Introduction
Coronary artery disease has one of the highest
morbidity and mortality rates of any disease worldwide, and
myocardial infarction is the most common coronary artery disease
(1,2). Percutaneous coronary intervention
(PCI) is the primary effective treatment for myocardial infarction
in clinical practice; it can clear narrow lumens and occlude the
coronary lumen, but ischemia-reperfusion (I/R) injury is the most
important obstacle to PCI treatment (1,2). I/R
injury is one of the main mechanisms of arrhythmia, myocardial
contractile dysfunction and irreversible damage of cardiomyocytes
(3). Increased inflammation
induced by myocardial I/R is one of the main causes of myocardial
cell apoptosis (4). However, the
release of inflammatory mediators also initiates the repair of
damaged tissues in the body (5,6).
Moreover, inflammatory cytokines can induce cardiomyocyte
apoptosis, which further promotes increases in inflammatory
cytokine levels (7,8).
Long non-coding RNA (lncRNA) is a type of RNA that
is >200 bp in length with no or little open reading frame, which
does not encodes a protein (9,10).
With the development of gene sequencing, gene chips and genomics,
numerous lncRNAs have been revealed to be involved in the
regulation of inflammation (9,11).
Previous studies had reported that lncRNAs are not only involved in
the development of cardiovascular diseases, such as cardiac
hypertrophy, myocardial infarction, heart failure and myocardial
fibrosis (12,13), but also in inflammation and
inflammatory diseases through the regulation of numerous gene
expression and signaling pathways (14).
lncRNA ZNFX1 antisense RNA 1 (ZFAS1) is abnormally
expressed in patients with acute myocardial infarction (15) and in atherosclerotic model rats
(16). Furthermore, lncRNA ZFAS1
was reported to contribute to the impairment of cardiac contractile
function in myocardial infarction (17,18).
The aim of the present study was to investigate the relationship
between lncRNA ZFAS1 and I/R injury using hypoxia/reoxygenation
(H/R)-induced H9c2 cardiomyocytes as an in vitro model to
examine apoptosis and inflammation. It was found that H/R increased
the expression level of ZFAS1 in H9c2 cells, and that ZFAS1
knockdown reduced inflammation and apoptosis by targeting the
microRNA (miR)-590-3p/NF-κB pathway.
Materials and methods
Cell culture and H/R stress
Rat H9c2 cardiomyocyte cells (cat. no. CRL-1446;
American Type Culture Collection) were cultured with DMEM (cat. no.
12491-15; Thermo Fisher Scientific, Inc.) with 10% of FBS (cat. no.
10100-147; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (cat. no. 15640055; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. H/R treatment was
used to establish an I/R injury model in H9c2 cells. H9c2 cells
were sequentially exposed to hypoxia for 2, 4, 8, 12, 18 and 24 h
(95:5 CO2:N2 ratio) at 37°C and re-oxygenated
for (95:5 O2:CO2 ratio) 2 h at 37°C (19).
Cell transfection
Small interfering (si)RNA for ZFAS1 knockdown
(si-ZFAS1 forward, 5′-UGGAUUUGUACCAUUCUUCUG-3′ and reverse,
5′-GAAGAAUGGUACAAAUCCAAG-3′), negative control knockdown (si-NC
forward, 5′-AGUUUCAACCGUCUUAAUCAG-3′ and reverse,
5′-GAUUAAGACGGUUGAAACUAG-3′), hsa-miR-590-3p-inhibitor
(5′-AAUUUUCAUAUUCGAUCA-3′), hsa-miR-590-3p-mimic
(5′-UUAAAAGUAUAAGCUAGU-3′) and hsa-miR-590-5p-NC
(5′-GGAUGGCCAAUCUUCGCGGGCU-3′) were designed and synthesized by
Shenggong Bioengineering Co., Ltd. Cells (1×106) were
directly transfected with 25 nmol si-RNA, si-NC, miR-inhibitor,
miR-mimic or miR-NC using Lipofectamine® 2000
transfection reagent (cat. no. 11668019; Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 72 h. Subsequent experiments were
performed at 72 h post-transfection.
Dual-luciferase reporter assay
The StarBase database
(starbase.sysu.edu.cn/index.php) was used to identify the binding
sites between miR-590-3p and ZFAS1. The wild-type (WT) or mutant
(MUT) mRNA 3′-untranslated regions (UTRs) of ZFAS1 and p50 were
cloned into the psiCHECK2 vector (Promega Corporation). Cells
(5×106) were transfected with psiCHECK2 vectors using
Lipofectamine® 2000. The Dual-Lucy Assay kit (cat. no.
D00100; Beijing Solarbio Science & Technology Co., Ltd.) was
used to detect luciferase activities according to the
manufacturer's protocol. Firefly luciferase activity was normalized
to Renilla luciferase activity.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to detect the mRNA expression
levels of U6, miR-590-3p and lncRNA ZFAS1 in cells.
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to extract the total RNA from H9c2 cells. The extracted RNA
was reverse transcribed into cDNA using PrimeScript RT Master mix
RT kit (cat. no. RR036B; Takara Bio, Inc.) at 37°C for 15 min and
85°C for 15 sec. qPCR was set up and conducted according to the
SYBR Green qPCR Master Mix kit instructions (cat. no. 638320;
Takara Bio, Inc.) and amplified using an ABI 7500 fluorescence qPCR
instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 94°C for 30 sec; 40 cycles of 93°C for 2 min, 93°C
for 1 min and 55°C for 2 min; followed by final extension at 72°C
for 1.5 min. PCR primers were as follows: U6, forward
5′-AUAAAUCCCUUUACACCUCTT-3′, reverse 5′-AAUAAAUCCCUUUACACCUCTT-3′;
GAPDH, forward 5′-AGGTCGGTGTGAACGGATTTG-3′, reverse,
5′-GGGGTCGTTGATGGCAACA-3′; miR-590-3p, forward
5′-ACACTCCAGCTGGGTGATCGAATATGTAT-3′, reverse 5-TGGTGTCGTGGAGTCG-3;
and ZFAS1, forward 5′-ACGTGCAGACATCTACAACCT-3′, reverse
5′-TACTTCCAACACCCGCAT-3′. miRNA and mRNA expression levels were
quantified using the 2−∆∆Cq method (20) and normalized to the internal
reference genes U6 and GAPDH, respectively.
Western blotting
Total protein was extracted from H9c2 cells using
RIPA lysis buffer (cat. no. P0013K; Beyotime Institute of
Biotechnology) and quantified using the BCA Protein Assay kit (cat.
no. P0010S; Beyotime Institute of Biotechnology). Proteins (50 µg)
were separated via 12% SDS-PAGE and then transferred to PVDF
membranes. The membranes were blocked with 5% skimmed milk powder
at room temperature for 2 h. Membranes were incubated overnight at
4°C with the following primary antibodies: Anti-p50 (1:1,000; cat.
no. ab32360; Abcam), anti-tumor necrosis factor-α (TNF-α; 1:2,000;
cat. no. ab6671; Abcam), anti-interleukin (IL)-6 (1:1,000; cat. no.
12912; Cell Signaling Technology, Inc.), anti-Bax (1:3,000; cat.
no. ab32503; Abcam), anti-Bcl-2 (1:500; cat. no. ab692; Abcam),
anti-cleaved-caspase 3 (1:5,000; cat. no. ab2302; Abcam),
anti-pro-caspase-3 (1:10,000; cat. no. ab32499; Abcam) and
anti-GAPDH (1:3,000; cat. no. ab9484; Abcam). The membranes were
subsequently incubated with the following horseradish
peroxidase-conjugated secondary antibodies for 1 h at room
temperature: Goat anti-mouse (cat. no. ab6789; 1:3,000; Abcam) or
goat anti-rabbit (cat. no. ab6721; 1:3,000; Abcam). Protein bands
were visualized using the BeyoECL Plus kit (cat. no. P0018S;
Beyotime Institute of Biotechnology). Protein expression levels
were quantified using ImageJ software (version 1.8.0; National
Institutes of Health) with GAPDH as the loading control.
MTT assay
H9c2 cells (2×104 cells/well) were seeded
into a 96-well plate and cultured for 12 h in 5% CO2 at
37°C. Cells were subjected to H/R exposure and then washed twice
with PBS. Cell viability was measured using an MTT assay kit (cat.
no. C0009; Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. The absorbance was measured in a
Bio-Rad 680 microplate reader at 490 nm (Bio-Rad Laboratories,
Inc.).
Flow cytometry
H9c2 cells (2×106) were treated with 0,
1, 2 and 4 µg/ml TNF-α (cat. no. P6231; Beyotime Institute of
Biotechnology) at 37°C for 12 h. H9c2 cells (1×106) were
collected and an Annexin V FITC/PI kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for flow cytometric analysis to detect
apoptosis, according to the manufacturer's protocol. A Beckman
CytoFLEX flow cytometer (Beckman Coulter, Inc.) and FlowJo software
(version 10.0.7; FlowJo LLC) were used to analyze the rate of
apoptosis.
Statistical analysis
Data are presented as the mean ± SD, and were
analyzed using SPSS 20.0 (IBM Corp.). Student's t-test was used to
compare differences between two groups, and one-way ANOVA with
Tukey's test was used to compare the difference between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
H/R induces cell apoptosis and gene
expression changes in H9c2 cells
H9c2 cells were cultured under anoxic conditions for
2, 4, 8, 12, 18 and 24 h, and then re-oxygenated for 2 h to
establish an in vitro I/R model. MTT assay (Fig. 1A) and flow cytometry (Fig. 1B) results suggested that
H/R-induced cell injury decreased cell viability and increased
apoptotic rates in a time-dependent manner.
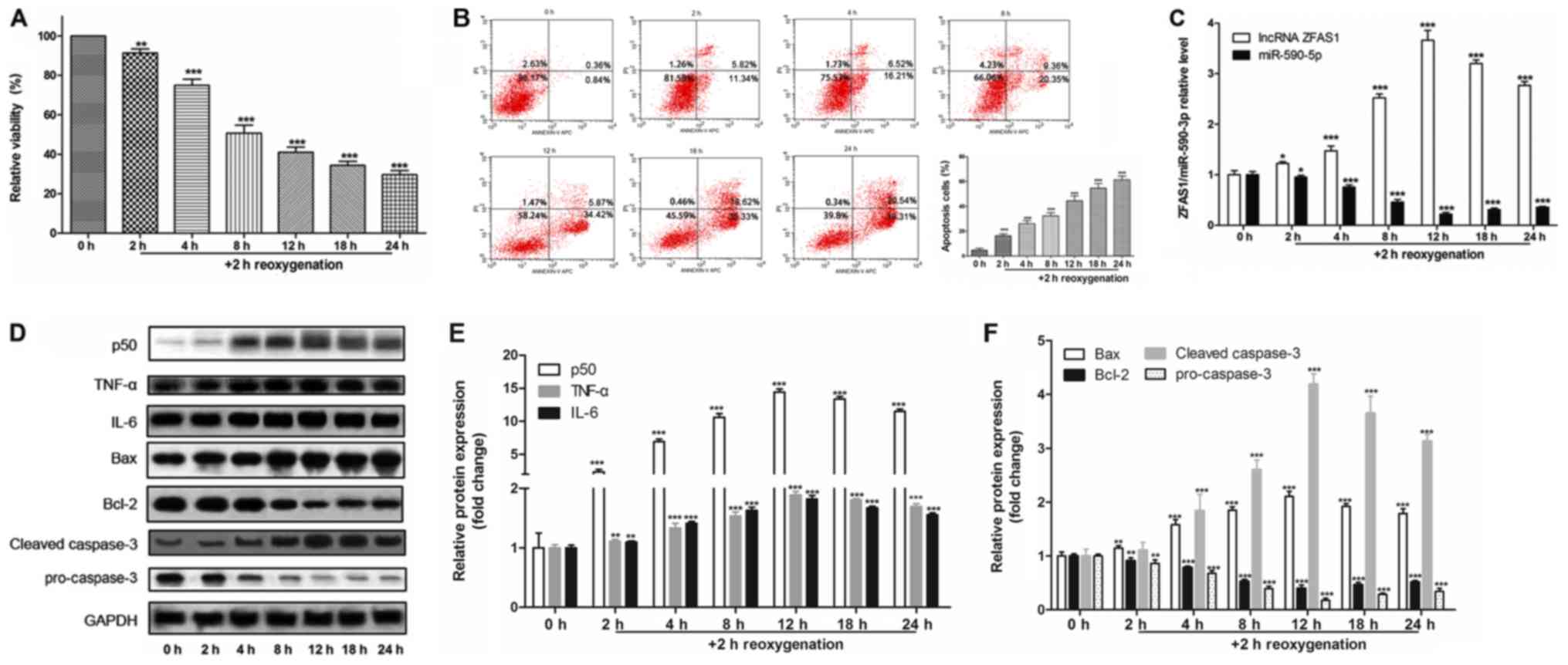 | Figure 1.H/R induces cell apoptosis and gene
expression changes in H9c2 cells. (A) MTT assay for cell viability
following H/R exposure. (B) Apoptotic rate of cells was detected by
flow cytometry. (C) Reverse transcription-quantitative PCR was used
to detect the expression level of ZFAS1, which was normalized by
GAPDH, and of miR-590-5p, which was normalized by U6. (D)
Representative western blotting images used to determine protein
expression levels of (E) p50, TNF-α and IL-6, and (F) Bax, Bcl-2,
pro-caspase-3 and cleaved caspase-3. *P<0.05, **P<0.01,
***P<0.001 vs. 0 h. H/R, hypoxia/reoxygenation; IL, interleukin;
miR, microRNA; TNF-α, tumor necrosis factor-α; ZFAS1, ZNFX1
antisense RNA 1. |
To investigate the underlying molecular mechanisms,
the expression levels of genes of interest were assessed. It was
demonstrated that, compared with control cells (0 h), H/R increased
the expression levels of ZFAS1 (Fig.
1C), as well as the protein expression levels of p50, TNF-α,
IL-6, Bax and cleaved-caspase-3 (Fig.
1D-F). Furthermore, H/R decreased the expression levels of
miR-590-3p (Fig. 1C), as well as
the protein expression levels of Bcl-2 and pro-caspase-3 (Fig. 1D and F). Therefore, the present
results suggested that lncRNA ZFAS1 and miR-590-3p may be related
to H/R-induced apoptosis of H9c2 cells.
lncRNA ZFAS1 regulates apoptosis in
H9c2 cells
The StarBase database was used to identify the
binding sites between miR-590-3p and ZFAS1 (Fig. 2A). miR-590-3p-mimic significantly
increased the expression of miR-590-3p, whereas miR-590-3p-mimic
significantly decreased the expression of miR-590-3p compared with
miR-590-3p-NC. Moreover, si-ZFAS1 significantly decreased the
expression of ZFAS1 compared with si-NC (Fig. 2B). Results from the luciferase gene
reporter assay indicated that infection with miR-590-3p mimic or
miR-590-3p inhibitor did not change the expression level of ZFAS1
(Fig. 2C). However, ZFAS1
knockdown increased the expression level of miR-590-3p (Fig. 2D). Furthermore, flow cytometry
results suggested that lncRNA ZFAS1 knockdown could affect the
protein expression levels of Bax, Bcl-2, cleaved-caspase-3 and
pro-caspase-3 (Fig. 2E), as well
as decrease H/R-induced apoptosis in H9c2 cells (Fig. 2F). Collectively, the results
indicated that ZFAS1 knockdown reduced H/R-induced H9c2 apoptosis,
and ZFAS1 knockdown increased miR-590-3p expression.
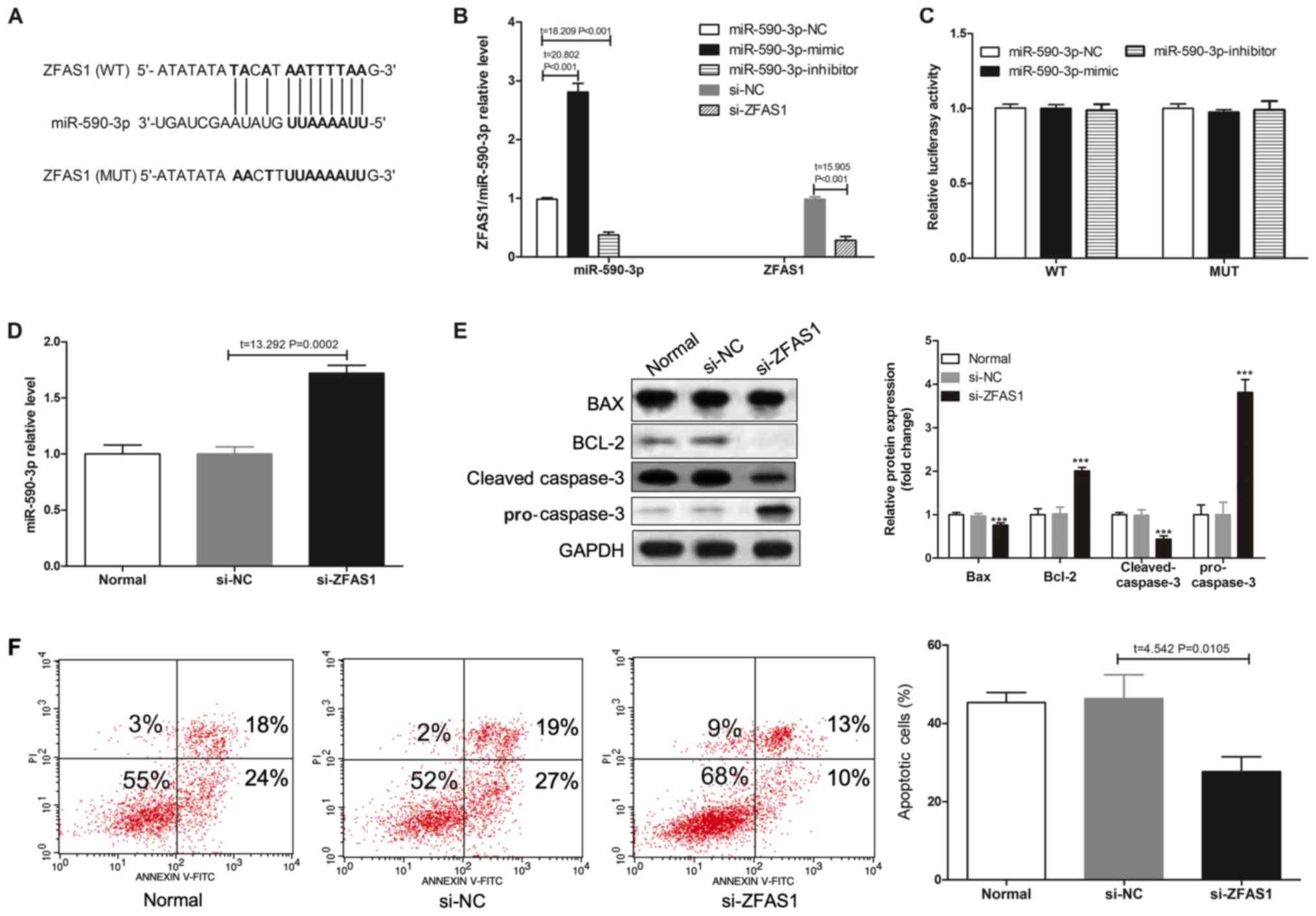 | Figure 2.ZAFS1 knockdown reduces H/R-induced
H9c2 apoptosis. (A) A WT-ZFAS1 3′UTR luciferase reporter vector and
a MUT-ZSAF1 3′UTR luciferase reporter vector with mutations on the
miR-590-3p binding sites of the ZFAS1 3′UTR were constructed. (B)
RT-qPCR was used to detect the expression levels of ZAFS1 or
miR-590-3p in H9c2 cells without H/R induction. (C) miR-590-3p-NC,
miR-590-3p-mimic and miR-590-3p-inhibitor were transected into H9c2
cells, and luciferase activity was determined. (D) RT-qPCR results
of miR-590-3p expression level in H9c2 cells transfected with
si-ZFAS1. (E) Western blotting was used to detect the protein
expression levels of Bax, Bcl-2, pro-caspase-3 and cleaved
caspase-3 in H9c2 cells after si-ZFAS1 transfection and H/R injury.
(F) Flow cytometry results of the percentage of apoptotic cells of
different transfected groups following H/R induction. ***P<0.001
vs. si-NC group. 3′UTR, 3′untranslated regions; H/R,
hypoxia/reoxygenation; miR, microRNA; MUT, mutation; NC, negative
control; RT-qPCR, reverse transcription-quantitative PCR; WT,
wild-type; ZFAS1, ZNFX1 antisense RNA 1. |
miR-590-3p regulates inflammation and
apoptosis in H9c2 cells
The StarBase was also used to searched for target
sites of miR-590-3p in p50 3′UTR (Fig.
3A). To assess whether miR-590-3p can regulate p50 expression
level, a luciferase gene reporter assay was performed. It was
demonstrated that transfection of miR-590-3p mimic significantly
decreased WT type 3′UTR luciferase activity in H9c2 cells
(P<0.001; Fig. 3B); however, no
effect was observed with the MUT in any group. Furthermore, the
miR-590-3p mimic transfection could decrease the protein expression
levels of Bax and cleaved-caspase-3, and increase the protein
expression levels of Bcl2 and pro-caspase-3 (Fig. 3C). Furthermore, miR-590-3p mimic
decreased the expression levels of p50, TNF-α, IL-6 in H9c2 cells
following H/R injury (Fig. 3D). In
addition, transfection with the miR-590-3p mimic decreased
H/R-induced apoptosis (Fig. 3E).
Therefore, these results suggested that miR-590-3p may have a
protective effect in reducing H/R-induced apoptosis by targeting
p50.
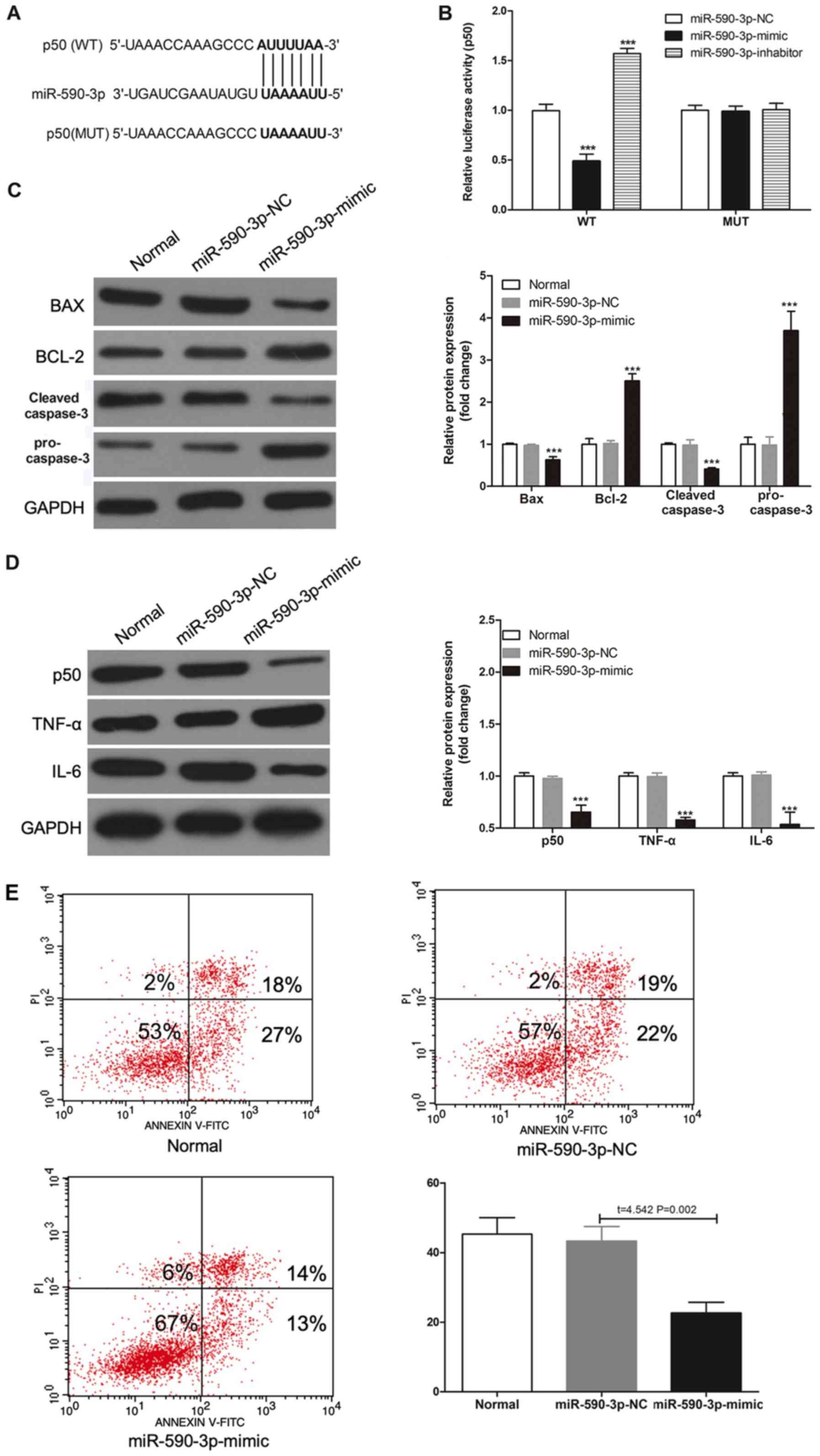 | Figure 3.miR-590-3p targets p50 and reduces
H/R-induced apoptosis and inflammation. (A) A WT-p50 3′UTR and a
MUT-p50 3′UTR luciferase reporter vector, with mutations on
miR-590-3p binding sites of the p50 3′UTR were constructed. (B)
miR-590-3p-NC, miR-590-3p-mimic or miR-590-3p-inhibitor were
transected into H9c2 cells, and luciferase activity was detected.
(C) Protein expression levels of Bax, Bcl-2, pro-caspase-3 and
cleaved-caspase-3, and (D) p50, TNF-α and IL-6 were detected by
western blotting in H/R-induced H9c2 cells. (E) Flow cytometry
results of the percentage of apoptotic cells in different
transfected groups with H/R. ***P<0.001 vs. miR-590-3p-NC.
3′UTR, 3′untranslated region; H/R, hypoxia/reoxygenation; IL,
interleukin; miR, microRNA; MUT, mutation; NC, negative control;
TNF-α, tumor necrosis factor-α; WT, wild-type. |
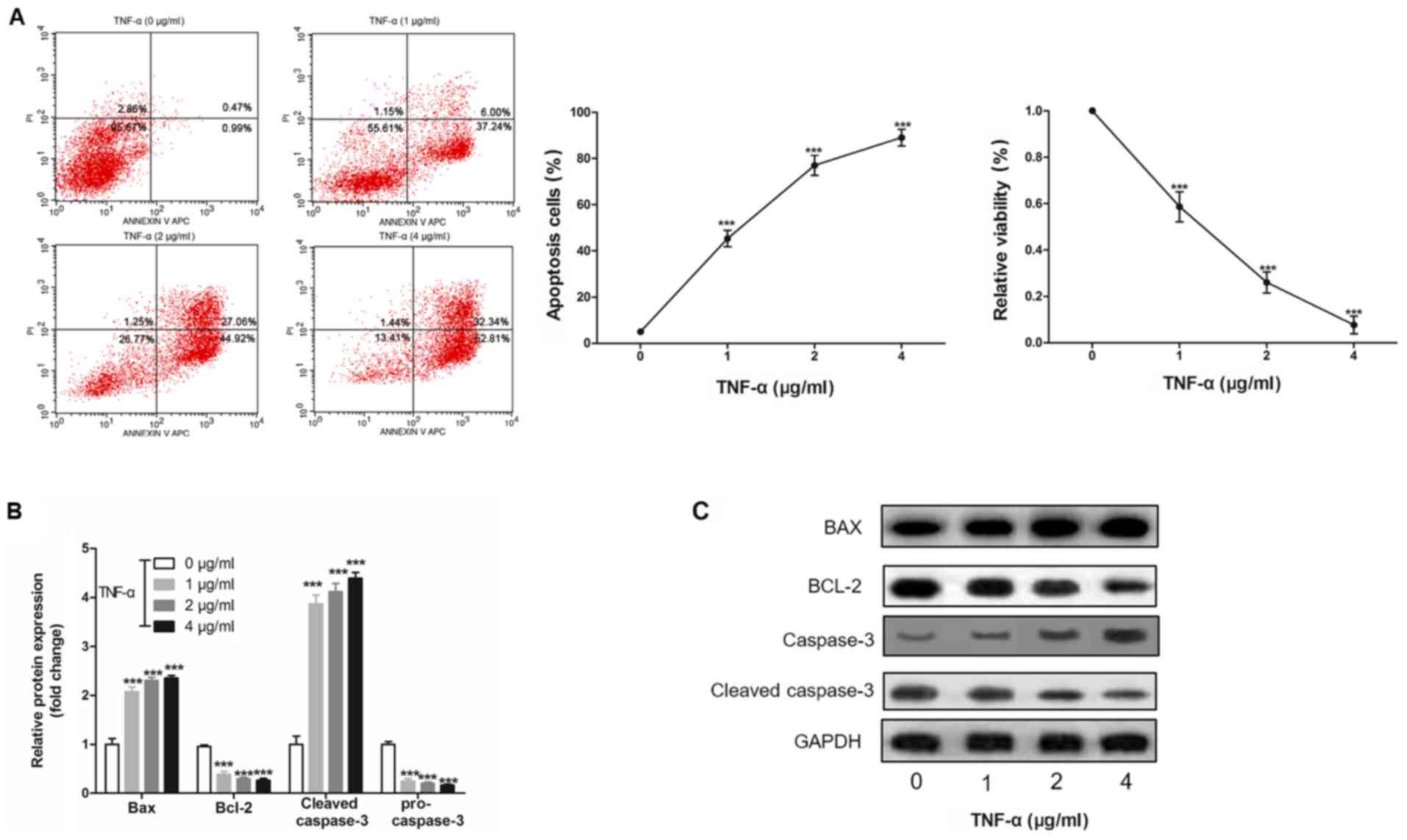
H9c2 cell apoptosis is induced by
TNF-α in a dose-dependent manner
To investigate the effect of inflammation on
apoptosis, H9c2 cells were treated with TNF-α, and it was revealed
that TNF-α induced H9c2 cell apoptosis and cell viability in a
dose-dependent manner (Fig. 4A and
B). Furthermore, it was demonstrated that TNF-α altered the
protein expression levels of Bax, Bcl-2, cleaved-caspase-3 and
pro-caspase-3 in a dose-dependent manner (Fig. 4C).
Discussion
The present study established an I/R injury in
vitro model by H/R exposure in H9c2 rat cardiomyocytes, and
found that H/R increased the expression levels of ZFAS1, p50, TNF-α
and IL-6, decreased miR-590-3p expression, and induced apoptosis of
H9c2 cells in a time-dependent manner. There have been an
increasing number of studies investigating the potential role of
lncRNA in heart disease, which have reported that lncRNAs serve a
key role in the regulation of heart disease, such as cardiac
hypertrophy, cardiac graft rejection and ischemic heart failure
(21,22). Moreover, lncRNA ZFAS1 is abnormally
expressed in patients with acute myocardial infarction (15) and atherosclerotic model rats
(16), and contributes to the
impairment of cardiac contractile function in myocardial infarction
(17,18).
lncRNAs are non-coding RNAs that exert biological
functions by regulating the expression levels of other genes
(9,10). Previous studies have shown that
there are several mechanisms by which lncRNAs can regulate gene
expression (23,24). The interaction mechanism between
lncRNAs and miRNAs is an important way that lncRNAs regulate gene
expression (25). However, lncRNA
not only acts as a target for miRNAs to inhibit their binding to
target genes, but also an endogenous miRNA sponge that can inhibit
the expression of miRNAs and indirectly inhibits the negative
control of miRNAs to target genes (9,10).
Moreover, RNA competes with miRNAs to bind to the 3′UTR of target
gene mRNA (9,10). In addition, miRNAs can target a
large number of protein-coding genes and lncRNAs. The present
results suggested that ZFAS1 could directly bind to miR-590-3p and
inhibit its expression. There had been a number of previous studies
investigating the relationship between miR-590-3p and the NF-κB
signaling pathway. For example, Zhao et al (26) found that miR-590-3p is a novel
miRNA in myocarditis by targeting NF-κB in vivo. In
addition, Bao et al (27)
showed that miR-590 protects against oxidized low-density
lipoprotein-induced endothelial cell apoptosis via the p53/NF-κB
pathway. The present study results indicated that p50 was a target
gene of miR-590-3p, and that the miR-590-3p-mimic could
downregulate the protein expression levels of p50, TNF-α and IL-6.
Moreover, it was found that ZFAS1 knockdown or miR-590-3p
overexpression attenuated H/R-induced apoptosis in H9c2
cardiomyocytes.
The important role of lncRNA regulation of the NF-κB
signaling pathway has been a focus of research into inflammatory
diseases. The lncRNA Lethe binds directly to the NF-κB
heterodimeric subunit RelA and inhibits the DNA-binding activity of
NF-κB (28). Thus, Lethe acts as a
negative feedback regulator of the TNF-α pathway and regulates the
inflammatory response (29).
lncRNA metastasis associated lung adenocarcinoma transcript 1
(MALAT1) inhibits DNA binding activity of NF-κB, reduces
inflammatory cytokine production and downregulates the autoimmune
inflammatory response (30).
Furthermore, knockdown of MALAT1 upregulates
lipopolysaccharide-induced TNF-α and IL-6 expression (30). In I/R injury, the inflammatory
response plays a key role in myocardial I/R injury and occurs
during the whole process of myocardial cell injury (31). Moreover, adhesion molecules and
cytokines involved in the inflammatory reaction have the same NF-κB
gene initiation site (32), and
NF-κB is activated to mediate overexpression of these factors, thus
aggravating the inflammatory response after myocardial I/R
(33).
p50 protein is an important part of the NF-κB
signaling pathway, and the dimeric complex consisting of p50-p65 is
called the standard NF-κB protein complex (34). A previous study found that deletion
of the NF-κB subunit p50 reduces I/R injury in vivo
(35). The present results
suggested that the miR-590-3p-mimic decreased the protein
expression level of p50, thus miR-590-3p may inhibit the activation
of the NF-κB signaling pathway. In addition, it was found that H9c2
cardiomyocyte apoptosis was induced by TNF-α in a dose-dependent
manner. Therefore, downregulation of lncRNA ZFAS1 may protect H9c2
cardiomyocytes from I/R-induced apoptosis via the miR-590-3p/NF-κB
pathway. However, there are some limitations to the present study,
including the lack of in vivo experiments and the absence of
clinical data.
In conclusion, it was demonstrated that ZFAS1 was
upregulated and miR-590-3p was downregulated in H9c2 cells
subjected to I/R injury. Furthermore, the present results suggested
that downregulation of ZFAS1 protected against I/R-induced
myocardial cell apoptosis by increasing miR-590-3p expression via
the NF-κB signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY and YS conceived and designed the present study.
PH and DY performed the experiments and analyzed the data. LY
substantially contributed to drafting the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for Percutaneous
Coronary Intervention: Executive summary: A report of the American
College of Cardiology Foundation/American Heart Association Task
Force on Practice Guidelines and the Society for Cardiovascular
Angiography and Interventions. Circulation. 124:2574–2609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim
MH, Loo G, Koo CY, Gao XF, Chandra S, et al: Obstructive sleep
apnea and cardiovascular events after percutaneous coronary
intervention. Circulation. 133:2008–2017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang C, Batkai S, Dangwal S, Gupta SK,
Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al:
Cardiac fibroblast-derived microRNA passenger strand-enriched
exosomes mediate cardiomyocyte hypertrophy. J Clin Invest.
124:2136–2146. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michaels AD, Gibson CM and Barron HV:
Microvascular dysfunction in acute myocardial infarction: Focus on
the roles of platelet and inflammatory mediators in the no-reflow
phenomenon. Am J Cardiol. 85:50B–60B. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maier W, Altwegg LA, Corti R, Gay S,
Hersberger M, Maly FE, Sütsch G, Roffi M, Neidhart M, Eberli FR, et
al: Inflammatory markers at the site of ruptured plaque in acute
myocardial infarction: Locally increased interleukin-6 and serum
amyloid A but decreased C-reactive protein. Circulation.
111:1355–1361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pop M, Qi X, Barry J, Strauss BH, Wright
GA and Ghugre NR: Hemorrhage promotes inflammation and myocardial
damage following acute myocardial infarction. J Cardiovascular
Magnetic Resonance. 16:1–2. 2014. View Article : Google Scholar
|
|
8
|
Westman PC, Lipinski MJ, Luger D, Waksman
R, Bonow RO, Wu E and Epstein SE: Inflammation as a driver of
adverse left ventricular remodeling after acute myocardial
infarction. J Am Coll Cardiol. 67:2050–2060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hon CC, Ramilowski JA, Harshbarger J,
Bertin N, Rackham OJ, Gough J, Denisenko E, Schmeier S, Poulsen TM,
Severin J, et al: An atlas of human long non-coding RNAs with
accurate 5′ends. Nature. 543:199–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta SC and Tripathi YN: Potential of
long non-coding RNAs in cancer patients: From Bio-markers to
therapeutic targets. Int J Cancer. 140:1955–1967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Archer K, Broskova Z, Bayoumi AS, Teoh JP,
Davila A, Tang Y, Su H and Kim IM: Long non-coding RNAs as master
regulators in cardiovascular diseases. Int J Mol Sci.
16:23651–23667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uchida S and Dimmeler S: Long noncoding
RNAs in cardiovascular diseases. Circ Res. 116:737–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathy NW and Chen XM: Long non-coding RNAs
(lncRNAs) and their transcriptional control of inflammatory
responses. J Biol Chem. 292:12375–12382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Sun L, Xuan L, Pan Z, Li K, Liu
S, Huang Y, Zhao X, Huang L, Wang Z, et al: Reciprocal changes of
circulating long non-coding RNAs ZFAS1 and CDR1AS predict acute
myocardial infarction. Sci Rep. 6:223842016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Yao H, Hui JY, Ding SH, Fan YL,
Pan YH, Chen KH, Wan JQ and Jiang JY: Global transcriptomic study
of atherosclerosis development in rats. Gene. 592:43–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun G, Wang Y, Zhang J, Lin N and You Y:
MiR-15b/HOTAIR/p53 form a regulatory loop that affects the growth
of glioma cells. J Cell Biochem. 119:4540–4547. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vervliet T, Robinson EL and Roderick HL:
Lnc'ing Ca2+, SERCA and cardiac disease. Cell Calcium.
72:132–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu Y, Xing Y, Xu Y, Huang C, Bao H, Hong K
and Cheng X: Pim-2 protects H9c2 cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis via downregulation of Bim
expression. Environ Toxicol Pharmacol. 48:94–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu G, Huang Y, Wu C, Guo Z, Ma Y, Xia Q,
Awasthi A and He X: Differential expression of long noncoding RNAs
during cardiac allograft rejection. Transplantation. 101:83–91.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kühl C and Frey N: Long noncoding RNAs in
heart disease. Springer International Publishing. 2016. View Article : Google Scholar
|
|
23
|
Yang L, Lin C, Jin C, Yang JC, Tanasa B,
Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, et al:
LncRNA-dependent mechanisms of androgen receptor-regulated gene
activation programs. Nature. 500:598–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Necsulea A, Soumillon M, Warnefors M,
Liechti A, Daish T, Zeller U, Baker JC, Grützner F and Kaessmann H:
The evolution of lncRNA repertoires and expression patterns in
tetrapods. Nature. 505:635–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol.
1402:271–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao S, Yang G, Liu PN, Deng YY, Zhao Z,
Sun T, Zhuo XZ, Liu JH, Tian Y, Zhou J, et al: miR-590-3p is a
novel MicroRNA in myocarditis by targeting nuclear factor Kappa-B
in vivo. Cardiology. 132:182–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao MH, Li JM, Zhou QL, Li GY, Zeng J,
Zhao J and Zhang YW: Effects of miR-590 on oxLDL-induced
endothelial cell apoptosis: Roles of p53 and NF-κB. Mol Med Rep.
13:867–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rapicavoli NA, Qu K, Zhang J, Mikhail M,
Laberge RM and Chang HY: A mammalian pseudogene lncRNA at the
interface of inflammation and anti-inflammatory therapeutics.
elife. 2:e007622013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wallach D: The cybernetics of TNF: Old
views and newer ones. Semin Cell Dev Biol. 50:105–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao G, Su Z, Song D, Mao Y and Mao X: The
long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced
inflammatory response through its interaction with NF-κB. FEBS
Lett. 590:2884–2895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang R, Zhao Q, Jian G, Cheng D, Wang N,
Zhang G and Wang F: Tanshinone IIA attenuates contrast-induced
nephropathy via Nrf2 activation in rats. Cell Physiol Biochem.
46:2616–2623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang F, Zhang G, Lu Z, Geurts AM, Usa K,
Jacob HJ, Cowley AW, Wang N and Liang M: Antithrombin III/SerpinC1
insufficiency exacerbates renal ischemia/reperfusion injury. Kidney
Int. 88:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi Z, Lian A and Zhang F: Nuclear
factor-κB activation inhibitor attenuates ischemia reperfusion
injury and inhibits Hmgb1 expression. Inflamm Res. 63:919–925.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghosh G, Van Duyne G, Ghosh S and Sigler
PB: Structure of NF-kappaB p50 homodimer bound to a kappa B site.
Nature. 373:303–310. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frantz S, Tillmanns J, Kuhlencordt PJ,
Schmidt I, Adamek A, Dienesch C, Thum T, Gerondakis S, Ertl G and
Bauersachs J: Tissue-specific effects of the nuclear Factor kappaB
subunit p50 on myocardial ischemia-reperfusion injury. Am J Pathol.
171:507–512. 2007. View Article : Google Scholar : PubMed/NCBI
|