Introduction
The human body has multiple barriers between the
internal and external environment, including the gastrointestinal
barrier, which serves key functions. The intestinal epithelial
barrier (IEB) is the line of defense in the intestine that prevents
harmful substances from passing through the intestinal epithelium
and entering other tissues, organs or the blood stream. The
intestinal immune system serves a vital role in maintaining body
health; however, it may be dysregulated in response to various
diseases (1,2).
Rho is a small G protein with GTPase activity.
Rho-associated kinase (ROCK) acts as a downstream effector of Rho
signaling and belongs to the serine/threonine protein kinase family
(3). The ROCK catalytic center is
exposed and activated when the Rho-binding domain binds to Rho-GTP
(4). In addition, our previous
study using Caco-2 cells in vitro demonstrated that Rho A is
involved in ethanol-induced IEB permeability increase and inhibits
tight junction protein (5). The
Rho effector molecule ROCK can affect the function of IEB by
adjusting the function of tight junctions (6). Dysfunction can occur when IEB is
disabled. Based on this research, it was hypothesized that ROCK may
participate in the IEB dysfunction process via a signaling
pathway.
Currently, two Rho subtypes have been identified,
namely ROCK1 and ROCK2. Both isoforms share an amino acid sequence
homology of 64–65%, with the highest homology (80-92%) within the
kinase domain (7). Although the
two ROCK isoforms are structurally similar, they may have different
functions. ROCK1 is associated with cell migration, whereas ROCK2
is associated with vimentin and actin tension fibers (8,9).
Therefore, it was hypothesized that they serve different roles in
IEB dysfunction.
Nuclear factor κB (NF-κB) is an important
transcription factor regulating the expression of several
inflammatory factors. It has been suggested that ROCK activates the
NF-κB signaling pathway (10).
Thus, ROCK may accelerate the development and progression of IEB
dysfunction by activating NF-κB. ROCK may also activate NF-κB p65
by downregulating NF-κB inhibitor α (IκBα) expression, which in
turn mediates the transcription of multiple inflammatory
factors.
Aquaporins (AQPs), a family of homologous water
transporters in mammals, mainly mediate the passive trans-biofilm
transport of free water and regulate water absorption (11). To date, 13 members of the AQP
family have been identified (AQP 0–12) (12). Previous studies have shown that
AQP8 is downregulated in the intestinal epithelial cells and is
associated with water transport in the human colon (13–15).
The Caco-2 cell model was first proposed by Hidalgo
et al (16) in 1989. Under
normal cell culture conditions, Caco-2 cells can differentiate into
a polar monolayer on the porous membrane after 21 days and express
certain structural and functional characteristics of intestinal
epithelial cells. Since then, a number of studies have used this
model. Nighot et al (17)
used a Caco-2 monolayer model system to investigate the role of
autophagy in regulating the function of the intestinal tight
junction barrier. The Caco-2 cell model was also used by Lin et
al (18) and Elamin et
al (19) to study factors that
affect intestinal epithelial barrier function. Therefore, the
Caco-2 cell model was selected as a normal human intestinal
epithelial cell model in the present study.
Therefore, the aim of the present study was to
investigate whether AQP8 was involved in the downstream mechanisms
of the ROCK/NF-κB pathway in IEB dysfunction using Caco-2 cell
model.
Materials and methods
Caco-2 monolayer cell culture
The human colon adenocarcinoma cell line Caco-2 was
purchased from the American Type Culture Collection. Caco-2 cells
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences) at
37°C with 5% CO2 atmosphere. When Caco-2 cells had grown
to 80% confluence, they were plated onto Transwell filters (Corning
Inc.) and the medium was changed every two days. The cells were
visually monitored using an inverted microscope and epithelial
resistance measurements at 21 days.
Measurement of transepithelial
electrical resistance (TEER)
TEER values were measured at when the monolayer
formed at 21 days, as previously described (20). The TEER of Caco-2 cell monolayers
was measured using a Millicell®-electrical resistance
system (EMD Millipore) at 37°C (21). The electrical resistance was
expressed in ohm (Ω)•cm2 using the surface area of the
Transwell insert.
Gene silencing using small interfering
RNA (siRNA)
For knockdown experiments, Caco-2 cells were
transfected with 100 pmol ROCK1- and ROCK2-specific siRNAs
(Shanghai GenePharma Co., Ltd.), and 100 pmol inducible control
siRNA (Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 24 h. Subsequent experiments were
performed 48 h after transfection. The ROCK1 and ROCK2 siRNA
sequences are listed in Table
I.
 | Table I.Sequences of siRNA. |
Table I.
Sequences of siRNA.
| Gene | Sequence (5→3) |
|---|
| ROCK1 siRNA-1 | F:
UCCACCAGGAAGGUAUAUGTT |
|
| R:
CAUAUACCUUCCUGGUGGATT |
| ROCK1 siRNA-2 | F:
GCCUGAUAACAUGCUGCUGTT |
|
| R:
CAGCAGCAUGUUAUCAGGCTT |
| ROCK1 siRNA-3 | F:
GGCAUGGUACGAUGUGAUATT |
|
| R:
UAUCACAUCGUACCAUGCCTT |
| ROCK2 siRNA-1 | F:
AUCAGAGGUCUACAGAUGATT |
|
| R:
UCAUCUGUAGACCUCUGAUTT |
| ROCK2 siRNA-2 | F:
CUCAGCAGUGACAUAGACATT |
|
| R:
UGUCUAUGUCACUGCUGAGTT |
| ROCK2 siRNA-3 | F:
GUGACUCUCCAUCUUGUAGTT |
|
| R:
CUACAAGAUGGAGAGUCACTT |
| Control siRNA | F:
UUCUCCGAACGUGUCACGUTT |
|
| R:
ACGUGACACGUUCGGAGAATT |
Experimental groups and cell
treatment
Our previous study demonstrated that following 21
days of cell cultivation, TEER values were significantly increased
and stabilized compared with day 0 (20). This finding confirmed the
successful establishment of the monolayer epithelial cell model.
For the ethanol-treatment group, ethanol was added to the culture
medium at a concentration of 5% (v/v) for 1 h. ROCK1 and ROCK2
siRNA with high transfection efficiency, as determined by
preliminary experiments (Figs. 1
and 2), were selected to transfect
Caco-2 monolayer cells. Subsequently, ammonium pyrrolidine
dithiocarbamate (PDTC; 100 µM), an NF-κB inhibitor, was added to
Transwell chamber medium. The upper chamber medium was removed
after 30 min and then ethanol was added. The cells were incubated
with DAPI (1:5; Beijing Solarbio Science & Technology Co.,
Ltd.) at 37°C for 10 min. Finally, the glass slides were observed
in all seven groups with three visual fields in each group using
immunofluorescence microscopy (Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Following Caco-2 cell transfection, the expression
levels of ROCK1, ROCK2, NF-κB and AQP8 were determined using
RT-qPCR. Briefly, total RNA was extracted from Caco-2 cells with
the TRIpure reagent (BioTeke Corporation) and the purity and
concentration were measured. Subsequently, RNA was reverse
transcribed into cDNA using an RT-PCR kit (v3.0; Takara Bio, Inc.)
in a final volume of 20 µl. PCR amplification was performed using
cDNA as a template in 50 µl reactions. PCR was performed using SYBR
GREEN master kit (Beijing Solarbio Co., Ltd.) running the
thermocycling programs: 94°C for 5 min, then 40 two-step cycles of
72°C for 2.5 min, 40°C for 1.5 min and 25°C for 2 min. The specific
PCR primers were designed with the Primer Express v2.0 software
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and
synthesized by Sangon Biotechnology Co., Ltd. The primer sequences
are listed in Table II. Relative
gene expression levels were analyzed using the 2−ΔΔCq
method (22).
 | Table II.Primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5→3) |
|---|
| ROCK1 | F:
AGGTTAGGGCGAAATGGT |
|
| R:
GAATGTTTCTTCCTCTCCTT |
| ROCK2 | F:
CAACTGTGAGGCTTGTATGA |
|
| R:
CAACCGACTAACCCACTTCT |
| NF-κB p65 | F:
TGCCGAGTGAACCGAAAC |
|
| R:
TGGAGACACGCACAGGAG |
| AQP8 | F:
TGAGAATGGGACGGACACTG |
|
| R:
AGTACGGGAGGAGCATCACC |
| β-actin | F:
ACCCTGAAGTACCCCATCGA |
|
| R:
CAAACATGATCTGGGTCATCT |
Western blot analysis
Caco-2 cells were plated onto glass coverslips and
cultured until 70% confluency was reached. Subsequently, cells were
washed with PBS containing 0.1 mM EDTA (without calcium and
magnesium ions), scraped from the slides using a scraper,
homogenized in 1 ml lysis buffer A (2 mM EDTA, 10 mM EGTA, 0.4%
NaF, 20 mM Tris-HCl, protease inhibitor, phenylmethylsulfonyl
fluoride, protease inhibitor cocktail, pH 7.5) and centrifuged at
10,000 × g at 4°C for 10 min. Finally, the supernatant containing
the total cellular proteins was collected. Equal amounts of
proteins (40 µg) were separated by SDS-PAGE on 8% polyacrylamide
gels. After transferring the gel to PVDF membrane, 5% (M/V) skimmed
milk powder was used for blocking at 37°C for 1 h. The membrane was
incubated with the primary antibodies against ROCK1, ROCK2, NF-κB
p65 (all 1:500; cat. nos. WL01761, WL00550 and WL01980,
respectively; all Wanleibio Co., Ltd.) and AQP8 (1:1,000;
AB2768409; ABclonal Biotech Co., Ltd.) overnight at 4°C. Then, the
membranes were incubated with β-actin antibody (1:1,000; cat. no.
WL01845; Wanleibio Co., Ltd.) and Histone H3 antibody (1:1,000;
cat. no. WL0984a; Wanleibio Co., Ltd.) overnight at 4°C.
Subsequently, proteins were incubated with a secondary antibody
(anti-rabbit; 1:5,000; cat. no. WLA023; Wanleibio Co., Ltd.) at 37
°C for 45 min. The protein bands were visualized using a Gel
Imaging Analysis System (cat. no. WD-9413B; Beijing Liuyi
Biotechnology Co., Ltd.), and the integrated density values (IDV)
for each protein were calculated using the Gel-Pro-Analyzer (v6.0;
Media Cybernetics, Inc.) and were normalized to the IDV of
β-actin.
Electrophoretic mobility shift assay
(EMSA)
EMSA binding reactions were performed using a
Lighshift Chemiluminescent EMSA kit (Pierce; Thermo Fisher
Scientific, Inc.). The double-stranded oligonucleotide probe
(5′-AGTTGAGGGGACTTTCCCAGGC-3) (Sangon Biotechnology Co., Ltd) was
labeled with biotin. Nuclear proteins were extracted using a
Nuclear and Cytoplasmic Protein Extraction kit (Pulilai Gene
Technology Co., Ltd.) according to the manufacturers protocol,
rotated at maximum speed every 2 min for a total of 30 min and
centrifuged in 10,000 × g at 4°C for 5 min. The proteins
concentration was determined by bicinchoninic acid method. The
DNA-nucleoprotein binding reaction using a BCA protein
concentration determination kit (A:B=50:1; cat. no. WLA004;
Wanleibio Co., Ltd.) was carried out for 20 min at room
temperature. The DNA-protein complexes were then separated using a
6% non-denaturing polyacrylamide gel by electrophoresis and
transferred onto a nylon membrane. Following transfer, the nylon
membrane was exposed to 254 nm UV light to cross-link using an
ultraviolet lamp in the darkroom for 15 min. The bands were
visualized with enhanced chemiluminescence reagents (cat. no.
WLA003; Wanleibio Co., Ltd.). Finally, the membrane was exposed to
X-ray film and the binding activity of NF-κB was analyzed using a
Gel Imaging Analysis System (cat. no. WD-9413B; Beijing Liuyi
Biotechnology Co., Ltd.).
Statistical analysis
All data are expressed as mean ± SD of at least
three independent experiments. Multiple comparisons between groups
were performed using one-way ANOVA followed by post hoc analysis
(Bonferroni). All statistical analyses were performed with SPSS
v22.0 (IBM Corp.). P<0.01 and P<0.05 were considered to
indicate a statistically significant difference.
Results
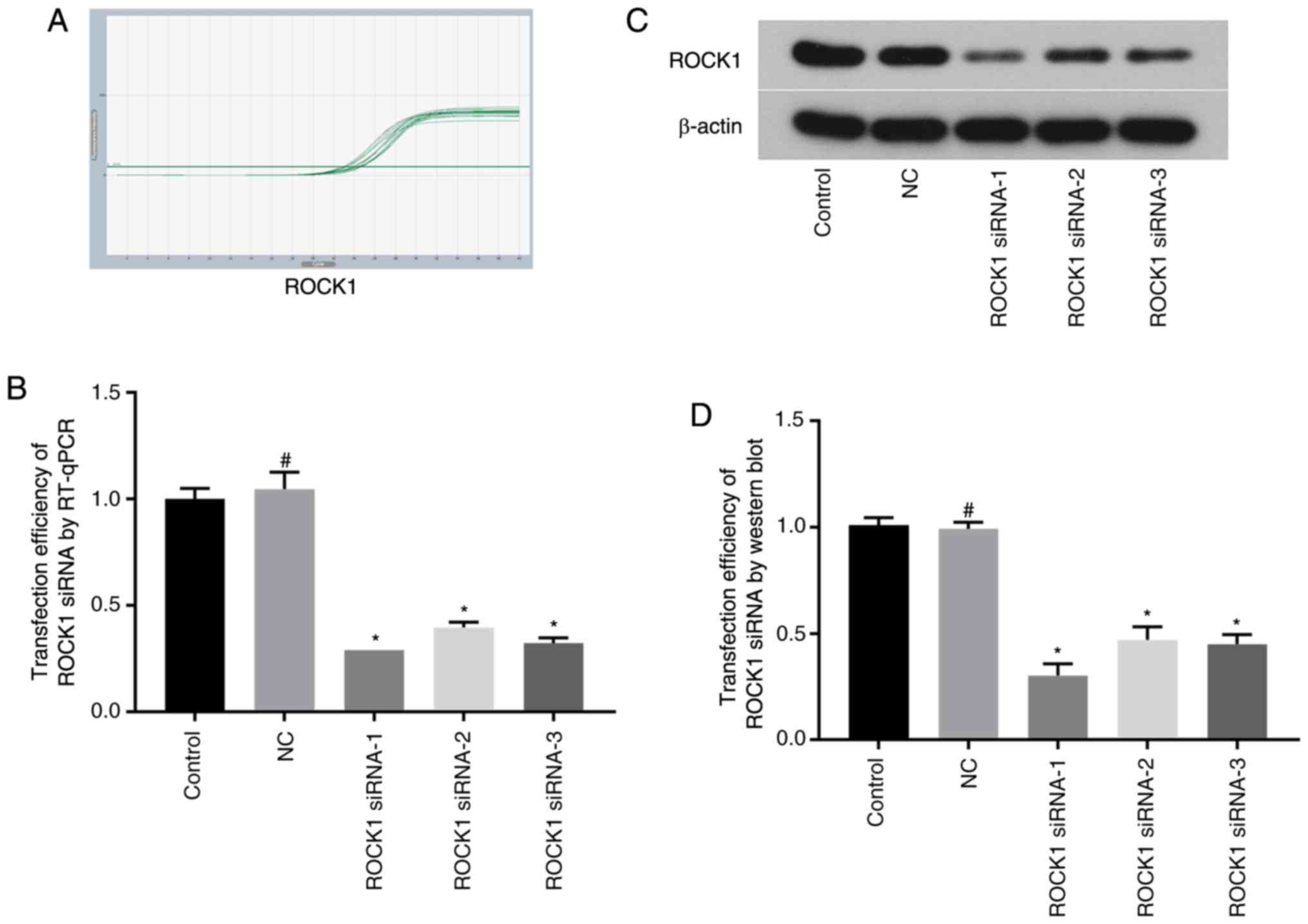
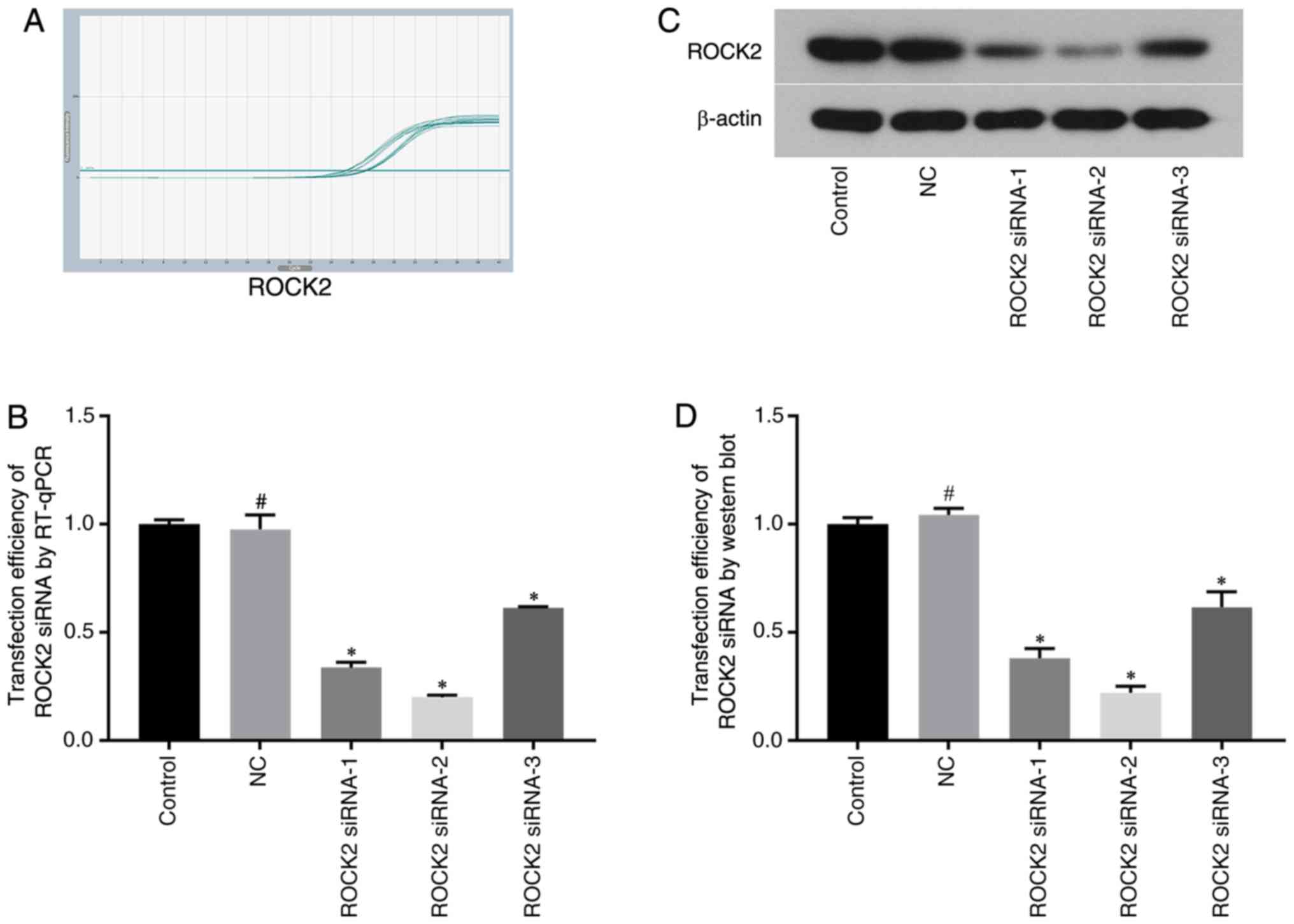
Selection of ROCK1/ROCK2 siRNAs and
their transfection efficiency by RT-qPCR and western blotting
The mRNA and protein expression levels of ROCK1 and
ROCK2 were measured by RT-qPCR and western blot analysis,
respectively, following siRNA transfection. ROCK1 and ROCK2
expressions were reduced following transfections, indicating the
transfection was successful. ROCK1 mRNA and protein expression
levels were significantly decreased in the ROCK1 siRNA-1 group,
with the reduction in expression up to 71 and 70%, respectively
(Fig. 1). ROCK2 mRNA and protein
expression levels were significantly decreased in ROCK2 siRNA-2
group, with the reduction in expression up to 80 and 78%,
respectively (Fig. 2). As the
results showed significantly downregulated protein and mRNA
expression levels in the ROCK1 siRNA-1 group and ROCK2 siRNA-2
group compared with expressions in the control group, these siRNAs
were chosen for use in subsequent experiments.
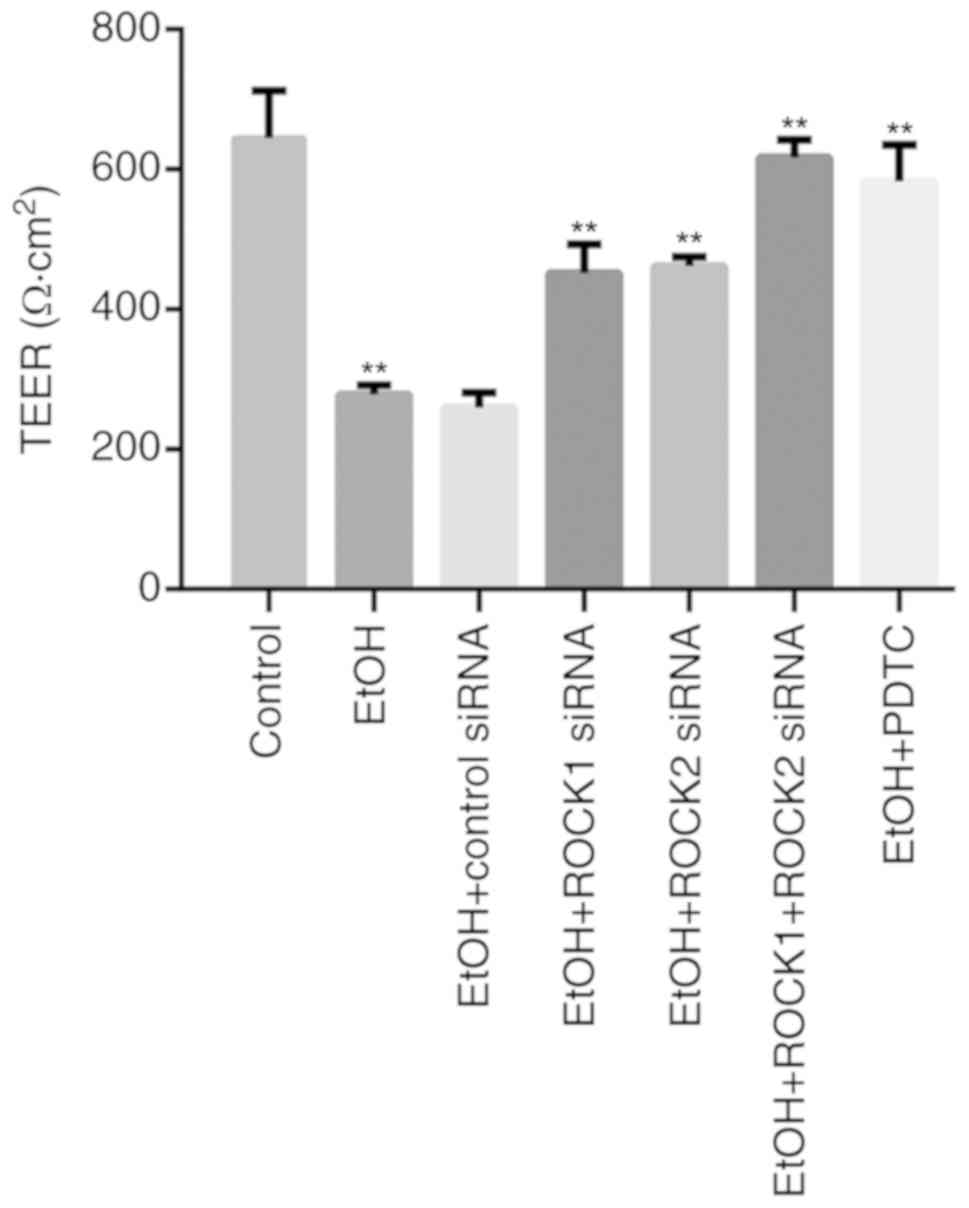
Effects of ROCK1 and ROCK2 knockdown
or PDTC treatment on ethanol-induced IEB permeability in Caco-2
cells
Treatment of Caco-2 cells with 5% ethanol for 1 h
significantly reduced TEER values compared with the control group
(Fig. 3). Furthermore, TEER values
were partially recovered in the ethanol + ROCK1 siRNA and in the
ethanol + ROCK2 siRNA groups, and significantly affected in the
ethanol + ROCK1 + ROCK2 siRNA group. When compared with the ethanol
+ ROCK1 siRNA (P=0.002) and ethanol + ROCK2 siRNA (P=0.004) groups,
TEER values of ethanol + ROCK1 + ROCK2 siRNA group were more
significantly recovered. In addition, compared with the
ethanol-treated group, treatment of Caco-2 cells with PDTC also
restored the decreased TEER values induced by ethanol.
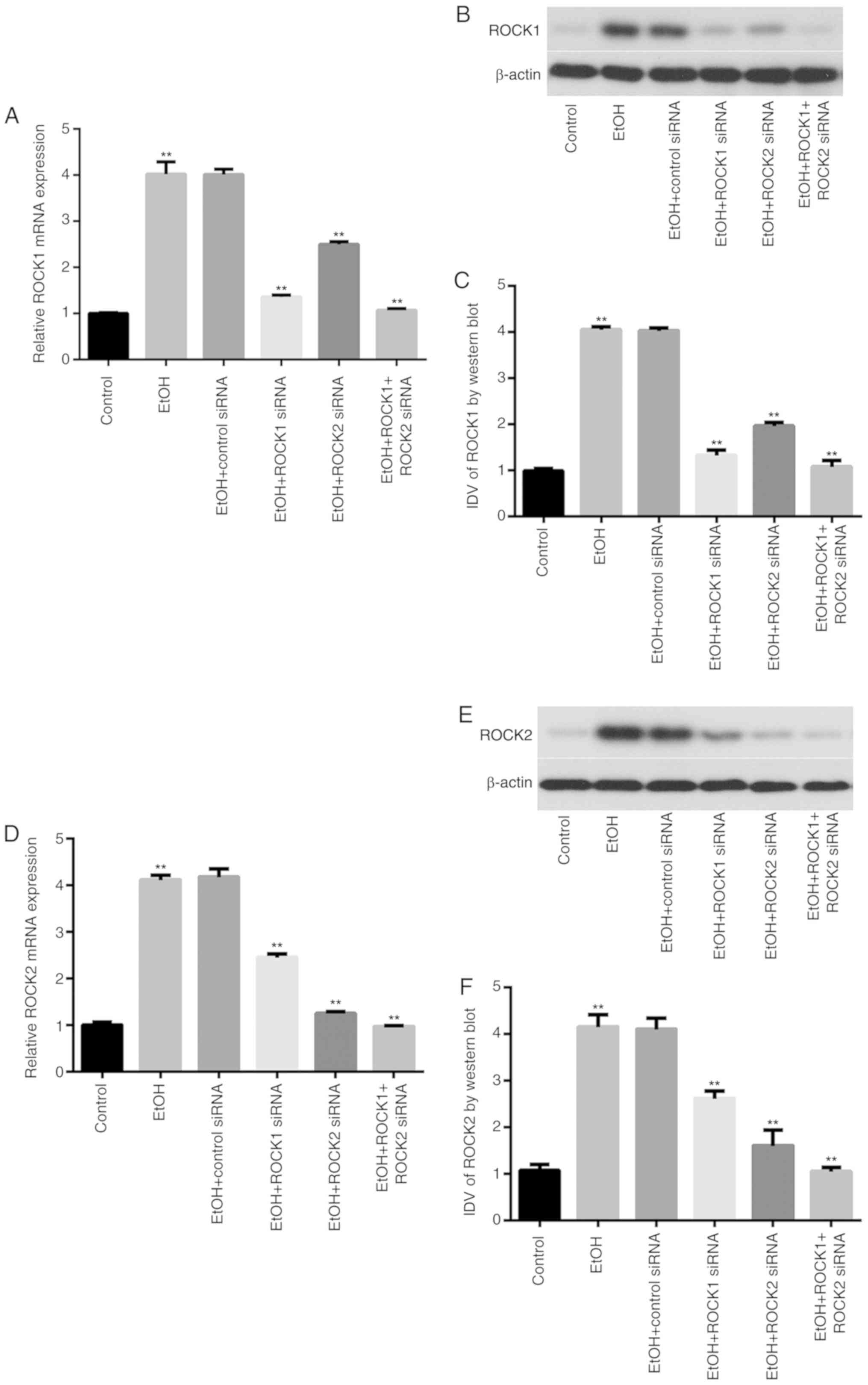
Effects of ethanol exposure on ROCK
isoform expression in Caco-2 cells
The mRNA and protein expression levels of ROCK1 and
ROCK2 in Caco-2 cells from control, ethanol-treated and ROCK siRNA
groups were determined by RT-qPCR and western blot analysis,
respectively (Fig. 4). The results
showed that exposure of intestinal epithelial cells to ethanol
significantly increased ROCK1 and ROCK2 mRNA and protein expression
in the ethanol-treated group compared with the control group. In
addition, ROCK1 and ROCK2 knockdown significantly reduced their
respective mRNA expression levels in the ethanol-treated group
compared with the ethanol-only group. Furthermore, ethanol-treated
cells transfected with the combination of ROCK1 + ROCK2 siRNAs
exhibited further reduction of ROCK1 and ROCK2 mRNA and protein
expression levels.
NF-κB activity in Caco-2 cells
NF-κB p65 mRNA expression levels were determined
using RT-qPCR among the different groups. No statistically
significant changes were observed in the total NF-κB p65 mRNA
expression levels in control, ROCK siRNAs and NF-κB
inhibitor-treated groups compared the with ethanol-treated group
(Fig. 5A). However, the
intracellular distribution and activity of NF-κB p65 protein was
significantly altered in each group. For example, the protein
expression of NF-κB p65 was significantly reduced in the cytoplasm
of intestinal epithelial cells following ethanol exposure (Fig. 5B); however, the cytoplasmic
expression of NF-κB p65 in ethanol-treated cells transfected with
ROCK siRNAs, or co-treated with PDTC, was significantly increased
compared with expressions in the ethanol-only group (Fig. 5B). Additionally, ethanol exposure
significantly upregulated NF-κB p65 in intestinal epithelial cells,
whereas it was downregulated in cells transfected with ROCK siRNAs
or PDTC-treated cells (Fig. 5C).
NF-κB p65 was also significantly increased in cell nuclei in the
ethanol-treated group compared with the ethanol-only group
(Fig. 5D).
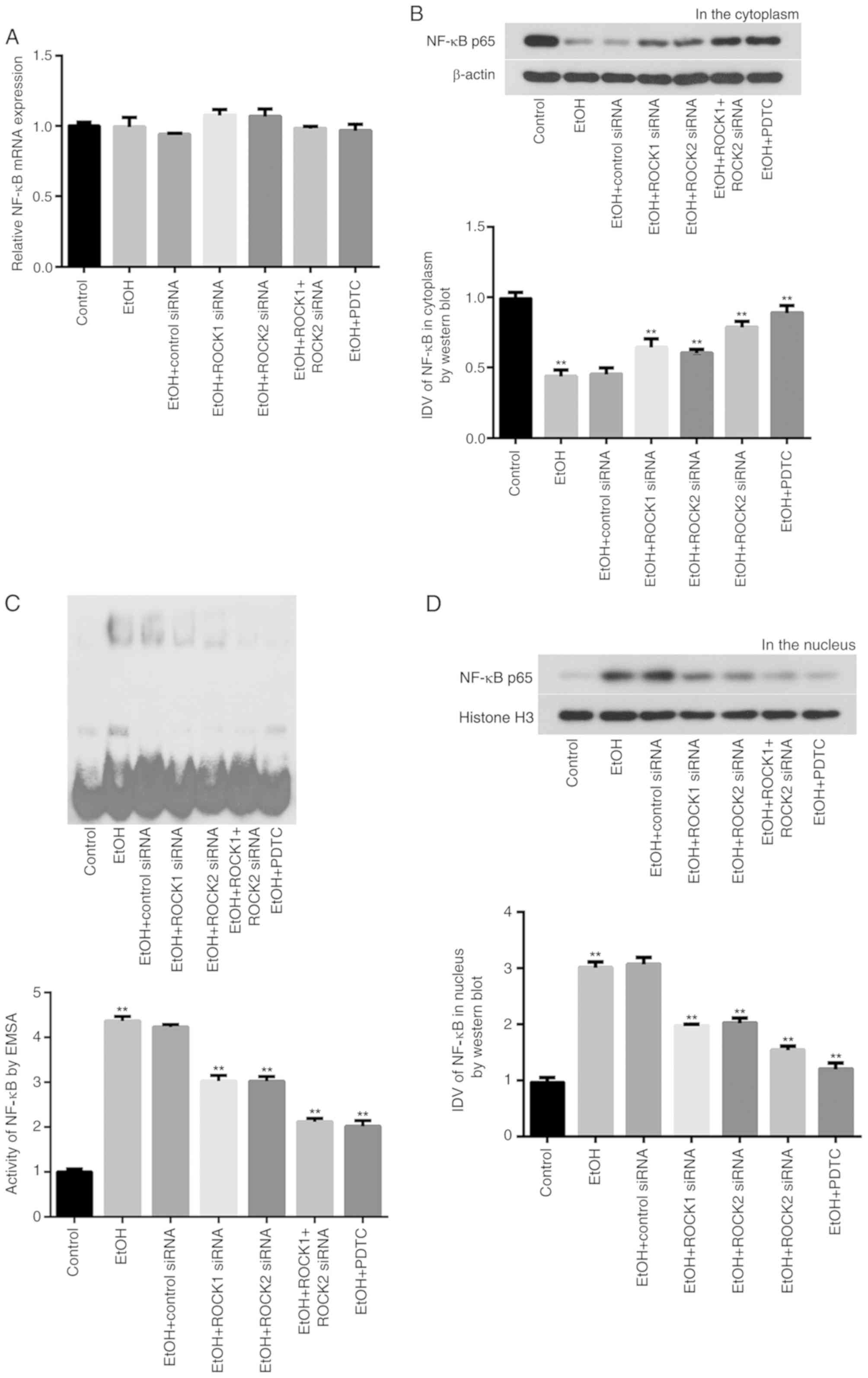 | Figure 5.NF-κB p65 expression levels in Caco-2
cells treated with ethanol, ROCK1 or ROCK2 siRNAs or the NF-κB
inhibitor PDTC. (A) No statistically significant changes were
observed in the total NF-κB p65 mRNA expression in the ethanol,
ROCK1 siRNA, ROCK2 siRNA, ROCK1 + ROCK2 siRNAs or PDTC treatment
groups. (B) Reduced NF-κB p65 protein expression levels in the
cytoplasm of ethanol-treated Caco-2 cells were detected using
western blot analysis. (C) Increased activity of NF-κB p65 in
cultured ethanol-treated Caco-2 cells was evaluated using EMSA. (D)
Increased NF-κB p65 protein expression levels in the nuclei of
cultured ethanol-treated Caco-2 cells were detected using western
blot analysis. All results were compared with the ethanol group and
expressed as mean ± SD; n=3; **P<0.01. EtOH, ethanol; EMSA,
electrophoretic mobility shift assay; NF-κB, nuclear factor κB;
PDTC, ammonium pyrrolidine dithiocarbamate; ROCK, Rho-associated
kinase; siRNA, small interfering RNA; IDV, integrated density
values. |
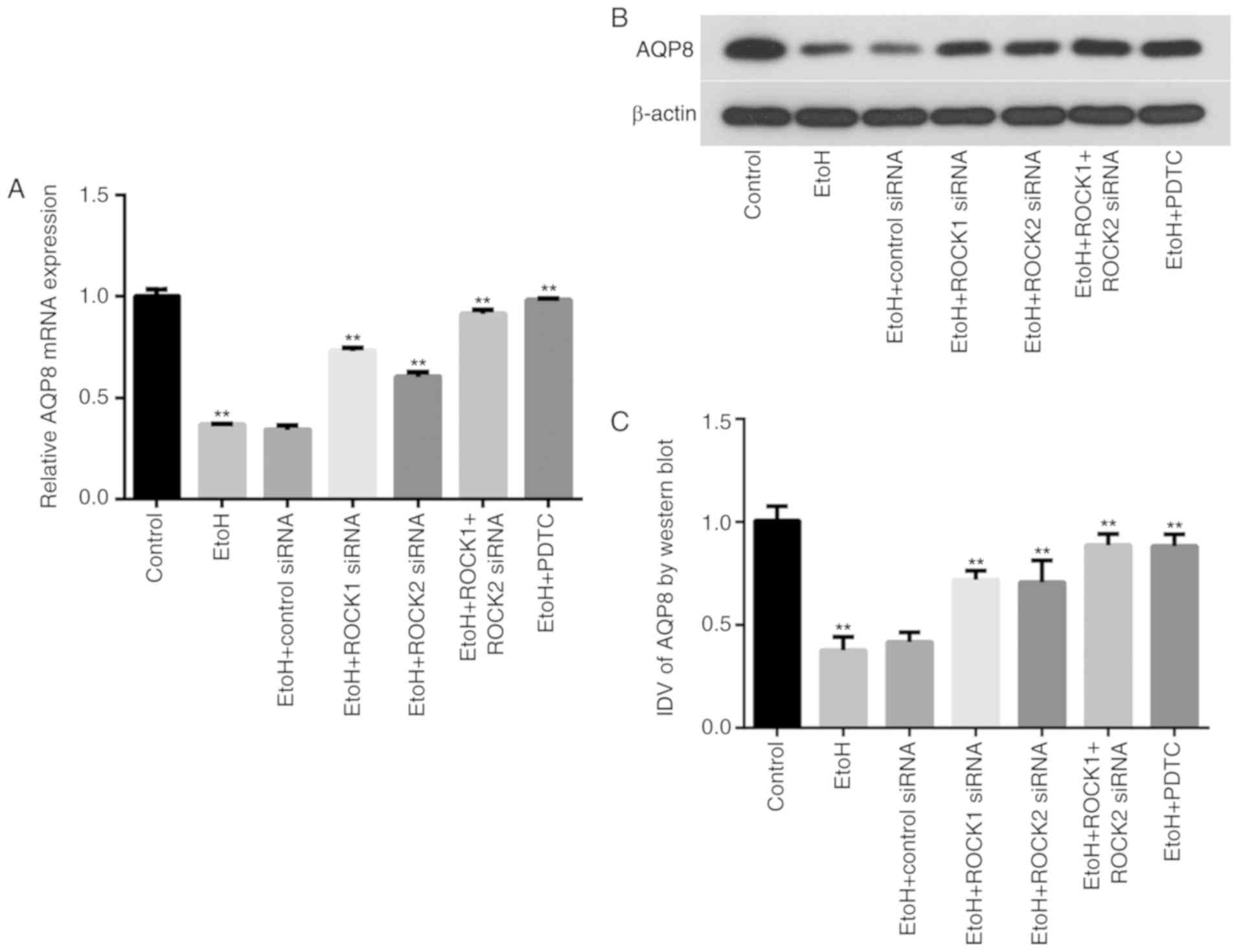
Ethanol exposure affects AQP8 mRNA and
protein expression in Caco-2 cells
AQP8 mRNA and protein expression levels in the
various treatment groups were determined by RT-qPCR and western
blot analysis, respectively (Fig.
6). The results demonstrated that exposure of intestinal
epithelial cells to ethanol significantly downregulated AQP8 mRNA
and protein expression in the ethanol-treated group compared with
the control group. ROCK1 and ROCK2 siRNAs, as well as PDTC
treatment, partially restored AQP8 mRNA and protein expression
levels in the ethanol-treated group compared with the ethanol-only
group. Furthermore, AQP8 expression levels were also recovered in
ethanol-treated cells transfected with the combination of ROCK1 +
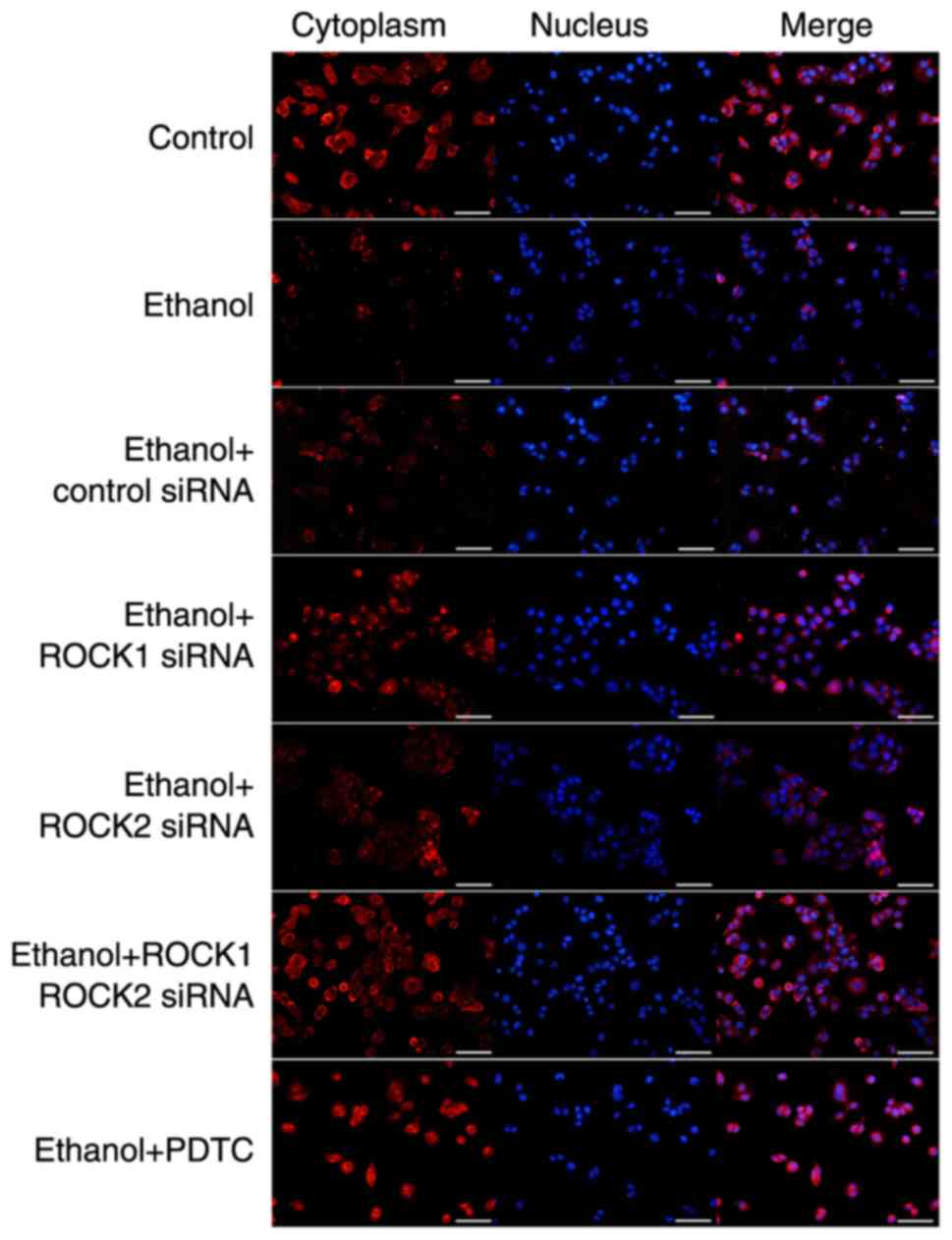
ROCK2 siRNAs. The distribution of AQP8 in the IEB model from each
group was detected using immunofluorescence (Fig. 7), which showed that the expression
level was recovered in the ethanol-treated group compared with the
ethanol-only group and that the AQP8 cell distribution pattern was
consistent with the aforementioned results.
Discussion
The effects of Rho/ROCK pathway in IEB dysfunction
have already been reported in the literature. Mihaescu et al
(23), demonstrated that the ROCK
signaling pathway was involved in the IEB dysfunction in an
experimental C57BL/6J mouse model of radiation enteritis. In
addition, our previous studies confirmed that the Rho/ROCK
signaling pathway was associated with ethanol-induced IEB
dysfunction (5,24). The effects of Rho/ROCK have also
been found to be reversed following treatment with the ROCK
inhibitor Y-27632 (25).
NF-κB is a nuclear transcription factor that
regulates the expression of several genes. In addition, several
studies have shown that Rho/ROCK signaling pathway may also
activate the NF-κB pathway (26).
Segain et al (27) and
Anwar et al (28)
demonstrated that Rho/ROCK signaling served a role in the
activation of NF-κB in peripheral blood mononuclear cells and
endothelial cells, respectively. Other studies in endothelial cells
also confirmed that the Rho/ROCK pathway could mediate NF-κB p65
activation (29). Furthermore,
Rodriguez et al (10)
showed that RhoB activated NF-κB via upregulating ROCK1 expression.
Therefore, it was hypothesized that ROCK activated NF-κB p65,
thereby resulting in IEB dysfunction.
The ROCK protein family comprises two members, ROCK1
and ROCK2. Both members share an overall 64–65% homology in amino
acid sequence and 80–92% homology in their kinase domains (30). Although ROCK1 and ROCK2 are
structurally similar, it is hypothesized that both isoforms may
exhibit different functions. However, their functions have not yet
been reported. In the present study, ROCK1 and ROCK2 siRNAs and a
NF-κB inhibitor, PDTC, were used to investigate the expression
levels of ROCK1, ROCK2, NF-κB and AQP8 in Caco-2 cells. Consistent
with our previous study (5), the
results demonstrated that ethanol treatment reduced the TEER values
in the in vitro IEB model, which were restored following
transfection of cells with ROCK siRNAs and then ethanol treatment,
thus suggesting a recovered intestinal epithelial permeability.
Furthermore, ROCK siRNAs and PDTC downregulated the expression of
NF-κB p65 in the cell nucleus, while its expression was increased
in the cytoplasm. This finding indicated that both treatments
affected the distribution and activity of NF-κB p65 in Caco-2
cells. In addition, the results revealed that both ROCK isoforms
had the same effects on NF-κB expression in ethanol-treated Caco-2
cells.
It has also been reported that NF-κB inhibitors
upregulate AQP1, AQP3 and AQP8 expression in irritable bowel
syndrome (IBS) (13). This finding
suggested that the NF-κB pathway regulated the expression of AQP1,
AQP3 and AQP8, and that abnormal water metabolism and intestinal
permeability could mediate IBS pathogenesis. However, NF-κB
inhibition in IBS model rats did not fully restore the intestinal
epithelium (13). Furthermore,
Duan et al (31) found that
the early acute receptor-interacting protein kinase 1
(RIPK1)/NF-κB/AQP8 axis mediated the RIPK1-dependent acinar cell
necrosis. Consistent with this, the present study suggested that
the NF-κB-mediated regulation of AQP8 expression was likely to be
involved in ethanol-induced IEB dysfunction. Following treatment of
Caco-2 cells with ROCK siRNAs or PDTC, the expression of AQP8 was
significantly increased, indicating that ROCK1, ROCK2 and NF-κB
were involved in the regulation of the ethanol-induced increase in
IEB permeability. These findings were consistent with the results
observed in the IBS model (13).
Taken together, the aforementioned results indicated that ROCK may
regulate the NF-κB pathway, which in turn mediates the activation
AQP8, resulting in the regulation of ethanol-induced IEB
dysfunction. However, further research is required to determine the
exact molecular mechanism.
There were some limitations in the present study.
For example, it could not be verified whether NF-κB was the only
intermediate pathway between ROCK and AQP8. In addition, the
application of ROCK siRNAs or PDTC did not completely restore the
ethanol-induced IEB dysfunction, thus indicating that there are
some unknown factors involved in this process. Furthermore, the
involvement of a feedback pathway must be investigated. Therefore,
the authors aim to explore the role of ROCK/NF-κB/AQP8 signaling
pathway and its mechanism of action in future experiments.
In the present study, Caco-2 monolayers were used as
a model of the IEB. The study of the role of ROCK and its
downstream targets, NF-κB and AQP8, in regulating IEB may lay the
foundation for the treatment of ethanol-induced IEB
dysfunction.
Taken together, results from the present study
suggested that the activation of ROCK/NF-κB/AQP8 signaling pathway
in Caco-2 cells may be involved in ethanol-induced IEB
dysfunction.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81700453).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
HZ and JT designed the experiment together. HZ
conducted experiments, collected and analyzed data, and wrote the
manuscript. XS provided technical support for experiments. JT
helped analyze results and gave useful suggestions for
modification. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AQP8
|
aquaporin 8
|
|
EMSA
|
electrophoretic mobility shift
assay
|
|
IκBα
|
NF-κB inhibitor α
|
|
IEB
|
intestinal epithelial barrier
|
|
NF-κB
|
nuclear factor κB
|
|
TEER
|
transepithelial electrical
resistance
|
|
RIPK1
|
receptor interacting protein kinase
1
|
|
ROCK
|
Rho-associated kinase
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Cheru L, Saylor CF and Lo J:
Gastrointestinal barrier breakdown and adipose tissue inflammation.
Curr Obes Rep. 8:165–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao H, Gokulan K, Piñeiro SA, Williams KM,
Yuan Z, Cerniglia CE and Khare S: Effects of Acute and Chronic
Exposure to Residual Level Erythromycin on Human Intestinal
Epithelium Cell Permeability and Cytotoxicity. Microorganisms.
7:72019. View Article : Google Scholar
|
|
3
|
Sunico CR, González-Forero D, Domínguez G,
García-Verdugo JM and Moreno-López B: Nitric oxide induces
pathological synapse loss by a protein kinase G-, Rho
kinase-dependent mechanism preceded by myosin light chain
phosphorylation. J Neurosci. 30:973–984. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao JK, Seto M and Noma K: Rho kinase
(ROCK) inhibitors. J Cardiovasc Pharmacol. 50:17–24. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tong J, Wang Y, Chang B, Zhang D and Wang
B: Evidence for the involvement of RhoA signaling in the
ethanol-induced increase in intestinal epithelial barrier
permeability. Int J Mol Sci. 14:3946–3960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou Y, Ma L, Zhao Y, Zhang S, Zhou C and
Cai Y: Inhibition of Rho kinase protects against colitis in mice by
attenuating intestinal epithelial barrier dysfunction via MLC and
the NF-κB pathway. Int J Mol Med. 41:430–438. 2018.PubMed/NCBI
|
|
7
|
Pan P, Shen M, Yu H, Li Y, Li D and Hou T:
Advances in the development of Rho-associated protein kinase (ROCK)
inhibitors. Drug Discov Today. 18:1323–1333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bauer PO, Hudec R, Goswami A, Kurosawa M,
Matsumoto G, Mikoshiba K and Nukina N: ROCK-phosphorylated vimentin
modifies mutant huntingtin aggregation via sequestration of IRBIT.
Mol Neurodegener. 7:432012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farber MJ, Rizaldy R and Hildebrand JD:
Shroom2 regulates contractility to control endothelial
morphogenesis. Mol Biol Cell. 22:795–805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez PL, Sahay S, Olabisi OO and
Whitehead IP: ROCK I-mediated activation of NF-kappaB by RhoB. Cell
Signal. 19:2361–2369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verkman AS: Physiological importance of
aquaporin water channels. Ann Med. 34:192–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Q, Yang ZF, Wang KJ, Feng XY, Lv ZJ, Li
Y and Jian ZX: AQP8 inhibits colorectal cancer growth and
metastasis by down-regulating PI3K/AKT signaling and PCDH7
expression. Am J Cancer Res. 8:266–279. 2018.PubMed/NCBI
|
|
13
|
Chao G and Zhang S: Aquaporins 1, 3 and 8
expression in irritable bowel syndrome rats colon via NF-κB
pathway. Oncotarget. 8:47175–47183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JP, Hou XH and Ma RJ: The clinical
features and colonic epithelium AQP8 expression in
diarrhea-irritable bowel syndrome. Zhonghua Nei Ke Za Zhi.
45:1000–1003. 2006.(In Chinese). PubMed/NCBI
|
|
15
|
Escudero-Hernández C, Münch A and Koch S:
The water channel aquaporin 8 is a critical regulator of intestinal
fluid homeostasis in collagenous colitis. J Crohns Colitis. Feb
4–2020.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Hidalgo IJ, Raub TJ and Borchardt RT:
Characterization of the human colon carcinoma cell line (Caco-2) as
a model system for intestinal epithelial permeability.
Gastroenterology. 96:736–749. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nighot PK, Hu CA and Ma TY: Autophagy
enhances intestinal epithelial tight junction barrier function by
targeting claudin-2 protein degradation. J Biol Chem.
290:7234–7246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin S, Han Y, Jenkin K, Lee SJ, Sasaki M,
Klapproth JM, He P and Yun CC: Lysophosphatidic Acid Receptor 1 Is
Important for Intestinal Epithelial Barrier Function and
Susceptibility to Colitis. Am J Pathol. 188:353–366. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elamin E, Jonkers D, Juuti-Uusitalo K, van
Ijzendoorn S, Troost F, Duimel H, Broers J, Verheyen F, Dekker J
and Masclee A: Effects of ethanol and acetaldehyde on tight
junction integrity: In vitro study in a three dimensional
intestinal epithelial cell culture model. PLoS One. 7:e350082012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong J, Wang Y, Chang B, Zhang D and Wang
B: Y-27632 inhibits ethanol-induced increase in intestinal
epithelial barrier permeability. Mol Med Rep. 9:2357–2361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turner JR, Angle JM, Black ED, Joyal JL,
Sacks DB and Madara JL: PKC-dependent regulation of transepithelial
resistance: Roles of MLC and MLC kinase. Am J Physiol.
277:C554–C562. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mihaescu A, Santén S, Jeppsson B and
Thorlacius H: Rho kinase signalling mediates radiation-induced
inflammation and intestinal barrier dysfunction. Br J Surg.
98:124–131. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong J, Wang Y, Chang B, Zhang D, Liu P
and Wang B: Activation of RhoA in alcohol-induced intestinal
barrier dysfunction. Inflammation. 36:750–758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Narumiya S, Ishizaki T and Uehata M: Use
and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol.
325:273–284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z, Gao M, Yang B, Zhang H, Wang K, Liu
Z, Xiao X and Yang M: Naringin attenuates MLC phosphorylation and
NF-kappaB activation to protect sepsis-induced intestinal injury
via RhoA/ROCK pathway. Biomed Pharmacother. 103:50–58. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Segain JP, Raingeard de la Blétière D,
Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P,
Galmiche JP and Loirand G: Rho kinase blockade prevents
inflammation via nuclear factor kappa B inhibition: Evidence in
Crohns disease and experimental colitis. Gastroenterology.
124:1180–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anwar KN, Fazal F, Malik AB and Rahman A:
RhoA/Rho-associated kinase pathway selectively regulates
thrombin-induced intercellular adhesion molecule-1 expression in
endothelial cells via activation of I kappa B kinase beta and
phosphorylation of RelA/p65. J Immunol. 173:6965–6972. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimada H and Rajagopalan LE: Rho kinase-2
activation in human endothelial cells drives lysophosphatidic
acid-mediated expression of cell adhesion molecules via NF-kappaB
p65. J Biol Chem. 285:12536–12542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duan PY, Ma Y, Li XN, Qu FZ, Ji L, Guo XY,
Zhang WJ, Xiao F, Li L, Hu JS, et al: Inhibition of RIPK1-dependent
regulated acinar cell necrosis provides protection against acute
pancreatitis via the RIPK1/NF-κB/AQP8 pathway. Exp Mol Med.
51:1–17. 2019. View Article : Google Scholar : PubMed/NCBI
|