Introduction
One of the common health issues both in developed
and developing countries around the world is the increasing
epidemic of obesity-related chronic diseases, including
non-alcoholic fatty liver disease (NAFLD), type 2 diabetes (T2D)
and cardiovascular disease, which results in high costs in terms of
health-care expenditure and quality of life (1–3). In
the Western world and Asia, the prevalence of NAFLD is estimated as
15–30% of the population (4,5).
NAFLD is described as a presence of non-alcoholic hepatic steatosis
and liver tissue inflammation (6).
Patients with NAFLD have an increase in liver fat (triglyceride;
TG) accumulation and hepatic insulin resistance (7). In addition, NAFLD has been associated
with the increased risks of T2D, cardiovascular disease and
metabolic syndrome (1). However,
the exact molecular mechanisms of the regulation remain unclear and
effective drugs for the treatment of NAFLD have yet to be
found.
Trimetazidine (TMZ) is known to protect against
myocardial ischemia injury, which inhibits fatty acid β-oxidation
and enhances glucose oxidation to optimize cardiac energy
metabolism (8,9). TMZ has been used in the treatment of
coronary heart disease in China. In previous years, Chinese
researchers have found that TMZ has a synergistic hypolipidemic
effect with statins (10,11). However, the detailed molecular
mechanism of TMZ remains unclear.
AMP-activated protein kinase (AMPK), a crucial
cellular senor of energy homeostasis, can influence metabolism and
energy balance at the whole body level by regulating metabolic and
non-metabolic processes (12,13).
The AMPK signal pathway is also closely related to NAFLD (14,15).
As AMPK activity is reduced by inflammation, obesity and diabetes,
increasing AMPK activity has been regarded as a viable therapeutic
strategy to improve NAFLD (16).
Some studies suggest that the enhanced AMPK signaling may attenuate
liver lipid accumulation and hepatic fibrosis in mice (17,18).
AMPK and AKT signaling pathways can increase forkhead box O1
(FOXO1) expression in a number of tissues and cells (19,20).
The carbohydrate-responsive element-binding protein
(ChREBP) has a crucial role in the regulation of hepatic
lipogenesis, hepatic steatosis and insulin resistance (21,22).
ChREBP is a major mediator in glycolysis and lipogenesis via
binding to the promoter region of lipogenic genes.
The present study aimed to investigate the effect of
TMZ on NAFLD induced by a high-fat diet (HFD). It demonstrated that
administration of TMZ alleviated hepatic steatosis and insulin
resistance in HFD mice. The curative effects could be achieved by
attenuating hepatic lipogenesis via the AMPK-ChREBP signaling
pathway.
Materials and methods
Animal experiments and study
design
The animal experiments were approved by the
Institutional Animal Care and Research Advisory Committee at
Guizhou Medicine University, Guiyang, China. A total of 18
8-week-old (weight, 18–20 g) male C57BL/6 mice (Experimental Animal
Center of Hubei) were randomly divided and fed with a normal chow
diet containing 4% fat (NCD group, n=6) or a HFD containing 60% fat
and 2% cholesterol (HFD group, n=6) (23), or a HFD plus daily infused TMZ (40
mg/kg) by gavage (HFD + TMZ group, n=6) for 8 weeks before
sacrifice. All mice were housed in a temperature-controlled
environment in 12/12 h light/dark cycles with free access to food
and water.
Cell culture and treatments
HepG2 cells were maintained in Dulbecco's modified
Eagle's medium (DMEM, Sigma-Aldrich; Merck KGaA) with 10% fetal
bovine serum (FBS, Beijing Transgen Biotech Co., Ltd.), in an
incubator kept at 37°C with 5% CO2. At 70–80%
confluence, the cells were incubated in serum-free medium or
serum-free medium containing 0.25 mM palmitic acid (PA;
Sigma-Aldrich; Merck KGaA), or 10 µM TMZ (Laboratoires Servier) or
1 µM Compound C (MedChemExpress) for another 24 h.
Histological analysis
The livers were fixed in 4% formaldehyde at room
temperature and embedded in paraffin, sections were at a thickness
of 5 µm. All staining was performed at room temperature. The
hepatic morphology was detected by haematoxylin and eosin (H&E)
staining and Oil Red O (ORO) staining. Staining with Sirius red was
performed to visualize liver tissue fibrosis.
Biochemical parameters
TG and total cholesterol (TC) levels in plasma or
HepG2 cells were determined by GRO-PAP analysis kits (Nanjing
Jiancheng Bioengineering Institute). TG and TC in liver were
measured on an AEROSET Clinical Chemistry System (Abbott
Laboratories).
Small interfering RNA (siRNA)
siRNA targeting FOXO1 (NM_002015) and the negative
control (NC) were purchased from Guangzhou RiboBio Co., Ltd. siRNA
transfections were performed with Lipofectamine® 2000
(Invitrogen, Thermo Fisher Scientific, Inc.) at a final
concentration of 100 nM. After 48 h cells were used for subsequent
experiments.
RNA extraction and reverse
transcription-quantitative (RT-q) PCR
Total RNA was isolated using a TRIzol reagent kit
(Invitrogen, Thermo Fisher Scientific, Inc.) and quantified by qPCR
using a SYBR Premix Ex Taq II mix (Takara Bio, Inc.), according to
the manufacturer's instructions. The quantitative PCR was performed
in an ABI 7900 Fast Real-Time PCR System (Applied Biosystems Inc.),
following the manufacturer's protocols. mRNA levels were quantified
using the 2−ΔΔCq method (24) and normalized to the internal gene
β-actin, primers are listed in Table
I.
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Gene name | Genera | Primer sequences
(5′-3′) |
|---|
| ChREBPa | Mouse | F:
CGACACTCACCCACCTCTTC |
|
|
| R:
TTGTTCAGCCGGATCTTGTC |
| ACC | Mouse | F:
CTTCGGGGTGGTTCTTGGGTTGTG |
|
|
| R:
CCTGCATCCGGCCTGGTGTG |
| FASN | Mouse | F:
ATCCTGGAACGAGAACACGATCT |
|
|
| R:
AGAGACGTGTCACTCCTGGACTT |
| TGF-β | Mouse | F:
GCAGTGGCTGAACCAAGGA |
|
|
| R:
AGCAGTGAGCGCTGAATCG |
| Collagen IV | Mouse | F:
CAACCTGGACGCCATCAAG |
|
|
| R:
CAGACGGCTGAGTAGGGAACA |
| ACTB | Mouse | F:
CAGCCTTCCTTCTTGGGTAT |
|
|
| R:
TGGCATAGAGGTCTTTACGG |
| ChREBP | Human | F:
AGTGCTTGAGCCTGGCCTAC |
|
|
| R:
TTGTTCAGGCGGATCTTGTC |
| LXRα | Human | F:
AGAAGAACAGATCCGCCTGAAG |
|
|
| R:
GGCAAGGATGTGGCATGAG |
| SREBP1 | Human | F:
GGGCGTGAAGACTGAGGTG |
|
|
| R:
CTGCTGCCAAGGGACAAG |
| TRAK2 | Human | F:
ATGAGTCAATCCCAGAATGCAA |
|
|
| R:
TCAGTCCTCCTTCAGGACACC |
| ABCA1 | Human | F:
TACAGCCAGAAAGACACCAG |
|
|
| R:
CACAGTAGACTTTGGGAGAG |
| ABCG1 | Human | F:
TCGGTGGATGAGGTGGTG |
|
|
| R:
TGGGCTTCCGTGAGGTTA |
| PKLR | Human | F:
GTGGACATCGTCTTTGCCT |
|
|
| R:
TCTTGATGCCGTGTCCTTC |
| FASN | Human | F:
CGCTCGGCATGGCTATCT |
|
|
| R:
CTCGTTGAAGAACGCATCCA |
| ACC | Human | F:
GGATGGTGTTCACTCGGTAATAG |
|
|
| R:
GGGTGATATGTGCTGCGTCAT |
| ACTB | Human | F:
TGGATCAGCAAGCAGGAGTATG |
|
|
| R:
GCATTTGCGGTGGACGAT |
Western blot analysis
Protein was extracted using RIPA lysis buffer (Wuhan
Boster Biological Technology, Ltd.). Protein (20 µg) was separated
by 8% SDS-PAGE and transferred on to PVDF membrane. The membranes
were incubated with antibodies of ChREBP (1:1,000, Abcam, cat. no.
ab92809), fatty acid synthase (FASN; 1:500, Wuhan Boster Biological
Technology, Ltd., cat. no. PB0909), acetyl-CoA carboxylase (ACC;
1:500, Wuhan Boster Biological Technology, Ltd., cat. no. BM4414),
AMPK (1:1,000, Cell Signaling Technology, Inc., cat. no. 2532),
phosphorylated (p)-AMPK (Thr172; 1:1,000, Cell Signaling
Technology, Inc., cat. no. 2535), AKT (1:1,000, Cell Signaling
Technology, Inc., cat. no. 9272), p-AKT (Ser473; 1:1,000, Cell
Signaling Technology, Inc., cat. no. 9271), GAPDH (1:2,000, Wuhan
Boster Biological Technology, Ltd., cat. no. BM1623) and FOXO1
(1:500, Wuhan Boster Biological Technology, Ltd., BM4249) overnight
at 4°C. Then, membranes were incubated with rabbit anti-mouse lgG
(cat. no. BA1048) and goat anti-rabbit lgG antibodies (cat. no.
BA1039; both from Wuhan Boster Biological Technology, Ltd.).
Finally, the results were detected by ECL reagents (Beyotime
Institute of Biotechnology) and semi-quantified by densitometry
using the Tanon 5200 automatic chemiluminescent imaging system
(Tanon Science and Technology Co., Ltd).
Cholesterol efflux
The HepG2 cells were subjected to DMEM with 5 nM
22-NBD cholesterol (Invitrogen; Thermo Fisher Scientific, Inc.) for
6 h. The cholesterol efflux was measured following 2-h incubation
with apolipoprotein (apo)A-I (25 mg/ml) or high-density lipoprotein
(HDL; Guangzhou Yiyuan Biotech Co., Ltd.) particles isolated from
human plasma (50 mg/ml).
Luciferase assay
Bioinformatics analyses (http:///jaspar.genereg.net/) demonstrated that there
were a number of FOXO1 binding sites in the ChREBP promoter region.
The 2,256 bp ChREBP promoter sequence was amplified from human DNA
by PCR, with Mlu1 or HindIII restriction enzyme
cutting site at 5′ and 3′ end, respectively. The primers used were
5′-CTCTGAACCCATCTTTGAGGC-3′ and 5′-AGTCTGTGTCCGAGTCCGAgT-3′. PCR
products were digested and inserted into a pGL-3 luciferase
reporter vector (Beijing Augct DNA-Syn Biotechnology Co., Ltd.).
293T cells (1×106 cells per well) in 24-well plates were
co-transfected with 400 ng of plasmid, 50 ng of Renilla
luciferase plasmid and 100 nM si-FOXO1 or 100 nM si-NC, using
Lipofectamine® 2000 (Invitrogen, Thermo Fisher
Scientific, Inc.). Cells were incubated at 37°C and 5%
CO2. After 48 h, the cells were washed using cold PBS
and lysed with passive lysis buffer (Promega Corporation).
Luciferase activities were then measured by a luminometer (Sirius;
Berthold Detection Systems GmbH) according to the manufacturer's
instructions. Luciferase expression levels were adjusted with
reference to Renilla luciferase activity. Six independent
experiments were performed for each reporter vector.
Statistical analysis
Statistical analyses were performed with SPSS 24.0
(IBM Corp.). Quantitative variables were expressed as mean ±
standard deviation of n experiments. Comparisons of quantitative
variables between two groups were performed by the paired or
unpaired Student's t-test. One-way ANOVA was used to compare
multiple variables followed by a Tukey's post hoc test. The
differences among three or more groups were analyzed by a multiple
comparisons test. All probability values were two-sided and
P<0.05 was considered to indicate a statistically significant
difference.
Results
TMZ attenuates symptoms in HFD
mice
Metabolic disorder was induced in mice with
continuous HFD exhibiting body weight gain, hyperlipidemia,
hyperglycemia and glucose intolerance compared with NCD mice
(Fig. 1). To clarify the potential
effect of TMZ on HFD-mediated metabolic disorder, the
administration of TMZ by gavage was used. The TMZ significantly
decreased the liver weight of HFD + TMZ mice compared with the HFD
mice, but it did not change the whole body weight as shown in
Fig. 1A and B. HFD + TMZ mice
exhibited 25–30% lower plasma TG/TC levels than those of HFD mice
(Fig. 1C and D) and improved
glucose tolerance compared with the HFD mice (Fig. 1E and F). All these data led to the
hypothesis that TMZ might ameliorate hepatic lipid metabolism in
HFD mice.
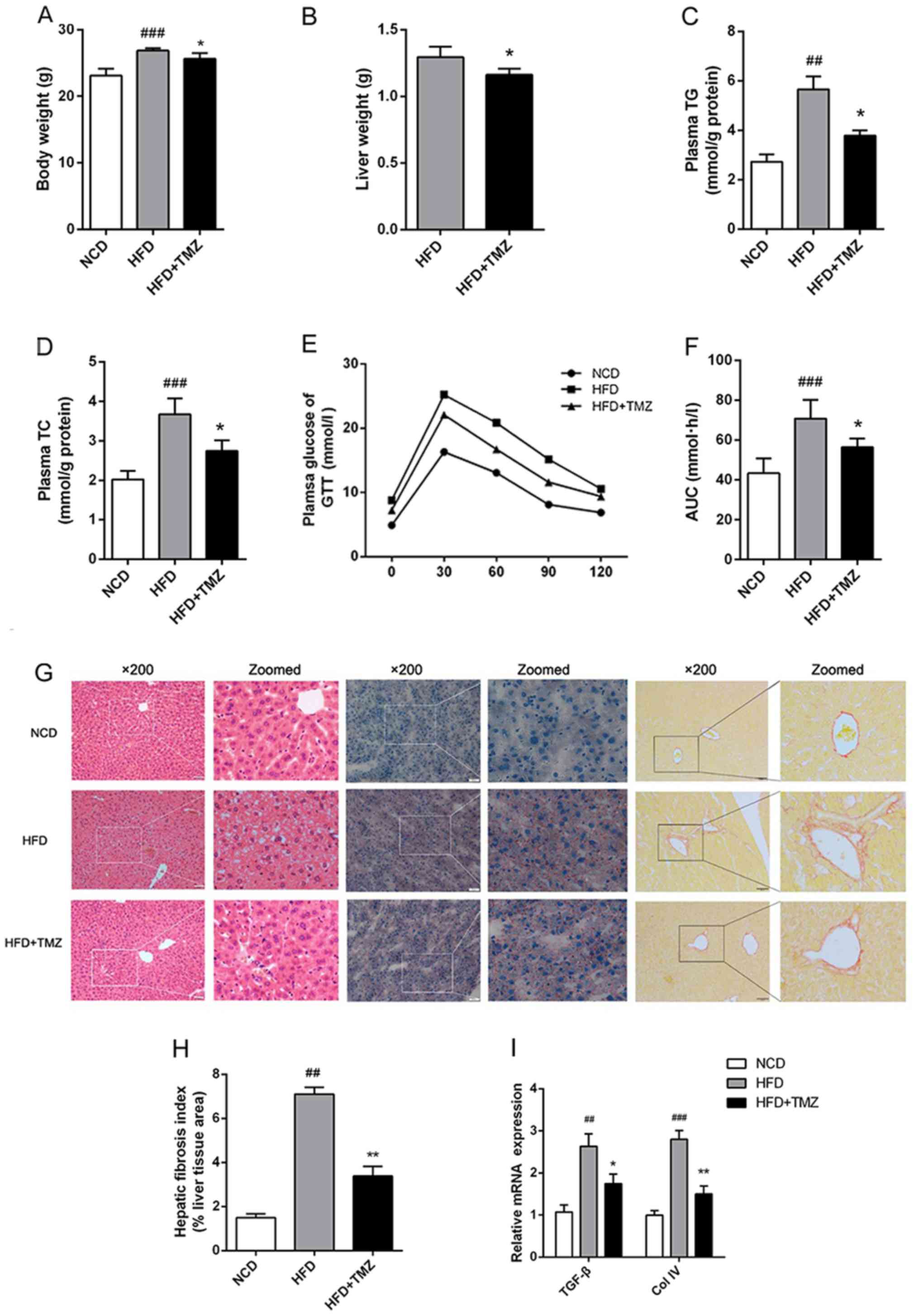 | Figure 1.TMZ improved metabolic
characteristics and hepatic fibrosis in treated mice. The C57BL/6J
mice were fed with a NCD, HFD or HFD + daily infused TMZ (40 mg/kg)
by gavage (HFD + TMZ) for 8 weeks (n=6). (A and B) Body weight and
liver weight are shown. (C and D) Mice plasma TG and TC were
detected by the GRO-PAP method. (E and F) After an overnight, fast
and serial tail blood glucose was measured before and after glucose
administration (2 g/kg, intraperitoneal injection). (G)
Representative images of H&E staining, Oil Red O staining and
Sirius red staining of mouse livers (magnification, ×200). (H)
Hepatic fibrosis index. (I) Relative mRNA levels of TGF-β and Col
IV. (##P<0.01 vs. NCD group, ###P<0.001
vs. NCD group, *P<0.05 vs. HFD group, **P<0.05 vs. HFD
group). TMZ, trimetazidine; NCD, normal chow diet; HFD, high fat
diet; TG, triglyceride; TC, total cholesterol; GTT, glucose
tolerance test; TGF-β, transforming growth factor-β; Col IV,
Collagen I. |
TMZ ameliorates hepatic fibrosis in
vivo
To investigate the hypothesis above, the liver was
investigated. As shown in Fig. 1G,
H&E and ORO staining demonstrated excessive lipid accumulation
in HFD mice liver compared with the NCD mice liver. TMZ markedly
decreased hepatocyte bullous steatosis and hepatic fibrosis in HFD
+ TMZ mice liver compared with the HFD mice (Fig. 1G and H). In addition, TMZ decreased
the expression of transforming growth factor-β and Collagen IV in
livers of HFD + TMZ mice compared with those of the HFD + TMZ mice,
which also suggested that TMZ protected hepatic fibrosis in the
livers of HFD + TMZ mice compared with those of the HFD + TMZ mice
(Fig. 1I).
TMZ ameliorates hepatic lipid
synthesis and suppressed HFD-related activation of AMPK in
vivo
The hepatic TG/TC level in HFD + TMZ mice was
markedly decreased compared with HFD mice (Fig. 2A and B). Notably, TMZ treatment
demonstrated a lower mRNA and protein expression level of ChREBP,
which was a critical transcription factor in hepatic lipogenesis in
the liver of HFD + TMZ mice compared with those of the HFD mice
(Fig. 2C and D). As expected, TMZ
had the same effects on mRNA expression of ACC and FASN in livers
of HFD + TMZ mice compared with those of the HFD mice. Furthermore,
HFD mice demonstrated decreased phosphorylation of AMPK (Thr172)
compared with NCD mice (Fig. 2D).
TMZ treatment significantly reversed the reduced phosphorylation of
AMPK.
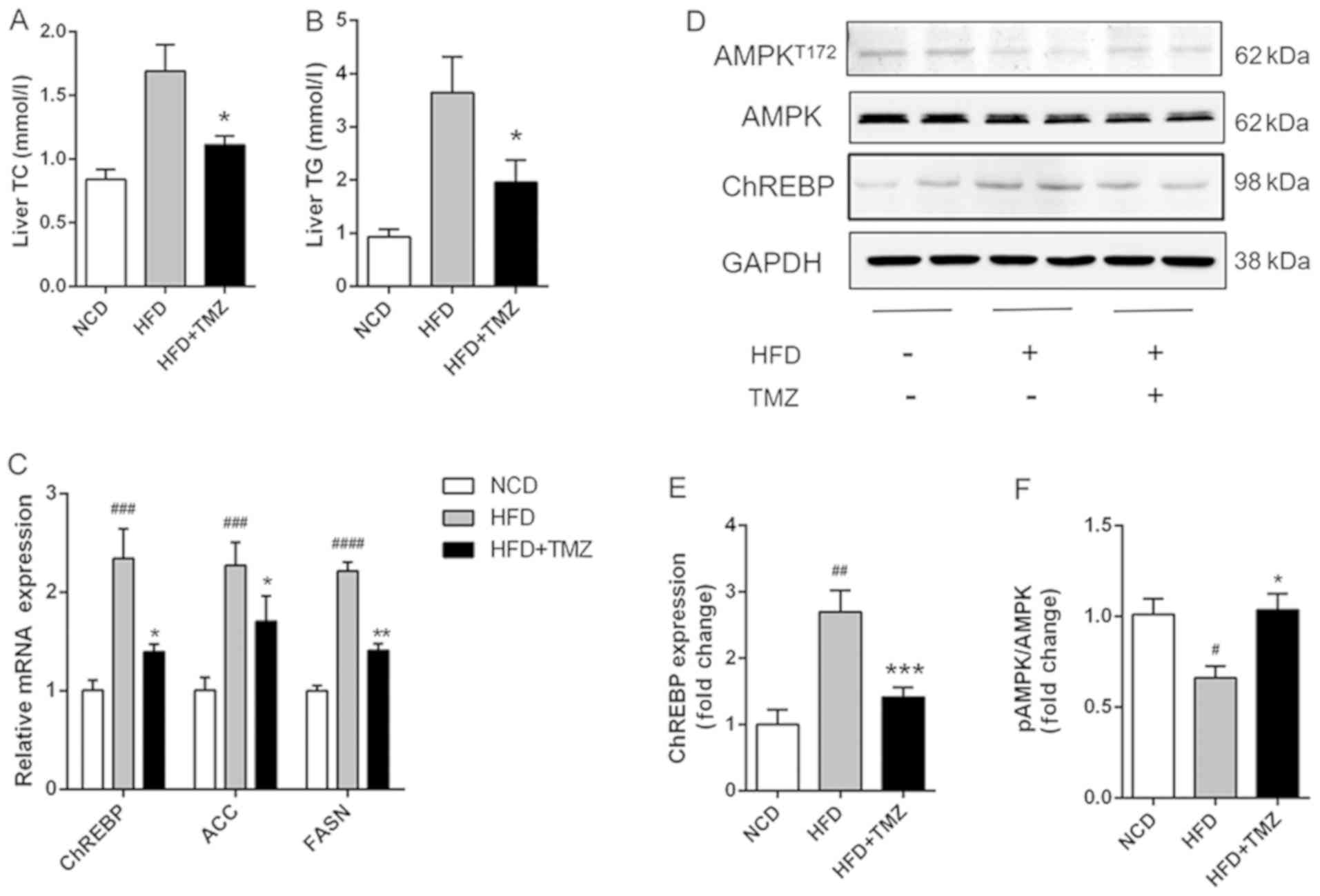 | Figure 2.TMZ attenuated hepatic lipid
accumulation and lipogenic gene expression in vivo. (A and
B) Hepatic TG and TC in treated mice. (C) Relative mRNA levels of
ChREBP, ACC and FASN in liver of treated mice. (D-F) Protein levels
of ChREBP and AMPK in liver of treated mice. (#P<0.05
vs. NCD group, ##P<0.01 vs. NCD group,
###P<0.001 vs. NCD group, ####P<0.0001
vs. NCD group, *P<0.05 vs. HFD group, **P<0.05 vs. HFD group,
***P<0.05 vs. HFD group.) TMZ, trimetazidine; NCD, normal chow
diet; HFD, high fat diet; TG, triglyceride; TC, total cholesterol;
ChREBP, carbohydrate-responsive element-binding protein; ACC,
acetyl-CoA carboxylase; FASN, fatty acid synthase; AMPK,
AMP-activated protein kinase. |
TMZ reduces the lipogenic gene
expression in vitro
To verify the protective action of TMZ on NAFLD
in vitro, the palmitate-treated liver cancer cell line HepG2
was used to confirm the animal experiment results. As demonstrated
in Fig. 3A and B, ORO staining
demonstrated that TMZ reduced excessive lipid accumulation, as
before. There was no evidence to show that TMZ effected cholesterol
efflux to apoA-I and HDL in liver cancer cell lines (Fig. 3C). A number of fat
synthesis-related genes and cholesterol efflux-related genes were
detected in liver cancer cells treated with TMZ and palmitate. TMZ
only decreased the ChREBP mRNA expression. Liver X receptor (LXR)α,
sterol regulatory element-binding 1 (SREBP1), CD36, SCARB1,
trafficking kinesin protein 2, ATP-binding cassette subfamily A
member 1 and ATP-binding cassette subfamily G member 1 demonstrated
no significant difference in expression (Fig. 3D). In addition, TMZ also
significantly decreased the mRNA and protein expression of key
lipogenic genes, such as pyruvate kinase L/R, ACC and FASN
(Fig. 3E and G).
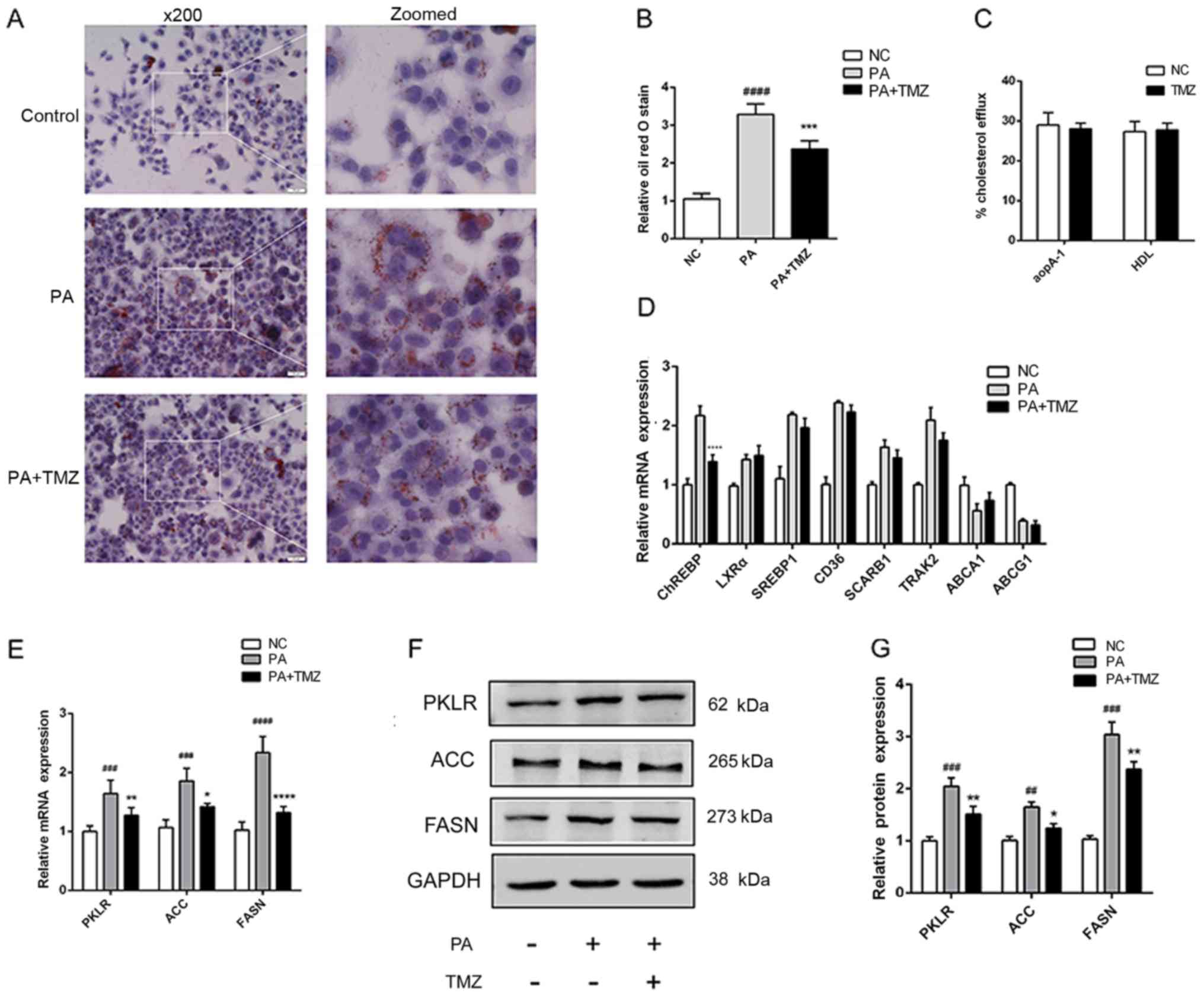 | Figure 3.TMZ attenuated hepatic lipid
accumulation and lipogenic gene in vitro. (A)
Histopathological examination of palmitate-treated HepG2 cells with
Oil Red O staining (×200). (B) Hepatic fibrosis index. (C)
Cholesterol efflux to apoA-I and HDL. (D) Relative mRNA levels of
key transcription factors in lipogenesis and other cholesterol
efflux regulatory protein. (E) Relative mRNA and (F and G) protein
levels of PKLR, ACC and FASN in HepG2 cells.
(##P<0.01 vs. NC group, ###P<0.001 vs.
NC group, ####P<0.0001 vs. NC group. *P<0.05 vs.
PA group, **P<0.01 vs. PA group, ****P<0.0001 vs. PA group).
TMZ, trimetazidine; apoA-I, apolipoprotein A1; HDL, high-density
lipoprotein; PKLR, pyruvate kinase L/R; ACC, acetyl-CoA
carboxylase; FASN, fatty acid synthase; PA, palmitic acid; NC,
negative control. |
TMZ alleviates hepatic lipogenesis via
AMPK-FOXO1 pathway
It was further investigated how TMZ affected fatty
acid and fat synthesis in the liver cancer cell line. The level of
TG and cholesterol in HepG2 cells are shown in Fig. 4A and B. The reduced TG and
cholesterol level in TMZ-treated liver cancer cells were both
increased following intervention with the AMPK inhibitor Compound
C. It was then established that TMZ increased the PA-related
activation of AMPK and suppressed the PA-related activation of AKT
in vitro. All these effects were reversed when the Compound C was
added (Fig. 4C-E). The expression
of FOXO1 was associated with the AMPK/AKT pathway (Fig. 4F). Nevertheless, the relationship
between the AMPK/AKT pathway and ChREBP-mediated lipid accumulation
remains to be determined. As shown in Fig. 4G and H, suppression of FOXO1
decreased the expression of ChREBP in the HepG2 and TMZ-PA-treated
HepG2 cells. Furthermore, the suppression of FOXO1 decreased the
luciferase reporter activity of the vector in 293T cells (Fig. 4I). These results suggested that TMZ
suppressed hepatic lipogenesis by regulating ChREBP expression via
AMPK-FOXO1 pathway.
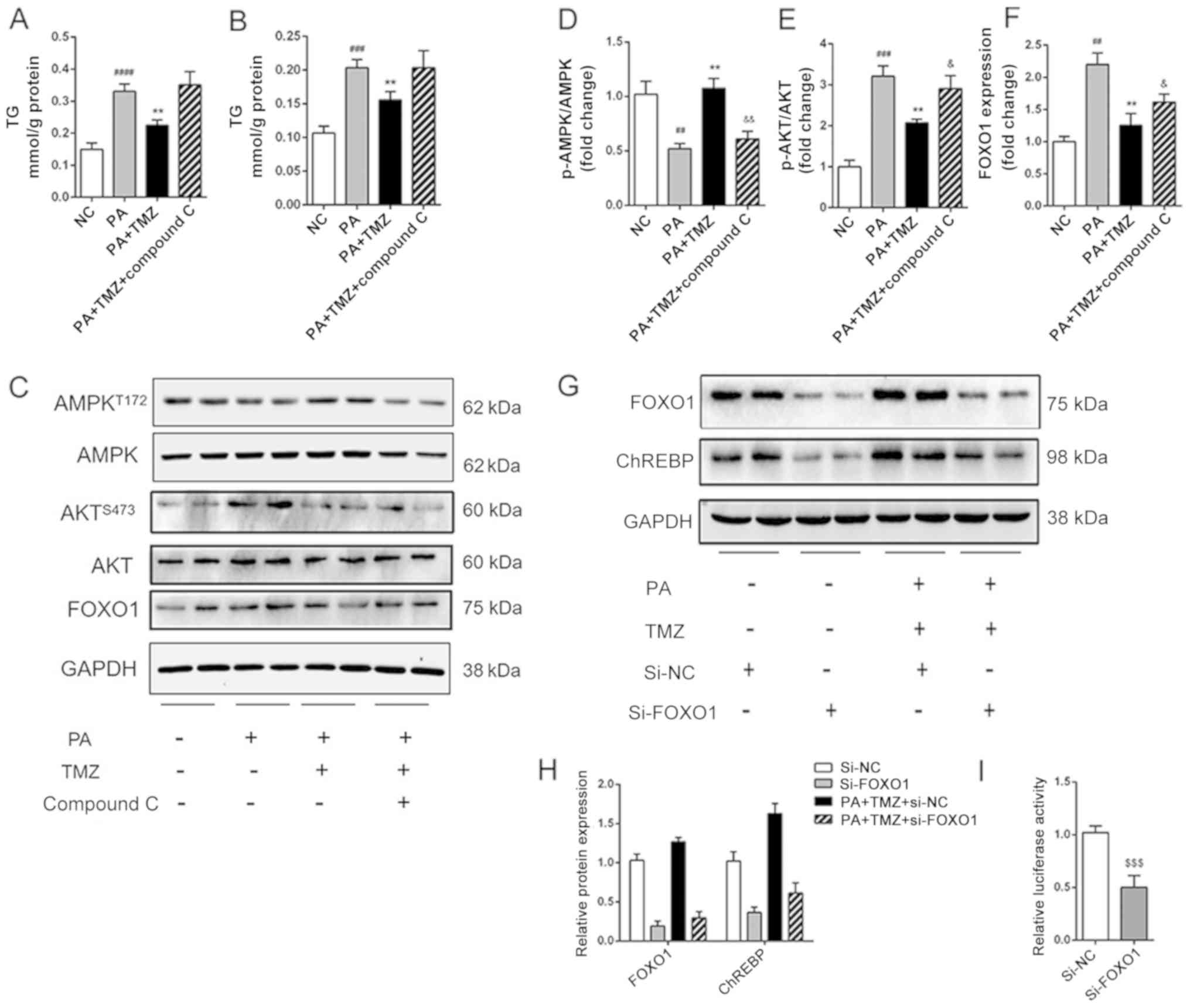 | Figure 4.TMZ reduced hepatic de novo
lipogenesis by activating AMPK-FOXO1-ChREBP signaling pathway in
vitro. (A and B) TG and TC accumulation in HepG2 cells. (C-F)
Protein levels of p-AMPK, AMPK, p-AKT, AKT and FOXO1 in HepG2
cells. (G and H) Protein levels of FOXO1 and ChREBP in
si-FOXO1-treated HepG2 cells. (I) Effects of si-FOXO1 on ChREBP
promoter constructs activity in HepG2 cells.
(##P<0.01 vs. NC group, ###P<0.001 vs.
NC group, ####P<0.0001 vs. NC group, **P<0.01 vs.
HFD group, &P<0.05 vs. PA + TMZ group
&&P<0.01 vs. PA + TMZ group,
$$$P<0.001). TMZ, trimetazidine; FOXO1, forkhead box
O1; chREBP, carbohydrate-responsive element-binding protein; TG,
triglyceride; TC, total cholesterol; p-, phosphorylated; si-, small
interfering RNA; NC, negative control; HFD, high fat diet; PA,
palmitic acid; AMPK, AMP-activated protein kinase; NCD, normal chow
diet. |
Discussion
The present study conducted a mouse model mimicking
human NAFLD with continuous HFD. The HFD mice demonstrated obesity,
hyperlipidemia, hyperglycemia and glucose intolerance. The TMZ
treatment alleviated the clinical phenotypes and pathophysiological
changes, including liver weight gain, hyperlipidemia, glucose
intolerance and hepatic steatosis. Furthermore, TMZ also
demonstrated the same effects in palmitate-treated HepG2 cells.
These effects were associated with the reduction of hepatic
lipogenesis. TMZ restrained the downregulation of phosphorylation
of AMPK (Thr172) and AKT (Ser473) led by PA, downregulated ChREBP
and ChREBP downstream genes and reduced the synthesis of lipids. In
addition, the AMPK/AKT pathway inhibited FOXO1 expression.
Furthermore, the suppression of FOXO1 was involved in the
downregulation via decrease in the transcriptional activity of
ChREBP promoter region.
The deposition of lipid droplets in the cells of the
liver (that is, hepatic steatosis) is the hallmark of NAFLD. This
deposition is strongly associated with the increasing fat
synthesis. Up to 30% of patients with NAFLD develop non-alcoholic
steatohepatitis (NASH), which depends on the presence of
inflammation, oxidative stress and injury to liver cells, and a
patient with NASH may progress to developing liver cancer (25). In human and mouse models, NAFLD
increases the risk of liver cancer (26). It is important to understand the
mechanisms of NAFLD and develop new intervention strategies
(27).
TMZ has already been widely used in the treatment of
stable angina pectoris, where it mobilizes myocardial fatty acid
oxidation and glucose metabolism (11). A previous study suggests that TMZ
can change energy production from fatty acid oxidation to glucose
oxidation by inhibiting the enzymatic activity of long-chain
3-ketoacyl CoA thiolase and increasing the activity of pyruvate
dehydrogenase to facilitate the transformation of pyruvate to
acetyl Co-A (28). Chen et
al (29) demonstrate that
pressure overload-induced early cardiac energy dysfunction is
ameliorated with the treatment of TMZ at 40 mg/kg/day. Although TMZ
has been regarded as a metabolic medicine, with a synergistic
hypolipidemic effect with statins, the effect of TMZ on lipid
metabolism remains to be elucidated.
The present study, for the first time to the best of
the authors' knowledge, established the relationship between TMZ
and NAFLD. HFD mouse model or palmitate-treated cell model
demonstrated TG accumulation. ChREBP plays important roles in
modulating the process of glycolysis and lipogenesis (30). Some key transcription factors,
including PPAR, LXR, RXR, SREBP1 and ChREBP, link the molecular and
metabolic bases of fatty acid regulation to NAFLD (31). ChREBP expression is positively
related to the degree of hepatic steatosis in human and mice with
NAFLD (32). Our previous study
also confirmed the decreased expression of ChREBP attenuated lipid
synthesis and accumulation (33).
As expected, the HFD mouse demonstrated increased ChREBP-mediated
fat syntheses. In addition, the model also demonstrated decreased
phosphorylation of AMPK (Thr172). The TG accumulation and liver
steatosis were alleviated with the addition of TMZ in a 40
mg/kg/day dose in vivo and 10 µM in vitro as in the
experimental design of Chen et al (29). The reduced phosphorylation of AMPK
was increased by TMZ. The ChREBP expression was also influenced by
the drug, but the other vital transcription factors in lipogenesis
and other cholesterol efflux regulatory protein expression were not
affected. The present study suggested that the ChREBP gene and the
AMPK pathway might be involved in this novel role for TMZ.
The AMPK inhibitor, Compound C, was used to verify
the connection between ChREBP and AMPK in vitro. As
expected, Compound C inhibited the phosphorylation of AMPK and AKT.
The inhibitor also alleviated the protective effect of TMZ in
palmitate-treated HepG2 cells. These observations corroborated the
hypothesis that TMZ ameliorates hepatic steatosis via the AMPK/AKT
pathway. Furthermore, the present study reported that the AMPK/AKT
pathway altered FOXO1 expression, which plays an important role in
the processes in NAFLD (34). The
transcription factor FOXO1 is the main target of insulin signaling,
and contributes to the regulation of lipid and glucose metabolism
(35). Bioinformatics analyses
(http:///jaspar.genereg.net/)
demonstrated that there were a number of FOXO1 binding sites in the
ChREBP promoter region. As expected, the suppression of FOXO1
resulted in a reduction of ChREBP protein expression and
post-transcriptional activity. The present study confirmed the
relationship between the AMPK/AKT pathway and NAFLD, and
demonstrated that TMZ ameliorated ChREBP-induced de novo
lipogenesis by activating AMPK-FOXO1 signaling.
The present study has several limitations with a few
factors uninterpreted. As mentioned above, the experiments were
designed to test the role of TMZ in disease. Thus the role of TMZ
in a control group was not tested, which led to the lack of a sham
+ TMZ group. It was observed that the liver weight of NAFLD was
significantly attenuated by TMZ, but due to some unavoidable
factors, the original data of normal liver weight was lost. The
function of the AMPK pathway was also not confirmed in vivo.
TMZ treatment on NAFLD should have been confirmed by combination
therapy with the Compound C or AMPK gene deficiency mice. Another
limitation was the mechanism of the regulation of AMPK by TMZ,
which requires further experiments. Furthermore, the cell line used
(HepG2) is a problematic cell line, it was originally identified as
a hepatocellular carcinoma cell line, however it has since been
shown to be from a hepatoblastoma (PubMed =19751877).
In summary, the present study reveals a novel role
for TMZ: Protecting hepatic steatosis in NAFLD. The protective
action of TMZ depends on its ability to reduce ChREBP-mediated
de novo lipogenesis via the AMPK/AKT/FOXO1 signaling
pathway. The findings of the present study show that TMZ may
provide potentially therapeutic strategies for NAFLD.
Acknowledgements
The authors would like to thank Dr Jin Li (Tsinghua
University, Beijing, China) for his English editing of the
manuscript.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 31760294), the Science and
Technology Fund of Guizhou Provincial Health Department [grant nos.
31760294 qiankehejichu (2016) 1120, qiankehejichu (2018) 1137)],
and the Fund of Guiyang Science and Technology department [grant
nos. (2017) 5–14, (2017) 30–10], the Health and Family Planning
Commission of Guizhou Province (grant no. gzwjkj2017-1-016), the
Fund of Qiannan Science and Technology department [grant no.
qiannankeheshezi (2017) 73].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ participated in the research design, performed
experiments, conducted statistical analyses and drafted the
manuscript. CL performed experiments, performed statistical
analyses and drafted the manuscript. CW performed the animal
experiments. HZ performed statistical analyses. SL performed
statistical analyses and edited the final manuscript. XDL conceived
the study and edited the final manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Research Advisory Committee at
Guizhou Medicine University, Guiyang, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yki-Järvinen H: Non-alcoholic fatty liver
disease as a cause and a consequence of metabolic syndrome. Lancet
Diabetes Endocrinol. 2:901–910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malik VS, Willett WC and Hu FB: Global
obesity: Trends, risk factors and policy implications. Nat Rev
Endocrinol. 9:13–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alberti KGMM, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM and
Smith SC Jr; International Diabetes Federation Task Force on
Epidemiology and Prevention; National Heart, Lung, and Blood
Institute; American Heart Association; World Heart Federation;
International Atherosclerosis Society; International Association
for the Study of Obesity, : Harmonizing the metabolic syndrome: A
joint interim statement of the International Diabetes Federation
Task Force on Epidemiology and Prevention; National Heart, Lung,
and Blood Institute; American Heart Association; World Heart
Federation; International Atherosclerosis Society; and
International Association for the Study of Obesity. Circulation.
120:1640–1645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong VW-S: Nonalcoholic fatty liver
disease in Asia: A story of growth. J Gastroenterol Hepatol.
28:18–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazo M, Hernaez R, Eberhardt MS, Bonekamp
S, Kamel I, Guallar E, Koteish A, Brancati FL and Clark JM:
Prevalence of nonalcoholic fatty liver disease in the United
States: The Third National Health and Nutrition Examination Survey,
1988–1994. Am J Epidemiol. 178:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marengo A, Jouness RIK and Bugianesi E:
Progression and natural history of nonalcoholic fatty liver disease
in adults. Clin Liver Dis. 20:313–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chalasani N, Younossi Z, Lavine JE, Diehl
AM, Brunt EM, Cusi K, Charlton M and Sanyal AJ; American
Gastroenterological Association; American Association for the Study
of Liver Diseases; American College of Gastroenterologyh, : The
diagnosis and management of non-alcoholic fatty liver disease:
Practice guideline by the American Gastroenterological Association,
American Association for the Study of Liver Diseases, and American
College of Gastroenterology. Gastroenterology. 142:1592–1609. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaswal JS, Keung W, Wang W, Ussher JR and
Lopaschuk GD: Targeting fatty acid and carbohydrate oxidation--a
novel therapeutic intervention in the ischemic and failing heart.
Biochim Biophys Acta. 1813:1333–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopaschuk GD, Barr R, Thomas PD and Dyck
JRB: Beneficial effects of trimetazidine in ex vivo working
ischemic hearts are due to a stimulation of glucose oxidation
secondary to inhibition of long-chain 3-ketoacyl coenzyme a
thiolase. Circ Res. 93:e33–e37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H and Xu Y: Clinical trial of
trimetazidine combined with atorvastatin calcium in the treatment
of coronary heart disease and angina pectoris with dyslipidemia.
Zhongguo Lin Chuang Yao Li Xue Za Zhi. 32:966–968.
2016.(Chinese).
|
|
11
|
Zhou X and Huang H: Therapeutic effect of
atorvastatin combined trimetazidine on blood lipids, inflammatory
factors and cardiac function in patients with coronary heart
disease. Chin J Cardiovasc Rehabil Med. 25:276–279.
2016.(Chinese).
|
|
12
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hardie DG: AMPK: Positive and negative
regulation, and its role in whole-body energy homeostasis. Curr
Opin Cell Biol. 33:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ideta T, Shirakami Y, Miyazaki T, Kochi T,
Sakai H, Moriwaki H and Shimizu M: The dipeptidyl peptidase-4
inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK
activation in non-alcoholic fatty liver disease model mice. Int J
Mol Sci. 16:29207–29218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng S, Liang S, Liu Q, Deng Z, Zhang Y,
Du J, Zhang Y, Li S, Cheng B and Ling C: Diosgenin prevents
high-fat diet-induced rat non-alcoholic fatty liver disease through
the AMPK and LXR signaling pathways. Int J Mol Med. 41:1089–1095.
2018.PubMed/NCBI
|
|
16
|
Smith BK, Marcinko K, Desjardins EM, Lally
JS, Ford RJ and Steinberg GR: Treatment of nonalcoholic fatty liver
disease: Role of AMPK. Am J Physiol Endocrinol Metab.
311:E730–E740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZQ, Zhang XH, Yu Y, Tipton RC, Raskin
I, Ribnicky D, Johnson W and Cefalu WT: Artemisia scoparia extract
attenuates non-alcoholic fatty liver disease in diet-induced
obesity mice by enhancing hepatic insulin and AMPK signaling
independently of FGF21 pathway. Metabolism. 62:1239–1249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Woods A, Williams JR, Muckett PJ, Mayer
FV, Liljevald M, Bohlooly-Y M and Carling D: Liver-specific
activation of AMPK prevents steatosis on a high-fructose diet. Cell
Rep. 18:3043–3051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Postic C, Dentin R, Denechaud P-D and
Girard J: ChREBP, a transcriptional regulator of glucose and lipid
metabolism. Annu Rev Nutr. 27:179–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Viscarra J, Kim S-J and Sul HS:
Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell
Biol. 16:678–689. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saline M, Badertscher L, Wolter M, Lau R,
Gunnarsson A, Jacso T, Norris T, Ottmann C and Snijder A: AMPK and
AKT protein kinases hierarchically phosphorylate the N-terminus of
the FOXO1 transcription factor, modulating interactions with 14-3-3
proteins. J Biol Chem. 294(35): 13106–13116. 2019.pii:
jbc.RA119.008649. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bandyopadhyay GK, Lu M, Avolio E, Siddiqui
JA, Gayen JR, Wollam J, Vu CU, Chi NW, O'Connor DT and Mahata SK:
Pancreastatin-dependent inflammatory signaling mediates
obesity-induced insulin resistance. Diabetes. 64:104–116. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Zhang C, Luo X, Wang P, Zhou W,
Zhong S, Xie Y, Jiang Y, Yang P, Tang R, et al: CD36 palmitoylation
disrupts free fatty acid metabolism and promotes tissue
inflammation in non-alcoholic steatohepatitis. J Hepatol.
69:705–717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta DeltaC (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DeWeerdt S: Disease progression. Divergent
paths. Nature. 551:S92–S93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michelotti GA, Machado MV and Diehl AM:
NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol.
10:656–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong VW-S and Wong GL-H: A LEAN treatment
for non-alcoholic steatohepatitis. Lancet. 387:628–630. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kantor PF, Lucien A, Kozak R and Lopaschuk
GD: The antianginal drug trimetazidine shifts cardiac energy
metabolism from fatty acid oxidation to glucose oxidation by
inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase.
Circ Res. 86:580–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Lai J, Yang L, Ruan G, Chaugai S,
Ning Q, Chen C and Wang DW: Trimetazidine prevents
macrophage-mediated septic myocardial dysfunction via activation of
the histone deacetylase sirtuin 1. Br J Pharmacol. 173:545–561.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdul-Wahed A, Guilmeau S and Postic C:
Sweet sixteenth for ChREBP: Established roles and future goals.
Cell Metab. 26:324–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jump DB, Tripathy S and Depner CM: Fatty
acid-regulated transcription factors in the liver. Annu Rev Nutr.
33:249–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benhamed F, Denechaud P-D, Lemoine M,
Robichon C, Moldes M, Bertrand-Michel J, Ratziu V, Serfaty L,
Housset C, Capeau J, et al: The lipogenic transcription factor
ChREBP dissociates hepatic steatosis from insulin resistance in
mice and humans. J Clin Invest. 122:2176–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Hu SL, Hu D, Jiang JG, Cui GL,
Liu XD and Wang DW: miR-1322 regulates ChREBP expression via
binding a 3′-UTR variant (rs1051943). J Cell Mol Med. 22:5322–5332.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie X, Yan D, Li H, Zhu Q, Li J, Fang YP,
Cheung CW, Irwin MG, Xia Z and Lian Q: Enhancement of adiponectin
ameliorates nonalcoholic fatty liver disease via inhibition of
FoxO1 in Type I Diabetic Rats. J Diabetes Res. 2018:62543402018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O-Sullivan IS, Zhang W, Wasserman DH, Liew
CW, Liu J, Paik J, DePinho RA, Stolz DB, Kahn CR, Schwartz MW, et
al: FoxO1 integrates direct and indirect effects of insulin on
hepatic glucose production and glucose utilization. Nat Commun.
6:70792015. View Article : Google Scholar : PubMed/NCBI
|


















