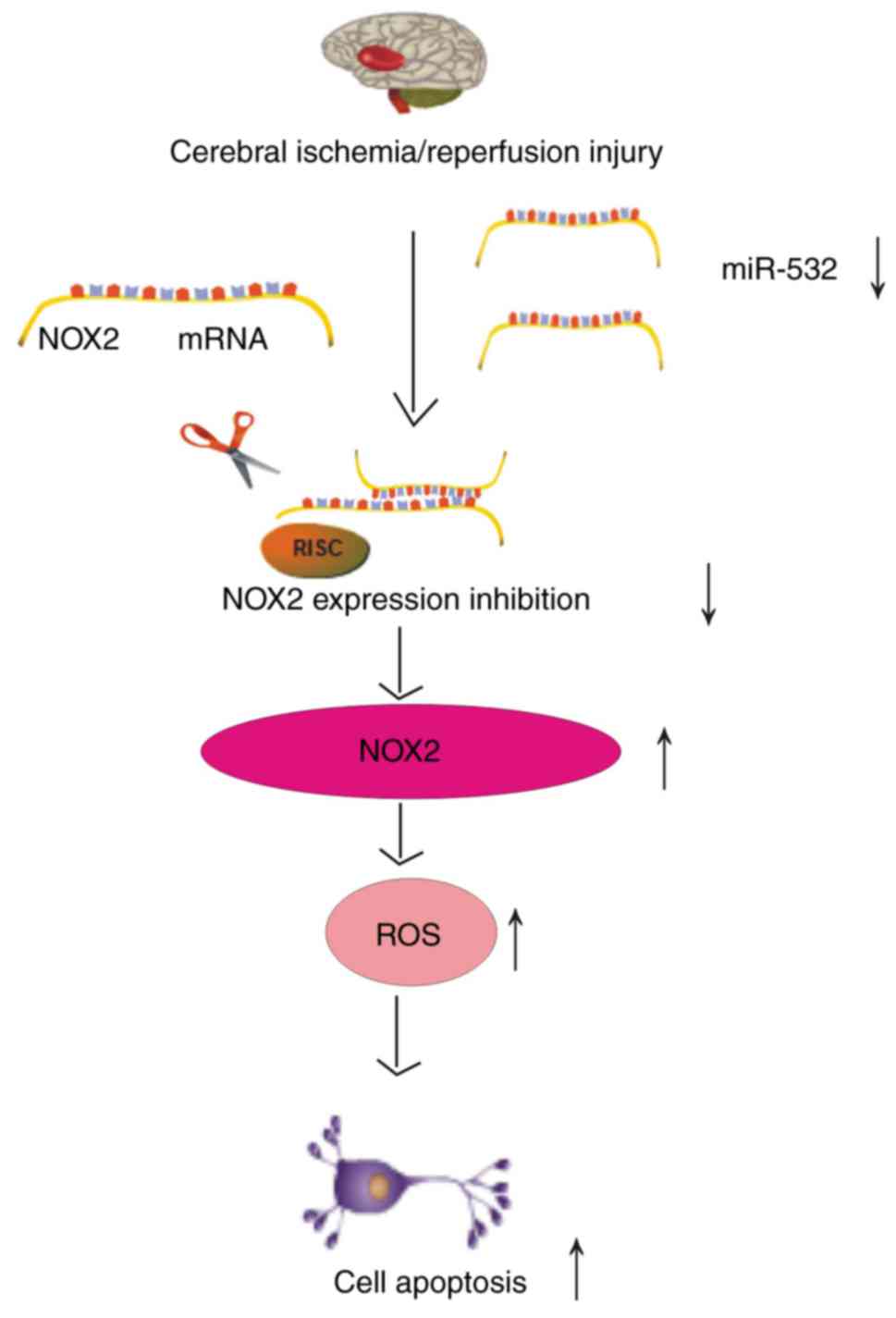
Introduction
Ischemic stroke (IS) is an age-related central
nervous system disease that occurs worldwide, and is associated
with high mortality and morbidity rates, as well as large economic
and psychological burdens to patients and their families (1). When the blood supply of the brain
vessels is interrupted, the affected brain tissues that lack an
adequate oxygen and glucose supply suffer severe damage and death,
which leads to IS (2). Currently,
the most common treatment strategy for IS is recovering the blood
supply of the brain using methods such as thrombolysis (1). However, reperfusion of blood to the
brain results in severe injury of the affected neuron cells, which
is termed cerebral ischemia/reperfusion (CI/R) injury (3). Numerous factors contribute to the
pathogenesis of CI/R injury, such as oxidative stress,
excitotoxicity and edema (2,4).
Moreover, oxidative stress is reported to serve pivotal roles in
CI/R injury (4); however, its
exact mechanism is yet to be elucidated. Therefore, identifying the
mechanism of oxidative stress in CI/R injury has become an
important topic of research in IS therapy and drug development.
NADPH oxidase (NOX) is a critical enzyme for the
production of reactive oxygen species (ROS) in several cell types,
such as neutrophils, white blood cells and neurons (5). Furthermore, NOX mediates the electron
transport between NADPH and oxygen and generates superoxide anion
(a reactive free-radical), which is the primary cause of oxidative
stress injury (6). Previous
studies have reported that seven isoforms of NOX exist in mammalian
cells, including NOX1-NOX5, Dual Oxidase (DUOX)1 and DUOX2
(5,6). In addition, our previous study
revealed that NOX4, and particularly NOX2, were the primary
subtypes responsible for ROS generation in CI/R injury (6). When subjected to I/R injury, the
expression of NOX2 in affected brain tissues is significantly
upregulated and is followed by ROS overproduction (6). In addition, middle cerebral artery
occlusion (MCAO) rats administrated with a NOX2 inhibitor, such as
apocynin, exhibit reduced cerebral infarction and improved
neurological function (7). These
findings suggest that NOX2 is a promising target for the treatment
of CI/R injury. Therefore, elucidating the regulatory mechanism of
NOX2 expression is important for the understanding of CI/R injury,
and thus requires further investigation.
MicroRNAs (miRNAs/miRs) are a class of epigenetic
regulators, with a length of 21–24 nucleotides, which serve a role
in gene expression and are involved in the pathogenesis of numerous
diseases, including IS (8). The
expression levels of miRNAs have been identified to be altered in
the brain tissues of rats when subjected to MCAO, such as miR-107
(9–11). Although previous studies have shown
that miR-126a-3p and miR-138-5p are targets of NOX2 (12), further research is required to
examine the target miRNAs of NOX2 in CI/R injury. miR-532 is a
multifunctional molecule and has important roles in multiple
biological processes. For example, miR-532 mitigates cardiomyocyte
apoptosis caused by hypoxia by targeting the pyruvate dehydrogenase
complex associated with energy homeostasis in the heart (13,14).
In addition, miR-532 is an important molecule in the pathogenesis
of several central neuronal system diseases, including IS (15,16).
However, to the best of our knowledge, whether there is an
interaction between miR-532 and NOX2 has not yet been investigated.
Therefore, examining the roles of miR-532 in the regulation of NOX2
is important for the understanding and treatment of IS.
The present study was conducted to assess whether
miR-532-3P is a target miRNA of NOX2 and to identify the role of
miR-532-3p in CI/R oxidative stress injury. To the best of our
knowledge, the present study is the first to investigate the role
of the miR-532-3p/NOX2 axis in CI/R injury. Therefore, the results
may provide a novel target for drug development and IS therapy.
Materials and methods
Animal experiments
A total of 16 male Sprague-Dawley rats (weight,
240–260 g; age, 8 weeks) were purchased from the Hunan SJA
Laboratory Animal Co., Ltd., and a rat CI/R injury model was
established following a previously described surgical method
(11). Rats were subjected to a 2
h occlusion of the left internal carotid artery and a 24 h
reperfusion. During the whole experimental process, the animals
were housed under standard conditions (25°C; 65 % humidity; an
alternative of 12 h light/12 h dark cycle) with free access to food
and water. In total, 3% pentobarbital sodium (40 mg/kg weight) was
used for light anesthesia. During the surgical course, the body
temperature of the rat was maintained at ~37°C. The duration of the
experiments was ~26 h (including 2 h ischemia and 24 h
reperfusion). During ischemia, vital signs of rats were monitored,
such as body temperature and respiration. During reperfusion, vital
signs of rats were monitored every 6 h. Animal experiments were
conducted following the Guide for the Care and Use of Laboratory
Animals published by the National Institute of Health (17), and were approved by the Animal
Ethics Committee of Hunan Normal University. There were eight rats
used in each group (I/R and sham). The sham group was subjected to
a skin incision without occlusion of the left internal carotid
artery.
At the end of the 24 h reperfusion, all rats (16
rats) were sacrificed by decapitation after loss of consciousness
following anesthesia via intraperitoneal injection of 3%
pentobarbital sodium (40 mg/kg weight). The neurological function
was evaluated following a 24 h reperfusion, and subsequently the
brain tissue was collected for 2,3,5-tripenyltetrazolium chloride
(TTC) staining or other assays, including the detect of protein and
mRNA expression levels.
Cell culture
The SH-SY5Y cell line (neuroblastoma) was provided
by the Committee on Type Culture Collection of Chinese Academy of
Sciences of Shanghai. Short-tandem repeat profiling of SH-SY5Y cell
line was performed prior to experiments. A compound culture was
used for cells culture, which consisted of DMEM (Thermo Fisher
Scientific, Inc.), 10% FBS and penicillin/streptomycin (100 U/ml).
Cells were maintained in a cell incubator at standard conditions
(37°C, 5% CO2 and 95% air). Cells (5×105)
were cultured with 12-well plates for miRNA functional assays and
the luciferase reporter gene experiment.
Cell model of hypoxia/reoxygenation
(H/R)
An in vitro SH-SY5Y cell H/R model was
established to mimic IS or CI/R injury. When cells reached 70%
confluence, DMEM culture was removed and replaced with Dulbecco's
phosphate-buffered saline (DPBS; Sigma-Aldrich; Merck KGaA). Then,
cells were maintained at 37°C in a hypoxic condition (95%
N2 and 5% CO2) for 5 h. After the 5 h hypoxia
process, DPBS was removed and the compound DMEM culture was added
and cells were maintained under standard conditions (37°C, 5%
CO2 and 95% air) for 20 h reoxygenation.
Assessment of brain injury
To evaluate the degree of brain injury, a 5-point
rating scale was used for neurological function assessment.
According to the scale, 0 indicated the rat had no deficit, 1
indicates the rat was unable to spread the left forepaw, 2
indicates the rat's grasp strength of the left forepaw was reduced,
3 indicates the rat circling to the left on pulling of the tail and
4 indicates the rat is spontaneously circling (6).
Brain infarct was assessed using TTC staining
(ischemic hemisphere and non-ischemic contralateral side appear
while or red, respectively). Brain slices were prepared by
coronally cutting brain tissues into sections with a thickness of
0.2–0.3 cm. Then, the slices were immersed in 2% TTC and maintained
in dark (37°C) for 0.5 h. Stained brain tissues were scanned and
the infarct volume was calculated using ImageJ software (version
1.4; National Institutes of Health). The infarction volume
(cm3) of each slice was calculated using the following
equation, which was described in our previous study (11): Infarction volume of each
slice=infarct size (cm2) of each slice × thickness.
Determination of NOX activity
To measure the total NOX enzyme activity, a
colorimetric commercial kit (cat. no. GMS50095.1; Genmed
Pharmaceutical Technology Co., Ltd.) was used according to the
manufacturer's instructions. A mixture (90 µl) of cell or tissues
lysates (Cell lysis buffer for Western and IP; cat. no. P0013;
Beyotime Institute of Biotechnology) and oxidized cytochrome c (2
µl) was prepared and aliquoted into a quartz cuvette. After the
mixture was reacted for 3 min at 30°C, 2 µl NADPH was added to
create a reaction solution. Then, the reaction solution was
incubated at 30°C for 15 min. Subsequently, NOX activity was
determined by measuring the absorbance of the solution at 550 nm
using a spectrophotometer.
Determination of caspase-3
activity
To measure caspase-3 activity, a commercial kit
(cat. no. C1116; Beyotime Institute of Biotechnology) was used
according to the manufacturer's instructions. A mixture of cell or
tissue lysate and caspase-3 substrate (Ac-DEVD-pNA; 10 µl) was
prepared and incubated at 37°C for 60 min. Then, caspase-3
enzymatic activity was determined by measuring the absorbance of
the reaction solution at 405 nm using a multiscan spectrum (Thermo
Fisher Scientific, Inc.). In total, one caspase-3 enzymatic
activity unit refers to the quantity of enzyme required to catalyze
1.0 nM substrate in 1 h at 37°C (6).
TUNEL/Hoechst double-labeling
A TUNEL assay kit (cat. no. C1086; Beyotime
Institute of Biotechnology), and a Hoechst 33342 kit (cat. no.
C1027; Beyotime Institute of Biotechnology) were used for the brain
tissue cellular apoptosis assay. The assay process was conducted
according to the method (TUNEL/Hoechst double-labeling) established
by Whiteside et al (18).
The sections of brain tissue (thickness, 5 µm) underwent the
following steps: Fixed with formaldehyde (4% w/v) at 25°C for 10
min, rinsed with PBS and post-fixed with formaldehyde and acetic
acid at 4°C for 5 min, washed with PBS, incubated with
equilibration buffer and working strength deoxynucleotide
transferase at 37°C for 1 h, washed with PBS and incubated with
Hoechst 33342 at 25°C for 5 min. Slices were sealed using neutral
balsam and examined using an epifluorescence microscope (Nikon
Eclipse 80i) at ×200 magnification in eight randomly selected
fields of view. TUNEL-positive cells indicated apoptotic cells.
Hoechst staining
A commercial kit (Hoechst 33258; cat. no. C1017;
Beyotime Institute of Biotechnology) was used to evaluate the
apoptosis of SH-SY5Y cell. According to the manufacture's protocol,
SH-SY5Y cells underwent the following steps: Fixed with
formaldehyde (4%) at 25°C for 10 min, rinsed with PBS, stained with
Hoechst 33258 at 25°C for 5 min and imaged using a fluorescent
microscope (magnification, ×200). Cellular apoptosis was evaluated
by calculating apoptotic percentage. Cellular apoptosis was
calculated using the following formula: Number of apoptosis
bodies/(number of apoptotic cells + number of cells).
Flow cytometry analysis
An Annexin V-FITC kit (cat. no. C1062M; Beyotime
Institute of Biotechnology) was used to evaluate apoptosis of
SH-SY5Y cells. According to the manufacturer's instruction, SH-SY5Y
cells underwent the following steps: Incubated with FITC-conjugated
Annexin V (2.5%, v/v) and propidium iodide (PI; 5%, v/v) in the
dark at 25°C for 20 min, and then flow cytometry (BD FACSCalibur;
BD Biosciences) was used to analyze cellular apoptosis. Cell
apoptosis was analyzed using CellQuest Pro software (version 4.02;
BD Biosciences). The rate of apoptosis was defined as the
percentage of early and late apoptotic cells.
Bioinformatic prediction
TargetScan (www.targetscan.org/vert-72) and miRbase (www.mirbase.org) were used to predict the target
miRNAs of NOX2.
Transfection with miR-532-3p
mimics
To observe the function of miR-532-3p, an in
vitro gain-of function experiment was performed using
miR-532-3p mimics (5′-CCUCCCACACCCAAGGCUUGCA-3′) and negative
control miRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) (Guangzhou RiboBio Co.,
Ltd.). According to the manufacturer's protocols, a reaction
mixture consisting of Lipofectamine® 2,000 (Invitrogen;
Thermo Fisher Scientific, Inc.) and miR-532-3p mimic was prepared
and the final concentration of miR-532-3p mimic was 100 nmol. After
6 h of miR-532-3p mimic transfection, the mimic mixture was removed
and replaced with standard media (DMEM supplement with 10% FBS) for
another 48 h. Then, cells were collected for various analysis, such
as western blotting and reverse transcription-quantitative PCR
(RT-qPCR).
Luciferase reporter gene
experiment
To determine the relationship between miR-532-3p and
NOX2, a luciferase reporter gene plasmid (pGL6; Promega
Corporation) containing a wild-type (WT) sequence (5′-GUGGGAGA-3′)
for miR-532-3p (defined as NOX2-WT) or a mutated (MU) sequence
(5′-GUGAAAGA-3′) for miR-532-3p (NOX2-MU) was constructed. The
plasmids were purified by electrophoresis (0.8% agarose gel
supplemented with 0.5% ethidium bromide). Then, SH-SY5Y cells were
co-transfected with 100 ng plasmid (NOX2-WT or NOX2-MU) and
miR-532-3p mimics or negative control miRNAs using
Lipofectamine® 2000. At 6 h post-transfection, cells
were incubated with DMEM supplemented with 10% FBS for another 24
h. Subsequently, cells were collected to assess enzyme activity. To
evaluate the interaction between miR-532-3p and NOX2, a Dual
Luciferase Reporter Gene Assay Kit (Beyotime Institute of
Biotechnology) was used to measure the relative luciferase activity
of SH-SY5Y cells. Firefly luciferase activities were normalized to
Renilla luciferase activities.
Determination of ROS levels
To determine the intracellular total ROS levels in
SH-SY5Y cells, a Dichloro-dihydro-fluorescein diacetate (DCFH-DA)
commercial kit (Beyotime Institute of Biotechnology) was used
according to the manufacturer's instructions. SH-SY5Y cells or
tissue homogenization were immersed in DCFH-DA (10 µM) and
maintained in dark for 20 min (37°C). The fluorescence signal of
DCFH-DA at 502 nm was measured and the results are presented as
arbitrary units.
Plasma
To obtain the plasma, blood was centrifuged at 3000
r/min for 10 min at 4°C. The plasma was used for RNA extraction and
RT-qPCR.
RT-qPCR
TRIzol® reagent (Takara Biotechnology
Co., Ltd.) was used for RNA extraction. Total RNA of each sample
was obtained and purified prior to its concentration determination.
A reverse transcription kit (cat. no. DRR037A; Takara Bio, Inc.)
was used to obtain cDNA. The temperature protocol used for reverse
transcription was as follows: 37°C for 5 min and 85°C for 5 sec.
The RT PCR amplification reaction of NOX2 and miRNA was performed
using ABI 7300 plus system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). SYBR Green (cat. no. SC200N/SC210N; Takara Bio,
Inc.) was used for RT PCR. The following thermocycling conditions
were used for qPCR: 95°C for 30 sec; 95°C for 5 sec; and 60°C for
31 sec for 40 cycles. The PCR primers used were as follows: NOX2
forward, 5′-ACAAGGTTTATGACGATGAGCC-3′ and reverse,
5′-TTGAGCAACACGCACTGGAA-3′); β-actin forward,
5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′; miR-15a forward,
5′-UAGCAGCACAUAAUGGUUUGUG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
miR-16 forward, 5′-CGCGCTAGGAGCACGTAAATA-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; miR-30a forward, 5′-GGTTGGCGCTCTGAGAGGTA-3′
and reverse, 5′-GTGCAGGGGTCCGAGGT-3′; miR-125b forward,
5′-UCACCGGGUGUAAAUCAGCUU-3′ and reverse, 5′-GTGCAGGGGTCCGAGGT-3′;
miR-146a forward, 5′-GGGTGAGAACTGAATTCCA-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; miR-153 forward,
5′-GGGATGGAGTCGAGGTGCGGCTAAT-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′, miR-223 forward,
5′-UGUCAGUUUGUCAAAUACCCCA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
miR-532-3p forward, 5′-ACACTCCCCTCCCACACCCAAGG-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The 2−ΔΔCq method
was used for data analysis and results were expressed as the ratio
of NOX2 mRNA to β-actin mRNA or miR-532-3p to U6 (19).
Western blotting
Cell lysis buffer (cat. no. P0013; Beyotime
Institute of Biotechnology) was used for total protein extraction
of each sample and the bicinchoninic acid protein assay kit (cat.
no. P0009; Beyotime Institute of Biotechnology) was used for
protein concentration determination. Protein was incubated at 99°C
for 5 min for denaturation. Subsequently, 40 µg protein from each
sample was separated via 10% SDS-PAGE, which was then transferred
onto PVDF membranes. Membranes were then incubated with primary
antibodies (1:1,000) against rabbit anti-NOX2 (cat. no. sc-130543;
Santa Cruz Biotechnology, Inc.) anti-caspase-3 (cat. no. sc-7272;
Santa Cruz Biotechnology, Inc.) and β-actin (cat. no. sc-47778;
Santa Cruz Biotechnology, Inc.), followed by incubation with a
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat. no. A0208; Beyotime Institute of Biotechnology;
1:2,000). Enhanced chemiluminescence solutions (BeyoECL Plus kit;
Amersham; Cytiva) and a ChemiDox XRS+ Imaging System
(Bio-Rad Laboratories, Inc.) were used for protein visualization
and imaging. ImageJ 1.43 (NIH) was used for densitometric analysis
of protein bands. Results were expressed as the ratio to
β-actin.
Statistical analysis
SPSS software (version 19; IBM Corp.) was used for
statistical analysis. Data are presented as the mean ± standard
deviation. One-way ANOVA followed by Tukey's post hoc test was used
for statistical analysis among multiple groups. All experiments
were repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
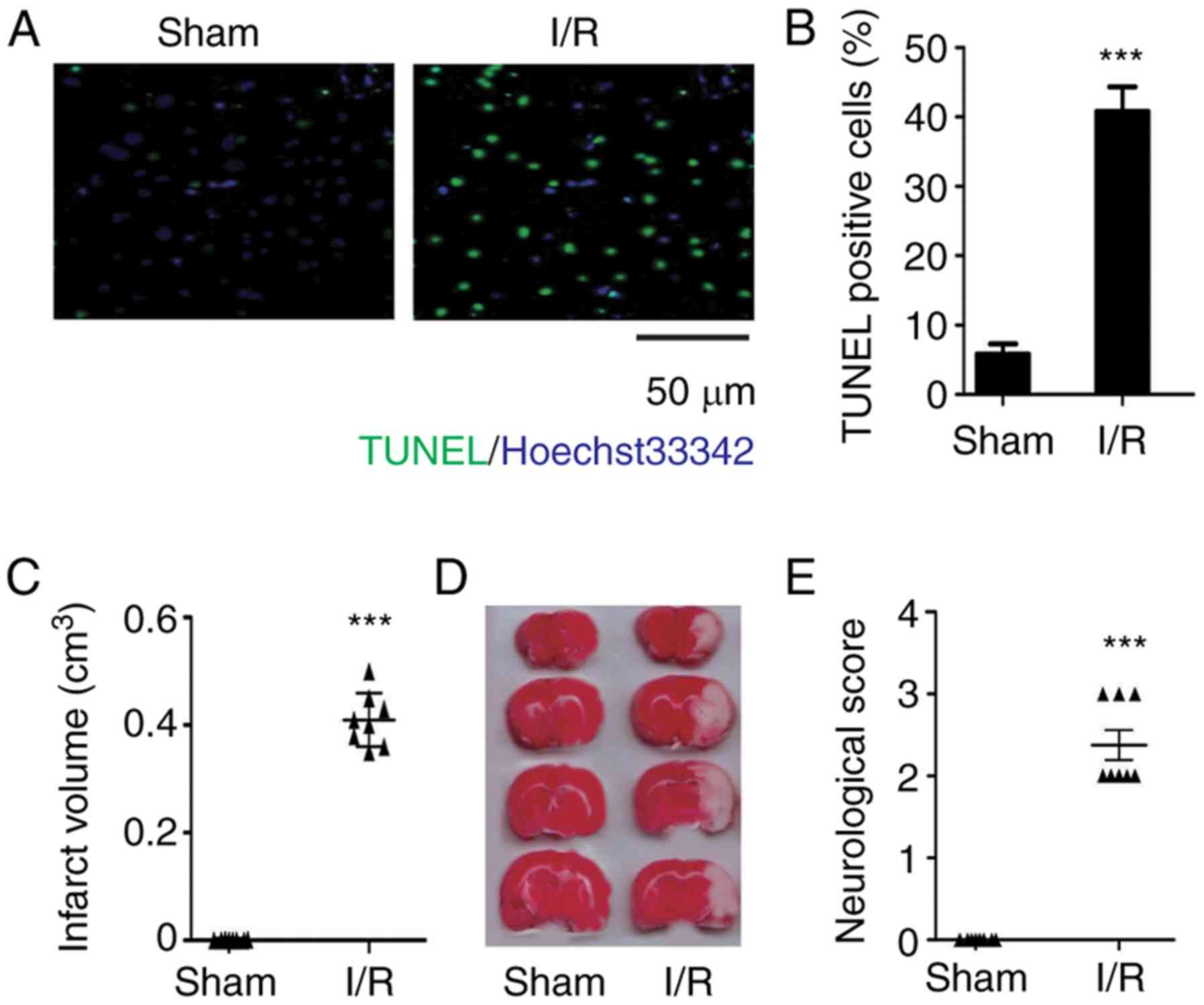
CI/R causes obvious brain injury in
rats
TUNEL/Hoechst double staining was performed to
observe cell apoptosis in brain tissues of rats subjected to I/R
injury. It was found that there were significantly more TUNEL
positive cells in the I/R brain tissues compared with the sham
group, which suggested that I/R contributes to cell apoptosis in
brain tissues (Fig. 1A and B).
In accordance with the TUNEL/Hoechst double staining
results, the rat brains subjected to I/R were stained white
following TTC, which indicated that the brains had undergone
cerebral infarction (Fig. 1C and
D). Moreover, the neurological scores of rats subjected to I/R
were significantly increased compared with the shams, demonstrating
reduced neurological function (Fig.
1E). Therefore, the data suggested that CI/R contributes to
severe brain injury.
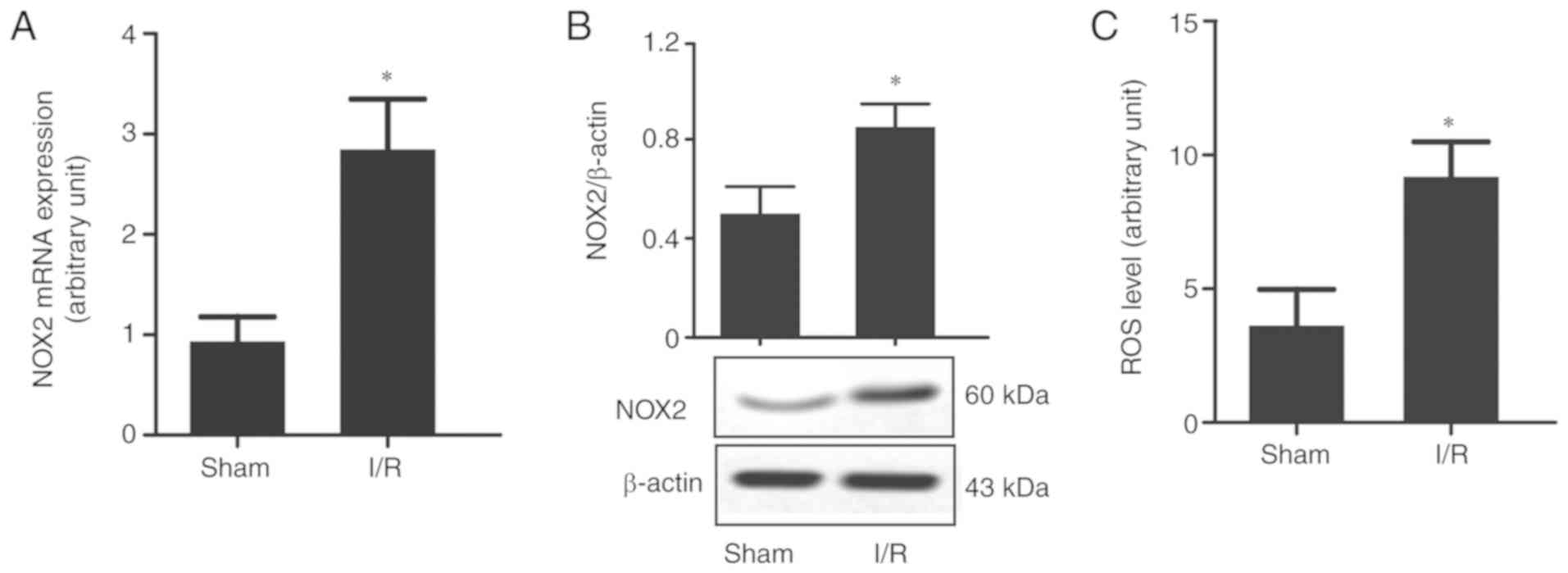
CI/R causes high expression of NOX2
and accumulation of ROS in rat brain tissues
Since oxidative stress is one of the main causes of
CI/R injury, and NOX expression changes following CI/R injury
(5), the present study focused on
NOX2, a marker for ROS production. It was demonstrated that the
mRNA and protein expression levels of NOX2 were significantly
upregulated in the brain tissues subjected to I/R injury (Fig. 2A and B), which was accompanied by a
significant increase in ROS levels compared with the sham group
(Fig. 2C). This suggested that
NOX2 may be an important gene associated with oxidative stress in
CI/R injury.
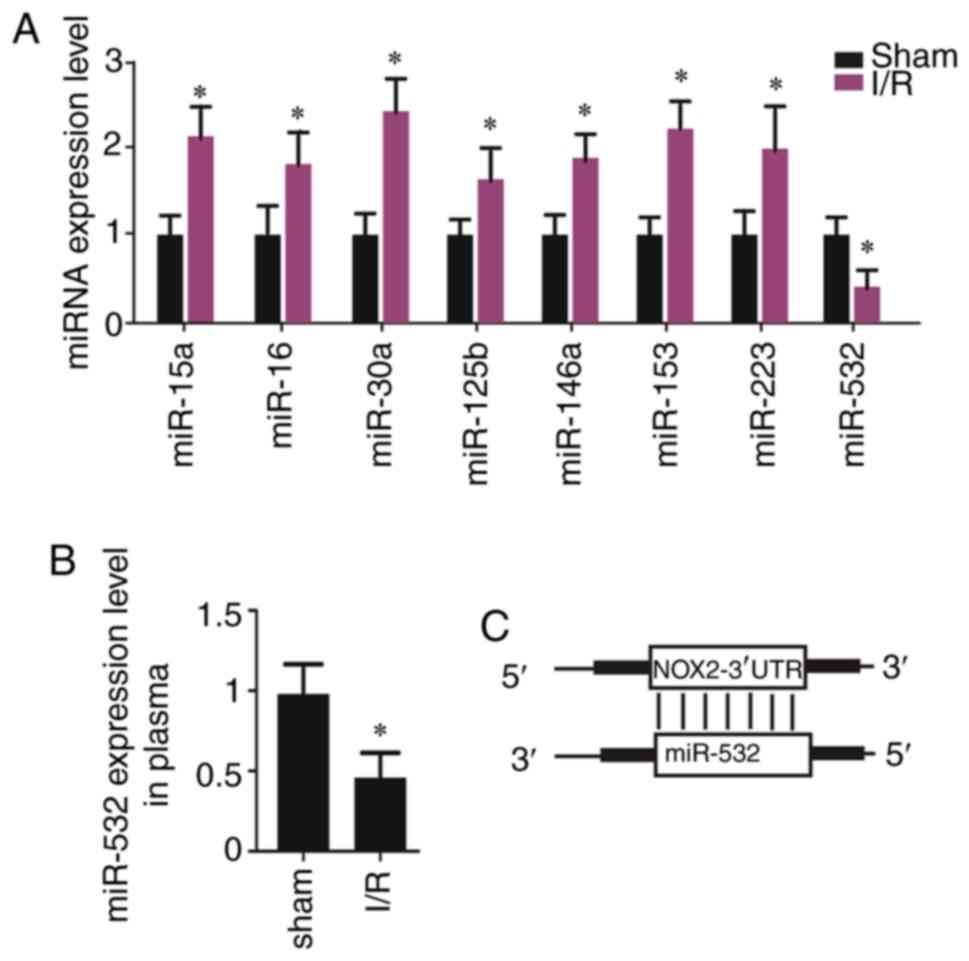
CI/R causes low expression of
miR-532-3p in rat brain tissues
Subsequently, the target miRNAs of NOX2 were
assessed. miR-15a, miR-16, miR-30a, miR-125b, miR-146a, miR-153,
miR-223 and miR-532-3p were predicted targets of NOX2 and it was
identified that only miR-532-3p was significantly decreased (the
other miRs were increased) in the brain tissues subjected to I/R
injury (Fig. 3A). Moreover, the
plasma expression levels of miR-532-3p were significantly decreased
in rats with I/R (Fig. 3B). Thus,
miR-532-3p may be a potential target of NOX2 (Fig. 3C). In addition, these data
indicated that low expression of miR-532-3p may result in
upregulation of NOX2.
Overexpression of miR-532-3p
contributes to the expression inhibition of NOX2 in SH-SY5Y
cells
miR-532-3p mimics were transfected into SH-SY5Y
cells to assess its transfection efficiency, and it was identified
that miR-532-3p mimics transfection significantly increased
miR-532-3p expression in cells (Fig.
S1), thus indicating successful transfection.
The effect of gain-of function of miR-532-3p on NOX2
expression and ROS level was examined in SH-SY5Y cells subjected to
H/R injury. Compared with the control group, H/R significantly
decreased the expression of miR-532-3p in SH-SY5Y cells, while
miR-532-3p mimics transfection significantly increased the
expression of miR-532-3p in SH-SY5Y cells subjected to H/R
(Fig. 4A). Subsequently, the
expression of NOX2 was measured and it was demonstrated that the
mRNA and protein expression levels of NOX2 were significantly
increased in SH-SY5Y cells subjected to H/R, but miR-532-3p mimics
significantly suppressed the expression of NOX2 in these cells
(Fig. 4B and C). There was also a
significant increase in the total NOX activity and ROS levels in
SH-SY5Y cells subjected to H/R compared with control; however,
miR-532-3p mimics significantly decreased these effects in the H/R
group (Fig. 4D-F). These results
suggested that low-expression of miR-532-3p contributed, at least
partly, to upregulation of NOX2.
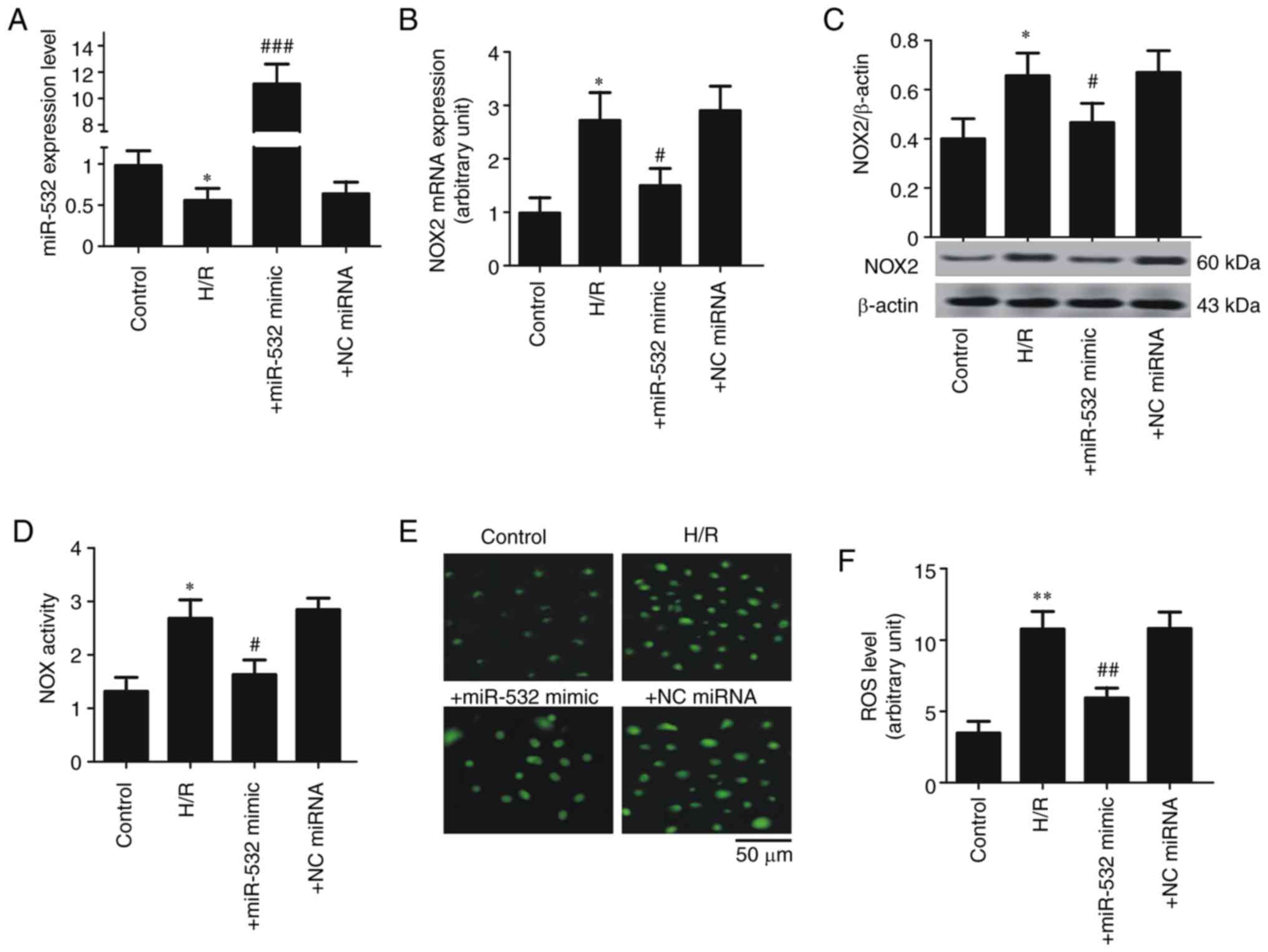 | Figure 4.Overexpression of miR-532-3p
contributes to the expression inhibition of NOX2 in SH-SY5Y cells.
(A) miR-532-3p expression. (B) NOX2 mRNA expression. (C) NOX2
protein expression. (D) Total NOX enzyme activity. (E)
Representative image of ROS level. Scale bar, 50 µm. (F) ROS level.
Data are presented as the mean ± standard deviation. H/R, cells
subjected to 5 h of hypoxia followed by 20 h of reoxygenation;
+miR-532-3p mimics, cells subjected to H/R and treated with
miR-532-3p mimic (50 µM); +NC miRNA, cells subjected to H/R and
treated with NC miRNA (50 µM). *P<0.05 and **P<0.01 vs.
control; #P<0.05, ##P<0.01 and
###P<0.001 vs. H/R. H/R, hypoxia/ reoxygenation;
miR/miRNA, microRNA; NOX, NADPH oxidase; NC, negative control; ROS,
reactive oxygen species. |
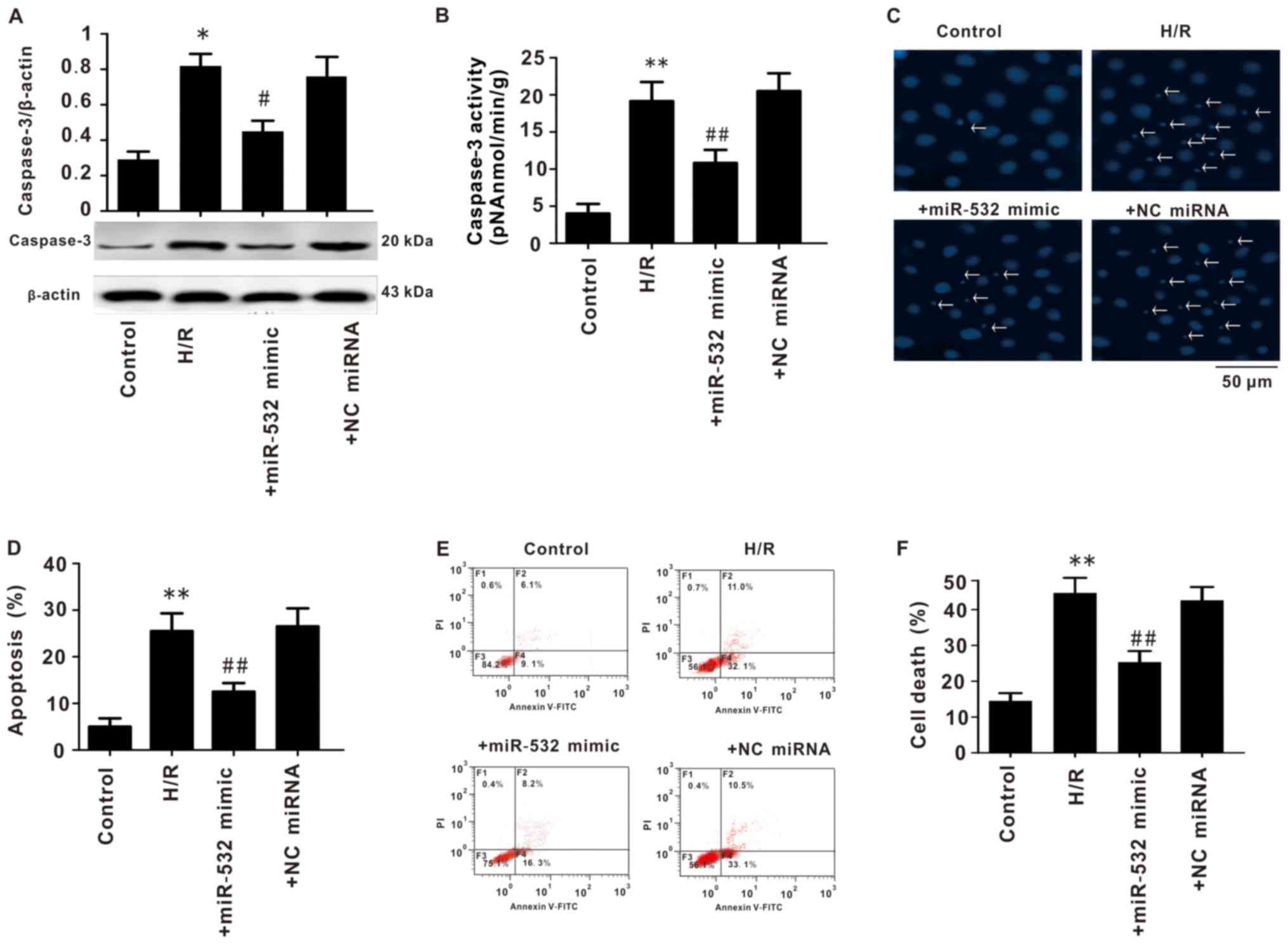
Overexpression of miR-532-3p decreases
the apoptosis of SH-SY5Y cells
Apoptosis of SH-SY5Y cells was also measured. The
expression of caspase-3 was significantly increased in cells
subjected to H/R, while miR-532-3p mimic transfection significantly
decreased caspase-3 expression (Fig.
5A). Furthermore, H/R treatment significantly increased
caspase-3 enzyme activity in SH-SY5Y cells, and miR-532-3p mimics
intervention significantly decreased this enzyme activity (Fig. 5B).
Hoechst staining and flow cytometry results
demonstrated that H/R significantly increased apoptosis and
cellular death of SH-SY5Y cells; however, transfection with
miR-532-3p mimics significantly reduced apoptosis and cell death
caused by H/R (Fig. 5C-F).
Collectively, these data indicated that miR-532-3p may protect
cells from apoptosis and cell death.
miR-532-3p decreases the relative
luciferase activity
A reporter gene was constructed by cloning the
sequence of 3′untranslated region (UTR) of NOX2 (WT or MU) into a
vector plasmid (Fig. 6A and B).
Then, these plasmids containing NOX2-WT or NOX2-MU were purified
and isolated by electrophoresis (Fig.
6C). The relative luciferase activity was measured and it was
demonstrated that miR-532-3p mimics lead to a significant decrease
in the relative luciferase activity in cells transfected with the
NOX2-WT plasmid, but not with NOX2-MU (Fig. 6D). Collectively, these results
suggested that miR-532-3p suppressed the expression of NOX2 by
directly binding to its 3′UTR.
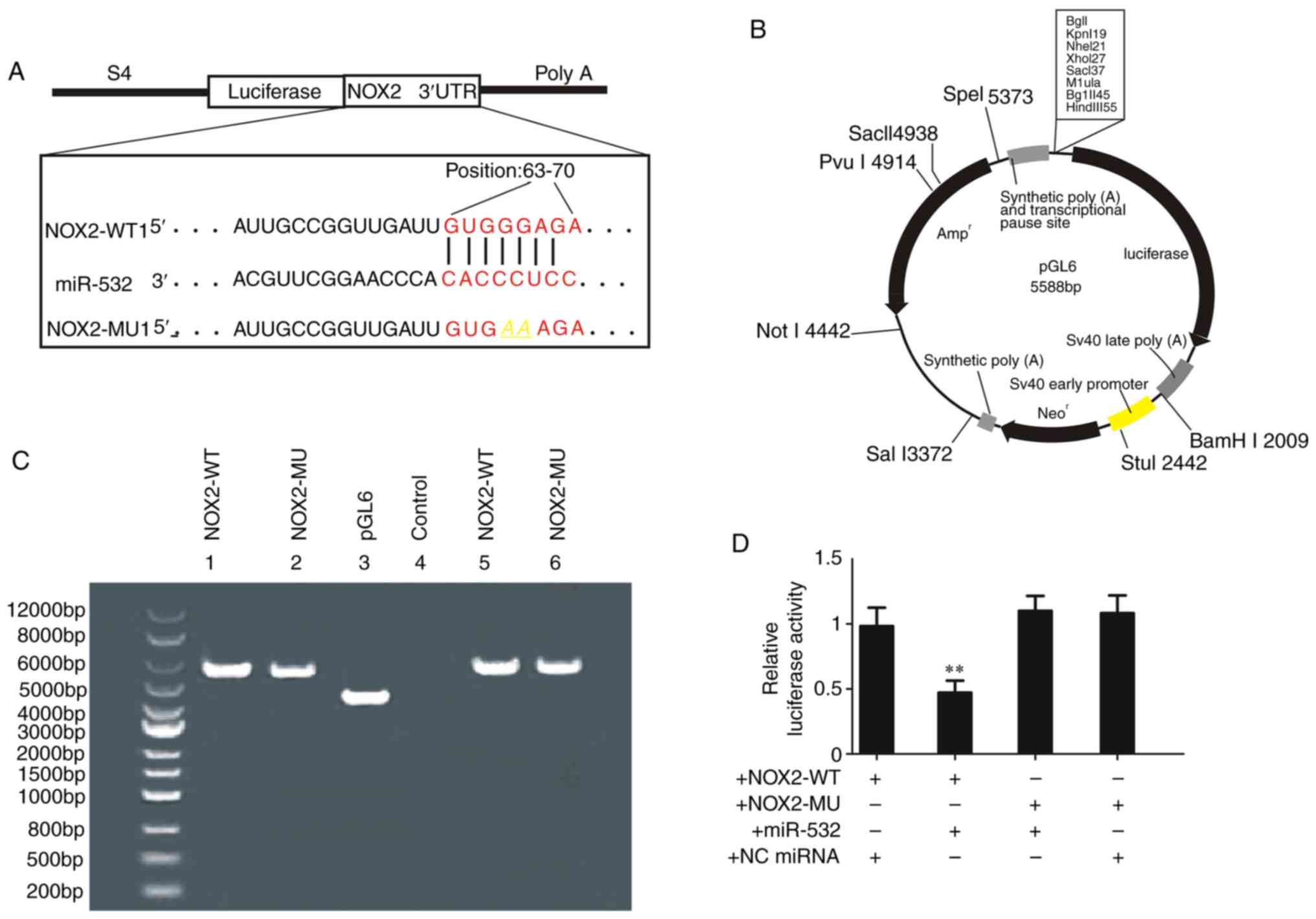 | Figure 6.miR-532-3p decreases the relative
luciferase activity. (A) Schematic diagram of the reporter gene
plasmid containing the sequence of NOX2 targeted by miR-532-3p. (B)
Schematic of the plasmid pGL6. (C) Bands of plasmid
electrophoresis. (D) Relative luciferase activity. Data are
presented as the mean ± standard deviation. +NOX2-WT, cells treated
with WT reporter gene plasmid NOX2-WT1 (100 ng); +NOX2-MU, cells
treated with MU reporter gene plasmid NOX2-MU1 (100 ng);
+miR-532-3p mimics, cells treated with miR-532-3p mimic (50 µM);
+NC miRNA, cells with NC miRNA (50 µM). **P<0.01 vs. NOX2-WT +
miR-532-3p mimics. H/R, hypoxia/reoxygenation; miR/miRNA, microRNA;
NC, negative control; WT, wild-type; MU, mutant; NOX, NADPH
oxidase; UTR, untranslated region. |
Discussion
NOX-derived ROS-mediated oxidative stress serves an
important role in CI/R injury (5).
The aim of the present study was to identify the role of miR-532-3p
in the regulation of NOX2 expression. The results indicated that
miR-532-3p directly suppressed the expression of NOX2 by targeting
its 3′UTR, which contributed to oxidative stress injury (Fig. 7). It was demonstrated that brain
tissues subjected to I/R or SH-SY5Y cells exposed to H/R had
significantly increased expression levels of NOX2, as well as
decreased miR-532-3p expression. Bioinformatics analysis identified
that there was an interaction between miR-532-3p and NOX2.
Moreover, miR-532 mimics significantly increased the expression of
miR-532-3p in SH-SY5Y cells subjected to H/R, but decreased the
expression of NOX2. Luciferase activity was also significantly
decreased in cells co-transfected with NOX2-WT plasmid and
miR-532-3p, but was not affected in cells co-transfected with the
NOX2-MU plasmid. Therefore, these findings suggested that
miR-532-3p may be a target for NOX2, as well as a potential
biomarker and a novel target for IS.
Oxidative stress is defined as a state of ROS
accumulation and an imbalance of ROS production and clearance in
cells (20). ROS serve a role in
the pathogenesis of numerous neurological disorders, particularly
IS (21). Furthermore, ROS damage
cellular macromolecules, including lipids, proteins and nucleic
acids, which leads to cell injury and death (20). ROS also damage antioxidant enzymes
responsible for ROS scavenging, such as glutathione peroxidase,
superoxide dismutase and catalases, which results in a continuous
chain reaction (5). Severe
oxidative stress occurs in brain tissues subjected to ischemia for
2 h followed by reperfusion for 24 h (6). Previous studies have shown that brain
tissues subjected to I/R injury undergo an increase in ROS during
the reperfusion process (7,22).
Reoxygenation during reperfusion provides a substrate for several
enzymes in the oxidation reaction, such as xanthine oxidase, NOX
and cytochromes P450, which produce numerous ROS, including
H2O2, O2.− and OH (5,22).
Gene expression alteration is a common mechanism for
cells to respond to oxidative stress and one of the affected genes
is NOX. Previous studies have reported that NOX is the main enzyme
for ROS production during CI/R injury (5,6,22).
Of the seven subtypes of NOX, NOX4 and particularly NOX2 are the
most important for CI/R injury (5,22).
It has been revealed that knockdown of NOX2 expression in rodents
significantly decreases the cerebral infarction caused by MCAO
(23–25). In addition, rats subjected to MCAO
treated with a general or specific NOX inhibitor, such as vs2870,
present with reduced brain infarction and improved neurological
function (26–28). However, Kelly et al
(29) reported that NOX2
inhibition with apocynin worsens stroke outcome in older rats.
Possible reasons for these findings are as follows: i) ROS have
dual roles (protective or harmful), if the balance between these
roles becomes dysregulated, cells or tissues will be subjected to
damage. For instance, NOX2 inhibition may lead to a sharp decrease
in ROS level, resulting in dysregulation (5); and ii) apocynin itself exerts both
therapeutic and toxic side effects, which are associated with the
administration time, dosage and route. Thus, this suggests that
NOX2 is a major source of ROS in brain tissues (5). In line with these previous findings,
the present results indicated that CI/R significantly increased
NOX2 expression in brain tissues. Collectively, these results
demonstrated that NOX2 serves a key role in CI/R oxidative stress
injury. Therefore, elucidating the regulatory mechanism of NOX2
expression is important for understanding the pathogenesis of CI/R
injury, particularly for the development of novel drugs and disease
therapy.
Previous studies have reported that differences in
the expression patterns of miRNAs are associated with various
pathophysiological processes of IS, including oxidative stress
(miR-101, miR-146a, miR-410, miR-424, miR-106-5p and miR-34b),
inflammation (miR-125b, miR-26a, miR-145, miR-34a, miR-9 and
let-7b), excitotoxicity (miR-125b and miR-107), edema (miR-320a and
miR-29a) and apoptosis (miR-125b, miR-15, miR-16, miR-34, miR-25
and miR-497) (30–38). This suggests that miRNAs have
important roles in IS, particularly in the regulation of oxidative
stress-related genes. NOX, especially NOX2, serves a role in the
generation of ROS, which contributes to CI/R oxidative stress
injury (39). Therefore,
investigating the roles of miRNAs in the regulation of NOX2
expression is of great significance for the understanding and
therapy of IS. The majority of studies examining the associations
of miRNAs with NOX2 have focused on cancer and cardiovascular
disease (miR-34a/27b/106b/148b/204/155/17/34a), and few studies
have investigated their role in CI/R oxidative stress injury
(39–43). Thus, identifying the target miRNAs
of NOX2 is key to improving the understanding of CI/R injury and
facilitate the development of novel treatments. The present study
identified that I/R significantly decreased miR-532-3p expression
in brain tissues, as well as significantly increased the expression
levels of NOX2. A previous study also demonstrated that I/R
contributed to increased expression levels of NOX4 (6). However, the bioinformatics analysis
indicated that miR-532-3p may be a potential target for NOX2, but
not NOX4. Therefore, the present study focused on the relationship
between miR-532-3p and NOX2. It was found that miR-532-3p mimics
significantly decreased the expression of NOX2 in cells subjected
to H/R, as well as the relative luciferase activity. Thus, it was
demonstrated that miR-532-3p may be a target of NOX2 and serves key
roles in oxidative stress of I/R injury. As the roles of miRNAs in
gene regulation are complex, for example, a certain gene can be
targeted by different miRNAs and a specific miRNA may target
different genes, the mechanisms of miRNAs targeting NOX2 remain
unknown (44). In addition,
whether the regulatory mechanism of miRNAs and NOX2 under normal
conditions is the same as during CI/R injury requires further
investigation. Furthermore, the present study only observed the
effect of miR-532-3p on oxidative stress, without observing the
effect of oxidative stress on miR-532-3p. Another limitation of
this study is the lack of in vivo experiments to confirm the
function of miR-532-3p in CI/R injury. Therefore, further research
is required prior to use of miR-532-3p for clinical therapy.
In conclusion, by directly targeting the 3′UTR of
NOX2, miR-532-3p may be a regulator of NOX2 and serves important
roles in CI/R injury. To the best of our knowledge, the present
study was the first to investigate the role of the miR-532-3p/NOX2
axis in CI/R oxidative stress injury, which may provide a novel
target for drug development and IS treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was funded by the National Natural Science
Foundation of China (grant no. 81603107), the Education Department
of HuNan Province (grant no. 18C1853) and ChangSha Science &
Technology Bureau (grant no. kq1801125).
Availability of data and materials
The datasets used and /or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
LM, MLZ, APW, YT and LCD performed the experiments.
TML and DBK contributed to the data analysis and manuscript
drafting. GLS and ZBY contributed to the design of the experiments
and drafting of the manuscript. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
Animal experiments were conducted following the
Guide for the Care and Use of Laboratory Animals, and were approved
by the Animal Ethics Committee of Hunan Normal University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Randolph SA: Ischemic Stroke. Workplace
Health Saf. 64:4442016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Enzmann G, Kargaran S and Engelhardt B:
Ischemia-reperfusion injury in stroke: Impact of the brain barriers
and brain immune privilege on neutrophil function. Ther Adv Neurol
Disord. 11:17562864187941842018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siti HN, Kamisah Y and Kamsiah J: The role
of oxidative stress, antioxidants and vascular inflammation in
cardiovascular disease (a review). Vascul Pharmacol. 71:40–56.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma MW, Wang J, Zhang Q, Wang R, Dhandapani
KM, Vadlamudi RK and Brann DW: NADPH oxidase in brain injury and
neurodegenerative disorders. Mol Neurodegener. 12:72017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lou Z, Wang AP, Duan XM, Hu GH, Song GL,
Zuo ML and Yang ZB: Upregulation of NOX2 and NOX4 mediated by TGF-β
signaling pathway exacerbates cerebral ischemia/reperfusion
oxidative stress injury. Cell Physiol Biochem. 46:2103–2113. 2015.
View Article : Google Scholar
|
|
7
|
Sun JB, Li Y, Cai YF, Huang Y, Liu S,
Yeung PK, Deng MZ, Sun GS, Zilundu PL, Hu QS, et al: Scutellarin
protects oxygen/glucose-deprived astrocytes and reduces focal
cerebral ischemic injury. Neural Regen Res. 13:1396–1407. 2015.
|
|
8
|
Xu W, Gao L, Zheng J, Li T, Shao A, Reis
C, Chen S and Zhang J: The roles of microRNAs in stroke: Possible
therapeutic targets. Cell Transplant. 27:1778–1788. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Majdi A, Mahmoudi J, Sadigh-Eteghad S,
Farhoudi M and Shotorbani SS: The interplay of microRNAs and
post-ischemic glutamate excitotoxicity: An emergent research field
in stroke medicine. Neurol Sci. 37:1765–1771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao L, Zuo ML, Hu GH, Duan XM and Yang ZB:
mir-193 targets ALDH2 and contributes to toxic aldehyde
accumulation and tyrosine hydroxylase dysfunction in cerebral
ischemia/reperfusion injury. Oncotarget. 8:99681–99692. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang ZB, Zhang Z, Li TB, Lou Z, Li SY,
Yang H, Yang J, Luo XJ and Peng J: Up-regulation of brain-enriched
miR-107 promotes excitatory neurotoxicity through down-regulation
of glutamate transporter-1 expression following ischaemic stroke.
Clin Sci. 127:679–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Tuo YH, Chen JW, Wang QY, Li S, Li
MC, Dai G, Wang JS, Zhang YL, Feng L and Shi ZS: NADPH oxidase
inhibitor regulates microRNAs with improved outcome after
mechanical reperfusion. J Neurointerv Surg. 9:702–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J, Zhang J, Wang Y, Long K, Wang X, Jin
L, Tang Q, Zhu L, Tang G, Li X and Li M: MiR-532-5p alleviates
hypoxia-induced cardiomyocyte apoptosis by targeting PDCD4. Gene.
675:36–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun W, Liu Q, Leng J, Zheng Y and Li J:
The role of Pyruvate Dehydrogenase Complex in cardiovascular
diseases. Life Sci. 121:97–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li P, Teng F, Gao F, Zhang M, Wu J and
Zhang C: Identification of circulating microRNAs as potential
biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol.
35:433–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sepramaniam S, Tan JR, Tan KS, DeSilva DA,
Tavintharan S, Woon FP, Wang CW, Yong FL, Karolina DS, Kaur P, et
al: Circulating microRNAs as biomarkers of acute stroke. Int J Mol
Sci. 15:1418–1432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academicals Press (US). (Washington, DC).
2011.
|
|
18
|
Whiteside G, Cougnon N, Hunt SP and
Munglani R: An improved method for detection of apoptosis in tissue
sections and cell culture, using the TUNEL technique combined with
Hoechst stain. Brain Res Protoc. 2:160–164. 1998. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brandes RP, Weissmann N and Schröder K:
Redox-mediated signal transduction by cardiovascular Nox NADPH
oxidases. J Mol Cell Cardio. 73:70–79. 2014. View Article : Google Scholar
|
|
21
|
Patel M: Targeting oxidative stress in
central nervous system disorders. Trends Pharmacol Sci. 37:768–778.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Awooda HA, Lutfi MF, Sharara GGM and Saeed
AM: Oxidative/nitrosative stress in rats subjected to focal
cerebral ischemia/reperfusion. Int J Health Sci (Qassim). 9:17–24.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis SM and Pennypacker KR: Targeting
antioxidant enzyme expression as a therapeutic strategy for
ischemic stroke. Neurochem Int. 107:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Silva TM, Brait VH, Drummond GR, Sobey
CG and Miller AA: Nox2 oxidase activity accounts for the oxidative
stress and vasomotor dysfunction in mouse cerebral arteries
following ischemic stroke. PLoS One. 6:e283932011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Wei X, Liu K, Zhang X, Yang F,
Zhang H, He Y, Zhu T, Li F, Shi W, et al: NOX2 deficiency
ameliorates cerebral injury through reduction of complexin
II-mediated glutamate excitotoxicity in experimental stroke. Free
Radic Biol Med. 65:942–951. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jackman KA, Miller AA, De Silva TM, Crack
PJ, Drummond GR and Sobey CG: Reduction of cerebral infarct volume
by apocynin requires pretreatment and is absent in Nox2-deficient
mice. Br J Pharmacol. 156:680–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wingler K, Altenhoefer SA, Kleikers PWM,
Radermacher KA, Kleinschnitz C and Schmidt HH: VAS2870 is a
pan-NADPH oxidase inhibitor. Cell Mol Life Sci. 69:3159–3160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Surace MJ and Block ML: Targeting
microglia-mediated neurotoxicity: The potential of NOX2 inhibitors.
Cell Mol Life Sci. 69:2409–2427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly KA, Li X, Tan Z, VanGilder RL, Rosen
CL and Huber JD: NOX2 inhibition with apocynin worsens stroke
outcome in aged rats. Brain Res. 1292:165–172. 2009.PubMed/NCBI
|
|
30
|
Liu NN, Dong ZL and Han LL: MicroRNA-410
inhibition of the TIMP2-dependent MAPK pathway confers
neuroprotection against oxidative stress-induced apoptosis after
ischemic stroke in mice. Brain Res Bull. 143:45–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang SW, Liu Z and Shi ZS: Non-Coding RNA
in acute ischemic stroke: Mechanisms, biomarkers and therapeutic
targets. Cell Transplant. 27:1763–1777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang R, Ma J, Niu B, Li J, Chang J, Zhang
Y, Liu P and Luan X: MiR-34b protects against focal cerebral
ischemia-reperfusion (I/R) injury in rat by targeting keap1. J
Stroke Cerebrovasc Dis. 28:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng Y, Pan C, Chen M, Pei A, Xie L and
Zhu S: miR-29a ameliorates ischemic injury of astrocytes in vitro
by targeting the water channel protein aquaporin 4. Oncol Rep.
41:1707–1717. 2019.PubMed/NCBI
|
|
34
|
Deng Y, Ma G, Dong Q, Sun X, Liu L, Miao Z
and Gao F: Overexpression of miR-224-3p alleviates apoptosis from
cerebral ischemia reperfusion injury by targeting FIP200. J Cell
Biochem. 1200:17151–17158. 2019. View Article : Google Scholar
|
|
35
|
Fu C, Chen S, Cai N, Liu Z, Wang P and
Zhao J: Potential neuroprotective effect of miR-451 against
cerebral ischemia/reperfusion injury in stroke patients and a mouse
model. World Neurosurgery. 130:e54–e61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rink C and Khanna S: MicroRNA in ischemic
stroke etiology and pathology. Physiol Genomics. 43:521–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan JR, Koo YX, Kaur P, Liu F, Armugam A,
Wong PT and Jeyaseelan K: microRNAs in stroke pathogenesis. Curr
Mol Med. 11:76–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Han Z, Ji X and Luo Y: Epigenetic
regulation of oxidative stress in ischemic stroke. Aging Dis.
7:295–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kahles T and Brandes RP: Which NADPH
oxidase isoform is relevant for ischemic stroke? The case for nox
2. Antioxid Redox Signal. 18:1400–1417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Hui L, Kang Q and Li R:
Down-regulation of microRNA-27b promotes retinal pigment epithelial
cell proliferation and migration by targeting Nox2. Pathol Res
Pract. 214:925–933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim SM, Hur DY, Hong SW and Kim JH:
EBV-encoded EBNA1 regulates cell viability by modulating
miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem Biophys
Res Commun. 494:550–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang J, Brown ME, Zhang H, Martinez M,
Zhao Z, Bhutani S, Yin S, Trac D, Xi JJ and Davis ME:
High-throughput screening identifies microRNAs that target Nox2 and
improve function after acute myocardial infarction. Am J Physiol
Heart Circ Physio. 312:H1002–H1012. 2017. View Article : Google Scholar
|
|
43
|
Wang XH and Wang TL: MicroRNAs of
microglia: Wrestling with central nervous system disease. Neural
Regen Res. 13:2067–2072. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|