Introduction
Severe acute pancreatitis (SAP) is the most serious
type of pancreatitis. SAP is frequently accompanied by systemic
inflammatory response syndrome (SIRS) and multiple organ
dysfunction syndromes (MODS) and is associated with high morbidity
and mortality rates (1). Although
gallstones are the most common cause of pancreatitis, and increase
in alcohol misuse has been linked to the increased incidence of
pancreatitis in several European countries and the USA (2). The pathogenic mechanisms underlying
the development of SAP have been extensively studied but remain
incompletely understood. Pancreatitis-associated ascitic fluid
(PAAF) contains high concentrations of pancreatic enzymes, free
fatty acids, inflammatory cytokines and endotoxins (3). The removal of PAAF by abdominal
paracentesis drainage and peritoneal lavage exerts a beneficial
effect on patients with SAP, reducing multiple organ failure and
mortality rates (4,5). However, the mechanisms underlying the
effectiveness of this procedure have remained elusive.
Toll-like receptors (TLRs) are innate
pattern-recognition receptors that detect distinct
pathogen-associated molecules and induce the transcription and
release of inflammatory cytokines (6). TLR4 in particular recognizes
lipopolysaccharide present on Gram-negative bacteria (7). TLR4 is also required for the
phagocytosis of E. coli in murine macrophages (8). Stimulation of TLR4 is required for
the activation of pro-inflammatory pathways, thereby inducing the
production of inflammatory cytokines in a variety of cell types
(9). Excess release of
pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, is the
underlying mechanism of MODS in patients with SAP (10). Several studies have confirmed that
TLR4-mediated activation of the NF-κB signaling pathway is involved
in pancreatic injury in a mouse model of SAP (11,12).
Thus, inhibition of TLR4-activated NF-κB signaling may represent a
potential approach for the prevention of SAP (11).
Single immunoglobulin and Toll-interleukin-1
receptor domain-containing molecule (SIGIRR), which is also known
as Toll-IL-1R (TIR)-8 or IL-1R8, is a member of the IL-1R family
(13). It is widely expressed in
several epithelial tissues, particularly in epithelial cells of the
kidney, digestive tract, liver, lung and lymphoid organs. Among
leukocytes, SIGIRR is expressed in monocytes, B and T lymphocytes,
dendritic cells and natural killer cells (14). SIGIRR functions as a negative
regulator of IL-1R and TLR signaling by inhibiting TIR
domain-containing receptors and adapter proteins, thus blocking
Akt, JNK, mitogen-activated protein kinases, TGF-β-activated kinase
1, mTOR and NF-κB activation (15). SIGIRR may have a beneficial or
detrimental role in the innate immunity against pathogens, emerging
as a key modulator of the equilibrium between protective immune
responses and inflammation and host injury (16).
PAAF is one of the initial presentations of acute
pancreatitis (17), and
PAAF-induced inflammatory responses are involved in the development
of SAP (18). Furthermore,
inflammatory cytokines involved in the TLR4 signaling pathway are
associated with pancreatic injury (11). However, whether an association
exists between PAAF and the activation of the TLR4-mediated
inflammatory pathways in patients with SAP has remained to be
determined. In addition, little is known about the role of SIGIRR
in SAP. Therefore, in the present study, it was hypothesized that
PAAF-mediated activation of the TLR4 signaling pathway contributes
to the development of SAP. Accordingly, SIGIRR may negatively
regulate this process to protect against SAP. The aim of the
present study was to investigate the role of SIGIRR in regulating
the TLR4 signaling pathway in an in vitro SAP model of
PAAF-treated macrophages. To the best of our knowledge, the present
study was the first to examine the association between SIGIRR and
SAP in vitro. The results may provide insight into the
pathogenesis of SAP and help identify therapeutic targets for this
condition.
Materials and methods
Sources of PAAF
PAAF was obtained by collection of abdominal
drainage fluid from two patients with untreated SAP under aseptic
conditions who were admitted to the First Affiliated Hospital of
Nanchang University (Nanchang, China) in January 2019. The
diagnosis was based on at least two of the following parameters: i)
Amylasemia >3 times the upper limit of normal; ii) abdominal
pain compatible with acute pancreatitis; and iii) computed
tomography or magnetic resonance imaging features compatible with
acute pancreatitis. The diagnostic criteria for SAP were according
to the 2012 revision of the Atlanta Classification of Acute
Pancreatitis (1). The general
laboratory characteristics of the PAAF from the two patients are
presented in Table I. A total of
50 ml PAAF was collected from both patients, then pooled in a
centrifuge tube and centrifuged at 11,180 × g for 15 min at 4°C.
The PAAF supernatant was collected and stored at −80°C after
sequential filtration through membranes with pore sizes of 3.0,
2.0, 1.0 and 0.45 µm, as well as 0.22 µm. Written informed consent
for the use of PAAF samples was obtained from both patients
enrolled in this study. The study was approved by The Ethics
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China; approval no. EL20180027) and was performed in
compliance with the Declaration of Helsinki.
 | Table I.General laboratory characteristics of
pancreatitis-associated ascitic fluid. |
Table I.
General laboratory characteristics of
pancreatitis-associated ascitic fluid.
| Parameter | Patient A | Patient B |
|---|
| Disease
information |
|
Age | 47 | 51 |
|
Sex | Male | Female |
|
Severity | Severe | Severe |
| Disease
duration | 5 days | 4 days |
|
Relevant medical history | None | None |
| Routine
analysis |
|
Colour | Red | Yellow |
|
Turbidity | Turbid | Slightly
turbid |
|
Clot | Flocculation | None |
| Rivalta
test | Positive | Positive |
|
Nucleated cells (1/µl) | 6,720 | 3,800 |
|
Segmented neutrophils (%) | 90 | 70 |
|
Lymphocytes (%) | 10 | 28 |
| Biochemistry |
| Total
bilirubin (µmol/l) | 27.9 | 37.9 |
| Total
proteins (g/l) | 38.5 | 44.5 |
| Albumin
(g/l) | 28.6 | 32.9 |
|
Globulin (g/l) | 9.9 | 11.6 |
| Glucose
(mmol/l) | 5.8 | 14.3 |
| Lactate
dehydrogenase (U/l) | 895 | 1718 |
| Amylase
(U/l) | 1,438 | 1,248 |
|
Adenosine deaminase (U/l) | 1 | 2 |
| Cytokines |
| IL-1
(pg/ml) | 231 | 257 |
| IL-6
(pg/ml) | 1,051 | 998 |
Cell culture and treatment
RAW264.7 murine macrophage cells were obtained from
Nanjing KeyGen Biotech Co., Ltd. and cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Zhejiang Tianhang Biotechnology Co., Ltd.) at 37°C with 5%
CO2. RAW264.7 cells in the logarithmic growth phase were
treated with titrated doses of PAAF (1.25, 2.5, 5.0 and 10.0%
diluted in RPMI-1640 medium) harvested from the patients with SAP
for 6, 12 or 24 h. Untreated cells were used as a negative
control.
Plasmid extraction and
transfection
The GT110 E. coli strains containing the pUNO
plasmid or pUNO-mSIGIRR plasmid (4199 bp) with two restriction
sites were purchased from InvivoGen. Plasmids were extracted from
GT110 cells in the logarithmic growth phase using a Plasmid Mini
kit (Tiangen Biotech Co., Ltd.), then identified by digestion with
the EcoRI restriction endonuclease (Thermo Fisher
Scientific, Inc.) at 37°C for 3 h, resulting in two fragments.
RAW264.7 cells were separately transfected with pUNO or
pUNO-mSIGIRR (InvivoGen) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. After 24 h, the transfected RAW264.7
cells in the logarithmic growth phase were transferred to six-well
tissue culture plates at a density of 2×105 cells/well.
Certain designated wells of transfected cells were treated with 5%
PAAF for 24 h. Furthermore, untreated and untransfected cells were
used as negative controls.
Semi-quantitative reverse
transcription (RT-PCR)
Total RNA was extracted from RAW264.7 cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RT was performed with PrimeScript RT Reagent kit (Takara
Bio, Inc.) to obtain complementary DNA according to the
manufacturer's protocol. Briefly, genomic DNA was eliminated using
gDNA Eraser treatment for 2 min at 42°C. RT was then carried out at
37°C for 15 min, followed by 85°C for 5 sec. The resulting cDNA
which was then used as a template for amplification of the target
genes with 2X Taq PCR MasterMix (Tiangen Biotech Co., Ltd.)
including TLR2, TLR4, myeloid differentiation factor 88 (MyD88),
IL-1R-associated kinase-1 (IRAK-1) and TNF receptor-associated
factor-6 (TRAF-6) and SIGIRR. Primer sequences specific for these
genes are presented in Table II.
The thermocycling conditions for each target gene are described in
Table III. All RT-PCRs were set
up in triplicate. Amplification products were resolved by
electrophoresis using 1% agarose gels stained with ethidium
bromide. Electrophoresis images were acquired using the ChemiDoc MP
system (Bio-Rad Laboratories, Inc.) and the gray-scale value of
bands was determined to quantify mRNA expression levels using the
BandScan software (version 5.0; Glyko, Inc.).
 | Table II.Primer sequences used for
semi-quantitative PCR and resulting product sizes. |
Table II.
Primer sequences used for
semi-quantitative PCR and resulting product sizes.
| Target gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| β-actin | Forward:
TGGAATCCTGTGGCATCCATGAAAC | 234 |
|
| Reverse:
TAAAACGCAGCTCAGTAACAGTCCG |
|
| SIGIRR | Forward:
GTGGCTGAAAGATGGTCTGGCATTG | 123 |
|
| Reverse:
CAGGTGAAGGTTCCATAGTCCTCTGC |
|
| TLR4 | Forward:
CAGCTTCAATGGTGCCATCA | 438 |
|
| Reverse:
CTGCAATCAAGAGTGCTGAG |
|
| TLR2 | Forward:
TCAAAAGTCGATCGGCGACAT | 340 |
|
| Reverse:
TACCCAGCTCGCTCATCACGT |
|
| MyD88 | Forward:
AGAGCTGCTGGCCTTGTTA | 265 |
|
| Reverse:
TCATCTCCTGCACAAACTCG |
|
| IRAK-1 | Forward:
GCCTCAACGACTGGACATTC | 589 |
|
| Reverse:
GCCTCTTCTTGGCCCGACGGT |
|
| TRAF-6 | Forward:
GAGGAGATCCAGGGCTACGA | 292 |
|
| Reverse:
ATGTACTTGATGATCCTCGA |
|
 | Table III.Semi-quantitative PCR thermocycling
conditions. |
Table III.
Semi-quantitative PCR thermocycling
conditions.
| Genes | Initial
denaturation, temperature (°C)/duration (min) | Denaturation,
temperature (°C)/duration (min) | Annealing
temperature (°C)/duration (min) | Extension,
temperature (°C)/duration (min) | Terminal extension,
temperature (°C)/duration (min) | Number of
cycles |
|---|
| β-actin | 94/5 | 94/0.5 | 56/0.5 | 72/1 | 72/5 | 30 |
| SIGIRR | 94/5 | 94/0.5 | 56/0.5 | 72/1 | 72/5 | 30 |
| TLR4 | 94/5 | 94/0.5 | 48/0.5 | 72/1 | 72/5 | 30 |
| TLR2 | 94/5 | 94/0.5 | 53/0.5 | 72/1 | 72/5 | 30 |
| MyD88 | 94/5 | 94/0.5 | 49/0.5 | 72/1 | 72/5 | 30 |
| IRAK-1 | 94/5 | 94/0.5 | 54/0.5 | 72/1 | 72/5 | 30 |
| TRAF-6 | 94/5 | 94/0.5 | 47/0.5 | 72/1 | 72/5 | 30 |
ELISA
The concentrations of IL-2, IL-4, IL-10, IL-12,
IL-17 and IFN-γ in the culture supernatant of RAW264.7 cells were
determined using ELISA kits (cat. nos. BMS601 for IL-2; BMS613 for
IL-4; BMS614/2 for IL-10; BMS616 for IL-12; BMS6001 for IL-17;
BMS606 for IFN-γ; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. All assays were repeated at least three
times.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation of at least three independent experiments.
Multigroup comparisons were performed using two-way ANOVA, followed
by Tukey's Honestly Significant Difference test. Paired data were
analyzed using two-way repeated-measures ANOVA, followed by Tukey's
post hoc test. Statistical analysis was performed using SPSS 24.0
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of PAAF on TLR4 signaling and
SIGIRR expression in PAAF-treated macrophages
RAW264.7 cells were treated with 1.25, 2.5, 5 or 10%
PAAF for 6, 12 or 24 h. TLR2 mRNA expression was not significantly
altered following PAAF treatment (Fig.
1A). By contrast, TLR4 mRNA levels in macrophages significantly
were increased in response to incubation with 2.5-10% PAAF for 24 h
(Fig. 1B). Furthermore, in cells
stimulated with 5% PAAF for 24 h, for instance, the mRNA levels of
TLR4, MyD88, IRAK-1 and TRAF-6 increased in a time-dependent manner
(Fig. 1B-E; P<0.05 vs. the same
PAAF concentration in the 6-h group). However, the expression of
TLR4 and downstream key molecules did not further increase with
increasing PAAF concentration. Compared with 5% PAAF stimulation,
the mRNA levels of TLR2, TLR4, MyD88, IRAK-1 and TRAF-6 were
decreased following stimulation with 10% PAAF in the same subgroup
at 24 h.
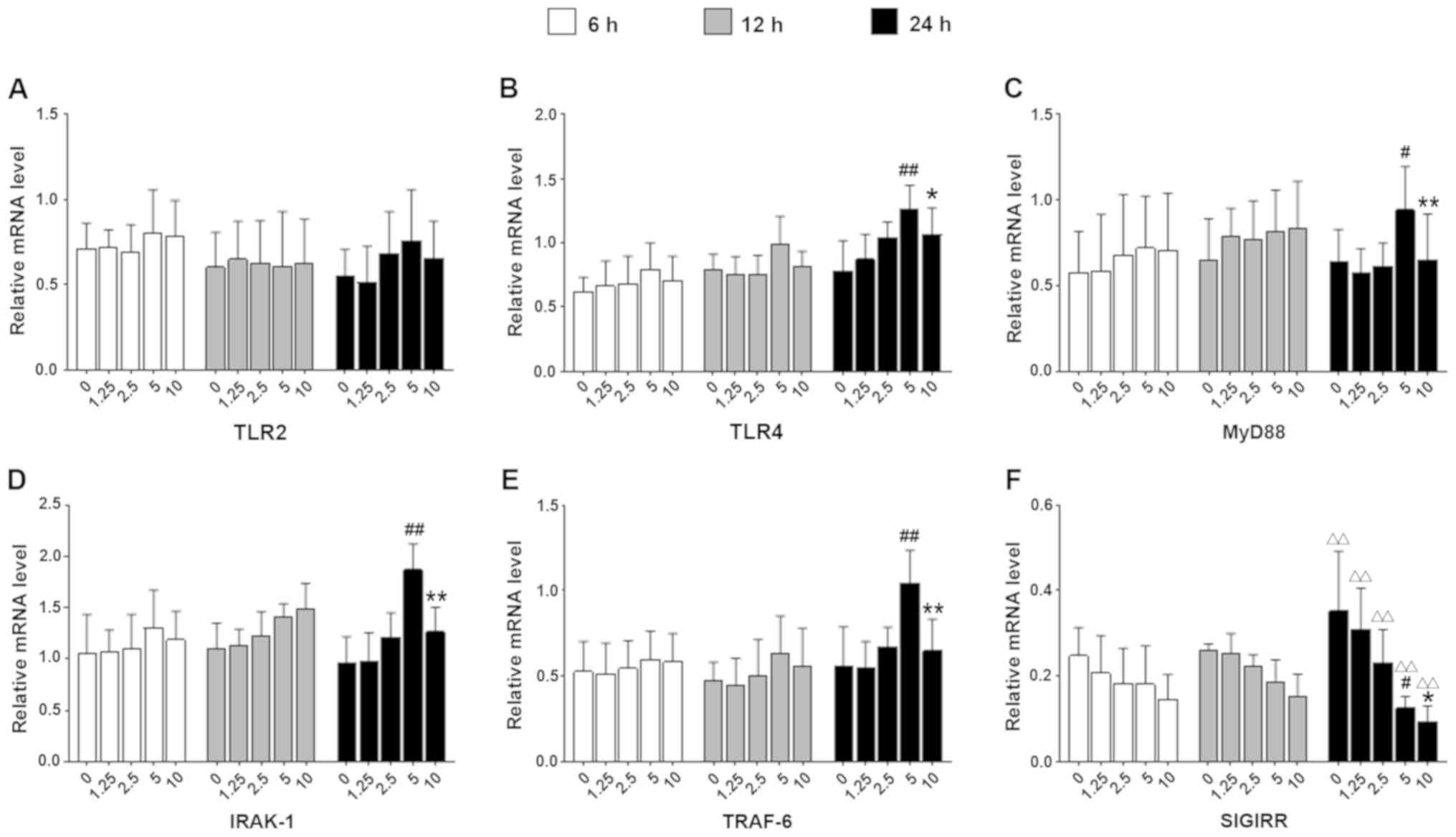 | Figure 1.Effect of PAAF on the TLR4 signaling
pathway and expression of SIGIRR in macrophages in vitro.
The RAW264.7 cells were stimulated with 1.25, 2.5, 5 and 10% PAAF.
The mRNA levels of (A) TLR2, (B) TLR4, (C) MyD88, (D) IRAK-1, (E)
TRAF-6 and (F) SIGIRR were determined. Values are expressed as the
mean ± standard deviation from six independent experiments.
#P<0.05, ##P<0.01 vs. corresponding
concentration at the 6-h time-point. *P<0.05, **P<0.01 vs.
the same group treated with 5% PAAF at 24 h. ∆∆P<0.01
vs. the same PAAF concentration in the 6 h group. IL-1R, IL-1
receptor; SIGIRR, Single Ig and Toll-IL-1 receptor domain
containing molecule; TLR, Toll-like receptor; MyD88, myeloid
differentiation factor 88; IRAK-1, IL-1R-associated kinase-1;
TRAF-6, TNF receptor-associated factor-6; PAAF,
pancreatitis-associated ascitic fluid. |
In addition, SIGIRR mRNA expression was reduced with
increasing PAAF concentration, but only in a significant manner
following 24 h of incubation (Fig.
1F; P<0.01 vs. the same PAAF concentration in 6 h group).
Therefore, the optimal concentration of 5% PAAF and a stimulus
duration of 24 h were used in subsequent experiments.
Effect of SIGIRR on the TLR4 signaling
pathway in PAAF-treated macrophages
SIGIRR overexpression plasmid was successfully
constructed and validated using RT-PCR and restriction enzyme
digestion (Fig. 2). A clear 123-bp
band was observed for the PCR product from the pUNO-mSIGIRR
plasmid, but not the empty plasmid, which was consistent with the
expected size of the SIGIRR PCR product (Fig. 2A). In addition, the pUNO-mSIGIRR
plasmid was cleaved into two fragments using a single restriction
enzyme, a 1,911-bp SIGIRR-containing fragment and a 2,288-bp vector
fragment (Fig. 2B). SIGIRR gene
expression was increased in macrophages transfected with
pUNO-mSIGIRR as compared with that in untransfected macrophages and
macrophages transfected with the empty vector (Fig. 2C).
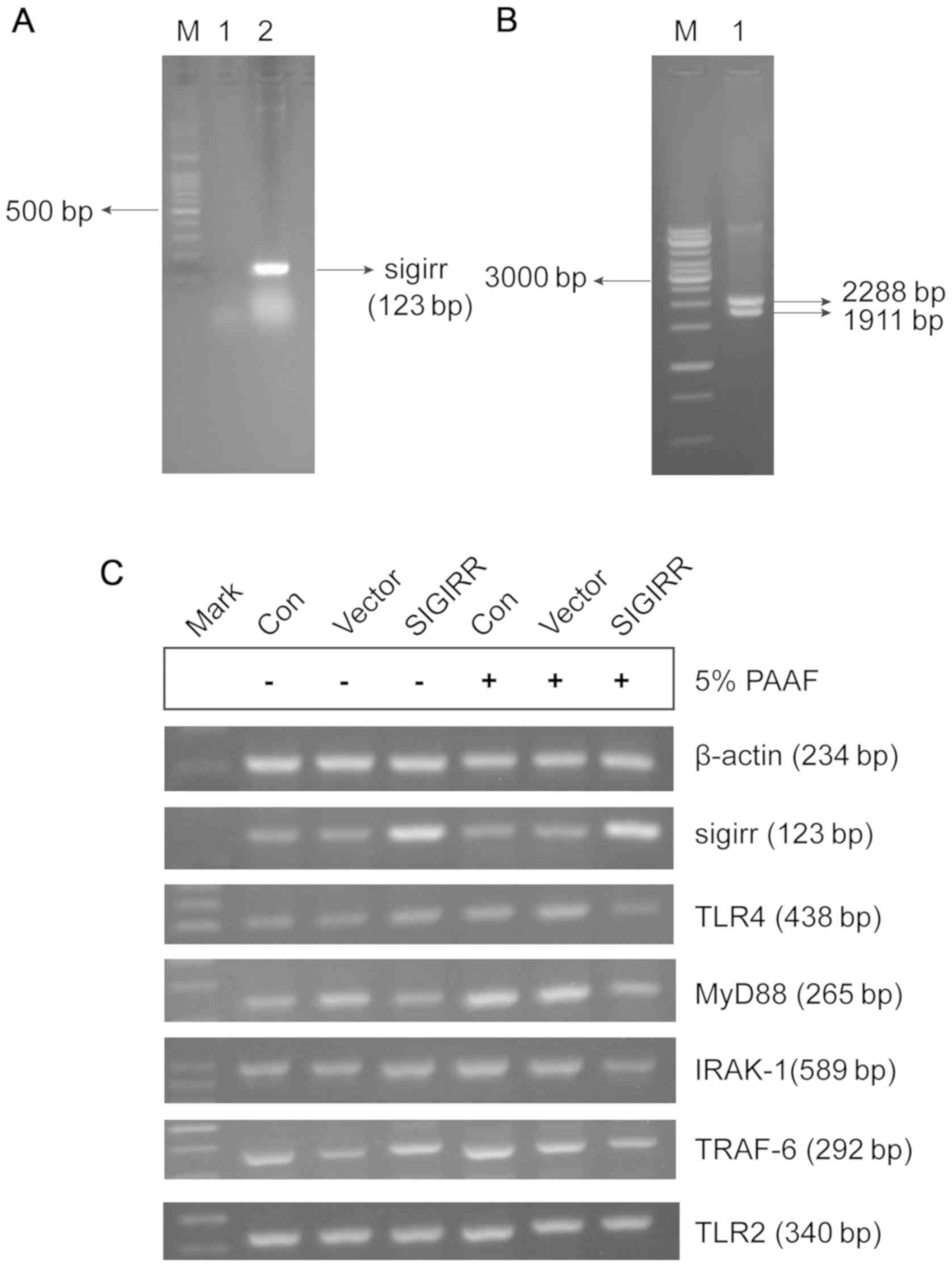 | Figure 2.Plasmid transfection and
identification. (A) SIGIRR gene products in pUNO-mSIGIRR plasmids
and pUNO plasmids were amplified and detected by PCR. Lanes: M,
100-bp DNA ladder; 1, SIGIRR PCR product from empty plasmids; 2,
SIGIRR PCR product from SIGIRR-containing plasmids. (B) Results of
restriction enzyme digestion. The SIGIRR-containing plasmid was
cleaved into a 1,911-bp gene-containing fragment and a 2,288-bp
vector fragment. (C) Representative agarose gel electrophoresis of
SIGIRR, TLR4, MYD88, IRAK-1, TRAF-6 and TLR2 expression. RAW264.7
macrophages were transfected with pUNO-mSIGIRR overexpression
vector and treated with 5% PAAF. Control cells were left
untransfected and the vector group was transfected with empty pUNO
plasmid. Con, control; Mark, marker; IL-1R, IL-1 receptor; SIGIRR,
single immunoglobulin and Toll-interleukin-1 receptor domain
containing molecule; TLR, Toll-like receptor; MyD88, myeloid
differentiation factor 88; IRAK-1, IL-1R-associated kinase-1;
TRAF-6, TNF receptor-associated factor-6. |
Transfection with pUNO-mSIGIRR resulted in
significantly increased levels of SIGIRR as compared with those in
untransfected macrophages and the empty vector group, regardless of
PAAF stimulation (P<0.05 vs. the control group; Fig. 3A). By contrast, the mRNA levels of
key molecules in the TLR4 signaling pathway, including TLR4, MyD88,
IRAK-1 and TRAF-6, were significantly decreased in the
SIGIRR-transfected group stimulated with 5% PAAF (P<0.01 vs. the
control group with 5% PAAF). There was a further decrease in the
presence of PAAF in the SIGIRR group (P<0.05 vs. SIGIRR group
without 5% PAAF). However, the presence of PAAF caused an increase
both in the control and vector groups (P<0.05 vs. the same
sub-group without 5% PAAF; Fig.
3B-E). However, there was no significant change in the mRNA
levels of TLR2 (Fig. 3F).
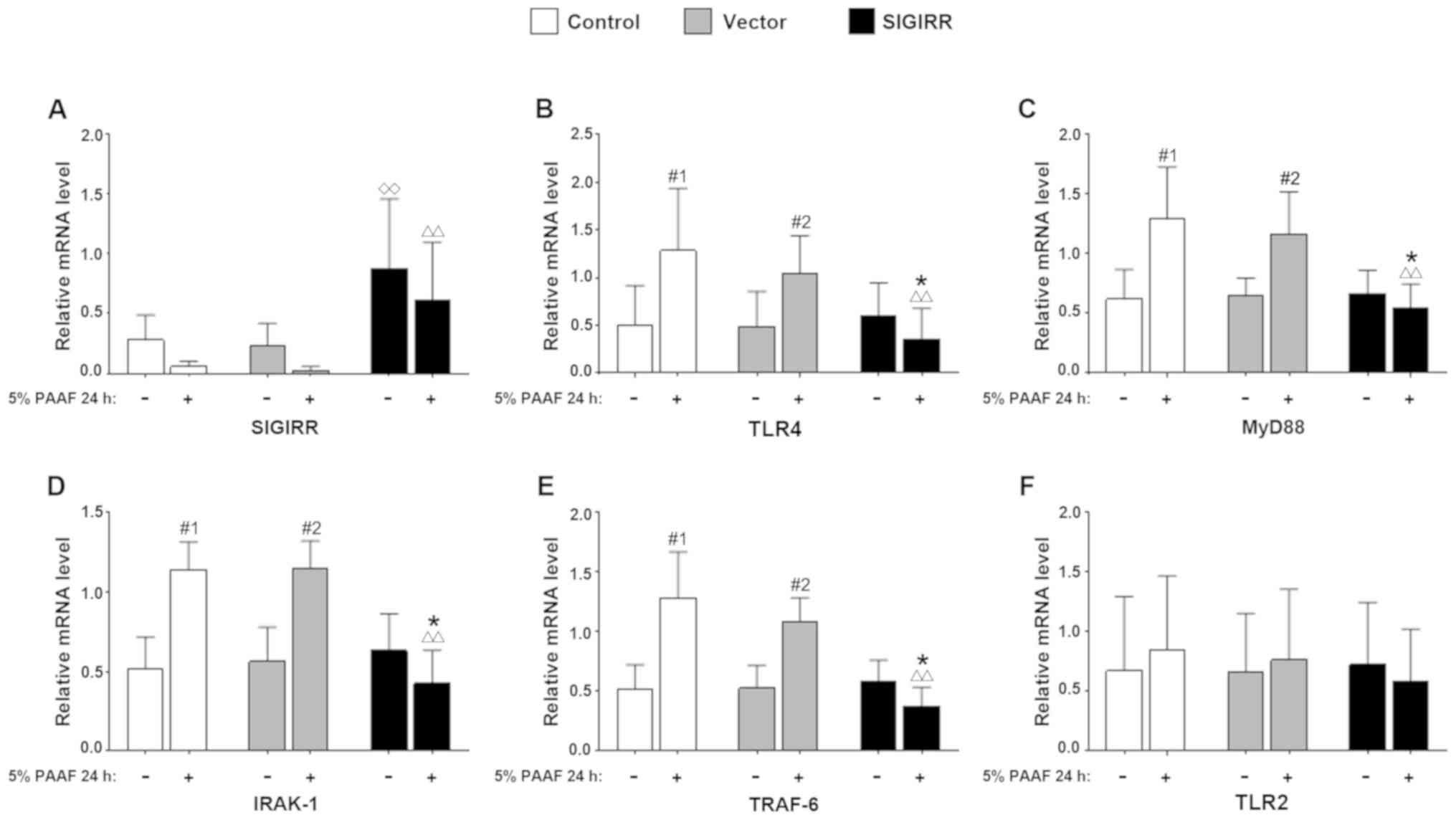 | Figure 3.Effect of SIGIRR on the TLR4
signaling pathway in macrophages with PAAF stimulation. RAW264.7
macrophages were transfected with either the pUNO-mSIGIRR or pUNO
vector and treated for 24 h with 5% PAAF. Control cells were left
untransfected. The transcription levels of (A) SIGIRR, (B) TLR4,
(C) MyD88, (D) IRAK-1, (E) TRAF-6 and (F) TLR2 were measured by
semi-quantitative reverse transcription PCR. Values are expressed
as the mean ± standard deviation from six independent experiments.
◊◊P<0.01 vs. control group-5% PAAF,
∆∆P<0.01 vs. control group + 5% PAAF. *P<0.05 vs.
SIGIRR group-5% PAAF. #1P<0.05 vs. control group-5%
PAAF. #2P<0.05 vs. vector group-5% PAAF. IL-1R, IL-1
receptor; SIGIRR, single immunoglobulin and Toll-interleukin-1
receptor domain containing molecule; TLR, Toll-like receptor;
MyD88, myeloid differentiation factor 88; IRAK-1, IL-1R-associated
kinase-1; TRAF-6, TNF receptor-associated factor-6; PAAF,
pancreatitis-associated ascitic fluid. |
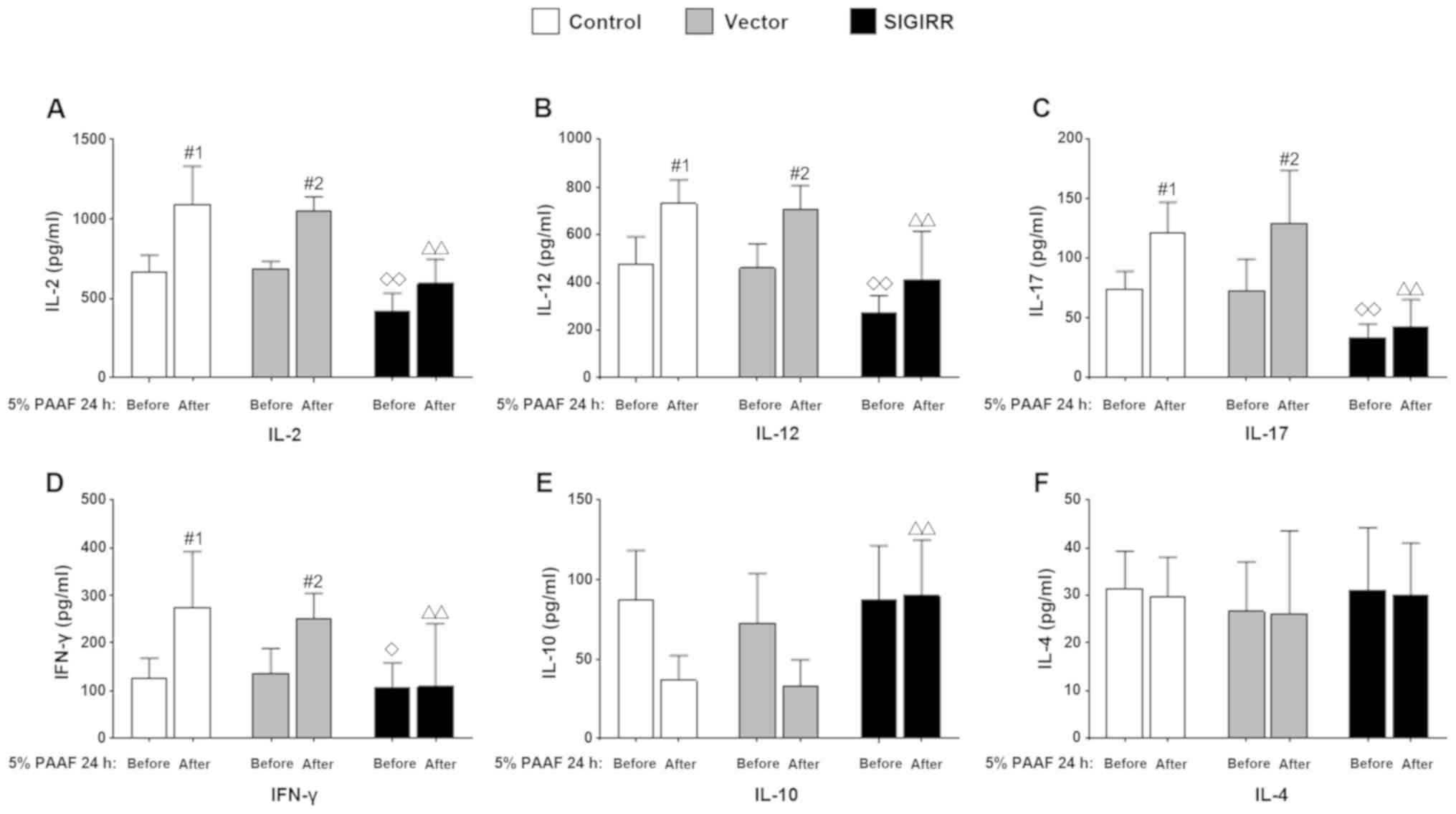
Effect of SIGIRR overexpression on
cytokine secretion in PAAF-treated macrophages
The levels of the pro-inflammatory cytokines IL-2,
IL-12, IL-17 and IFN-γ were significantly decreased in the
supernatant of PAAF-treated macrophages transfected with SIGIRR
overexpression vector (P<0.05 vs. the control group; Fig. 4A-D). Conversely, increased
concentrations of the anti-inflammatory cytokine IL-10 were
observed in the SIGIRR group with or without 5% PAAF stimulation
for 24 h (P<0.01 vs. the control or vector group with 5% PAAF
for 24 h; Fig. 4E). However, for
IL-2 and IL-12, the levels increased in the presence of PAAF. This
effect was also observed in the control group (IL-2, IL-12, IL-17,
and IFN-γ; P<0.05 vs. the control group without 5% PAAF for 24
h). However, there were no significant changes in IL-4 expression
(Fig. 4F).
Discussion
Previous studies have documented the importance of
PAAF-induced inflammatory responses in the development of acute
pancreatitis (19–22). Inflammatory responses mediated by
the TLR4 signaling pathway are associated with SAP (11,23–25).
SIGIRR overexpression inhibits TLR-induced cytokine production in
macrophages, while SIGIRR knockdown results in increased cytokine
production following TLR stimulation (13,15,26,27).
TLR-mediated activation of NF-κB promotes the transcription of
genes encoding pro-inflammatory cytokines, such as TNF-α, IL-1,
IL-6, IL-8, IL-12 and IFN-γ, their protein secretion and
inflammation (28), which is an
important mechanism of SIRS in acute pancreatitis (10).
PAAF contains high concentrations of pancreatic
enzymes, free fatty acids, inflammatory cytokines and endotoxins.
PAAF resembles the environment in the pancreas during SAP that may
contain inflammatory messengers. Thus, in the present study, it was
used to trigger TLR4 signaling/inflammatory response in macrophages
as an in vitro model of SAP (19,20).
However, whether a direct association exists between PAAF and the
activation of the TLR4 signaling pathway in patients with SAP has
remained elusive. In addition, the potential protective effects of
SIGIRR have remained to be evaluated in an in vitro model of
SAP. Therefore, the aim of the present study was to determine the
effect of PAAF on TLR4 signaling and the expression of SIGIRR in
macrophages in vitro. Furthermore, the effect of SIGIRR on
the TLR4 signaling pathway in macrophages stimulated with PAAF was
assessed. PAAF was collected aseptically from two patients with
untreated SAP who were diagnosed using the criteria of the 2012
revision of the Atlanta Classification of Acute Pancreatitis
(1). The effect of PAAF treatment
on SIGIRR-overexpressing murine RAW264.7 macrophages was evaluated.
The major results of the present study were that TLR4, MyD88,
IRAK-1 and TRAF-6 mRNA levels in RAW264.7 cells increased following
PAAF stimulation, compared with those in untreated cells. However,
these molecules were significantly downregulated in
SIGIRR-overexpressing RAW264.7 cells stimulated with PAAF as
compared with those in untransfected and untreated cells.
Furthermore, the concentrations of IL-2, IL-12, IL-17 and IFN-γ in
the culture supernatant decreased, while IL-10 levels
increased.
PAAF contributes to the pathogenesis of acute
pancreatitis, as it stimulates the synthesis of TNF-α by pancreatic
acinar cells and induces macrophage activation (19). PAAF increases the production of
pro-inflammatory cytokines by interfering with pro-inflammatory and
anti-inflammatory signaling pathways (29) involved in the pathogenesis of SIRS
and MODS in patients with acute pancreatitis (30). In addition, the expression of TLR4
in pancreatic tissue was significantly increased in murine models
of SAP, compared with control mice receiving normal saline
injections (31,32). However, the molecular mechanisms
underlying the role of SIGIRR in regulating the TLR4 signaling
pathway in an in vitro SAP model of PAAF-treated macrophages
have remained largely elusive. In the present study, TLR4 and key
downstream molecules were upregulated in PAAF-stimulated
macrophages, except for TLR2. Awla et al (33) demonstrated that TLR4, but not TLR2,
regulates inflammation and tissue damage in individuals with acute
pancreatitis induced by a retrograde infusion of taurocholate. A
similar explanation for this result is that PAAF may be involved in
the development of acute pancreatitis and may be involved in the
activation of the TLR4, but not TLR2, signaling pathway. In the
present study, the expression of key molecules in the TLR4
signaling pathway was upregulated after treatment with PAAF up to a
concentration of 5%. The expression of TLR4 also increased in a
time-dependent manner. Thus, the ascites from patients with SAP
activate the TLR signaling pathway in macrophages, which may have
an important role in the development of SIRS and SAP.
Of note, SIGIRR mRNA was downregulated in
PAAF-stimulated macrophages. Previous studies have suggested that
SIGIRR acts as a key modulator of inflammation in several
pathological conditions, ranging from infectious and sterile
inflammation, to autoimmunity and cancer-related inflammation
(16,34). SIGIRR exerts a protective effect on
keratitis and gastroenteritis by negatively regulating the
activation of inflammatory cytokines in the TLR4 signaling pathway
(35,36). PAAF contains a large number of
ligands for TLRs, such as IL-1 and LPS, which are able to directly
activate TLR signaling pathways (20). In this study, PAAF could inhibit
the negative regulation of TLR signaling pathways by downregulating
the expression of SIGIRR, which may be one of the mechanisms
underlying the early hyperactivation of TLR signaling pathways in
patients with SAP. Thus, it may be hypothesized that SIGIRR was
able to attenuate PAAF-mediated activation of the TLR4 signaling
pathway in the present study.
In SIGIRR-overexpressing macrophages, the mRNA
levels of TLR4 and key downstream molecules in the signaling
pathway decreased following PAAF treatment. Furthermore, the levels
of the pro-inflammatory factors IL-2, IL-12, IL-17, and IFN-γ also
decreased as compared with those in untransfected cells. IL-2,
IL-12 and IFN-γ promote T-helper type 1 (Th1) cell-mediated immune
responses and exacerbate inflammation, which has a role in the
onset and development of SAP (37). IL-17 levels increase significantly
in individuals with early-stage acute pancreatitis and are a
prognostic factor for SAP (38).
IL-10 is an anti-inflammatory cytokine that inhibits the production
of the pro-inflammatory cytokines IL-1, TNF-α and IL-6 in response
to LPS stimulation but also inhibits the activation of Th1 cells
and the synthesis of IL-6 and IL-8 by downregulating major
histocompatibility complex class II on monocytes and macrophages,
thus inhibiting a cascade of inflammatory mediators and blocking or
delaying the occurrence of SIRS (39). Thus, the results of the present
study suggest that ascites from patients with SAP promote the
production of pro-inflammatory cytokines and inhibit the secretion
of anti-inflammatory cytokines in macrophages. In addition, SIGIRR
is involved in acute pancreatitis and negatively regulates the TLR4
signaling pathway, which is partially consistent with the results
of a previous study by our group (40).
The expression levels of TLR4 and its key downstream
molecules did not increase with the increase in the concentration
of PAAF. When the concentration of PAAF used to stimulate cells
reached 10%, the expression level of these factors did not
increase, but decreased. The potential explanation for this result
is that high concentrations of PAAF may inhibit the proliferation
and function of macrophages. Hyperactivation of the TLR signaling
pathway has been demonstrated to occur in the early stages of SAP
in several studies (11,31). In the present study, PAAF
stimulation for 6 h altered the expression of TLR4, but not that of
SIGIRR, MyD88, IRAK-1 and TRAF-6, further confirming an important
role of TLR4 in pancreatitis. In addition, the expression of these
molecules was increased by PAAF in the control and
vector-transfected cells but decreased in SIGIRR-overexpressing
RAW264.7 cells, suggesting that SIGIRR influence the expression of
molecules in the TLR4 signaling pathway in the absence of exogenous
stimulation. Thus, the inhibitory effect of SIGIRR on mediators of
the TLR signaling pathway was hypothesized to mainly occur in the
presence of exogenous stimuli. These results further support the
hypothesis that SIGIRR suppresses inflammatory responses and
alleviates the inflammatory injury in tissues by exerting a
negative regulatory effect on pancreatitis.
Of note, the present preliminary study has certain
limitations. The major limitation is that the protein
concentrations of key molecules in the TLR4 signaling pathway were
not detected. In addition, the mRNA expression levels of TLR4 and
key downstream molecules and concentrations of inflammatory factors
were not detected in the peripheral blood from the patients.
Despite these limitations, the present study certainly improves the
current understanding of the association between PAAF and the TLR4
signaling pathway, as well as the role of SIGIRR in the
PAAF-mediated activation of the TLR4 signaling pathway.
In summary, the present study was the first in
vitro study on the effects of SIGIRR as an inhibitor of the
TLR4 signaling pathway in macrophages stimulated with PAAF. The
negative regulatory effect of SIGIRR on the in vitro model
of PAAF-mediated activation of the TLR4 signaling pathway may be a
mechanism of action and a potential therapeutic target for SAP.
Further studies are necessary to determine whether the function of
endogenously expressed SIGIRR is compromised in patients with acute
pancreatitis. An understanding of the mechanisms by which SIGIRR
regulates inflammation may lead to novel therapeutic opportunities
for immune-mediated diseases, including acute pancreatitis.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Key
Research and Development Program of China (grant. no.
2016YFC1302201), the National Natural Science Foundation of China
(grant. no. 81860099), the Science and Technology Supporting
Projects of Jiangxi Province (grant. no. 20151BDH80076), the
Science and Technology Supporting Projects of Jiangxi Province
(grant. no. 20161BBG70113) and the Leading Talent Training Plan of
the Gan-Po Outstanding Talents 555 Project of Jiangxi Province
(grant. no. 20100361).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and YX designed the study. RZ and CS performed
the experiments. LL, QL and NZ collected and interpreted the
patient data regarding severe acute pancreatitis and
pancreatitis-associated ascitic fluid. CS performed the statistical
analysis. CS wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First Affiliated Hospital of Nanchang University
(approval no. EL20180027) and was carried out in compliance with
the Helsinki Declaration. Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IL-1R
|
IL-1 receptor
|
|
SIGGIR
|
single immunoglobulin and
Toll-interleukin-1 receptor domain containing molecule
|
|
TLR
|
Toll-like receptor
|
|
MyD88
|
myeloid differentiation factor 88
|
|
IRAK-1
|
IL-1R-associated kinase-1
|
|
TRAF-6
|
TNF receptor-associated factor-6
|
|
MODS
|
multiple organ dysfunction
syndrome
|
|
PAAF
|
pancreatitis-associated ascitic
fluid
|
|
SAP
|
severe acute pancreatitis
|
|
SIRS
|
systemic inflammatory response
syndrome
|
References
|
1
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS; Acute Pancreatitis
Classification Working Group, : Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:2851–111. 2013.
View Article : Google Scholar
|
|
2
|
Roberts SE, Akbari A, Thorne K, Atkinson M
and Evans PA: The incidence of acute pancreatitis: Impact of social
deprivation, alcohol consumption, seasonal and demographic factors.
Aliment Pharmacol Ther. 38:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gou S, Yang C, Yin T, Liu T, Wu H, Xiong
J, Yang Z and Wang C: Percutaneous catheter drainage of
pancreatitis-associated ascitic fluid in early-stage severe acute
pancreatitis. Pancreas. 44:1161–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Xia C, Zhang L, Zhang Y, Liu Z and
Qiu F: Peritoneal lavage for severe acute pancreatitis: A
meta-analysis and systematic review. Pancreas. 45:806–813. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Z, Yu SH, Liang HY, Zhou J, Yan HT,
Chen T, Cheng L, Ning L, Wang T, Luo ZL, et al: Outcome benefit of
abdominal paracentesis drainage for severe acute pancreatitis
patients with serum triglyceride elevation by decreasing serum
lipid metabolites. Lipids Health Dis. 15:1102016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Nardo D: Toll-like receptors:
Activation, signalling and transcriptional modulation. Cytokine.
74:181–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molteni M, Gemma S and Rossetti C: The
role of toll-like receptor 4 in infectious and noninfectious
inflammation. Mediators Inflamm. 2016:69789362016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skjesol A, Yurchenko M, Bösl K,
Gravastrand C, Nilsen KE, Grøvdal LM, Agliano F, Patane F, Lentini
G, Kim H, et al: The TLR4 adaptor TRAM controls the phagocytosis of
Gram-negative bacteria by interacting with the Rab11-family
interacting protein 2. PLoS Pathog. 15:e10076842019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Cui Y, Cao F, Qin Y, Li W and
Zhang J: Ganglioside GD1a suppresses LPS-induced pro-inflammatory
cytokines in RAW264.7 macrophages by reducing MAPKs and NF-κB
signaling pathways through TLR4. Int Immunopharmacol. 28:136–145.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minkov GA, Halacheva KS, Yovtchev YP and
Gulubova MV: Pathophysiological mechanisms of acute pancreatitis
define inflammatory markers of clinical prognosis. Pancreas.
44:713–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li G, Wu X, Yang L, He Y, Liu Y, Jin X and
Yuan H: TLR4-mediated NF-κB signaling pathway mediates
HMGB1-induced pancreatic injury in mice with severe acute
pancreatitis. Int J Mol Med. 37:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong K: Curcumin mediates a protective
effect via TLR-4/NF-κB signaling pathway in rat model of severe
acute pancreatitis. Cell Biochem Biophys. 73:175–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drexler SK, Kong P, Inglis J, Williams RO,
Garlanda C, Mantovani A, Yazdi AS, Brennan F, Feldmann M and
Foxwell BM: SIGIRR/TIR-8 is an inhibitor of Toll-like receptor
signaling in primary human cells and regulates inflammation in
models of rheumatoid arthritis. Arthritis Rheum. 62:2249–2261.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fawley J, Cuna A, Menden HL, McElroy S,
Umar S, Welak SR, Gourlay DM, Li X and Sampath V:
Single-immunoglobulin interleukin-1-related receptor regulates
vulnerability to TLR4-mediated necrotizing enterocolitis in a mouse
model. Pediatr Res. 83:164–174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wald D, Qin J, Zhao Z, Qian Y, Naramura M,
Tian L, Towne J, Sims JE, Stark GR and Li X: SIGIRR, a negative
regulator of Toll-like receptor-interleukin 1 receptor signaling.
Nat Immunol. 4:920–927. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Molgora M, Supino D, Mantovani A and
Garlanda C: Tuning inflammation and immunity by the negative
regulators IL-1R2 and IL-1R8. Immunol Rev. 281:233–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landahl P, Ansari D and Andersson R:
Severe acute pancreatitis: Gut barrier failure, systemic
inflammatory response, acute lung injury, and the role of the
mesenteric lymph. Surg Infect (Larchmt). 16:651–656. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Huang Z, Lin N, Liu W, Yang G, Wu
D, Xiao H, Sun H and Tang L: Abdominal paracentesis drainage
protects rats against severe acute pancreatitis-associated lung
injury by reducing the mobilization of intestinal XDH/XOD. Free
Radic Biol Med. 99:374–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh A, Shimosegawa T, Masamune A, Fujita
M, Koizumi M and Toyota T: Ascitic fluid of experimental severe
acute pancreatitis modulates the function of peritoneal
macrophages. Pancreas. 19:268–275. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramudo L, Manso MA and De Dios I: Biliary
pancreatitis-associated ascitic fluid activates the production of
tumor necrosis factor-alpha in acinar cells. Crit Care Med.
33:143–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gutierrez PT, Folch-Puy E, Bulbena O and
Closa D: Oxidised lipids present in ascitic fluid interfere with
the regulation of the macrophages during acute pancreatitis,
promoting an exacerbation of the inflammatory response. Gut.
57:642–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pérez S, Finamor I, Martí-Andrés P, Pereda
J, Campos A, Domingues R, Haj F, Sabater L, de-Madaria E and Sastre
J: Role of obesity in the release of extracellular nucleosomes in
acute pancreatitis: A clinical and experimental study. Int J Obes
(Lond). 43:158–168. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hietaranta A, Mustonen H, Puolakkainen P,
Haapiainen R and Kemppainen E: Proinflammatory effects of
pancreatic elastase are mediated through TLR4 and NF-kappaB.
Biochem Biophys Res Commun. 323:192–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue J and Habtezion A: Carbon
monoxide-based therapy ameliorates acute pancreatitis via TLR4
inhibition. J Clin Invest. 124:437–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan LF, Yu L, Wang LM, He JT, Sun JL, Wang
XB, Wang H, Bai ZH, Feng H and Pei HH: Augmenter of liver
regeneration (ALR) regulates acute pancreatitis via inhibiting
HMGB1/TLR4/NF-κB signaling pathway. Am J Transl Res. 10:402–410.
2018.PubMed/NCBI
|
|
26
|
Xiao H, Yin W, Khan MA, Gulen MF, Zhou H,
Sham HP, Jacobson K, Vallance BA and Li X: Loss of single
immunoglobulin interleukin-1 receptor-related molecule leads to
enhanced colonic polyposis in Apc(min) mice. Gastroenterology.
139:574–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Zepp J, Bulek K and Li X: SIGIRR,
a negative regulator of colon tumorigenesis. Drug Discov Today Dis
Mech. 8:e63–e69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anders HJ: Pseudoviral immunity-a novel
concept for lupus. Trends Mol Med. 15:553–561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Xu P, Hou YQ, Xu K, Li QH and
Huang L: Pancreatitis-associated ascitic fluid induces
proinflammatory cytokine expression in THP-1 cells by inhibiting
anti-inflammatory signaling. Pancreas. 42:855–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hongliang T, Rong Z, Xiaojing W, Rao S,
Lun L, Jinhui T, Nong C and Kehu Y: The effects of continuous blood
purification for SIRS/MODS patients: A systematic review and
meta-analysis of randomized controlled trials. ISRN Hematol.
2012:9867952012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharif R, Dawra R, Wasiluk K, Phillips P,
Dudeja V, Kurt-Jones E, Finberg R and Saluja A: Impact of toll-like
receptor 4 on the severity of acute pancreatitis and
pancreatitis-associated lung injury in mice. Gut. 58:813–819. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sawa H, Ueda T, Takeyama Y, Yasuda T,
Shinzeki M, Nakajima T and Kuroda Y: Role of toll-like receptor 4
in the pathophysiology of severe acute pancreatitis in mice. Surg
Today. 37:867–873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Awla D, Abdulla A, Regnér S and Thorlacius
H: TLR4 but not TLR2 regulates inflammation and tissue damage in
acute pancreatitis induced by retrograde infusion of taurocholate.
Inflamm Res. 60:1093–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riva F, Bonavita E, Barbati E, Muzio M,
Mantovani A and Garlanda C: TIR8/SIGIRR is an interleukin-1
receptor/Toll like receptor family member with regulatory functions
in inflammation and immunity. Front Immunol. 3:3222012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang X, Hazlett LD, Du W and Barrett RP:
SIGIRR promotes resistance against Pseudomonas aeruginosa
keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4
signaling. J Immunol. 177:548–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stahl M, Ries J, Vermeulen J, Yang H, Sham
HP, Crowley SM, Badayeva Y, Turvey SE, Gaynor EC, Li X and Vallance
BA: A novel mouse model of Campylobacter jejuni
gastroenteritis reveals key pro-inflammatory and tissue protective
roles for Toll-like receptor signaling during infection. PLoS
Pathog. 10:e10042642014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong D, Zhang P, Ji D, Chen Z, Li W, Li J,
Li L and Liu Z: Improvement of immune dysfunction in patients with
severe acute pancreatitis by high-volume hemofiltration: A
preliminary report. Int J Artif Organs. 33:22–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dai SR, Li Z and Zhang JB: Serum
interleukin 17 as an early prognostic biomarker of severe acute
pancreatitis receiving continuous blood purification. Int J Artif
Organs. 38:192–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Keceli M, Kucuk C, Sozuer E, Kerek M, Ince
O and Arar M: The effect of interleukin-10 on acute pancreatitis
induced by cerulein in a rat experimental model. J Invest Surg.
18:7–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu J, Chen Y, Liu D, Liu W, Hu S, Zhou N
and Xie Y: Ectopic expression of SIGIRR in the colon ameliorates
colitis in mice by downregulating TLR4/NF-κB overactivation.
Immunol Lett. 183:52–61. 2017. View Article : Google Scholar : PubMed/NCBI
|