Introduction
Thiopurines have been widely applied in clinical
practice; among these, 6-thioguanine (6-TG), 6-thiopurine (6-MP)
and its pro-drug azathioprine (AZA) are the most commonly used
(1,2). 6-TG and 6-MP have been demonstrated
to induce and maintain the stable stage of inflammatory bowel
disease (3,4) and are also the most widely used drugs
in the treatment of children with acute lymphoblastic leukemia
(5,6). AZA plays an important role in the
treatment of autoimmune diseases and acute rejection after organ
transplantation (7). In
vivo, thiopurines are catalyzed by metabolic enzymes into the
active metabolite 6-TG nucleotides (6-TGNs), which participate in
the synthesis of DNA and RNA molecules to exert cytotoxic and
pharmacological effects by inhibiting DNA replication and RNA
transcription as an antagonist of guanine nucleoside (8–10).
In addition, thiopurines can also be metabolized by thiopurine
S-methyltransferase (TPMT) into inactive 6-methyl-thioinosine
monophosphate, which prevents the formation of 6-TGNs, thereby
affecting the clinical efficacy and adverse drug reactions of
thiopurines (8–10). Changes in TPMT activity are closely
related to the concentration balance between 6-MP methyltransferase
and 6-TGNs, and thus to the efficacy of thiopurine drugs (2,11,12).
With research into the mechanism underlying the
metabolic processing of thiopurine drugs, as well as the continuous
disclosure of TPMT genetic polymorphisms, it has been revealed that
TPMT genetic polymorphisms are single nucleotide polymorphisms
(SNPs) that serve an important role in thiopurine therapies
(13,14). The pre-detection of TPMT genotypes
should thus predict the toxicity of thiopurines and drug
administration schedules can be adjusted accordingly. This serves
to achieve individualized medicine and avoids palindromia during
maintenance treatment caused by insufficient dosing and avoids the
serious side effects caused by excessive doses. For patients
lacking TPMT enzyme activity, other treatment options can also be
considered. Numerous institutions and organizations (15,16)
have suggested that for patients who possess a heterozygous mutant
(MT) TPMT genotype (i.e. intermediate activity), the initial dose
of AZA or 6-MP should be reduced by 30–70%. However, for patients
who possess a homozygous MT TPMT genotype (i.e. MT variants with
low or deficient activity), the initial dose of AZA, 6-MP or 6-TG
should be reduced by 10-fold, and an extended dosing frequency or
alternative drugs should be selected. Therefore, TPMT genotyping
should be considered in the process of thiopurine therapy, in order
to tailor individual treatments to avoid toxicity and adverse
reactions (4,17,18).
With the development of gene detection technologies,
the technologies for detection of gene polymorphisms include
first-generation sequencing [i.e. DNA sequencing and quantitative
PCR (qPCR)] (14,19–21)
and next-generation sequencing (NGS; i.e. gene microarray and
Genome Sequencer FLX) (22–24).
Although direct sequencing remains the gold standard for the
detection of SNPs, it also requires contact with potentially toxic
chemicals (i.e. SYBR Green, Gold View, Gel Red or Acrylamide) or UV
light for agarose gel electrophoresis, meaning there is still a
risk of mutagenesis for the technician performing the sequencing,
and the procedure and interpretation of results are time-consuming
and labor-intensive. Therefore, it is necessary to develop methods
for rapid and simple determination of SNPs without gene sequencing.
NGS is high-throughput and has high accuracy. Moreover, NGS can be
fully automated (22–24), allowing hundreds of thousands to
millions of DNA molecules to be analyzed at a time, but it is not
necessary for clinical patients for which only one or two SNPs
require detection, and its use increases the financial burden on
clinical patients. qPCR is a conventional gene detection technique
that is convenient and quick to operate (14). Unfortunately, due to the
thermodynamic driving force of thermophilic DNA polymerases,
non-specific amplification commonly occurs (25,26).
These methods are complex, costly and time-consuming. Therefore,
innovative detection methods for TPMT genotyping avoiding these
shortcomings are urgently needed.
Our group previously delineated TPMT*2 mutations
through competitive real-time fluorescent allele-specific PCR
(CRAS-PCR) (19); the single-tube
detection of the TPMT*2 polymorphism only required 1.5 h. The
relative fluorescence intensity is related to the gene copy number,
meaning that results can be directly judged by amplification
curves. In general, the CRAS-PCR method is more specific and
sensitive than qPCR, particularly for large quantities of samples.
Therefore, this study further developed a CRAS-PCR system targeting
TPMT*3, which has higher MT frequency than TPMT*2. In this paper,
based on the Web-based Allele-Specific PCR (WASP) principle
(25), Scorpion primers suitable
for polymorphism detection of TPMT*3B and *3C were designed.
Wild-type (WT)-allele-specific forward (ASF) and MT-ASF Scorpion
primers were used for TPMT*3B detection in a single reaction
mixture, and WT-allele-specific reverse (ASR) and MT-ASR Scorpion
primers targeting TPMT*3C were used in another single reaction
mixture. Application of this analysis to 226 samples confirmed that
CRAS-PCR was suitable for genotyping TPMT*3B and *3C variants.
Materials and methods
Instruments and reagents
The traditional end-point PCR instrument used for
the construction of the quality control (QC) plasmids was the C1000
Touch Thermal cycler (Bio-Rad Laboratories, Inc.). qPCR data were
collected and analyzed using a ViiA™ 7 Real-Time PCR system with a
96-well block (Applied Biosystems; Thermo Fisher Scientific, Inc.).
DNA was collected using a QIAamp DNA Blood Mini kit (Qiagen GmbH)
and analyzed using a NanoDrop ND-1000 Spectrophotometer (NanoDrop;
Thermo Fisher Scientific, Inc.).
Ex Taq DNA Polymerase Hot-Start Version and
MutanBEST kit (Takara Biotechnology Co., Ltd.) were used to
construct WT-QC and MT-QC plasmids. qPCR was performed with 2X
Premix Ex Taq™ (Probe qPCR) master mix (Takara Biotechnology Co.,
Ltd.). QC plasmids were sequenced using a Big Dye Terminator V3.1
Cycler Sequencing kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on an ABI Prism 3500 Genetic Analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Oligonucleotides were
synthesized at General Biosystems (Anhui) Corporation, Ltd.
Samples and genomic DNA
extraction
The present study was carried out in accordance with
the Declaration of Helsinki and was approved by the ethics
committee of Southwest Hospital (Chongqing, China). In total, 226
samples (53.6% male; age, 32±11 years; all Han Chinese) were
collected from healthy volunteers living in Chongqing. Before
peripheral blood was collected, the volunteers were informed of the
possible risks (i.e., pain, bleeding, redness and swelling) and
signed informed consent documents. A peripheral blood sample was
taken from each individual and added to a tube with EDTA before
being identified with a unique code. Genomic DNA was extracted
using the QIAamp DNA Blood Mini kit, according to the
manufacturer's protocol. The genomic DNA concentration of each
sample was quantified using a NanoDrop ND-1000 Spectrophotometer,
following the manufacturer's instructions.
Construction of QC plasmids
The PCR reaction mixture (20 µl) used to amplify
whole exon 7 fragments containing TPMT*3B or exon 10 fragments
containing TPMT*3C included 1X ExTaq Buffer (Mg2+ plus),
250 µM each dNTP, 0.5 units Ex Taq HS, 200 nM each forward and
reverse primer (Table I) and 10 ng
human genomic DNA. Reactions were performed under the following
conditions: An initial denaturation at 95°C for 3 min; 39 cycles at
95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec; and final
extension at 72°C for 5 min. Sanger sequencing of the PCR products
was performed using the BigDye Terminator V3.1 Cycler Sequencing
kit. After the WT alleles of TPMT*3B and TPMT*3C were confirmed,
the PCR products were cloned to construct the WT-QC plasmids. The
WT-QC plasmids of TPMT*3B and TPMT*3C were subsequently used to
prepare the MT-QC plasmids using the MutanBEST kit, according to
the manufacturer's instructions.
 | Table I.Oligonucleotides used in the present
study. |
Table I.
Oligonucleotides used in the present
study.
| ID | Name | Sequence
(5′-3′) |
|---|
| HQ-593 | Exon-7.F |
CCTAATACCTTGACGATTGTTG |
| HQ-594 | Exon-7.R |
TAGAAGTCTAAGCTGATTTTCT |
| HQ-611 | Exon-10.F |
CCCTGATGTCATTCTTCATAG |
| HQ-612 | Exon-10.R |
GGCTTTAGCCATAATTTTCAAT |
| HQ-1742 | TPMT*3B.WT-ASF |
(VIC)ACCGCGCGCGATCACCTGGATTGATGGCAACTAAGCGCGGT(BHQ1)(HEG)ACATGATTTGGGATAGAGGTG |
| HQ-1743 | TPMT*3B.MT-ASF |
(FAM)ACCGCGCGCGATCACCTGGATTGATGGCAACTAAGCGCGGT(BHQ1)(HEG)GACATGATTTGGGATAGAGGTA |
| HQ-1744 | TPMT*3C.WT-ASR |
(VIC)ACCGCGCGAACGACATAAAAGTTGGGGAATTGACTGTCTTTTGCGCGGT(BHQ1)(HEG)CTCATTTACTTTTCTGTAAGTAGCT |
| HQ-1745 | TPMT*3C.MT-ASR |
(FAM)ACCGCGCGAACGACATAAAAGTTGGGGAATTGACTGTCTTTTGCGCGGT(BHQ1)(HEG)GTCTC
ATTTACTTTTCTGTAAGTAGTC |
| HQ-1608 | TPMT*3B.COR |
CAAACTCATAGAAGTCTAAGCTGATTTTCT |
| HQ-1609 | TPMT*3C.COF |
CAATATACGTTGTCTTGAGAAGGTTGA |
qPCR
Three types of primers were designed according to
WASP principles (25) for TPMT*3B:
WT-ASF and MT-ASF Scorpion primers, and a common reverse primer
(Table I). Another three types of
primers were designed for TPMT*3C: WT-ASR and MT-ASR Scorpion
primers, and a common forward primer (Table I). The qPCR mixture (20 µl) for
TPMT*3B or TPMT*3C contained 1X Premix Ex Taq™, a fixed
concentration of primers and QC plasmid (Tables SI and SII). Reactions were performed under the
following cycling conditions: An initial denaturation at 95°C for 2
min, followed by 50 cycles at 95°C for 15 sec and at 59°C for 1 min
(with double fluorescence acquisition). The quantification cycle
(Cq) values were automatically determined using the ViiA™ 7
Real-Time PCR system with a 96-well block. All reactions were
performed in duplicate with no-template controls to monitor for
contamination and non-specific products. A 1:1 mixture of WT-QC and
MT-QC plasmids was used as MIX-QC plasmids for TPMT*3B and TPMT*3C,
respectively.
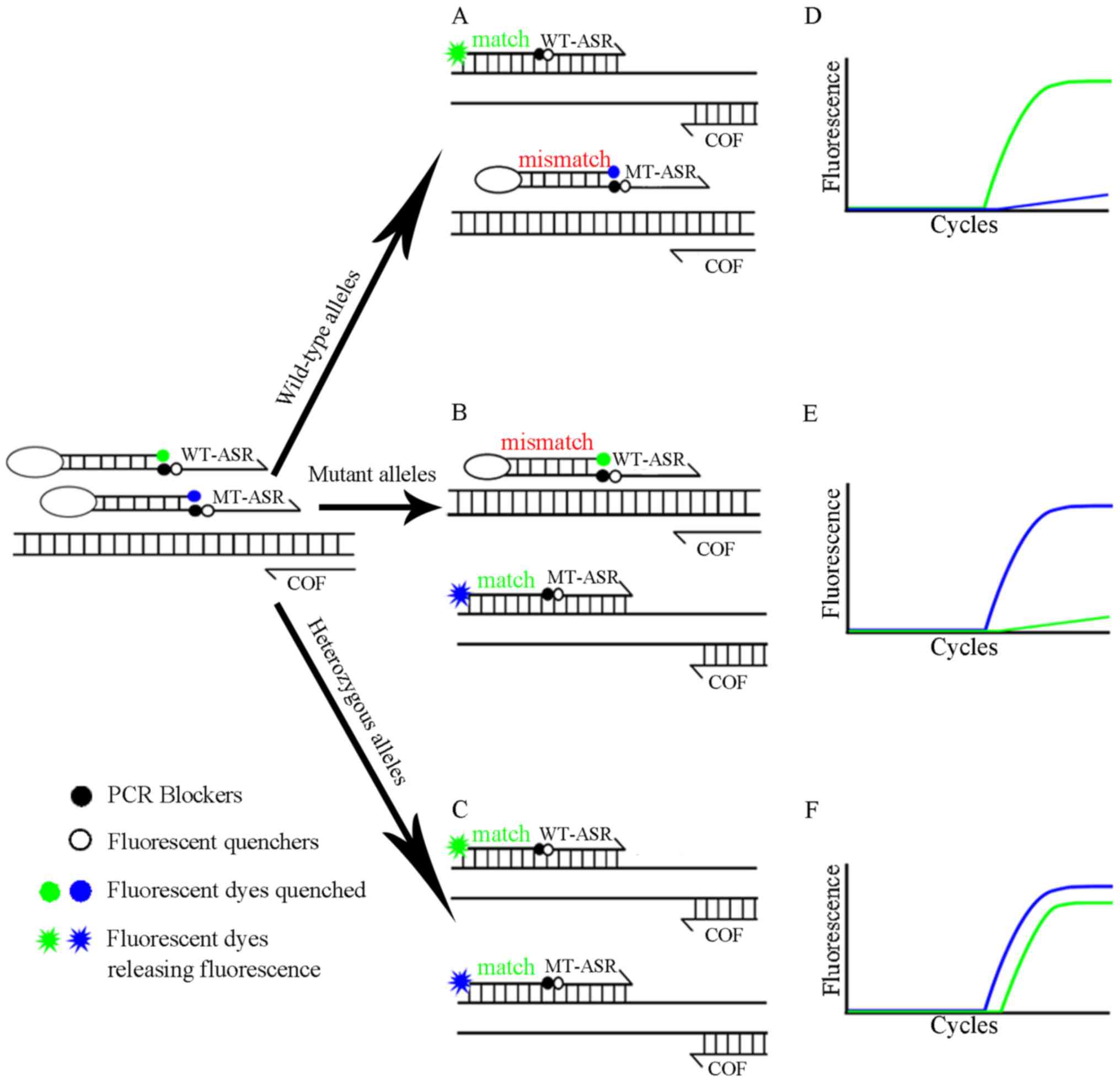
Principles of CRAS-PCR
A schematic diagram illustrating the protocol for
CRAS-PCR is presented in our previous study (19). Following the WASP principles, two
pairs of forward (or reverse) primers and a common reverse (or
forward) primer were designed for TPMT*3B and TPMT*3C genotyping.
The primers in the CRAS-PCR system are constructed in the shape of
a scorpion structure, in which a unique fluorescent reporter (VIC
or FAM) and a corresponding quencher (BH1) are labeled at the start
and end of a stem-loop structure of the Scorpion primers separately
(Table I). In the process of PCR
extension, based on fluorescence resonance energy transfer
(27), the DNA polymerase could
not extend to the probe attached to the primer due to the steric
resistance effect of 18 carbon atoms provided by hexa-ethylene
glycol (28); thus, the integrity
of the copied target DNA chain is ensured (Figs. 1 and 2).
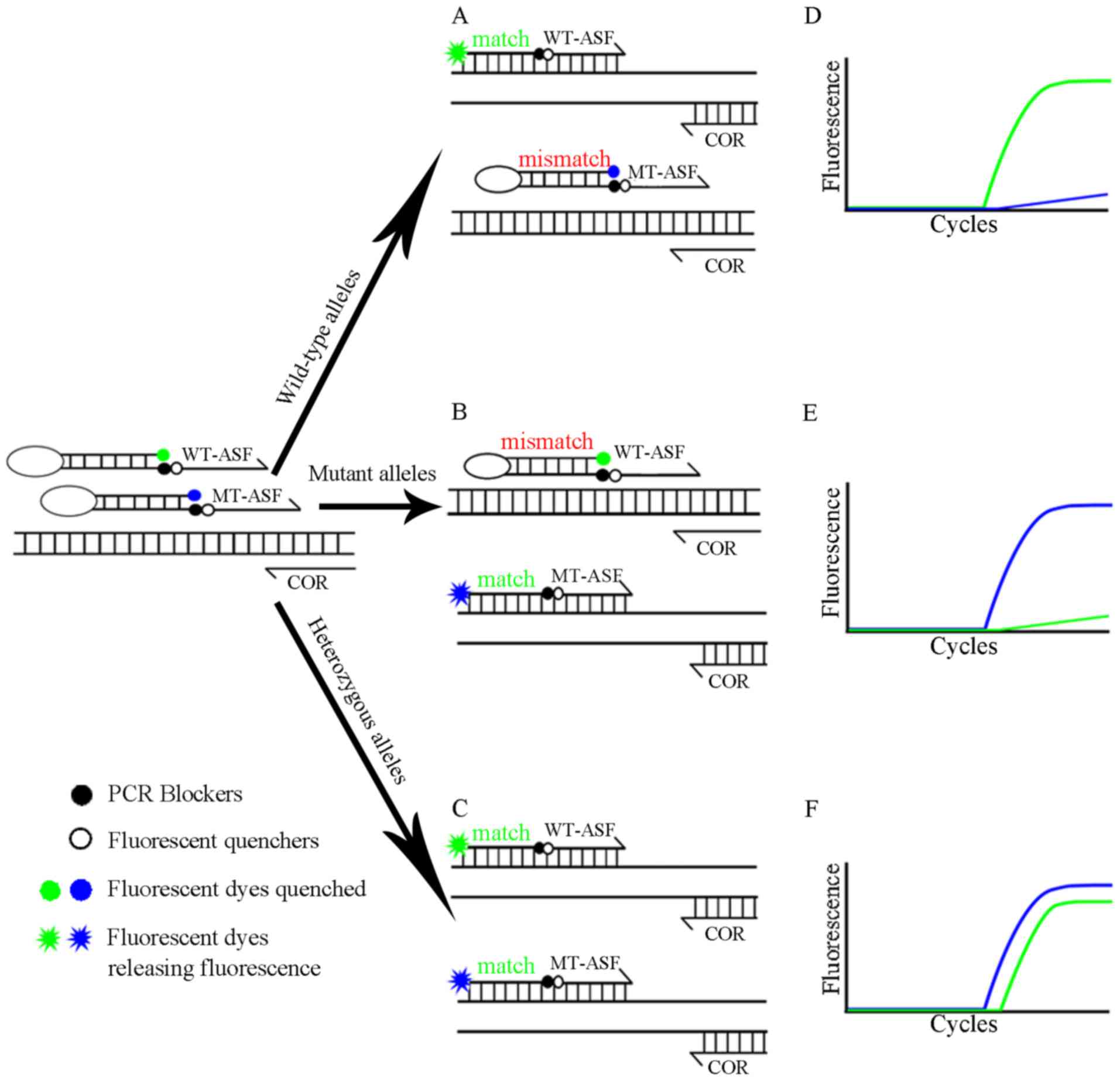 | Figure 1.CRAS-PCR schematic diagram for
TPMT*3B. TPMT*3B polymorphism detected by WT-ASF and MT-ASF
Scorpion primers and a COR primer. The central image depicts the
process of (A) WT, (B) MT and (C) mixed plasmid pairings with
primers. The images on the right show the predicted amplification
curves for (D) WT homozygous, (E) MT homozygous and (F) WT/MT
heterozygous. The green (VIC fluorescent channel) and blue (FAM
fluorescent channel) lines indicate the amplification signals of WT
and MT alleles, respectively. WT, wild-type; MT, mutant; ASF,
allele-specific forward; COR, common reverse\CRAS-PCR, competitive
real-time fluorescent allele-specific-PCR; TPMT, thiopurine
S-methyltransferase; MIX, mixed. |
Statistical analysis
qPCR data were analyzed by ABI 7500 v2.0 (Applied
Biosystems; Thermo Fisher Scientific Inc.); the statistical
threshold was automatically set by ViiA™ 7 Real-Time PCR system,
the difference in the Cq value between FAM and VIC was denoted ΔCq
(29). Distribution frequencies of
alleles and genotypes were analyzed by using a XY model of GraphPad
Prism 5.01 software (GraphPad Software, Inc.), in which the
fluorescence units of VIC and FAM of each reaction were inputted to
automatically analyze and count the number of genotypes.
Results
Construction of TPMT*3B and *3C
QC-plasmids
The PCR products of the TPMT exon 7 and exon 10 were
cloned to prepare the WT-QC plasmids for TPMT*3B and TPMT*3C,
respectively. The plasmids were further used to prepare a
corresponding MT-QC by site-directed mutagenesis; the 141st base
guanine in exon 7 was mutated into adenine (TPMT*3B, G460A) and the
147th base adenine in exon 10 was mutated into guanine (TPMT*3C,
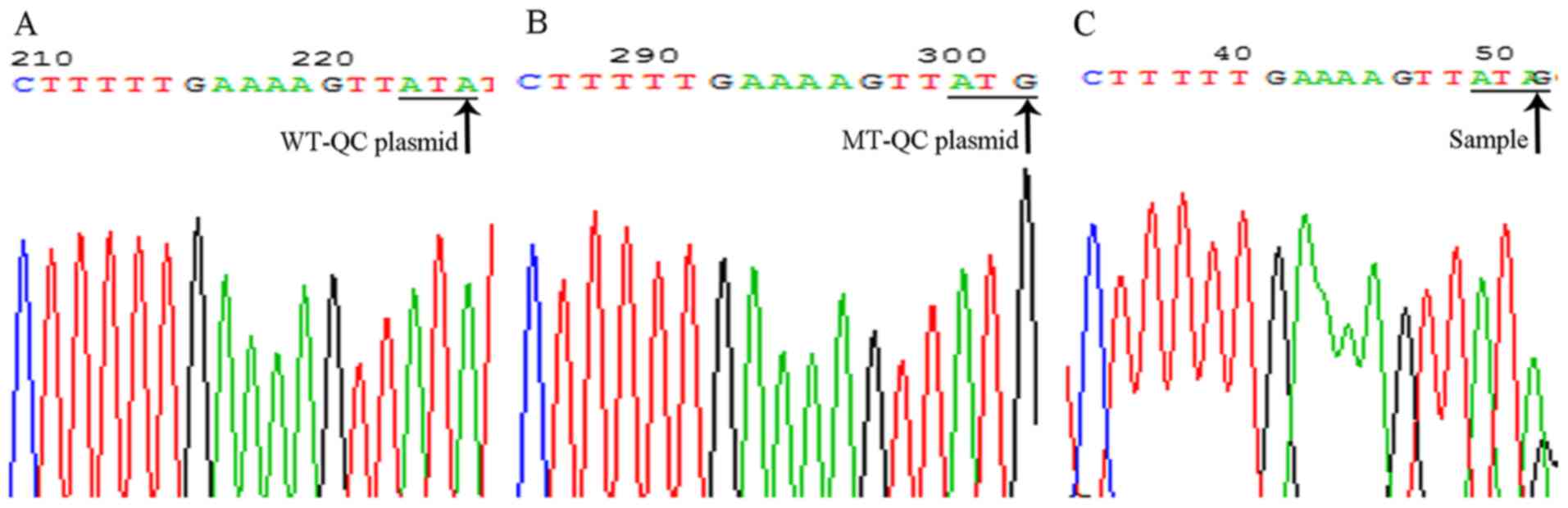
A719G). All plasmids were sequenced to confirm the TPMT*3B and
TPMT*3C genotypes (Figs.
S1–S4).
Evaluation of the CRAS-PCR system
using TPMT*3B and *3C QC-plasmids
Based on a previous study of duplex-crossed
allele-specific-PCR (30), where
TPMT*3B and *3C were identified successfully, Platinum®
Quantitative PCR Supermix-UDG was tested as the reaction buffer. No
amplification curves were observed in WT, MT or a MIX plasmid
reaction system, for either TPMT*3B or TPMT*3C (Fig. S5; Appendix S1). Premix Ex Taq™ Hot
Start was also tested as reaction buffer; once again, gene
polymorphisms could not be distinguished from a single reaction
tube (Fig. S6). Therefore, Premix
Ex Taq™ (Probe qPCR), which was previously used for successfully
genotyping TPMT*2 (19), was used
for TPMT*3B and TPMT*3C genotyping in the present study. TPMT*3B
and *3C QC-plasmids were used to test the specificity of Scorpion
primers targeting WT/MT TPMT*3B and *3C alleles. The amplification
system contained WT and MT primers simultaneously that only had
mononucleotide differences between the defined and opposite
genotype primers. Although non-specific amplification was observed
in the TPMT*3B and TPMT*3C QC plasmid amplification reactions, the
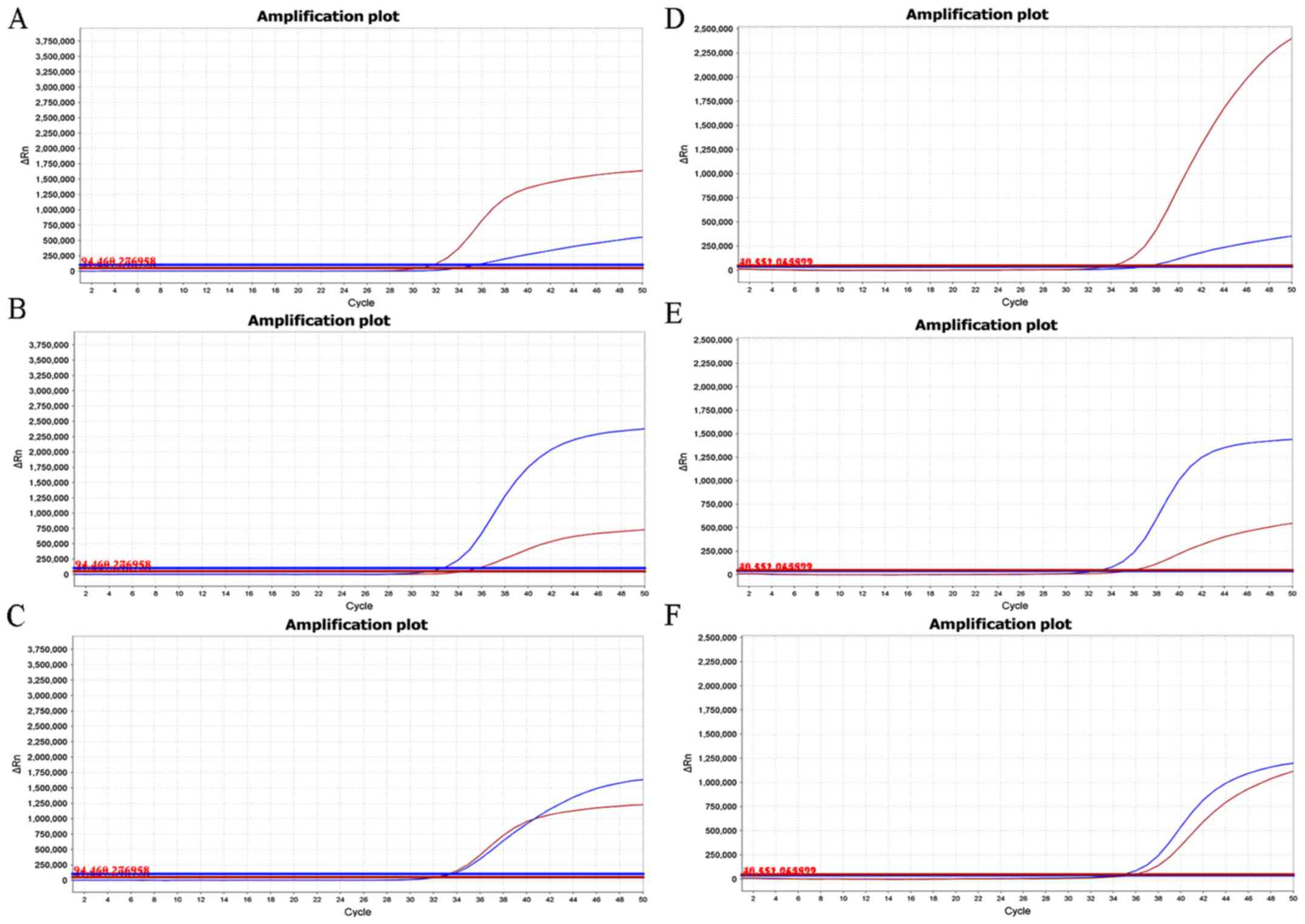
experimental judgment was not affected (Fig. 3). The positive control results
showed that Scorpion primers were able to identify TPMT*3B and *3C
polymorphisms specifically. When the WT plasmid was used in the
reaction system, the ΔCq value between FAM and VIC was found to be
≥3, and the relative fluorescence units (RFU) value of VIC was
three times that of FAM for TMPT*3B (Fig. 3A) and TPMT*3C (Fig. 3D). In the case of the MT plasmid in
the reaction system, the ΔCq value between VIC and FAM was ≥3 and
the RFU value of FAM was three times that of VIC for TPMT*3B
(Fig. 3B) and TPMP*3C (Fig. 3E). MIX plasmid demonstrated equal
Cq values of FAM and VIC and equal RFU values in both TPMT*3B
(Fig. 3C) and TPMT*3C (Fig. 3F). In summary, the Scorpion primers
in our CRAS-PCR system could identify WT, MT and heterozygous
genotypes of TPMT*3B and *3C.
Analysis of blood samples from
volunteers using CRAS-PCR
TPMT*3B and TPMT*3C frequencies were analyzed using
the CRAS-PCR system; the reaction system was used to analyze the
TPMT*3B and TPMT*3C genotypes of 226 blood samples from individuals
of Han Chinese ethnicity living in Chongqing. To analyze TPMT*3B or
TPMT*3C, each sample was analyzed in duplicate, and WT-, MT- and
MIX-QC plasmids were tested in parallel to monitor the
amplification quality. In this study, the rule that if both
MT-TPMT*3B and MT-TPMT*3C were detected in the sample, the sample
was identified as a variant of TPMT*3A was adopted (31).
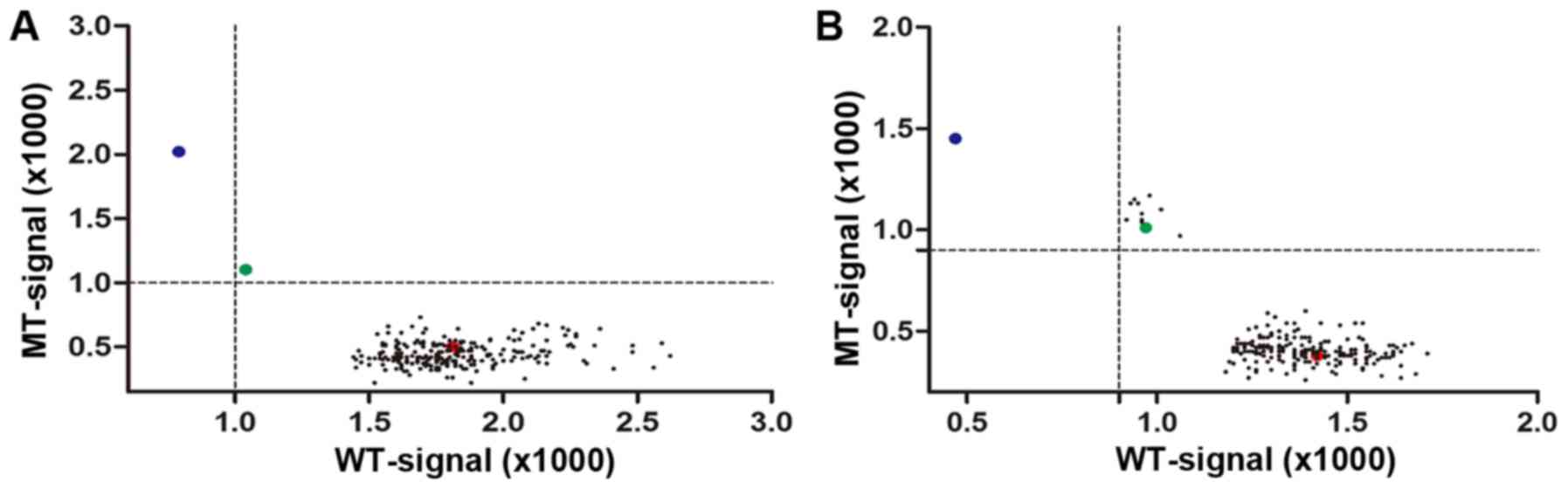
The results revealed that of the 226 samples, all
were WT homozygous for TPMT*3A and *3B (Fig. 4A). A total of 217 samples were WT
homozygous (96.02%), nine (3.98%) were MT heterozygous and no
samples were MT homozygous (Fig.
4B) for TPMT*3C. A mutation frequency of 3.98% is higher than
that discovered in other published work for the Han Chinese
population (32). Three cases of
TPMT*3C MT heterozygotes were randomly selected for DNA sequencing,
and the sequencing results were consistent with our CRAS-PCR
results (Fig. 5).
Discussion
TPMT plays a crucial role in the metabolism of
thiopurine drugs. Before treatment, TPMT genotyping is an important
step for avoiding adverse reactions. In this study, a new method
for detecting polymorphisms in TPMT*3B and TPMT*3C was explored,
CRAS-PCR. The primers for TPMT*3B and TPMT*3C were designed using
the WASP principle (25), with the
3′-end terminal codon indicating allele-distinguishable variants,
and the codon in the penultimate position indicating a mismatch
introduced to increase primer specificity. Although non-specific
amplification was still present in this study, the genotype
variants were correctly identified through prior optimization of
the reaction conditions.
When establishing CRAS-PCR to identify TPMT*3B and
*3C, the annealing temperature, number of amplification cycles, and
template amount were all systematically optimized in this study
(data not shown). Different annealing temperatures may alter
primer-binding kinetics in a reaction mixture, and influence
amplification curves and RFU values (33). In general, the annealing
temperature was 55–65°C, but when the temperature was <57°C or
>63.3°C, the specific amplification and non-specific
amplification curves yielded the same Cq and RFU value, thus the
results could not be assessed accurately. However, when the
temperature was within 57–63.3°C, the differences between Cq and
RFU values for specific and non-specific amplification were
relatively clear; therefore, an annealing temperature of 59°C was
chosen for this reaction system, as the ΔCq and RFU ratios were
clearly different between specific and non-specific amplification
and allowed for interpretation of the results. Through a gradient
screening of amplification of 104−109 plasmid
copies, it was found that with 106 copies of plasmids,
the Cq value and RFU values were consistent with that of 100 ng
genomic DNA. Therefore, a positive control concentration of
106 copies plasmids was selected. Under these
conditions, the Cq value was 32–36, and the amplification was
stopped after the amplification curve became stable. To identify
genetic polymorphisms accurately, the total number of cycles was
set to 50 in our PCR amplification procedure.
At present, >30 SNPs of TPMT genes have been
identified globally, and the main MT genotypes of TPMT are
TPMT*1/*2, TPMT*1/*3C, TPMT*1/*3B and TPMT*l/*3A, which account for
~90% of the known variant genotypes of this gene (13,17,31,34,35).
In Caucasian, African and Asian populations, the overall prevalence
of TPMT loss-of-function variants is 0.2–0.4% for TPMT*2, up to
2.6% for TPMT*3B, 1.2–6.2% for TPMT*3C and up to 5.7% for TPMT*3A
(31,36,37),
with the TPMT*3C variant being predominant. In the Han Chinese
population, TPMT*3C alleles are 1.4–3.2%, but TPMT*3B and TPMT*3A
alleles have not been detected (32,38–40).
In the present study, CRAS-PCR was used to detect TPMT*3 mutations
in 226 Han Chinese individuals living in Chongqing. Mutation
detection results showed that the frequencies were 3.98% for
TPMT*3C, which was higher than other published results for Han
Chinese populations (32). The
TPMT*3A and TPMT*3B mutations were not found, which was consistent
with published data on the Han Chinese population (32).
The main limitation of this study was that due to
the rarity of these alleles in the test population, as well as the
limited number of samples, it was not possible to validate the
TPMT*3A or TPMT*3B MTs or the homozygous TPMT*3C MT. However,
Sanger sequencing for TPMT*3C confirmed that the sample limitation
did not affect the interpretation or validity of the results. As
such, it was extrapolated that the TPMT*3C mutation was the
dominant genotype in the Han Chinese population living in the
Chongqing territory. This further verified that CRAS-PCR could
identify TPMT*3B, *3C and *3A mutations successfully.
A simple and specific CRAS-PCR assay was established
and optimized herein for the commonly found MT alleles of TPMT*3A
(G460A and A719G), TPMT*3B (G460A) and TPMT*3C (A719G). The
presented results confirmed that CRAS-PCR was an accurate,
time-saving and simple method for the detection of mutations in
TPMT*3, and may be applied in clinical trials of TPMT*3 genotyping.
Further prospective studies may establish the roles of TPMT*3 in
genotype-informed dosage adjustment of thiopurines, the use of
alternative agents in selected patient subgroups, and the
prevention of morbidity and mortality due to thiopurine
intolerance.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Sangon Biotech
(Shanghai) Co., Ltd., for experimental assistance in DNA
sequencing.
Funding
Professor Qing Huang received support from the
Chongqing Social and People's Livelihood Science and Technology
Innovation Special Project (grant no. cstc2015shmszx120102) and
Major Military Logistics Scientific Research Projects (grant no.
AWS17J010) for the present study.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding authors upon reasonable
request.
Authors' contributions
QH planned and managed the project. NZ, BBW and LC
recruited volunteers. QH, PY, XMQ, NS, LW, NZ, BBW, XDR and SR were
involved in designing the study, performing the experiments and
collecting the data. PY, XMQ, LC, FJS and QH conducted statistical
analyses, interpreted the results, searched the literature and
wrote parts of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was carried out in accordance with the
Declaration of Helsinki and was approved by the ethics committee of
Southwest Hospital (Chongqing, China). All individuals signed
informed consent documents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TPMT
|
thiopurine S-methyltransferase
|
|
CRAS-PCR
|
competitive real-time fluorescent
allele-specific PCR
|
|
6-TG
|
6-thioguanine
|
|
6-MP
|
6-thiopurine
|
|
AZA
|
azathioprine
|
|
6-TGNs
|
6-thioguanine nucleotides
|
|
SNPs
|
single nucleotide polymorphisms
|
|
WT
|
wild-type
|
|
MT
|
mutant
|
|
ASF
|
allele-specific forward
|
|
ASR
|
allele-specific reverse
|
|
COR
|
common reverse
|
|
COF
|
common forward
|
|
WASP
|
Web-based Allele-Specific PCR
|
|
RFU
|
relative fluorescence units
|
References
|
1
|
Appell M, Hindorf U, Almer S and Peterson
C: Thiopurines in inflammatory bowel disease-the role of
pharmacogenetics and therapeutic drug monitoring. Curr
Pharmacogenomics. 4:285–300. 2006. View Article : Google Scholar
|
|
2
|
Ford LT and Berg JD: Thiopurine
S-methyltransferase (TPMT) assessment prior to starting thiopurine
drug treatment; A pharmacogenomic test whose time has come. J Clin
Pathol. 63:288–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Florin THJ, Wright JD, Jambhrunkar SD,
Henman MG and Popat A: A well-tolerated and rapidly acting
thiopurine for IBD? Drug Discov Today. 24:37–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saibeni S, Kohn A, Meucci G and Papi C;
Italian Group for Inflammatory Bowel Disease, : How thiopurines are
used for the treatment of inflammatory bowel diseases: An Italian
survey. Dig Liver Dis. 47:170–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Estlin EJ: Continuing therapy for
childhood acute lymphoblastic leukaemia: Clinical and cellular
pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanine.
Cancer Treat Rev. 27:351–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sobiak J, Skalska-Sadowska J, Chrzanowska
M, Resztak M, Koltan S, Wysocki M and Wachowiak J: Thiopurine
methyltransferase activity in children with acute myeloid leukemia.
Oncol Lett. 16:4699–4706. 2018.PubMed/NCBI
|
|
7
|
Hollander AA, van Saase JL, Kootte AM, van
Dorp WT, van Bockel HJ, van Es LA and van der Woude FJ: Beneficial
effects of conversion from cyclosporin to azathioprine after kidney
transplantation. Lancet. 345:610–614. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hogarth LA, Redfern CP, Teodoridis JM,
Hall AG, Anderson H, Case MC and Coulthard SA: The effect of
thiopurine drugs on DNA methylation in relation to TPMT expression.
Biochem Pharmacol. 76:1024–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larussa T, Suraci E, Lentini M, Nazionale
I, Gallo L, Abenavoli L, Imeneo M, Costanzo FS, Cuda G and Luzza F:
High prevalence of polymorphism and low activity of thiopurine
methyltransferase in patients with inflammatory bowel disease. Eur
J Intern Med. 23:273–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matalon ST, Ornoy A and Lishner M: Review
of the potential effects of three commonly used antineoplastic and
immunosuppressive drugs (cyclophosphamide, azathioprine,
doxorubicin on the embryo and placenta). Reprod Toxicol.
18:219–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dervieux T, Blanco JG, Krynetski EY, Vanin
EF, Roussel MF and Relling MV: Differing contribution of thiopurine
methyltransferase to mercaptopurine versus thioguanine effects in
human leukemic cells. Cancer Res. 61:5810–5816. 2001.PubMed/NCBI
|
|
12
|
Coulthard SA, Hogarth LA, Little M,
Matheson EC, Redfern CPF, Minto L and Hall AG: The effect of
thiopurine methyltransferase expression on sensitivity to
thiopurine drugs. Mol Pharmacol. 62:102–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang RS and Ratain MJ: Pharmacogenetics
and pharmacogenomics of anticancer agents. CA Cancer J Clin.
59:42–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lorenz M, Weise A, Prause S, Klemm M,
Eidens M, Luchi M, Forst T, Pfützner A and Weber MM: Development
and validation of a rapid and reliable method for TPMT genotyping
using real-time PCR. Clin Lab. 58:959–971. 2012.PubMed/NCBI
|
|
15
|
Maitland ML, Vasisht K and Ratain MJ:
TPMT, UGT1A1 and DPYD: Genotyping to ensure safer cancer therapy?
Trends Pharmacol Sci. 27:432–437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen CM, Mendes MAS and Ma JD:
Thiopurine methyltransferase (TPMT) genotyping to predict
myelosuppression risk. PLoS Current. 3:RRN12362011. View Article : Google Scholar
|
|
17
|
Hindorf U and Appell ML: Genotyping should
be considered the primary choice for pre-treatment evaluation of
thiopurine methyltransferase function. J Crohns Colitis. 6:655–659.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Relling MV, Gardner EE, Sandborn WJ,
Schmiegelow K, Pui CH, Yee SW, Stein CM, Carrillo M, Evans WE and
Klein TE; Clinical Pharmacogenetics Implementation Consortium, :
Clinical Pharmacogenetics Implementation Consortium guidelines for
thiopurine methyltransferase genotype and thiopurine dosing. Clin
Pharmacol Ther. 89:387–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen D, Yang Z, Xia H, Huang JF, Zhang Y,
Jiang TN, Wang GY, Chuai ZR, Fu WL and Huang Q: Enhanced
specificity of TPMT*2 genotyping using unidirectional wild-type and
mutant allele-specific scorpion primers in a single tube. PLoS One.
9:e918242014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain-terminating inhibitors. Proc Natl Acad Sci
USA. 74:5463–5467. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Román M, Cabaleiro T, Ochoa D, Novalbos J,
Chaparro M, Gisbert JP and Abad-Santos F: Validation of a
genotyping method for analysis of TPMT polymorphisms. Clin Ther.
34:878–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shendure J, Porreca G, Reppas N, Lin X,
McCutcheon J, Rosenbaum A, Wang M, Zhang K, Mitra R and Church G:
Accurate multiplex polony sequencing of an evolved bacterial
genome. Science. 309:1728–1732. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shendure J, Mitra RD, Varma C and Church
GM: Advanced sequencing technologies: Methods and goals. Nat Rev
Genet. 5:335–344. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shendure J and Ji H: Next-generation DNA
sequencing. Nat Biotechnol. 26:1135–1145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wangkumhang P, Chaichoompu K, Ngamphiw C,
Ruangrit U, Chanprasert J, Assawamakin A and Tongsima S: WASP: A
Web-based Allele-Specific PCR assay designing tool for detecting
SNPs and mutations. BMC Genomics. 8:2752007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collie-Duguid ES, Pritchard SC, Powrie RH,
Sludden J, Collier DA, Li T and McLeod HL: The frequency and
distribution of thiopurine methyltransferase alleles in Caucasian
and Asian populations. Pharmacogenetics. 9:37–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tyagi S, Bratu DP and Kramer FR:
Multicolor molecular beacons for allele discrimination. Nat
Biotechnol. 16:49–53. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ng CT, Gilchrist CA, Lane A, Roy S, Haque
R and Houpt ER: Multiplex real-time PCR assay using Scorpion probes
and DNA capture for genotype-specific detection of Giardia lamblia
on fecal samples. J Clin Microbiol. 43:1256–1260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang K, Niu Y, Wang Q, Liu H, Jin Y and
Zhang S: Cloning and evaluation of reference genes for quantitative
real-time PCR analysis in Amorphophallus. PeerJ. 5:e32602017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qu XM, Zhao N, Mo QY, Yao P, Su N, Wei K,
Wang L, Huang JF, Ren XD, Ren S, et al: Development of
duplex-crossed allele-specific PCR targeting of TPMT*3B and *3C
using crossed allele-specific blockers to eliminate non-specific
amplification. Anal Biochem. 575:54–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burchard PR, AbouTayoun AN, Lefferts JA,
Lewis LD, Tsongalis GJ and Cervinski MA: Development of a rapid
clinical TPMT genotyping assay. Clin Biochem. 47:126–129. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang JP, Zhou SF, Chen X and Huang M:
Determination of intra-ethnic differences in the polymorphisms of
thiopurine S-methyltransferase in Chinese. Clin Chim Acta.
365:337–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lueders T and Friedrich MW: Evaluation of
PCR amplification bias by terminal restriction fragment length
polymorphism analysis of small-subunit rRNA and mcrA genes by using
defined template mixtures of methanogenic pure cultures and soil
DNA extracts. Appl Environ Microbiol. 69:320–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cabaleiro T, Roman M, Gisbert JP and
Abad-Santos F: Utility of assessing thiopurine S-methyltransferase
polymorphisms before azathioprine therapy. Curr Drug Metab.
13:1277–1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Robert J, Morvan VL, Smith D, Pourquier P
and Bonnet J: Predicting drug response and toxicity based on gene
polymorphisms. Crit Rev Oncol Hematol. 54:171–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamdy SI, Hiratsuka M, Narahara K, Endo N,
El-Enany M, Moursi N, Ahmed MSE and Mizugaki M: Genotype and allele
frequencies of TPMT, NAT2, GST, SULT1A1 and MDR-1 in the Egyptian
population. Br J Clin Pharmacol. 55:560–569. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teresa C, Manuel R, Javier PG and
Francisco AS: Utility of assessing Thiopurine S-methyltransferase
polymorphisms before azathioprine therapy. Curr Drug Metab.
13:1277–1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kham SK, Tan PL, Tay AH, Heng CK, Yeoh AE
and Quah TC: Thiopurine methyltransferase polymorphisms in a
multiracial asian population and children with acute lymphoblastic
leukemia. J Pediatr Hematol Oncol. 24:353–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang LR, Song DK, Zhang W, Zhao J, Jia LJ
and Xing DL: Efficient screening method of the thiopurine
methyltransferase polymorphisms for patients considering taking
thiopurine drugs in a Chinese Han population in Henan Province
(central China). Clinica Chimica Acta. 376:45–51. 2007. View Article : Google Scholar
|
|
40
|
Liu L, Yang L, Zhang YC, Ai XF, Wang JX
and Xiao ZJ: Polymorphisms of drug-metabolizing enzymes genes in a
Han Chinese population. Zhonghua Yi Xue Za Zhi. 89:2675–2681.
2009.(In Chinese). PubMed/NCBI
|