Introduction
Blunt chest trauma is commonly associated with a
wide range of injuries, a number of which are life-threatening with
high mortality and require immediate medical attention (1). Hemorrhagic shock (HS), which
endangers patients' lives and causes a high trauma-induced
mortality rate of 30–40%, is a common complication in patients who
suffer from blunt chest trauma (2,3).
Furthermore, the ensuing hypoxemia and ischemia during HS, and the
resulting ischemia-reperfusion injury following HS commonly
aggravate tissue damage (4,5). The
lungs are frequently the first organ to become injured; acute lung
injury (ALI) is the most common lung injury (6). ALI is secondary to HS in thoracic
trauma and may lead to acute respiratory distress syndrome (ARDS)
(6). An epidemiological study
estimated that the annual prevalence of ARDS in the USA is 5–35
cases for every 100,000 individuals, depending on the definitions
utilized and the design of study methodology (7). Therefore, the pathogenesis and
pathophysiology of ALI/ARDS require elucidation to identify and
develop novel targeted therapies for this disorder. ALI/ARDS is
closely associated with oxidative stress and inflammatory
responses; however, no widely available, effective and specific
interventions have been established for ALI/ARDS treatment
(7).
As an important mediator in HS and various types of
ALI/ARDS (8,9), the nucleotide binding and
oligomerization domain-like receptor family pyrin domain-containing
protein 3 (NLRP3) inflammasome is an oligomeric molecular complex
composed of the NLRP3 scaffold, adapter protein
apoptosis-associated speck-like protein (ASC) and caspase-1
(10). The NLRP3 inflammasome
plays an important role in immune responses and disease processes
such as type II diabetes (11),
atherosclerosis (12) and
inflammatory bowel disease (13).
MCC950, which can block both canonical and non-canonical NLRP3
inflammasome activation, was reported to lower pulmonary
inflammation in mice (14).
Furthermore, activation of the NLRP3 inflammasome was demonstrated
to be involved in excessive inflammatory responses, and to be
closely associated with ALI/ARDS pathogenesis (15). Therefore, as an important component
of innate immunity, the NLRP3 inflammasome may provide novel
targets for the treatment of various inflammatory diseases.
Dexmedetomidine (Dex) is a short-acting, highly
selective α-2 adrenoreceptor agonist that is extensively applied in
clinical anesthesia and intensive care (16,17).
A number of basic research experiments and clinical trials have
reported that Dex can protect various organs, including the lungs,
by inhibiting oxidative stress, inflammatory responses and
apoptosis in a range of diseases (18–20).
The protective effects of Dex were recently demonstrated in a
two-hit rat model (21). A recent
study has shown that Dex is relevant to the inhibition of NLRP3
inflammasome activity (19). Two
recent studies reported that Dex can inhibit the NF-κB pathway and
NLRP3 inflammasome to suppress the inflammatory response (22,23).
However, the question of whether Dex exerts protective effects on
trauma-induced ALI has not been investigated, and the underlying
molecular mechanisms remain unclear. Therefore, the present study
focused on the NLRP3 inflammasome in order to elucidate the
possible cellular and molecular events involving NLRP3 inflammasome
in Dex-mediated alleviation of THSR-induced ALI in a rat model.
The present study aimed to test the hypothesis that
Dex exerts protective effects on THSR-induced ALI in rats by
alleviating the inflammatory response and inhibiting NLRP3
signaling pathways.
Materials and methods
Ethics statement
All laboratory and animal experiments were approved
by the Medical Ethics Committee of Renmin Hospital of Wuhan
University in accordance with the Guide for the Care and Use of
Laboratory Animals of the National Research Council (US) Committee
(24).
Animals
Healthy adult male Sprague-Dawley rats (age, 8
weeks; weight, 200–220 g) were obtained from Vital River Laboratory
Animal Technology Co., Ltd. (certificate no. SYXK 2014-0080) and
maintained under specific pathogen-free conditions (12:12-h
light/dark cycle, 20–24°C and 40–60% humidity) with food and water
ad libitum.
Experimental protocols
A total of 40 rats were randomly divided into four
equal groups: Sham, Dex, ALI and Dex + ALI groups. Rats in the ALI
and Dex + ALI groups underwent THSR surgery, whereas those in the
sham and Dex groups were subjected to sham surgery, including
femoral arterial and venous cannulation, although neither blunt
chest trauma nor HS-resuscitation were induced. In addition, rats
in the Dex and Dex + ALI groups were administered with 5 µg/kg/h
Dex for 30 min via intraperitoneal injection (cat. no. 14030332;
Jiangsu Hengrui Medicine Co., Ltd.) immediately after the sham or
blunt chest traumatic surgery. At 6 h after the surgery, all
animals were sacrificed under anesthesia via intraperitoneal
injection of 50 mg/kg pentobarbital sodium and exsanguination from
the right carotid artery. At the same time, arterial blood,
bronchoalveolar lavage fluid and lung tissue samples were
collected. Lung tissues were snap-frozen in liquid nitrogen and
stored at −80°C for subsequent analysis. All animal experiments
were performed from 08:00 to 12:00.
Animal model of THSR-induced ALI
The rat model of THSR-induced ALI adopted in the
present study was described in mice by Seitz et al (25). This model was successfully
established in rats in our previous studies (26,27).
In brief, the rats were provided free access to
water and fasted for 12 h prior to surgery. After induction of
anesthesia via intraperitoneal injection of pentobarbital sodium
(2%; 50 mg/kg), sham or THSR surgery was performed according to the
groupings. A polyethylene tube filled with heparinized saline was
used for cannulation of the femoral artery and vein to monitor
continuous invasive pressure [in the form of mean arterial pressure
(MAP)] and heart rate using a monitor (IntelliVue MP20; Philips
Healthcare), as well as to establish venous access. As previously
described, blunt chest trauma was induced with a fixed 2.45-J chest
impact by dropping a hollow cylindrical encased in a vertical
stainless steel tube which was positioned onto a lexon platform
from a definite height (26). A
precordial shield directed the impact force to the lungs
bilaterally; thus, cardiac trauma was prevented. After 5 min, blood
was withdrawn into a heparinized syringe until the MAP reached
35–45 mmHg in 15 min to induce HS, and the MAP was maintained for 1
h by drawing blood via transfusion. Then, it was determined that
the MAP met the criteria for HS as previously described (26). Furthermore, the rats were
transfused with a 1:1 mix of all the withdrawn blood and Ringer's
lactate solution (Baxter Healthcare Co., Ltd.) to induce
resuscitation during the next 1 h.
Blood gas analysis
At 6 h following surgery, blood samples were
collected from animals under anesthesia from the right femoral
artery (0.5 ml/animal) and then immediately assayed with an i-STAT
portable clinical analyzer (Abbott Point Of Care, Inc.; Abbott
Pharmaceutical Co., Ltd.). Arterial partial pressure of oxygen
(PaO2) was measured, and the oxygenation index
[PaO2/fraction of inspired oxygen (FiO2)] was
calculated.
Measurement of lung wet/dry (W/D)
weight ratio
The W/D ratio is used as an index of the severity of
pulmonary edema. The W/D ratio was determined by measuring the
water content in the lungs 6 h after THSR challenge. The right
middle lobe of the lungs was dissected from non-pulmonary tissues
and then accurately weighed to determine wet weight using an
electronic balance after the surface blood and water were wiped
off. Afterward, the lungs were incubated for 72 h in an oven at
60°C and then reweighed to determine the dry weight. Finally, the
W/D ratio was calculated.
Hematoxylin and eosin (H&E)
staining and lung injury score
Lung tissue samples were collected and fixed in 4%
paraformaldehyde at 4°C for 48 h. Subsequently, the samples were
dehydrated, embedded in paraffin and sectioned routinely (5 µm
thickness). Finally, H&E staining was performed with the
following steps at room temperature using H&E solution
(Sigma-Aldrich; Merck KGaA): Firstly, paraffin sections were
incubated at 60°C for 30 min, twice immersed in xylene for 15 min
at room temperature and then treated with a descending ethanol
series for 5 min each at room temperature. The sections were then
treated with 0.5% hematoxylin for 1–5 min at room temperature and
then rinsed in tap water for 1 min. Sections were incubated with
PBS for 8 sec until a blue color was observed, and then the
sections were washed using tap water for 1 min and then distilled
water for 8 sec. Sections were then stained with 1% eosin for 3 min
at room temperature and then washed with tap water. Then, sections
were treated with an ascending ethanol series for 1 min each at
room temperature.
Pathological alterations of the lung tissues were
observed under a light microscope (magnification, ×200; BX51;
Olympus Corporation). Simultaneously, lung injury scores were
calculated by applying the histological scoring system designed for
mice by Belperio et al (28). Histological scoring of 10 fields of
each 5-µm paraffin-embedded tissue section was performed blindly
for the following parameters: Congestion and hemorrhage of the
alveoli; airway epithelial cellular damage; neutrophil infiltration
or aggregation in airspace or vessel wall; and thickness of the
alveolar wall or formation of hyaline membrane. These parameters
were scored from grade 0 to 4 using specific criteria: Normal or
little damage, 0; mild damage (<25%), +1; moderate damage
(25-50%), +2; severe damage (50-75%), +3; and maximal damage
(>75%), +4. The pathological score of each field was measured as
the total score of all four items. The lung injury score of each
section was expressed as the average value of the 10 fields.
Transmission electron microscopy
Fragments of the right middle lung tissues were cut
into 1-mm slices and then fixed in 2.5% glutaraldehyde solution for
2 h at 0–4°C. Afterward, the specimens were rinsed with PBS three
times, fixed with 1% osmic acid at room temperature for 1 h, rinsed
with PBS three times and stained with 2% uranium acetate solution
at room temperature for 30 min, and then dehydrated with dimethyl
ketone. The specimens were embedded in Epon-812 at 60°C for 2 days,
cut into ultrathin sections (60 nm), and stained at room
temperature with 1% uranyl acetate for 30 min and lead citrate for
15 min. The sections were observed under a transmission electron
microscope (magnification, ×10,000; Hitachi H-600; Hitachi,
Ltd.).
Measurement of malondialdehyde (MDA),
superoxide dismutase (SOD), lactate dehydrogenase (LDH) and
myeloperoxidase (MPO) activities in lung tissues
MDA reflects the degree of lipid peroxidation,
whereas SOD protects cells from superoxide damage (29). LDH and MPO activities are reported
as indices of intracellular injury and neutrophil accumulation,
respectively (29). LDH, MPO, SOD
and MDA activity levels were determined via colorimetry. In brief,
100 mg frozen (4°C) lung tissue was cut, weighed, homogenized and
centrifuged at 1,500 × g for 10 min at 4°C. The supernatants were
collected, and MDA, SOD, LDH and MPO activities were determined
using MDA, SOD, LDH and MPO assay kits (MDA, cat. no. A003-1-2;
SOD, cat. no. A001-3-2; LDH, cat. no. A020-2-2; MPO, cat. no.
A044-1-1; Nanjing Jiancheng Bioengineering Institute Co., Ltd.)
according to their respective manufacturer's protocols.
ELISA determination of serum
interleukin (IL)-1β, IL-18, IL-6, and tumor necrosis factor (TNF)-α
expression levels
The blood samples were immediately centrifuged at
1,500 × g for 10 min at 4°C after collection to obtain the serum.
The supernatant fluid was harvested and assayed using ELISA kits
(IL-1β, cat. no. RLB00; IL-18, cat. no. DY521-05; IL-6, cat. no.
R6000B; TNF-α, cat. no. RTA00; all R&D Systems, Inc.) to
measure the levels of proinflammatory cytokines in serum according
to the manufacturer's protocols. The absorbance was measured at 450
nm using an ELISA reader (BioTek Instruments, Inc.).
Western blot analysis of NLRP3, ASC
and caspase-1
The lung tissue samples were homogenized with RIPA
lysis buffer (25 mM Tris-HCl, pH 7.6, 1% NP-40, 0.5% sodium
deoxycholate and 0.1% SDS) supplemented with 1% PMSF. Lysates were
sonicated and centrifuged at 3,000 × g at 4°C for 10 min, and then
the homogenate supernatant was centrifuged again at 10,000 × g at
4°C for 10 min to obtain the final lung homogenate supernatant,
which was used to detect proteins. The concentrations of the
proteins were measured using a bicinchoninic acid protein assay
kit. On the basis of the protein concentrations, an equal quantity
of protein (30 µg) was loaded into each well and then separated via
10% SDS-PAGE. The proteins were subsequently electrotransferred to
PVDF membranes. The membranes were blocked with 5% fat-free milk at
room temperature for 1 h and then incubated with different primary
polyclonal antibodies (rabbit anti-rat) at 4°C overnight. The
primary polyclonal antibodies used in the present study were NLRP3
(1:1,000; cat. no. ab214185; Abcam), ASC (1:1,000; cat. no. D2W8U;
Cell Signaling Technology, Inc.), caspase-1 (1:300; cat. no.
ab1872; Abcam) and GAPDH (1:1,000; cat. no. sc-137179; Santa Cruz
Biotechnology, Inc.). Afterward, the membranes were incubated with
secondary antibody (horseradish peroxidase-conjugated goat
anti-rabbit IgG; 1:2,000; cat. no. 7074; Cell Signaling Technology,
Inc.) for 1 h at room temperature. The immunoreactive protein bands
were visualized by enhanced chemiluminescence (cat. no. 32132;
Thermo Fisher Scientific, Inc.) with an Odyssey color infrared
laser scan-imaging instrument (LI-COR Biosciences). The quantities
of the target proteins were analyzed using Image Lab software
(version 5.2.1; National Institutes of Health) and reported as the
densitometric ratios between the target protein and GAPDH, which
was used as a loading control.
Statistical analysis
All quantitative data are presented as the mean ± SD
(n=10/group). Multiple comparisons were performed for statistical
analysis via one-way ANOVA by using GraphPad Prism Software 7.0
(GraphPad Software, Inc.). Bonferroni post hoc test was used to
test differences between individual means when the F-statistic was
significant. P<0.05 was considered to indicate a statistically
significant difference.
Results
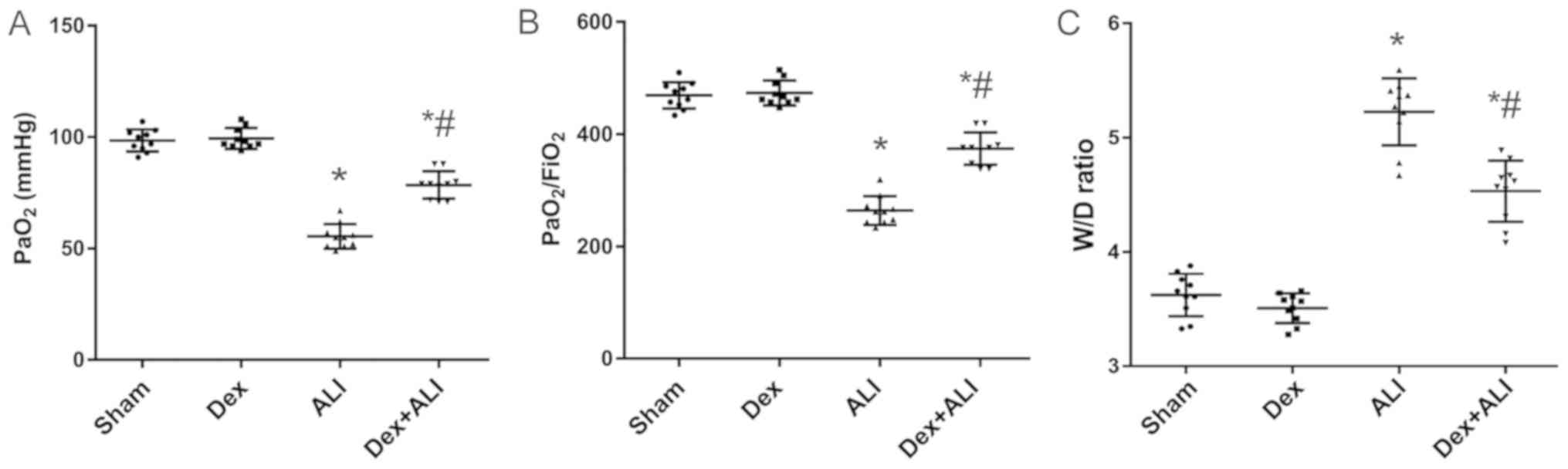
Effects of Dex on PaO2 and
PaO2/FiO2 in THSR-induced ALI rats
The results of arterial blood gas analysis revealed
that the levels of PaO2 significantly decreased in the
ALI group 6 h after THSR procedure (Fig. 1A). Furthermore, the
PaO2/FiO2 ratios in the ALI group
significantly decreased significantly (Fig. 1B). Dex treatment significantly
attenuated the decreases induced by THSR challenge (Fig. 1A and B).
Dex decreases the lung W/D ratio in
THSR-induced ALI
The lung W/D ratios in the ALI group were
significantly increased compared with those in the sham group. Dex
treatment significantly reversed the increase induced by THSR
(Fig. 1C).
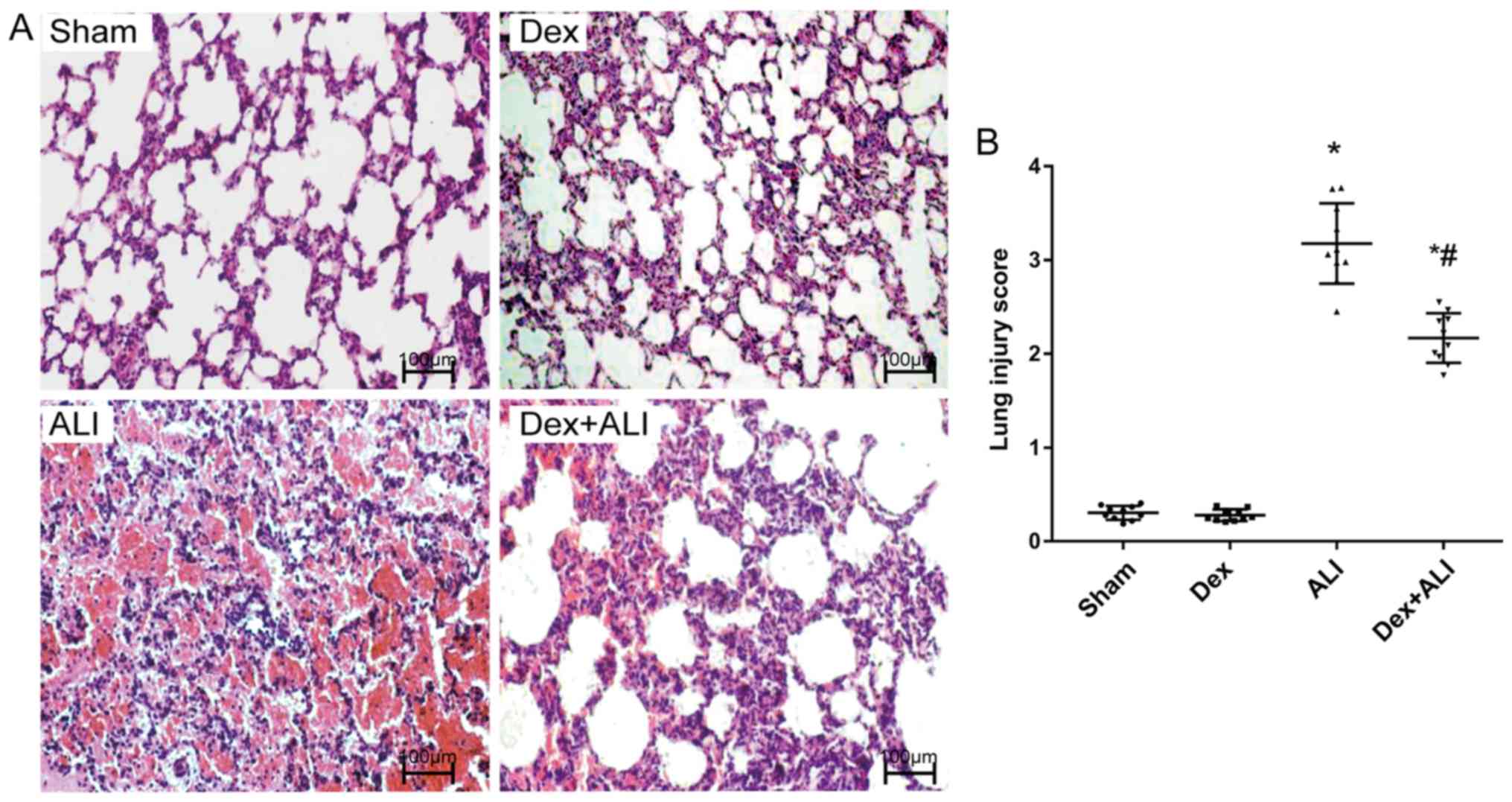
Dex attenuates lung pathological
alterations in THSR-induced ALI
H&E staining of the lung sections in the sham
group showed parenchymal microscopic findings of normal lungs: The
alveolar structure of the sham group was intact, the alveolar wall
was smooth and the pulmonary interstitial showed no evident
exudation (Fig. 2A). No notable
changes were observed in the Dex group. However, the ALI group
showed a notable disruption to normal alveolar structure, severe
alveolar congestion, hemorrhage, rupture, and infiltration with
exuded and accumulated inflammatory cells; the intervals of lung
tissues were markedly widened and thickened, and pulmonary
interstitial edema and alveolar cavity fusion were also shown. By
contrast, the Dex + ALI group exhibited relatively mild
intra-alveolar hemorrhage, infiltration of inflammatory cells,
pulmonary interstitial edema, and narrower alveolar intervals
(Fig. 2A).
Lung injury scores were calculated to indicate the
lung pathological alterations based on the observations of lung
damage parameters under a light microscope. The ALI group reported
significantly higher lung injury scores than the sham group.
Conversely, the Dex + ALI group exhibited significantly reduced
lung injury scores compared with the ALI group (Fig. 2B).
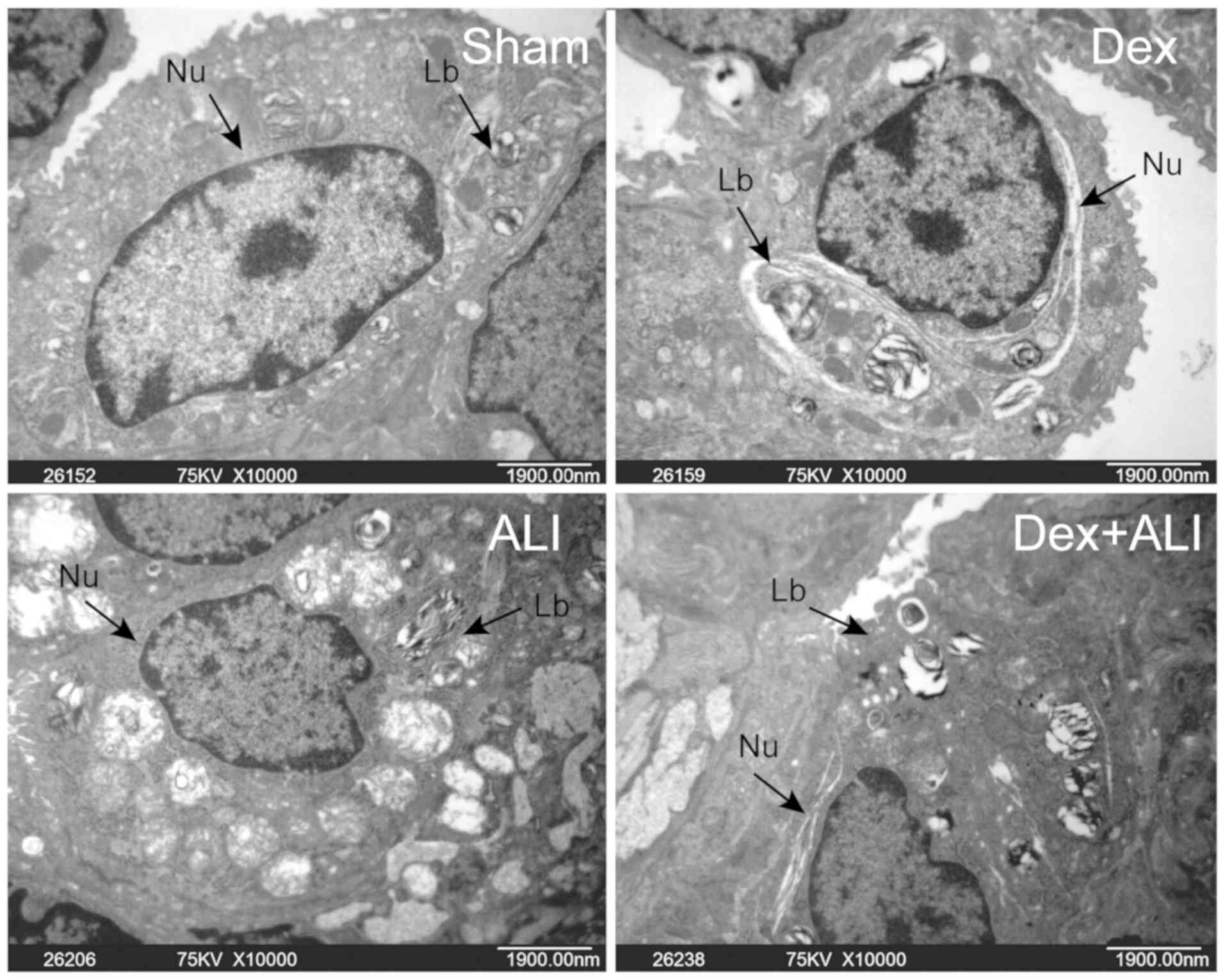
Transmission electron microscopy was used to examine
the ultrastructural changes in lung tissues. Electron microscopy of
the lung tissues in the sham or Dex group demonstrated clear edges
of cells, nuclear membranes and certain osmiophilic lamellar
bodies, whereas the ALI group showed significant pathological
alterations and decreased number of lamellar bodies. Moreover, the
Dex + ALI group exhibited a substantial attenuation of THSR-induced
pulmonary injury compared with the ALI group (Fig. 3).
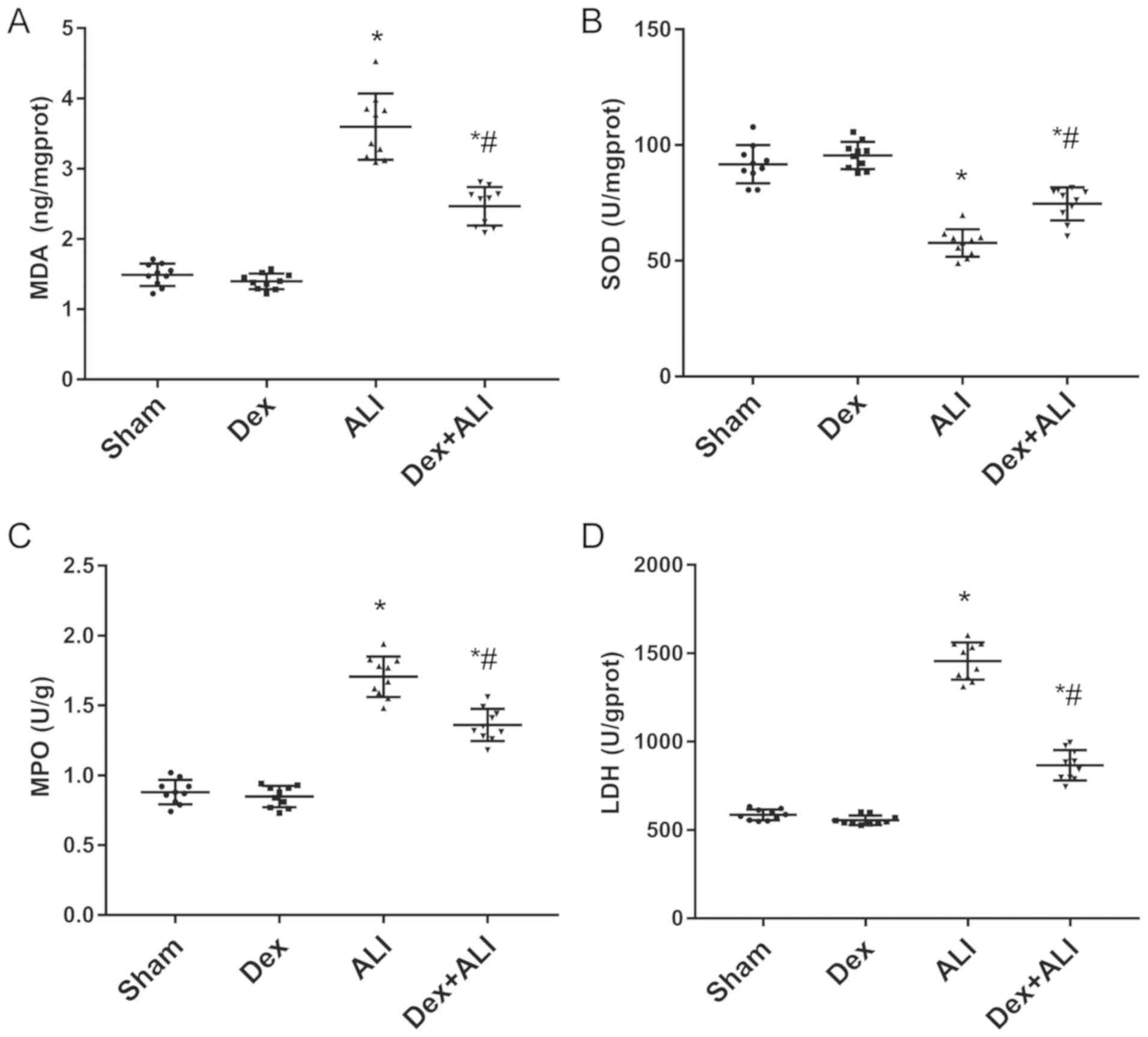
Dex decreases LDH levels, MDA and MPO
activity, and increases SOD activity to inhibit THSR-induced
ALI
Oxidative stress indices (MPO, SOD, LDH and MDA)
were selected to determine ALI severity and explore the mechanisms
of THSR-induced ALI. As presented in Fig. 4, MDA, LDH and MPO levels in the ALI
group were significantly increased compared with those in the sham
group. Dex treatment resulted in significant decreases compared
with the ALI group (Fig. 4A, C and
D). By contrast, SOD activity was significantly increased in
the ALI group compared with the sham group, which was attenuated by
Dex (Fig. 4B).
Dex inhibits inflammatory responses by
decreasing serum IL-1β, IL-18, IL-6 and TNF-α levels in
THSR-induced ALI
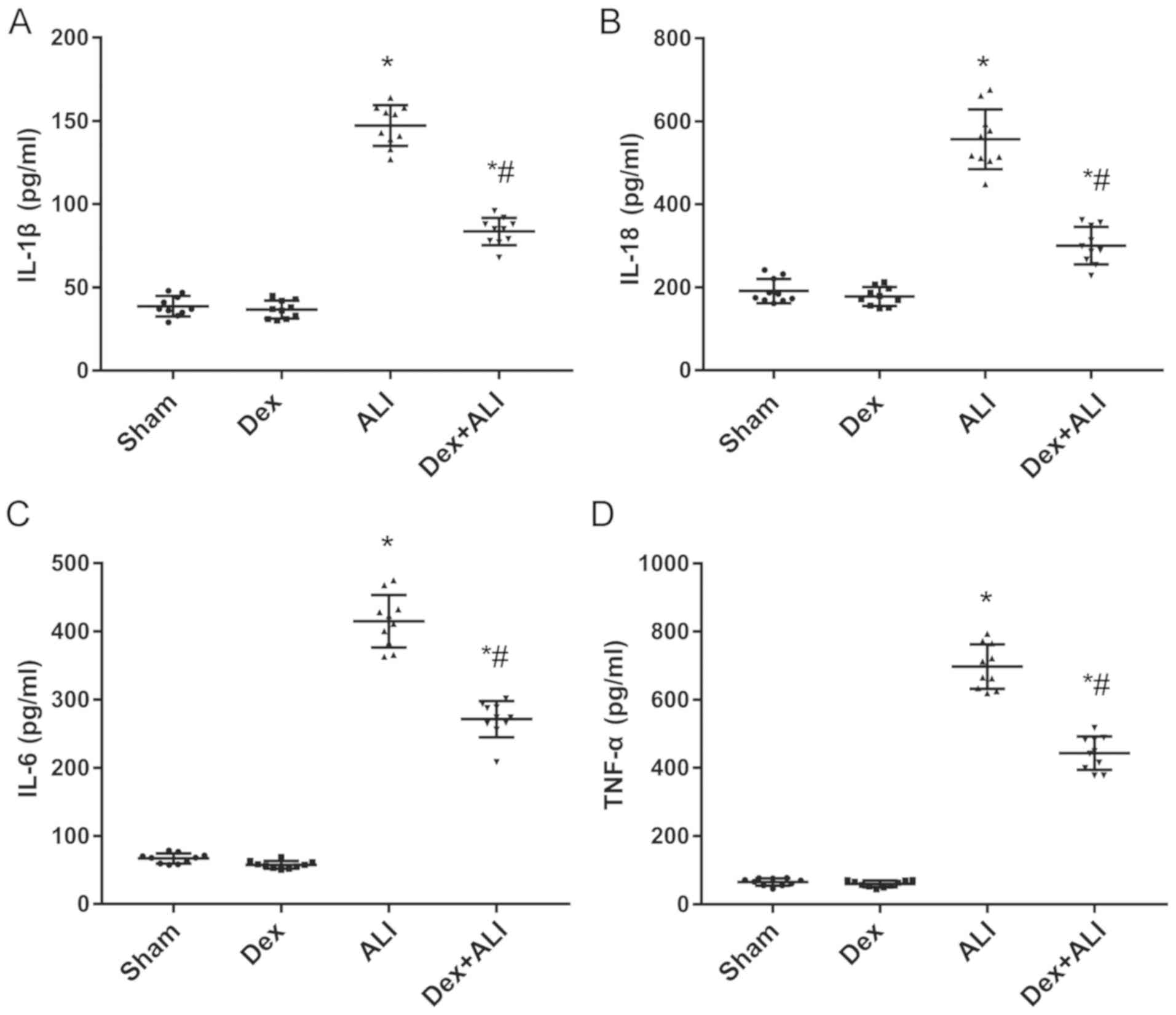
The serum expression levels of the proinflammatory
cytokines IL-1β, IL-18, IL-6 and TNF-α in the ALI group were
significantly increased compared with those in the sham group.
Conversely, Dex treatment resulted in a significant decrease in
their expression levels compared with the ALI group (Fig. 5A-D).
Dex attenuates THSR-induced ALI by
upregulating NLRP3, ASC, and caspase-1 protein expression in lung
tissues
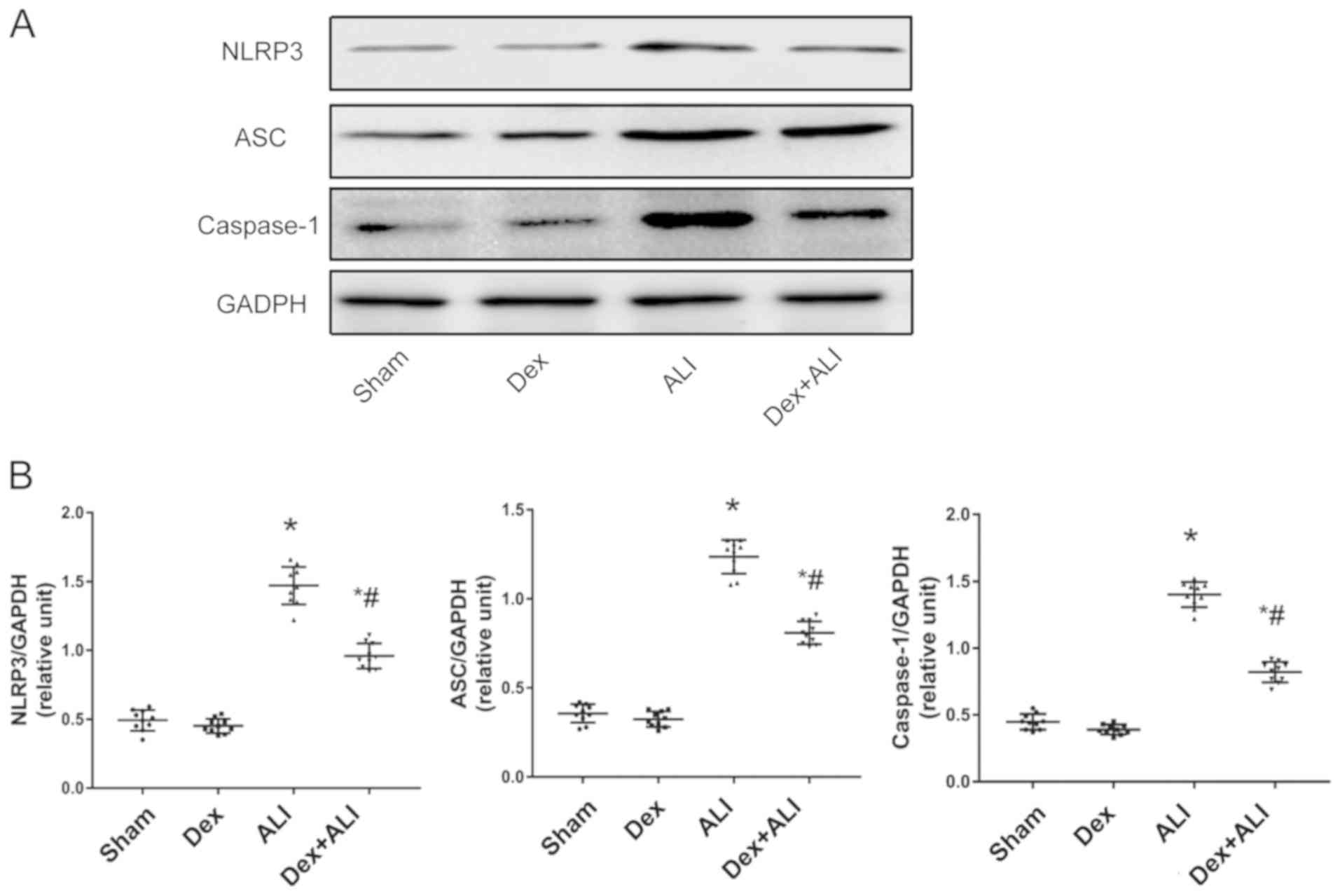
Western blot analysis was performed to determine
NLRP3, ASC and caspase-1 protein expression levels (Fig. 6A). Following THSR, NLRP3, ASC and
caspase-1 levels were significantly increased compared with the
sham group. Dex treatment significantly decreased NLRP3, ASC and
caspase-1 protein expression levels in ALI model rats (Fig. 6B).
Discussion
In a previous study, a rat model of ALI was
successfully established using THSR (26). We did not induce HS by purely
referring to the relative blood volume loss per kilogram because
blunt chest trauma itself could exert a considerable effect on
blood pressure, which also contributes to the induction of HS. This
hypothesis was confirmed in a model of blunt chest trauma in rats
(30). The results of the present
study were consistent with those of previous studies (25–27,30).
The findings indicated that the rat ALI model was successfully
established based on the arterial blood gases, which were improved
by Dex in the rat model of THSR-induced ALI. Additionally, the lung
W/D ratios showed the severity of pulmonary edema in different
groups; the results showed that the condition of pulmonary edema
was worse in the THSR-induced ALI group, and that treatment of Dex
alleviated the severity of pulmonary edema in ALI rats. As for the
condition of the lungs, the ALI group showed extensive pulmonary
injury, whereas Dex ameliorated pathological alterations to the
lung in the model.
Severe THSR damage often triggers systemic
inflammatory response syndrome and multiple organ dysfunction
syndrome (1). ALI or ARDS are
common pathophysiological processes due to ‘waterfall’ inflammatory
reactions resulting from traumatic pulmonary contusion (1,31).
Lung tissue damage is associated with inflammatory reactions,
particularly when infection secondary to trauma plays an important
role in the process of ALI; the production of oxygen radicals by
the respiratory burst of granulocytes or monocytes is also an
important part of defense processes (32). MDA, LDH and MPO activity levels
reflect the degree of cell injury and inflammatory responses,
whereas SOD is a pivotal antioxidant enzyme reflecting the ability
to scavenge the oxygen-free radicals and protects cells from
superoxide damage (29). The
measurement of SOD activity and MDA levels in lung tissue revealed
the balance between antioxidant action and oxidation. As observed
in previous studies (32,33), oxidative system activation and
excessive free radical production were observed, but the
antioxidant system was partially destroyed and deactivated in
response to trauma, which were demonstrated by detection of MDA and
SOD levels. In the present study, THSR-induced ALI increased MDA
levels and decreased SOD activity in lung tissue; at the same time,
the expression levels of the proinflammatory cytokines IL-1β,
IL-18, IL-6 and TNF-α were significantly increased in the ALI
group. However, treatment with Dex alleviated the degree of
oxidative stress and improved the antioxidant reaction to the
overproduction of oxygen radicals in the experimental model.
As the core of innate immunity, NLRP3 forms an
oligomeric molecular complex called an inflammasome, which responds
to harmful stimuli, such as pathogen-associated molecular patterns
and danger-associated molecular patterns (34). After NLRP3 activation, ASC is
recruited, caspase-1 is activated, and pro-IL-1β and pro-IL-18 are
processed into mature IL-1β and IL-18, respectively (34). These cytokines are released to
induce selective recruitment of monocytes, neutrophils and
lymphocytes, eventually causing pyroptosis, a novel form of
programed cell death (34,35), which leads to tissue damage as a
consequence. ALI/ARDS pathogenesis is closely associated with the
NLRP3 inflammasome in lipopolysaccharide (LPS)-induced mouse and
macrophage models (36), in
LPS-induced animal models of ALI (36,37),
and in cecal ligation perforation (38). The present study demonstrated that
NLRP3, ASC and caspase-1 expression levels were elevated in the ALI
group, which was consistent with the aforementioned studies. These
results suggested that the NLRP3 pathway was activated in
THSR-induced ALI, and that Dex inhibited the NLRP3 inflammasome in
this model, indicating that NLRP3 inflammasome signaling activation
may serve an important role in ALI.
An increasing number of studies have reported that
Dex exerts protective effects on the lungs (18,39–41).
A recent study discovered that Dex alleviates multiple organ damage
by inhibiting the inflammatory response and oxidative stress in a
two-hit hemorrhagic, resuscitation and subsequent endotoxemia model
in rats; these effects were partially mediated by α2-adrenoceptors
(20). Based on these findings,
the effects of Dex on the lungs were investigated in a rat model of
THSR-induced ALI. Another study found that Dex inhibits the NLRP3
inflammasome and sympathetic nerve activity in mice with acute
pancreatitis (42). However, the
underlying mechanisms remain unclear. A previous study reported
that clinically relevant doses of Dex do not significantly affect
the attenuation of ventilator-induced lung injury (VILI) (43). However, Dex at a dose that is
~10-fold higher than the recommended clinical dose had a
significant effect on VILI attenuation (43,44).
On the basis of these findings, 5.0 µg/kg/h was used as the dose of
Dex in the present study to explore the underlying mechanisms. The
findings of the present study indicated that Dex improved arterial
blood gas levels, alleviated the severity of pulmonary edema,
ameliorated pathological lung alterations, attenuated inflammatory
responses, alleviated the degree of oxidative stress and inhibited
the NLRP3 inflammasome signaling pathway at the same time. These
results confirmed that Dex treatment may contribute to exert
protective effects on THSR-induced ALI in rats.
However, the present study contains several
limitations. The regulatory mechanism of NLRP3 inflammasome
activation and the role of Dex in this mechanism remain unclear.
Moreover, the question of whether Dex directly interacts with the
NLRP3 inflammasome or via other indirect mechanisms that trigger
signaling remains unanswered. Furthermore, there remain unresolved
issues regarding whether the interaction was through the α2
adrenergic receptor pathway and whether other drugs aside from Dex
could exert similar protective effects. If so, whether these
effects were different, and whether the NLRP3 inflammasome plays
the same role in ALI attenuation by different drugs, require
further verification. Further studies are required to address these
issues and questions.
The present study demonstrated that Dex inhibits
NLRP3 inflammasome signaling pathways and alleviates THSR-induced
ALI in rats. This pathomechanism needs to be further analyzed in
mechanistic and dose-related studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81901952
and 81970722).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TQM and MY drafted the manuscript. TQM and QK
analyzed and interpreted the data and all manuscript figures. MY
and QH performed the transmission electron microscopy and lung
injury score analysis. ZYX and XJW conceived and designed the
study, reviewed the data and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was provided by the Medical Ethics
Committee of Renmin Hospital of Wuhan University. All surgical
procedures were performed in accordance with the Guide for the Care
and Use of Laboratory Animals of the National Research Council (US)
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALI
|
acute lung injury
|
|
Dex
|
dexmedetomidine
|
|
THSR
|
blunt chest trauma and hemorrhagic
shock-resuscitation
|
|
NLRP3
|
nucleotide binding and oligomerization
domain-like receptor family pyrin domain-containing protein 3
|
|
ASC
|
apoptosis-associated speck-like
protein containing a caspase recruitment domain
|
|
W/D
|
wet/dry
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
LDH
|
lactate dehydrogenase
|
|
MPO
|
myeloperoxidase
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
HS
|
hemorrhagic shock
|
|
MAP
|
mean arterial pressure
|
|
VILI
|
ventilator-induced lung injury
|
References
|
1
|
Ehrnthaller C, Flierl M, Perl M, Denk S,
Unnewehr H, Ward PA, Radermacher P, Ignatius A, Gebhard F,
Chinnaiyan A and Huber-Lang M: The molecular fingerprint of lung
inflammation after blunt chest trauma. Eur J Med Res. 20:702015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kauvar DS and Wade CE: The epidemiology
and modern management of traumatic hemorrhage: US and international
perspectives. Crit Care 9 Suppl. 5 (Suppl 5):S1–S9. 2005.
View Article : Google Scholar
|
|
3
|
Sauaia A, Moore EE, Johnson JL, Chin TL,
Banerjee A, Sperry JL, Maier RV and Burlew CC: Temporal trends of
postinjury multiple-organ failure: Still resource intensive,
morbid, and lethal. J Trauma Acute Care Surg. 76:582–593. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Angele MK, Schneider CP and Chaudry IH:
Bench-to-bedside review: Latest results in hemorrhagic shock. Crit
Care. 12:2182008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah CV, Localio AR, Lanken PN, Kahn JM,
Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD and Christie
JD: The impact of development of acute lung injury on hospital
mortality in critically ill trauma patients. Crit Care Med.
36:2309–2315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Villar J, Blanco J and Kacmarek RM:
Current incidence and outcome of the acute respiratory distress
syndrome. Curr Opin Crit Care. 22:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu P, Wen Z, Shi X, Li Y, Fan L, Xiang M,
Li A, Scott MJ, Xiao G, Li S, et al: Hemorrhagic shock augments
Nlrp3 inflammasome activation in the lung through impaired pyrin
induction. J Immunol. 190:5247–5255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grailer JJ, Canning BA, Kalbitz M,
Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS and Ward PA:
Critical role for the NLRP3 inflammasome during acute lung injury.
J Immunol. 192:5974–5983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mangan MSJ, Olhava EJ, Roush WR, Seidel
HM, Glick GD and Latz E: Targeting the NLRP3 inflammasome in
inflammatory diseases. Nat Rev Drug Discov. 17:588–606. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ and
Jo EK: Upregulated NLRP3 inflammasome activation in patients with
type 2 diabetes. Diabetes. 62:194–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duewell P, Kono H, Rayner KJ, Sirois CM,
Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr
M, et al: NLRP3 inflammasomes are required for atherogenesis and
activated by cholesterol crystals. Nature. 464:1357–1361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhen Y and Zhang H: NLRP3 inflammasome and
inflammatory bowel disease. Front Immunol. 10:2762019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Primiano MJ, Lefker BA, Bowman MR, Bree
AG, Hubeau C, Bonin PD, Mangan M, Dower K, Monks BG, Cushing L, et
al: Efficacy and pharmacology of the NLRP3 inflammasome inhibitor
CP-456,773 (CRID3) in murine models of dermal and pulmonary
inflammation. J Immunol. 197:2421–2433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizushina Y, Karasawa T, Aizawa K, Kimura
H, Watanabe S, Kamata R, Komada T, Mato N, Kasahara T, Koyama S, et
al: Inflammasome-independent and atypical processing of IL-1β
contributes to acid aspiration-induced acute lung injury. J
Immunol. 203:236–246. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Afonso J and Reis F: Dexmedetomidine:
Current role in anesthesia and intensive care. Rev Bras Anestesiol.
62:118–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carollo DS, Nossaman BD and Ramadhyani U:
Dexmedetomidine: A review of clinical applications. Curr Opin
Anaesthesiol. 21:457–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Zhang R, Li C, Yin X, Lv C, Wang Y,
Zhao W and Zhang X: Dexmedetomidine attenuates acute lung injury
induced by lipopolysaccharide in mouse through inhibition of MAPK
pathway. Fundam Clin Pharmacol. 29:462–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Wu D, Yang Y, Liu T and Liu H:
Dexmedetomidine alleviates hyperoxia-induced acute lung injury via
inhibiting NLRP3 inflammasome activation. Cell Physiol Biochem.
42:1907–1919. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang K, Gao Y, Wang SC, Liu HT, Kong WL,
Zhang X, Huang R, Qi ZD, Zheng JB, Qu JD, et al: Dexmedetomidine
protects against lipopolysaccharide-induced sepsis-associated acute
kidney injury via an α7 nAChR-dependent pathway. Biomed
Pharmacother. 106:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Xia M, Huang Q, Ding D, Li Y,
Zhang Z and Zhang X: Protective effect of dexmedetomidine against
organ dysfunction in a two-hit model of hemorrhage/resuscitation
and endotoxemia in rats. Braz J Med Biol Res. 52:e79052019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng F, Yan FF, Liu YP, Cong Y, Sun KF
and He XM: Dexmedetomidine inhibits the NF-κB pathway and NLRP3
inflammasome to attenuate papain-induced osteoarthritis in rats.
Pharm Biol. 57:649–659. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin D, Zhou S, Xu X, Gao W, Li F, Ma Y,
Sun D, Wu Y, Guo Q, Liu H, et al: Dexmedetomidine attenuated early
brain injury in rats with subarachnoid haemorrhage by suppressing
the inflammatory response: The TLR4/NF-κB pathway and the NLRP3
inflammasome may be involved in the mechanism. Brain Res.
1698:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals (8th).
National Academies Press (US). Washington (DC): 2011.PubMed/NCBI
|
|
25
|
Seitz DH, Perl M, Liener UC, Tauchmann B,
Braumüller ST, Brückner UB, Gebhard F and Knöferl MW: Inflammatory
alterations in a novel combination model of blunt chest trauma and
hemorrhagic shock. J Trauma. 70:189–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu XJ, Liu HM, Song XM, Zhao B, Leng Y,
Wang EY, Zhan LY, Meng QT and Xia ZY: Penehyclidine hydrochloride
inhibits TLR4 signaling and inflammation, and attenuates blunt
chest trauma and hemorrhagic shock-induced acute lung injury in
rats. Mol Med Rep. 17:6327–6336. 2018.PubMed/NCBI
|
|
27
|
Wu X, Song X, Li N, Zhan L, Meng Q and Xia
Z: Protective effects of dexmedetomidine on blunt chest
trauma-induced pulmonary contusion in rats. J Trauma Acute Care
Surg. 74:524–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belperio JA, Keane MP, Burdick MD, Londhe
V, Xue YY, Li K, Phillips RJ and Strieter RM: Critical role for
CXCR2 and CXCR2 ligands during the pathogenesis of
ventilator-induced lung injury. J Clin Invest. 110:1703–1716. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun K, Fan J and Han J: Ameliorating
effects of traditional Chinese medicine preparation, Chinese
materia medica and active compounds on ischemia/reperfusion-induced
cerebral microcirculatory disturbances and neuron damage. Acta
Pharm Sin B. 5:8–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kao RL, Huang W, Martin CM and Rui T: The
effect of aerosolized indomethacin on lung inflammation and injury
in a rat model of blunt chest trauma. Can J Surg. 61:S208–S218.
2018.PubMed/NCBI
|
|
31
|
Rendeki S and Molnár TF: Pulmonary
contusion. J Thorac Dis. 11 (Suppl 2):S141–S151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X and Chen Z: The pathophysiological
role of mitochondrial oxidative stress in lung diseases. J Transl
Med. 15:2072017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Torun AC, Tutuncu S, Ustun B and Akdemir
HU: A study of the therapeutic effects of resveratrol on blunt
chest trauma-induced acute lung injury in rats and the potential
role of endocan as a biomarker of inflammation. Inflammation.
40:1803–1810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamkanfi M and Dixit VM: Mechanisms and
functions of inflammasomes. Cell. 157:1013–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pedraza-Alva G, Pérez-Martínez L,
Valdez-Hernández L, Meza-Sosa KF and Ando-Kuri M: Negative
regulation of the inflammasome: Keeping inflammation under control.
Immunol Rev. 265:231–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y and Li X, Grailer JJ, Wang N, Wang
M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX and Li X: Melatonin
alleviates acute lung injury through inhibiting the NLRP3
inflammasome. J Pineal Res. 60:405–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang H, Chen S, Zeng M, Lin D, Wang Y,
Wen X, Xu C, Yang L, Fan X, Gong Y, et al: Apelin-13 administration
protects against LPS-induced acute lung injury by inhibiting NF-κB
pathway and NLRP3 inflammasome activation. Cell Physiol Biochem.
49:1918–1932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Z, Jia Y, Feng Y, Cui R, Miao R, Zhang
X, Qu K, Liu C and Zhang J: Methane alleviates sepsis-induced
injury by inhibiting pyroptosis and apoptosis: In vivo and in vitro
experiments. Aging (Albany NY). 11:1226–1239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deletombe B, Trouve-Buisson T, Godon A,
Falcon D, Giorgis-Allemand L, Bouzat P, Bosson JL and Payen JF:
Dexmedetomidine to facilitate non-invasive ventilation after blunt
chest trauma: A randomised, double-blind, crossover,
placebo-controlled pilot study. Anaesth Crit Care Pain Med.
38:477–483. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu C, Dai X, Yang Y, Lin M, Cai Y and Cai
S: Dexmedetomidine attenuates lipopolysaccharide-induced acute lung
injury by inhibiting oxidative stress, mitochondrial dysfunction
and apoptosis in rats. Mol Med Rep. 15:131–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB
and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Pan Y, Gao L, Lu G, Zhang J, Xie X,
Tong Z, Li B, Li G and Li W: Dexmedetomidine attenuates pancreatic
injury and inflammatory response in mice with pancreatitis by
possible reduction of NLRP3 activation and up-regulation of NET
expression. Biochem Biophys Res Commun. 495:2439–2447. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang CL, Tsai PS and Huang CJ: Effects of
dexmedetomidine on regulating pulmonary inflammation in a rat model
of ventilator-induced lung injury. Acta Anaesthesiol Taiwan.
46:151–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tasdogan M, Memis D, Sut N and Yuksel M:
Results of a pilot study on the effects of propofol and
dexmedetomidine on inflammatory responses and intraabdominal
pressure in severe sepsis. J Clin Anesth. 21:394–400. 2009.
View Article : Google Scholar : PubMed/NCBI
|