Taurine synthesis
Taurine (2-aminoethanesulphonic acid) is one of the
major free β amino acids, which is normally localized in
high amounts in various mammalian tissues. It is believed that
taurine exerts a wide range of physiological functions, including
conjugation with bile acids (1),
osmoregulation (2), antioxidation
(3), neuronal development
(4) and membrane stabilization
(5). Many of the cytoprotective
properties of taurine are based on its intracellular levels, which
are dependent on its synthesis and transport and these processes
are primarily controlled by the taurine biosynthetic enzymes
cysteine dioxygenase (CDO), cysteine sulfinate decarboxylase (CSAD)
and taurine transporter (TauT transporter) (6).
In most species, taurine is well-known as a
non-essential amino acid, and its distribution is regulated through
four key mechanisms: i) synthesis from methionine/cysteine; ii)
intake from food in the intestine; iii) excretion as bile salt
(taurocholate); and iv) unconjugated taurine in urine via the
kidneys. The major site for taurine biosynthesis has been reported
to be the liver (7), but it is
also synthesized by plethora of other tissues like the brain
(8), lungs (9), skeletal muscle (9), adipose tissue (10) and mammary glands (11) to a lesser extent. Endogenously,
there are two distinct routes for taurine biosynthesis. Regarding
taurine biosynthesis, methionine, or cysteine are the main
substrates on which CDO and CSAD enzymes exert their action towards
taurine biosynthesis (9).
Initially, the CDO enzyme plays a key role in the oxidation of
cysteine to cysteine sulfinic acid, on which CSAD enzyme displays
its activity, converting cysteine sulfinic acid to hypotaurine and
the last step is the transformation of hypotaurine to taurine
(12). Alternatively, cysteine can
be conjugated to coenzyme A (CoA), cysteamine is released during
CoA turnover and cysteamine dioxygenase (ADO) enzyme converts
cysteamine to hypotaurine (13).
Besides, the dietary intake of cysteine has been considered as an
important factor that affects taurine biosynthesis in the liver
(7). In particular, a diet
enriched with sulfur amino acids (methionine, cysteine) caused a
100-fold increase of CDO activity, without any alteration at the
CDO mRNA levels, whereas CSAD activity and CSAD mRNA levels
appeared to be compromised under these conditions (14). The results highlighted the ability
of cysteine to upregulate CDO activity in the liver (9). Of great interest was the phenotypic
outcome of cysteine dioxygenase deficiency (CDO KO) in mice, which
developed severe taurine elimination and increased catabolism of
cysteine to hydrogen sulfide instead of cysteine sulfinic acid
formation, causing pulmonary and pancreatic toxicity (15,16).
Towards this direction, the importance of CSAD enzyme was
confirmed, since third-generation (G3) of cysteine sulfinic acid
decarboxylase deficient (CSAD KO) mice died within 24 h after birth
and this result was avoided by the addition of 0.05% taurine added
to the drinking water (17).
Since taurine biosynthesis is low in the vast
majority of organisms, except for rabbits, intracellular taurine
levels are maintained by taurine intake through the action of TauT
transporter (18). TauT
transporter mediates the translocation of taurine across tissues
throughout the body, thus ensuring cytoprotection (19). The high taurine intake is balanced
through two major pathways of taurine excretion. The first released
form is in the urine, which is excreted by the kidney and the other
one is taurine conjugated to bile acids, that are in turn released
by the feces (20).
Taurine transport
Taurine biosynthesis is high during prenatal life
and declines during adulthood with the lowest concentrations
emerging normally in the elderly and also in certain pathologic
conditions (trauma, sepsis) (13).
Humans exhibit reduced activity of the biosynthetic enzyme CSAD and
obtain the required amount of taurine from food (21). Taurine that derives from food is
absorbed by the small intestine. Following absorption, active
transport in the brush border membrane directs taurine to
enterocytes, which can then deliver it to the portal vein (19). Then, taurine is imported to liver
cells where taurine exerts its action, regulating hepatic
metabolism, with the final step being the transport of taurine to
circulatory cells. There are two types of transporters (TauT
transporter or PAT1 transporter), that deliver taurine from hepatic
tissue to different sites. The TauT transporter (SLC6A6
gene) is considered the major transporter, which is characterized
by its dependence on ions (sodium or chloride), the high affinity
and the low capacity against its substrates. On the contrary, the
PAT1 transporter (SLC36A1) is considered as a
proton-coupled/pH-dependent transporter, equipped with high
capacity and low affinity for substrates. Several common features
characterize both transporters. However, based on studies on their
Km values, it has been shown that the concentration range of PAT1
Km is 7–4 mM, whereas the values of TauT transporter Km is (Km
<60 µM) (22).
Concerning the localization of the two taurine
transporters, TauT and PAT1, they are mainly localized on the
plasma membrane. However, the location of taurine transporters in
not limited to the cell membrane, but also expanded to the nucleus
and other intracellular sites. The nuclear localization of taurine
transporter potentially contributes to nuclear swelling/shrinking
elicited by taurine (23,24). When taurine is eliminated, using
the competitive taurine uptake inhibitor β-alanine, the
mitochondrial taurine content remains intact despite the total
observed low taurine content (25), suggesting that taurine transporter
can be also located in the mitochondria (26). In line with the above, at least one
taurine transporter has been proven to be present in the
mitochondria to import taurine (27). Furthermore, the PAT1 transporter
has been reported to be localized on endosomal and lysosomal
membranes (28).
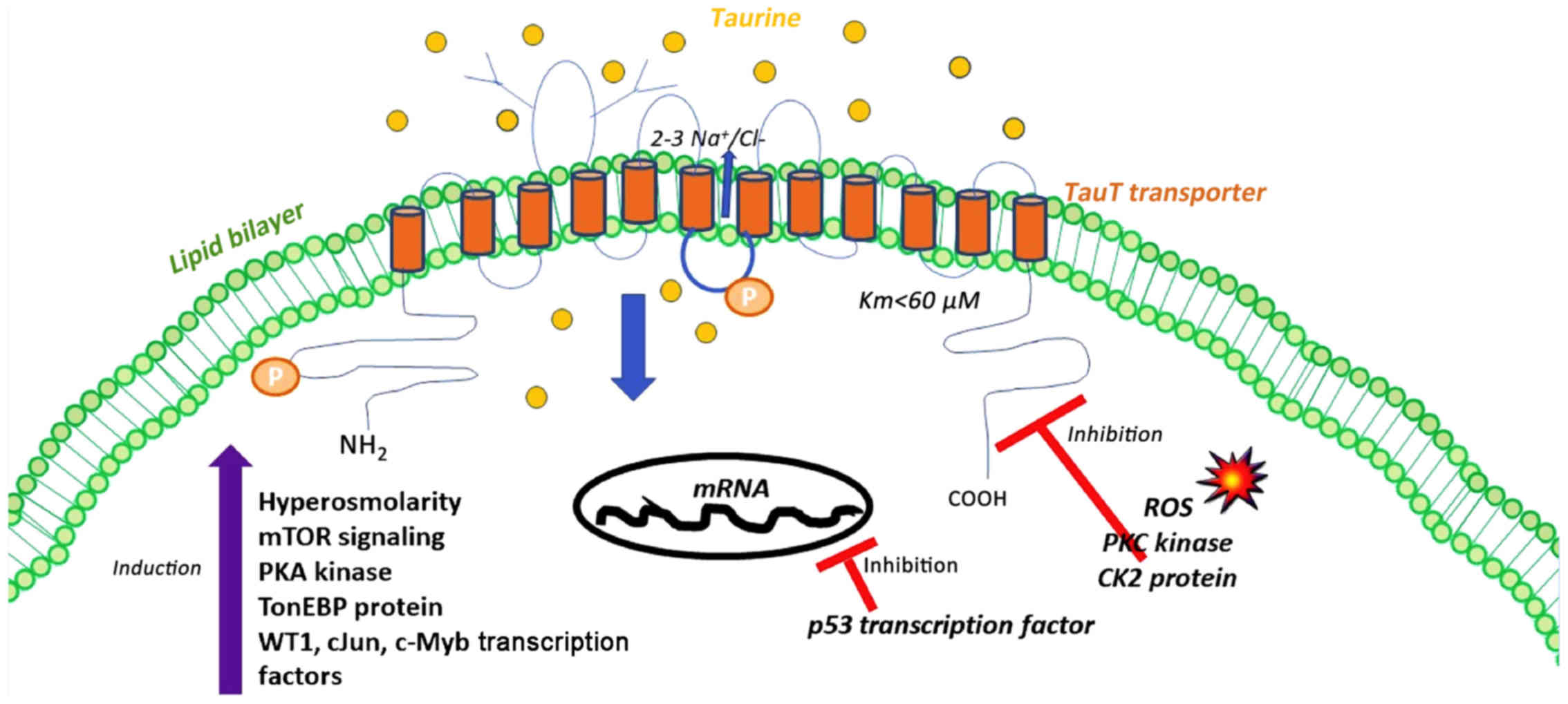
From a structural perspective, it has been
substantiated that TauT transporter has 12 hydrophobic
transmembrane (TM) domains, with the N- and C-terminal being
exposed to the cytosolic compartment. In particular, sodium
(Na+) and chloride (Cl−) are required for
taurine transport, since these ions (Na+ and
Cl−) bind to the first N-terminal, extracellular loop
(29), suggesting strong
dependence of TauT transporter on ions. More precisely, one to
three Na+ ions are required to elicit taurine transport
(30). The importance of sodium is
confirmed through the fact that any reduction in the sodium
gradient can disable further binding of taurine to TauT transporter
(31). The ionic binding and the
gating of taurine to TauT transporter relies on the presence of
arginine (Arg-324) at the fourth intracellular segment of TauT
transporter (32).
Researchers have examined the significance of TauT
transporter using taurine-deficient models and they have shown that
up to 90% reduction in taurine content takes place in most tissues,
demonstrating that TauT transporter is the main non-redundant
transporter for taurine and that PAT1 transporter was unable to
compensate for the loss of TauT transporter (33). Many researchers have focused on the
mechanisms that regulate the function of the TauT transporter,
since a complete understanding of its regulation will provide
compelling evidence on how to use taurine synergistically with
other drugs for the identification of appropriate therapeutic
strategies against complex diseases.
Regulation of TauT transporter
Various parameters determine the concentration of
taurine: biosynthesis (9), TauT
transporter activity (34), volume
sensitive flux pathways (35),
liver diseases (36), age
(37), high-fat diet (HFD)
(38), high-arginine diet
(39), high/low-protein diet
(40,41) and ethanol-containing diet (42). Taurine homeostasis at the organism
level is controlled by multiple regulatory factors, it can
essentially be categorized into taurine biosynthesis, absorption
from intestinal cells and excretion from renal cells. Among them,
the delivery of taurine from the extracellular to the intracellular
environment is the most significant, which is mediated by the TauT
transporter. TauT transporter belongs to a family of Na+
Cl−-dependent transporters, which regulates taurine
movement based on the ionic environment, electrochemical charge,
pH, and temperature (23).
Initially, taurine is transported across the
brush-border membrane of the small intestine through the action of
the TauT transporter and the H+/amino acid transporter 1
(22). The brush-border-mediated
taurine uptake was generally proposed to be non-regulated; however,
it was suggested that inflammation might increase taurine transport
(43). Indicatively,
lysophosphatidylcholine (44) and
pro-inflammatory mediators (TNFα, IL-1β) (45) have been considered responsible for
the regulation of TauT transporter. On the contrary, conditions
such as type 2 diabetes have appeared to be of great importance for
the downregulation of intestinal taurine absorption (46).
In order to elucidate the determining factors for
the regulation of taurine transport, we have used kidney as a model
system. Interestingly, taurine's significance in renal cells was
evidenced by the phenotype of the F1 generation of inbred
taurine-deficient cats. Taurine was proven to be of outmost
importance for renal cells, since its ablation lead to kidney
damage (47). Upon taurine
deficiency, dysregulated differentiation of epithelial cells,
enlarged glomeruli, and ureteral dilatation were observed (47).
There are multiple distinct levels of TauT
transporter regulation. Taurine transporter is a membrane-bound
channel and its distribution appears to adapt to taurine levels,
sharing common features with the ligand-dependent receptor
(48). Renal reabsorption of
taurine ranges from 40 to 99.5% as opposed to reabsorption of most
amino acids (98-99%). As a general note, TauT transporter
expression is ubiquitous (49),
due to the acknowledged function of taurine as an organic osmolyte
(50). The main function of TauT
transporter is osmoregulation which contributes to cell volume
regulation, utilizing a chemiosmotic Na+ gradient to
couple ‘downhill’ transport of Na+ with ‘uphill’
transport of taurine across the membrane (51), under isotonic and hypertonic
conditions (52). The external to
internal downhill Na+/Cl− gradient determines
the uptake of taurine from cells through the action of TauT
transporter and taurine is moved through the following
stoichiometry 2 Na+:1 taurine:1 Cl−,
according to Hill model (53). In
this way, sodium has been highlighted as the cation that plays a
determinant role in the renal adaptive response of TauT transporter
(54), showing that there are no
other cations that are sufficient to replace the importance of
sodium for taurine transport (Fig.
1). Another research group has highlighted the importance of
chloride for taurine transport, considering that chloride ions
facilitate the binding of the second sodium to TauT transporter
(53). The same research group has
supported that bromide can partially compensate for the lack of
chloride, aiding taurine uptake (55). In order to maintain the normal cell
volume, sodium is pumped out of the cell by the Na+
K+-dependent ATPase, as shown in Ehrlich ascites tumor
cells (EATC) (56). Even though
sodium or chloride ions are important in taurine transport, these
ions do not play the most determinant role in regulating taurine
transport. These ions are crucial for the maintenance of the
electrochemical gradient, which in turn determines proper taurine
transport. Besides, taurine transport is a stereospecific and
membrane surface-specific procedure. In support of this, taurine
transport can be suppressed only by the presence of other β-amino
acids and β-aminobutyric acid (GABA) but not by α-amino acids
(57).
Taurine concentration has also been classified as a
factor of great importance for the regulation of TauT transporter.
The type of regulation was clarified in two continuous renal
epithelial cell lines, porcine kidney-proximal tubule cells
(LLCPK1) and Madin-Darby canine kidney, mainly distal tubule cells
(MDCK), in which augmented taurine uptake was observed in
taurine-deficient conditions. When low taurine concentrations
occurred, the activity of TauT transporter was upregulated to
compensate for the loss of intracellular taurine levels and
consequently taurine reabsorption occurred through circulation
through the renal tubules (58).
In contrast, taurine-rich conditions led to reduced taurine
transport as compared to cells cultured in normal medium (47). Importantly, the transcription of
TauT reporter was reduced in response to excess taurine whereas any
alteration in the transcription of taurine biosynthetic enzymes
(CDO, CSAD) was not observed (47).
Polarity has been acknowledged as another factor
capable of influencing TauT transporter regulation. For example,
MDCK cells mediated taurine transport across their basolateral
surface rather than the apical surface (59). In particular, TauT transporter
expression was accumulated in the apical surface of LLC-PK1 cells
compared to MDCK cells (60–62).
In this manner, LLC-PK1 cells transported taurine from the apical
to the basolateral surface whereas MDCK cells directed taurine
transport from the basolateral surface into the cells. The main
principle for that renal adaptive response was the response of
cells to existent osmoregulatory needs. At the molecular level, the
underlying transcription factor that determined the appropriate
taurine transport was the tonicity-responsive enhancing binding
protein (TonEBP), which was recruited to the promoter region
(tonicity response element-TonE) of the TauT transporter gene. In
support of the above, Handler and Kwon (63) reported that the protein expression
of TauT transporter was enriched under hyperosmolar stress
conditions (like extracellular administration of sucrose or
raffinose), in which TonEBP protein was recruited to the tonicity
response element (TonE) (64).
When elevated synthesis of TauT transporter was observed in
response to hyperosmolar conditions, taurine transport within the
cell was increased (65).
Considering that hypertonicity conditions are present within cells,
the transactivation capacity of TauT transporter gene was activated
through the binding of TonEBP protein to the TonE site of TauT
transporter promoter (65). In
this sense, the tonicity-responsive enhancer-binding protein
(TonEBP/NFAT5) binds to the tonicity response element (TonE) in the
promoter region of the TauT transporter gene (65), proposing that TauT transporter is
dependent on the recruitment of TonEBP protein to its promoter,
following hypertonic exposure. Since, mTOR facilitates
transcription of several osmostress response genes including TonEBP
(66), it has been proposed that
elevated TauT activity is derived from an increased mTOR-dependent
TonEBP activity upon hypertonic response (2). For example, the mTOR signaling
cascade has been proposed as a determinant factor for augmenting
the activity of TauT transporter in primary human trophoblasts
(67), non-malignant NIH3T3 and
carcinoma Ehrlich ascites (ELA) mouse fibroblasts (2), thereby increasing the activity of
TauT transporter following hypertonic exposure (2). In contrast, inhibition of the
mammalian target of rapamycin mTOR has been reported to
downregulate TauT mRNA abundance, translation, as well as TauT
activity in primary human trophoblasts (67), non-malignant NIH3T3 and carcinoma
Ehrlich Lettre ascites (ELA) mouse fibroblasts (2). In line with the above, the tonicity
susceptibility of taurine biosynthetic enzymes (CDO, CSAD) has been
observed but to a lesser extent compared to that of TauT
transporter. Therefore, the TonE/TonEBP system appears to be an
essential mechanism in regulating cell volume in hyperosmolar
conditions through mTOR signaling, causing the activity of TauT
transporter to be increased (Fig.
1).
Furthermore, the transcription levels of the TauT
transporter are under the control of p53 and c-Jun transcription
factors, which are commonly implicated in renal damage induced by
chemotherapeutic drugs (68). The
balance of this regulation determines the rate of synthesis of TauT
transporter protein, thereby influencing the fate of renal cells
(68). Indeed, WT1, c-Jun, and
c-Myb potentiate TauT transporter expression whilst the p53 tumor
suppressor gene hinders TauT transporter expression (68) (Fig.
1). The inverse association of TauT transporter with p53
explains the way by which renal cells are protected from
cisplatin-induced nephrotoxicity, suggesting the cytoprotective
role of taurine against renal side effects induced by cisplatin
(68). In particular, transgenic
TauT transporter mice have been shown capable of bypassing
cisplatin induced renal dysfunction, potentially through
suppressing the p53 signaling pathway rather than altering the
cisplatin delivery, as it was indicated at both in vitro and
in vivo settings (69). The
results were in line with previous ones that have supported that
the phenotype of TauT transporter deficient mice was identical to
phenotypes from either taurine-deficient kittens or transgenic p53
mice because TauT transporter constitutes a transcriptional target
of p53 (47). It is worth noting
that regulation of TauT transporter takes place in S3 fragments of
renal proximal tubule cells where cisplatin exerts its action,
given that surviving renal proximal tubule cells are considered of
crucial importance in maintaining normal renal function following
acute kidney injury (70). During
renal development, TauT transporter is transcriptionally regulated
by WT1 and Sp1 whereas the p53 transcription factor is the crucial
modulator of TauT transporter in case of kidney injury (47). The response of renal cells to the
nephrotoxic chemotherapeutic agent (cisplatin) is dependent on the
regulation of TauT transporter transcription (69). As a result, the functional TauT
transporter gene plays a determinant role, protecting renal cells
from cisplatin-induced damage. Since then, other researchers have
shown that taurine ameliorates the symptoms of renal dysfunction
mediated by cisplatin, through its anti-inflammatory and
anti-oxidant properties (71).
Apart from the significance of TauT transporter in
overcoming cisplatin resistance through its interaction with p53
tumor suppressor protein, TauT transporter transactivation capacity
has been suggested to be reduced by doxorubicin-induced activation
of p53 (72). In this way,
researchers have examined how transcriptional regulation of TauT
transporter affected the response of cancer cells to
chemotherapeutic drugs. For example, researchers have provided
convincing evidence that human breast cancer MCF-7 TauT
transporter-deficient cells were vulnerable to chemotherapeutic
drug-induced apoptotic effects (72). Along with in vitro results,
the in vivo experiments proved that the phenotype of
heterozygous p53 mutant (p53+/−) and p53 null
(p53−/−) thymic lymphoma-bearing mice was recovered
using TauT transporter vaccine, causing prolonged survival of
tumor-bearing mice by 1.5-fold (72). Therefore, the TauT transporter
vaccine has been proposed as a new therapeutic option to forestall
cancer growth both in in vitro and in vivo
conditions, independent of the p53 transcriptional status (72).
Besides, the reduced activity of TauT transporter
may be the direct result of either activation of the tumor
suppressor protein p53 or treatment with high extracellular taurine
levels (23) or hypotonicity
(73) (Fig. 1). It is commonly accepted that
increased taurine intake is accompanied by the downregulation of
TauT transporter, in order to respond to the changes of chemical
gradient and to maintain the cell volume (49). In this context, many studies have
revealed that the activity of TauT transporter is under the control
of acidification, incubation with oxidative molecules [reactive
oxygen species (ROS)] (73) and
osmotic cell swelling (56,73).
Importantly, the transport of taurine is regulated by
phosphorylation signaling cascades. The amino acid sequence of TauT
transporter contains several putative consensus sites for
phosphorylation through Ca2+/diacyglycerol-dependent
protein kinase C (PKC) and cAMP-dependent protein kinase A (PKA)
within the intracellular domains. In particular, the maximal
activity of TauT transporter was inhibited in a wide range of cells
through stimulation of the serine/threonine kinase protein kinase C
(PKC) activity (23,74–76).
Moreover, PKC is sufficient to mediate phosphorylation at Ser-332
residue of TauT transporter, which in turn neutralizes ionic
binding of taurine to Arg-324 and abolishes the activity of TauT
transporter, through structural conformational changes (23). Accordingly, it has been reported
that the effect of cAMP-dependent protein kinase A (PKA) is
eradicated following PKC activation since PKA activation is
required for Na+-dependent taurine uptake by cells
(30,74). The fourth segment of the TauT
transporter contributes to taurine propagation across the cell
membrane due to PKC phosphorylation at serine 322 (50,60,77).
In line with these data, casein kinase 2 (CK2) has been
demonstrated to inhibit the activity of TauT transporter,
presumably through phosphorylation at Thr-28. When CK2 is
suppressed, TauT transporter affinity is promoted towards sodium
and the Na+/taurine stoichiometry for taurine transport
is suppressed (Fig. 1) (76).
Hence, taurine transport can be regulated by
hypoxia. The effect of hypoxia on the activity of TauT transporter
has been documented in brain microvascular endothelial cells
(blood-brain barrier, BBB) since hyperglycemia accounts for the
loss of microvascular barrier integrity (78–80).
Researchers examined the association of TauT transporter with
hypoxia-inducible factor-1 (HIF-1), which is the major causal
factor for the induction of BBB disruption and increased BBB
permeability (81,82), and is also related to diabetic
retinopathy or neuropathy through vascular endothelial growth
factor (VEGF) upregulation (83).
Moreover, it should be taken into consideration that PKC kinase
exerts its regulatory activity on both VEGF and TauT transporter
(32,84).
So, researchers proved that brain vascular
endothelial cells exhibited high expression levels of HIF-1 and
VEGF, by reducing the activity of TauT transporter in high-glucose
conditions. The results were confirmed when HIF-1 inhibitors
rescued BBB cells from hypoxia due to TauT transporter upregulation
(78).
In addition to changes in osmolarity, hyperglycemia
and oxidative stress conditions have also been reported to modulate
gene expression of TauT transporter. Interestingly, transcriptional
levels of TauT transporter were induced in healthy subjects, as
compared to type 1 diabetes patients, accompanied by low plasma
homocysteine levels (85). In
another study, mononuclear peripheral blood cells of patients
bearing diabetic retinopathy were characterized by reduced TauT
transporter expression (86). In
line with the above, an outstanding study provided convincing
evidence that taurine status determined the development of diabetic
nephropathy in diabetic animal models. The underlying concept
relied on three lines of evidence: i) taurine deficiency was
apparent in diabetic patients (46); ii) taurine excess provided
protection against acute kidney injury (87); and iii) taurine provided
cytoprotection against high-glucose-induced oxidative stress,
through preventing protein carbonyl content (88). Accordingly, the upregulation of
TauT transporter in diabetic conditions was also observed in fat
tissues from obese mice caused by a high-fat diet or genetic
mutations (ob/ob and db/db mice). Following in
vivo observations, the commitment of human adipose-derived stem
cells (hASCs) to adipogenic progenitors was only promoted, when
TauT transporter levels were high. Among the molecules that are
regulated by TauT transporter, hypotaurine and β-alanine promoted
adipocyte formation, whereas taurine inhibited the process. In this
manner, it was proved that impaired hASCs differentiation
accompanied by eliminated TauT transporter activity was recovered
by hypotaurine and β-alanine through impeding β-catenin
transcriptional transactivation. The results proposed that taurine
transport can act as a new therapeutic strategy against obesity
(89). Additional evidence
supporting the functional importance of TauT transporter in
diabetes, emerged through the phenotype of TauT transporter
deficient animal models, in which researchers observed that
characteristics of TauT transporter null animals comprised
mesangial cell expansion, glomerular basement thickening, nodular
sclerosis with expansion of the glomerulus beyond Bowman's capsule,
and juxtaglomerular tuft sclerosis (90). Since, typical features of human
diabetic nephropathy involved changes in ischemic heart,
atherosclerosis induction, glomerular dropout, and interstitial
fibrosis, it was plausible that TauT transporter null mice mimicked
human diabetic nephropathy. Histologically, TauT transporter null
mice presented increased deposition or abundance of SMA, collagen
IV, CD34 (as a marker of endothelial injury), and Ki67.
A large number of studies have suggested that
taurine transport may be enhanced by inflammation (45). It has been highlighted that
inflammatory cytokines contribute to the induction of TauT
transporter activity to maintain taurine levels. Interestingly,
intestinal epithelial cells increased TauT transporter levels to
respond under inflammatory conditions. For example,
anti-inflammatory properties of taurine were manifested through the
regulation of TauT transporter in leukocytes of patients with
depression. It was observed that the antidepressant mirtazapine
diminished CD4+ T and CD8+ T leukocytes that
contained TauT transporter to 77 and 80%, respectively. Notably,
TauT transporter distribution in all cells did not change following
anti-depressant therapy, suggesting that reduced taurine transport
in circulating immune specific leukocytes potentially accounted for
high extracellular taurine content, which might be involved in
protection against oxidants and inhibition of pro-inflammatory
cytokine-mediated damage through the formation of N-Chlorotaurine
(TauCl) (91).
Volume-insensitive and -sensitive taurine
transport
During isotonic conditions taurine is released to
the extracellular compartment, and it is upregulated when
cholesterol-depleting agents (92), lysophosphatidylcholine (93) are administered, and during the
apoptotic process (94). The
release of taurine in unstimulated cells through the action of TauT
transporter does not seem feasible, considering the low
intracellular sodium (94). On the
contrary, taurine can be released via TauT transporter, as observed
in Ehlrich ascites tumor cells (EATC) in response to arachidonic
acid/eicosapentaenoic acid (95),
prostaglandin E2 (96)
or cisplatin (94) which increased
the sodium conductance.
Increased release of taurine via the
volume-sensitive taurine release pathway, designated
volume-sensitive organic anion channel (VSOAC) has been observed
following hypotonic exposure. The threshold for the activation of
taurine release has been estimated at 15% of cell swelling in
NIH-3T3 cells, and there is a positive relationship between efflux
and reduced osmolality in an exponential manner (97).
Taurine deficient conditions
To determine the functional significance of taurine,
models of taurine deficiency have been developed by knocking out
the TauT transporter gene (33,98)
nutritional depletion, an inhibitor of taurine intake, such as
taurine transporter antagonist (guanidinoethane sulfonate)
(99) or β-alanine (100) and carbon tetrachloride
(CCL4) (101), all of
which contribute to the elimination of taurine values. For example,
taurine transport inhibitor (β-alanine) can cause the elimination
of mitochondrial taurine content by 60% after supplementation with
β-alanine (100).
Taurine Transporter deficient mice (TauT
KO)
Towards delineating the impact of taurine on the
cardiac muscle, researchers proceeded to the elimination of taurine
transporter, generating TauT KO mice. In one case, Ito et al
managed to ablate exons 2–4 of TauT transporter, causing a decrease
in taurine in both cardiac and skeletal muscle, respectively, of
100 and 96% (98). In another
case, Heller-Stilb et al (33) generated TauT KO mice by elimination
of exon 2 of taurine transporter. Similarly, the phenotype of
latter TauT KO mice showed a decrease at cardiac and muscle taurine
levels at a rate of 98%. They developed a mouse model with a
disrupted gene encoding the taurine transporter
(TauT−/−) and observed that higher amounts of taurine
loss occurred in skeletal muscle and heart (95% decrease), followed
by lower amounts of taurine plasma, kidney, liver, and the eye (74%
decrease). Interestingly, the mice presented severe retinal
degeneration due to vision loss (33). Undoubtedly, in both cases, there
was a remarkable decline of taurine and abnormal function in
tissues in which taurine is necessary. More specifically, the
phenotypic characteristics of TauT KO mice were: lower body weight,
exercise intolerance and muscle atrophy (98,102), loss of retinal photoreceptor
function, reduced responsiveness to nociceptive stimulation
(103), degeneration of their
inner ear, alteration of renal development and function, unspecific
hepatitis/liver fibrosis and cardiomyopathy (87,104), as well as reduced T-cell memory
generation (105) and blunted
apoptosis in erythrocytes accompanied by alterations in the balance
of blood cells (91). All the
disturbances in different organs were exacerbated with aging,
implying the regulation of taurine transporter during aging. Based
on the above, we can conclude that the intracellular taurine levels
in cardiac and skeletal muscle are dependent on taurine transport
activity. However, Warskulat et al (102) contributed to causing a debate
regarding the phenotype of TauT KO mouse models, because TauT KO
mice had a normal function with high susceptibility towards heart
failure, as indicated by high expression of fetal genes such as
atrial natriuretic peptide (ANP), and brain natriuretic peptide
(BNP). Consequently, taurine is fundamental in cardiac homeostasis
and the differential results of deficiency ultimately depend on the
genetic background of the animal models used. Focusing on the
generation of former TauT KO mouse models, it was observed that
signs of dilated cardiomyopathy (levels of ANP, BNP) were more
intense in 9-month-old TauT KO mouse models compared to 5-month-old
TauT KO animals, implying the regulation of taurine in
age-sensitive manner (98). In
general, TauT KO mice exerted high percentages of necrotic cells
and disorganization of myofilaments in skeletal muscle (104).
Studies from TauT KO mice proved that low
intracellular taurine levels caused a variety of dysfunctions in
different organs in an age-dependent way (14–20).
Within 1 year after birth, TauT KO mice manifested vision, suffered
from auditory, olfactory malfunctions accompanied by muscle
impairment, and altered synaptic transmission in the brain
(21). At greater age (beyond 15
months), TauT KO mice presented liver manifestations, such as
fibrosis, unspecific hepatitis, and tumor formation through
lowering mitochondrial respiratory chain activity (17,21).
Even though earlier studies have reported the great implication of
TauT transporter in hepatocytes, the latest studies highlighted
that TauT transporter was crucial in hepatic non-parenchymal cells
(56,57). Interestingly, differences in the
taurine distribution of TauT KO mice were observed, underscoring
the complete taurine loss in Kupffer and sinusoidal endothelial
cells but not in parenchymal cells (106). For instance, hepatic parenchymal
cells of TauT KO mice presented a decline of Tau transporter by
30%, whereas hepatic non-parenchymal cells of TauT KO mice had a
marked decline of TauT transporter (52).
Furthermore, the research team led by Häussinger
provided convincing evidence for the presence of apoptotic cells in
taurine-transporter deficient tissues. Apoptosis emerged in
photoreceptor cells, leading to taurine transporter deficient mice
to blindness at an early age, given that the differentiation of
cells was not closely dependent on taurine transporter.
Accordingly, the olfactory receptor neurons of TauT KO mice
followed the same apoptotic trend, displaying signs of immaturity.
Importantly, taurine caused hepatocyte destruction which was
manifested by a high proportion of hepatic apoptotic cells, thereby
leading moderate unspecific hepatitis and liver fibrosis in TauT KO
mice beyond 1 year of age (107).
For many years, researchers were not able to reveal
the mechanisms underlying structural liver dysfunction in aged TauT
KO mice. Recently, Qvartskhava et al (108) provided the instructive principles
of taurine loss by which liver dysfunction of TauT KO mice emerged.
Researchers attributed the liver malfunction of TauT KO mice to
altered hepatic ammonia handling and hepatic glutamine synthesis in
a time-dependent manner. In particular, it was suggested that TauT
transporter deficiency disturbed ammonia detoxification by
perivenous scavenger cells in the liver, thereby inducing systemic
hyperammonemia through impaired hepatic glutamine synthesis. The
underlying mechanisms varied according to the age of TauT KO mice.
That was reflected by the inactivation of the ammonium transporter
rhesus type glycoprotein B (RhBG) in 3-month-old TauT KO mice and a
tyrosine nitration-mediated elimination of glutamine synthesis at
12-month-old because of oxidative/nitrative stress. The results of
the 3-month-old TauT KO mice could be explained, taking into
consideration that hepatic RhBG expression is restricted to
glutamine synthetase-expressing hepatocytes. As a result,
3-month-old TauT KO mice indirectly impaired glutamine synthesis
and doubled the ammonia concentration required for half maximal
glutamine synthesis while 12-month-old TauT KO mice directly
disturbed glutamine synthesis.
Concerning the effects of taurine elimination in the
myocardium, H NMR spectroscopy experiments substantiated that TauT
KO mice presented significant taurine loss, rendering them energy
starved. In that sense, taurine elimination was responsible for
cardiomyopathy with concomitant cardiac atrophy, which was
manifested by remarkable signs of cardiac myofibrillar
fragmentation, mitochondrial disruption, and swelling of the outer
mitochondrial membrane (98,100,102). In support of the above, the
results of liquid chromatography-mass spectrometry (LC-MS)
distinguished the range of metabolites that were present between
the hearts of wild-type and taurine transporter deficient mice.
Initially, it was observed that there was no difference in
taurine-modified bile acids, comprising taurocholate,
taurodeoxycholate, and taurohyocholate and those findings were
consistent with previous ones (106). Importantly, mass spectrometry
classified a great number of metabolites that were differentially
distributed in the hearts of KO or wild-type mice. As expected,
methionine levels were elevated in TauT KO hearts probably due to
protein turnover, since methionine is required for taurine
biosynthesis (109). The
identification of differential metabolites derived from two groups
provided the information that impaired fatty acid oxidation and
dysregulated osmoregulation were the prevalent consequences of
taurine elimination. For the maintenance of organic osmolyte
homeostasis, the amino acid transporter Slc38a2 and betaine, as
well as glycerophosphocholine, were found to be enriched in the
hearts of taurine transporter deficient mice compared to hearts of
control mice. Notably, Slc38a2 constituted a transporter of
glutamine, proline, alanine (110) and it was stimulated upon
hypertonic exposure, through the action of NFAT5/TonEBP
transcription factor (98). TauT
transporter was upregulated via NFAT/TonEBP in hypertonic stress
conditions, with the ultimate aim of regulating intracellular
osmolytes (65,111). Consistent with the above, betaine
and glycerophosphocholine were of utmost importance in regulating
the concentrations of organic osmolytes (112). Therefore, these constitute
irrefutable evidence that the enrichment of the molecules was to
compensate for the taurine deficiency, thereby restoring the defect
in intracellular levels of osmolytes.
Taurine is essential not only for the maintenance of
organic osmolytes but also for fatty acid metabolism. As expected,
analysis of metabolites derived from TauT KO hearts indicated that
there was an inhibition in b oxidation of fatty acids and in the
Krebs cycle (100,113). Specifically, dysfunctional
mitochondrial respiration was shown by downregulation of long-chain
acyltaurines and upregulation of short-chain acyltaurines in TauT
KO hearts (109). Consistent with
the central role of taurine in the respiratory chain, it was
reasonable that TauT KO mice displayed impaired complex I activity,
accompanied by dysfunctional NADH dehydrogenase. Mass spec results
confirmed the significance of taurine in mitochondria, showing that
TauT KO hearts had high levels of NADH due to inactivation of NADH
dehydrogenase and acetylcarnitine, which was contributed to
inefficient use of acetyl CoA as a substrate in citric acid cycle
(109). Metabolome analysis also
provided an interesting explanation concerning the phenotype of old
TauT KO mice (beyond 15 months), that displayed symptoms of chronic
liver disease (106). Urea
metabolites (ornithine, citrulline, and arginosuccinate) prevailed
in TauT KO hearts, that were probably released from the surplus of
hearts to livers of TauT KO mice. Additional search related to
heart changes of taurine loss proved that the hearts of KO mice
presented significant alterations in their glutathione metabolism,
as indicated by higher amounts of pyroglutamate and undetectable
levels of glutamyltaurine, compared to normal hearts. These results
were expected given that there is a competitive relationship
between pyroglutamate and glutamyltaurine (114).
Given the osmoregulatory activity of taurine, its
anti-oxidant activity, and its capacity to maintain protein
folding/phosphorylation, it is reasonable to suggest that taurine
not only plays a key role in cellular homeostasis, but it also
potentially contributes to the rescue of the organism from the
aging process. However, there is only a small number of studies
that support the beneficial action of taurine on health, modulating
skeletal muscle senescence, and protein folding. In this direction,
Ito et al (116) observed
a couple of aging-related functional defects in skeletal muscle of
(TauT KO mice aged 18 to 22-months). At a rapid pace, the mice
developed aging-related hallmarks that were evidenced by diminished
mitochondrial complex I activity and excessive formation of muscle
fibers with central nuclei, implying the contribution of taurine
loss to sarcopenia. Taurine deficiency in aged mice caused a
decline in respiratory chain complex 1 activity, accompanied by an
enhancement in unfolded protein response (URP) and in
Cyclin-dependent kinase 4 inhibitor (p16INK4a), which constitutes
an aging biomarker (115).
Specifically, it was observed that aged TauT KO mice displayed
increased levels of p16INK4A up to 10 times compared to aged
wild-type mice. Similar enrichment of p16INK4A was observed in the
lung and kidney of aged TauT KO mice to a lesser extent compared to
that of skeletal muscle of aged TauT KO mice. There was no
difference in p16INK4A protein content between young wild-type and
TauT KO mice, suggesting that a pronounced increase in p16INK4A
protein content was tightly related to aging (116). Furthermore, aging was related to
taurine loss through their effect on UPR signaling cascades which
served as a protective response against ER stress. Both aging and
taurine deficiency was reported to display decreased GRP78
expression and downregulation of several ER membrane sensor
proteins (PERK, eIF2α, IREα) that are associated to UPR. Our
current findings in the taurine-depleted hearts show a close
resemblance to accelerated aging (117,118), ER (119,120). As a consequence, taurine
deficient and aged hearts seemed to be very susceptible to
accumulating oxidation-mediated protein aggregates (121). Transcriptome data derived from
the skeletal muscle of old TauT KO mice revealed induction of SMAD3
and β-catenin signal transduction pathway compared to old wild-type
mice (122).
Mitochondrial myopathy, encephalopathy,
lactic acidosis and stroke-like episodes (MELAS) syndrome
The normal development of animals is dependent on
adequate taurine levels. Interestingly, if animals were fed with a
taurine-deficient diet, they would exhibit a plethora of
malfunctions: metabolic and endocrine changes and renal
dysfunction. The underlying cause of abnormalities was attributed
to impaired biosynthesis of mitochondria-encoded protein subunits
of the respiratory chain complexes, resulting in disturbed electron
transport flux. Essentially, the biosynthesis of
mitochondria-encoded protein subunits of the respiratory chain
complexes exhibited an absolute dependence on a specific
post-translational modification, which is manifested at tRNA Leu
(UUR), contributing to the formation of 5 taurinomethyluridine-tRNA
Leu (UUR). When animals were fed a taurine-deficient diet, the
formation of mitochondrial 5-taurinomethyluridine in the wobble
position of tRNA Leu (UUR) was hindered due to loss of taurine
which was used as a substrate for the post-translational
modification of the uridine base in the wobble position. As a
result, the production of mitochondria-encoded protein subunits of
the respiratory chain complexes was prevented, given that the
electron transport chain subunits contained multiple UUG codons,
directed for the translation of leucine and lysine residues
(123). Interestingly, it is
worth mentioning that MELAS (mitochondrial myopathy,
encephalopathy, lactic acidosis, and stroke-like episodes) is a
mitochondrial disease that might be caused by impaired UUG
decoding, which is often observed in taurine-deficient conditions
(124). Following extensive
research, it was suggested that there were no significant
differences between the phenotype of MELAS syndrome and taurine
deficiency since MELAS symptoms are nearly indistinguishable from
those of taurine deficiency. In MELAS conditions, impaired UUG
decoding, diminished respiratory chain flux accompanied by
decreased adenosine triphosphate (ATP) synthesis, superoxide anion
overproduction and stimulation of anaerobic metabolism were
observed, thus essentially impairing the viability and
functionality of cells of all organs in the body (125,126). Certainly, MELAS patients are
deprived of post-translational modification in the wobble position
of tRNA Leu (UUR), which is required for efficient decoding of both
UUG and UUA involved in mitochondrial proteins of electron
transport chain. However, the biosynthesis of mitochondria encoded
proteins of MELAS patients is also diminished due to impaired
aminoacylation rendering mitochondrial-encoded proteins more
susceptible to the wobble defect. As a result, it is concluded that
the aminoacylation defect of MELAS patients orchestrates the whole
landscape of electron transport flux, reducing the activities of
complexes I and III–V whereas wobble defect of taurine deficient
subjects only causes diminished synthesis of complex I
proteins.
Vionnet et al (127) proposed that the endocrine system
was tightly associated with MELAS syndrome through the emergence of
diabetes. Initially, researchers observed that 2% of type 2
diabetic patients harbored the primary MELAS-linked tRNA Leu(UUR)
mutation. In particular, MELAS patients with type 2 diabetes
exhibited impaired insulin secretion due to disturbance of
pancreatic β cells mimicking the glucose intolerance and impaired
insulin secretion of type 2 diabetes patients (128). The metabolic pattern of MELAS
patients with type 2 diabetes favored anaerobic metabolism (the
enhanced conversion of glucose to lactate) than glucose oxidation,
which is a predominant procedure of aerobic metabolism, thereby
culminating in lactic acidosis. In other words, the accumulation of
lactate was the direct result of increased NADH/NAD+
ratio and decreased respiratory chain activity since pyruvate is
not easily incorporated in the citric acid cycle (125).
Conclusions
Taurine transport can be regulated at multiple
levels, ranging from cellular stimuli to transcription factors.
This explains why taurine transporter null mice display dysfunction
in several tissue types. The impaired homeostasis of taurine
transporter deficient mice has mainly been proposed to be ascribed
to disturbed energy metabolism, as shown by a dysfunctional
respiratory chain in various organs. Notably, the phenotype of
taurine transporter null mice exerts various indiscernible
similarities with a phenotype of mitochondrial diseases such as
MELAS. These findings are of great significance, raising important
questions for further research. It will be remarkably interesting
to study the consequences of specific taurine transporter
inactivation in single tissues, to better understand the function
of the taurine transporter and in this manner to conclude if the
phenotype of taurine transporter null mice is the result of
synergistic interactions among organs. In the future, it is
important to determine if the function of taurine transporter in
one organ is sufficient to orchestrate the energy metabolism in all
organs.
Acknowledgements
Not applicable.
Funding
This research was supported by I.K.Y State
Scholarship Foundation for S. Baliou's Ph.D. studies. The IKY code
is no. 2018-050-0502-13155.
Availability of data and materials
Not applicable.
Authors' contributions
All authors were involved in the conception and
design of the study. SB performed the literature search, wrote the
manuscript, critically analyzing the existing knowledge and
designed the figures. SB, MG, AMK and VZ contributed to editing the
manuscript concerning the regulation of TauT transporter; SB, MIP,
DAS and VZ contributed to editing the manuscript concerning taurine
deficient conditions. All the authors approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Falany CN, Johnson MR, Barnes S and Diasio
RB: Glycine and taurine conjugation of bile acids by a single
enzyme. Molecular cloning and expression of human liver bile acid
CoA:amino acid N-acyltransferase. J Biol Chem. 269:19375–19379.
1994.PubMed/NCBI
|
|
2
|
Lambert IH, Jensen JV and Pedersen PA:
mTOR ensures increased release and reduced uptake of the organic
osmolyte taurine under hypoosmotic conditions in mouse fibroblasts.
Am J Physiol Cell Physiol. 306:C1028–C1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Espe M and Holen E: Taurine attenuates
apoptosis in primary liver cells isolated from Atlantic salmon
(Salmo salar). Br J Nutr. 110:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trenkner E: Possible role of glutamate
with taurine in neuron-glia interaction during cerebellar
development. Prog Clin Biol Res. 351:133–140. 1990.PubMed/NCBI
|
|
5
|
Schaffer SW, Azuma J and Madura JD:
Mechanisms underlying taurine-mediated alterations in membrane
function. Amino Acids. 8:231–246. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tappaz ML: Taurine biosynthetic enzymes
and taurine transporter: Molecular identification and regulations.
Neurochem Res. 29:83–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stipanuk MH: Role of the liver in
regulation of body cysteine and taurine levels: A brief review.
Neurochem Res. 29:105–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palkovits M, Elekes I, Láng T and Patthy
A: Taurine levels in discrete brain nuclei of rats. J Neurochem.
47:1333–1335. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stipanuk MH, Londono M, Lee JI, Hu M and
Yu AF: Enzymes and metabolites of cysteine metabolism in nonhepatic
tissues of rats show little response to changes in dietary protein
or sulfur amino acid levels. J Nutr. 132:3369–3378. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueki I and Stipanuk MH: 3T3-L1 adipocytes
and rat adipose tissue have a high capacity for taurine synthesis
by the cysteine dioxygenase/cysteinesulfinate decarboxylase and
cysteamine dioxygenase pathways. J Nutr. 139:207–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueki I and Stipanuk MH: Enzymes of the
taurine biosynthetic pathway are expressed in rat mammary gland. J
Nutr. 137:1887–1894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park E, Park SY, Wang C, Xu J, LaFauci G
and Schuller-Levis G: Cloning of murine cysteine sulfinic acid
decarboxylase and its mRNA expression in murine tissues. Biochim
Biophys Acta. 1574:403–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marcinkiewicz J and Kontny E: Taurine and
inflammatory diseases. Amino Acids. 46:7–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jerkins AA and Steele RD: Quantification
of cysteine sulfinic acid decarboxylase in male and female rats:
Effect of adrenalectomy and methionine. Arch Biochem Biophys.
294:534–538. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ueki I, Roman HB, Valli A, Fieselmann K,
Lam J, Peters R, Hirschberger LL and Stipanuk MH: Knockout of the
murine cysteine dioxygenase gene results in severe impairment in
ability to synthesize taurine and an increased catabolism of
cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab.
301:E668–E684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roman HB, Hirschberger LL, Krijt J, Valli
A, Kožich V and Stipanuk MH: The cysteine dioxgenase knockout
mouse: Altered cysteine metabolism in nonhepatic tissues leads to
excess H2S/HS(−) production and evidence of pancreatic and lung
toxicity. Antioxid Redox Signal. 19:1321–1336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sturman JA: Taurine in development. J
Nutr. 118:1169–1176. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyamoto Y, Tiruppathi C, Ganapathy V and
Leibach FH: Active transport of taurine in rabbit jejunal
brush-border membrane vesicles. Am J Physiol. 257:G65–G72.
1989.PubMed/NCBI
|
|
19
|
O'Flaherty L, Stapleton PP, Redmond HP and
Bouchier-Hayes DJ: Intestinal taurine transport: A review. Eur J
Clin Invest. 27:873–880. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glass EN, Odle J and Baker DH: Urinary
taurine excretion as a function of taurine intake in adult cats. J
Nutr. 122:1135–1142. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schuller-Levis G and Park E: Is taurine a
biomarker. Advances in Clinical Chemistry. 41:Elsevier. 1–21. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson CMH, Howard A, Walters JRF,
Ganapathy V and Thwaites DT: Taurine uptake across the human
intestinal brush-border membrane is via two transporters:
H+-coupled PAT1 (SLC36A1) and Na+- and
Cl−-dependent TauT (SLC6A6): intestinal taurine
transport via PAT1 (SLC36A1) and TauT (SLC6A6). J Physiol.
587:731–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voss JW, Pedersen SF, Christensen ST and
Lambert IH: Regulation of the expression and subcellular
localization of the taurine transporter TauT in mouse NIH3T3
fibroblasts. Eur J Biochem. 271:4646–4658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jensen A, Figueiredo-Larsen M, Holm R,
Broberg ML, Brodin B and Nielsen CU: PAT1 (SLC36A1) shows nuclear
localization and affects growth of smooth muscle cells from rats.
Am J Physiol Endocrinol Metab. 306:E65–E74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jong CJ, Ito T, Mozaffari M, Azuma J and
Schaffer S: Effect of β-alanine treatment on mitochondrial taurine
level and 5-taurinomethyluridine content. J Biomed Sci. 17 (Suppl
1):S252010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ubuka T, Okada A and Nakamura H:
Production of hypotaurine from L-cysteinesulfinate by rat liver
mitochondria. Amino Acids. 35:53–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki T, Suzuki T, Wada T, Saigo K and
Watanabe K: Taurine as a constituent of mitochondrial tRNAs: New
insights into the functions of taurine and human mitochondrial
diseases. EMBO J. 21:6581–6589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ögmundsdóttir MH, Heublein S, Kazi S,
Reynolds B, Visvalingam SM, Shaw MK and Goberdhan DCI:
Proton-assisted amino acid transporter PAT1 complexes with Rag
GTPases and activates TORC1 on late endosomal and lysosomal
membranes. PLoS One. 7:e366162012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takeuchi K, Toyohara H and Sakaguchi M: A
hyperosmotic stress-induced mRNA of carp cell encodes
Na+- and Cl−-dependent high affinity taurine
transporter1 the sequence reported in this paper has been deposited
in the DDBJ/EMBL/GenBank database with accession no. AB006986.1.
Biochim Biophys Acta Biomembr. 1464:219–230. 2000. View Article : Google Scholar
|
|
30
|
Mollerup J and Lambert IH: Calyculin a
modulates the kinetic constants for the Na+-coupled
taurine transport in Ehrlich ascites tumour cells. Biochim Biophys
Acta Biomembr. 1371:335–344. 1998. View Article : Google Scholar
|
|
31
|
Sakai S, Tosaka T, Tasaka J, Hashiguchi T
and Yoshihama I: Taurine uptake by glial cells in the bullfrog
sympathetic ganglia. Neurochem Int. 14:193–198. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han X, Patters AB, Jones DP, Zelikovic I
and Chesney RW: The taurine transporter: Mechanisms of regulation.
Acta Physiol (Oxf). 187:61–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heller-Stilb B, van Roeyen C, Rascher K,
Hartwig HG, Huth A, Seeliger MW, Warskulat U and Häussinger D:
Disruption of the taurine transporter gene (taut) leads to retinal
degeneration in mice. FASEB J. 16:231–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han X, Patters AB and Chesney RW:
Transcriptional repression of taurine transporter gene (TauT) by
p53 in renal cells. J Biol Chem. 277:39266–39273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lambert IH: Regulation of the cellular
content of the organic osmolyte taurine in mammalian cells.
Neurochem Res. 29:27–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyazaki T and Matsuzaki Y: Taurine and
liver diseases: A focus on the heterogeneous protective properties
of taurine. Amino Acids. 46:101–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hammarqvist F, Angsten G, Meurling S,
Andersson K and Wernerman J: Age-related changes of muscle and
plasma amino acids in healthy children. Amino Acids. 39:359–366.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Y, Hu Z, Chen B, Bu Q, Lu W, Deng Y,
Zhu R, Shao X, Hou J, Zhao J, et al: Taurine attenuates
methamphetamine-induced autophagy and apoptosis in PC12 cells
through mTOR signaling pathway. Toxicol Lett. 215:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Holecek M and Sispera L: Effects of
arginine supplementation on amino acid profiles in blood and
tissues in fed and overnight-fasted rats. Nutrients. 8:2062016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Li F, Wu L, Wei H, Liu Y, Li T, Tan
B, Kong X, Yao K, Chen S, et al: Effects of dietary protein
restriction on muscle fiber characteristics and mTORC1 pathway in
the skeletal muscle of growing-finishing pigs. J Anim Sci
Biotechnol. 7:472016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holecek M and Kovarik M: Alterations in
protein metabolism and amino acid concentrations in rats fed by a
high-protein (casein-enriched) diet - effect of starvation. Food
Chem Toxicol. 49:3336–3342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Sebastian BM, Tang H, McMullen MM,
Axhemi A, Jacobsen DW and Nagy LE: Taurine supplementation prevents
ethanol-induced decrease in serum adiponectin and reduces hepatic
steatosis in rats. Hepatology. 49:1554–1562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mochizuki T, Satsu H, Nakano T and Shimizu
M: Regulation of the human taurine transporter by TNF-α and an
anti-inflammatory function of taurine in human intestinal Caco-2
cells. Biofactors. 21:141–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ishizuka K, Miyamoto Y, Satsu H, Sato R
and Shimizu M: Characteristics of lysophosphatidylcholine in its
inhibition of taurine uptake by human intestinal Caco-2 cells.
Biosci Biotechnol Biochem. 66:730–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mochizuki T, Satsu H and Shimizu M:
Signaling pathways involved in tumor necrosis factor α-induced
upregulation of the taurine transporter in Caco-2 cells. FEBS Lett.
579:3069–3074. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Merheb M, Daher RT, Nasrallah M, Sabra R,
Ziyadeh FN and Barada K: Taurine intestinal absorption and renal
excretion test in diabetic patients: A pilot study. Diabetes Care.
30:2652–2654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han X, Budreau AM and Chesney RW: The
taurine transporter gene and its role in renal development. Amino
Acids. 19:499–507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chesney RW, Scriver CR and Mohyuddin F:
Localization of the membrane defect in transepithelial transport of
taurine by parallel studies in vivo and in vitro in hypertaurinuric
mice. J Clin Invest. 57:183–193. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Satsu H, Miyamoto Y and Shimizu M:
Hypertonicity stimulates taurine uptake and transporter gene
expression in Caco-2 cells. Biochim Biophys Acta. 1419:89–96. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Uchida S, Kwon HM, Yamauchi A, Preston AS,
Marumo F and Handler JS: Molecular cloning of the cDNA for an MDCK
cell Na(+)- and Cl(−)-dependent taurine transporter that is
regulated by hypertonicity. Proc Natl Acad Sci USA. 89:8230–8234.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pramod AB, Foster J, Carvelli L and Henry
LK: SLC6 transporters: Structure, function, regulation, disease
association and therapeutics. Mol Aspects Med. 34:197–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kubo Y, Akanuma SI and Hosoya KI: Impact
of SLC6A transporters in physiological taurine transport at the
blood-retinal barrier and in the liver. Biol Pharm Bull.
39:1903–1911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zelikovic I and Chesney RW: Sodium-coupled
amino acid transport in renal tubule. Kidney Int. 36:351–359. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Silbernagl S: The renal handling of amino
acids and oligopeptides. Physiol Rev. 68:911–1007. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zelikovic I and Chesney RW: Ionic
requirements for amino acid transport. Am J Kidney Dis. 14:313–316.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hoffmann EK and Lambert IH: Amino acid
transport and cell volume regulation in Ehrlich ascites tumour
cells. J Physiol. 338:613–625. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Han X, Budreau AM and Chesney RW:
Identification of promoter elements involved in adaptive regulation
of the taurine transporter gene: Role of cytosolic Ca2+
signaling. Adv Exp Med Biol. 483:535–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte
A, Costa M and Chen Y: The potential protective effects of taurine
on coronary heart disease. Atherosclerosis. 208:19–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jones DP, Miller LA and Chesney RW:
Polarity of taurine transport in cultured renal epithelial cell
lines: LLC-PK1 and MDCK. Am J Physiol. 265:F137–F145.
1993.PubMed/NCBI
|
|
60
|
Jones DP, Miller LA, Dowling C and Chesney
RW: Regulation of taurine transporter activity in LLC-PK1 cells:
Role of protein synthesis and protein kinase C activation. J Am Soc
Nephrol. 2:1021–1029. 1991.PubMed/NCBI
|
|
61
|
Jones DP, Miller LA and Chesney RW:
Adaptive regulation of taurine transport in two continuous renal
epithelial cell lines. Kidney Int. 38:219–226. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jones DP, Miller LA, Budreau A and Chesney
RW: Characteristics of taurine transport in cultured renal
epithelial cell lines: asymmetric polarity of proximal and distal
cell lines. Adv Exp Med Biol. 315:405–411. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Handler JS and Kwon HM: Transcriptional
regulation by changes in tonicity. Kidney Int. 60:408–411. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ito T, Fujio Y, Schaffer SW and Azuma J:
Involvement of transcriptional factor TonEBP in the regulation of
the taurine transporter in the cardiomyocyte. Adv Exp Med Biol.
643:523–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ito T, Fujio Y, Hirata M, Takatani T,
Matsuda T, Muraoka S, Takahashi K and Azuma J: Expression of
taurine transporter is regulated through the TonE
(tonicity-responsive element)/TonEBP (TonE-binding protein) pathway
and contributes to cytoprotection in HepG2 cells. Biochem J.
382:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ortells MC, Morancho B, Drews-Elger K,
Viollet B, Laderoute KR, López-Rodríguez C and Aramburu J:
Transcriptional regulation of gene expression during osmotic stress
responses by the mammalian target of rapamycin. Nucleic Acids Res.
40:4368–4384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Roos S, Kanai Y, Prasad PD, Powell TL and
Jansson T: Regulation of placental amino acid transporter activity
by mammalian target of rapamycin. Am J Physiol Cell Physiol.
296:C142–C150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Han X and Chesney RW: The role of taurine
in renal disorders. Amino Acids. 43:2249–2263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han X, Yue J and Chesney RW: Functional
TauT protects against acute kidney injury. J Am Soc Nephrol.
20:1323–1332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Matsell DG, Bennett T, Han X, Budreau AM
and Chesney RW: Regulation of the taurine transporter gene in the
S3 segment of the proximal tubule. Kidney Int. 52:748–754. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shalby AB, Assaf N and Ahmed HH: Possible
mechanisms for N-acetyl cysteine and taurine in ameliorating acute
renal failure induced by cisplatin in rats. Toxicol Mech Methods.
21:538–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Han X: Targeting taurine transporter
(TauT) for cancer immunotherapy of p53 mutation mediated cancers -
molecular basis and preclinical implication. Adv Exp Med Biol.
1155:543–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hansen DB, Friis MB, Hoffmann EK and
Lambert IH: Downregulation of the taurine transporter TauT during
hypo-osmotic stress in NIH3T3 mouse fibroblasts. J Membr Biol.
245:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mollerup J and Lambert IH: Phosphorylation
is involved in the regulation of the taurine influx via the
β-system in Ehrlich ascites tumor cells. J Membr Biol. 150:73–82.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Qian X, Vinnakota S, Edwards C and Sarkar
HK: Molecular characterization of taurine transport in bovine
aortic endothelial cells. Biochim Biophys Acta. 1509:324–334. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jacobsen JH, Clement CA, Friis MB and
Lambert IH: Casein kinase 2 regulates the active uptake of the
organic osmolyte taurine in NIH3T3 mouse fibroblasts. Pflugers
Arch. 457:327–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Smith KE, Borden LA, Wang CH, Hartig PR,
Branchek TA and Weinshank RL: Cloning and expression of a high
affinity taurine transporter from rat brain. Mol Pharmacol.
42:563–569. 1992.PubMed/NCBI
|
|
78
|
Kang YS, Ohtsuki S, Takanaga H, Tomi M,
Hosoya K and Terasaki T: Regulation of taurine transport at the
blood-brain barrier by tumor necrosis factor-α, taurine and
hypertonicity. J Neurochem. 83:1188–1195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Frank RN: Diabetic retinopathy. N Engl J
Med. 350:48–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lorenzi M, Healy DP, Hawkins R, Printz JM
and Printz MP: Studies on the permeability of the blood-brain
barrier in experimental diabetes. Diabetologia. 29:58–62. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Schoch HJ, Fischer S and Marti HH:
Hypoxia-induced vascular endothelial growth factor expression
causes vascular leakage in the brain. Brain. 125:2549–2557. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yeh WL, Lu DY, Lin CJ, Liou HC and Fu WM:
Inhibition of hypoxia-induced increase of blood-brain barrier
permeability by YC-1 through the antagonism of HIF-1α accumulation
and VEGF expression. Mol Pharmacol. 72:440–449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Nicholson BP and Schachat AP: A review of
clinical trials of anti-VEGF agents for diabetic retinopathy.
Graefes Arch Clin Exp Ophthalmol. 248:915–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Poulaki V, Qin W, Joussen AM, Hurlbut P,
Wiegand SJ, Rudge J, Yancopoulos GD and Adamis AP: Acute intensive
insulin therapy exacerbates diabetic blood-retinal barrier
breakdown via hypoxia-inducible factor-1α and VEGF. J Clin Invest.
109:805–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Napoli Z, Seghieri G, Bianchi L, Anichini
R, De Bellis A, Campesi I, Carru C, Occhioni S, Zinellu A and
Franconi F: Taurine transporter gene expression in mononuclear
blood cells of type 1 diabetes patients. J Diabetes Res.
2016:73131622016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bianchi L, Lari R, Anichini R, De Bellis
A, Berti A, Napoli Z, Seghieri G and Franconi F: Taurine
transporter gene expression in peripheral mononuclear blood cells
of type 2 diabetic patients. Amino Acids. 42:2267–2274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Han X and Chesney RW: Knockdown of TauT
expression impairs human embryonic kidney 293 cell development. Adv
Exp Med Biol. 776:307–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Son HY, Kim H and Kwon YH: Taurine
prevents oxidative damage of high glucose-induced cataractogenesis
in isolated rat lenses. J Nutr Sci Vitaminol (Tokyo). 53:324–330.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hou X, Wang Z, Ding F, He Y, Wang P, Liu
X, Xu F, Wang J and Yang Y: Taurine transporter regulates
adipogenic differentiation of human adipose-derived stem cells
through affecting Wnt/β-catenin signaling pathway. Int J Biol Sci.
15:1104–1112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tervaert TWC, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al Renal Pathology Society, : Pathologic classification of
diabetic nephropathy. J Am Soc Nephrol. 21:556–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lang PA, Warskulat U, Heller-Stilb B,
Huang DY, Grenz A, Myssina S, Duszenko M, Lang F, Häussinger D,
Vallon V, et al: Blunted apoptosis of erythrocytes from taurine
transporter deficient mice. Cell Physiol Biochem. 13:337–346. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Villumsen KR, Duelund L and Lambert IH:
Acute cholesterol depletion leads to net loss of the organic
osmolyte taurine in Ehrlich Lettré tumor cells. Amino Acids.
39:1521–1536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lambert IH, Nielsen JH, Andersen HJ and
Ørtenblad N: Cellular model for induction of drip loss in meat. J
Agric Food Chem. 49:4876–4883. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Poulsen KA, Andersen EC, Hansen CF,
Klausen TK, Hougaard C, Lambert IH and Hoffmann EK: Deregulation of
apoptotic volume decrease and ionic movements in
multidrug-resistant tumor cells: Role of chloride channels. Am J
Physiol Cell Physiol. 298:C14–C25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lambert IH: Effect of arachidonic acid on
conductive Na, K and anion transport in Ehlrich ascites tumor cells
under isotonic and hypotonic conditions. Cell Physiol Biochem.
1:177–194. 1991. View Article : Google Scholar
|
|
96
|
Lambert IH: Effect of arachidonic acid,
fatty acids, prostaglandins, and leukotrienes on volume regulation
in Ehrlich ascites tumor cells. J Membr Biol. 98:207–221. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lambert IH: Reactive oxygen species
regulate swelling-induced taurine efflux in NIH3T3 mouse
fibroblasts. J Membr Biol. 192:19–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ito T, Kimura Y, Uozumi Y, Takai M,
Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, et al:
Taurine depletion caused by knocking out the taurine transporter
gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell
Cardiol. 44:927–937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Izumi K, Kishita C, Nakagawa K, Huxtable
RJ, Shimizu T, Koja T and Fukuda T: Modification of the
antiepileptic actions of phenobarbital and phenytoin by the taurine
transport inhibitor, guanidinoethane sulfonate. Eur J Pharmacol.
110:219–224. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Schaffer SW, Shimada-Takaura K, Jong CJ,
Ito T and Takahashi K: Impaired energy metabolism of the taurine
deficient heart. Amino Acids. 48:549–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Choi D, Kim SJ, Kwon DY, Lee SY and Kim
YC: Taurine depletion by beta-alanine inhibits induction of
hepatotoxicity in mice treated acutely with carbon tetrachloride.
Adv Exp Med Biol. 643:305–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Warskulat U, Flögel U, Jacoby C, Hartwig
HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J
and Häussinger D: Taurine transporter knockout depletes muscle
taurine levels and results in severe skeletal muscle impairment but
leaves cardiac function uncompromised. FASEB J. 18:577–579. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lötsch J, Hummel T, Warskulat U, Coste O,
Häussinger D, Geisslinger G and Tegeder I: Congenital taurine
deficiency in mice is associated with reduced sensitivity to
nociceptive chemical stimulation. Neuroscience. 259:63–70. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ito T, Oishi S, Takai M, Kimura Y, Uozumi
Y, Fujio Y, Schaffer SW and Azuma J: Cardiac and skeletal muscle
abnormality in taurine transporter-knockout mice. J Biomed Sci. 17
(Suppl 1):S202010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kaesler S, Sobiesiak M, Kneilling M, Volz
T, Kempf WE, Lang PA, Lang KS, Wieder T, Heller-Stilb B, Warskulat
U, et al: Effective T-cell recall responses require the taurine
transporter Taut. Eur J Immunol. 42:831–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Warskulat U, Borsch E, Reinehr R,
Heller-Stilb B, Mönnighoff I, Buchczyk D, Donner M, Flögel U,
Kappert G, Soboll S, et al: Chronic liver disease is triggered by
taurine transporter knockout in the mouse. FASEB J. 20:574–576.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Warskulat U, Borsch E, Reinehr R,
Heller-Stilb B, Roth C, Witt M and Häussinger D: Taurine deficiency
and apoptosis: Findings from the taurine transporter knockout
mouse. Arch Biochem Biophys. 462:202–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Qvartskhava N, Jin CJ, Buschmann T,
Albrecht U, Bode JG, Monhasery N, Oenarto J, Bidmon HJ, Görg B and
Häussinger D: Taurine transporter (TauT) deficiency impairs ammonia
detoxification in mouse liver. Proc Natl Acad Sci USA.
116:6313–6318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ito T, Okazaki K, Nakajima D, Shibata D,
Murakami S and Schaffer S: Mass spectrometry-based metabolomics to
identify taurine-modified metabolites in heart. Amino Acids.
50:117–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Franchi-Gazzola R, Gaccioli F, Bevilacqua
E, Visigalli R, Dall'Asta V, Sala R, Varoqui H, Erickson JD,
Gazzola GC and Bussolati O: The synthesis of SNAT2 transporters is
required for the hypertonic stimulation of system a transport
activity. Biochim Biophys Acta Biomembr. 1667:157–166. 2004.
View Article : Google Scholar
|
|
111
|
Trama J, Go WY and Ho SN: The
osmoprotective function of the NFAT5 transcription factor in T cell
development and activation. J Immunol. 169:5477–5488. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Burg MB and Ferraris JD: Intracellular
organic osmolytes: Function and regulation. J Biol Chem.
283:7309–7313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Schaffer SW, Jong CJ, Ito T and Azuma J:
Role of taurine in the pathologies of MELAS and MERRF. Amino Acids.
46:47–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Board PG, Moore KA and Smith JE:
Purification and properties of γ-glutamylcyclotransferase from
human erythrocytes. Biochem J. 173:427–431. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Krishnamurthy J, Torrice C, Ramsey MR,
Kovalev GI, Al-Regaiey K, Su L and Sharpless NE: Ink4a/Arf
expression is a biomarker of aging. J Clin Invest. 114:1299–1307.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ito T, Yoshikawa N, Inui T, Miyazaki N,
Schaffer SW and Azuma J: Tissue depletion of taurine accelerates
skeletal muscle senescence and leads to early death in mice. PLoS
One. 9:e1074092014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Back SH, Scheuner D, Han J, Song B, Ribick
M, Wang J, Gildersleeve RD, Pennathur S and Kaufman RJ: Translation
attenuation through eIF2α phosphorylation prevents oxidative stress
and maintains the differentiated state in β cells. Cell Metab.
10:13–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol Cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Araki K and Nagata K: Protein folding and
quality control in the ER. Cold Spring Harb Perspect Biol.
4:a015438. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Salminen A and Kaarniranta K: ER stress
and hormetic regulation of the aging process. Ageing Res Rev.
9:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jong CJ, Ito T, Azuma J and Schaffer S:
Taurine depletion decreases GRP78 expression and downregulates
Perk-dependent activation of the unfolded protein response. Adv Exp
Med Biol. 803:571–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ito T, Yamamoto N, Nakajima S and Schaffer
SW: Beta-catenin and SMAD3 are associated with skeletal muscle
aging in the taurine transporter knockout mouse. Adv Exp Med Biol
975 (Pt 1). 497–502. 2017. View Article : Google Scholar
|
|
123
|
Kirino Y, Yasukawa T, Ohta S, Akira S,
Ishihara K, Watanabe K and Suzuki T: Codon-specific translational
defect caused by a wobble modification deficiency in mutant tRNA
from a human mitochondrial disease. Proc Natl Acad Sci USA.
101:15070–15075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yasukawa T, Suzuki T, Ueda T, Ohta S and
Watanabe K: Modification defect at anticodon wobble nucleotide of
mitochondrial tRNAs(Leu)(UUR) with pathogenic mutations of
mitochondrial myopathy, encephalopathy, lactic acidosis, and
stroke-like episodes. J Biol Chem. 275:4251–4257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mozaffari MS, Tan BH, Lucia MA and
Schaffer SW: Effect of drug-induced taurine depletion on cardiac
contractility and metabolism. Biochem Pharmacol. 35:985–989. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jong CJ, Azuma J and Schaffer S: Mechanism
underlying the antioxidant activity of taurine: Prevention of
mitochondrial oxidant production. Amino Acids. 42:2223–2232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Vionnet N, Passa P and Froguel P:
Prevalence of mitochondrial gene mutations in families with
diabetes mellitus. Lancet. 342:1429–1430. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Maassen JA, 'T Hart LM, Van Essen E, Heine
RJ, Nijpels G, Jahangir Tafrechi RSJ, Raap AK, Janssen GMC and
Lemkes HHPJ: Mitochondrial diabetes: Molecular mechanisms and
clinical presentation. Diabetes. 53 (Suppl 1):S103–S109. 2004.
View Article : Google Scholar : PubMed/NCBI
|