Introduction
Cadmium (Cd) is an environmental pollutant and an
industrial heavy metal that significantly endangers public health,
and exhibits a long biological half-life of 10–30 years (1). Moreover, cigarette smoke and
Cd-contaminated food and drinking water are the principal exposure
routes for Cd, which is absorbed via the respiratory and digestive
tract, respectively, resulting in the accumulation of Cd in the
organism (2). The kidney has been
considered to be particularly sensitive to Cd (3,4),
which accumulates in different nephron segments via the blood
circulation, and the proximal tubules have been indicated to be
more sensitive during the later stage of intoxication (5). A short-term exposure of cells to Cd
has been indicated to cause a destruction of tight and gap
junctions, and result in an increase of cell viability without
causing cell death (6,7). By contrast, a long-term exposure to
Cd has been revealed to result in a decreased resistance to
oxidation and oxidative stress which is reflected by the levels of
reactive oxygen species (ROS). ROS are formed as a by-product of
the normal metabolism of oxygen, and a notable increase in ROS
levels can hinder cell function, leading to mitochondrial damage
and ultimately, apoptosis (8,9).
Indeed, a previous study has suggested that apoptosis was primarily
responsible for Cd-induced cell death (10).
Autophagy is an evolutionarily conserved metabolic
process, via which the intracellular components that are enveloped
by the autophagy membrane are transported to the lysosome and
eventually degraded for recycling (11). Autophagy is a cellular adaptive
response, which is regulated by a variety of autophagy-related
genes (12,13). In addition, autophagy has been
indicated to exert two opposing functions: i) An adaptive mechanism
to remove damaged organelles or proteins to maintain cellular
homeostasis; and ii) autophagic cell death, which is caused by
excessive autophagy (14–16). It has been reported that autophagy
promoted cell survival or induced cell death depending on the
different growth conditions, cell types and type of stimulus
(17). Autophagosomes are
spherical structures with double layer membranes serve a key role
in autophagy. Accumulation of autophagosomes may be associated with
increased autophagosome synthesis or obstruction of autophagosome
degradation by lysosomes, i.e. blockade of the autophagic flux
(12). Autophagosomes are degraded
following fusion with lysosomes, a process which is regulated by
RAB7 (18). Numerous studies have
suggested that autophagy may be induced via an exposure to Cd
(19–22). Endoplasmic reticulum (ER) stress
(ERS) has been indicated to cause apoptosis in a variety of
pathological conditions, including neurodegenerative disorders,
infections, drug intoxication, metabolic diseases and heavy metal
intoxication (23–25). ER is a network structure, which is
connected by membranes, and is primarily responsible for the
synthesis of biological macromolecules, such as proteins, lipids
and sugars (26). A variety of
stimuli, such as oxidative and glycosylation stress, can cause
protein unfolding and misfolding, resulting in the accumulation of
proteins in the ER lumen, which triggers ERS (27). In vertebrates, ERS is characterized
by three different types of protein sensors located at the ER inner
membrane, protein kinase R (PKR)-like ER kinase (PERK), eukaryotic
initiation factor 2α (eIF2α) and inositol-requiring enzyme 1
(IRE1). ERS has been indicated to promote restoration of ER
homeostasis and cell survival, while excessive ERS has been
revealed to result in cell injury and death (28,29).
Autophagy and ERS are critical mechanisms that are associated with
various physiological and pathological processes, such as the
physical activity of neurons and chronic obstructive pulmonary
disease (26). An interaction
between autophagy and ERS has been revealed in previous studies
(30–32). A number of studies have reported
that NRF2, a transcription factor that regulates the anti-oxidative
stress response, is associated with both autophagy and ERS
(33,34). Under normal conditions, KEAP1
interacts with NRF2 and forms protein complexes in the cytoplasm,
thereby inhibiting the activity of NRF2. However, under oxidative
stress NRF2 has been reported to be released from KEAP1 and
transferred to the nucleus, where it regulates the expression
levels of antioxidant enzyme genes (35). A number of studies have reported
that the NRF2/KEAP1 pathway regulates autophagy under oxidative
stress (36–38). It has been reported that autophagy
may be triggered or regulated by ERS (39). By contrast, another study suggested
that autophagy may confer cellular protection via suppressing ERS
(40). This discrepancy may depend
on the different cell types or stimuli, and the regulatory
mechanism is still unclear.
In recent years, extracts from a variety of Chinese
medicinal herbs have been widely studied as therapeutic drugs for
different diseases. Puerarin
(C21H20O9) is a plant isoflavone,
which is extracted from the dried root of the Chinese medicinal
herb kudzu. Several studies have reported that puerarin exhibited a
wide range of pharmacological effects, such as anti-oxidant,
anti-apoptotic and anti-inflammatory functions, decreasing the
blood pressure, and improving the microcirculation and
neuroprotection (41–43). However, the protective mechanism of
puerarin in Cd-induced cytotoxicity is largely unknown, to the best
of our knowledge, and requires additional studies. Therefore, in
the present study, the immortalized normal rat renal proximal
tubular cell line NRK-52E was used as a research model to
investigate whether puerarin relieves ERS via regulating autophagy
in Cd-induced nephrotoxicity.
Materials and methods
Chemicals and reagents
All chemicals were of the highest purity grade
available. DMEM, Opti-MEM® I Reduced-Serum Medium, FBS,
trypsin-EDTA, Lipofectamine® 3000 Transfection Reagent
and Lipofectamine® RNAiMAX Transfection Reagent were
purchased from Thermo Fisher Scientific, Inc. Puerarin, cadmium
chloride, 2′,7′-dichlorofluorescein diacetate (DCFH-DA), DMSO and
Cell Counting Kit-8 (CCK-8) were purchased from Merck KGaA. The
malondialdehyde (MDA) detection kit and all antioxidant enzyme
detection kits, including Glutathione Peroxidase (GSH-px) (cat. no.
A005-1-1), Reduced GSH (cat. no. A006-2-1), Superoxide Dismutase
(SOD) (cat. no. A001-3-2) and Catalase (CAT) (cat. no. A007-1-1)
assay kits, were purchased from Nanjing Jiancheng Bio-Engineering
Institute Co., Ltd. The short hairpin RNA (shRNA) targeting
autophagy-related protein 7 (ATG7; cat no. RSH046234) and the
plasmid encoding the open reading frame (ORF) of Ras-related
protein Rab-7 (RAB7; cat. no. Rn25016) were purchased from
GeneCopoeia, Inc. The following primary antibodies were used:
Microtubule-associated protein 1 light chain 3 β (LC3B; cat. no.
L7543) and p62/sequestosome-1 (SQSTM1; cat. no. P0067), were
purchased from Merck KGaA. Binding-immunoglobulin protein (BIP;
cat. no. ab227865), CCAAT-enhancer-binding protein homologous
protein (CHOP; cat. no. ab11419), nuclear factor erythroid
2-related factor 2 (NRF2; cat. no. ab137550), kelch-like
ECH-associated protein 1 (KEAP1; cat. no. ab139729), heme
oxygenase-1 (HO-1; cat. no. ab189491), NAD(P)H dehydrogenase
[quinone] 1 (NQO1; cat. no. ab80588), superoxide dismutase (SOD) 1
(SOD1; cat. no. ab16831), SOD2 (cat. no. ab13533) and catalase
(CAT; cat. no. ab16731) were purchased from Abcam. Phosphorylated
(p)-eIF2α (cat. no. 3398S), activating transcription factor 4
(ATF-4; cat. no. 11815), cleaved caspase-3 (cat. no. 9661), histone
H3 (cat. no. 4499S), β-actin (cat. no. 4970L) and horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. 7074) or horse
anti-mouse (cat. no. 7076) IgG secondary antibodies were purchased
from Cell Signaling Technology, Inc. Bicinchoninic acid (BCA)
protein assay kit was purchased from Beyotime Institute of
Biotechnology. All other chemicals were purchased from Merck
KGaA.
Cell culture
The NRK-52E cell line was cultured in DMEM with 5%
FBS and 100 U/ml penicillin-streptomycin at 37°C in humidified 5%
CO2 atmosphere. The cells were sub-cultured with
trypsin-EDTA digestion, incubated at 37°C for 45 sec. When the
cells reached 75% confluence, they were used for subsequent
experiments.
Cell viability assay
The NRK-52E cells were seeded in 96-well plates at a
density of 1×104 cells/well. The cells were treated with
0.0, 2.5, 5.0, 10.0, 20.0 and 40.0 µM Cd for 12 h when the
confluence reached 75%, to assess cell viability. Reagent from Cell
Counting Kit-8 was added to each well and incubated in a 37°C
CO2 incubator for 1–2 h in the dark, according to the
manufacturer's instructions. The optical density was measured using
a full-wavelength microplate reader at a wavelength of 450 nm.
ROS measurement
DCFH-DA was diluted 1:1,000 with serum-free DMEM to
a final concentration of 100 µM. NRK-52E (0.4×106) cells
were seeded in 6-well plate, treated with 0.0, 2.5, 5.0, 10.0, 20.0
and 40.0 µM Cd in a 37°C CO2 incubator for 12 h, then
1.5×106 cells were collected and suspended in 1 ml
diluted DCFH-DA and incubated in a 37°C CO2 incubator
for 30 min. The 0 µM Cd treated NRK-52E cells suspended in PBS were
used as the negative control. The solutions were mixed by inversion
every 3–5 min to fully integrate the probe with the cells. The
cells were washed three times with PBS to remove excess DCFH-DA,
and the fluorescence intensity (FL-1; 530 nm) of 10,000 cells was
measured using a flow cytometer.
Detection of MDA and antioxidant
enzyme activities
The NRK-52E cells were seeded at a density of
6–8×105 in 60 mm dishes and were exposed to 0.0, 2.5,
5.0, 10.0, 20.0 and 40.0 µM Cd in a 37°C CO2 incubator
for 12 h. The cells were collected and lysed in ice-cold PBS on ice
by sonication. The parameters of the sonicator were adjusted to 35%
amplitude, 30 sec sonication and 5 sec sonication with 5 sec
between pulses. Following lysis, the supernatant was centrifuged at
15,000 × g for 5 min at 4°C for subsequent determination. The level
of MDA and the activities of GSH-Px, SOD, CAT and GSH were
evaluated using commercial kits according to the manufacturer's
protocols. BCA protein assay kit was used to quantify the protein
concentration of the samples.
Red fluorescent protein (RFP)-LC3 and
enhanced green fluorescent protein (EGFP)-RFP-LC3 transfection
RFP-LC3 and EGFP-RFP-LC3 plasmids were gifts from
Dr. Lin Wang (College of Animal Science and Veterinary Medicine,
Shandong Agricultural University, Tai'an, China). NRK-52E cells
(2×105 cells/well) were seeded onto sterile coverslips
in 24-well plates before treatment. Subsequently, the cells were
transfected with RFP-LC3 or EGFP-RFP-LC3 followed by 0, 10 and 20
µM Cd treatment in a 37°C CO2 incubator for 12 h.
Briefly, the cells were cultured in Opti-MEM for 2 h in a 37°C
CO2 incubator, followed by culture in transfection
medium (Opti-MEM, 50 µl; plasmid, 0.75 µg;
Lipofectamine® 3000 Reagent, 1.5 µl; P3000 Reagent, 1
µl) for 3 days in a 37°C CO2 incubator according to the
manufacturer's protocol. The coverslips were washed in PBS, mounted
onto slides and inspected under a 1.4 phase-contrast oil-immersion
lens, Leica laser scanning confocal microscope (Leica Microsystems
GmbH) (magnification, ×63).
shRNA and plasmid transfection
NRK-52E cells (2×105 cells/well) were
seeded in 24-well plates before transfection. For each well, the
cells were transfected with 60 pmol shRNA against ATG7 or 1 µg
plasmid (ORF Rab7) in a 37°C CO2 incubator for 6 h using
Lipofectamine® RNAiMAX or Lipofectamine® 3000
Transfection Reagent according to the manufacturer's protocol.
Scrambled control (CSHCTR001-nU6; GeneCopoeia, Inc.) and empty
control vector (EX-NEG-M39; GeneCopoeia, Inc.) were used as
controls. Subsequently, the culture medium was replaced with
complete DMEM medium for another 6 h. The cells were subjected to a
second transfection, as described above, for 12 h. At 24 h after
transfection, the cells were treated with 20 Cd and/or 100 µM
puerarin in 37°C CO2 incubator for 12 h
Western blot analysis
NRK-52E cells were lysed in RIPA lysis buffer to
obtain the total protein extract. Nuclear proteins were obtained
using CelLytic™ NuCLEAR™ Extraction kit according to the
manufacturer's protocol. The BCA protein assay kit was used to
quantify the protein concentration. Equal amounts (20 µg) of
protein lysates were separated via SDS-PAGE on an 8–15% gel and
were transferred to 0.22 or 0.45-µm PVDF membranes. The membranes
were subsequently blocked for 60 min with 5% skimmed milk in TBS +
0.1% Tween-20 at room temperature. The membranes were then
incubated overnight at 4°C with the primary antibodies (1:1,000).
Next, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit or horse anti-mouse IgG
secondary antibody (1:2,000) at room temperature for 60 min,
followed by incubation with ECL reagent. The level of protein
expression was determined via computer-assisted densitometric
analysis (GS-800™ Densitometer; Quantity One v4.6.6 software;
Bio-Rad Laboratories, Inc.). Protein expression was quantified
using Image Lab v6.0.1 software (Bio-Rad Laboratories, Inc.).
Histone H3 or β-actin (1:1,000) was used as the loading control.
Each blot was performed in triplicate.
Statistical analysis
The data were analyzed via one-way ANOVA followed by
Scheffe's test using SPSS Statistics v19.0 software (IBM Corp.) and
are presented as the mean ± standard error of the mean of at least
three independent experiments. P<0.05 was considered to indicate
a statistically significant difference.
Results
Cd induces oxidative stress
Alterations in morphology and viability are
considered to be direct indexes of cell injury (44). Fig.
1A depicts the cell morphology, which was associated with Cd
toxicity, as observed under a Nikon ECLIPSE TE200 light microscope
(magnification, ×20). The control group exhibited confluence, a
uniform distribution, and complete morphology without shrinking and
swelling. Compared with the control, the cells treated with
increasing doses of Cd exhibited a decreased density, shrinkage and
a round shape. Moreover, compared with the control, cell viability
was decreased following treatment with 5–40 µM Cd for 12 h
(Fig. 1B). As illustrated in
Fig. 1C, the levels of ROS
increased up to 14.96-fold under increasing concentrations of Cd,
compared with control cells. As indicated in Fig. 1D, MDA levels increased up to
7.86-fold under increasing concentrations of Cd, compared with
control cells. These data indicated that Cd treatment induced
oxidative stress, decreased cell viability and increased cellular
damage in NRK-52E cells.
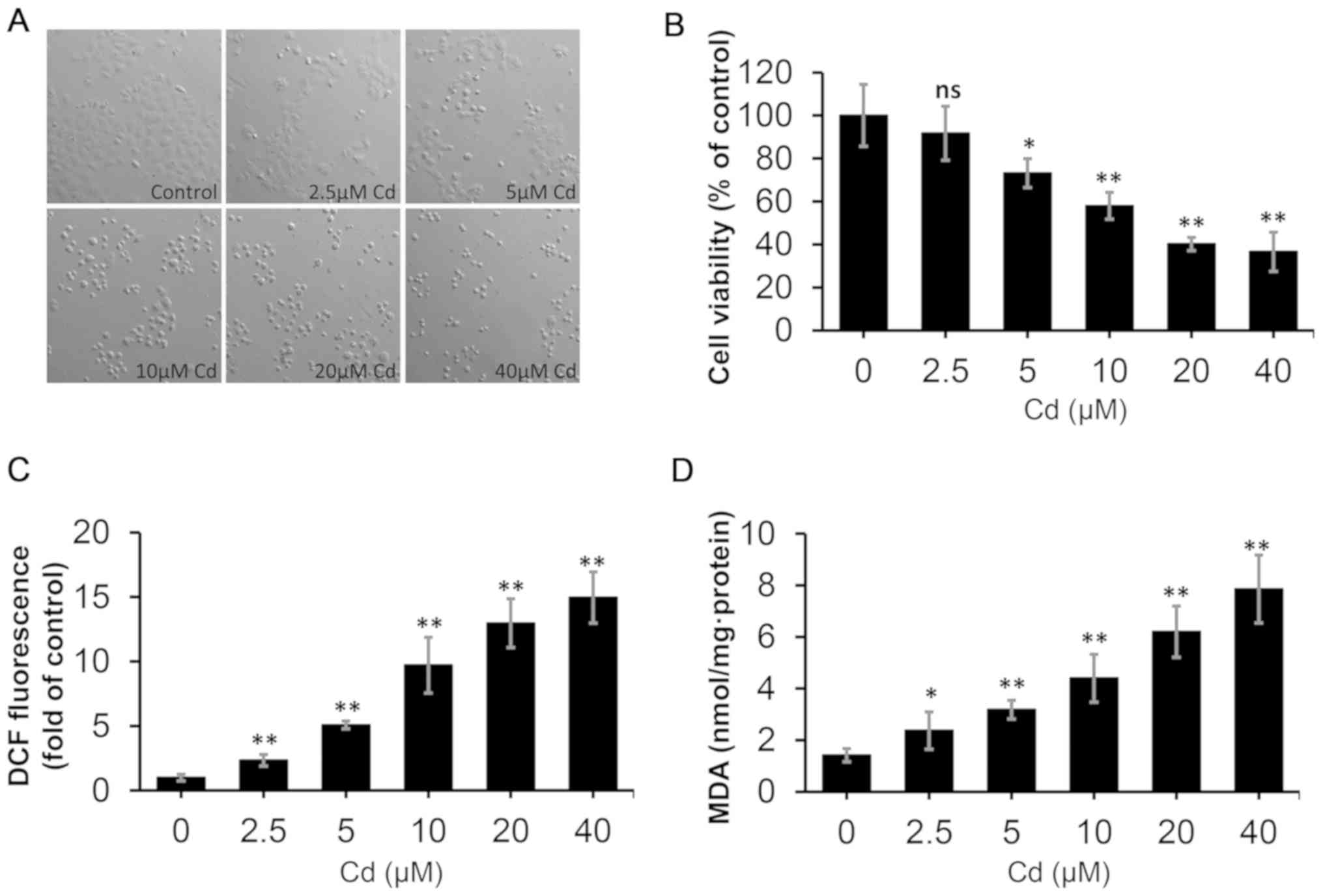 | Figure 1.Effect of Cd on the morphology,
viability and oxidative stress status of NRK-52E cells. The cells
were treated with different concentrations of Cd (0, 2.5, 5.0, 10,
20 and 40 µM) for 12 h. (A) Cell morphology was examined under
Nikon ECLIPSE TE200 light microscope (magnification, ×200). (B)
Cell Counting Kit-8 reagent was used to determine cell viability.
(C) DCF fluorescence was measured to reflect ROS levels using flow
cytometry. (D) MDA levels were measured using a commercial kit.
n=6. *P<0.05 and **P<0.01 vs. 0 µM Cd. Cd, cadmium; DCF,
dichlorofluorescein; MDA, malondialdehyde; ROS, reactive oxygen
species; ns, not significant. |
Cd decreases the antioxidant capacity
and promotes apoptosis
The activities of antioxidant enzymes and the levels
of antioxidants were examined to evaluate the antioxidant status of
NRK-52E cells following exposure to various doses of Cd for 12 h.
As demonstrated in Fig. 2, the
activities of GSH-Px, SOD and CAT were reduced in the Cd-treated
groups, exhibiting a reduction up to 0.44, 0.45 and 0.22-fold,
respectively, compared with the control. Moreover, a decrease in
GSH levels up to 0.59-fold compared with the control was observed.
The data illustrated in Fig. 3
indicate that Cd at 5–40 µM reduced the protein levels of SOD1 and
SOD2, increased the protein level of cleaved caspase-3, and Cd at
10–40 µM decreased the protein levels of CAT, resulting in
oxidative stress. These data revealed that Cd treatment resulted in
a decreased antioxidant capacity and increased apoptosis in NRK-52E
cells.
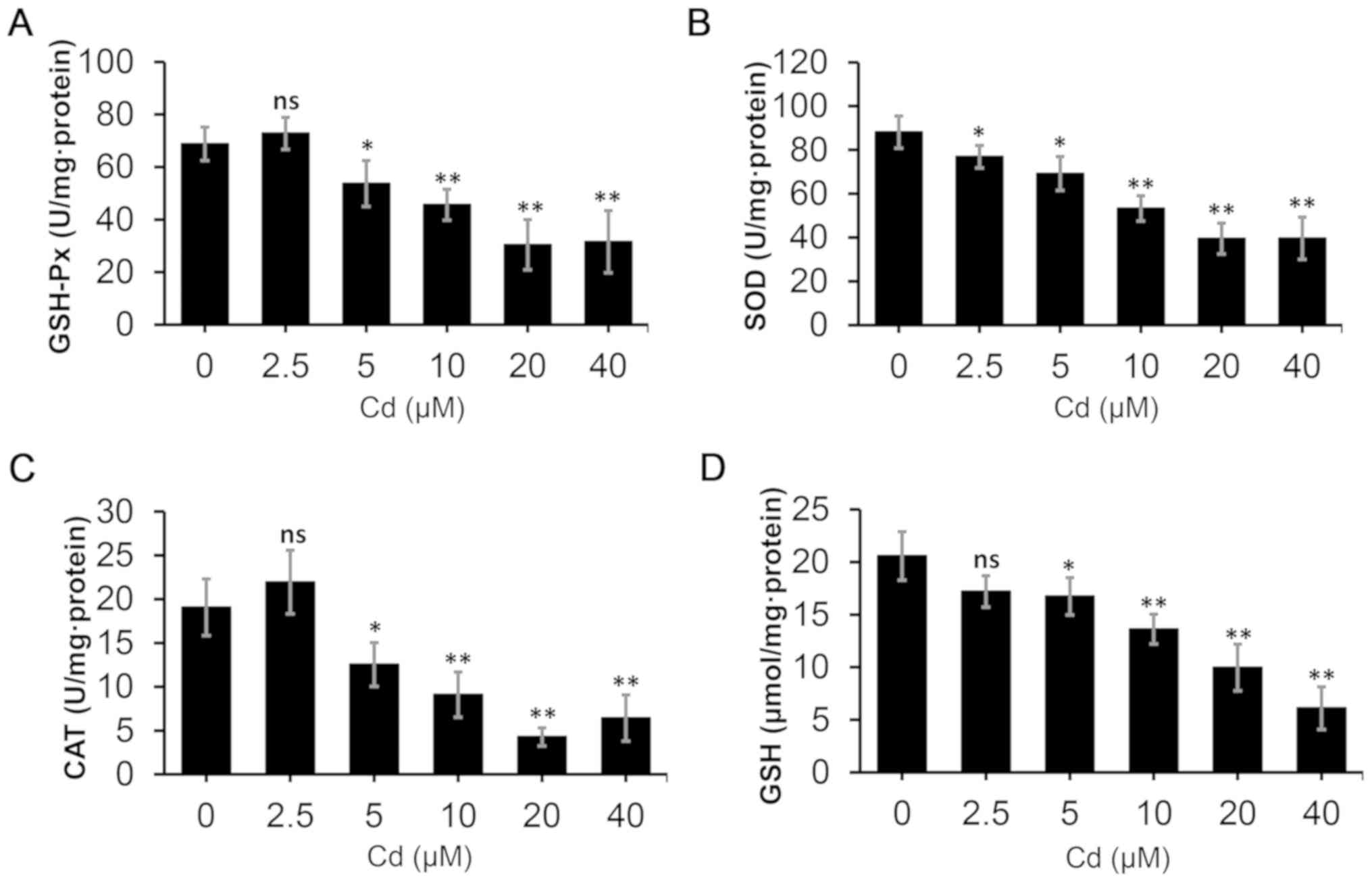 | Figure 2.Oxidative stress assays in NRK-52E
cells. The cells were treated with different concentrations of Cd
(0, 2.5, 5.0, 10, 20 and 40 µM) for 12 h, and subsequently the
cells were collected to examine the levels of (A) GSH-Px, (B) SOD,
(C) CAT and (D) GSH. n=6. *P<0.05 and **P<0.01 vs. 0 µM Cd.
Cd, cadmium; GSH-Px, glutathione peroxidase; SOD, superoxide
dismutase; CAT, catalase; GSH, glutathione; ns, not
significant. |
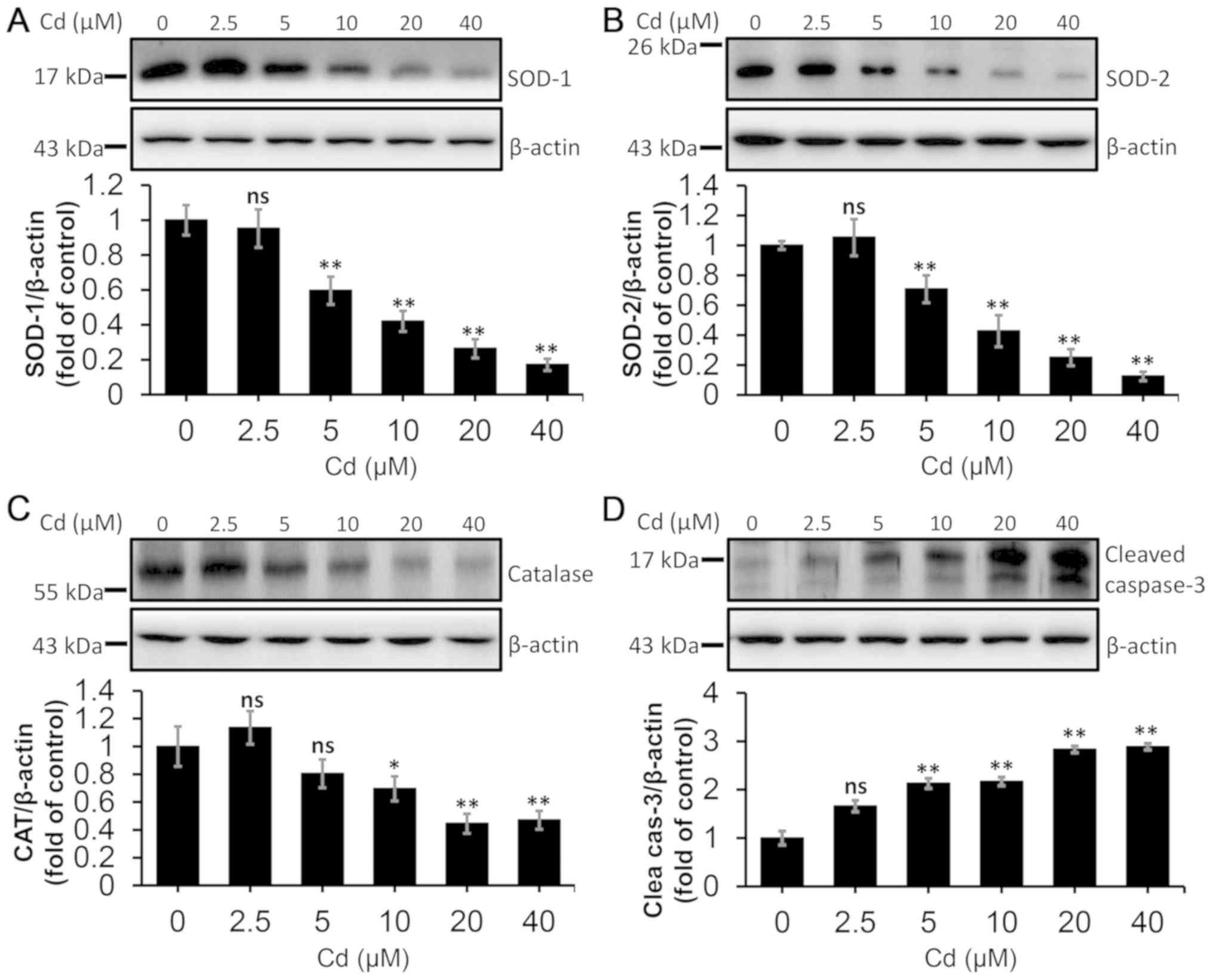 | Figure 3.Effect of Cd on protein levels of
antioxidant enzymes and cleaved caspase-3 in NRK-52E cells. The
cells were collected and lysed following treatment with a range of
Cd concentrations (0, 2.5, 5.0, 10, 20 and 40 µM) for 12 h. The
protein levels of (A) SOD-1, (B) SOD-2, (C) CAT and (D) cleaved
caspase-3 were quantified using densitometry following western
blotting. The upper panels depict representative western blotting
images and the lower panels indicate the quantitative analysis.
n=3. *P<0.05 and **P<0.01 vs. 0 µM Cd. Cd, cadmium; SOD,
superoxide dismutase; CAT, catalase; ns, not significant. |
Cd inhibits the autophagic flux and
promotes ERS
Cd treatment was indicated to induce ROS
accumulation, which has been associated with autophagy and ERS;
however, the regulatory mechanism between them is controversial
(45). To examine this mechanism,
the effect of Cd on autophagy and ERS was assessed. Firstly, the
number of RFP-LC3 puncta was observed after transient transfection
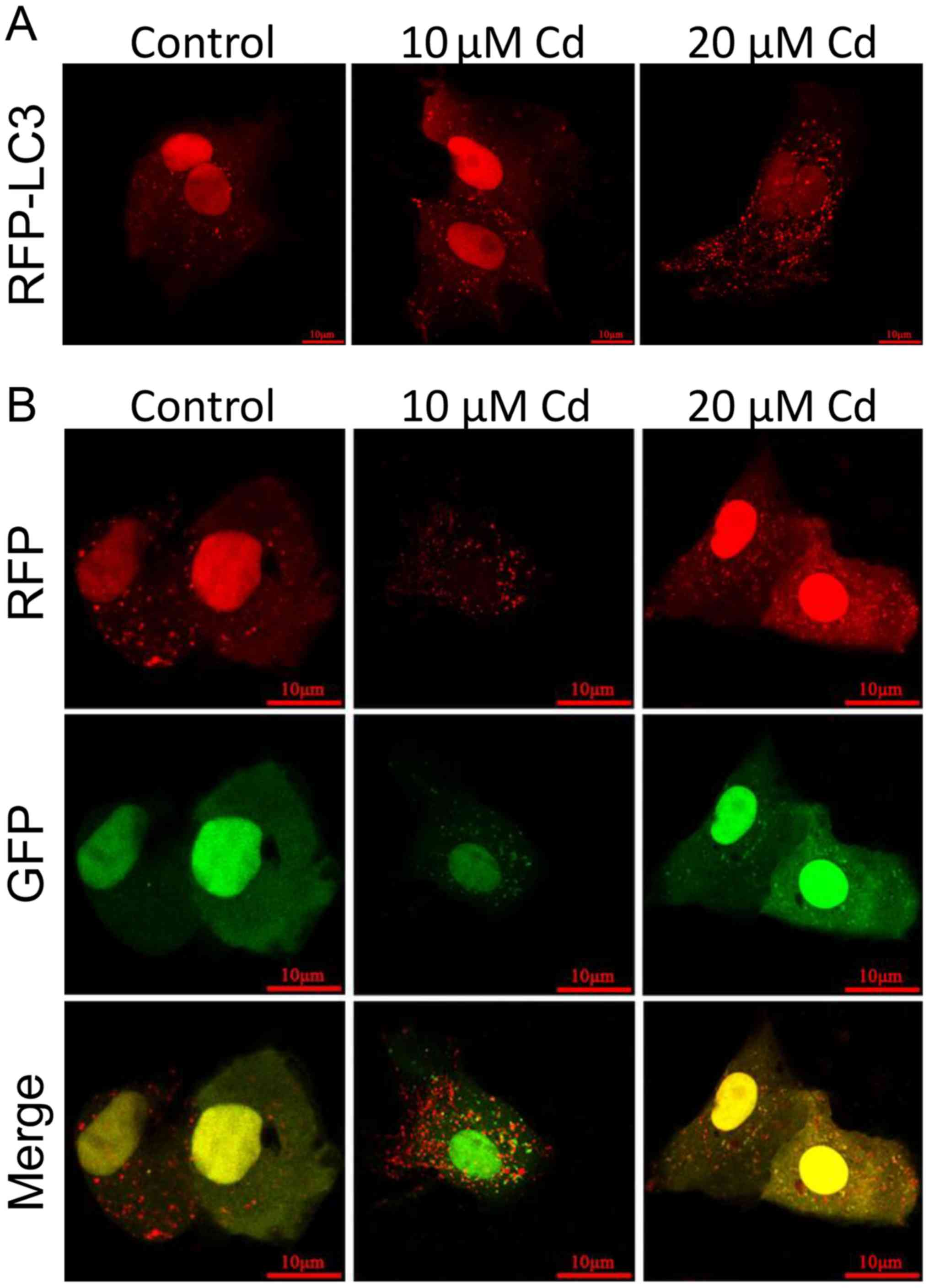
of the RFP-LC3 plasmid into NRK-52E cells. As illustrated in
Fig. 4A, compared with the control
group, the number of RFP-LC3 puncta in the Cd-treated group was
increased, indicating an accumulation of autophagosomes, which is a
key element of the autophagy process (11). To further clarify the cause of
autophagosome accumulation, the EGFP-RFP-LC3 plasmid was
transfected into NRK-52E cells to detect the autophagic flux. Under
normal conditions, the LC3-II-positive autophagosomes are labeled
with yellow color (GFP and RFP signals), while autophagosomes are
presented as red puncta only after fusion with lysosomes, as GFP is
sensitive to the low lysosomal pH and is rapidly quenched (46). The data in Fig. 4B indicate that, compared with the
control group, the accumulation of yellow puncta increased, whereas
the accumulation of red puncta decreased after exposure to Cd,
revealing that the autophagic flux was blocked. Subsequently, the
protein levels of LC3-II and p62 were detected (Fig. 5A and B). The levels of both
proteins increased in the 20 µM Cd treatment group, which further
suggested that the autophagic flux was inhibited by Cd treatment.
The expression levels of BIP, p-eIF2α, ATF-4 and CHOP were detected
via western blotting to examine Cd-induced ERS. As presented in
Fig. 5C-F, the levels of these
proteins increased in a dose-dependent manner after treatment with
10 and 20 µM Cd compared with the control group. Collectively,
these data demonstrated that the autophagic flux was impaired and
ERS was induced by Cd treatment in NRK-52E cells.
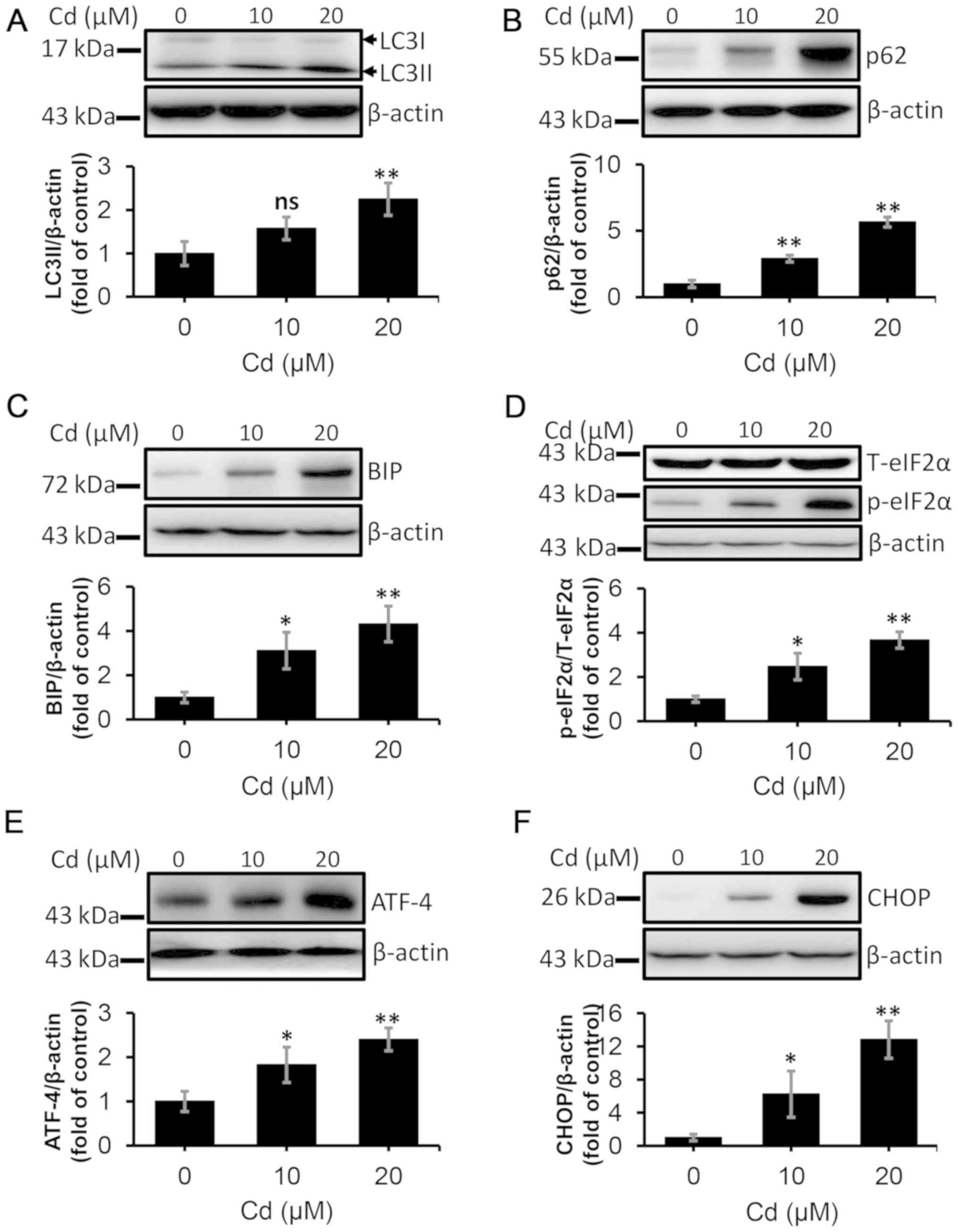 | Figure 5.Cd induces autophagic flux blockade
and endoplasmic reticulum stress in NRK-52E cells. The cells were
collected and lysed following treatment with 10 and 20 µM Cd for 12
h. The protein levels of (A) LC3II, (B) p62, (C) BIP, (D) p-eIF2α,
(E) ATF-4 and (F) CHOP were quantified using densitometry following
western blotting. n=3. *P<0.05 and **P<0.01 vs. 0 µM Cd. Cd,
cadmium; LC3, microtubule-associated protein 1 light chain 3; BIP,
binding-immunoglobulin protein; p-eIF2α, phosphorylated eukaryotic
initiation factor 2α; T-eIF2α, total eukaryotic initiation factor
2α; ATF-4, activating transcription factor 4; CHOP,
CCAAT-enhancer-binding protein homologous protein; ns, not
significant. |
Puerarin prevents Cd-induced
activation of the NRF2/KEAP1 pathway
Western blotting analysis was used to determine the
effect of puerarin on the NRF2/KEAP1 pathway in the current study.
The results indicated that puerarin inhibited Cd-induced NRF2
translocation into the nucleus (Fig.
6A). Consistently, puerarin attenuated the Cd-mediated decrease
in KEAP1 in the cytoplasm, as depicted in Fig. 6B. Moreover, the expression levels
of NRF2/KEAP1 downstream proteins were examined via western
blotting. The results indicated that the Cd-induced increase in
HO-1 and NQO1 levels were prevented via puerarin administration
(Fig. 6C and D). These data
suggested that Cd-induced activation of the NRF2/KEAP1 pathway may
be inhibited via puerarin administration.
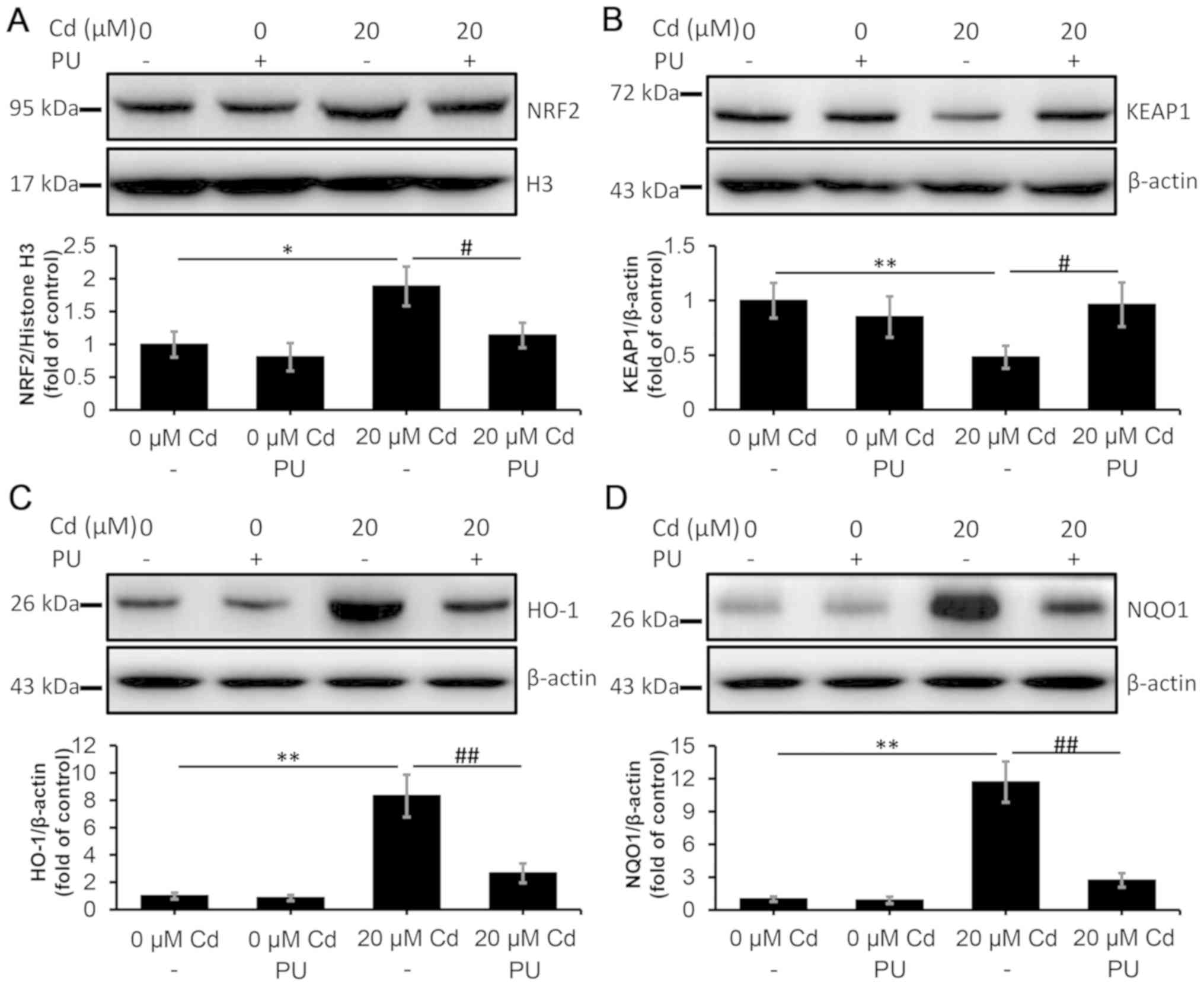 | Figure 6.Inhibitory effect of PU on the
Cd-induced NRF2/KEAP1 pathway in NRK-52E cells. The cells were
treated with 20 µM Cd in the absence or presence of 100 µM PU for
12 h, and they were subsequently collected and lysed. The protein
levels of (A) nuclear NRF2, (B) KEAP1, (C) HO-1 and (D) NQO1 in
total cell lysates were quantified using densitometry following
western blotting. The upper panels depict representative western
blotting images and the lower panels indicate the quantitative
analysis. n=3. *P<0.05 and **P<0.01 vs. 0 µM Cd;
#P<0.05 and ##P<0.01 vs. 20 µM Cd and
puerarin co-treatment. Cd, cadmium; PU, puerarin; NRF2, nuclear
factor erythroid 2-related factor 2; KEAP1, kelch-like
ECH-associated protein 1; HO-1, heme oxygenase-1; NQO1, NAD(P)H
dehydrogenase [quinone] 1; H3, histone H3. |
Puerarin restores the autophagic flux
and alleviates ERS
The results illustrated in Fig. 6 suggested that the NRF2/KEAP1
pathway was regulated by puerarin. NRF2 is a key regulator of
autophagy that is associated with ERS (33). In the current study, the effect of
puerarin on autophagy and ERS was examined. Consistently with
previous findings (47), puerarin
aided in the restoration of the autophagic flux following Cd
exposure (Fig. 7A and B).
Moreover, the levels of BIP, p-eIF2α, ATF-4 and CHOP were decreased
in the Cd and puerarin-treated group compared with those in the
Cd-treated group, indicating that Cd-induced ERS was alleviated by
puerarin (Fig. 7C-F). Furthermore,
puerarin alleviated the oxidative stress and the decrease in the
antioxidant capacity and cell viability induced by Cd treatment
(Fig. S1). Subsequently, it was
examined whether puerarin-induced autophagy is involved in ERS
attenuation. ATG7 shRNA was used to construct an
autophagy-deficient cell model, and ATG7 protein level was
determined in ATG7-deficient NRK-52E cells. The ATG7 level in cells
treated with ATG7 shRNA decreased by 65% (Fig. S2). In the autophagy-deficient
cells, puerarin co-treatment was not efficient in decreasing the
levels of BIP, p-eIF2A, ATF-4 and CHOP that were induced by Cd
(Fig. 8A-D), which indicated that
puerarin cannot alleviate the Cd-induced ERS in the absence of
autophagy. In conclusion, ERS attenuation following exposure to Cd
was dependent on the restored autophagic flux, which was induced by
puerarin. In addition, ATG7 knockdown further exacerbated
Cd-induced oxidative stress and the decreasing of antioxidant
capacity and cell viability (Fig.
S3). These data indicated that puerarin alleviated the
Cd-induced ERS primarily via autophagy regulation in NRK-52E
cells.
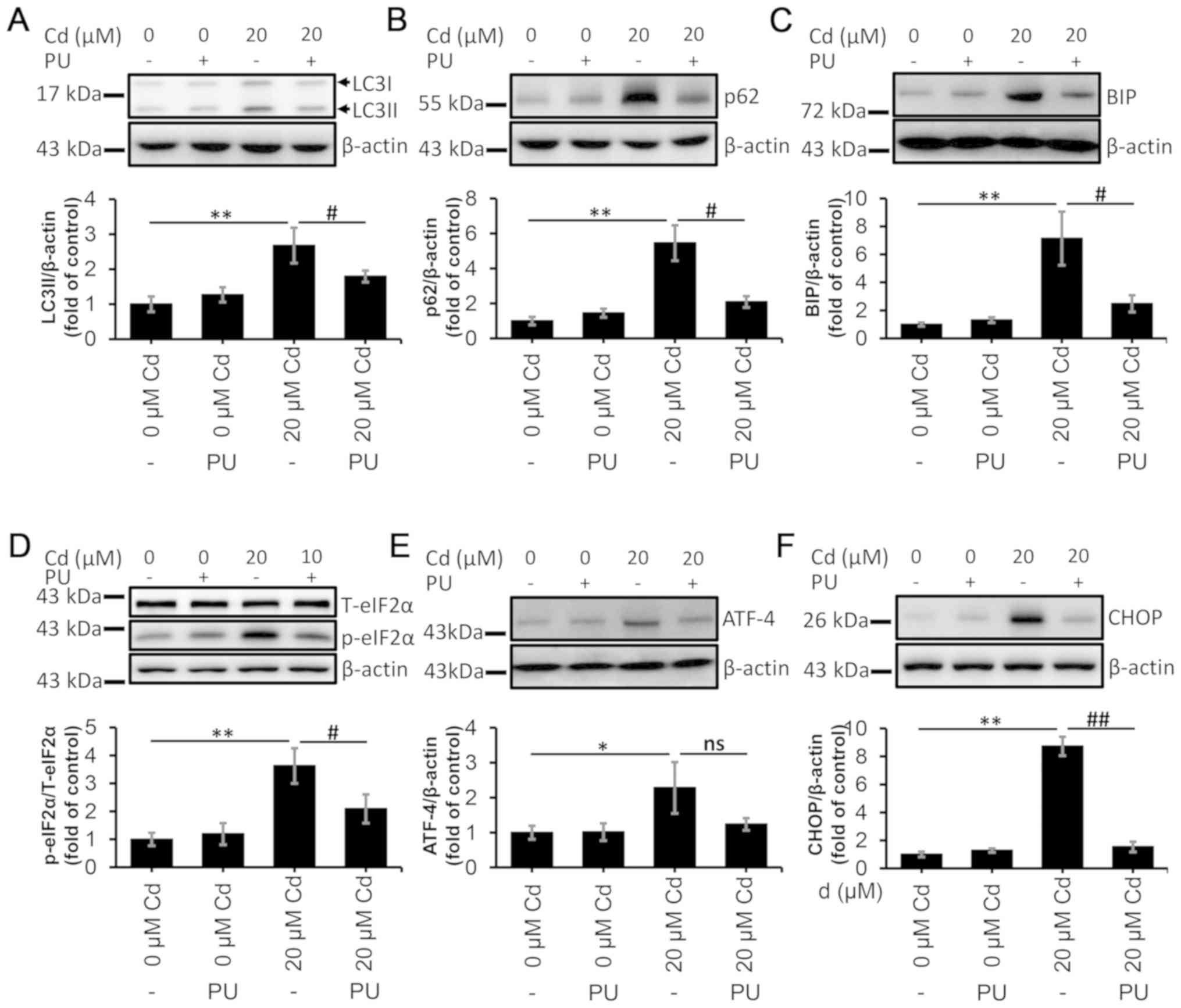 | Figure 7.PU restores the autophagic flux and
alleviates endoplasmic reticulum stress. The cells were treated
with 20 µM Cd in the absence or presence of 100 µM PU for 12 h, and
they were subsequently collected and lysed. The protein levels of
(A) LC3II, (B) p62, (C) BIP, (D) p-eIF2α, (E) ATF-4 and (F) CHOP
were quantified using densitometry. n=3. *P<0.05 and **P<0.01
vs. 0 µM Cd; #P<0.05 and ##P<0.01 vs.
20 µM Cd and puerarin co-treatment. Cd, cadmium; PU, puerarin; LC3,
microtubule-associated protein 1 light chain 3; BIP,
binding-immunoglobulin protein; p-eIF2α, phosphorylated eukaryotic
initiation factor 2α; T-eIF2α, total eukaryotic initiation factor
2α; ATF-4, activating transcription factor 4; CHOP,
CCAAT-enhancer-binding protein homologous protein; ns, not
significant. |
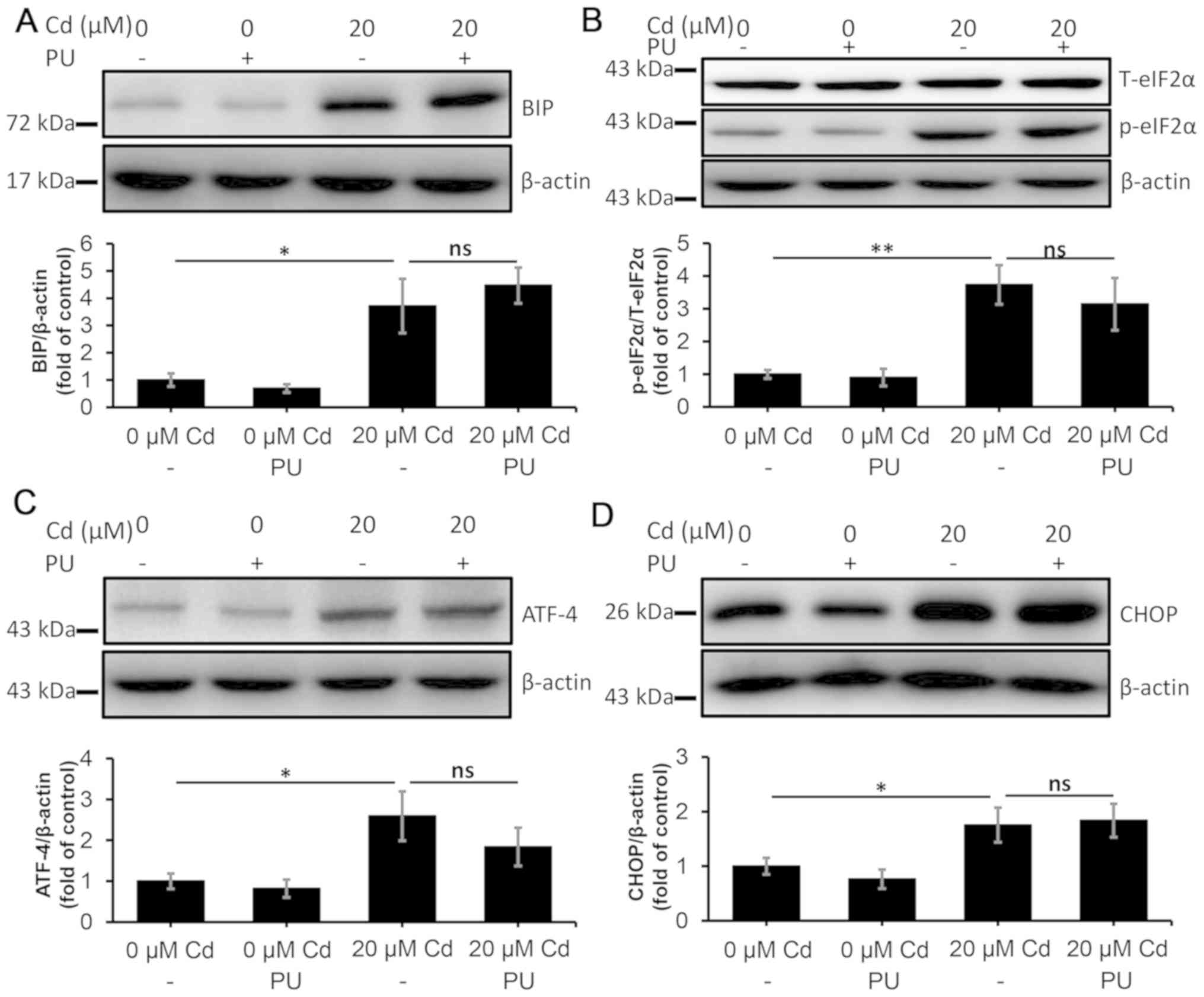 | Figure 8.PU fails to alleviate endoplasmic
reticulum stress in NRK-52E cells following ATG7 knockdown. The
cells were transfected with a short hairpin RNA targeting ATG7, and
they were subsequently treated with 20 µM Cd in the absence or
presence of 100 µM PU for 12 h. The protein levels of (A) BIP, (B)
p-eIF2α, (C) ATF-4 and (D) CHOP were quantified using densitometry
following western blotting. n=3. *P<0.05 and **P<0.01 vs. 0
µM Cd. Cd, cadmium; PU, puerarin; ATG7, autophagy-related protein
7; BIP, binding-immunoglobulin protein; p-eIF2α, phosphorylated
eukaryotic initiation factor 2α; T-eIF2α, total eukaryotic
initiation factor 2α; ATF-4, activating transcription factor 4;
CHOP, CCAAT-enhancer-binding protein homologous protein; ns, not
significant. |
RAB7-induced autophagic flux
restoration is involved in alleviating ERS
In cells overexpressing RAB7 (Fig. S4), the Cd-induced blockade of the
fusion between autophagosomes and lysosomes was restored (Fig. S5). Moreover, the levels of BIP,
p-eIF2α, ATF-4 and CHOP, which were upregulated by Cd, were
downregulated in RAB7-overexpressing cells, which indicated that
ERS was alleviated by RAB7 overexpression (Fig. 9A-D). In addition, RAB7
overexpression exhibited a positive effect on alleviating oxidative
stress, and enhancing antioxidant capacity and cell viability
(Fig. S6), which is similar to
puerarin (Fig. S1). Additionally,
Cd-induced activation of p38 and JNK suppression of ERK were
reversed by puerarin administration (Fig. S7). Taken together, these data
demonstrated that the restoration of the autophagic flux alleviated
ERS, which was indicated to serve a critical role in
puerarin-mediated protection against cell injury following exposure
to Cd.
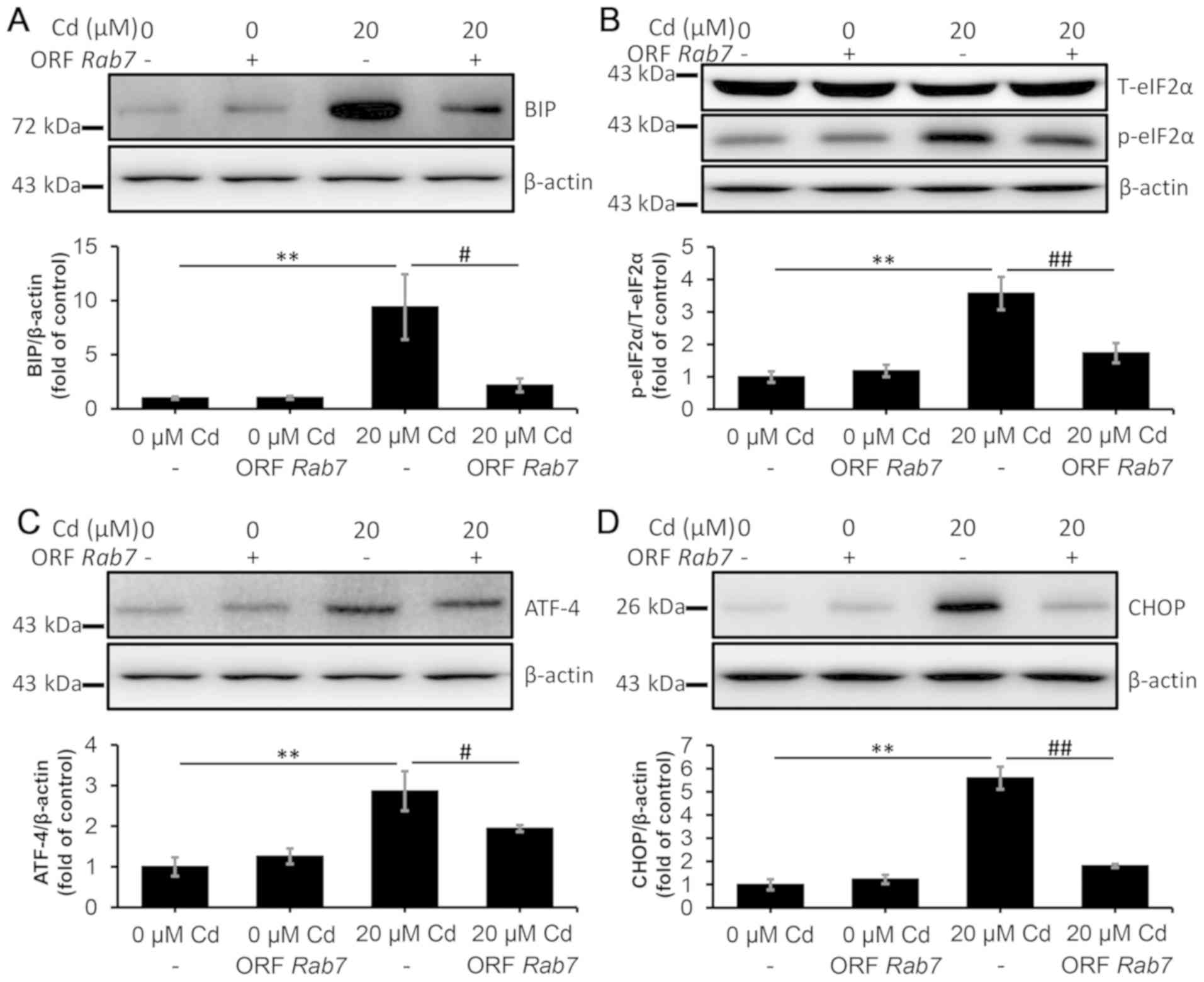 | Figure 9.RAB7 overexpression alleviates
endoplasmic reticulum stress in NRK-52E cells. The cells were
transfected with ORF RAB7 and they were subsequently treated with
20 µM Cd for 12 h. The protein levels of (A) BIP, (B) p-eIF2α, (C)
ATF-4 and (D) CHOP were quantified using densitometry following
western blotting. n=3. **P<0.01 vs. 0 µM Cd;
#P<0.05 and ##P<0.01 vs. 20 µM Cd and
puerarin co-treatment. Cd, cadmium; BIP, binding-immunoglobulin
protein; p-eIF2α, phosphorylated eukaryotic initiation factor 2α;
T-eIF2α, total eukaryotic initiation factor 2α; ATF-4, activating
transcription factor 4; CHOP, CCAAT-enhancer-binding protein
homologous protein; ORF, open reading frame; RAB7, Ras-related
protein Rab-7. |
Discussion
In the present study, the effect of autophagy on ERS
in Cd-mediated nephrotoxicity was investigated using an NRK-52E
cell model. Firstly, it was demonstrated that Cd treatment induced
oxidative damage and apoptosis in NRK-52E cells in a dose-dependent
manner. Secondly, Cd was indicated to inhibit the autophagic flux
and promote ERS. Thirdly, puerarin was revealed to restore the
autophagic flux, which was induced by Cd, and alleviate ERS;
however, this effect was not observed in the absence of autophagy
in ATG7-deficient NRK-52E cells. Lastly, the NRF2/KEAP1 pathway was
indicated to be involved in the process of autophagic flux
restoration that prevented ERS in NRK-52E cells following exposure
to Cd. Collectively, the results of the current study indicated
that Cd-induced nephrotoxicity was attenuated by puerarin, which
inhibited the NRF2/KEAP1 pathway to restore the autophagic flux and
alleviate ERS.
Autophagy and apoptosis are two distinct
physiological responses of cells, which regulate cell survival and
death after stimulation of cells by different signals, and have
been indicated to be associated with ERS and be involved in several
kidney diseases, such as diabetes, ischemia-reperfusion injury and
kidney stones (48). ERS has been
revealed to exhibit a dual function by promoting cell survival or
apoptosis, and may be associated with autophagy under a variety of
pathological conditions (49).
Autophagy has been reported to serve a cytoprotective role in acute
kidney injury, as blockade of the autophagic flux has been
indicated to deteriorate the pathological process of acute kidney
injury (50). Autophagy and ERS
have been indicated to interact and are implicated in numerous
pathological processes, including neurodegenerative disorder,
cancer, diabetic nephropathy and renal fibrosis (30,48,51).
However, the interaction between autophagy and ERS requires
additional elucidation. The present study explored whether a
crosstalk between ERS and autophagy inhibition in Cd-treated
NRK-52E cells exists, and determined the implicated mechanism.
Moreover, the results of the current study provided insight into
the signaling mechanisms via which puerarin administration restores
the autophagic flux to alleviate ERS during Cd-induced
nephrotoxicity.
Both apoptosis and autophagy have been associated
with Cd-induced nephrotoxicity (20,52).
Moreover, ERS has been indicated to promote renal disease via
inducing apoptosis (52). All
these processes are closely associated with oxidative stress, which
has been reported to be a principal mechanism of Cd-induced
toxicity (53,54). The results of the present study
indicated that both ROS and MDA levels were increased in NRK-52E
cells following treatment with 2.5–40 µM Cd. On the contrary, cell
viability, GSH and the levels of antioxidant enzymes were decreased
following treatment with 5–40 µM Cd. Moreover, the levels of
cleaved caspase-3, which is a marker of apoptosis, were increased
in Cd-treated cells. These results are consistent with that of
previous reports (55,56) and revealed that Cd induced
oxidative stress and apoptosis in NRK-52E cells. Kidney injury is
induced following chronic accumulation of Cd, and this process may
last for decades (57). It would
be unrealistic to treat the cells with the same Cd concentration
(0.22-1.03 µM) as that in blood of patients with renal disease
(58,59), which would not be efficient in
inducing renal cell injury in the limited time of the in
vitro study. Therefore, the doses of Cd that were used to treat
NRK-52E cells in the current study were higher than the
concentration of free Cd in blood (0.22-1.03 µM) and locally in the
kidney, in order to simulate renal cell injury, which is induced
following decades of Cd accumulation, and affect the associated
mechanisms, such as oxidative stress, organelle damage and
apoptosis.
In the present study, ERS was activated and the
autophagic flux was inhibited in NRK-52E cells treated with 10 and
20 µM Cd. However, the effect of Cd on autophagy may vary in
different cell types. In addition to proximal tubular cells, renal
mesangial cells may also be affected by Cd-induced renal injury.
Fujishiro et al (20),
reported a protective effect of Cd-induced autophagy in rat renal
mesangial cells, which is contradictory to the results of the
current study. Fujishiro et al (20), used cells that were cultured in
medium with 0.2% FBS for 48 h before transfer to serum-free medium
with Cd. This prolonged serum starvation may have induced
additional effects on autophagy, especially in the presence of
other stimuli. Moreover, it was suggested that autophagy was
primarily regulated by JNK-mediated signaling in rat renal
mesangial cells exposed to Cd (16). However, another study has revealed
that in addition to JNK, the ERK and p38 pathways are also involved
in Cd-induced mouse proximal tubular cell injury (60). Therefore, in the current study,
activation of p38, but not JNK, and the ERS-induced inhibition of
the autophagic flux mediated the effect of Cd on autophagy.
Previous studies have indicated that the NRF2/KEAP1
axis is an important endogenous antioxidant signaling pathway
(35,61). NRF2 has been reported to be
activated following translocation to the nucleus, where it has been
indicated to interact with the antioxidant response element (ARE)
to regulate the transcription and expression of antioxidant
enzymes, thereby enhancing the resistance to oxidative stress
(56). In the present study, the
Cd-induced nuclear accumulation of NRF2 was inhibited by puerarin.
Moreover, the expression levels of KEAP1, which is an inhibitory
receptor of NRF2, and two downstream targets of NRF2, HO-1 and
NQO1, were detected via western blotting in the current study to
indirectly assess the activation status of NRF2. The results
indicated that compared with the Cd group, puerarin treatment
ablated the downregulation of KEAP1 and the upregulation of HO-1
and NQO1 protein levels. Activation of NRF2 has been indicated to
be associated with a series of pathological and physiological
processes including respiratory distress syndrome,
neurodegeneration, and carcinogenesis and tumor development
(62,63), and participates in renal pathology
(64). Puerarin has been revealed
to protect against CCl4-induced oxidative stress and
inflammation via inhibiting the ERK/NRF2/ARE pathway in rat kidneys
(65). In addition, puerarin
treatment has been indicated to enhance the mRNA expression levels
of anti-oxidant enzymes, such as NRF2, HO-1 and SOD2 (66). As oxidative stress and ERS have
been indicated to positively regulate the NRF2 signaling pathway
(67), they may account for the
upregulation of NRF2 in the present study. However, since ERS has
not been reported to inhibit the NRF2 signaling pathway, to the
best of our knowledge, puerarin-induced NRF2 inhibition may be
attributed to the antioxidant capacity of puerarin.
A crosstalk between NRF2 and autophagy under stress
conditions has been reported in previous studies (36,68).
In addition, both persistent ERS and the inhibition of autophagic
flux have been indicated to mediate cell injury, and although ERS
may result in autophagy (69), the
effect of autophagic flux on ERS is largely unknown. Therefore, the
autophagic flux was examined in the NRK-52E cell model in the
current study. The blockade of the autophagic flux, which was
induced by Cd, was indicated to be attenuated by puerarin.
Moreover, Cd-induced ERS was alleviated when NRK-52E cells were
treated with both Cd and puerarin. Collectively, puerarin was
indicated to attenuate ERS following Cd exposure, via preventing
the activation of NRF2 signaling to restore autophagic flux.
ATG7 knockdown has been indicated to result in
autophagy deficiency (70) and
RAB7 overexpression has been revealed to enhance the fusion of
autophagosomes and lysosomes (18). Consequently, both were used in the
current study to further examine the effect of autophagy on ERS. In
a previous study, ATG7 gene knockout in a EPCK-Cre Atg7
mouse model has been reported to result in autophagy deficiency,
which was indicated by a lack of LC3II and p62/SQSTM1 accumulation
(70). In the autophagy deficient
cell model of the current study, the Cd-induced upregulation of
BIP, p-eIF2α, ATF4 and CHOP was not inhibited by puerarin. This
suggested that puerarin alleviated ERS during Cd exposure primarily
via regulating autophagy. As Cd inhibits, and RAB7 has been
indicated to induce, the autolysosome formation (30), the effect of RAB7 was examined in a
RAB7 overexpression cell model in the current study. In the
overexpressing cells, the Cd-induced increase in ERS markers was
attenuated. Moreover, for untreated cells, compared with the empty
control vector group, the levels of the ERS markers was slightly
increased in the RAB7 overexpression group. This additionally
suggested that the autophagic flux serves a critical role in the
puerarin-induced alleviation of ERS following treatment with Cd,
and excessive autophagy may induce ERS and subsequent cell injury.
Autophagy has been revealed to serve a critical role in regulating
oxidative stress, and reactive oxygen/nitrogen species may induce
ERS and autophagy, indicating that an interaction among oxidative
stress, ERS and autophagy exists (26). However, among these three cellular
processes, only autophagy has been indicated to exhibit a
protective effect against cell damage (71). The results of the present study
suggested that the puerarin-mediated restoration of the autophagic
flux alleviated Cd-induced ERS. Autophagy is a regulatory mechanism
of the cell that removes unnecessary or dysfunctional components,
which allows the degradation and recycling of cellular proteins and
organelles, especially under stress conditions (11). ERS, which is primarily induced by
the accumulation of unfolded or misfolded proteins, is an important
factor that promotes cell damage (27). In the present study, the autophagic
flux, which was restored via the puerarin-mediated inhibition of
the NRF2 signaling pathway, was indicated to promote the
degradation of misfolded proteins to alleviate ERS.
Furthermore, ERS can also be activated by oxidative
stress, which is induced by ROS, one of its primary sources being
dysfunctional organelles, such as mitochondria (27). Therefore, the clearance of
dysfunctional organelles to restore the autophagic flux may
represent another key mechanism responsible for alleviating ERS
during Cd exposure.
In conclusion, the findings of the present study
demonstrated that Cd induced oxidative stress, apoptosis,
activation of NRF2 signaling, obstruction of the autophagic flux
and ERS in NRK-52E cells. Moreover, puerarin administration
inhibited NRF2 signaling to restore the autophagic flux, via which
misfolded proteins and dysfunctional organelles are degraded to
alleviate ERS. In the present study, the association between
autophagy and ERS was elucidated in the context of Cd-induced renal
damage, and it was indicated that puerarin may potentially
alleviate environmental Cd-induced nephrotoxicity and improve
kidney health in affected populations.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Key
Research and Development Program of China (grant no.
2016YFD0501208), the National Natural Science Foundation of China
(grant nos. 31101866, 31872533 and 31702305), Jiangsu Provincial
Natural Science Foundation of China (grant no. BK20150447),
Postdoctoral Research Funding of Yangzhou University (grant no.
137070430) and the Priority Academic Program Development of Jiangsu
Higher Education Institutions.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and GL contributed to the study design and
obtained funding. GL and KZ drafted the manuscript. KZ, WD and YT
performed the experiments. ML, HZ, WD and YT performed data
acquisition, data analysis and interpretation. ML and HZ reviewed
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jarup L, Rogenfelt A, Elinder CG, Nogawa K
and Kjellstrom T: Biological half-time of cadmium in the blood of
workers after cessation of exposure. Scand J Work Environ Health.
9:327–331. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tchounwou PB, Yedjou CG, Patlolla AK and
Sutton DJ: Heavy metal toxicity and the environment. Exp Suppl.
101:133–164. 2012.PubMed/NCBI
|
|
3
|
Programme UNE and World Health
Organization: Cadmium: Environmental aspects. Environmental Health
Criteria. 1992.
|
|
4
|
Lind Y, Engman J, Jorhem L and Glynn AW:
Cadmium accumulation in liver and kidney of mice exposed to the
same weekly cadmium dose continuously or once a week. Food Chem
Toxicol. 35:891–895. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernard A: Renal dysfunction induced by
cadmium: Biomarkers of critical effects. Biometals. 17:519–523.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prozialeck WC, Wellington DR, Mosher TL,
Lamar PC and Laddaga RA: The cadmium-induced disruption of tight
junctions in LLC-PK1 cells does not result from apoptosis. Life
Sci. 57:199–204. 1995. View Article : Google Scholar
|
|
7
|
Dong Z, Wang L, Xu J, Li Y, Zhang Y, Zhang
S and Miao J: Promotion of autophagy and inhibition of apoptosis by
low concentrations of cadmium in vascular endothelial cells.
Toxicol In Vitro. 23:105–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Cao J, Chen D, Liu X, Lu H and Liu
Z: Role of oxidative stress, apoptosis, and intracellular
homeostasis in primary cultures of rat proximal tubular cells
exposed to cadmium. Biol Trace Elem Res. 127:53–68. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen R, Liu D, Hou C, Liu D, Zhao L, Cheng
J, Wang D and Bai D: Protective effect of Potentilla anserina
polysaccharide on cadmium-induced nephrotoxicity in vitro
and in vivo. Food Funct. 8:3636–3646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Wang H, Li J, Chen D and Liu Z:
Simultaneous effects of lead and cadmium on primary cultures of rat
proximal tubular cells: Interaction of apoptosis and oxidative
stress. Arch Environ Contam Toxicol. 61:500–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Duve C: The lysosome. Sci Am.
208:64–72. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terman A and Brunk UT: Autophagy in
cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res.
68:355–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang LY, Wu KH, Chiu WT, Wang SH and Shih
CM: The cadmium-induced death of mesangial cells results in
nephrotoxicity. Autophagy. 5:571–572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin Y, Tanaka A, Choi AM and Ryter SW:
Autophagic proteins: New facets of the oxygen paradox. Autophagy.
8:426–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Miao G, Xue X, Guo X, Yuan C, Wang
Z, Zhang G, Chen Y, Feng D, Hu J and Zhang H: The vici syndrome
protein EPG5 Is a Rab7 effector that determines the fusion
specificity of autophagosomes with late Endosomes/Lysosomes. Mol
Cell. 63:781–795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen M, Li X, Fan R, Yang J, Jin X, Hamid
S and Xu S: Cadmium induces BNIP3-dependent autophagy in chicken
spleen by modulating miR-33-AMPK axis. Chemosphere. 194:396–402.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujishiro H, Liu Y, Ahmadi B and Templeton
DM: Protective effect of cadmium-induced autophagy in rat renal
mesangial cells. Arch Toxicol. 92:619–631. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
So KY, Lee BH and Oh SH: The critical role
of autophagy in cadmium-induced immunosuppression regulated by
endoplasmic reticulum stress-mediated calpain activation in
RAW264.7 mouse monocytes. Toxicology. 393:15–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thevenod F and Lee WK: Cadmium and
cellular signaling cascades: Interactions between cell death and
survival pathways. Arch Toxicol. 87:1743–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee AS: The glucose-regulated proteins:
Stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaufman RJ: Orchestrating the unfolded
protein response in health and disease. J Clin Invest.
110:1389–1398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo B, Lin Y, Jiang S, Huang L, Yao H,
Zhuang Q, Zhao R, Liu H, He C and Lin Z: Endoplasmic reticulum
stress eIF2α-ATF4 pathway-mediated cyclooxygenase-2 induction
regulates cadmium-induced autophagy in kidney. Cell Death Dis.
7:e22512016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Z and Chen L: Endoplasmic reticulum
stress and autophagy. Adv Exp Med Biol. 1206:167–177. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang SW and Hegde RS: Lighting up the
stressed ER. Cell. 135:787–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oslowski CM and Urano F: Measuring ER
stress and the unfolded protein response using mammalian tissue
culture system. Methods Enzymol. 490:71–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rivas A, Vidal RL and Hetz C: Targeting
the unfolded protein response for disease intervention. Expert Opin
Ther Targets. 19:1203–1218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Y, Arikkath J, Yang L, Guo ML,
Periyasamy P and Buch S: Interplay of endoplasmic reticulum stress
and autophagy in neurodegenerative disorders. Autophagy.
12:225–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khaminets A, Heinrich T, Mari M, Grumati
P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N,
et al: Regulation of endoplasmic reticulum turnover by selective
autophagy. Nature. 522:354–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Xia Q, Zhou Y and Li J:
Endoplasmic reticulum stress and autophagy contribute to
cadmium-induced cytotoxicity in retinal pigment epithelial cells.
Toxicol Lett. 311:105–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hernandez-Gea V, Hilscher M, Rozenfeld R,
Lim MP, Nieto N, Werner S, Devi LA and Friedman SL: Endoplasmic
reticulum stress induces fibrogenic activity in hepatic stellate
cells through autophagy. J Hepatol. 59:98–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Digaleh H, Kiaei M and Khodagholi F: Nrf2
and Nrf1 signaling and ER stress crosstalk: Implication for
proteasomal degradation and autophagy. Cell Mol Life Sci.
70:4681–4694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang Z, Hu B, Zang F, Wang J, Zhang X and
Chen H: Nrf2 drives oxidative stress-induced autophagy in nucleus
pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect
intervertebral disc from degeneration. Cell Death Dis. 10:5102019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dodson M, Redmann M, Rajasekaran NS,
Darley-Usmar V and Zhang J: KEAP1-NRF2 signalling and autophagy in
protection against oxidative and reductive proteotoxicity. Biochem
J. 469:347–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ichimura Y, Waguri S, Sou YS, Kageyama S,
Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et
al: Phosphorylation of p62 activates the Keap1-Nrf2 pathway during
selective autophagy. Mol Cell. 51:618–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li W, Yang Q and Mao Z: Signaling and
induction of chaperone-mediated autophagy by the endoplasmic
reticulum under stress conditions. Autophagy. 14:1094–1096.
2018.PubMed/NCBI
|
|
40
|
Ryabaya O, Prokofieva A, Khochenkov D,
Abramov I, Zasedatelev A and Stepanova E: Inhibition of endoplasmic
reticulum stress-induced autophagy sensitizes melanoma cells to
temozolomide treatment. Oncol Rep. 40:385–394. 2018.PubMed/NCBI
|
|
41
|
Liu CM, Ma JQ, Liu SS, Feng ZJ and Wang
AM: Puerarin protects mouse liver against nickel-induced oxidative
stress and inflammation associated with the TLR4/p38/CREB pathway.
Chem Biol Interact. 243:29–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang N, Zhang Y, Wu L, Wang Y, Cao Y, He
L, Li X and Zhao J: Puerarin protected the brain from cerebral
ischemia injury via astrocyte apoptosis inhibition.
Neuropharmacology. 79:282–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang B, Chen S, Yan X, Li M, Li D, Lv P
and Ti G: The therapeutic effect and possible harm of puerarin for
treatment of stage III diabetic nephropathy: A meta-analysis.
Altern Ther Health Med. 21:36–44. 2015.PubMed/NCBI
|
|
44
|
Shapiro DL: Morphological and biochemical
alterations in foetal rat brain cells cultured in the presence of
monobutyryl cyclic AMP. Nature. 241:203–204. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin YN, Jiang M, Chen WJ, Zhao TJ and Wei
YF: Cancer and ER stress: Mutual crosstalk between autophagy,
oxidative stress and inflammatory response. Biomed Pharmacother.
118:1092492019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou XL, Wan XM, Fu XX and Xie CG:
Puerarin prevents cadmium-induced hepatic cell damage by
suppressing apoptosis and restoring autophagic flux. Biomed
Pharmacother. 115:1089292019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cybulsky AV: Endoplasmic reticulum stress,
the unfolded protein response and autophagy in kidney diseases. Nat
Rev Nephrol. 13:681–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu SN, Kim SH, Kim KY, Ji JH, Seo YK, Yu
HS and Ahn SC: Salinomycin induces endoplasmic reticulum
stressmediated autophagy and apoptosis through generation of
reactive oxygen species in human glioma U87MG cells. Oncol Rep.
37:3321–3328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duann P, Lianos EA, Ma J and Lin PH:
Autophagy, innate immunity and tissue repair in acute kidney
injury. Int J Mol Sci. 17:6622016. View Article : Google Scholar
|
|
51
|
Coker-Gurkan A, Ayhan-Sahin B, Keceloglu
G, Obakan-Yerlikaya P, Arisan ED and Palavan-Unsal N: Atiprimod
induce apoptosis in pituitary adenoma: Endoplasmic reticulum stress
and autophagy pathways. J Cell Biochem. 120:19749–19763. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chou X, Ding F, Zhang XY, Ding XQ, Gao H
and Wu Q: Sirtuin-1 ameliorates cadmium-induced endoplasmic
reticulum stress and pyroptosis through XBP-1s deacetylation in
human renal tubular epithelial cells. Arch Toxicol. 93:965–986.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang JC, Zhu HL, Zhang C, Wang HW and Yang
ZJ: Baicalein ameliorates cadmium-induced hepatic and renal
oxidative damage in rats. Indian J Anim Res. 53:523–527. 2019.
|
|
54
|
Nair AR, Lee WK, Smeets K, Swennen Q,
Sanchez A, Thévenod F and Cuypers A: Glutathione and mitochondria
determine acute defense responses and adaptive processes in
cadmium-induced oxidative stress and toxicity of the kidney. Arch
Toxicol. 89:2273–2289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pathak N and Khandelwal S: Role of
oxidative stress and apoptosis in cadmium induced thymic atrophy
and splenomegaly in mice. Toxicol Lett. 169:95–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen J and Shaikh ZA: Activation of Nrf2
by cadmium and its role in protection against cadmium-induced
apoptosis in rat kidney cells. Toxicol Appl Pharmacol. 241:81–89.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Godt J, Scheidig F, Grosse-Siestrup C,
Esche V, Brandenburg P, Reich A and Groneberg DA: The toxicity of
cadmium and resulting hazards for human health. J Occup Med
Toxicol. 1:222006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Alli LA: Blood level of cadmium and lead
in occupationally exposed persons in Gwagwalada, Abuja, Nigeria.
Interdiscip Toxicol. 8:146–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Goyal T, Mitra P, Singh P, Sharma S and
Sharma P: Assessement of blood lead and cadmium levels in
occupationally exposed workers of Jodhpur, Rajasthan. Ind J Clin
Biochem. 2020.
View Article : Google Scholar
|
|
60
|
Gu J, Dai SY, Liu YM, Liu H, Zhang Y, Ji
X, Yu F, Zhou Y, Chen L, Tse WKF, et al: Activation of
Ca(2+)-sensing receptor as a protective pathway to reduce
Cadmium-induced cytotoxicity in renal proximal tubular cells. Sci
Rep. 8:10922018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tu W, Wang H, Li S, Liu Q and Sha H: The
anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE
signaling pathway in chronic diseases. Aging Dis. 10:637–651. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen DL, Tavana O, Chu B, Erber L, Chen Y,
Baer R and Gu W: NRF2 is a major target of ARF in p53-independent
tumor suppression. Mol Cell. 68:224–232.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Towers CG, Fitzwalter BE, Regan D,
Goodspeed A, Morgan MJ, Liu CW, Gustafson DL and Thorburn A: Cancer
cells upregulate NRF2 signaling to adapt to autophagy inhibition.
Dev Cell. 50:690–703.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Guerrero-Hue M, Farre-Alins V,
Palomino-Antolin A, Parada E, Rubio-Navarro A, Egido J, Egea J and
Moreno JA: Targeting Nrf2 in protection against renal disease. Curr
Med Chem. 24:3583–3605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ma JQ, Ding J, Xiao ZH and Liu CM:
Puerarin ameliorates carbon tetrachloride-induced oxidative DNA
damage and inflammation in mouse kidney through ERK/Nrf2/ARE
pathway. Food Chem Toxicol. 71:264–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ullah MZ, Khan AU, Afridi R, Rasheed H,
Khalid S, Naveed M, Ali H, Kim YS and Khan S: Attenuation of
inflammatory pain by puerarin in animal model of inflammation
through inhibition of pro-inflammatory mediators. Int
Immunopharmacol. 61:306–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen ZJ, Chen JX, Wu LK, Li BY, Tian YF,
Xian M, Huang ZP and Yu RA: Induction of endoplasmic reticulum
stress by cadmium and its regulation on Nrf2 signaling pathway in
kidneys of rats. Biomed Environ Sci. 32:1–10. 2019.PubMed/NCBI
|
|
68
|
Liao W, Wang Z, Fu Z, Ma H, Jiang M, Xu A
and Zhang W: p62/SQSTM1 protects against cisplatin-induced
oxidative stress in kidneys by mediating the cross talk between
autophagy and the Keap1-Nrf2 signalling pathway. Free Radic Res.
53:800–814. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Manley S, Ni HM, Kong B, Apte U, Guo G and
Ding WX: Suppression of autophagic flux by bile acids in
hepatocytes. Toxicol Sci. 137:478–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Khambu B, Huda N, Chen X, Antoine DJ, Li
Y, Dai G, Köhler UA, Zong WX, Waguri S, Werner S, et al: HMGB1
promotes ductular reaction and tumorigenesis in autophagy-deficient
livers. J Clin Invest. 129:21632019. View Article : Google Scholar
|
|
71
|
Nakka VP, Prakash-Babu P and Vemuganti R:
Crosstalk between endoplasmic reticulum stress, oxidative stress,
and autophagy: Potential therapeutic targets for acute CNS
injuries. Mol Neurobiol. 53:532–544. 2016. View Article : Google Scholar : PubMed/NCBI
|