Introduction
Chronic kidney disease (CKD) is a long-term
condition characterized by renal dysfunction that lasts 3 months
(1). CKD remains a leading public
health issue, and patients with CKD have poorer quality of life
compared with those of healthy individuals (2). There are numerous causes of CKD, the
most typical of which are hypertension and type 2 diabetes
(3). Although several studies on
CKD have been performed (4,5), the
molecular mechanisms involved in the occurrence and development of
this disease are not fully understood yet. Therefore, there is an
urgent need to understand the mechanism of CKD and to identify
appropriate strategies to manage it.
Renal interstitial fibrosis occurs in virtually
every type of CKD, and the severity of tubulointerstitial fibrosis
has long been considered a crucial factor of progressive renal
injury in glomerulonephritis (6).
The primary histopathological features of renal interstitial
fibrosis include marked accumulation of fibroblasts and deposition
of extracellular matrix (7).
Several cellular pathways, including the mesangial and tubular
epithelial-myofibroblast transdifferentiation (TEMT), have been
identified as important in renal interstitial fibrosis (8). The main feature of TEMT is the fact
that renal tubular epithelial cells acquire mesenchymal phenotypes
and myofibroblast functions. The transdifferentiation of TEMT
induces kidney epithelial cells to decrease the expression of
adherens junction proteins, such as E-cadherin, and to promote the
expression of numerous fibroblast markers, including α-smooth
muscle actin (α-SMA), transforming growth factor-β1 (TGF-β1) and
fibroblast growth factor-2 (FGF-2) (9). The inhibition of TEMT has also been
considered as an effective way to attenuate renal interstitial
fibrosis (10).
Heparan sulfate (HS) proteoglycans are important
constituents of the cell membrane, and they act as co-receptors for
cellular signaling. Cell surface HS proteoglycans are important
regulators of cellular migratory, mitogenic, secretory and
inflammatory activities (11).
Syndecan-1 (SDC1) is a member of the syndecan family, which
comprises a transmembrane HS proteoglycan. It can exert various
biological functions at the surface of renal epithelial cells, and
plays important roles in cell-cell or cell-matrix interactions
(12). Heparanase (HPSE), as an
endo-β-D-glucuronidase, can cleave HS at specific intrachain sites
(13). Precious studies reported
that increased levels of HPSE activity are associated with several
diseases, such as renal fibrosis, cancer (14) and Alzheimer's disease (15). Several stimulating factors, such as
ROS and angiotensin II (AngII), can promote the expression of HPSE,
which in turn cleaves the HS side-chains of SDC1 and promotes TEMT,
eventually leading to renal fibrosis (16).
Salvianolic acid B (Sal B) is a phenolic acid
isolated from Salvia miltiorrhiza Bge. It exhibits various
pharmacological activities, including antioxidant (17), anti-myocardial ischemia (18), antitumor (19), and anti-inflammatory (20) activities. A previous study has
demonstrated that Sal B has kidney-protective effects (21). However, whether Sal B could reduce
renal interstitial fibrosis by regulating the activation of the
HPSE/SDC1 axis remains unknown. It was hypothesized that the
mechanism of Sal B against renal interstitial fibrosis may be
associated with the inhibition of the HPSE/SDC1 axis. Therefore,
the present study was designed to investigate the renal-protective
effects of Sal B and to explore its underlying mechanism.
Materials and methods
Materials
Sal B (≥98% purity) was provided by Nanjing Hongqiao
Institute of Pharmaceutical Technology. Dulbecco's modified Eagle's
medium/Ham's F12 (DMEM/F12) was purchased from Wisent, Inc. Fetal
bovine serum (FBS) was purchased from Biological Industries.
Trypsin was purchased from Beyotime Institute of Biotechnology.
Serum creatinine (CR; cat. no. ANG-E12726H), blood urea nitrogen
(BUN; cat. no. ANG-E12727H), TGF-β1 (cat. no. ANG-E10095H) and
FGF-2 (cat. no. ANG-E12728H) ELISA kits were purchased from
AngleGene BioTechnology Co. Ltd, Nanjing, China. AngII was
purchased from Tocris Bioscience. Primary antibodies against HPSE
(cat. no. ab59787), SDC1 (cat. no. ab128936), TGF-β1 (cat. no.
ab92486), FGF-2 (cat. no. ab8880) and α-SMA (cat. no. ab5694) were
obtained from Abcam. Antibodies against E-cadherin (cat. no. 3195S)
and GAPDH (cat. no. 5174s) were obtained from Cell Signaling
Technology, Inc. The water used in this study was purified by a
Milli-Q® water purification system (EMD Millipore). All
culture plates were obtained from Corning, Inc.
Animals
A total of 35 2-month-old male C57BL/6 (C57) mice
(20±2 g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. The mice were housed under pathogen-free
conditions under a 12-h light/dark cycle at 22–24°C and had ad
libitum access to water and a regular pellet diet (22). The present study was carried out in
accordance with the principles of the Basel Declaration and the
recommendations of the Guidelines of Jiangsu Regulation for the
Administration of Laboratory Animals, and the protocols were
approved by the Animal Ethics Committee of Nanjing University of
Chinese Medicine (approval no. ACU-2320151203).
Experimental protocol and drug
administration
The mice were randomly divided into 5 groups (n=7
mice/group, the group size was decided based on the initial pilot
study), including the sham, unilateral ureteral obstruction (UUO),
UUO + Sal B (6.25 mg/kg), UUO + Sal B (12.5 mg/kg) and UUO + Sal B
(25 mg/kg) groups. The mice in the UUO and Sal B treatment groups
were subjected to partial UUO operation according to a previous
study (23). Briefly, sodium
pentobarbital (50 mg/kg) was used to anesthetize the mice, and then
the left ureter was isolated and a 0.20-mm steel wire segment was
placed next to the left ureter at the ureteropelvic junction. The
wire was ligated with the ureter using a single 6-0 silk suture,
and then the wire was removed. The mice in sham group underwent the
same operation without ligation. After the operation, the mice in
the Sal B treatment groups received a daily intraperitoneal
injection of Sal B, while the mice in the sham and UUO groups
received equal amounts of normal saline. All mice were sacrificed
14 days later. The anesthetic drug sodium pentobarbital (50 mg/kg)
was used to anesthetize the mice, then the blood was collected from
the mice retro-orbital sinus for a series of biochemical assays.
Then, CO2 (20% V/min; 1.2 l/min) was used to euthanize
mice at the endpoint, and the kidneys were harvested for
morphological and biochemical studies. Skillful operations were
applied to reduce animal suffering during the experiment.
Histopathological observation
The kidneys were immersed in 10% formaldehyde
solution for 24 h at room temperature immediately after isolation
and then embedded in paraffin. Next, the kidney tissues were cut
into 4-µm-thick sections (24).
The tissue sections were stained with hematoxylin and eosin
(H&E) for 5 min or Masson's stain for 10 min at room
temperature to observed the histopathological changes under a light
microscope at ×200 magnification.
Immunohistochemical analyses
Mice kidney sections were treated for 10 min with 3%
hydrogen peroxide/methanol and 30 min with 5% BSA (Solarbio Life
Sciences; cat. no. A8010) at room temperature. Sections were
incubated overnight at 4°C with primary antibodies against HPSE
(1:100) and SDC1 (1:100), and then labeled with
streptavidin-peroxidase (1:50; cat. no. A0208; Beyotime Institute
of Biotechnology) for 30 min at 37°C, followed by 5 min
3,3′-diaminobenzidine tetrahydrochloride solution staining, 8 min
hematoxylin counterstaining and mounting using neutral balsam.
Immunoreactive proteins were viewed with a light microscope at ×200
magnification.
Determination of CR, BUN, TGF-β1 and
FGF-2 levels in serum
Blood was collected from the mice retro-orbital
sinus on day 14 after surgery. Then, the serum CR, BUN, TGF-β1 and
FGF-2 levels were assessed with a microplate system (BioTek
Instruments, Inc.) according to the manufacturer's instructions
(25).
Cell culture and treatment
HK-2 cells were obtained from American Type Culture
Collection and routinely cultured in DMEM/F12 supplemented with 10%
FBS and incubated at 37°C with 5% CO2.
HK-2 cells were seeded on a 6-well plate a density
of 5×104 cells. Subsequently, 1 µM AngII was added to
stimulate the cells for 48 h at 37°C. Then, cells were treated with
different concentrations of Sal B (0.1, 1 and 10 µM) for 24 h at
37°C. The cells or the cell supernatants (centrifugation at 300 × g
at room temperature for 5 min) were then collected for various
analyses.
MTT assay
To determine the appropriate AngII treatment
concentration, an MTT assay was performed. Briefly, HK-2 cells were
seeded on a 96-well plate at a density of 1×104 cells.
After culture overnight, different concentrations of AngII (0,
0.0001, 0.001, 0.01, 0.1, 1, 10 and 100 µM) were added to each well
and stimulated for 48 h at 37°C. Then, MTT (5 mg/ml) prepared with
phosphate buffer (PBS) was added to each well (10 µl). After
incubation for 4 h at 37°C, the supernatant of each well was
discarded and the formazan was dissolved in 150 µl DMSO. Then, the
absorbance at 570 nm was read by BioTek Synergy™ 2 microplate
reader (BioTek Instruments, Inc.) and the cell viability of each
group was calculated. According to the experimental results, when
the concentration of AngII was ≥10 µM, the viability of HK-2 cells
declined markedly, and AngII stimulation (1 µM) could notably
promote the expression of key regulators of TEMT, such as TGF-β1
and FGF-2. So, 1 µM was selected as the treatment concentration of
AngII (data not shown).
Detection of TGF-β1 and FGF-2 in cell
supernatant
HK-2 cells were seeded on a 6-well plate at a
density of 5×104 cells. After overnight culture, AngII
(final concentration, 1 µM) was added to each well and stimulated
for 48 h at 37°C. Subsequently, cells were treated with different
concentrations of Sal B (0.1, 1 and 10 µM) for 24 h at 37°C, and
the cell supernatant of each group was collected for analysis
(centrifugation at 300 × g at room temperature for 5 min).
Immunofluorescence
HK-2 cells seeded on glass coverslips in 24-well
plates at a density of 2×104 cells were washed 3 times
with PBS after treatment, and then fixed for 30 min with 4%
paraformaldehyde at 37°C. Next, cells were washed twice with PBS,
incubated with 1% BSA (Beijing Solarbio Science & Technology
Co., Ltd., cat. no. A8010) for 1 h at room temperature, and
incubated with a primary rabbit anti-HPSE (1:100) or anti-SDC1
(1:100) antibody in blocking buffer for 12 h at 4°C. Then, cells
were washed twice with PBS and incubated with DAPI-goat anti-rabbit
antibody (1:500; Beijing Solarbio Science & Technology Co.,
Ltd., cat. no. A0562) for 2 h at 37°C. The images were acquired
using a fluorescence microscope (26).
Western blotting
The kidney tissues (including both the medulla and
cortex) and HK-2 cells were homogenized in ice-cold RIPA buffer
containing 2 mM PMSF. The samples were centrifuged at 12,000 × g
for 15 min at 4ºC, and the concentrations of total
protein were quantified using a NanoDrop instrument (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Total protein
samples (30 µg) were then separated by SDS-PAGE on 6–10% gels and
electro-transferred onto PVDF membranes. Then, the membranes were
blocked with 5% BSA for 1 h at room temperature and incubated
overnight at 4°C with primary antibodies against HPSE (1:1,000),
SDC1 (1:1,000), FGF-2 (1:1,000), TGF-β1 (1:1,000), E-cadherin
(1:1,000), α-SMA (1:1,000) and GAPDH (1:1,000). After washing 3
times with TBS-Tween-20 (1%), membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:1,000;
Beijing Solarbio Science & Technology Co., Ltd., cat. no.
A0208) in 5% BSA at room temperature for 1 h (27). The membranes were visualized using
an ECL advanced kit and target proteins were detected with a gel
imaging system (Image Lab software version 4.0.1; Bio-Rad
Laboratories, Inc.).
Cell transfection
Plasmids for overexpression of HPSE and empty
control plasmids were purchased from Shanghai GenePharma Co., Ltd.
HK-2 cells were seeded on a 6-well plate at a density of
5×104 cells and divided into five groups, including the
negative control (NC), Homo HPSE + AngII, Homo HPSE, NC + AngII and
Homo HPSE + AngII + Sal B groups. Transient transfection was
performed using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 3 µg overexpressed RNA (final concentration, 3 µg/ml), and 9 µl
Lipofectamine 2000 were added to the serum-free medium and
incubated at 25°C for 20 min. Then, the plasmids were mixed into
per group and cultured in serum-free DMEM at 37°C. After 6 h in
culture, the medium was replaced by DMEM containing 10% FBS and the
cells were cultured for 24 h at 37°C.
Statistical analysis
Data are presented as the mean ± SD. The statistical
significance of differences between the means of multiple groups
was analyzed by one-way ANOVA, followed by Tukey's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of Sal B on renal morphology in
mice with renal fibrosis
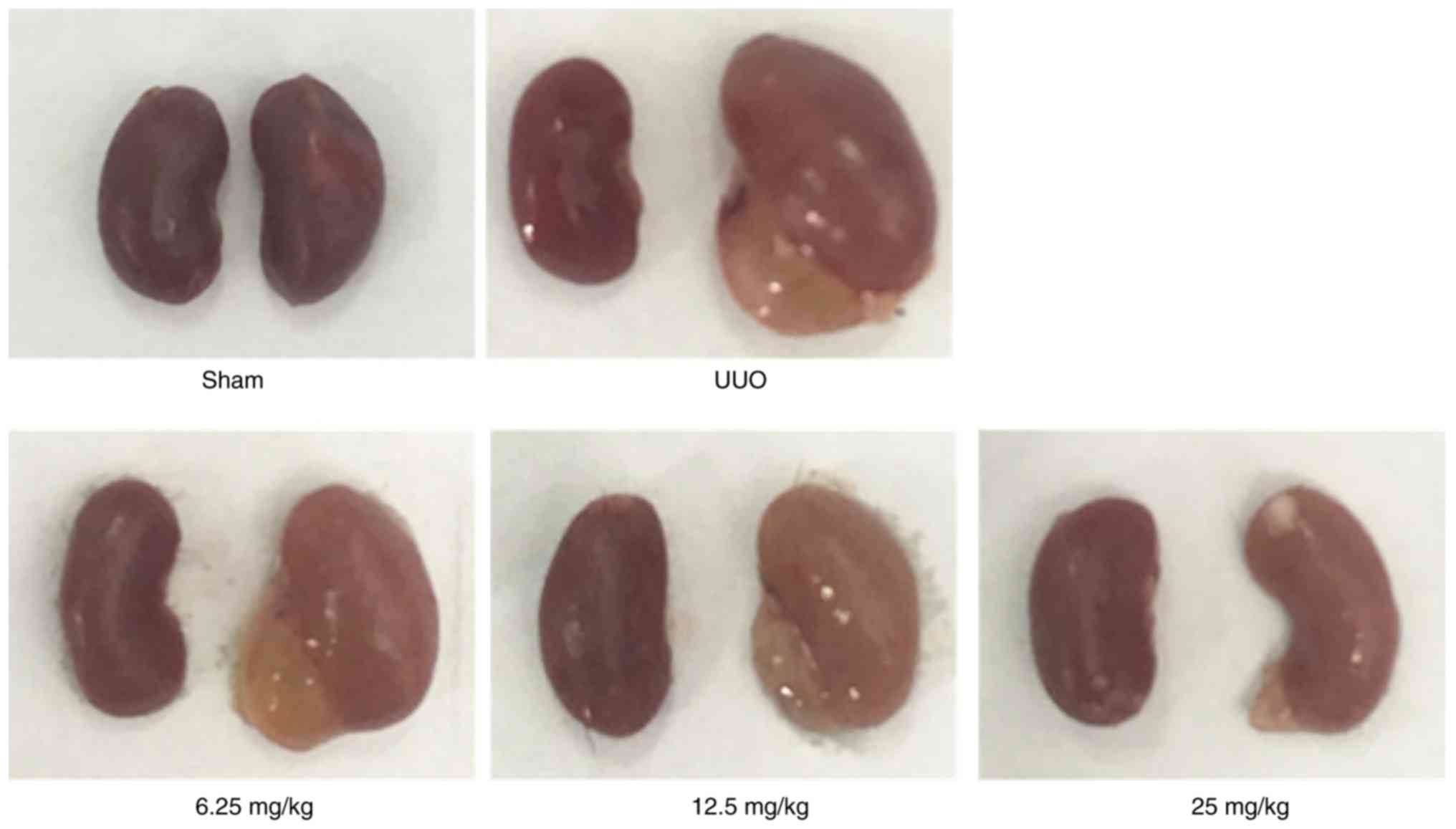
To explore the protective effects of Sal B on
UUO-induced mice kidneys, 3 mice were randomly selected 14 days
after modelling. As shown in Fig.
1, the sizes of the kidney in the UUO group were markedly
increased after left ureteral ligation, whereas Sal B treatment
notably reduced the kidney sizes.
Sal B alleviates the pathological
changes of mice kidneys
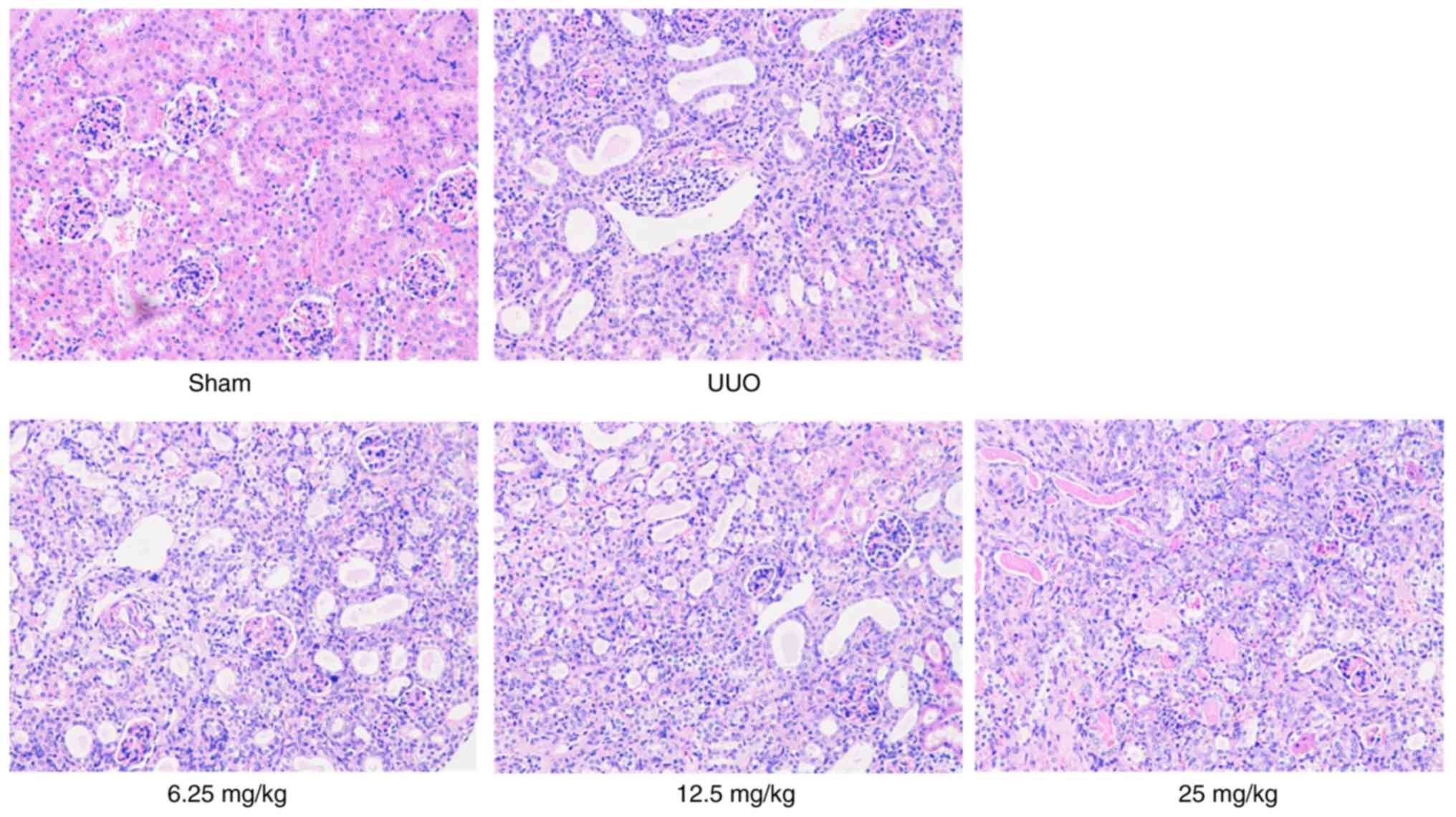
The results of H&E staining demonstrated that
the kidneys of the mice in the sham group maintained a normal
morphology and clear structure with no interstitial edema, while
those of the mice in the UUO group showed notable cavitation in the
renal tubular epithelium, evident inflammatory cell infiltration in
the renal tubulointerstitium and distinct tubular expansion. These
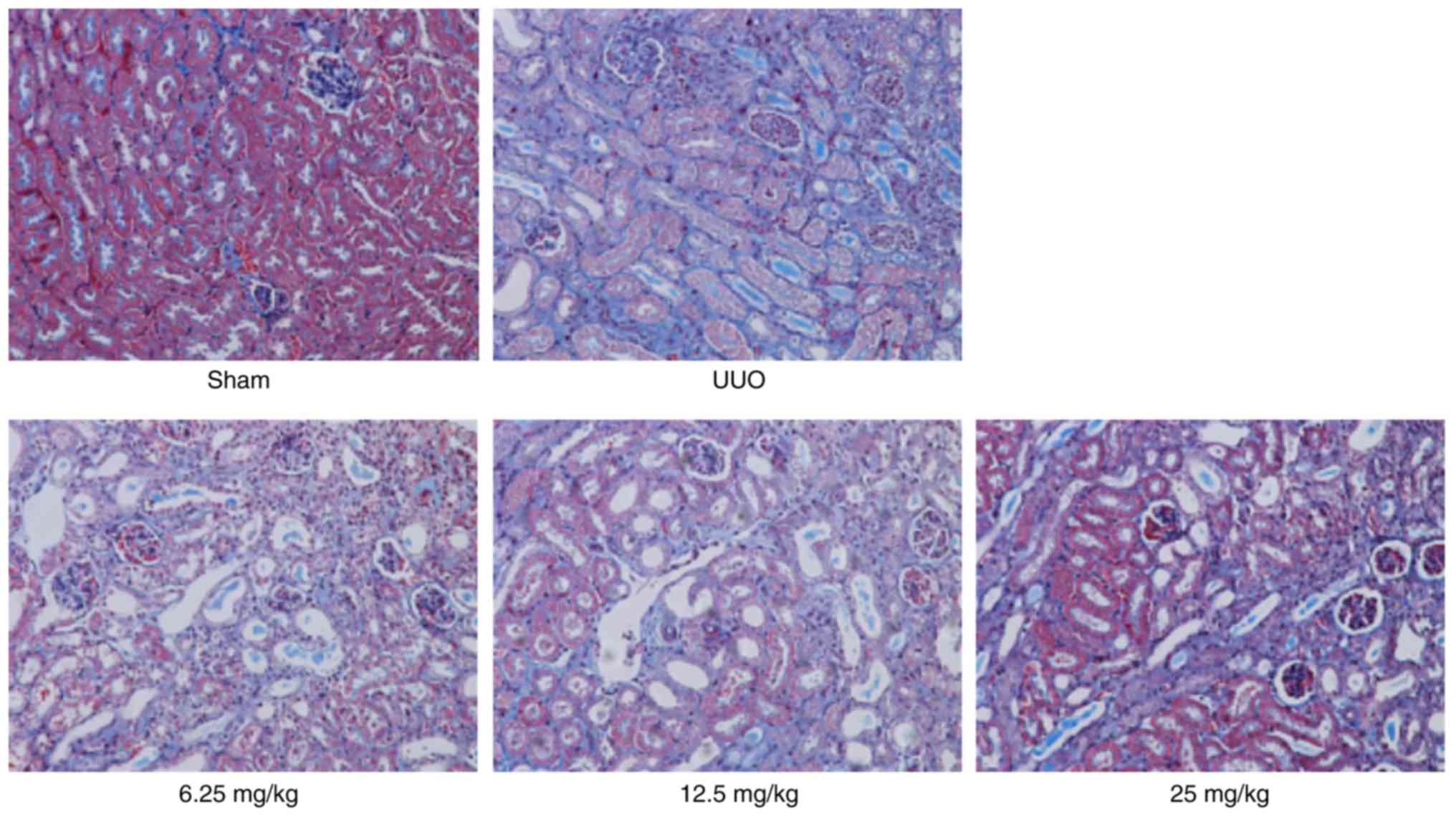
conditions could notably be restored by Sal B treatment (Fig. 2). Besides, the result of Masson's
staining showed that there were numerous collagen fiber streaks
stained blue with obvious collagen fiber hypertrophy in the UUO
group. However, fewer collagen fiber streaks were observed in the
Sal B treatment groups. These results indicated that Sal B could
alleviate renal injury and fibrosis in fibrotic mice (Fig. 3).
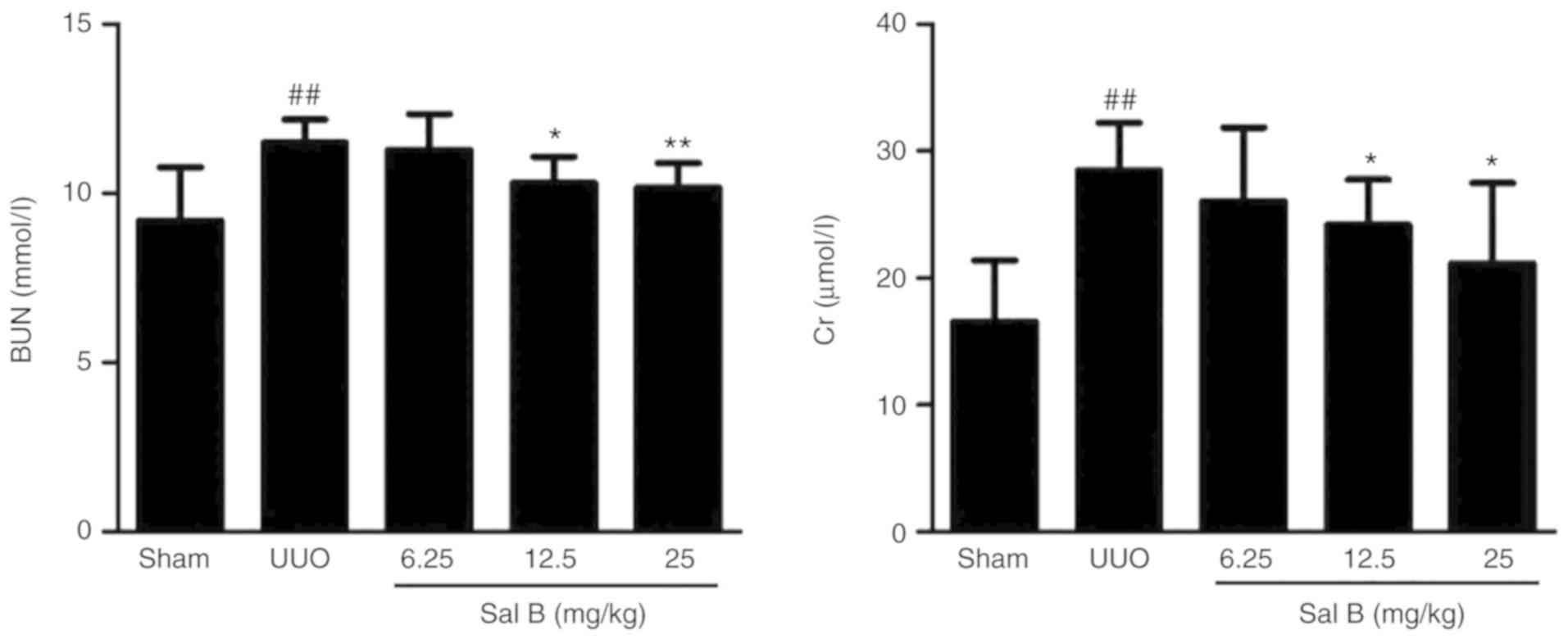
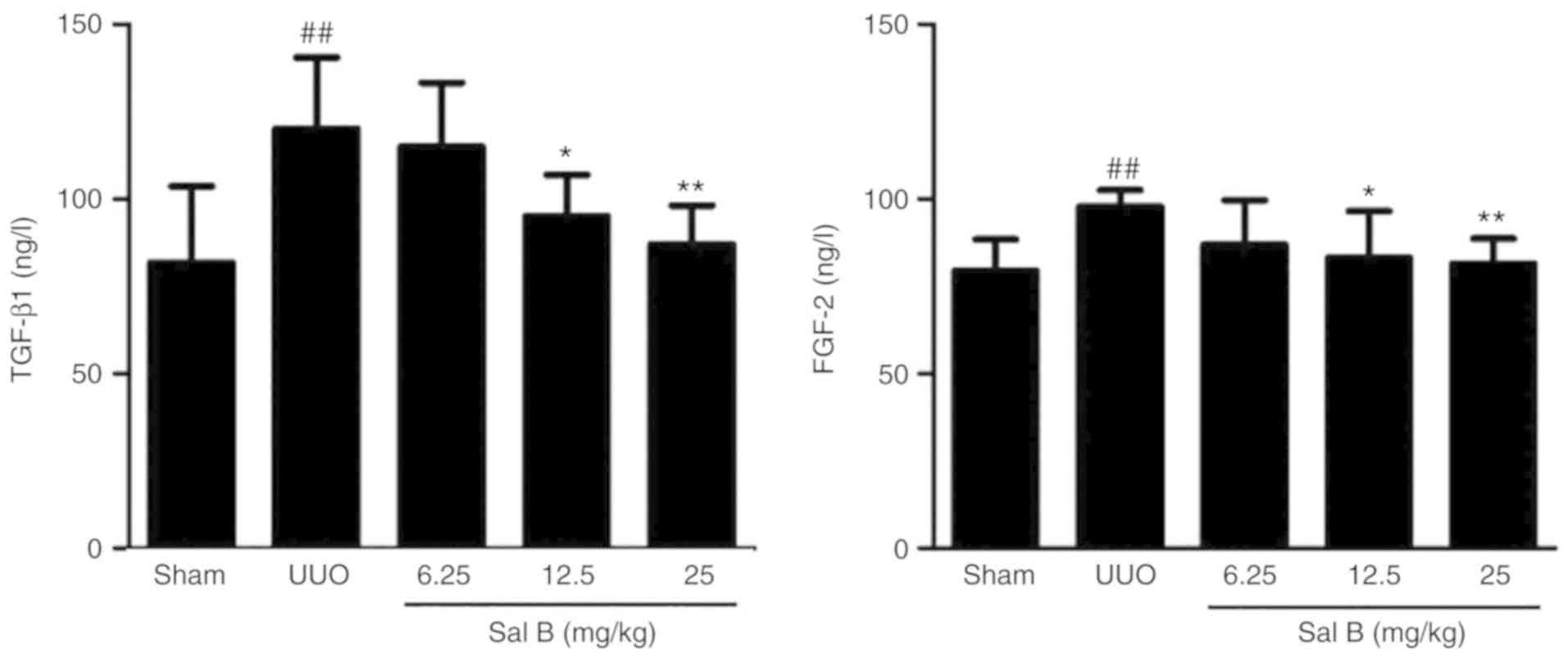
Effects of Sal B on BUN/CR in the
serum of renal fibrotic mice
The serum levels of BUN and CR are the classical
markers of renal function (28).
To evaluate the efficacy of Sal B on renal function after left
ureteral ligation, the expression levels of BUN and CR in serum
were determined in the present study. The results showed that left
ureteral ligation resulted in a significant increase in the levels
of BUN and CR (Fig. 4), but the
administration of Sal B (12.5 and 25 mg/kg) notably reduced the
expression of these cytokines.
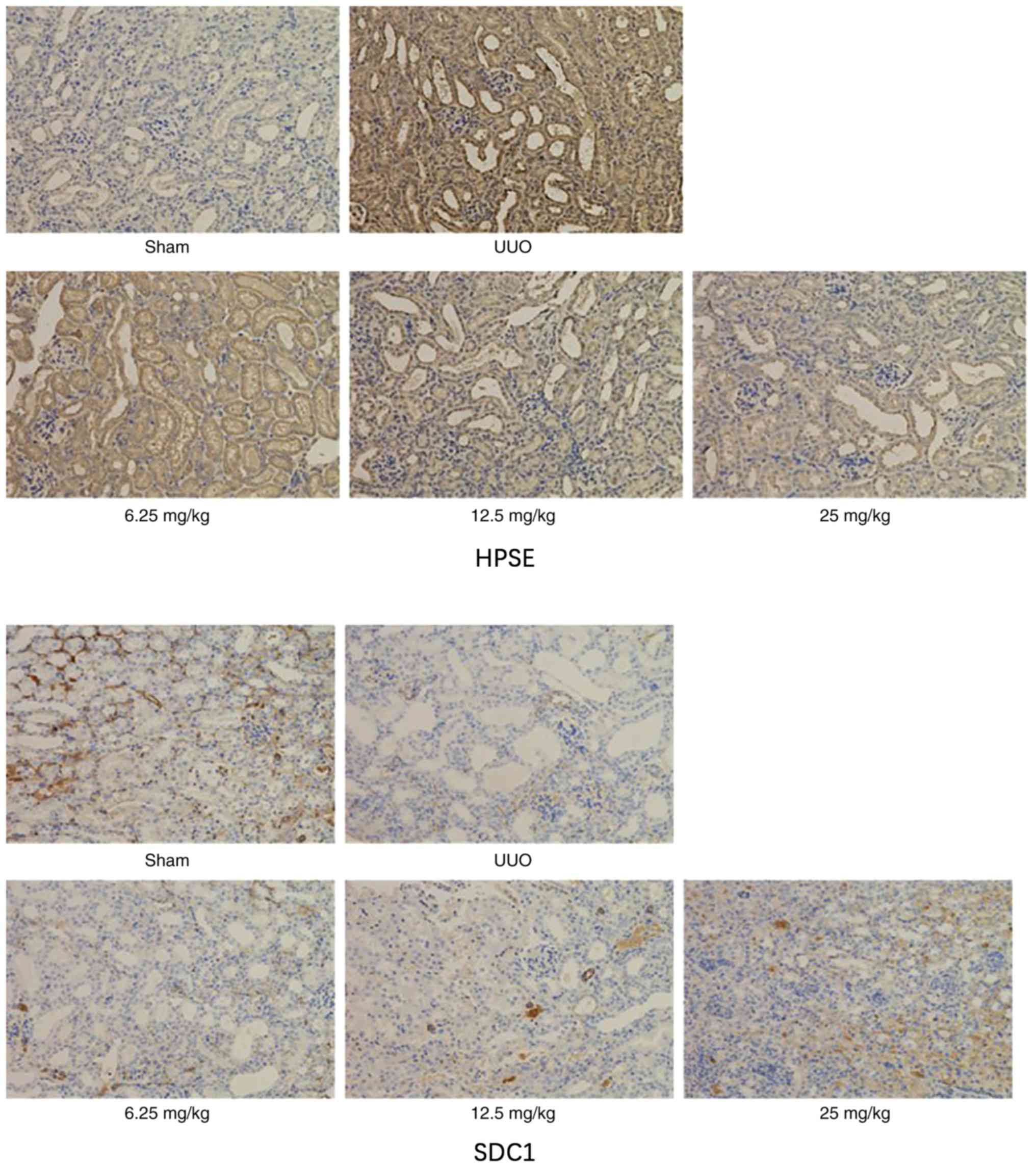
Sal B regulates the expression levels
of HPSE and SDC1 in mice kidneys
Using immunohistochemical analysis, the effect of
Sal B on the expression of HPSE and SDC1 in kidney tissues was
examined. As shown in Fig. 5, left
ureteral ligation notably downregulated the expression level of
HPSE and upregulated SDC1. However, these changes were notably
altered by Sal B treatment.
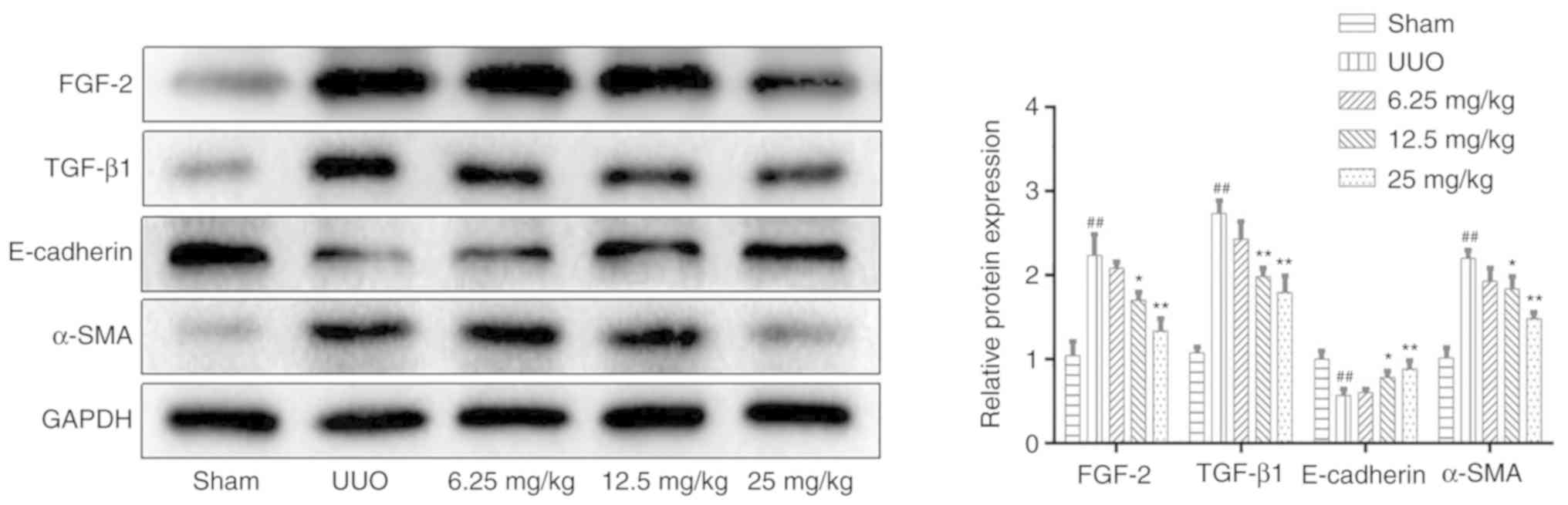
Sal B inhibits TEMT in renal fibrotic
mice
To evaluate whether Sal B could affect the
expression levels of fibrosis markers, ELISA and western blotting
were performed. The primary feature of renal interstitial fibrosis
is the accumulation of extracellular matrix components.
Myofibroblasts are considered to be primary renal interstitial
cells, which can produce large quantities of extracellular matrix
during fibrosis (29).
Furthermore, the activation of TEMT is closely associated with the
production of myofibroblasts (30). The present results showed that Sal
B could significantly downregulate the expression level of α-SMA
and upregulate the protein level of E-cadherin compared with the
UUO group (Fig. 6). TGF-β1 and
FGF-2 are key regulators of the TEMT process (31), the present study demonstrated that
Sal B attenuated the UUO-induced upregulation of these proteins in
UUO mice (Figs. 6 and 7).
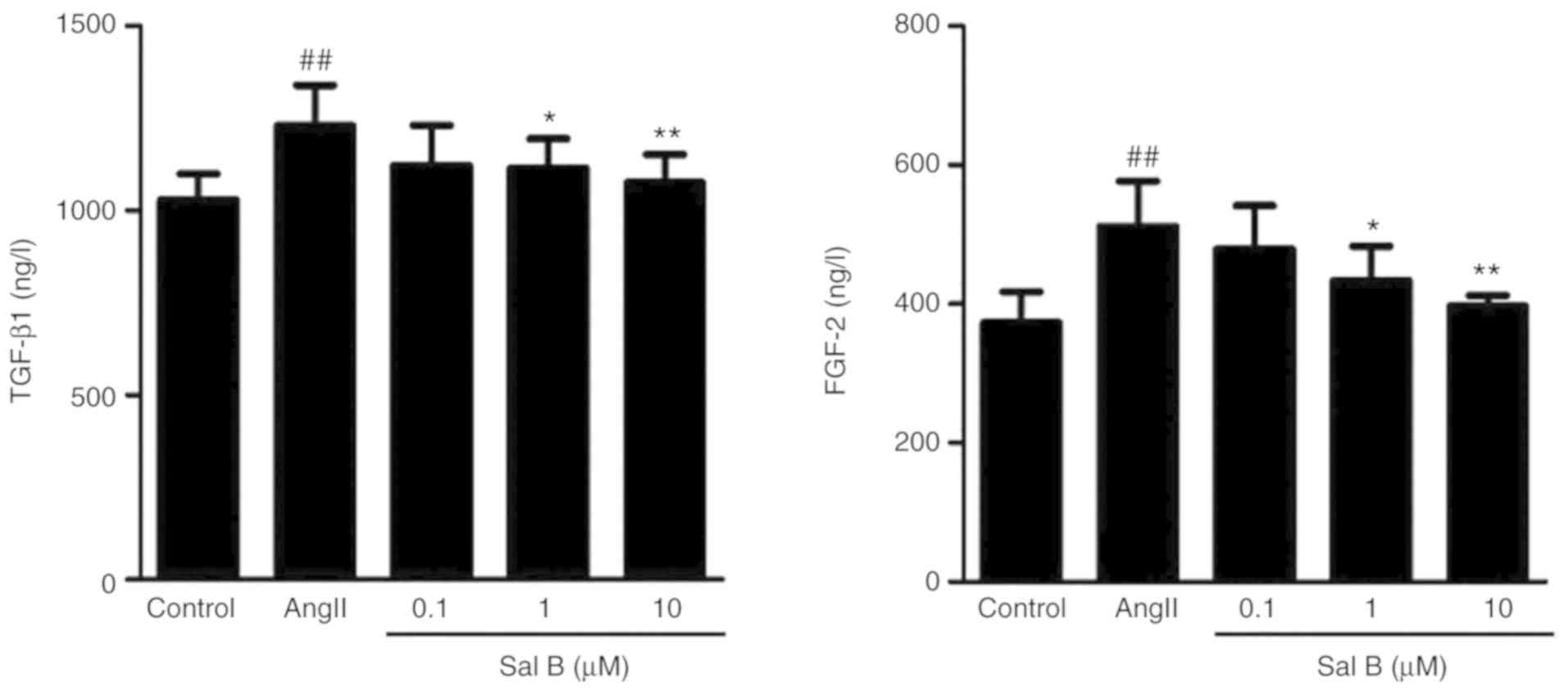
Effects of Sal B on TGF-β1/FGF-2 in
the supernatant of HK-2 cells
TGF-β1 and FGF-2 are key regulators of TEMT
(32). To evaluate the efficacy of
Sal B on TEMT, the expression levels of TGF-β1 and FGF-2 in HK-2
cell supernatant were determined. The results showed that AngII
stimulation significantly increased the expression levels of TGF-β1
and FGF-2 (Fig. 8). However, Sal B
treatment (1 and 10 µM) significantly reduced the expression levels
of these cytokines.
Sal B suppresses renal interstitial
fibrosis by inhibiting the HPSE/SDC1 axis
To explore the underlying mechanisms of Sal
B-mediated renal protection, the protein expression levels of the
HPSE/SDC1 axis were detected. The results showed that the protein
expression of HPSE, FGF-2, TGF-β1 and α-SMA were significantly
increased, while the expression levels of SDC1 and E-cadherin were
significantly reduced after modelling compared with those of the
control group. However, treatment with Sal B significantly reversed
these changes (Fig. 9).
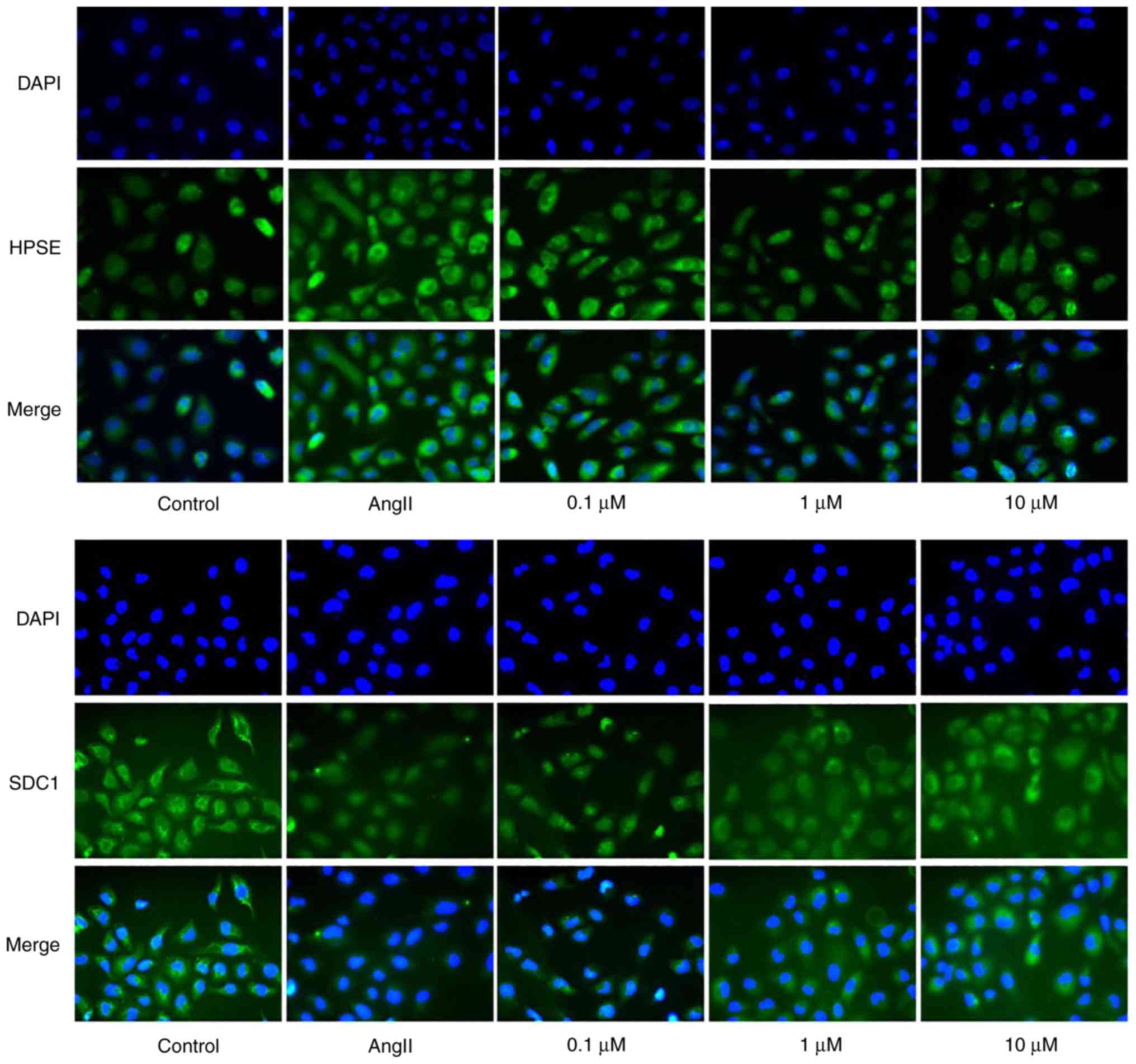
Furthermore, the results of immunofluorescence also showed that Sal
B could notably reduce the expression level of HPSE and promote the
expression level of SDC1 in HK-2 cells after treatment with AngII
(Fig. 10).
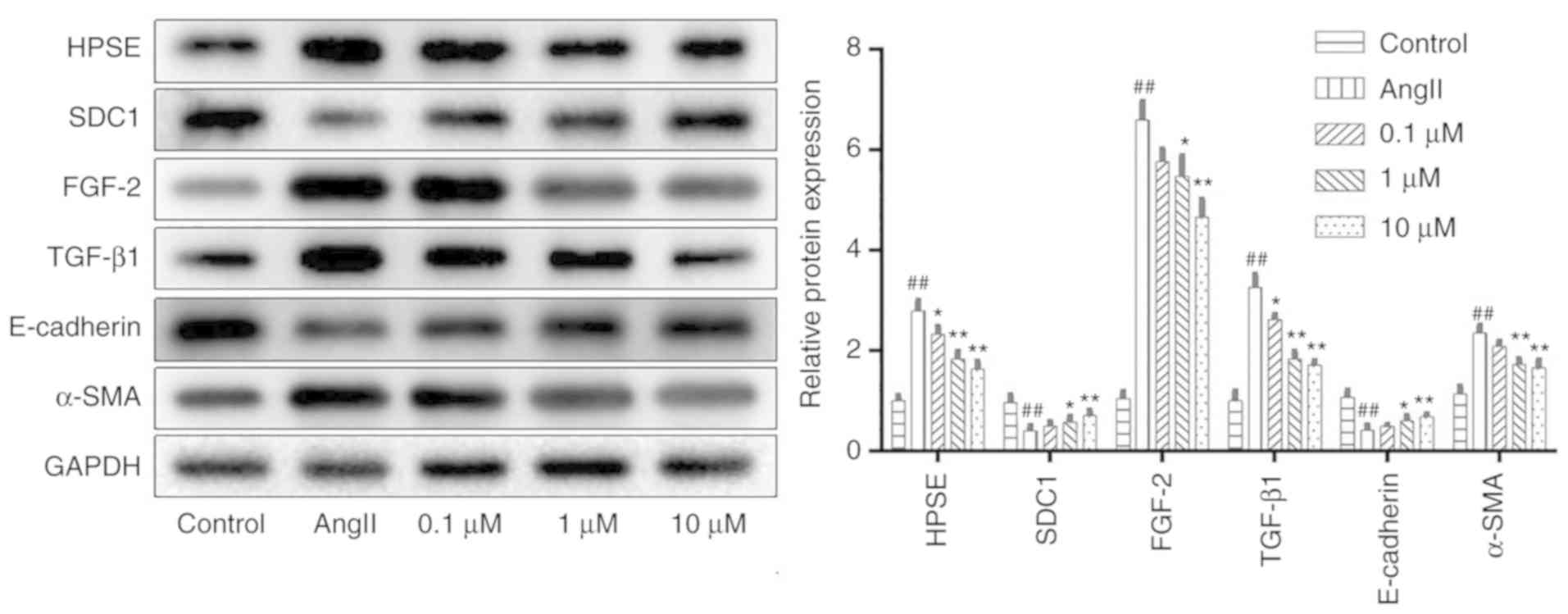 | Figure 9.Salvianolic acid B inhibits tubular
epithelial-myofibroblast transdifferentiation in HK-2 cells.
Western blotting was performed to determine the protein expression
levels of HPSE, SDC1, FGF-2, TGF-β1, E-cadherin and α-SMA in each
group. The expression was normalized to that of GAPDH and the data
are expressed as the mean ± SD of 3 independent experiments.
*P<0.05 and **P<0.01 vs. model group; ##P<0.01
vs. control group. HPSE, heparinase; SDC1, syndecan-1; FGF-2,
fibroblast growth factor-2; TGF-β1, transforming growth factor-β1;
α-SMA, α-smooth muscle actin; AngII, angiotensin II. |
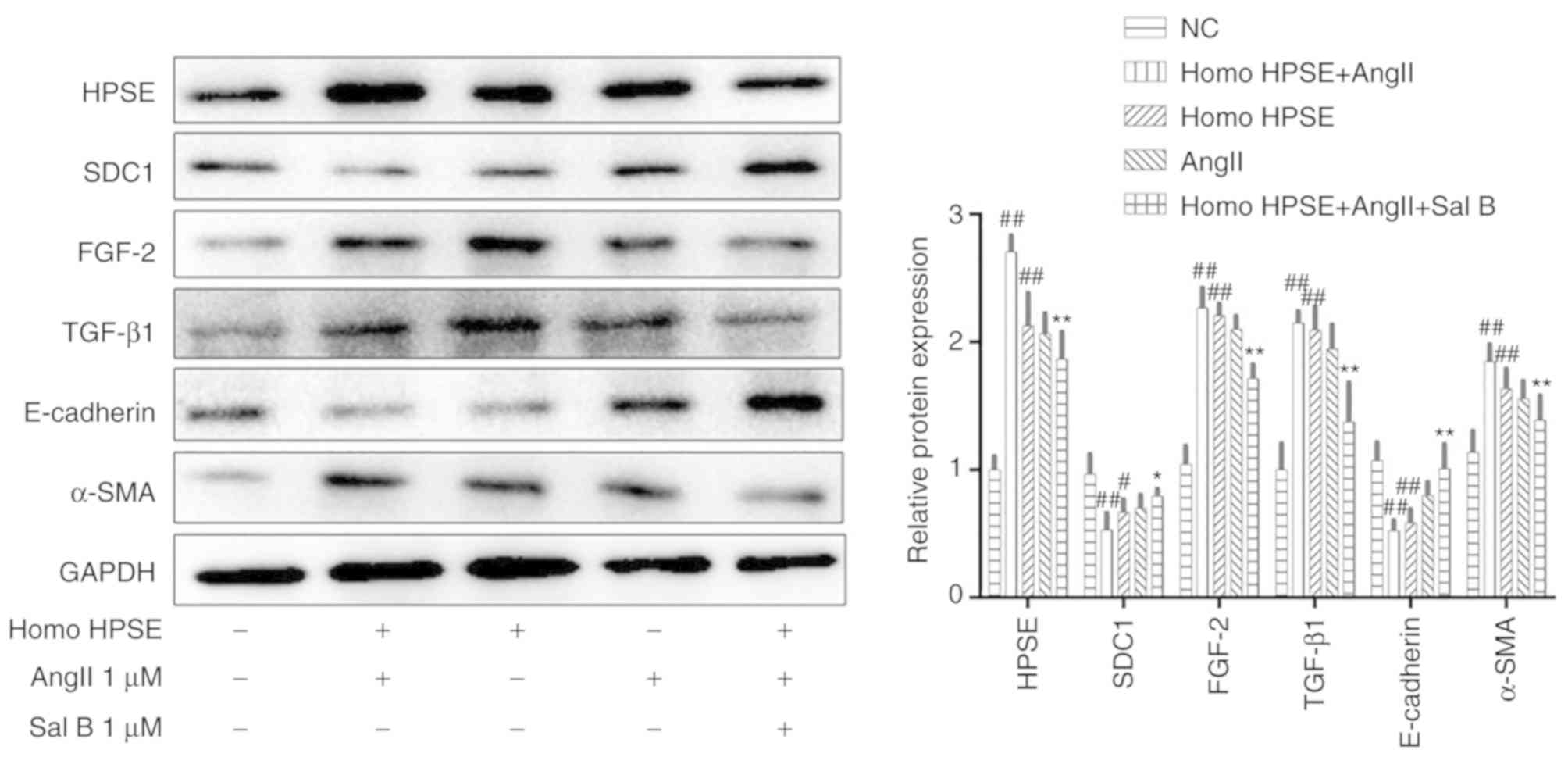
Overexpression of the HPSE gene
suppresses renal interstitial fibrosis by inhibiting the HPSE/SDC1
axis
In order to further assess the effects of the
overexpression of the HPSE gene on renal interstitial fibrosis, the
protein expression levels of the HPSE/SDC1 axis were detected using
western blotting. The findings showed that overexpression of HPSE
could significantly upregulate the expression levels of HPSE,
FGF-2, TGF-β1 and α-SMA, and downregulate the expression levels of
SDC1 and E-cadherin. However, treatment with Sal B could notably
restore these changes (Fig.
11).
Discussion
Various types of CKDs can lead to renal fibrosis and
eventually renal failure; thus, there are numerous studies on the
factors behind renal fibrosis. AngII is a key mediator that is
associated with the development of renal fibrosis (33,34),
it has been reported to promote TGF-β1 expression, leading to the
production of extracellular matrix and inhibition of the
extracellular matrix degradation (35). Moreover, TGF-β1 has been reported
to induce tubular epithelial cells to transdifferentiate into
myofibroblasts (36). In the
present study, AngII was used to promote the transdifferentiation
of HK-2 cells into myoblasts and to promote the expression of
TGF-β1. The results showed that AngII could promote TEMT via
activation of the HPSE/SDC1 axis. However, Sal B treatment notably
decreased the expression levels of extracellular matrix and TEMT
markers.
TEMT plays an important role in renal interstitial
fibrosis. Under the stimulation of numerous factors, renal tubular
epithelial cells could release cytokines (such as TGF-β1 and FGF-2)
into the tubulointerstitial microenvironment, and eventually lead
to TEMT (37,38), which is characterized by decreased
expression of E-cadherin and increased expression of α-SMA
(39). Sal B, as one of the main
water-soluble components of Salvia miltiorrhiza Bge, has
been shown by previous studies to be able to inhibit TEMT by
antioxidation (40) and to
decrease α-SMA expression (41).
The present study demonstrated that Sal B treatment could
significantly reduce the expression levels of TGF-β1 and FGF-2, and
regulate the expression of E-cadherin and α-SMA.
HS proteoglycans are important constituents of the
cell membrane and are associated with multiple physiological
processes (42). Previous reports
demonstrated that SDC1 in renal tubular epithelial cells is rich in
HS chains. HPSE could specifically cleave the HS side chain of SDC1
and lead to the release of several cytokines, such as TGF-β1 and
FGF-2. The present study showed that Sal B could significantly
reduce the expression of HPSE, which indicates that Sal B could
attenuate renal interstitial fibrosis by regulating the HPSE/SDC1
axis. However, considering the characteristics of natural products
with multiple targets, further in vitro and in vivo
research is required to investigate the underlying mechanisms of
Sal B in renal interstitial fibrosis.
In summary, the present study demonstrated that
activation of the HPSE/SDC1 axis, which promotes TEMT responses and
exacerbates renal injury, may play an important role in renal
interstitial fibrosis. Sal B, as a natural product with little
toxicity, may provide new prospects for the treatment of renal
interstitial fibrosis.
Acknowledgements
Not applicable.
Funding
This study was supported by Jiangsu Province
University Brand Professional Construction Project (grant no.
PPZY2015A070).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and MW contributed equally to this work. LX
designed the study. YH, MW, YP and QL performed the experiments and
analyzed the data. YH, MW and LX contributed to the writing of the
manuscript. All authors contributed to the revision of this
manuscript and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Animal Ethics
Committee of Nanjing University of Chinese Medicine (approval no.
ACU-2320151203).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levey AS, Eckardt KU, Tsukamoto Y, Levin
A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N and
Eknoyan G: Definition and classification of chronic kidney disease:
A position statement from Kidney Disease: Improving Global Outcomes
(KDIGO). Kidney Int. 67:2089–2100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parrish AR: Advances in chronic kidney
disease. Int J Mol Sci. 17:13142016. View Article : Google Scholar
|
|
3
|
Webster AC, Nagler EV, Morton RL and
Masson P: Chronic kidney disease. Lancet. 389:1238–1252. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilkinson TJ, Shur NF and Smith AC:
‘Exercise as medicine’ in chronic kidney disease. Scand J Med Sci
Sports. 26:985–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Bayliss G and Zhuang S: Cholesterol
crystal embolism and chronic kidney disease. Int J Mol Sci.
18:11202017. View Article : Google Scholar
|
|
6
|
Humphreys BD: Mechanisms of renal
fibrosis. Annu Rev Physiol. 80:309–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li R, Guo Y, Zhang Y, Zhang X, Zhu L and
Yan T: Salidroside ameliorates renal interstitial fibrosis by
inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int J Mol
Sci. 20:11032019. View Article : Google Scholar
|
|
8
|
Xie XS, Yang M, Liu HC, Zuo C, Li Z, Deng
Y and Fan JM: Influence of ginsenoside Rg1, a panaxatriol saponin
from Panax notoginseng, on renal fibrosis in rats with unilateral
ureteral obstruction. J Zhejiang Univ Sci B. 9:885–894. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li JH, Wang W, Huang XR, Oldfield M,
Schmidt AM, Cooper ME and Lan HY: Advanced glycation end products
induce tubular epithelial-myofibroblast transition through the
RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol.
164:1389–1397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu JH, He L, Zou ZM, Ding ZC, Zhang X,
Wang H, Zhou P, Xie L, Xing S and Yi CZ: A novel inhibitor of
homodimerization targeting MyD88 ameliorates renal interstitial
fibrosis by counteracting TGF-β1-induced EMT in vivo and in vitro.
Kidney Blood Press Res. 43:1677–1687. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waisberg J, Theodoro TR, Matos LL, Orlandi
FB, Serrano RL, Saba GT and Pinhal MA: Immunohistochemical
expression of heparanase isoforms and syndecan-1 proteins in
colorectal adenomas. Eur J Histochem. 60:25902016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palaiologou M, Delladetsima I and Tiniakos
D: CD138 (syndecan-1) expression in health and disease. Histol
Histopathol. 29:177–189. 2014.PubMed/NCBI
|
|
13
|
Masola V, Bellin G, Gambaro G and Onisto
M: Heparanase: A multitasking protein involved in extracellular
matrix (ECM) remodeling and intracellular events. Cells. 7:2362018.
View Article : Google Scholar
|
|
14
|
Jiao W, Chen Y, Song H, Li D, Mei H, Yang
F, Fang E, Wang X, Huang K, Zheng L and Tong Q: HPSE enhancer RNA
promotes cancer progression through driving chromatin looping and
regulating hnRNPU/p300/EGR1/HPSE axis. Oncogene. 37:2728–2745.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lorente-Gea L, Garcia B, Martin C, Quiros
LM and Fernandez-Vega I: Heparan sulfate proteoglycans and
heparanases in Alzheimer's disease: Current outlook and potential
therapeutic targets. Neural Regen Res. 12:914–915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masola V, Zaza G, Onisto M, Lupo A and
Gambaro G: Impact of heparanase on renal fibrosis. J Transl Med.
13:1812015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shu T, Pang M, Rong L, Liu C, Wang J, Zhou
W, Wang X and Liu B: Protective effects and mechanisms of
salvianolic acid B against H2O2-induced
injury in induced pluripotent stem cell- derived neural stem cells.
Neurochem Res. 40:1133–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z,
Kuai M, Sun X, Sun S, Jing Y, et al: Cardioprotective effect of
Salvianolic acid B on acute myocardial infarction by promoting
autophagy and neovascularization and inhibiting apoptosis. J Pharm
Pharmacol. 68:941–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katary MA, Abdelsayed R, Alhashim A,
Abdelhasib M and Elmarakby AA: Salvianolic acid B slows the
progression of breast cancer cell growth via enhancement of
apoptosis and reduction of oxidative stress, inflammation, and
angiogenesis. Int J Mol Sci. 20:56532019. View Article : Google Scholar
|
|
20
|
Wang B, Sun J, Shi Y and Le G: Salvianolic
acid B inhibits high-fat diet-induced inflammation by activating
the Nrf2 pathway. J Food Sci. 82:1953–1960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang QL, Tao YY, Yuan JL, Shen L and Liu
CH: Salvianolic acid B prevents epithelial-to-mesenchymal
transition through the TGF-beta1 signal transduction pathway in
vivo and in vitro. BMC Cell Biol. 11:312010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Li Q, Pan Y and Xu L: Sal B
alleviates myocardial ischemic injury by inhibiting TLR4 and the
priming phase of NLRP3 inflammasome. Molecules. 24:44162019.
View Article : Google Scholar
|
|
23
|
Schwalm S, Beyer S, Frey H, Haceni R,
Grammatikos G, Thomas D, Geisslinger G, Schaefer L, Huwiler A and
Pfeilschifter J: Sphingosine kinase-2 deficiency ameliorates kidney
fibrosis by up-regulating smad7 in a mouse model of unilateral
ureteral obstruction. Am J Pathol. 187:2413–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Y, Zhang J, Tan DQ, Chen XY, Ye DF,
Peng JP, Li JT, Zheng YQ, Fang L, Li YK and Fan MX: Interferon-γ
improves renal interstitial fibrosis and decreases intrarenal
vascular resistance of hydronephrosis in an animal model. Urology.
77:761.e8–e13. 2011. View Article : Google Scholar
|
|
25
|
Dou F, Liu Y, Liu L, Wang J, Sun T, Mu F,
Guo Q, Guo C, Jia N, Liu W, et al: Aloe-emodin ameliorates renal
fibrosis via inhibiting PI3K/Akt/mTOR signaling pathway in vivo and
in vitro. Rejuvenation Res. 22:218–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masola V, Zaza G, Granata S, Gambaro G,
Onisto M and Lupo A: Everolimus-induced epithelial to mesenchymal
transition in immortalized human renal proximal tubular epithelial
cells: Key role of heparanase. J Transl Med. 11:2922013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masola V, Gambaro G, Tibaldi E, Brunati
AM, Gastaldello A, D'Angelo A, Onisto M and Lupo A: Heparanase and
syndecan-1 interplay orchestrates fibroblast growth
factor-2-induced epithelial-mesenchymal transition in renal tubular
cells. J Biol Chem. 287:1478–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi M, Takayama S, Suga H, Kadomura
S, Kojima M, Iwao K, Takeda K, Sato H, Kobayashi M and Saitoh H:
Factors resulting correlation and differences in renal function
evaluation index using the serum cystatin c and creatinine as
measured by an enzymatic method. Yakugaku Zasshi. 140:81–90.
2020.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun YB, Qu X, Caruana G and Li J: The
origin of renal fibroblasts/myofibroblasts and the signals that
trigger fibrosis. Differentiation. 92:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HY, Zhang C, Xiao QF, Dou HC, Chen Y,
Gu CM and Cui MJ: Hepatocyte growth factor inhibits tubular
epithelial-myofibroblast transdifferentiation by suppression of
angiotensin II via the JAK2/STAT3 signaling pathway. Mol Med Rep.
15:2737–2743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Li X, Zhang Q and Zhao T: Low
molecular weight fucoidan and its fractions inhibit renal
epithelial mesenchymal transition induced by TGF-β1 or FGF-2. Int J
Biol Macromol. 105:1482–1490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan Q, Tan RJ and Liu Y: Myofibroblast in
kidney fibrosis: Origin, activation, and regulation. Adv Exp Med
Biol. 1165:253–283. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Ning X, Li R, Yang Z, Yang X, Sun S
and Qian Q: Signalling pathways involved in hypoxia-induced renal
fibrosis. J Cell Mol Med. 21:1248–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu X, Xia Y, Zeng L, Zhang X, Chen L, Yan
S, Zhang R, Zhao C, Zeng Z, Shu Y, et al: A blockade of PI3Kγ
signaling effectively mitigates angiotensin II-induced renal injury
and fibrosis in a mouse model. Sci Rep. 8:109882018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujimura K, Wakino S, Minakuchi H,
Hasegawa K, Hosoya K, Komatsu M, Kaneko Y, Shinozuka K, Washida N,
Kanda T, et al: Ghrelin protects against renal damages induced by
angiotensin-II via an antioxidative stress mechanism in mice. PLoS
One. 9:e943732014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thakur S, Viswanadhapalli S, Kopp JB, Shi
Q, Barnes JL, Block K, Gorin Y and Abboud HE: Activation of
AMP-activated protein kinase prevents TGF-β1-induced
epithelial-mesenchymal transition and myofibroblast activation. Am
J Pathol. 185:2168–2180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodriguez-Mateo C, Torres B, Gutierrez G
and Pintor-Toro JA: Downregulation of Lnc-Spry1 mediates
TGF-β-induced epithelial-mesenchymal transition by transcriptional
and posttranscriptional regulatory mechanisms. Cell Death Differ.
24:785–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Griggs LA, Hassan NT, Malik RS, Griffin
BP, Martinez BA, Elmore LW and Lemmon CA: Fibronectin fibrils
regulate TGF-β1-induced Epithelial-mesenchymal transition. Matrix
Biol 60–61. 157–175. 2017. View Article : Google Scholar
|
|
39
|
Lu HY, Zhou J, Lu M, Liu YM, Wang F, Lin M
and Zhang Y: Protection and mechanisms of salvianolic-acid B on
experimental renal interstitial fibrosis in rats. Zhong Yao Cai.
33:1755–1759. 2010.(In Chinese). PubMed/NCBI
|
|
40
|
Wang QL, Yuan JL, Tao YY, Hu YY and Liu
CH: Effect of salvianolic acid B on renal interstitial fibrosis in
rats induced by HgCl2. Pharmacol Clin Chin Materia Medica.
24:12–15. 2008.
|
|
41
|
Zhou J, Zhang Y, Lu H, Liu Y and Lin M:
Effect of salvianolic acid B on generation and activation of
myofibroblast in rat with renal interstitial fibrosis. Zhongguo
Zhong Yao Za Zhi. 34:2790–2793. 2009.(In Chinese). PubMed/NCBI
|
|
42
|
Motta G and Tersariol ILS: Modulation of
the plasma kallikrein-kinin system proteins performed by heparan
sulfate proteoglycans. Front Physiol. 8:4812017. View Article : Google Scholar : PubMed/NCBI
|