and prophase I progression in mammals.
Bioessays. 23:996–1009. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Western PS, Miles DC, van den Bergen JA,
Burton M and Sinclair AH: Dynamic regulation of mitotic arrest in
fetal male germ cells. Stem Cells. 26:339–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Drummond AE: The role of steroids in
follicular growth. Reprod Biol Endocrinol. 4:162006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elkhidir AE, Eltaher HB and Mohamed AO:
Association of lipocalin-2 level, glycemic status and obesity in
type 2 diabetes mellitus. BMC Res Notes. 10:2852017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Na GY, Yoon SR, An J, Yeo R, Song J, Jo
MN, Han S and Kim OY: The relationship between circulating
neutrophil gelatinase-associated lipocalin and early alteration of
metabolic parameters is associated with dietary saturated fat
intake in non-diabetic Korean women. Endocr J. 64:303–314. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rees WD and Hay SM: Lipocalin-2 (Lcn2)
expression is mediated by maternal nutrition during development of
the fetal liver. Genes Nutr. 9:3802014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Plagemann A: Perinatal programming and
functional teratogenesis: Impact on body weight regulation and
obesity. Physiol Behav. 86:661–668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gluckman PD and Hanson MA: The
developmental origins of the metabolic syndrome. Trends Endocrinol
Metab. 15:183–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Law IK, Xu A, Lam KS, Berger T, Mak TW,
Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B and Wang Y:
Lipocalin-2 deficiency attenuates insulin resistance associated
with aging and obesity. Diabetes. 59:872–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujino RS, Tanaka K, Morimatsu M, Tamura
K, Kogo H and Hara T: Spermatogonial cell-mediated activation of an
IkappaBzeta-independent nuclear factor-kappaB pathway in Sertoli
cell induces transcription of the lipocalin-2 gene. Mol Endocrinol.
20:904–915. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Westermeier F, Sáez PJ, Villalobos-Labra
R, Sobrevia L and Farías-Jofré M: Programming of fetal insulin
resistance in pregnancies with maternal obesity by ER stress and
inflammation. Biomed Res Int. 2014:9176722014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu G, Ahn J, Chang S, Eguchi M, Ogier A,
Han S, Park Y, Shim C, Jang Y, Yang B, et al: Lipocalin-2 induces
cardiomyocyte apoptosis by increasing intracellular iron
accumulation. J Biol Chem. 287:4808–4817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chella Krishnan K, Sabir S, Shum M, Meng
Y, Acín-Pérez R, Lang JM, Floyd RR, Vergnes L, Seldin MM, Fuqua BK,
et al: Sex-specific metabolic functions of adipose Lipocalin-2. Mol
Metab. 30:30–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roche J, Ramé C, Reverchon M, Mellouk N,
Cornuau M, Guerif F, Froment P and Dupont J: Apelin (APLN) and
apelin receptor (APLNR) in human ovary: Expression, signaling, and
regulation of steroidogenesis in primary human luteinized granulosa
cells. Biol Reprod. 95:1042016. View Article : Google Scholar : PubMed/NCBI
|