Introduction
Hand, foot and mouth disease (HFMD) is an infectious
illness, which predominantly affects infants and young children
(1). The HFMD epidemic is a
serious public health issue that could lead to an enormous economic
and social burden (2). One of the
causative agents of HFMD is enterovirus 71 (EV 71), a human virus
species of the Enterovirus genus in the
Picornaviridae family (3,4). EV
71-associated HFMD can be complicated by neurological
manifestations, including cardiopulmonary failure, myoclonus,
ataxia, polio-like paralysis, tremor and encephalomyelitis
(5–7).
The aquaporin (AQP) family of water channels is a
group of small membrane-spanning proteins that are vital for rapid
transport of water across the plasma membrane. AQP4, as a major
water channel of the central nervous system, is highly concentrated
on astrocyte endfeet (8,9). Previous studies demonstrated that
altered AQP4 expression serves a critical role in neuroinflammation
during brain injury (10–12). In cultured rat articular
chondrocytes, AQP4 downregulation attenuates interleukin
(IL)-1β-induced chondrocyte apoptosis by regulating the expression
of apoptosis-related genes and by inhibiting p38 MAPK (13). The levels of IL-6, IL-8, IL-1β,
interferon (IFN)-γ and monocyte chemotactic protein (MCP)-1 are
altered in the serum and cerebrospinal fluid of patients with
AQP4-antibody seropositive neuromyelitis optica spectrum disorder
(14–16). In astrocytes, AQP4 expression is
altered through the release of tumor necrosis factor (TNF)-α and
IL-6 by microglia during hypoxia-induced inflammatory responses
(17).
Proinflammatory cytokines are demonstrated to be
upregulated in the brains of EV 71-infected mice (18). EV 71 infection also directly
impacts the mitochondrial apoptotic pathway by modulating the
recruitment and activation of Bax (19). In addition, caspase-3 inhibition
protects host cells from the cytopathic effect of EV 71 infection
and prevents cell cycle arrest, leading to a decrease in EV 71
viral protein expression and viral production (20). The present study aimed to determine
whether AQP4 deletion could ameliorate EV 71 infection by
inhibiting inflammation and apoptosis in mice.
Materials and methods
In vivo experiments
All animal experiments were carried out in
accordance with The Guidelines of the Xuzhou Medical University
Institutional Committee for the Care and Use of Laboratory Animals
and approved by The Xuzhou Medical University Laboratory Animal
Management Ethics Committee. A total of 19 maternal mice (age, 8–10
weeks; weight, 210–240 g) were kept in a temperature (22±1°C) and
humidity (30-60%)-controlled room under a 12 h light-dark cycle
with free access to standard chow and tap water. To minimize animal
suffering and distress, the housing conditions, animal welfare, and
experimental procedures were in accordance with The Guide for The
Care and Use of Laboratory Animals (21). In addition, ≤8 newborn mice at a
time were fed by a maternal mouse. A total of 78 one-day-old mice
were used in the experiments. The sex of the pups was not
determined, due to their young age.
Mouse in vivo treatment
A total of 31 AQP4-knockout (KO) C57BL6/J mice
(Cyagen Biosciences, Inc.) were used as the experimental group, and
47 wild-type (WT; Cyagen Biosciences, Inc.) mice were treated as a
control group. Newborn mice were bred in-house. EV 71 was isolated
from a HFMD clinical specimen (male; age, 1 year) in 2018 in Xuzhou
Children's Hospital of Xuzhou Medical University, and written
consent was provided by the parents of the donor. The present study
was approved by The Ethics Committee of Xuzhou Children's
Hospital.
One-day-old mice were challenged intraperitoneally
with 50 µl EV 71 at a dose of 107 plaque-forming units
per mouse. The mice in the control group were treated with 50 µl
PBS (BioChannel; Nanjing Shenghang Biotechnology Co., Ltd.) and
kept in a separate cage from the infected mice. All the mice
received one EV 71 or PBS injection. Health was monitored and
scored as follows: i) 0, healthy; ii) 1, lethargic and inactive;
iii) 2, wasting; iv) 3, weak in limb; v) 4, paralyzed hind limb;
and vi) 5, moribund or dead. In the survival analysis and health
score experiments, WT-Control (n=8), KO-Control (n=8), WT-EV 71
(n=15) and KO-EV 71 (n=15) groups were used. For all other
experiments, there were eight animals in each group.
Determination of inflammatory factor
levels
The mice were sacrificed via 1.5% isoflurane
inhalation for 2 min. The brain and serum samples of the mice were
obtained 4 days after EV 71 injection. The brain tissues were
homogenized in lysis buffer (Thermo Fisher Scientific, Inc.). The
total protein in the homogenate was extracted and measured using a
BCA protein assay kit (BioChannel Biotechnology Co., Ltd.). The
levels of TNF-α, IL-1β, IL-6, MCP-1, IFN-α and IFN-γ (cat. nos.
SEA133Mu, SEA076Mu, SEA079Mu, SEA087Mu, SEA033Mu and SCA049Mu,
respectively) in the brain or serum were determined using ELISA
kits (all Wuhan USCN Business Co., Ltd.) following the
manufacturer's instructions.
Western blotting
The brain samples were sonicated in RIPA lysis
buffer (BioChannel; Nanjing Shenghang Biotechnology Co., Ltd.) and
homogenized. The debris was removed by centrifugation at 12,000 × g
for 10 min at 4°C and the supernatant was collected. Subsequently,
~30–40 µg protein (BCA protein assay kit) was separated by 8% gel
electrophoresis, transferred to PVDF membrane The membrane was
blocked with 5% skimmed milk powder at room temperature for 1 h and
probed with primary antibodies overnight at 4°C against AQP4, Bcl2
or Bax (all 1:1,000; cat. nos. 59678, 3498 and 14796, respectively;
all Cell Signaling Technology, Inc.). Then, horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:10,000; cat. no. ab7090; Abcam) was added and incubated at room
temperature for 1 h and GAPDH (1:10,000; cat. no. ab181602; Abcam)
was used as an internal control. The bands were visualized via ECL
(Beyotime, Shanghai, China). Images were analyzed using Image-Pro
Plus software (version 6.0; XRayScan; CAD/CAM Services, Inc.).
Measurement of AQP4 mRNA using reverse
transcription- quantitative PCR (RT-qPCR)
AQP4 mRNA levels in the brain were measured using an
RT-qPCR system (Roche Diagnostics). In brief, total RNA was
extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) cDNA was extracted from RNA via reverse
transcription using random primers in a total volume of 10 µl,
according to the instructions of the PrimeScript™ RT Master Mix
(37°C, 15 min; 85°C, 5 sec; Takara Biotechnology Co., Ltd.). mRNA
levels were determined via Power SYBR-Green PCR Master Mix (Thermo
Fisher Scientific, Inc.). All samples were amplified in triplicates
for 45 cycles in a 96-well plate (95°C, 15 sec; 60°C, 1 min). The
relative gene expression was determined using the 2−ΔΔCq
method (22). Primer sequences
were as follows: AQP4 forward, 5′-CGGCATCCTCTACCTGGTCACA-3′ and
reverse, 5′-GCCAGCGGTGAGGTTTCCAT-3′; and GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All data were analyzed using GraphPad Prism 7.0 (GraphPad
Software, Inc.). Survival rates were evaluated by the Mantel-Cox
log-rank test. Other data were analyzed using an unpaired t-test
for pairwise comparison, or one-way ANOVA, followed by Bonferroni's
correction when multiple comparisons were made. A two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
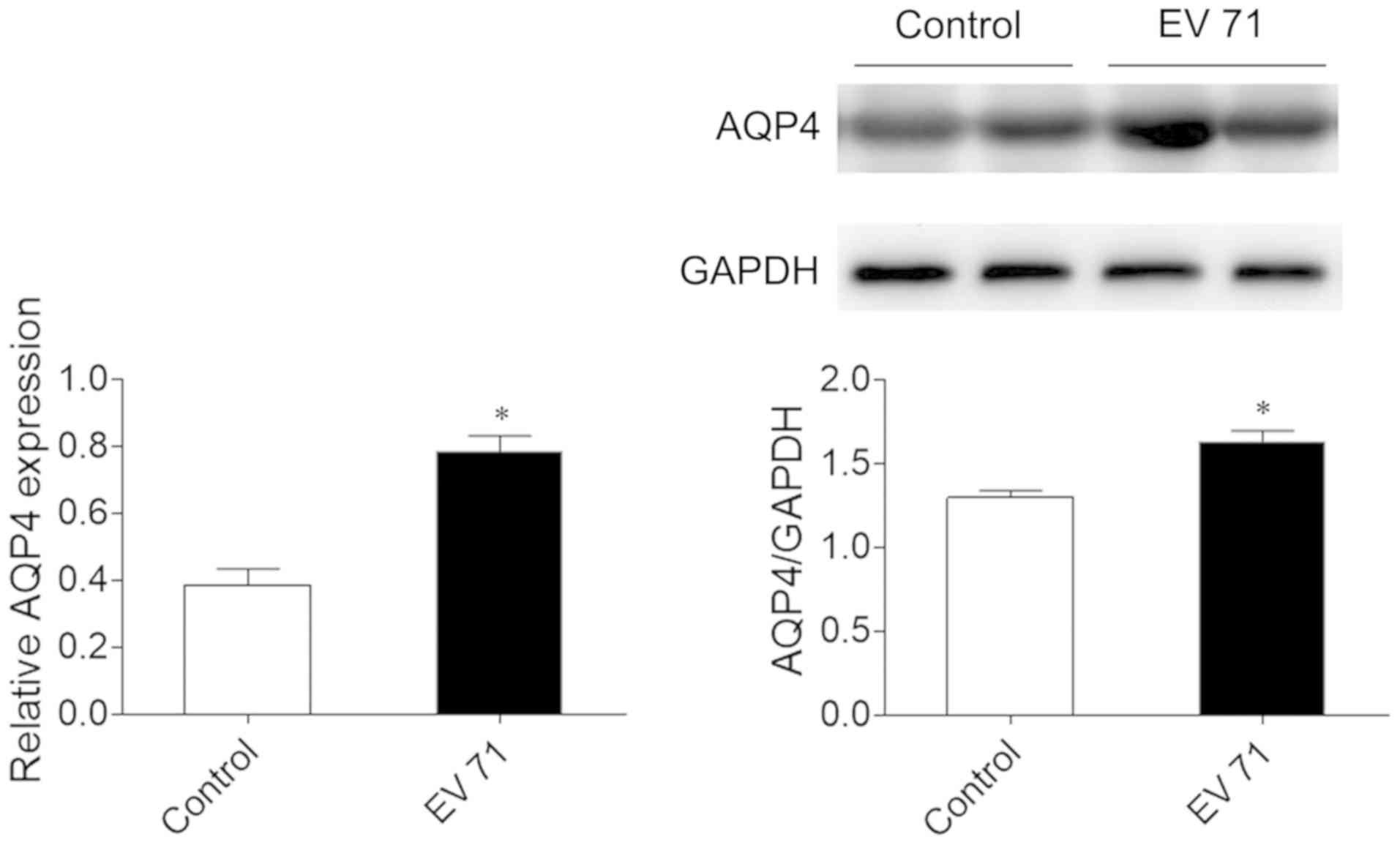
AQP4 levels in the brain
AQP4 mRNA level in brain was significantly higher in
EV 71-infected mice compared with in control mice
(2.04-fold-change). Furthermore, AQP4 protein levels were
significantly higher in the brains of EV 71-infected mice compared
with the control (Fig. 1).
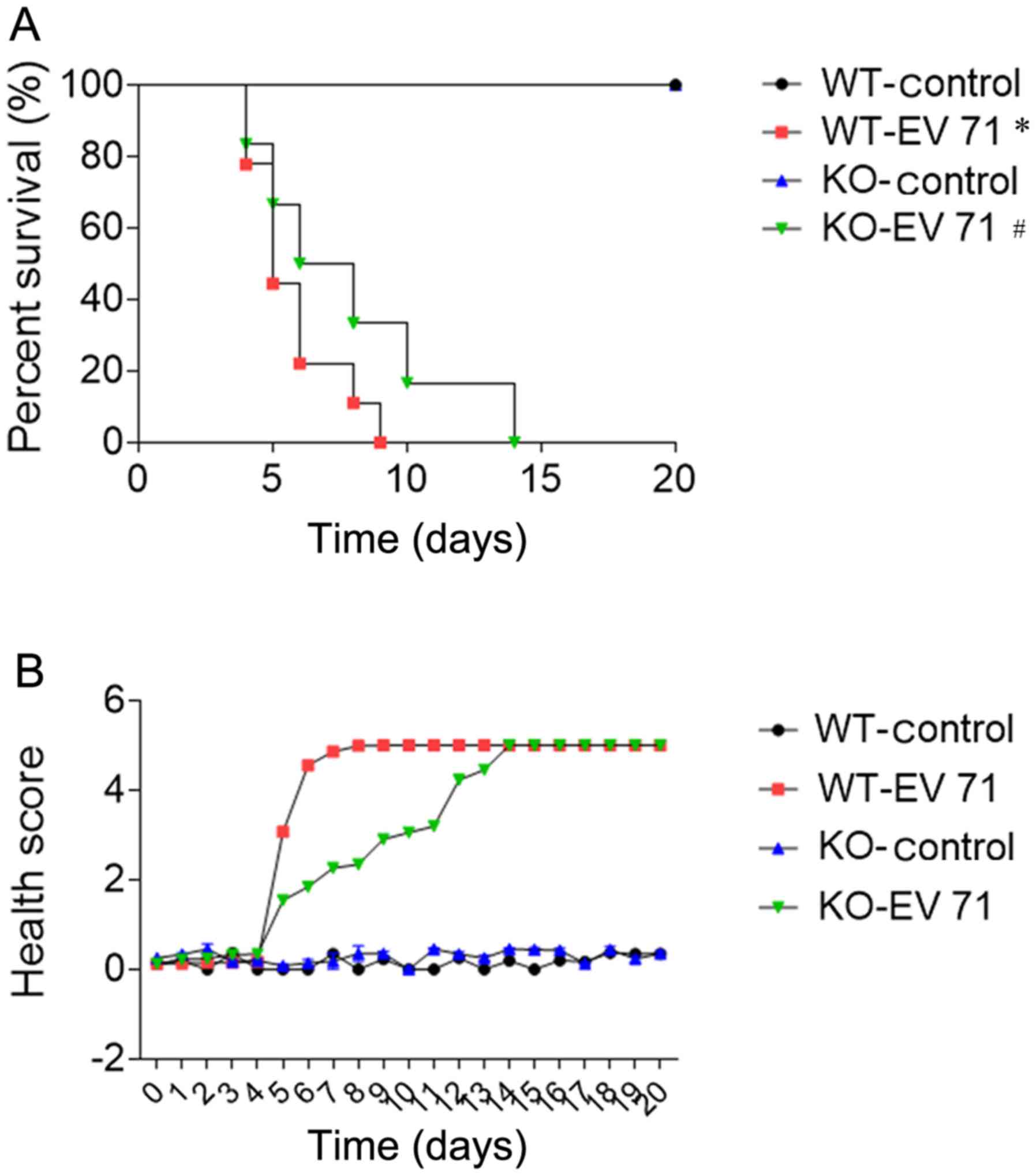
Effects of AQP4 deletion on survival
rate and clinical score in EV 71-infected mice
The survival rates and clinical scores of
one-day-old mice intraperitoneally injected with 107 PFU
EV 71 strain were recorded. In both the WT and KO groups, EV
71-inoculated mice exhibited shorter survival times compared with
the controls. In addition, survival time was improved in EV
71-inoculated AQP4-KO mice compared with EV 71-infected WT mice
(Fig. 2A). Furthermore, the mean
heath score was reduced in infected AQP4-KO mice in the first 14
days of infection compared with infected WT-controls. Thus, AQP4
deficiency can delay disease development in EV 71-infected mice
(Fig. 2B).
Effects of AQP4 deletion on
inflammatory factors in the serum and brain of EV 71-infected
mice
The levels of TNF-α, IL-1β, IL-6, MCP-1, IFN-α and
IFN-γ in the serum of EV 71-infected mice were significantly higher
compared with the control mice, both in WT and KO animals. However,
the increase in levels of these inflammatory factors following EV
71 infection were reduced in AQP4-KO mice compared with WT mice
(Fig. 3). These results also held
true in the brain (Fig. 4).
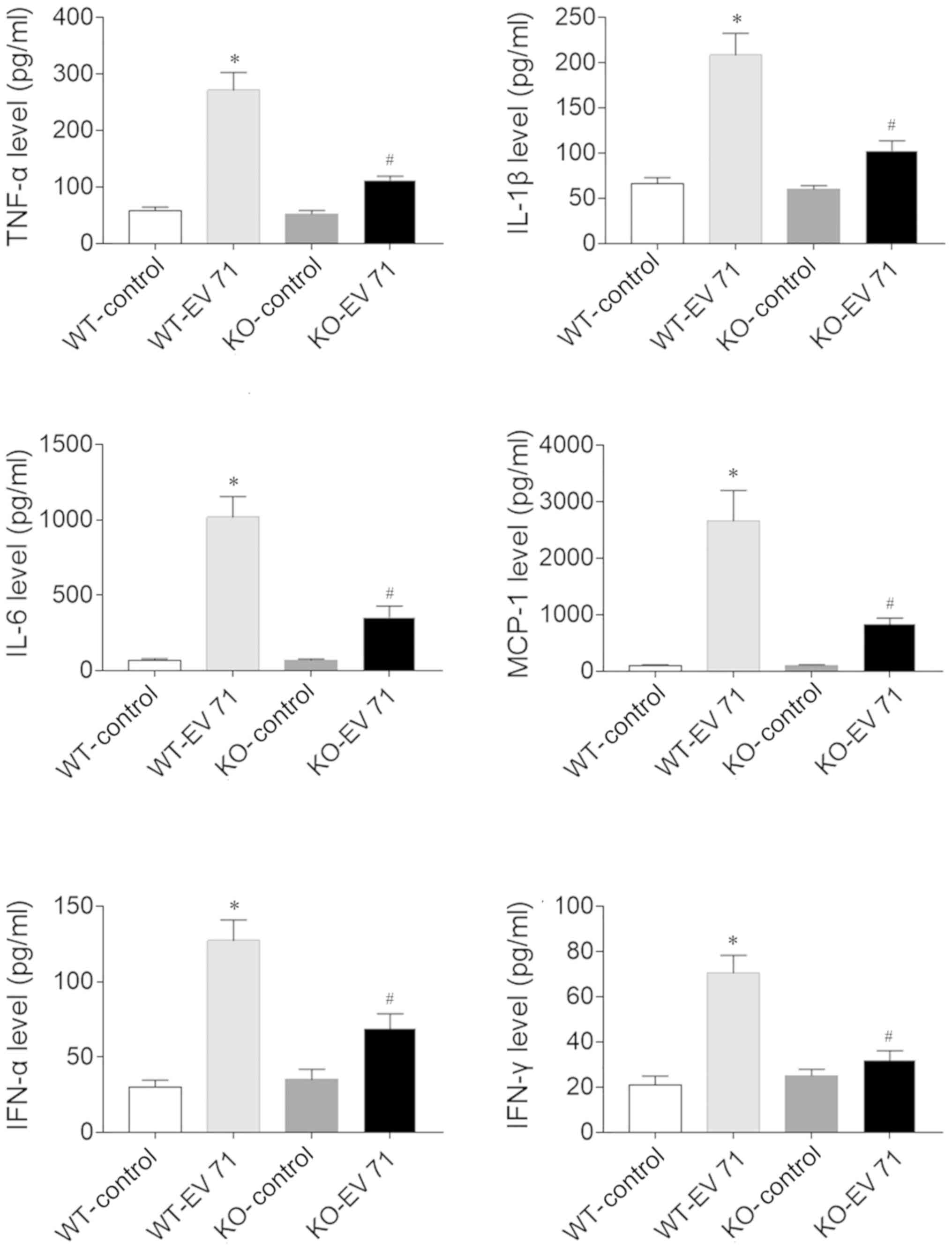 | Figure 3.AQP4 deletion affects inflammatory
factor levels in the serum of EV 71-infected mice. The EV
71-induced increases of TNF-α, IL-1β, IL-6, MCP-1, IFN-α and IFN-γ
in serum were inhibited in AQP4-KO mice. Data are presented as the
mean ± SEM. n=8 in each group. *P<0.05 vs. WT-Control;
#P<0.05 vs. WT-EV 71. AQP4, aquaporin-4; EV 71,
enterovirus 71; WT, wild-type; KO, knockout; IL, interleukin; IFN,
interferon; TNF-α, tumor necrosis factor-α; MCP-1, monocyte
chemotactic protein. |
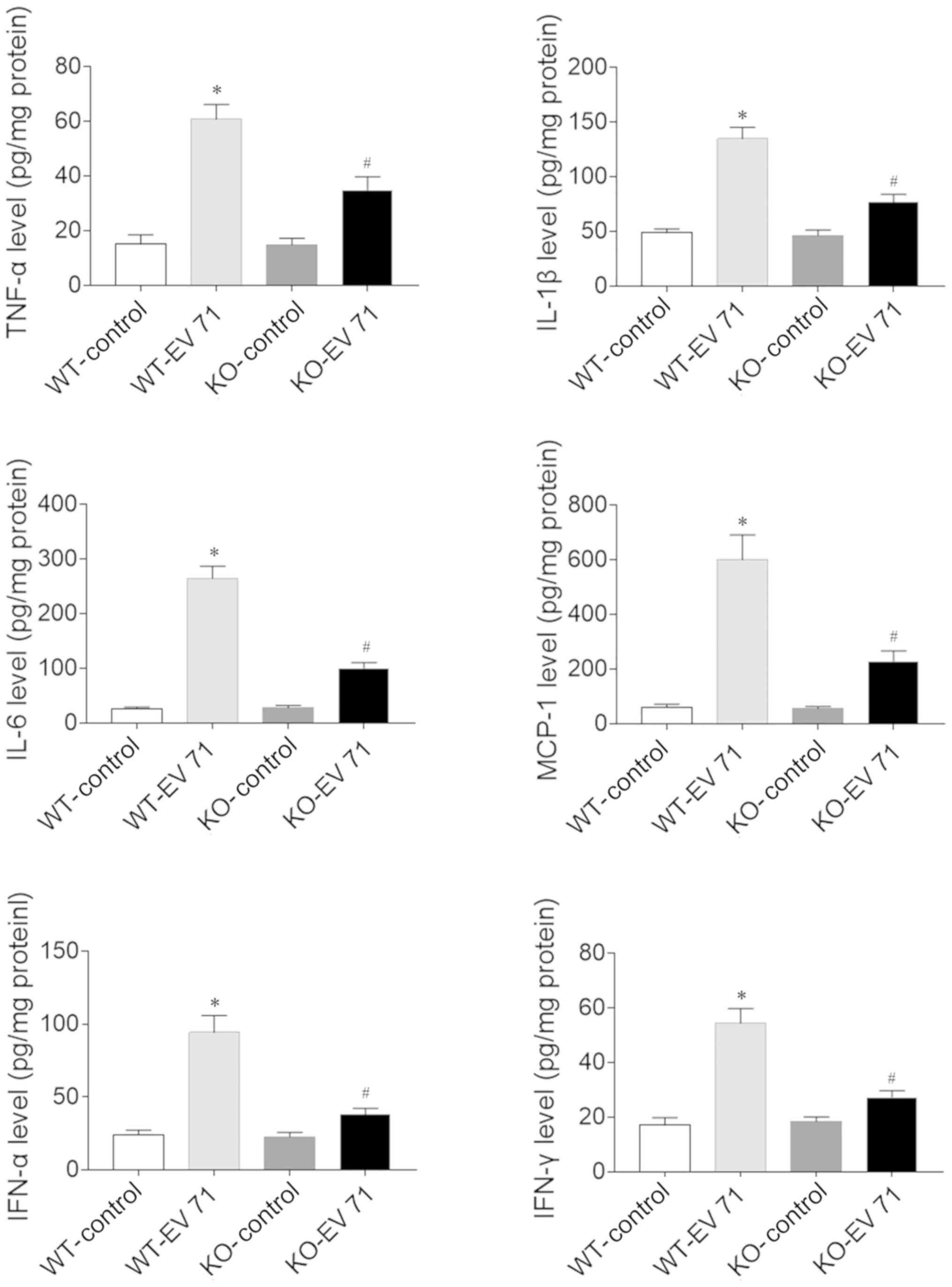 | Figure 4.AQP4 deletion affects inflammatory
factor levels in the brains of EV 71-infected mice. The EV
71-induced increases of TNF-α, IL-1β, IL-6, MCP-1, IFN-α and IFN-γ
in serum were inhibited in AQP4-KO mice. Data are presented as the
mean ± SEM. n=8 in each group. *P<0.05 vs. WT-Control;
#P<0.05 vs. WT-EV 71. AQP4, aquaporin-4; EV 71,
enterovirus 71; WT, wil-type; KO, knockout; IL, interleukin; IFN,
interferon; TNF-α, tumor necrosis factor-α; MCP-1, monocyte
chemotactic protein. |
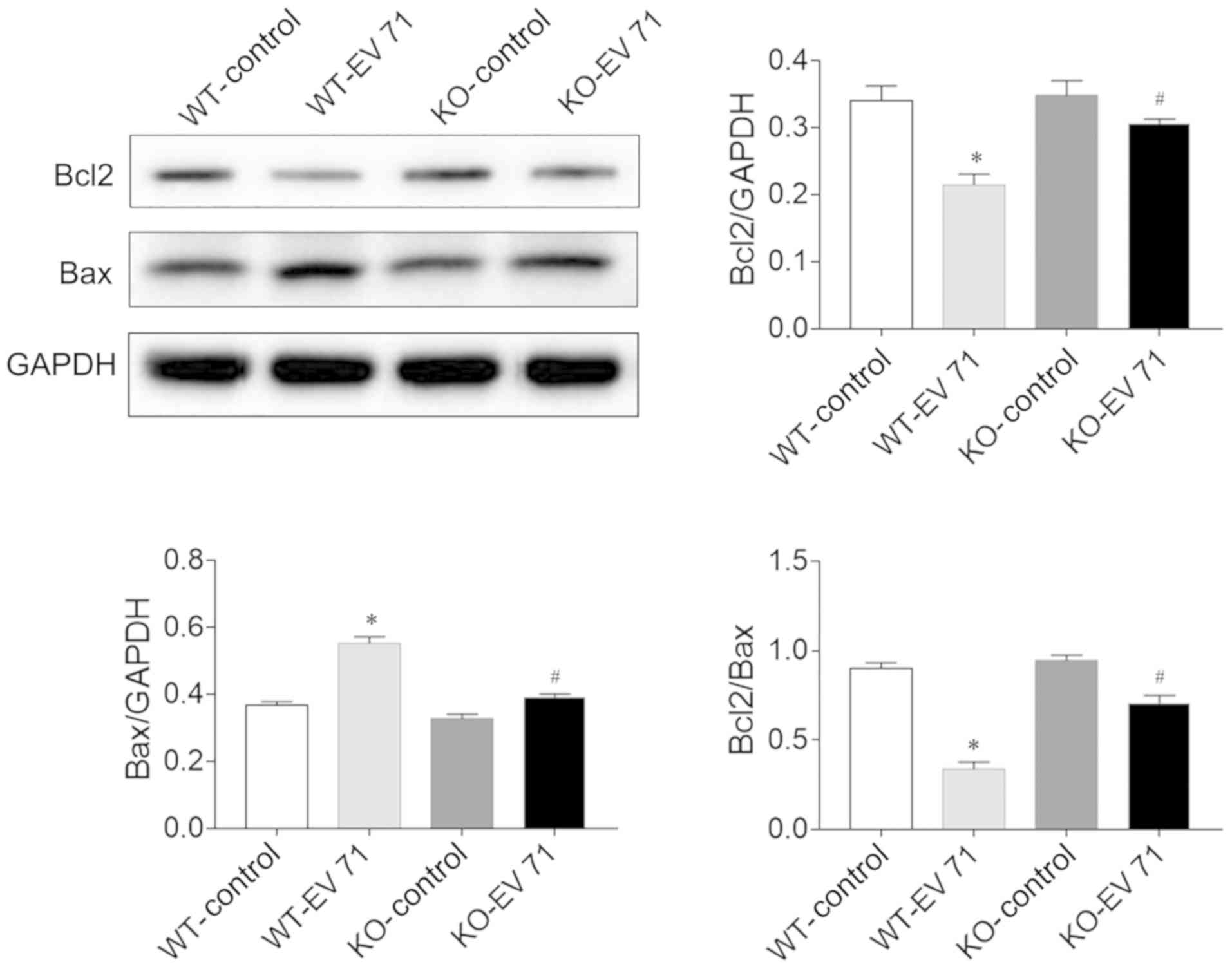
Effects of AQP4 deletion on apoptosis
in brain of EV 71-infected mice
The levels of Bcl2 in the brain were significantly
reduced in EV 71-infected mice compared with the respective control
group, while Bax levels were significantly increased. The Bcl2/Bax
ratios were also reduced accordingly. However, AQP4 deletion
limited the decrease in Bcl2 levels and the increase in Bax levels
induced by EV 71-infection in the brain compared with WT. AQP4
deficiency also decreased the decrease Bcl2/Bax ratios seen after
EV 71 infection (Fig. 5).
Discussion
The first case of EV 71 infection was reported more
than half a century ago (23), yet
specific therapy for this severe inflammatory disease is still
lacking. Human EV 71, a member of the Enterovirus genus of
the Picornaviridae family, is the main etiologic agent of
HFMD (24,25). The AQP4 water channel is widely
expressed in the brain and represents a potential drug target for
neurological disorders (26). The
present study demonstrated that, in addition to its potential for
the treatment of neurological disease, AQP4 deletion ameliorated EV
71 induced-HFMD by inhibiting inflammation and apoptosis in
mice.
A previous study suggested that serum AQP4 levels
significantly differed according to disease severity before and
after treatment in EV 71-associated HFMD (27). Similarly, in the present study, the
levels of AQP4 mRNA and protein increased in the brain of EV
71-induced HFMD mice. Indeed, AQP4 mRNA levels were doubled in EV
71-infected mice compared with control mice. Compared with EV
71-infected WT mice, AQP4-KO mice exhibited increased survival
time. In addition, the mean health scores were higher in EV
71-infected mice compared with the controls. However, in AQP4-KO
mice, the increase in health scores induced by EV 71 infection was
reduced. These results indicated that AQP4 may be a therapeutic
target for HFMD. A previous study suggested that
AQP4-overexpressing mice exhibit intracranial pressure elevation,
leading to brain herniation and death (28). Altogether, these results
demonstrated that AQP4 deficiency improved survival in diseases
such as HFMD.
AQP4 deletion protects the blood-brain barrier from
hypoglycemia by reducing inflammatory responses through the
inhibition of proinflammatory cytokine release (29). In a previous study, AQP4 deficiency
resulted in a significant increase in the production of IL-1β and
TNF-α in a murine model of Parkinson's disease (30). Furthermore, mice treated with the
AQP4 inhibitor TGN-020 displayed attenuated
lipopolysaccharide-induced lung injury, improved survival rates and
reduced proinflammatory cytokines release, including IL-1α, IL-1β,
IL-6, TNF-α, IL-23 and IL-17A (31). Whether downregulation of AQP4 also
attenuates inflammatory cytokine release in the brain of HFMD mice
is not well-studied. The present study demonstrated that the levels
of TNF-α, IL-1β, IL-6, MCP-1, IFN-α and IFN-γ in the serum and
brain of EV 71-infected mice were higher compared with those of the
control mice. In addition, the EV 71-induced increases in the
levels of these inflammatory factors were limited in AQP4-KO mice.
These findings indicated that AQP4 deletion could ameliorate
inflammation in EV 71-induced HFMD.
A previous study demonstrated that gene expression
levels of caspase-1 and active caspase-1 were significantly
increased in the SH-SY5Y human neuroblastoma cell line following EV
71 infection (32). EV 71 protease
3C plays an important role in EV 71-induced apoptosis (33). AQP4 small interfering RNA knockdown
markedly decreases Bax and caspase-3 and increases Bcl-2 mRNA
levels in joint diseases, such as rheumatoid arthritis (13). Knockdown of AQP4 significantly
decreases astrocyte apoptosis, and promotes viability of astrocytes
in anoxic conditions (34). In the
present study, the levels of Bcl2 and Bcl2/Bax ratios in the brain
were reduced in EV 71-infected mice, while AQP4 deletion limited
this EV 71-induced decrease. The levels of Bax were increased in
the brains of EV 71-infected mice, and AQP4 deletion inhibited this
EV 71-induced increase. These results indicated that AQP4 deletion
could attenuate apoptosis in EV 71-induced HFMD in mice.
AQP4 expression can be regulated by inflammatory
cytokines. High mobility group box-1, a mediator of inflammatory
responses, upregulates AQP4 expression and promotes cell swelling
in cultured spinal cord astrocytes after oxygen-glucose
deprivation/reoxygenation, which is mediated by IL-6 (35). AQP4 expression levels are also
increased in astrocytes following treatment with IL-1β, TNF-α and
IFN-γ (36). AQP4 and inflammatory
cytokines levels are changed in lipopolysaccharide-treated
astrocytes (37). Altogether,
previous studies and the present study indicate AQP4 regulates the
levels of several inflammatory cytokines. Inflammatory cytokines
also regulate AQP4 levels.
In conclusion, inflammation and apoptosis were
increased in HFMD. AQP4 expression levels were increased in the
brains of mice with of EV 71-induced HFMD. This increase in AQP4
levels may lead to the inflammation and apoptosis seen in EV 71
infection and HFMD. AQP4 deletion improved survival rates and
health scores, and attenuated inflammation and apoptosis in EV
71-infected mice. Thus, the findings of the present study suggested
that AQP4 deletion could ameliorate EV 71 infection and HFMD by
inhibiting inflammation and apoptosis in mice.
Acknowledgements
Not applicable.
Funding
The present work was supported by Jiangsu Provincial
Commission of Health and Family Planning Youth Project (grant no.
Q201617) and Xuzhou Clinical Skeleton Staff Training Project.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ and XWH conceptualized the study and performed
the experiments; XLW and BXQ performed the experiments and analyzed
data; LZ, TY and HJW wrote the manuscript. TY and HJW designed the
study and revised the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments in the present study were
carried out in accordance with The Guidelines of The Xuzhou Medical
University Institutional Committee for The Care and Use of
Laboratory Animals and were approved by The Xuzhou Medical
University Laboratory Animal Management Ethics Committee. The
present study was also approved by The Ethics Committee of Xuzhou
Children's Hospital. Written consent was obtained from the parents
of the children involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aswathyraj S, Arunkumar G, Alidjinou EK
and Hober D: Hand, foot and mouth disease (HFMD): Emerging
epidemiology and the need for a vaccine strategy. Med Microbiol
Immunol. 205:397–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koh WM, Badaruddin H, La H, Chen MI and
Cook AR: Severity and burden of hand, foot and mouth disease in
Asia: A modelling study. BMJ Global Health. 3:e0004422018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmidt NJ, Lennette EH and Ho HH: An
apparently new enterovirus isolated from patients with disease of
the central nervous system. J Infect Dis. 129:304–309. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ortner B, Huang CW, Schmid D, Mutz I,
Wewalka G, Allerberger F, Yang JY and Huemer HP: Epidemiology of
enterovirus types causing neurological disease in Austria
1999-2007: Detection of clusters of echovirus 30 and enterovirus 71
and analysis of prevalent genotypes. J Med Virol. 81:317–324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT,
Tsai SF, Wang JR and Shih SR: An epidemic of enterovirus 71
infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N
Engl J Med. 341:929–935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CC, Liu CC, Chang YC, Chen CY, Wang
ST and Yeh TF: Neurologic complications in children with
enterovirus 71 infection. N Engl J Med. 341:936–942. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang M, Du W, Liu J, Zhang H, Cao L, Yang
W, Zhang H, Wang Z, Wei P, Wu W, et al: Interleukin-27 as a novel
biomarker for early cardiopulmonary failure in enterovirus
71-infected children with central nervous system involvement.
Mediators Inflamm. 2016:40251672016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Filippidis AS, Carozza RB and Rekate HL:
Aquaporins in brain edema and neuropathological conditions. Int J
Mol Sci. 18:552016. View Article : Google Scholar
|
|
9
|
Hubbard JA, Hsu MS, Seldin MM and Binder
DK: Expression of the astrocyte water channel aquaporin-4 in the
mouse brain. ASN Neuro. 7:17590914156054862015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tourdias T, Mori N, Dragonu I, Cassagno N,
Boiziau C, Aussudre J, Brochet B, Moonen C, Petry KG and Dousset V:
Differential aquaporin 4 expression during edema build-up and
resolution phases of brain inflammation. J Neuroinflammation.
8:1432011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Badaut J, Ashwal S and Obenaus A:
Aquaporins in cerebrovascular disease: A target for treatment of
brain edema? Cerebrovasc Dis. 31:521–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Badaut J, Ashwal S, Adami A, Tone B,
Recker R, Spagnoli D, Ternon B and Obenaus A: Brain water mobility
decreases after astrocytic aquaporin-4 inhibition using RNA
interference. J Cereb Blood Flow Metab. 31:819–831. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai L, Lei C, Li R, Chen WN and Li CM:
Aquaporin-4 blockage by siRNA protects rat articular chondrocytes
from IL-1β-induced apoptosis by inhibiting p38 MAPK signal pathway.
Ann Clin Lab Sci. 47:563–571. 2017.PubMed/NCBI
|
|
14
|
Hofer LS, Mariotto S, Wurth S, Ferrari S,
Mancinelli CR, Delogu R, Monaco S, Gajofatto A, Schwaiger C,
Rostasy K, et al: Distinct serum and cerebrospinal fluid cytokine
and chemokine profiles in autoantibody-associated demyelinating
diseases. Mult Scler J Exp Transl Clin.
5:20552173198484632019.PubMed/NCBI
|
|
15
|
Gahlen A, Trampe AK, Haupeltshofer S,
Ringelstein M, Aktas O, Berthele A, Wildemann B, Gold R, Jarius S
and Kleiter I: Aquaporin-4 antibodies in patients treated with
natalizumab for suspected MS. Neurol Neuroimmunol Neuroinflamm.
4:e3632017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaneko K, Sato DK, Nakashima I, Ogawa R,
Akaishi T, Takai Y, Nishiyama S, Takahashi T, Misu T, Kuroda H, et
al: CSF cytokine profile in MOG-IgG+ neurological disease is
similar to AQP4-IgG+ NMOSD but distinct from MS: A cross-sectional
study and potential therapeutic implications. J Neurol Neurosurg
Psychiatry. 89:927–936. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang C, Yan M, Jiang H, Wang Q, He S, Chen
J and Wang C: Mechanism of aquaporin 4 (AQP 4) up-regulation in rat
cerebral edema under hypobaric hypoxia and the preventative effect
of puerarin. Life Sci. 193:270–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsiao HB, Chou AH, Lin SI, Chen IH, Lien
SP, Liu CC, Chong P and Liu SJ: Toll-like receptor 9-mediated
protection of enterovirus 71 infection in mice is due to the
release of danger-associated molecular patterns. J Virol.
88:11658–11670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han X and Cong H: Enterovirus 71 induces
apoptosis by directly modulating the conformational activation of
pro-apoptotic protein Bax. J Gen Virol. 98:422–434. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song F, Yu X, Zhong T, Wang Z, Meng X, Li
Z, Zhang S, Huo W, Liu X, Zhang Y, et al: Caspase-3 inhibition
attenuates the cytopathic effects of EV71 infection. Front
Microbiol. 9:8172018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guide for the Care and Use of Laboratory
Animals. Washington (DC): National Academies Press (US). 1996.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarma N: Hand, foot, and mouth disease:
Current scenario and Indian perspective. Indian J Dermatol Venereol
Leprol. 79:165–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Zhu C, Bao W, Zhao K, Niu J, Yu XF
and Zhang W: Characterization of full-length enterovirus 71 strains
from severe and mild disease patients in northeastern China. PLoS
One. 7:e324052012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng M, Zheng X, Wei R, Zhang N, Zhu K, Xu
B, Yang CH, Yang CF, Deng C, Pu D, et al: The cytokine and
chemokine profiles in patients with hand, foot and mouth disease of
different severities in Shanghai, China, 2010. PLoS Negl Trop Dis.
7:e25992013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verkman AS, Smith AJ, Phuan PW,
Tradtrantip L and Anderson MO: The aquaporin-4 water channel as a
potential drug target in neurological disorders. Expert Opin Ther
Targets. 21:1161–1170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu L, Yin H, Qian T, Qi GJ, Ren JS, Wang
Y and Qi BX: Distinct expression and clinical value of aquaporin 4
in children with hand, foot and mouth disease caused by enterovirus
71. J Med Virol. Apr 3–2019.doi: 10.1002/jmv.25475. Online ahead of
print. View Article : Google Scholar
|
|
28
|
Yang B, Zador Z and Verkman AS: Glial cell
aquaporin-4 overexpression in transgenic mice accelerates cytotoxic
brain swelling. J Biol Chem. 283:15280–15286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao F, Deng J, Xu X, Cao F, Lu K, Li D,
Cheng X, Wang X and Zhao Y: Aquaporin-4 deletion ameliorates
hypoglycemia-induced BBB permeability by inhibiting inflammatory
responses. J Neuroinflammation. 15:1572018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun H, Liang R, Yang B, Zhou Y, Liu M,
Fang F, Ding J, Fan Y and Hu G: Aquaporin-4 mediates communication
between astrocyte and microglia: Implications of neuroinflammation
in experimental Parkinson's disease. Neuroscience. 317:65–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo C, Wu T, Zhu H and Gao L: Aquaporin 4
blockade attenuates acute lung injury through inhibition of Th17
cell proliferation in mice. Inflammation. 42:1401–1412. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu X, Wu T, Chi Y, Ge Y, Wu B, Zhou M,
Zhu F, Ji M and Cui L: Pyroptosis induced by enterovirus A71
infection in cultured human neuroblastoma cells. Virology.
521:69–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Yao Y, Chen Y, Xu X, Lin Y, Yang Z,
Qiao W and Tan J: Enterovirus 71 3C promotes apoptosis through
cleavage of PinX1, a telomere binding protein. J Virol.
91:e02016–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng Y, Wang L, Chen M, Pei A, Xie L and
Zhu S: Upregulation of miR-130b protects against cerebral ischemic
injury by targeting water channel protein aquaporin 4 (AQP4). Am J
Transl Res. 9:3452–3461. 2017.PubMed/NCBI
|
|
35
|
Sun L, Li M, Ma X, Feng H, Song J, Lv C
and He Y: Inhibition of HMGB1 reduces rat spinal cord astrocytic
swelling and AQP4 expression after oxygen-glucose deprivation and
reoxygenation via TLR4 and NF-kappaB signaling in an IL-6-dependent
manner. J Neuroinflammation. 14:2312017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asai H, Kakita H, Aoyama M, Nagaya Y,
Saitoh S and Asai K: Diclofenac enhances proinflammatory
cytokine-induced aquaporin-4 expression in cultured astrocyte. Cell
Mol Neurobiol. 33:393–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu J, Ding D, Wang X, Li Q, Sun Y, Li L
and Wang Y: Regulation of aquaporin 4 expression by lipoxin A4 in
astrocytes stimulated by lipopolysaccharide. Cell Immunol.
344:1039592019. View Article : Google Scholar : PubMed/NCBI
|