Introduction
Neovascularization is associated with ocular
diseases, such as age-related macular degeneration (AMD) and
diabetic retinopathy (1). AMD
affects the macula, leading to significant vision loss (2). Diabetic retinopathy occurs in
patients with diabetes mellitus and affects the blood vessels of
the retina, ultimately causing vision loss through multiple
pathways (3). No surgery or drug
treatment is available for patients who have already lost their
vision.
A previous study have suggested that elevated levels
of vascular endothelial growth factor (VEGF) serve important roles
in the development of neovascularization (4). VEGF-A, the most common isoform of
VEGF, is predominantly produced by cells of the retinal pigment
epithelium (RPE) (5). A range of
stimuli increase VEGF release from the RPE (6), including the deprivation of adequate
oxygen levels, also termed hypoxia (7). Hypoxia triggers both the protein
synthesis and the release of VEGF (8).
VEGF is regulated by several factors under hypoxia.
Among them, signal transducer and activator of transcription 3
(STAT3) contributes to the regulation of the immune response and
inflammation in RPE cells (9).
Phosphorylated (p)-STAT3 translocates to the nucleus, where it
activates pro-survival genes, such as survivin and Bcl-x1 (10). In addition, STAT3 activation
mediates VEGF expression levels in human pancreatic cancer and
retina cells (11,12). Another major regulator of VEGF is
erb-B-2 receptor tyrosine kinase 2 (ERBB2), a member of the
epidermal growth factor family; high expression of ERBB2
contributes to tumor progression by upregulating VEGF, thus
accelerating tumor vascularization (13,14).
STAT3 interacts with ERBB2 in breast cancer cells (15). However, the function of ERBB2 in
ocular diseases remains unclear.
In the present study, a Janus kinase 2 (JAK2)
inhibitor that blocks STAT3 activation (16) was used to investigate whether STAT3
and ERBB2 may regulate VEGF release in an RPE cell line. The
present study also aimed to identify the proteins that regulate
VEGF release in the human RPE-derived cell line ARPE-19.
Materials and methods
Reagents
High-glucose Dulbecco's modified Eagle's medium
(DMEM), high-glucose DMEM F12, penicillin, streptomycin and FBS
were purchased from Gibco; Thermo Fisher Scientific, Inc.
Endothelial Cell Growth Medium 2 was purchased from PromoCell GmbH.
Cobalt II chloride hexahydrate (CoCl2), JAK2 inhibitor
AG490, poly-D-lysine, paraformaldehyde and DMSO were purchased from
Sigma-Aldrich; Merck KGaA. Antibodies specific for
hypoxia-inducible factor 1α (HIF-1α) (1:1,000; cat. no. 14179),
nuclear factor-κB (NF-κB) (1:1,000; cat. no. 8242), p-NF-κB
(1:1,000; cat. no. 3033), STAT3 (1:1,000; cat. no. 12640), p-STAT3
(1:2,000; cat. no. 9145), and p-ERBB2 (1:1,000; cat. no. 2243) were
purchased from Cell Signaling Technology, Inc. The antibody against
ERBB2 (MA5-13675; 1:1000) was purchased from Thermo Fisher
Scientific, Inc. The antibody against neuregulin 1 (NRG1) (1:1,000;
cat. no. ab53104) was purchased from Abcam. The antibody against
lamin A/C (1:1,000; cat. no. SC-7292) was purchased from Santa Cruz
Biotechnology, Inc. The antibody for α-tubulin (1:5,000; cat. no.
T5168) was purchased from Thermo Fisher Scientific, Inc. The
antibody against β-actin (1:10,000; cat. no. A2228) was purchased
from Sigma-Aldrich, Merck KGaA. Normal donkey serum (NDS) was
purchased from Bio-Rad Laboratories, Inc. Donkey anti-rabbit
immunoglobulin G secondary antibody (1:200; cat. no. A-21206; Alexa
Fluor® 488 conjugate) and ProLong Gold Antifade Mountant
with DAPI were purchased from Thermo Fisher Scientific, Inc. BD
Matrigel™ Basement Membrane Matrix was purchased from BD
Biosciences. CoCl2 was dissolved in water. AG490 was
dissolved in DMSO.
Cell culture and treatment
ARPE-19 cells (American Type Culture Collection)
were cultured at 37°C with 5% CO2 in DMEM supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. For
the HUVEC tube formation assay, ARPE-19 cells were cultured in DMEM
F12 with 0.1% FBS. Cells were exposed to 100 µM CoCl2
over a time course (1, 3, 6, 9, 12 and 24 h) or incubated at 1%
O2 in a hypoxic incubator (MCO-5M; Sanyo). Human
umbilical vein endothelial cells (HUVECs; PromoCell GmbH) were
seeded at a density of 1×105 cells/well in 96-well
plates coated with Matrigel™ and cultured at 37°C with 5%
CO2 in DMEM F12 and 0.1% FBS.
Western blot
A total of 1.5×106 ARPE-19 cells were
lysed in RIPA buffer (Thermo Fisher Scientific, Inc.) containing 25
mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS, protease inhibitor cocktail (Roche
Diagnostics) and phosphatase inhibitor cocktail (GenDEPOT).
Quantitative analysis of proteins was performed using a BCA Protein
Assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc). Then, a
total of 30 µg protein/lane was separated by SDS-PAGE on 8% gels
and transferred to PVDF membranes using a Trans-Blot Turbo™
Transfer System (Bio-Rad Laboratories, Inc.). The membranes were
blocked for 2 h at room temperature with 5% skim milk in
Tris-buffered saline containing 0.1% Tween-20 and subsequently
incubated overnight with anti-HIF-1α, anti-p-NF-κB, anti-NF-κB
anti-p-STAT3, anti-STAT3, anti-p-HER2/ERBB2, anti-ERBB2, or
anti-NRG1. Immunoreactivity was then detected using ECL reagent
(Thermo Fisher Scientific, Inc.) on an Image Reader LAS
4000(Fujifilm Wako Pure Chemical Corporation).
Cytoplasmic and nuclear protein
extraction
Cells were harvested and washed with cold PBS. The
cell pellet was resuspended in extraction buffer A (10 mM HEPES;
1.5 mM MgCl2; 10 mM KCl) containing 0.5 mM DTT, protease
inhibitors and phosphatase inhibitors. Following incubation for 20
min on ice, the cytoplasmic extract was separated by centrifugation
at 13,475 × g for 10 min at 4°C. The pellet was washed with cold
PBS and resuspended in extraction buffer C (20 mM HEPES; 1.5 mM
MgCl2; 420 mM NaCl; 0.2 mM EDTA) containing 25%
glycerol, 0.5 mM DTT, protease inhibitors and phosphatase
inhibitors. After incubation for 30 min on ice, the nuclear extract
was separated by centrifugation at 13,475 × g for 20 min at 4°C.
β-actin was used as a loading control. In addition, α-tubulin was
used as cytoplasmic protein marker, while lamin A/C served as
nuclear protein marker. Data were analyzed by western blot.
Immunocytochemistry
ARPE-19 cells were seeded at 3×104
cells/well and incubated for 12 h in poly-D-lysine-coated 24-well
plates for cell attachment, followed by incubation at 1%
O2 in a hypoxic incubator for 24 h. Cells were fixed
with 4% paraformaldehyde for 5 min at room temperature and blocked
with 5% NDS for 2 h in room temperature. Blocked cells were
incubated with anti-p-NF-κB (1:1,600; cat. no. 3033) for overnight
at 4°C. Subsequently, cells were incubated with donkey anti-rabbit
immunoglobulin G secondary antibodies (1:200; cat. no. A-21206) for
1 h at room temperature and then mounted with ProLong Gold Antifade
Mountant with DAPI overnight at room temperature (P36931). The
images of coverslips were captured using fluorescence microscopy
(magnification, ×200; Olympus Corporation). Data were analyzed
using the Olympus Fluoview 4.2 software (Olympus Corporation).
ELISA
ARPE-19 cells were seeded in 6-well plates at
5×104 cells/well and cultured in medium supplemented
with 1% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. VEGF
levels were measured using a commercial human VEGF ELISA kit (cat.
no. BMS277-2) from Invitrogen; Thermo Fisher Scientific, Inc..
HUVEC tube formation assay
HUVEC tube formation assays were carried out as
previously described (17–20). ARPE-19 cells were cultured in DMEM
F12 medium supplemented with 0.1% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C with 5% CO2 in the presence
of 10 µM AG490 and exposed to hypoxia for 24 h. The conditioned
medium (CM) obtained from ARPE-19 cells was added to HUVECs seeded
in 96-well plates coated with Matrigel™ at 1×105
cells/well. HUVECs were incubated for 48 h with CM. ARPE-19 cells
were divided into four groups as follows: i) Normoxia CM; ii)
normoxia + 10 µM AG490 CM; iii) hypoxia CM; and iv) hypoxia + 10 µM
AG490 CM. Tube formation (indicated by branch points at which at
least three tubes joined), was counted and analyzed using a
U-LH100HG light microscope (magnification, ×100) (Olympus
Corporation) with ImageJ 1.46r software (National Institutes of
Health).
Statistical analysis
All data are presented as the mean ± SEM.
Statistical analysis was performed using GraphPad Prism (version 5;
GraphPad Software, Inc.). The differences between groups were
analyzed using Student's t-test or one-way ANOVA followed by
Dunnett's or Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Hypoxic conditions alter the
expression of hypoxia-related proteins in ARPE-19 cells
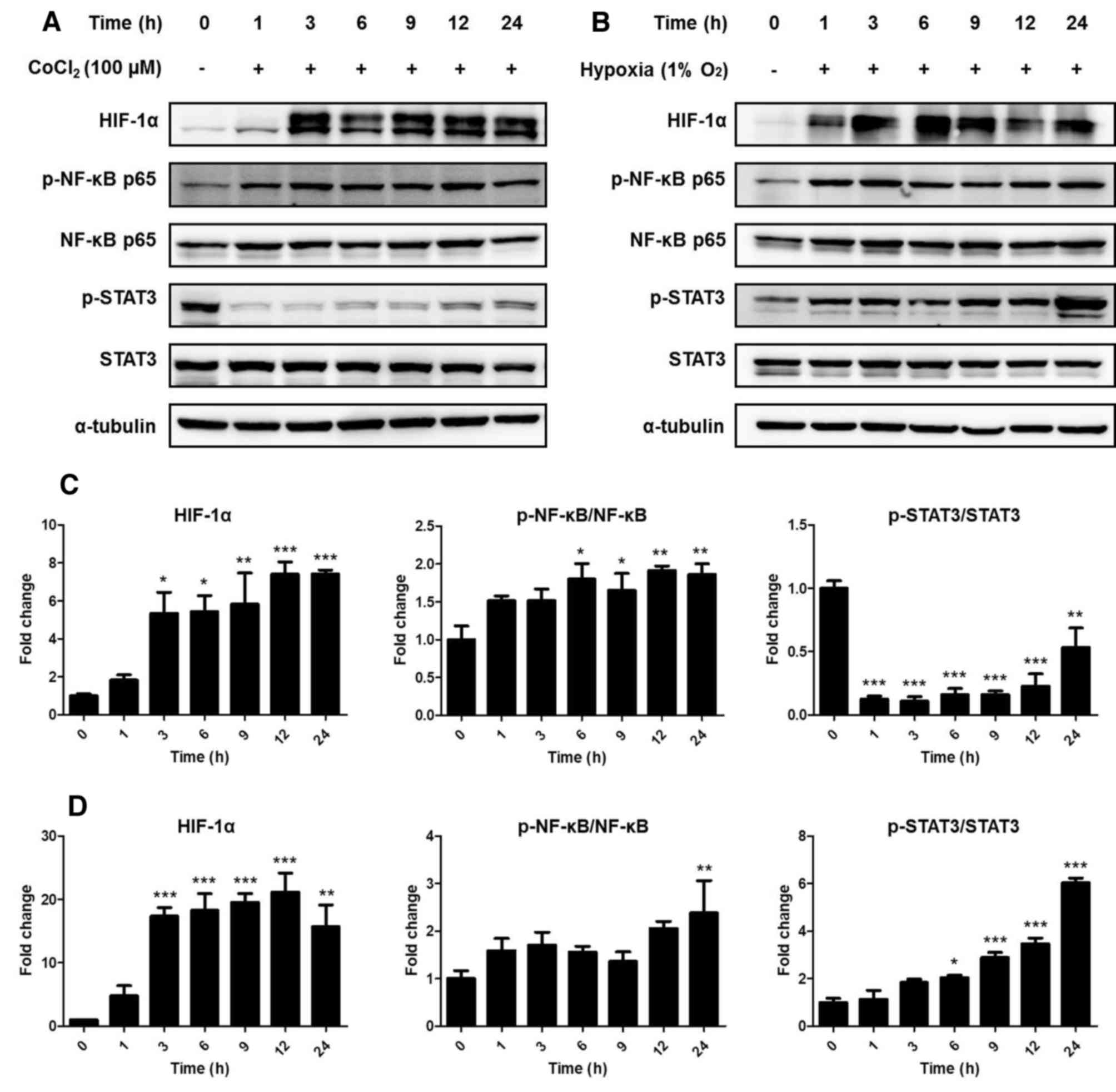
Experimental hypoxia was triggered in ARPE-19 cells
using treatment with 100 µM CoCl2 or a hypoxic incubator
(1% O2), and the levels of hypoxia-related proteins were
measured by western blotting. Compared with that in the control
group, the protein expression of HIF-1α was significantly
upregulated after 3 h both in the presence of CoCl2
treatment (P<0.05) and in hypoxic conditions (P<0.001)
(Fig. 1). In addition,
phosphorylation of NF-κB also increased 6 h after CoCl2
(P<0.05) treatment and following 24-h hypoxia (P<0.01).
By contrast, phosphorylation of STAT3 was decreased
by CoCl2 treatment (P<0.01) but increased by hypoxia
at the 24-h time point compared with that in the control group
(P<0.001). These results indicated that CoCl2
treatment could not identically replicate hypoxic conditions. Thus,
subsequent experiments were carried out under hypoxic conditions
using a 1% O2 incubator.
Hypoxia induces phosphorylation of
NF-κB and translocation of p-NF-κB from the cytoplasm to the
nucleus
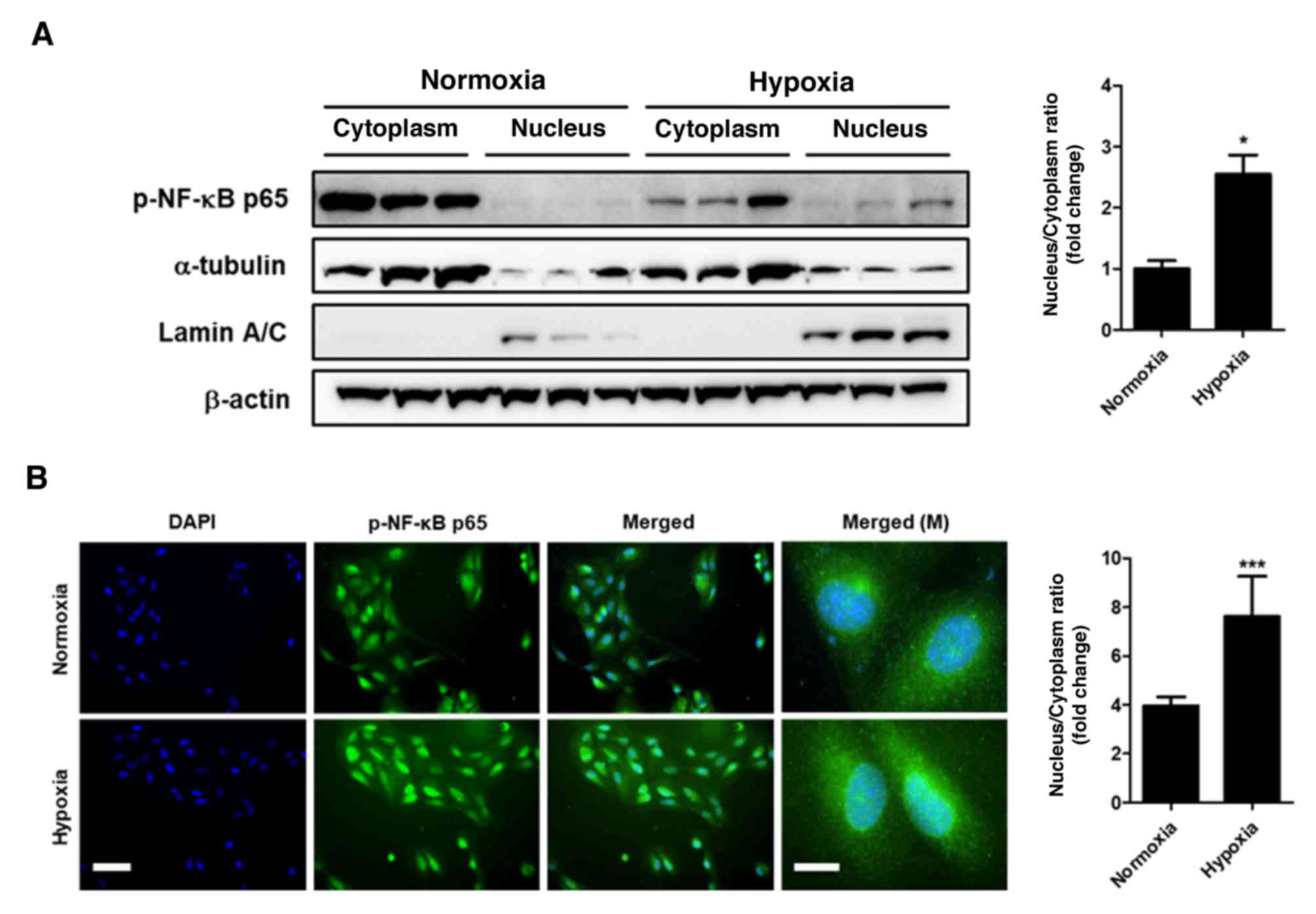
Since total protein analysis does not clarify the
location of p-NF-κB, cytoplasmic and nuclear protein fractions were
obtained from ARPE-19 cells. Western blot analysis of the nuclear
and cytoplasmic fractions demonstrated that the levels of p-NF-κB
significantly increased in the nucleus following 24-h hypoxia
compared with those observed under normoxia (P<0.05; Fig. 2A). Furthermore, immunocytochemistry
analysis also demonstrated the accumulation of p-NF-κB in the
nucleus under hypoxic conditions compared with normoxic conditions
(P<0.001; Fig. 2B). These
findings demonstrated that hypoxic conditions induced the nuclear
translocation of p-NF-κB.
Hypoxia induces the phosphorylation of
STAT3 and ERBB2 in ARPE-19 cells
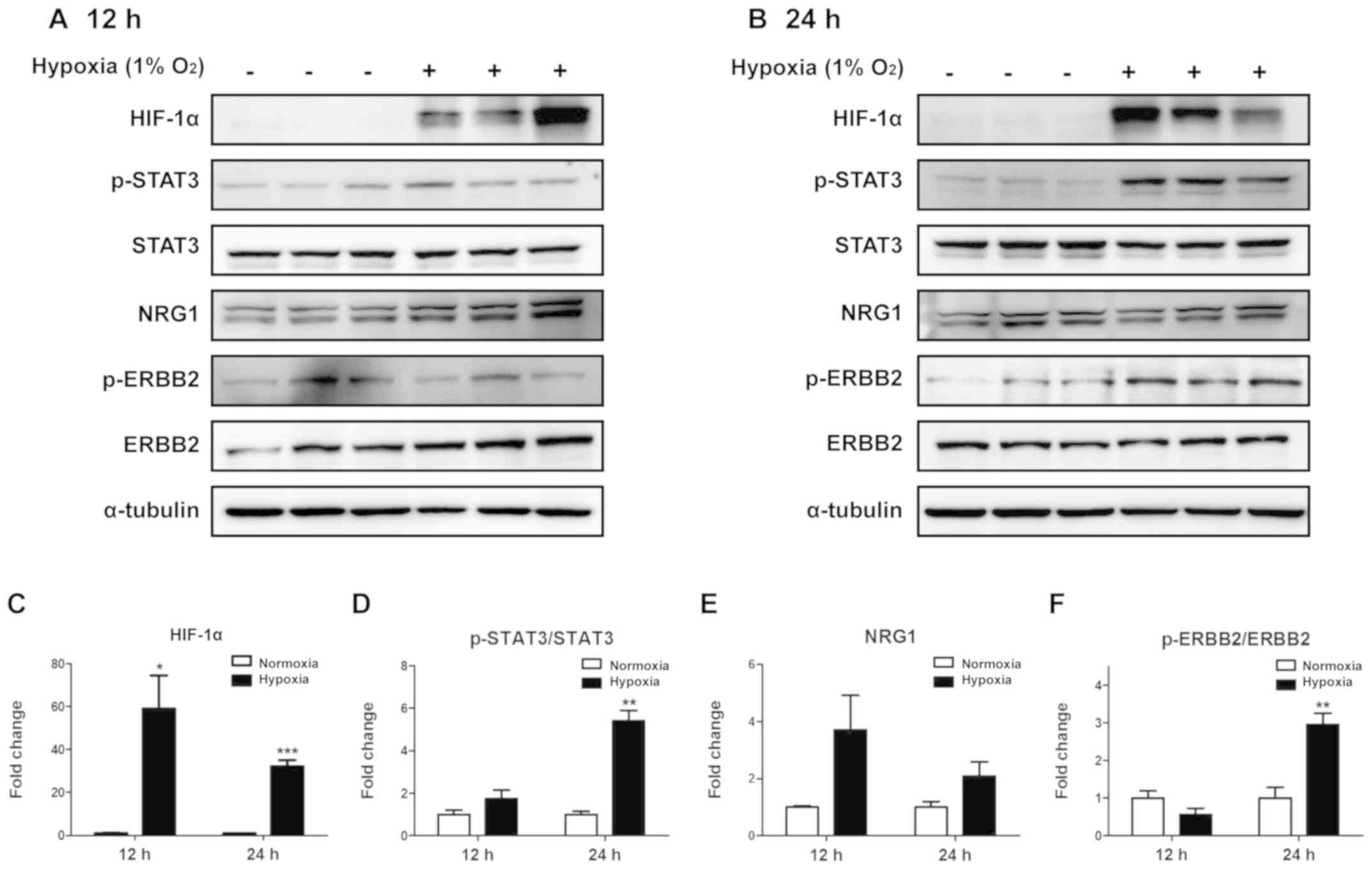
Phosphorylation of STAT3 and STAT3-related proteins
was assessed in ARPE-19 cells incubated under hypoxic conditions
for 12 (Fig. 3A) or 24 h (Fig. 3B). To determine whether hypoxia was
induced, HIF-1α was used as a hypoxic marker. Compared with that in
cells under normoxic conditions, HIF-1α expression increased after
12 h under hypoxia, and this increase was sustained at 24 h
(Fig. 3C). Phosphorylation of
STAT3 was significantly increased at the at the 24-h time point
(P<0.01), but not at 12 h (Fig.
3D) compared with that under normoxia. In addition, although
NRG1 expression appeared to increase at both time points, this
effect was not significant (Fig.
3E). Phosphorylation of ERBB2 significantly increased after 24
h of hypoxia compared with that under normoxia (Fig. 3F). These results demonstrated that
the phosphorylation of STAT3 and ERBB2 increased in ARPE-19 cells
following 24-h hypoxia.
AG490 suppresses hypoxia-induced
phosphorylation of STAT3 and ERBB2 in ARPE-19 cells
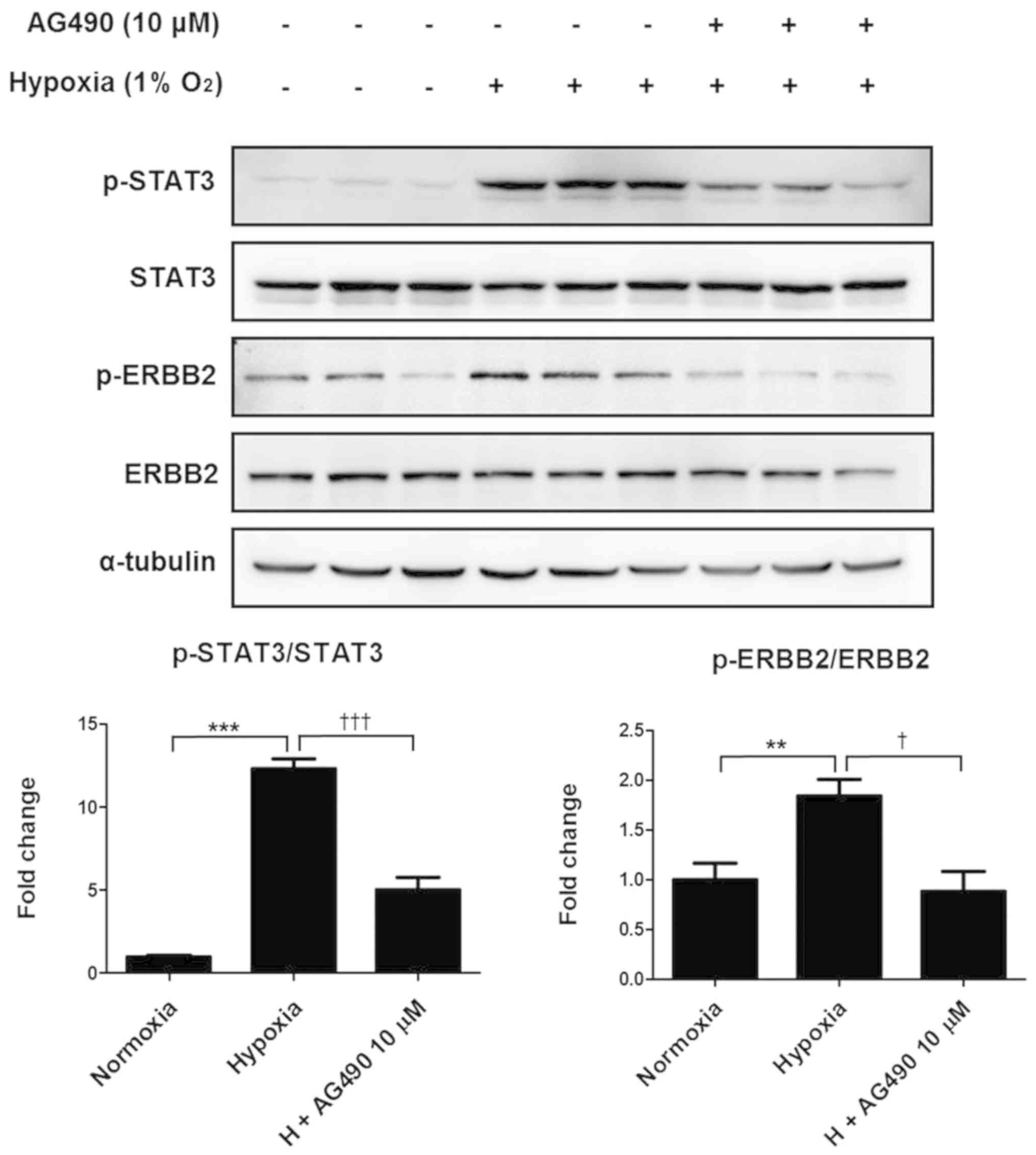
To examine the relationship between STAT3 and ERBB2,
ARPE-19 cells were treated with a commercial JAK2 inhibitor AG490
and incubated under hypoxic conditions for 24 h. Consistent with
the aforementioned results, hypoxic conditions significantly
increased phosphorylation of STAT3 and ERBB2 compared with that in
the control cells; however, the addition of 10 µM AG490 reversed
this effect, decreasing STAT3 and ERBB2 phosphorylation to levels
similar to those of the control (Fig.
4). These results suggested a possible interaction between
STAT3 and ERBB2, as AG490 is also a STAT3 inhibitor.
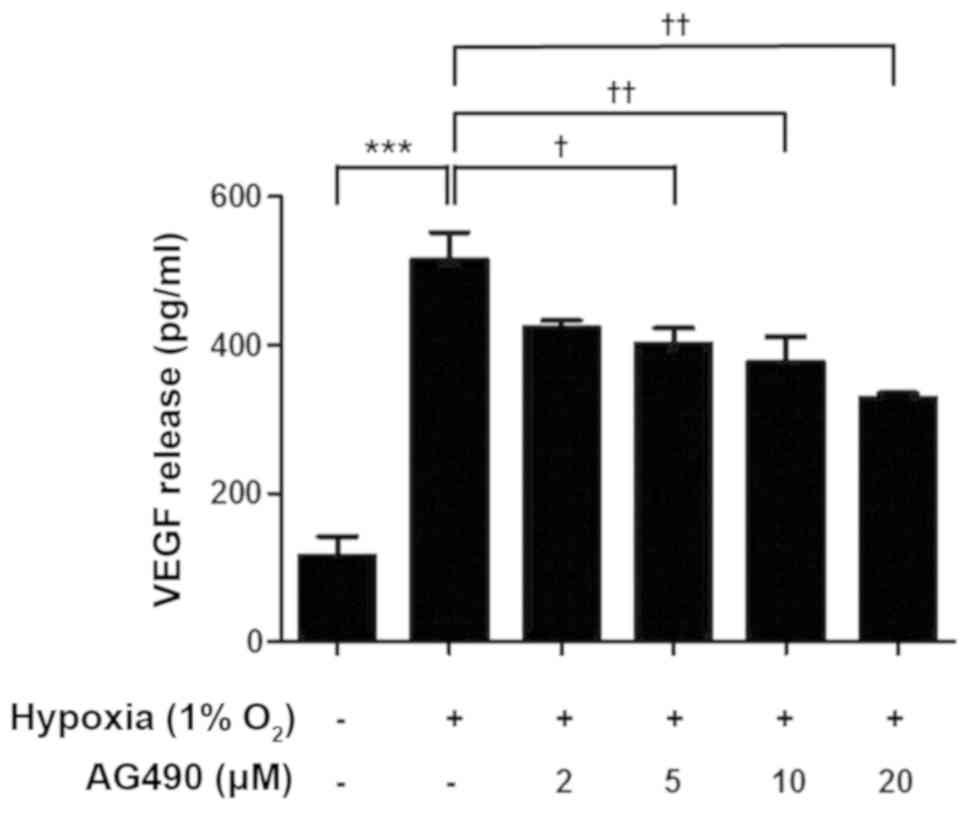
AG490 suppresses hypoxia-induced VEGF
release in ARPE-19 cells
VEGF levels in ARPE-19 cells cultured under hypoxic
conditions were evaluated using ELISA. Hypoxia significantly
increased VEGF release in ARPE-19 cells compared with those in the
control group (Fig. 5). In
addition, VEGF release decreased as the dose of AG490 increased
compared with untreated cells (Fig.
5). These results demonstrated STAT3 is involved in the
regulation of VEGF release.
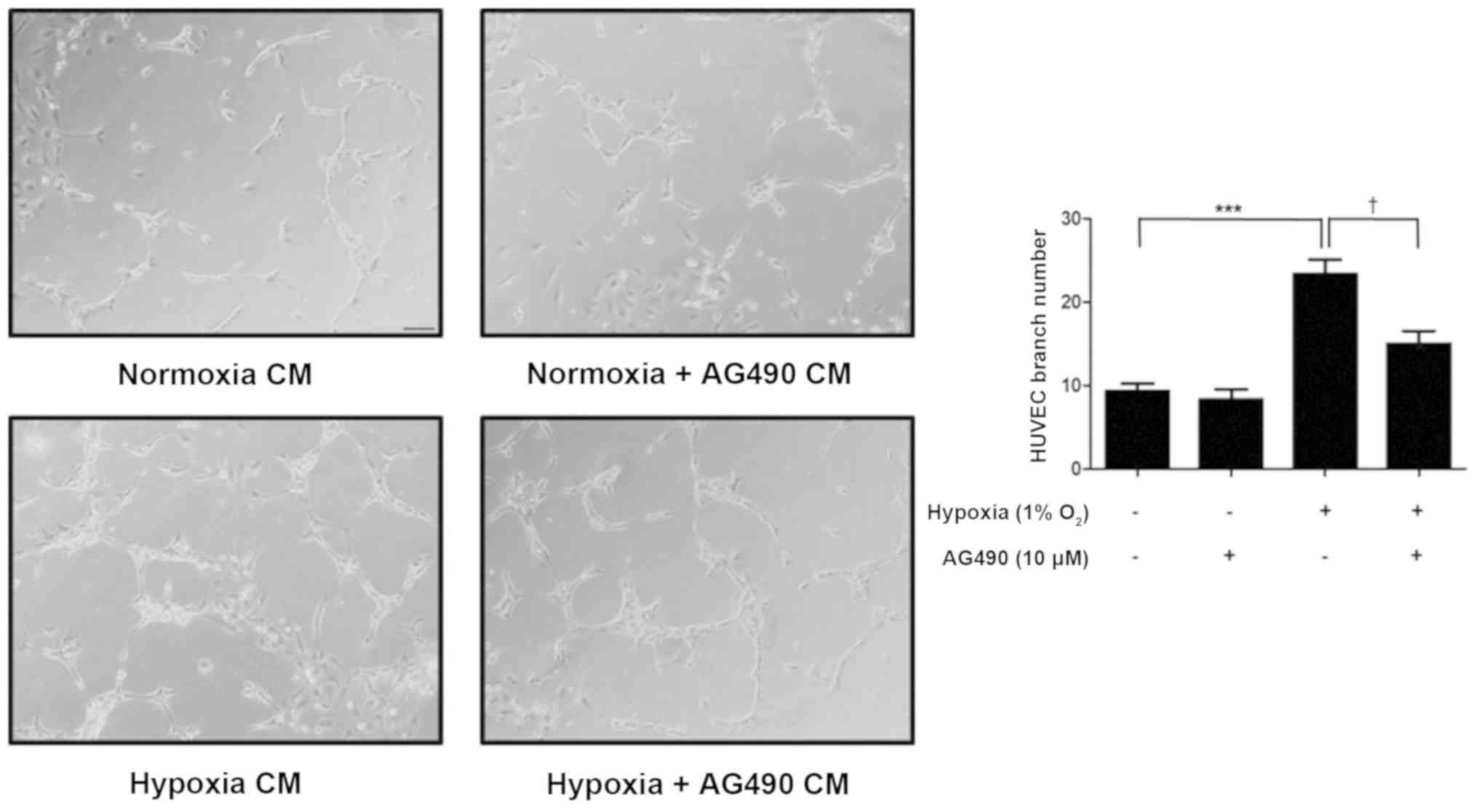
Conditioned medium (CM) from
AG490-treated ARPE-19 cells suppresses HUVEC tube formation
To assess the effects of AG490 on
neovascularization, a HUVEC tube formation assay was performed.
After a 24-h incubation, the CM was added to HUVECs, which were
incubated for an additional 48 h.
The number of branch points significantly increased
in HUVECs incubated in hypoxia CM compared with normoxia CM
(P<0.001). However, HUVECs incubated in hypoxia + AG490 CM
presented a significantly lower number of branch points compared
with those incubated with hypoxia CM (P<0.05). The number of
branch points in the normoxia + AG490 CM group was similar to the
normoxia CM controls, suggesting that in AG490 alone did not affect
HUVECs (Fig. 6). These findings
revealed that STAT3 affects branch formation via VEGF release.
Discussion
Neovascularization in the retina is induced by
multiple factors, and injury in newly formed blood vessels can
result in visual impairment (21).
Anti-VEGF therapy is widely used and highly effective for the
treatment of exudative AMD and diabetic retinopathy. However,
anti-VEGF agents are costly and are administered in clinic through
frequent injections (22). Thus,
alternative long-lasting therapeutic solutions for AMD and diabetic
retinopathy are urgently required. Furthermore, a number of
patients do not respond to anti-VEGF therapy or experience adverse
effects, such as intraocular infection, intraocular pressure
elevation, ocular hemorrhage and rhegmatogenous retinal detachment
(23). Identifying and inhibiting
the fundamental mechanisms of VEGF release represents a promising
strategy for treating retinal vascular disease.
VEGF is regulated by HIF-1α and NF-κB p65, which are
increased by hypoxia (24,25). NF-κB activation is associated with
phosphorylation during hypoxia (26,27).
The p65 subunit of NF-κB is the primary regulator of NF-κB activity
(28). In the present study, the
levels of p65 phosphorylation and nuclear translocation increased
under hypoxic conditions compared with those under normoxia.
CoCl2 treatment inhibits degradation of HIF-1α and is
therefore thought to mimic hypoxia (29); in the present study, HIF-1α
expression and the phosphorylation of NF-κB p65 were increased by
CoCl2 treatment and hypoxia. However, STAT3
phosphorylation decreased following CoCl2 treatment.
Although both HIF-1α and STAT3 regulate VEGF expression, they have
not been reported to interact with each other (30). Therefore, HIF-1α was used as a
marker of hypoxia in the present study. In addition, the potential
interaction between ERBB2 and STAT3 was evaluated using AG490. Both
ERBB2 and STAT3 were downregulated by AG490. ERBB2 overexpression
induces activation of STAT3 upon tumor vascularization (31). However, to the best of our
knowledge, this has not been documented in the retina. Thus, the
present study aimed to determine whether STAT3 and ERBB2 could
regulate VEGF release in the retina.
The RPE serves an important role in the physiology
of photoreceptors, including VEGF release (32). The human ARPE-19 cell line does not
develop typical epithelial cell polarization and may not be
entirely representative of the retinal pigment epithelium; however,
previous studies have demonstrated that APRE-19 cells display the
characteristics of retinal epithelial cells (33,34).
In addition, ARPE-19 is a commonly used human cell line due to the
difficulty in the culturing and extraction of specific RPE cells
from the murine retina (35,36).
In the present study, phosphorylation levels of STAT3 and ERBB2
increased in ARPE-19 cells after 24 h of hypoxia. ERBB2 forms
heterodimers with other ERBB receptors in a ligand-independent
manner, and autophosphorylation of ERBB dimers, in turn, initiates
several signaling pathways, such as RTK and MAPK pathways, which
are associated with neovascularization (37–39).
NRG1 is a member of the epidermal growth factor family that acts as
an essential paracrine regulator of cell growth through the
activation of ERBB tyrosine kinase receptors (40). Therefore, NRG1 may indirectly
interact with ERBB2. However, in the present study, although NRG1
levels appeared to increase after 12 h of hypoxia, this effect was
not significant.
AG490 is a specific inhibitor of the JAK2/STAT3
pathway (41,42). In the present study, AG490
suppressed the hypoxia-induced STAT3 and ERBB2 phosphorylation in
ARPE-19 cells, suggesting a potential association between STAT3 and
ERBB2. However, sinceAG490 is not a specific inhibitor of STAT3,
the exact upstream mediators of STAT3 and ERBB2 phosphorylation
under hypoxia remain unknown. Therefore, future studies should
further examine the relationship between STAT3 and ERBB2.
The RPE releases VEGF, which binds to VEGF receptors
on endothelial cells, promoting new blood vessel formation
(43). In the present study, AG490
suppressed hypoxia-induced VEGF release in ARPE-19 cells. In
addition, the effects of VEGF secretion from RPE cells on retinal
neovascularization were indirectly assessed in a HUVEC tube
formation assay; HUVEC tube formation was increased by conditioned
medium from ARPE-19 cells incubated under hypoxia compared with
cells incubated under normoxia. However, hypoxia was inhibited in
the presence of AG490. These results indicated that inhibition of
STAT3 leads to a decrease in VEGF release, which decreases
angiogenesis.
In conclusion, the results of the present study
demonstrated that the phosphorylation of STAT3 and ERBB2 in ARPE-19
cells was increased by hypoxia, accompanied by the induction of
VEGF release. AG490 suppressed these responses and ultimately
decreased HUVEC tube formation. Thus, targeting the STAT3 and ERBB2
pathway represents a new strategy for alleviating
neovascularization in the retina.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National
Research Foundation of Korea and the Ministry of Science and
Information and Communications Technologies (grant no.
NRF-2019R1F1A1061541) and by The Biomedical Research Institute Fund
at The Gyeongsang National University Hospital (grant no.
GNUHBRIF-2018-0002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH, SWS, YSH and SSK conceived the study. SH, SSK
and JR developed the methodology. SH, HS, JYJ, KYN and SJK carried
out the experiments. HS and TSK made contributions to the analysis
and interpretation of data. SH wrote the initial draft of the
manuscript. HS, TSK and YSH drafted and revised the manuscript
critically for intellectual content. SSK, YSH and TSK reviewed and
edited the manuscript. SSK and YSH supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neely KA and Gardner TW: Ocular
neovascularization: Clarifying complex interactions. Am J Pathol.
153:2733–670. 1998. View Article : Google Scholar
|
|
2
|
Chappelow AV and Kaiser PK: Neovascular
age-related macular degeneration. Drugs. 68:1029–1036. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial growth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwak N, Okamoto N, Wood JM and Campochiaro
PA: VEGF is major stimulator in model of choroidal
neovascularization. Invest Ophthalmol Vis Sci. 41:3158–3164.
2000.PubMed/NCBI
|
|
5
|
Klettner A, Westhues D, Lassen J, Bartsch
S and Roider J: Regulation of constitutive vascular endothelial
growth factor secretion in retinal pigment epithelium/choroid organ
cultures: p38, nuclear factor κB, and the vascular endothelial
growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol
Vis. 19:281–291. 2013.PubMed/NCBI
|
|
6
|
Klettner A, Kaya L, Flach J, Lassen J,
Treumer F and Roider J: Basal and apical regulation of VEGF-A and
placenta growth factor in the RPE/choroid and primary RPE. Mol Vis.
21:736–748. 2015.PubMed/NCBI
|
|
7
|
Shweiki D, Itin A, Soffer D and Keshet E:
Vascular endothelial growth factor induced by hypoxia may mediate
hypoxia-initiated angiogenesis. Nature. 359:843–845. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang X, Cao J, Du Y, Gong Q and Cheng Y:
Angiopoietin-like protein 4 (ANGPTL4) induces retinal pigment
epithelial barrier breakdown by activating signal transducer and
activator of transcription 3 (STAT3): Evidence from ARPE-19 cells
under hypoxic condition and diabetic rats. Med Sci Monit.
25:6742–6754. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arjamaa O, Aaltonen V, Piippo N, Csont T,
Petrovski G, Kaarniranta K and Kauppinen A: Hypoxia and
inflammation in the release of VEGF and interleukins from human
retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol.
255:1757–1762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel AK, Syeda S and Hackam AS: Signal
transducer and activator of transcription 3 (STAT3) signaling in
retinal pigment epithelium cells. JAKSTAT. 2:e254342013.PubMed/NCBI
|
|
11
|
Gutsaeva DR, Thounaojam M, Rajpurohit S,
Powell FL, Martin PM, Goei S, Duncan M and Bartoli M:
STAT3-mediated activation of miR-21 is involved in down-regulation
of TIMP3 and neovascularization in the ischemic retina. Oncotarget.
8:103568–103580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loureiro RM, Maharaj AS, Dankort D, Muller
WJ and D'Amore PA: ErbB2 overexpression in mammary cells
upregulates VEGF through the core promoter. Biochem Biophys Res
Commun. 326:455–465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W, Klos K, Yang Y, Smith TL, Shi D
and Yu D: ErbB2 overexpression correlates with increased expression
of vascular endothelial growth factors A, C, and D in human breast
carcinoma. Cancer. 94:2855–2861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venturutti L, Romero LV, Urtreger AJ,
Chervo MF, Cordo Russo RI, Mercogliano MF, Inurrigarro G, Pereyra
MG, Proietti CJ, Izzo F, et al: Stat3 regulates ErbB-2 expression
and co-opts ErbB-2 nuclear function to induce miR-21 expression,
PDCD4 downregulation and breast cancer metastasis. Oncogene.
35:2208–2222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Cai Y, Wang YS, Shi YY, Hou W, Xu
CS, Wang HY, Ye Z, Yao LB and Zhang J: Hyperglycaemia exacerbates
choroidal neovascularisation in mice via the oxidative
stress-induced activation of STAT3 signalling in RPE cells. PLoS
One. 7:e476002012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maisto R, Oltra M, Vidal-Gil L,
Martínez-Gil N, Sancho-Pellúz J, Filippo CD, Rossi S, D Amico M,
Barcia JM and Romero FJ: ARPE-19-derived VEGF-containing exosomes
promote neovascularization in HUVEC: The role of the melanocortin
receptor 5. Cell Cycle. 18:413–424. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Bai Y, Zhao M and Jiang Y: TLR4
inhibitor attenuates amyloid-β-induced angiogenic and inflammatory
factors in ARPE-19 cells: Implications for age-related macular
degeneration. Mol Med Rep. 13:3249–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Geng YU, Hua H, Cun B, Chen Q, Xi
X, Yang L and Li Y: Fenofibrate inhibits the expression of VEGFC
and VEGFR-3 in retinal pigmental epithelial cells exposed to
hypoxia. Exp Ther Med. 10:1404–1412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryu J, Seong H, Yoon NA, Seo SW, Park JW,
Kang SS, Park JM and Han YS: Tristetraprolin regulates the decay of
the hypoxia-induced vascular endothelial growth factor mRNA in
ARPE-19 cells. Mol Med Rep. 14:5395–5400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dorrell M, Uusitalo-Jarvinen H, Aguilar E
and Friedlander M: Ocular neovascularization: Basic mechanisms and
therapeutic advances. Surv Ophthalmol. 52 (Suppl 1):S3–S19. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gacche R and Meshram R: Angiogenic factors
as potential drug target: Efficacy and limitations of
anti-angiogenic therapy. Biochim Biophys Acta. 1846:161–179.
2014.PubMed/NCBI
|
|
23
|
Falavarjani KG and Nguyen Q: Adverse
events and complications associated with intravitreal injection of
anti-VEGF agents: A review of literature. Eye (Lond). 27:787–794.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
D'Ignazio L and Rocha S: Hypoxia induced
NF-κB. Cells. 5:102016. View Article : Google Scholar
|
|
25
|
Faridvand Y, Nozari S, Atashkhoei S, Nouri
M and Jodati A: Amniotic membrane extracted proteins protect H9c2
cardiomyoblasts against hypoxia-induced apoptosis by modulating
oxidative stress. Biochem Biophys Res Commun. 503:1335–1341. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kovacs K, Vaczy A, Fekete K, Kovari P,
Atlasz T, Reglodi D, Gabriel R, Gallyas F and Sumegi B: PARP
inhibitor protects against chronic hypoxia/reoxygenation-induced
retinal injury by regulation of MAPKs, HIF1α, Nrf2, and NFκB.
Invest Ophthalmol Vis Sci. 60:1478–1490. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Zheng D, Pu J, Dai J, Zhang Y,
Zhang W and Wu Z: MicroRNA-125b protects liver from
ischemia/reperfusion injury via inhibiting TRAF6 and NF-κB pathway.
Biosci Biotechnol Biochem. 83:829–835. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albensi B: What is nuclear factor kappa B
(NF-κB) doing in and to the mitochondrion? Front Cell Dev Biol.
7:1542019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YB, Wang X, Meister EA, Gong KR, Yan
SC, Lu GW, Ji XM and Shao G: The effects of CoCl2 on HIF-1α protein
under experimental conditions of autoprogressive hypoxia using
mouse models. Int J Mol Sci. 15:10999–11012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gray MJ, Zhang J, Ellis LM, Semenza GL,
Evans DB, Watowich SS and Gallick GE: HIF-1α, STAT3, CBP/p300 and
Ref-1/APE are components of a transcriptional complex that
regulates Src-dependent hypoxia-induced expression of VEGF in
pancreatic and prostate carcinomas. Oncogene. 24:3110–3120. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hawthorne VS, Huang WC, Neal CL, Tseng LM,
Hung MC and Yu D: ErbB2-mediated Src and signal transducer and
activator of transcription 3 activation leads to transcriptional
up-regulation of p21Cip1 and chemoresistance in breast cancer
cells. Mol Cancer Res. 7:592–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ford KM, Saint-Geniez M, Walshe T, Zahr A
and D'Amore PA: Expression and role of VEGF in the adult retinal
pigment epithelium. Invest Ophthalmol Vis Sci. 52:9478–9487. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li R, Du JH, Yao GM, Yao Y and Zhang J:
Autophagy: A new mechanism for regulating VEGF and PEDF expression
in retinal pigment epithelium cells. Int J Ophthalmol. 12:557–562.
2019.PubMed/NCBI
|
|
34
|
Li X, Zhao H, Wang Q, Liang H and Jiang X:
Fucoidan protects ARPE-19 cells from oxidative stress via
normalization of reactive oxygen species generation through the
Ca2+-dependent ERK signaling pathway. Mol Med Rep.
11:3746–3752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giddabasappa A, Bauler MN, Barrett CM,
Coss CC, Wu Z, Miller DD, Dalton JT and Eswaraka JR: GTx-822, an
ER{beta}-selective agonist, protects retinal pigment epithelium
(ARPE-19) from oxidative stress by activating MAPK and PI3-K
pathways. Invest Ophthalmol Vis Sci. 51:5934–5942. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dunn KC, Aotaki-Keen AE, Putkey FR and
Hjelmeland LM: ARPE-19, a human retinal pigment epithelial cell
line with differentiated properties. Exp Eye Res. 62:155–169. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olayioye MA: Intracellular signaling
pathways of ErbB2/HER-2 and family members. Breast Cancer Res.
3:385–389. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeltsch M, Leppänen VM, Saharinen P and
Alitalo K: Receptor tyrosine kinase-mediated angiogenesis. Cold
Spring Harb Perspect Biol. 5:a0091832013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jahejo AR, Niu S, Zhang D, Ning GB, Khan
A, Mangi RA, Qadir MF, Khan A, Li JH and Tian WX: Transcriptome
analysis of MAPK signaling pathway and associated genes to
angiogenesis in chicken erythrocytes on response to thiram-induced
tibial lesions. Res Vet Sci. 127:65–75. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang X, Ding Y, Zhang Y, Chai YH, He J,
Chiu SM, Gao F, Tse HF and Lian Q: Activation of NRG1-ERBB4
signaling potentiates mesenchymal stem cell-mediated myocardial
repairs following myocardial infarction. Cell Death Dis.
6:e17652015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Selvendiran K, Bratasz A, Kuppusamy ML,
Tazi MF, Rivera BK and Kuppusamy P: Hypoxia induces chemoresistance
in ovarian cancer cells by activation of signal transducer and
activator of transcription 3. Int J Cancer. 125:2198–2204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang GS, Qian GS, Zhou DS and Zhao JQ:
JAK-STAT signaling pathway in pulmonary arterial smooth muscle
cells is activated by hypoxia. Cell Biol Int. 29:598–603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, He S, Spee C, Ishikawa K and
Hinton DR: SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by
Resveratrol and its relevance to choroidal neovascularization.
Cytokine. 76:549–552. 2015. View Article : Google Scholar : PubMed/NCBI
|