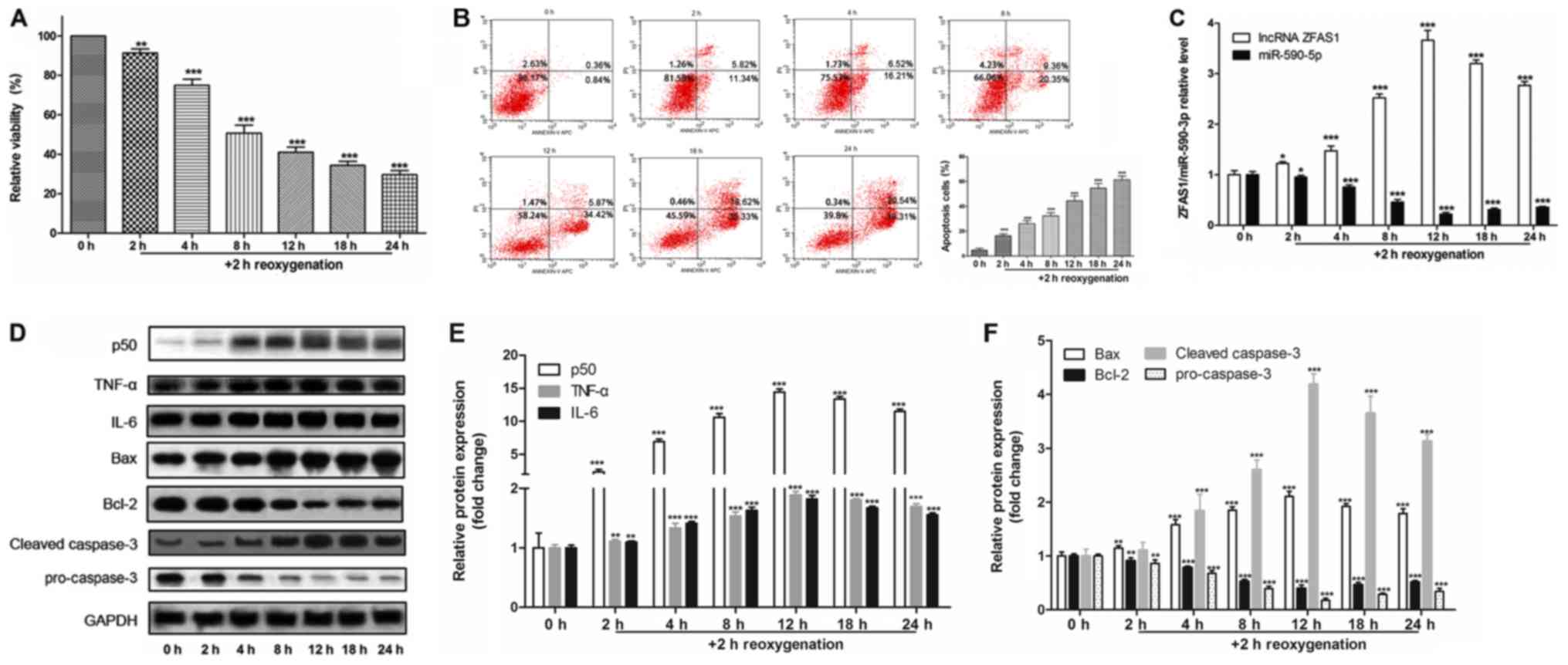
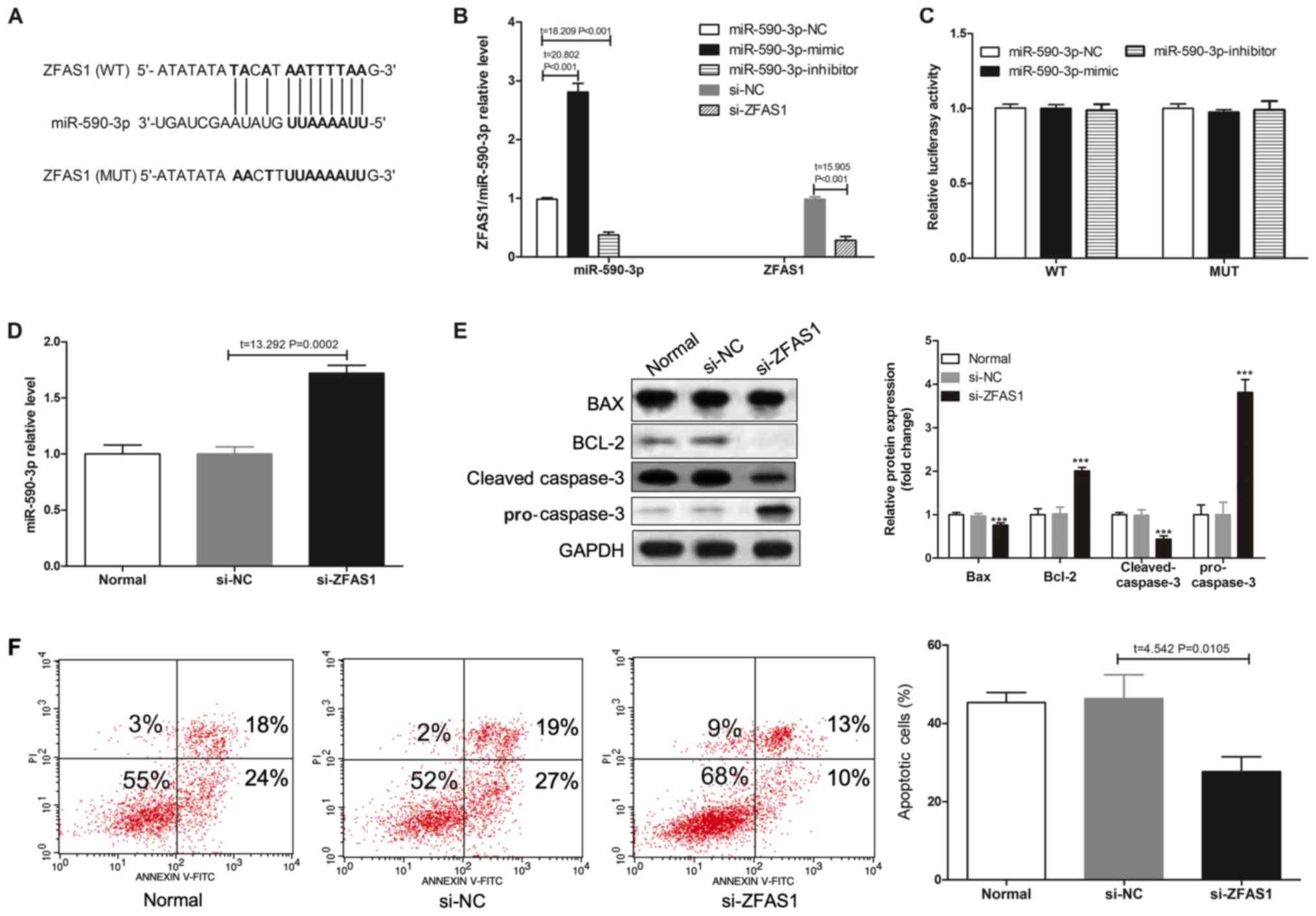
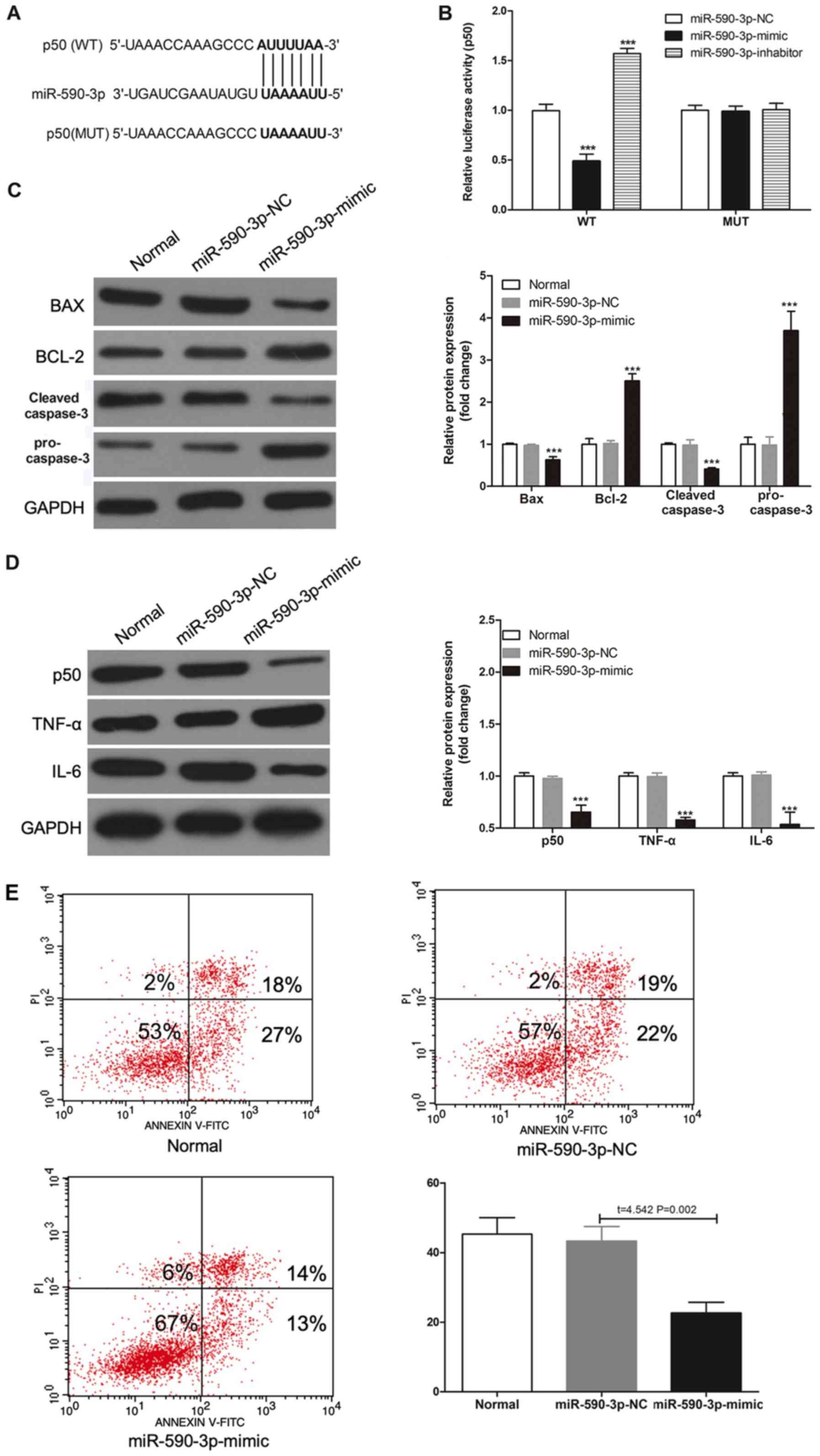
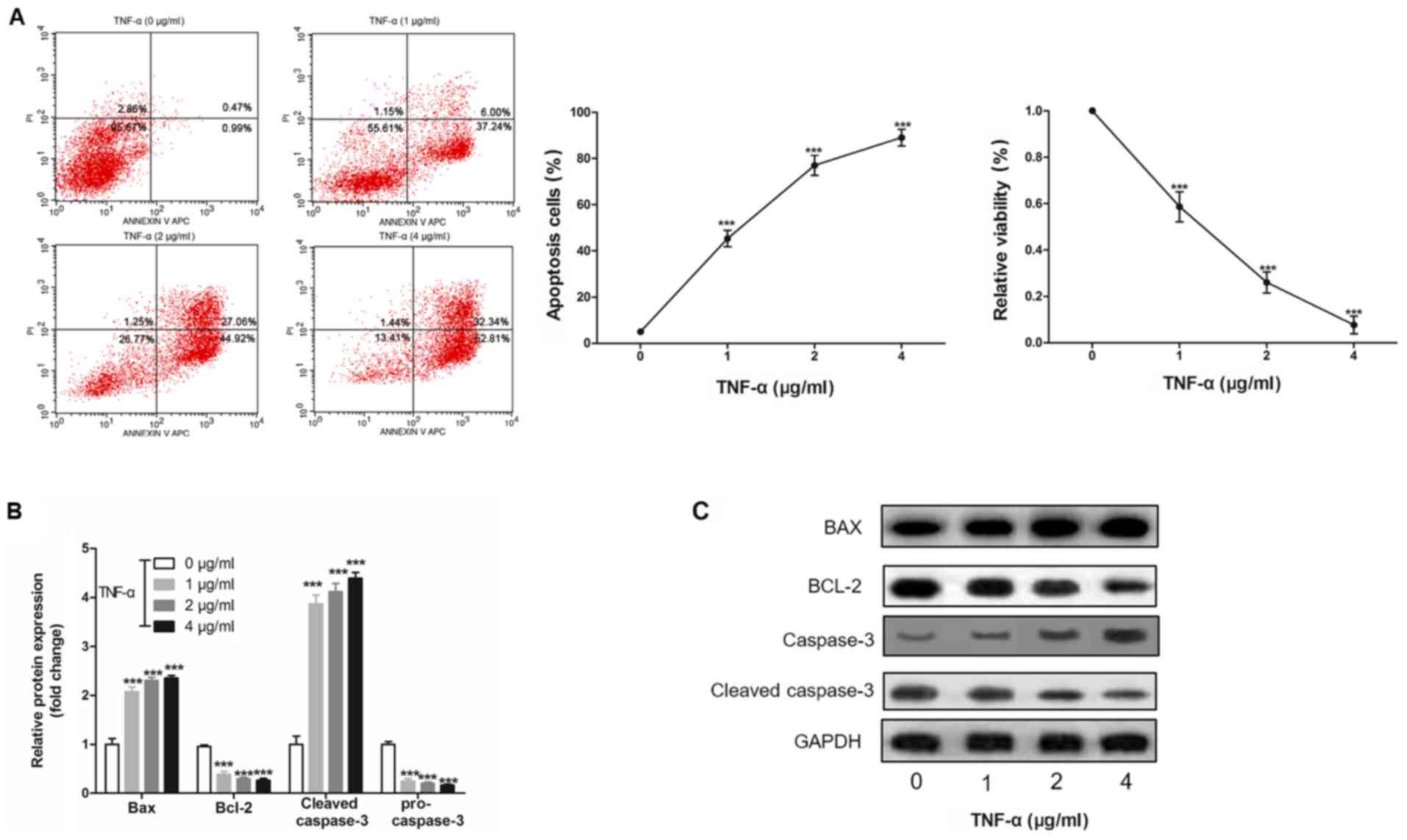
|
1
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for Percutaneous
Coronary Intervention: Executive summary: A report of the American
College of Cardiology Foundation/American Heart Association Task
Force on Practice Guidelines and the Society for Cardiovascular
Angiography and Interventions. Circulation. 124:2574–2609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim
MH, Loo G, Koo CY, Gao XF, Chandra S, et al: Obstructive sleep
apnea and cardiovascular events after percutaneous coronary
intervention. Circulation. 133:2008–2017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang C, Batkai S, Dangwal S, Gupta SK,
Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al:
Cardiac fibroblast-derived microRNA passenger strand-enriched
exosomes mediate cardiomyocyte hypertrophy. J Clin Invest.
124:2136–2146. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michaels AD, Gibson CM and Barron HV:
Microvascular dysfunction in acute myocardial infarction: Focus on
the roles of platelet and inflammatory mediators in the no-reflow
phenomenon. Am J Cardiol. 85:50B–60B. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maier W, Altwegg LA, Corti R, Gay S,
Hersberger M, Maly FE, Sütsch G, Roffi M, Neidhart M, Eberli FR, et
al: Inflammatory markers at the site of ruptured plaque in acute
myocardial infarction: Locally increased interleukin-6 and serum
amyloid A but decreased C-reactive protein. Circulation.
111:1355–1361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pop M, Qi X, Barry J, Strauss BH, Wright
GA and Ghugre NR: Hemorrhage promotes inflammation and myocardial
damage following acute myocardial infarction. J Cardiovascular
Magnetic Resonance. 16:1–2. 2014. View Article : Google Scholar
|
|
8
|
Westman PC, Lipinski MJ, Luger D, Waksman
R, Bonow RO, Wu E and Epstein SE: Inflammation as a driver of
adverse left ventricular remodeling after acute myocardial
infarction. J Am Coll Cardiol. 67:2050–2060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hon CC, Ramilowski JA, Harshbarger J,
Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM,
Severin J, et al: An atlas of human long non-coding RNAs with
accurate 5′ends. Nature. 543:199–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta SC and Tripathi YN: Potential of
long non-coding RNAs in cancer patients: From Bio-markers to
therapeutic targets. Int J Cancer. 140:1955–1967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Archer K, Broskova Z, Bayoumi AS, Teoh JP,
Davila A, Tang Y, Su H and Kim IM: Long non-coding RNAs as master
regulators in cardiovascular diseases. Int J Mol Sci.
16:23651–23667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uchida S and Dimmeler S: Long noncoding
RNAs in cardiovascular diseases. Circ Res. 116:737–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathy NW and Chen XM: Long non-coding RNAs
(lncRNAs) and their transcriptional control of inflammatory
responses. J Biol Chem. 292:12375–12382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Sun L, Xuan L, Pan Z, Li K, Liu
S, Huang Y, Zhao X, Huang L, Wang Z, et al: Reciprocal changes of
circulating long non-coding RNAs ZFAS1 and CDR1AS predict acute
myocardial infarction. Sci Rep. 6:223842016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Yao H, Hui JY, Ding SH, Fan YL,
Pan YH, Chen KH, Wan JQ and Jiang JY: Global transcriptomic study
of atherosclerosis development in rats. Gene. 592:43–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun G, Wang Y, Zhang J, Lin N and You Y:
MiR-15b/HOTAIR/p53 form a regulatory loop that affects the growth
of glioma cells. J Cell Biochem. 119:4540–4547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vervliet T, Robinson EL and Roderick HL:
Lnc'ing Ca2+, SERCA and cardiac disease. Cell Calcium.
72:132–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Xing Y, Xu Y, Huang C, Bao H, Hong K
and Cheng X: Pim-2 protects H9c2 cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis via downregulation of Bim
expression. Environ Toxicol Pharmacol. 48:94–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu G, Huang Y, Wu C, Guo Z, Ma Y, Xia Q,
Awasthi A and He X: Differential expression of long noncoding RNAs
during cardiac allograft rejection. Transplantation. 101:83–91.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kühl C and Frey N: Long noncoding RNAs in
heart disease. Springer International Publishing. 2016. View Article : Google Scholar
|
|
23
|
Yang L, Lin C, Jin C, Yang JC, Tanasa B,
Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, et al:
LncRNA-dependent mechanisms of androgen receptor-regulated gene
activation programs. Nature. 500:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Necsulea A, Soumillon M, Warnefors M,
Liechti A, Daish T, Zeller U, Baker JC, Grützner F and Kaessmann H:
The evolution of lncRNA repertoires and expression patterns in
tetrapods. Nature. 505:635–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol.
1402:271–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao S, Yang G, Liu PN, Deng YY, Zhao Z,
Sun T, Zhuo XZ, Liu JH, Tian Y, Zhou J, et al: miR-590-3p is a
novel MicroRNA in myocarditis by targeting nuclear factor Kappa-B
in vivo. Cardiology. 132:182–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao MH, Li JM, Zhou QL, Li GY, Zeng J,
Zhao J and Zhang YW: Effects of miR-590 on oxLDL-induced
endothelial cell apoptosis: Roles of p53 and NF-κB. Mol Med Rep.
13:867–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rapicavoli NA, Qu K, Zhang J, Mikhail M,
Laberge RM and Chang HY: A mammalian pseudogene lncRNA at the
interface of inflammation and anti-inflammatory therapeutics.
elife. 2:e007622013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wallach D: The cybernetics of TNF: Old
views and newer ones. Semin Cell Dev Biol. 50:105–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao G, Su Z, Song D, Mao Y and Mao X: The
long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced
inflammatory response through its interaction with NF-κB. FEBS
Lett. 590:2884–2895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang R, Zhao Q, Jian G, Cheng D, Wang N,
Zhang G and Wang F: Tanshinone IIA attenuates contrast-induced
nephropathy via Nrf2 activation in rats. Cell Physiol Biochem.
46:2616–2623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang F, Zhang G, Lu Z, Geurts AM, Usa K,
Jacob HJ, Cowley AW, Wang N and Liang M: Antithrombin III/SerpinC1
insufficiency exacerbates renal ischemia/reperfusion injury. Kidney
Int. 88:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi Z, Lian A and Zhang F: Nuclear
factor-κB activation inhibitor attenuates ischemia reperfusion
injury and inhibits Hmgb1 expression. Inflamm Res. 63:919–925.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghosh G, Van Duyne G, Ghosh S and Sigler
PB: Structure of NF-kappaB p50 homodimer bound to a kappa B site.
Nature. 373:303–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frantz S, Tillmanns J, Kuhlencordt PJ,
Schmidt I, Adamek A, Dienesch C, Thum T, Gerondakis S, Ertl G and
Bauersachs J: Tissue-specific effects of the nuclear Factor kappaB
subunit p50 on myocardial ischemia-reperfusion injury. Am J Pathol.
171:507–512. 2007. View Article : Google Scholar : PubMed/NCBI
|