Introduction
In previous studies, certain patients who suffered
from cytopenia did not exhibit characteristics concurrent with any
diagnostic standards of any hematological diseases. These patients,
however, may benefit from large doses of intravenous immunoglobulin
(Ig) and corticosteroid treatment (1–3).
Auto-antibodies are expressed on the membrane of hemopoietic cells
from bone marrow (BM) which can be detected using a BM mononuclear
cell (BMMNC) Coombs test, a modified version of the conventional
peripheral blood Coombs test (4–6).
Instead of peripheral erythrocytes, BMMNCs are used in BMMNC Coombs
test to detect auto-antibodies in the BM cells, and hemolysis (or
cytoclasis) can be detected at an early stage of hemopoiesis
(4). This abnormality has been
termed immuno-related pancytopenia (IRP) (1), which is also referred to as
immune-related hemocytopenia or cytopenia with positive results of
BMMNC-Coombs test (3).
Western blotting, immunofluorescence,
immunohistochemistry and flow cytometry have also been used to
detect auto-antibodies expressed on hemopoietic cells from the BM
(2,3,7–9). The
suppression of apoptosis in B lymphocytes and the abnormal counts
of B lymphocytes and their sub-types may be associated with the
presence of auto-antibodies (10,11).
IgG auto-antibodies stimulate the activation of macrophages, thus
inducing the phagocytic process of the hematopoietic cells
(12,13). IgM auto-antibodies stimulate the
activation of the complement system (14), thus inducing the lysis of
hematopoietic cells. The auto-antibodies can bind to the functional
antigens on the cell membrane, including the cytoplasmic domain of
human erythrocyte band-3 protein (based on the crystal structure)
(2), ubiquinol-cytochrome c
reductase, complex III subunit X (UQCR10), and chain P and
G-protein-coupled receptor 156 variant (15). In our previous studies, it was also
shown that T helper cells (Th)2 (16), Th17 (17), certain regulatory T cells (18), plasmacytoid dendritic cells
(19,20), memory B cells and T follicular
helper cells (21–23) participate in the pathogenic
mechanisms of IRP.
Autoimmune diseases are characterized by the
presence of auto-antibodies which are specific to the antigens from
the target organs; auto-antibodies can be detected in the serum and
may be used as biomarkers for autoimmune disorders (24). Therefore, the search for target
antigens on the cell membrane of hematopoietic cells in patients
with IRP and the auto-antibodies specific to antigens is crucial to
further determine the nature of IRP (15,25,26).
In our previous study, a K562 cDNA database was established which
was used to screen seven possible auto-antigens produced by
hematopoietic cells in patients with IRP, including ferritin light
chain (FTL) (15).
Ferritin, the primary protein involved in storing
iron, is predominantly expressed in the cytoplasm. The ferritin in
mammals is composed of 2 types of subunits: FTL and ferritin heavy
chain (FTH). The activity of ferroxidase is higher in FTH. FTH
participates in converting Fe (II) to Fe (III) (27). A more efficient iron nucleation
site in FTL can cooperate with the FTH-subunits which has
ferroxidase activity to improve the incorporation of ferritin iron
(27,28).
In the present study, FTL was produced and purified,
and the levels of the auto-antibodies specific to FTL were screened
for using K562 cells, a human leukemia cell line, using the
serologic analysis of recombinant cDNA expression libraries (SEREX)
(29).
Patients and methods
Patients
A total of 53 untreated patients with IRP were
selected, including 31 females and 22 males (median age, 34 years;
range, 10–78 years). Additionally, 60 patients who had recovered
from IRP were enrolled, including 33 females and 27 males (median
age, 26 years; range, 12–68 years). For the control groups, 14
patients with SAA and 14 patients with MDS were enrolled, and sera
from 34 healthy individuals matched for sex and age served as the
healthy controls. Serum samples from all the participants were
stored at −80°C for further detection of FTL antibodies. The
response criteria were set based on the response criteria of
aplastic anemia (AA) (30).
Among the above-mentioned patients, 35 patients with
IRP who did not receive treatment, 20 patients who had recovered
from IRP, 7 patients with MDS and 12 healthy patients were enrolled
and their fresh peripheral blood samples (5 ml) were collected to
detect FTL-mRNA levels by PCR.
Written informed consent was obtained from all
participants and the protocol was approved by the Ethics Committee
of the General Hospital of Tianjin Medical University. All the
experiments were performed in accordance with the approved
protocol.
Cell culture
Human leukemia K562 cells were provided by the
Institute of Biochemistry and Cell Biology of the Chinese Academy
of Sciences. RPMI-1640 medium supplemented with 10% FBS,
streptomycin (100 µg/ml) and penicillin (100 U/ml; all from Beijing
Solarbio Science & Technology Co., Ltd.) was used to culture
the cells in a humidified environment at 37°C with 5%
CO2.
Flow cytometric analysis
After heparinization (450 µl) (2), BM samples were rinsed with PBS three
times. All the samples were placed in separate tubes: One tube for
the control group and three tubes for the experiment groups.
Antibodies against mouse IgG1-allophycocyanin (APC) (20 µl/test,
cat. no. 555751, BD Pharmingen; BD Biosciences), mouse
IgG1-fluorescein isothiocyanate (FITC; 20 µl/test, cat. no. 551954,
BD Biosciences) and mouse IgG1-phycoerythrin (PE) (20 µl/test, cat.
no. 559320; BD Biosciences) were used as the negative controls.
Antibodies against GlycoA-FITC, CD34-FITC and CD15-FITC (20
µl/test, cat. nos. 559943, 348053 and 332778; all from BD
Biosciences) were added to the tubes separately. Anti-human IgM-APC
(20 µl/test, cat. no. 551062, BD Pharmingen; BD Biosciences) and
anti-human IgG-PE antibodies (20 µl/test, cat. no. 555787; BD
Pharmingen; BD Biosciences) were added to each tube. After
incubation for 30 min in the dark at 4°C, 2 ml erythrocyte lytic
solution (cat. no. 349202; BD Pharmingen; BD Biosciences) was added
to the cells for incubation at room temperature for 10 min.
Subsequently, the cells were rinsed with PBS twice. The density of
cells was adjusted to 1×106 cells/ml. Cells were
analyzed using a FACSCalibur flow cytometer (BD Biosciences) as
described previously (2).
Construction of the cDNA library and
serological screening of auto-antigens using SEREX
Total RNA was extracted from K562 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions.
The cDNA expression library was constructed using a
SMART cDNA Library Construction kit (Clontech Laboratories, Inc.),
according to the manufacturer's instructions. Briefly, total RNA
(2.6 g) was reverse transcribed to first-strand cDNA using the
SMART IV oligonucleotide and CDS III/3′ PCR primer, which harbor
SfiIA and SfiIB sites, respectively. The resulting full-length
first-strand cDNA was amplified to double-strand cDNA via
long-distance PCR using 5′ PCR primer and CDS III/3′ PCR primer
with the following thermocycling conditions: 95°C for 1 min;
followed by 22 cycles of 95°C for 15 sec; and a final extension at
68°C for 6 min. After excluding small DNA using CHROMA SPIN-400
tubes (Clontech Laboratories, Inc.), larger cDNAs were collected.
The product was ligated directionally into the λTripleEx2 vector
(Clontech Laboratories, Inc.), packaged into phage particles
(λ-phage) using Gigapack III Gold Packaging Extract (Agilent
Technologies, Inc.) and transformed into XL1-blue super competent
cells (Agilent Technologies, Inc.). The original library was
amplified and maintained at −80°C for immunoscreening.
The transformed bacteria were plated in top agarose
in a Petri dish. The plaques were transferred to presoaked
nitrocellulose filter. The pooled sera of untreated IRP patients
and the pooled sera of normal controls were preabsorbed with
bacteria lysate of XL1-blue E. coli transformed with phage
without cDNA inserts. The preabsorbed pooled sera of IRP and normal
controls was used as positive serum and negative serum,
respectively. The filter was incubated with positive serum
overnight at 4°C. The membranes were then washed, incubated with
horseradish peroxidase (HRP)-conjugated goat anti-human antibody
(cat. no. W403B; 1:5,000; Promega Corporation), washed with TBST
(0.05% Tween-20) and developed using TMB stabilized substrate for
HRP (Promega Corporation). The positive plaques were selected, and
were eluted, re-plated and re-screened with positive serum and
negative serum, respectively; targeted plaques were positive with
positive serum and negative with negative serum. The plaques which
were positive with IRP serum, but negative with normal control
serum, were identified as positive screened plaques (15).
PCR amplification and cloning
TransTaq DNA Polymerase High Fidelity (Beijing
Transgen Biotech Co., Ltd.) and pTriIEx2-FTL plasmid were used to
amplify the open reading frame (ORF) encoding FTL using the
following primers: Forward, ATGAGCTCCCAGATTCGTCA and reverse,
TTAGTCGTGCTTGAGAGTGAGC. To obtain the ORF of FTL, PCR was performed
using a Bio-Rad iQ5 Real-Time system (Bio-Rad Laboratories, Inc.)
with the following thermocycling conditions: 94°C for 5 min;
followed by 30 cycles of 94°C for 30 sec, 58°C for 30 sec, 72°C and
60 sec; and a final extension at 72°C for 10 min. Agarose gel
electrophoresis (2%) was used to detect the length of the PCR
products. The PCR products were cloned into the T7 RNA
polymerase-based expression vector pEASYTM-E1 (Beijing Transgen
Biotech Co., Ltd.) containing an N-terminal sequence that contained
a 6×His-tag. Competent E. coli Trans1-T1 were transformed
and selected for the LB-agar plates with ampicillin (60 mg/ml) used
to select for successfully transformed cells. Following PCR
screening using the primer FTL-R and vector primer T7-F, the
forward positive clones were incubated at 37°C and shaken for 6 h
at 220 rpm. The recombinant plasmid DNA in the cells was extracted
using an Plasmid extraction kit (Axygen; Corning, Inc.) according
to the manufacturer's protocol. The extracted DNA was sequenced
using a DNA sequencing assay with a T7 terminator primer and T7
promoter. The DNA sequences were analyzed using the BLAST algorithm
(ncbi.nlm.nih.gov/blast).
Expression of recombinant
proteins
The recombinant plasmid was inserted into BL21
(DE3), an E. coli strain. BL21 cells with the recombinant
plasmid were seeded in LB agar (agar 15 g/l, NaCl 5 g/l, yeast
extract 5 g/l and tryptone 10 g/l) and ampicillin (60 mg/ml) and
incubated at 37°C overnight. A single transformed E. coli
colony was added to LB broth (10 ml) supplemented with ampicillin
(60 mg/ml) and incubated at 37°C. The mixture was shaken for 12 h
at 220 rpm. Cells (5 ml) were added to the LB broth (100 ml)
supplemented with ampicillin (60 mg/ml) and incubated at 37°C, and
shaken at 220 rpm. When the optical density (OD) of the culture
medium was ~0.6 (at a wavelength of 600 nm),
isopropyl-β-D-thiogal-actopyranoside (IPTG; final concentration 1
mM) was added to the culture medium. Subsequently, the cells were
cultured at 37°C for 1 h and then centrifuged at 4°C, 11,200 × g
for 5 min) to collect the precipitate. The precipitate was used
immediately for purification or stored at −80°C. After
centrifugation (11,200 × g, 4°C for 5 min), the precipitate was
resuspended in 20 ml PBS and lysed on ice using lysozyme (4 mg/ml)
and Triton-X 100 (final concentration 3%) for 15 min, and then
disrupted by ultrasonication in ice for 8 min (8 sec pulses, with
an interval of 10 sec). After centrifugation for 20 min at 11,200 ×
g at 4°C, the supernatant was collected and stored at −20°C. Then,
the precipitate was resuspended in an equivalent volume of PBS
containing 6 mol/l urea, then disrupted by sonication until the
solution had turned clear and stored at −20°C. The supernatant and
precipitate of empty pEASY-E1 in E. coli BL21 (DE3) cells
were obtained using the same protocol. The protein in the
supernatant and precipitate was used for SDS-PAGE using a 15% SDS
gel, and the resolved protein bands were visualized using Coomassie
brilliant blue staining (31).
Purification of recombinant
proteins
The suspension of precipitate was added to
ProteinIso™ Ni-NTA agarose resin columns (Beijing Transgen Biotech
Co., Ltd.). The suspension was equilibrated using an equilibration
buffer (pH 8.0) supplemented with NaH2PO4
(100 mM), Urea (8 M), Trisbase (10 mM) and imidazole (20 mM). A
small portion of the protein was removed and eluted with 40, 62.5,
80, 100, 125, 160, 200, 250 or 500 mM imidazole in equilibration
buffer (pH 8.0). After elution, the protein was used for
electrophoresis on a 15% SDS gel. The remaining recombinant protein
was eluted with the optimum concentration of imidazole. An anti-His
antibody (cat. no. HT501-01, Beijing Transgen Biotech Co., Ltd.,
1:1,000, 4°C, incubated overnight) was used for western blotting to
detect the purified FTL protein which had a 6×His-tag. Urea-TBS
glycerol buffer [glycerol (10%), NaCl (50 mM), oxide glutathione
(0.2 mM), Tris HCl (50 mM) and reduced glutathione (2 mM); and a
decreasing concentration gradient of urea (6, 5, 4, 3, 2, 1 and 0
M), pH 8.0] was used to refold the purified protein at 4°C for 12
h. A BCA Protein assay kit (CoWin Biosciences) was used to quantify
the concentration of purified FTL, and the protein was stored at
−80°C for further use.
Detection of auto-antibodies in serum
using ELISA
Flat-bottom culture dishes were coated with the
recombinant target protein (2 µg/ml; diluted with 50 mM carbonate
buffer, pH 9.6) and were incubated in a humidified chamber at 4°C
overnight. After washing three times with PBS with 0.05% (vol/vol)
Tween 20 (pH 7.4; PBST), the protein in the plate was blocked with
300 µl 2% BSA (diluted in PBS) in each well at room temperature for
3 h. Plates were rinsed with PBST three times, and serum (1:100,
diluted in PBS containing 2% BSA) was added to the wells and
cultured at room temperature for 90 min. Subsequently, the protein
was washed six times and co-incubated with goat anti-human IgG
conjugated with horseradish peroxidase (1:2,500, cat. no. W4031,
Promega Corporation) diluted in PBS containing 2% BSA (100 µl/well)
at room temperature for 1 h. The plates were rinsed with PBST six
times, and the antibodies were measured using TMB Substrate
(Tiangen Biotech Co., Ltd.). The entire process was terminated by
the addition of 50 µl 2M H2SO4. The
absorbance was detected using a multi-scan microplate reader
(Metertech 960, Metertech Inc.) at 450 nm. The serum from 8 healthy
subjects was pooled and used as the control group. The
auto-antibody levels were measured using the binding index (BI),
which was calculated as follows: BI=(OD of the sample-OD of the
blank group)/(OD of the control group-OD of the blank group)
(32).
Detection of FTL mRNA levels using
semi-quantitative PCR
Using TRIzol® reagent (Thermo Fisher
Scientific, Inc.), total RNA was harvested from human BM according
to the manufacturer's protocol. According to the instructions of
the PrimeScript RT Master mix (cat. no. DRR036A, Takara Bio, Inc.),
reverse transcription was performed in a 20 µl system using 1 µg
total RNA, random primers and the PrimeScript RT Master mix
(Takara, Bio, Inc.). Pfu PCR MasterMix (Tiangen Biotech Co., Ltd.)
was used and the primers used for PCR are stated above. GAPDH was
used as the internal control. The sequence of the GAPDH primers
were: Forward, 5′-CCGGGAAACTGTGGCGTGATGG-3′ and reverse,
5′-AGGTGGAGGAGTGGGTGTCGCTGTT-3′. The thermocycling conditions were:
94°C for 3 min; followed by 30 cycles of 94°C for 30 sec and 60°C
for 30 sec; and a final extension step at 72°C for 2 min. Agarose
gel electrophoresis (2%) was used to detect the PCR products.
Semi-quantification was performed based on the gray value ratio of
FTL band/GAPDH band using GENETOOLS software (v1.0, Gene Company,
Ltd.).
Statistical analysis
Data are expressed as the mean ± standard deviation.
All the experiments were performed in triplicate. Statistical
analysis was performed using GraphPad Prism version 6 (GraphPad
Software, Inc.). The significance of multiple groups was assessed
by one-way ANOVA followed by Bonferroni correction post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
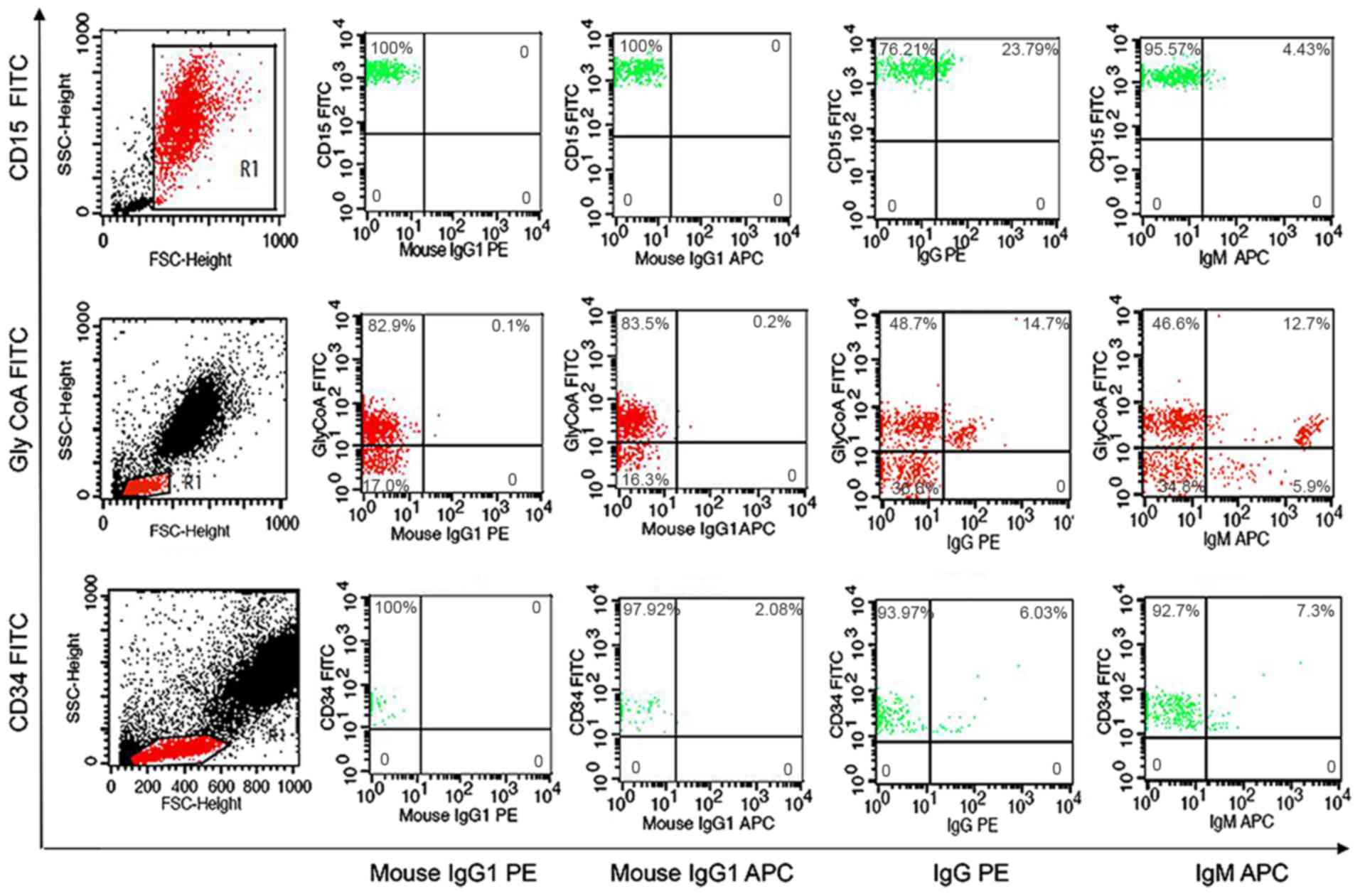
Detection of IgG and IgM on BM
hemopoietic cells of IRP patients by FCM
Flow cytometric analysis was used to detect the
expression of auto-antibodies on the cytoplasmic membrane of BM
hemopoietic cells. The positive rate of auto-antibodies on
nucleated erythrocytes (GlyCoA+), granulocytes (CD15+) and stem
cells (CD34+) was defined as >4.0% in flow cytometry analysis
(Fig. 1) (8).
Construction of the cDNA library
The cDNA library had good recombination efficiency,
capacity and ligation efficiency, sufficient for further
experiments (15).
SEREX and the serological screening of
the auto-antigens
A total of 11 bacterial clones were screened using
SEREX and 7 of these were successfully analyzed using the BLAST
algorithm, which included trafficking protein particle complex
subunit 4 (TRAPPC4), transcript variant 3, phosphoglycerate kinase
1 (PGK1), hemoglobin γ-G, stathmin 1 (STMN1), multifunctional
methyl-transferase subunit TRM112-like protein isoform 1 (TRMT112),
UQCR10, FTL and ferritin (15).
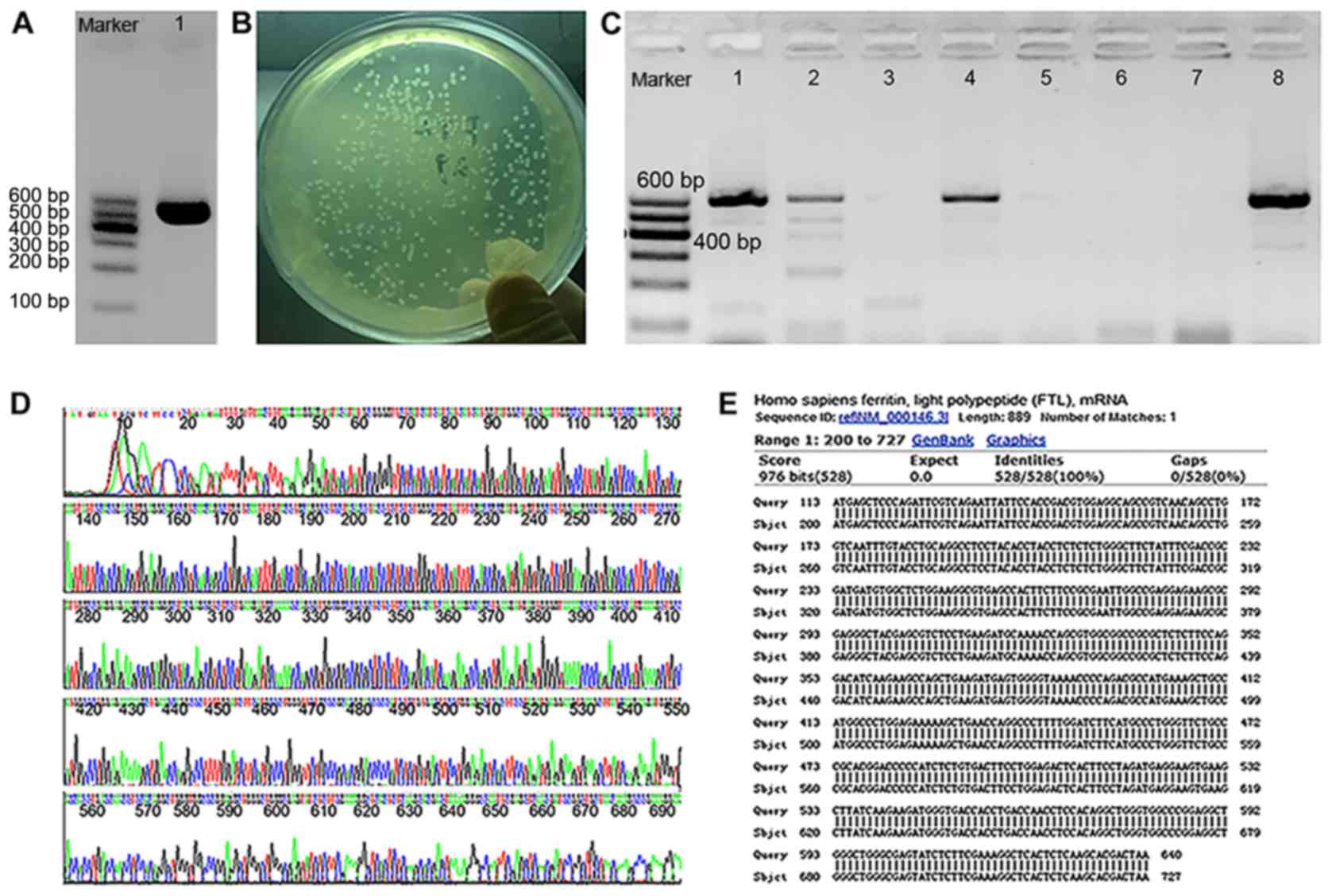
PCR amplification and cloning of the
FTL gene
After amplification, the quantity and quality of PCR
products were verified using agarose gel electrophoresis. In
agarose gel electrophoresis, a single band was obtained from the
PCR products and the length was in agreement with the expected gene
size of 528 bp (Fig. 2A). The
ligated mixture of PCR products and pEASYTM-E1 was inserted into
the competent E. coli Trans1-T1. Competent E. coli
Trans1-T1 colonies were well-defined and grew well (Fig. 2B), suggesting that the colonies
contained pEASYTM-E1-FTL although the insertion direction between
FTL and pEASYTM-E1 was unknown. Therefore, eight colonies were
randomly selected from the plate and screened using PCR with primer
FTL-R and the vector primer T7-F. The band in Fig. 2C lane 8 was clear without noise, so
the corresponding colony was selected as the forward-positive
clones. The size of the PCR products amplified with T7-F and FTL-R
was 89 bp which was larger than that of the target gene. Sequencing
results of the plasmid DNA extracted from the positive clones with
T7 promoter and T7 terminator primer are shown in Fig. 2D. In Fig 2E, DNA sequences matched the ORF of
FTL (200–727 bp), which was analyzed using the BLAST algorithm.
Expression, purification and
identification of FTL
After IPTG induction, the pEASY-E1-FTL in E.
coli BL21(DE3) cells was separated using SDS-PAGE. A band of
~19 kDa was present in the precipitate of transformed cells
(Fig. 3A, lane 4), which was
absent from the empty E. coli BL21(DE3) cells (Fig. 3A, lane 2). In order to increase
accuracy, SDS-PAGE analysis of precipitate of pEASY-E1-FTL in E.
coli BL21(DE3) cells was performed (Fig. 3B). Recombinant (r)FTL is an
insoluble protein primarily expressed in inclusion bodies with a
molecular weight of ~19.4 kDa. A small portion of the protein was
eluted with different concentrations of imidazole, and 500 mM was
identified as the optimum concentration (Fig. 3C, lane 9). The rest of the rFTL
protein was eluted with 500 mM imidazole. Three purified FTL
samples were initially loaded (lane 1–3), and sample 2 was
ultimately selected for subsequent experiments, due to its
relatively higher purity, compared with samples 1 and 3 (as
indicated by the black arrow pointing to the target protein rFTL;
Fig. 3D, lane 2). Fig. 3E shows western blot analysis of
recombinant HIS-FTL protein before purification (black arrow, lane
2). The rFTL protein after purification was detected by western
blotting with the use of the anti-histidine antibody conjugated to
horseradish peroxidase (Fig. 3F,
lane 1). Based on the BCA assay, the concentration of purified FTL
was 1,018.6 µg/ml.
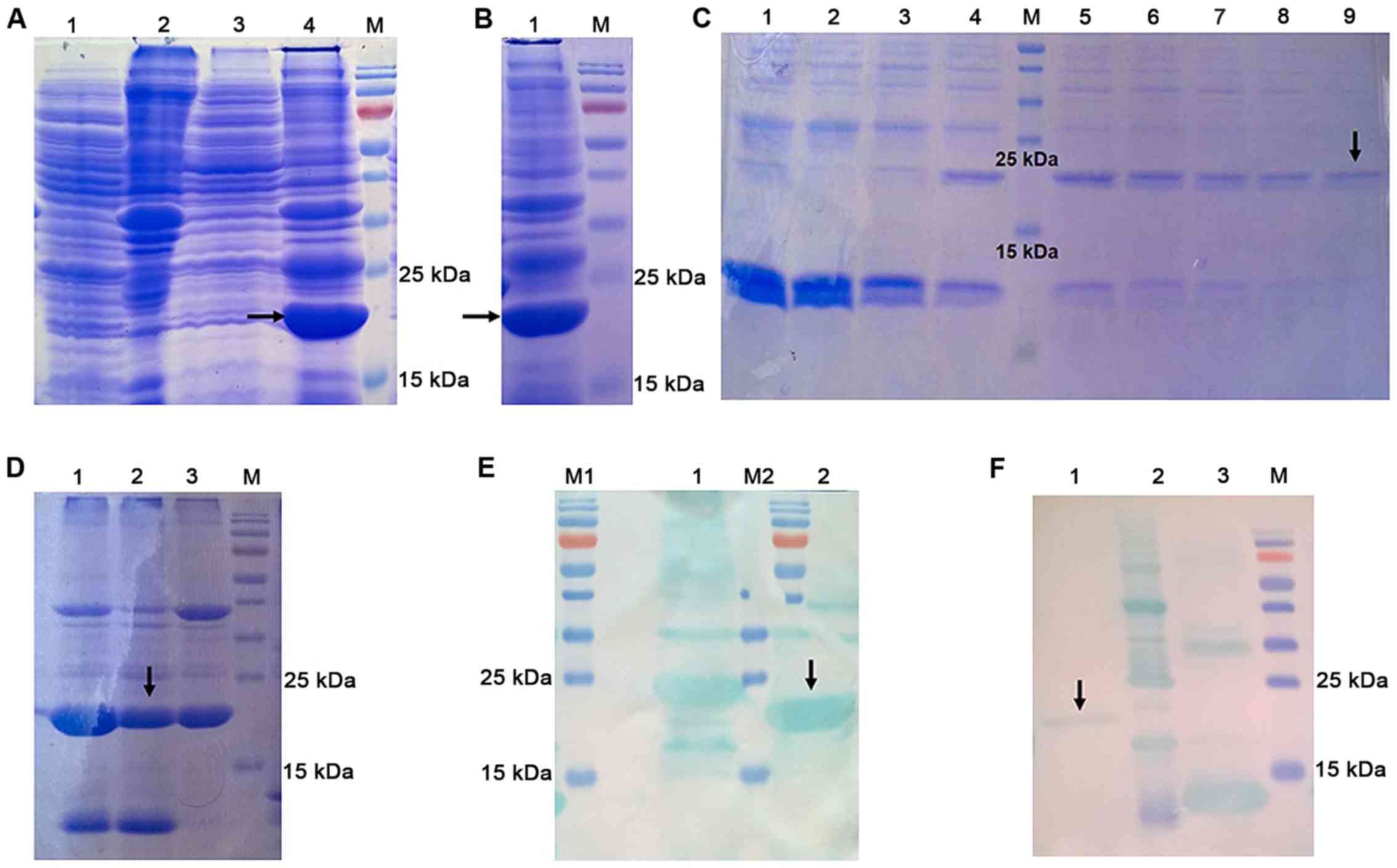 | Figure 3.Expression and purification of FTL.
(A) SDS-PAGE analysis of the pEASY-E1 in E. coli BL21(DE3)
cells and the pEASY-E1-FTL in E. coli BL21(DE3) cells. Lane
M, protein molecular marker; lane 1, supernatant of empty pEASY-E1
in E. coli BL21(DE3) cells; lane 2, precipitate of empty
pEASY-E1 in E. coli BL21(DE3) cells; lane 3, supernatant of
pEASY-E1-FTL in E. coli BL21(DE3) cells; lane 4, precipitate
of pEASY-E1-FTL in E. coli BL21(DE3) cells. rFTL was
expressed as an insoluble protein and accumulated in inclusion
bodies. It had a distinct band with a molecular weight of ~19 kDa
(black arrow). (B) SDS-PAGE analysis of precipitate of pEASY-E1-FTL
in E. coli BL21(DE3) cells. The concentrated band located
around 19 kDa in lane 1 represents rFTL protein (black arrow). (C)
SDS-PAGE analysis of purified rFTL eluted with different
concentration of imidazole. Lane M, protein molecular marker; Lanes
1–9, purified rFTL eluted with 40, 62.5, 80, 100, 125, 160, 200,
250 and 500 mM of imidazole in equilibration buffer (pH 8.0),
respectively. Purified rFTL eluted with 500 mM of imidazole (lane
9, black arrow) had relatively higher purity compared with others
and 500 mM was identified as the optimum concentration. (D)
SDS-PAGE analysis of purified rFTL. A total of three purified FTL
samples were initially loaded (lanes 1–3), and sample 2 is the one
that was ultimately selected for subsequent experiments, due to its
relatively higher purity, compared with samples 1 and 3 (the black
arrow points to the target protein rFTL). (E) Western blot analysis
of recombinant HIS-FTL protein before purification (black arrow).
(F) Western blot analysis of recombinant HIS-FTL protein after
purification (lane 1, black arrow). The other two proteins loaded
in lane 2 and 3 are not relevant to the present study. rFTL,
recombinant ferritin light chain. |
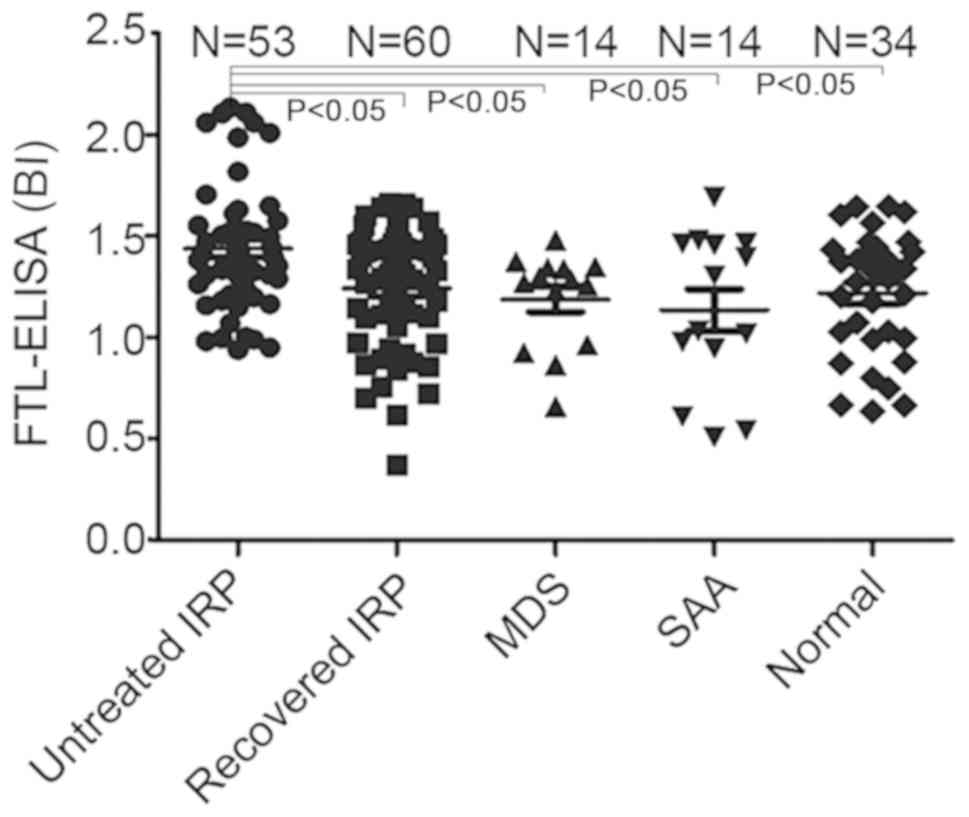
Anti-FTL antibodies levels in patients
with IRP
The anti-FTL antibodies levels in the serum of
untreated patients with IRP (1.44±0.32 BI) (32) were higher compared with the
patients who had recovered from IRP (1.24±0.29 BI), SAA patients
(1.14±0.39 BI), MDS patients (1.19±0.24 BI) and healthy controls
[(1.20±0.28 BI; all P<0.05)]. The levels of anti-FTL antibody
did not differ significantly between patients who had recovered
from IRP, SAA patients, MDS patients and normal controls
(P>0.05; Fig. 4).
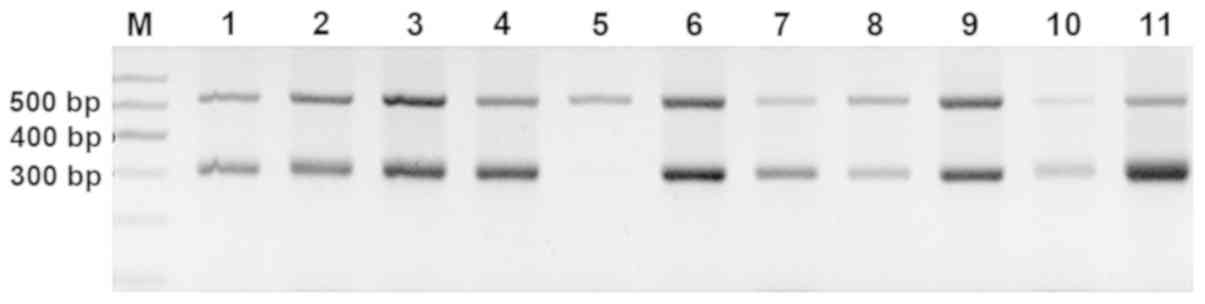
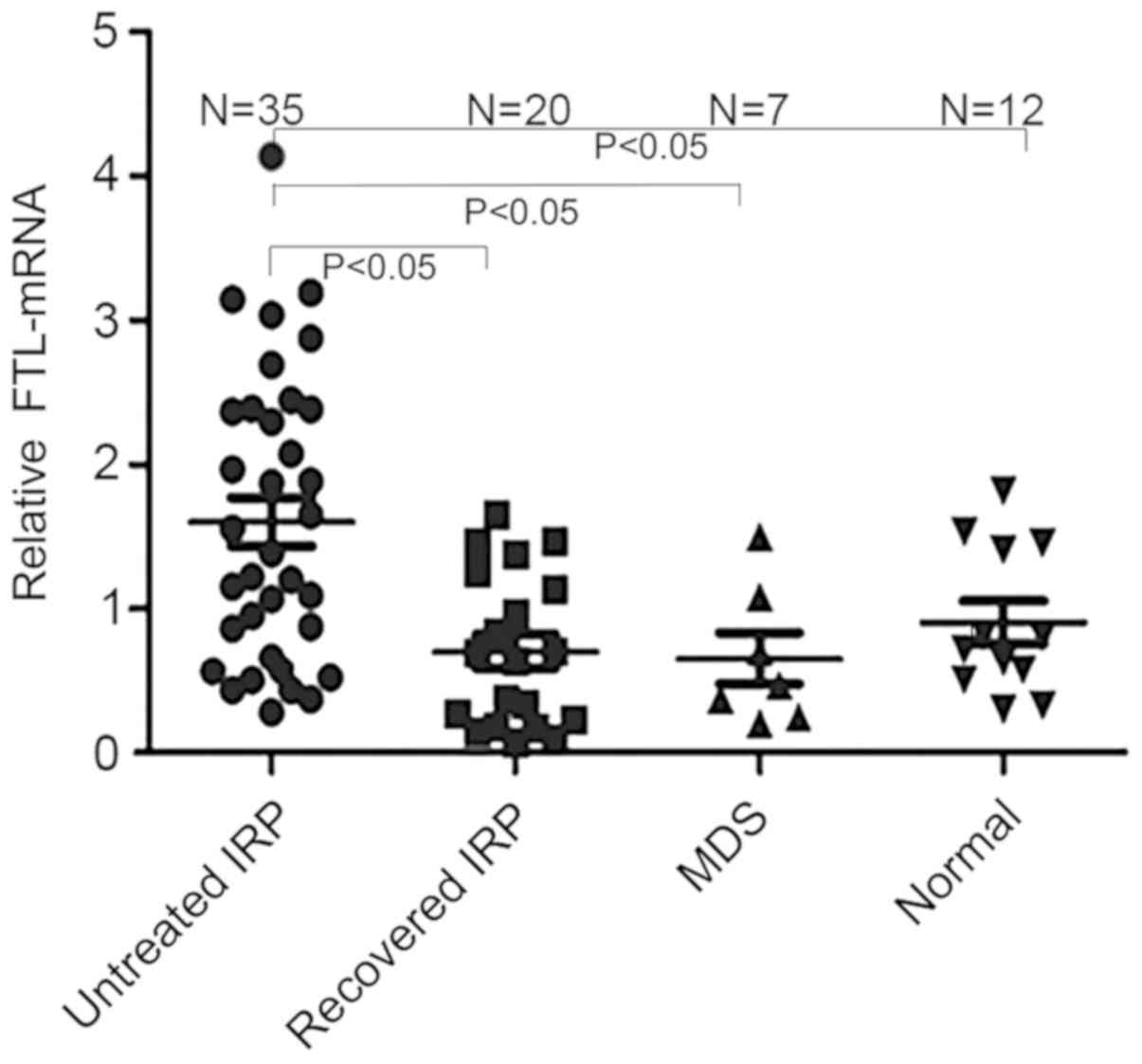
FTL-mRNA expression levels
The results of PCR were measured using agarose gel
electrophoresis (Fig. 5). The
relative levels of FTL-mRNA in untreated patients with IRP
(1.60±0.99) were significantly higher compared with the patients
who had recovered from IRP (0.70±0.53), MDS patients (0.65±0.48)
and healthy controls (0.91±0.51; all P<0.05). The FTL-mRNA
levels were also significantly increased in the untreated patients
with IRP compared with the patients who had recovered from IRP, MDS
patients and healthy subjects. However, there was no significant
difference in the FTL-mRNA expression levels among patients who had
recovered from IRP, SAA patients, MDS patients and healthy subjects
(all P>0.05; Fig. 6).
Discussion
Idiopathic acquired AA is a benign bone marrow
failure disease, in which type 1 cytokines and cytotoxic
lymphocytes are the main effector cells (33). MDS are a series of malignant
hematopoietic dysfunctions characterized by a tendency to acute
myeloid leukemia (AML), dysplastic changes in cell lineages and
ineffective hematopoietic function (34). However, their diagnostic criteria
do not necessarily reflect the pathophysiology of the disease. The
diagnosis of idiopathic AA and MDS both depend on exclusion of
other possible diseases rather than specific tests (34,35).
As such, it is difficult to diagnose MDS or AA when patients
exhibit unexplained cytopenia. In previous studies, multiple
definitions and classifications of pre-MDS were proposed, including
idiopathic cytopenia of undetermined significance (ICUS), clonal
cytopenia of unknown significance (CCUS), idiopathic dysplasia of
unknown significance (IDUS) and clonal hematopoiesis of
indeterminate (clinical) potential (CHIP) (36–38),
which allow diagnosis of in certain patients with unexplained
cytopenia.
In our previous study, it was shown that in some
patients, unexplained cytopenia was the result of BM suppression or
destruction mediated by auto-antibodies, and thus it was
temporarily termed IRP (1). The
clinical features of IRP consist of erythroid hyperplasia,
increased erythroblastic islands, hypoplastic or hyperplastic BM,
normal or high reticulocyte and neutrophil counts, and cytopenia or
pancytopenia (39).
Other studies have shown that different
auto-antibodies specific to kinectin (40), deficiency of ribosomal subunits
protein 1 (DRS1) (41), PMS1
protein homolog 1 (PMS1) (42),
moesin (43), heterogeneous
nuclear ribonucleoprotein K (hnRNP K) (44) and proteins involved in the
biogenesis of RNA, such as small nuclear ribonucleoprotein
polypeptide F (SNRPF), ribosomal protein S27 (RPS27), SRA stem-loop
interacting RNA binding protein (SLIRP) and ribosomal protein L41
(RPL41) (45), are found in the
serum of patients with AA consistently. Furthermore, the importance
of B cells and cluster of differentiation (CD)4+T cells
rather than CD8+T cells in the development of AA has
been demonstrated (41,43,44),
and raises the question of whether the cases with auto-antibodies
are different from typical AA cases without auto-antibodies in
terms of the pathophysiology.
Some patients with early MDS show favorable
responses to immunosuppressive therapy, and auto-antibodies have
been detected in patients with early MDS (46–50).
Stahl et al (46) demonstrated that the repertoires of
antibodies in self-reactive IgG and IgM in patients with MDS
exhibit notably different reactivity patterns compared with
antibodies from healthy individuals. Barcellini et al
(47) found that certain patients
with early MDS exhibit autoimmune reactions against erythroblasts,
which may contribute to the application of steroids used to treat
these patients. Zaninoni et al (50) showed that 66% of patients suffering
from MDS [refractory anemia (RA) and RA with ringed siderblasts]
have positive antibodies against erythroblasts, hemolysis in
peripheral blood and erythroid hyperplasia in BM during the early
phase. Mias et al (48)
showed that the reactivity of the antibodies against
ADP-ribosylation factor-like protein 8B (ARL8B), Akt3 and low
affinity immunoglobulin γ Fc region receptor III-A (FCGR3A) was
increased in patients with MDS. Increased reactivity of these
antibodies primarily occurs in patients with stable MDS which
suggests that the immune-related disorders of MDS during the early
phase were worse compared with the advanced stage MDS (48). Komrokji et al (49) assessed the occurrence of autoimmune
disorders among 1,408 patients with MDS in King's College Hospital
and Moffitt Cancer Center. A total of 28% of patients suffered from
an autoimmune disease (AID). This was more common in females, in
patients who were less reliant on erythrocyte transfusion and those
with refractory cytopenia with multilineage dysplasia or RA. The
median overall survival (OS) was 45 months in those without an AID
compared with 60 months in patients with an AID. According to the
results of the multivariate analysis, the presence of an AID was an
independent risk factor for OS. Furthermore, the rate of
transformation into AML was 30% in patients with MDS without
autoimmune-related diseases vs. 23% in those with
autoimmune-related diseases. The study suggested that the patients
with MDS complicated with an autoimmune-related disorder had better
prognosis and unique clinical features (49). The abovementioned studies suggest
that the diagnosis of cytopenia is gradually refined, and thus may
assist in improving our understanding of the underlying
pathogenesis and in the development of more accurate
treatments.
In our previous study, a K562 cDNA database was
established, which was used for screening seven possible
auto-antigens produced by hematopoietic cells in patients with IRP.
The auto-antigens included TRAPPC4, PGK1, hemoglobin γ-G, STMN1,
TRMT112, ubiquinol-cytochrome c reductase, light polypeptide,
ferritin and UQCR10 (15). In the
present study, the titers of antibodies against FTL were measured
indirectly using ELISA. There was a significant increase in serum
levels of FTL auto-antibodies in patients with IRP. Additionally,
the levels of anti-FTL antibodies in the serum of the untreated IRP
patients were significantly higher compared with the patients who
had recovered from IRP, patients with SAA, patients with MDS and
normal subjects. Therefore, it was also suggested that FTL was a
type of autoantigen which was targeted by IgG antibodies in
patients with IRP.
Ferritin consists of two subunits, FTL and FTH.
Mewar et al (51) used
immune-screening methods and a phage-displayed cDNA library to
select a cDNA clone which could encode the FTH. Antibodies against
ferritin exist in 2.1% of the patients with SLE, 2.1% of the
patients with osteoarthritis, 2.7% of the healthy subjects, 19% of
the patients with early RA and 16% of the patients with established
RA (51).
Baerlecken et al (52) using protein arrays found that FTH
may be an antigen associated with polymyalgia rheumatic (PMR) and
giant cell arteritis (GCA). Auto-antibodies against the full length
FTH, the 27 amino acids on the N terminus of FTH and the 27 amino
acids on the N terminus of the ferritin in Staphylococcus
epidermidis were detected. Antibodies against the ferritin
peptide and the Staphylococcus ferritin peptide were present in 89
and 92% of the untreated patients with PMR and GCA, respectively
(52).
Régent et al (53) detected antibodies against amino
acids 19–45 of FTH1 in patients with GCA, and found that 72.5%
patients suffering from GCA had anti-FTH1 antibodies, compared with
31.9% of the patients in the control group, and positive rates of
the antibody were higher prior to glucocorticoid therapy (53).
The capacity to detect human ferritin peptide
antibodies in GCA/PMR was increased, without notably changing the
false-positive rate in the diagnostic examinations, through a
combination of various ELISAs (epitopes A19-45, A79-104 and
A105-144) (54). Subsequently,
through the use of the combination of the various ELISAs, ferritin
peptide antibodies were detected in 30/48 (62%) patients with
Takayasu arteritis (55).
In the present study, FTL was expressed and purified
successfully. The serum levels of antibodies against FTL in the
patients with IRP without treatment were significantly higher
compared with the patients who recovered from IRP, patients with
SAA, patients with MDS and the healthy controls. Additionally, the
levels of FTL-mRNA were significantly upregulated in the patients
with IRP without treatment compared with the patients who recovered
from IRP, patients with MDS and the subjects in the control group.
It was hypothesized that this upregulation may be the result of a
negative feedback mechanism. However, anti-FTL antibodies were not
detected in all the serum samples in the present study, suggesting
that there may be other auto-antigens or auto-antibodies
interfering with the growth and differentiation of CD34+
cells. The diagnostic and prognostic value of anti-FTL antibodies
for IRP remains to be verified using larger, more varied cohorts
with larger control groups. Additionally, the identification of
FTL-specific T-helper cells and subsequent functional analysis may
further clarify the roles of FTL in the pathogenesis of IRP.
In conclusion, the present study demonstrated that
the levels of antibodies against FTL were increased in the
untreated patients with IRP and the antibody titers decreased
following immuno-suppressive treatment. These findings suggest that
detecting antibodies against FTL in patients with IRP may have
clinical significance.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81600088 and
81770118).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS, HW and SH conceived and designed the
experiments. SH, YZ and LH performed the experiments. NXie, RF and
NXia analyzed the data. SH wrote the manuscript. ZS approved the
final version of the manuscript to be published. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
the General Hospital of Tianjin Medical University. All the
experiments were performed in accordance with the approved
protocol. Written informed consent was obtained from all the
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He H, Shao Z, Liu H, Song L, Tian P, Cao
Z, Zhang Y, Li K, Zhao M, Shi J, et al: Immunorelated pancytopenia.
Zhonghua Xue Ye Xue Za Zhi. 22:79–82. 2001.(In Chinese). PubMed/NCBI
|
|
2
|
Liu H, Fu R, Wang Y, Liu H, Li L, Wang H,
Chen J, Yu H and Shao Z: Detection and analysis of autoantigens
targeted by autoantibodies in immunorelated pancytopenia. Clin Dev
Immunol. 2013:2976782013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu R, Liu H, Wang Y, Liu H, He H, Chen J,
Wang H, Yu H, Ding K, Huang L, et al: Distinguishing immunorelated
haemocytopenia from idiopathic cytopenia of undetermined
significance (ICUS): A bone marrow abnormality mediated by
autoantibodies. Clin Exp Immunol. 177:412–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He H, Shao Z, Cao Z, Tian P, Cui W and Liu
H: Category of bone marrow mononuclear cells Coombs test. Zhonghua
Xue Ye Xue Za Zhi 22. 5502000.(In Chinese).
|
|
5
|
He H and Shao ZH: Modified direct
antiglobulin test. Chin J Lab Med. 24:313–315. 2001.(In
Chinese).
|
|
6
|
Sun J, Cao Z and Tu M: Direct antiglobulin
test of bone marrow mononuclear cells: Results of 270 cases with
autoimmune hemocytopenia. Chin J Pract Internal Med. 26:12–14.
2006.
|
|
7
|
Fu R, Shao ZH, Liu H, He H, Jia HR, Sun J,
Zhao MF, He GS, Shi J, Bai J, et al: Category, quantity and
clinical significance of autoantibodies on bone marrow
hematopoietic cells in patients with immunorelated cytopenia.
Zhonghua Xue Ye Xue Za Zhi. 24:177–180. 2003.(In Chinese).
PubMed/NCBI
|
|
8
|
Wang YH, Fu R, Dong SW, Liu H and Shao ZH:
Erythroblastic islands in the bone marrow of patients with
immune-related pancytopenia. PLoS One. 9:e951432014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao Y, Fu R, Liu H, Wang Y, Ding S, Wang
H, Li L and Shao Z: IgG autoantibody subclasses altered in
immuno-related hemocytopenia. Cell Immunol. 294:13–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu R, Shao Z, He H, Liu H, Jia H, Sun J,
Zhao M, He G, Shi J, Bai J, et al: Quantity and apoptosis-related
protein level of B lymphocyte in patients with immunorelated
pancytopenia. Zhonghua Xue Ye Xue Za Zhi. 23:236–238. 2002.(In
Chinese). PubMed/NCBI
|
|
11
|
Fu R, Shao ZH, Liu H, Wu YH, Wang HQ and
Xing LM: Role of B lymphocyte and its subpopulations in
pathogenesis of immunorelated pancytopenia. Chin Med Sci J.
22:199–202. 2007.PubMed/NCBI
|
|
12
|
Wang YH, Fu R, Shao ZH, Wang HQ, Xing LM,
Liu H, Wu YH, Li LJ, Liu H, Wang J, et al: Study on quantity and
function of bone marrow macrophages in patients with BMMNC-Coombs
test(+) pancytopenia. Zhonghua Xue Ye Xue Za Zhi. 30:538–542.
2009.(In Chinese). PubMed/NCBI
|
|
13
|
Wang YH, Fu R, Shao ZH, Xing LM, Wang HQ,
Wu YH, Liu H, Liu H, Wang J and Chen J: Expression of bone marrow
macrophages antigen activation and its clinical significance in
pancytopenia patients with positive bone marrow mononuclear
cells-Coombs test. Zhonghua Nei Ke Za Zhi. 49:146–149. 2010.(In
Chinese). PubMed/NCBI
|
|
14
|
Chen J, Fu R, Li L, Liu H, Wang Y, Wang H
and Shao Z: Variation in complement level and its significance in
cytopenia patients with positive BMMNC-Coombs. Zhonghua Xue Ye Xue
Za Zhi. 30:454–457. 2009.(In Chinese). PubMed/NCBI
|
|
15
|
Hao S, Fu R, Wang H and Shao Z: Screening
novel autoantigens targeted by serum IgG autoantibodies in
immunorelated pancytopenia by SEREX. Int J Hematol. 106:622–630.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu R, Shao ZH, Liu H, He H, Sun J, Zhao
MF, He GS, Shi J, Bai J, Yang TY and Yang CI: Proliferation of bone
marrow hematopoietic stem cells and function of T helper
lymphocytes of patients with immuno-related pancytopenia. Zhong Hua
Xue Ye Xue Za Zhi. 25:213–216. 2004.(In Chinese).
|
|
17
|
Fu R, Wang HL, Chen J, Wang J, Li LJ, Liu
H, Wang YH, Ren Y and Shao ZH: Study of the quantity and function
of Th17 cells in the blood cytopenic patients with positive
BMMNC-Coombs test. Zhonghua Xue Ye Xue Za Zhi. 31:684–687. 2010.(In
Chinese). PubMed/NCBI
|
|
18
|
Fu R, Chen J, Wang HL, Wang J, Li LJ, Liu
H, Wang YH, Ren Y and Shao ZH: Quantity and function of regulatory
T cells in hemocytopenic patients with positive BMMNC-Coombs test.
Zhonghua Yi Xue Za Zhi. 90:2989–2993. 2010.(In Chinese). PubMed/NCBI
|
|
19
|
Teng GS, Fu R, Liu H, Wang HL, Wang YH,
Ruan EB, Qu W, Liang Y, Wang GJ, Wang XM, et al: Expression of CD80
and CD86 on dendritic cells of patients with immune related
pancytopenia and its clinical significance. Zhonghua Xue Ye Xue Za
Zhi. 33:865–868. 2012.(In Chinese). PubMed/NCBI
|
|
20
|
Teng GS, Fu R, Liu H, Wang HL, Wang YH,
Ruan EB, Qü W, Liang Y, Wang GJ, Wang XM, et al: Quantity and
subtypes of dendritic cells in patients with immune related
pancytopenia and their clinical significance. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 20:722–726. 2012.(In Chinese). PubMed/NCBI
|
|
21
|
Yu H, Fu R, Wang YH, Wang HQ, Liu H, Li
LJ, Wang HI, Ruan EB, Qu W, Wang XM, et al: Preliminary study on
the quantity and function of T follicular helper cells in the
cytopenic patients with positive BMMNC-Coombs test. Zhonghua Xue Ye
Xue Za Zhi. 34:606–609. 2013.(In Chinese). PubMed/NCBI
|
|
22
|
Yu H, Zhang J, Fu R, Liu H, Wang H, Ding
K, Wang Y, Li L, Wang H and Shao Z: Increased frequency of bone
marrow T follicular helper cells in patients with immune-related
pancytopenia. Clin Dev Immunol. 2013:7304502013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YH, Fu R and Shao ZH: A pilot study
of memory B lymphocytes in relapsed immune-related pancytopenia
patients. Clin Lab. 60:729–733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi MY and Fritzler MJ: Autoantibodies in
SLE: Prediction and the p value matrix. Lupus. 28:1285–1293. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu R, Liu H, Wang J, Li LJ, Wang HI, Wang
YH and Shao ZH: Preliminary study of autoantigens on the membrane
of erythropoietic cells of the patients with BMMNC-Coomb's test(+)
hemocytopenia. Zhonghua Yi Xue Za Zhi. 92:2689–2693. 2012.(In
Chinese). PubMed/NCBI
|
|
26
|
Wang T, Hao S, Fu R, Wang H and Shao Z:
Significance of anti-EPO receptor antibody in immune-related
pancytopenia. Zhonghua Yi Xue Za Zhi. 97:1406–1410. 2017.(In
Chinese). PubMed/NCBI
|
|
27
|
Santambrogio P, Levi S, Cozzi A, Corsi B
and Arosio P: Evidence that the specificity of iron incorporation
into homopolymers of human ferritin L- and H-chains is conferred by
the nucleation and ferroxidase centres. Biochem J. 314:139–144.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J and Pantopoulos K: Regulation of
cellular iron metabolism. Biochem J. 434:365–381. 1992. View Article : Google Scholar
|
|
29
|
Ganesan V, Ascherman DP and Minden JS:
Immunoproteomics technologies in the discovery of autoantigens in
autoimmune diseases. Biomol Concepts. 7:133–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
ZH S, R F, BZ S, DH L, GS H and LS Z:
Chinese consensus statement for the diagnosis and management of
aplastic anemia. Chin J Hematol. 31(11): 790–792. 2010.
|
|
31
|
Ren J, Guo Y, Shao L, Liu Y and Liu Q:
Capsid protein Vp1 from chlamydiaphage φCPG1 effectively alleviates
cytotoxicity induced by Chlamydia trachomatis. Exp Ther Med.
16:3286–3292. 2018.PubMed/NCBI
|
|
32
|
Luo XY, Yang MH, Peng P, Wu LJ, Liu QS,
Chen L, Tang Z, Liu NT, Zeng XF, Liu Y and Yuan GH:
Anti-erythropoietin receptor antibodies in systemic lupus
erythematosus patients with anemia. Lupus. 22:121–127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Young NS: Aplastic anemia. N Engl J Med.
379:1643–1656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Killick SB, Carter C, Culligan D, Dalley
C, Das-Gupta E, Drummond M, Enright H, Jones GL, Kell J, Mills J,
et al: Guidelines for the diagnosis and management of adult
myelodysplastic syndromes. Br J Haematol. 164:503–525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Killick SB, Bown N, Cavenagh J, Dokal I,
Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti
G, et al: Guidelines for the diagnosis and management of adult
aplastic anaemia. Br J Haematol. 172:187–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valent P, Horny HP, Bennett JM, Fonatsch
C, Germing U, Greenberg P, Haferlach T, Haase D, Kolb HJ, Krieger
O, et al: Definitions and standards in the diagnosis and treatment
of the myelodysplastic syndromes: Consensus statements and report
from a working conference. Leuk Res. 31:727–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwok B, Hall JM, Witte JS, Xu Y, Reddy P,
Lin K, Flamholz R, Dabbas B, Yung A, Al-Hafidh J, et al:
MDS-associated somatic mutations and clonal hematopoiesis are
common in idiopathic cytopenias of undetermined significance.
Blood. 126:2355–2361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Valent P: ICUS, IDUS, CHIP and CCUS:
Diagnostic criteria, separation from MDS and clinical implications.
Pathobiology. 86:30–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yue LZ and Shao ZH: Research progress on
the red cell diseases in China. Chin Med J. 125:2746–2751.
2012.PubMed/NCBI
|
|
40
|
Hirano N, Butler MO, Von Bergwelt-Baildon
MS, Maecker B, Schultze JL, O'Connor KC, Schur PH, Kojima S, Guinan
EC and Nadler LM: Autoantibodies frequently detected in patients
with aplastic anemia. Blood. 102:4567–4575. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng X, Chuhjo T, Sugimori C, Kotani T, Lu
X, Takami A, Takamatsu H, Yamazaki H and Nakao S: Diazepam-binding
inhibitor-related protein 1: A candidate autoantigen in acquired
aplastic anemia patients harboring a minor population of paroxysmal
nocturnal hemoglobinuria-type cells. Blood. 104:2425–2431. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hirano N, Butler MO, Guinan EC, Nadler LM
and Kojima S: Presence of anti-kinectin and anti-PMS1 antibodies in
Japanese aplastic anaemia patients. Br J Haematol. 128:221–223.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takamatsu H, Feng X, Chuhjo T, Lu X,
Sugimori C, Okawa K, Yamamoto M, Iseki S and Nakao S: Specific
antibodies to moesin, a membrane-cytoskeleton linker protein, are
frequently detected in patients with acquired aplastic anemia.
Blood. 109:2514–2520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qi Z, Takamatsu H, Espinoza JL, Lu X,
Sugimori N, Yamazaki H, Okawa K and Nakao S: Autoantibodies
specific to hnRNP K: A new diagnostic marker for immune
pathophysiology in aplastic anemia. Ann Hematol. 89:1255–1263.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goto M, Kuribayashi K, Takahashi Y, Kondoh
T, Tanaka M, Kobayashi D and Watanabe N: Identification of
autoantibodies expressed in acquired aplastic anaemia. Br J
Haematol. 160:359–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stahl D, Egerer G, Goldschmidt H,
Sibrowski W, Kazatchkine MD and Kaveri SV: Altered self-reactive
antibody repertoires are a general feature of patients with
myelodysplastic syndrome. J Autoimmun. 16:77–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barcellini W, Zaninoni A, Imperiali FG,
Boschetti C, Colombi M, Iurlo A and Zanella A: Anti-erythroblast
autoimmunity in early myelodysplastic syndromes. Haematologica.
92:19–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mias GI, Chen R, Zhang Y, Sridhar K,
Sharon D, Xiao L, Im H, Snyder MP and Greenberg PL: Specific plasma
autoantibody reactivity in myelodysplastic syndromes. Sci Rep.
3:33112013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Komrokji RS, Kulasekararaj A, Al Ali NH,
Kordasti S, Bart-Smith E, Craig BM, Padron E, Zhang L, Lancet JE,
Pinilla-Ibarz J, et al: Autoimmune diseases and myelodysplastic
syndromes. Am J Hematol. 91:E280–E283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zaninoni A, Imperiali FG, Cattaneo A,
Soverini G, Binda F, Porretti L, Cortelezzi A and Barcellini W:
Detection of erythroblast antibodies in mitogen-stimulated bone
marrow cultures from patients with myelodysplastic syndromes.
Transfusion. 56:2037–2041. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mewar D, Moore DJ, Young-Min S,
Bertolaccini ML, Khamashta MA, Watson PF and Wilson AG:
Antiferritin antibodies discovered by phage display expression
cloning are associated with radiographic damage in rheumatoid
arthritis. Arthritis Rheum. 52:3868–3872. 2010. View Article : Google Scholar
|
|
52
|
Baerlecken NT, Linnemann A, Gross WL,
Moosig F, Vazquez-Rodriguez TR, Gonzalez-Gay MA, Martin J, Kötter
I, Henes JC, Melchers I, et al: Association of ferritin
autoantibodies with giant cell arteritis/polymyalgia rheumatica.
Ann Rheum Dis. 71:9432012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Régent A, Ly KH, Blet A, Agard C, Puéchal
X, Tamas N, Le-Jeunne C, Vidal E, Guillevin L and Mouthon L:
Contribution of antiferritin antibodies to diagnosis of giant cell
arteritis. Ann Rheum Dis. 72:1269–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Grosse K, Schmidt RE, Witte T and
Baerlecken NT: Epitope mapping of antibodies against ferritin heavy
chain in giant cell arteritis and polymyalgia rheumatica. Scand J
Rheumatol. 42:215–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grosse K, Witte T, Moosig F, Hoyer BF,
Lansche C, Schmidt RE and Baerlecken NT: Association of ferritin
antibodies with Takayasu arteritis. Clin Rheumatol. 33:1523–1526.
2014. View Article : Google Scholar : PubMed/NCBI
|