Introduction
Pancreatic cancer (PC) is a lethal malignancy, with
a 91% mortality rate within five years of diagnosis worldwide
(1). Early stage diagnosis of PC
is rare, and the majority of patients are diagnosed when the tumor
has already metastasized, thereby precluding a curative surgical
resection (2,3). The high invasiveness of PC cells and
limited treatment options are the major factors underlying its poor
prognosis (4,5). Therefore, the present study aimed to
understand the mechanism underlying PC metastasis to identify novel
biomarkers, and improve the chances of early diagnosis and a
favorable clinical outcome.
Exosomes (EXOs) are small vesicles 40–100 nm in
diameter that either bleb directly from the plasma membrane or are
released when multivesicular bodies fuse with the cell membrane
(6,7). Studies have demonstrated that EXOs
serve important roles in intercellular signaling, and trafficking
of proteins and nucleic acids (8,9).
Certain exosomal proteins secreted from cancer cells actively
participate in tumor initiation, progression and metastasis
(10). In addition, cancer cell
EXOs shuttle signaling molecules that reflect their tissue origins
(11,12). For example, ovarian cancer cell
EXOs promote T cell expansion and protect them against apoptosis
(13), whereas mutant KRAS
shuttled by colon cancer EXOs induce malignant transformation of
wild-type KRAS colon epithelial cells (14). Therefore, the potential clinical
applications of EXOs, for example as biomarkers of various types of
pathology, have gained attention in recent years (15,16).
The present study demonstrated that receptor
tyrosine kinase Eph receptor A2 (EphA2)-expressing EXOs secreted by
the highly metastatic PC cell line Panc-1 notably altered the
function of low-metastatic BxPC-3 cells. The present results not
only provide novel insights into the pathological role of EphA2 in
PC but also provide an experimental basis for targeting EXO_EphA2
for early diagnosis of PC.
Materials and methods
Cell culture
The PC cell lines Panc-1 and BxPC-3 were purchased
from the American Type Culture Collection. Both cell lines were
maintained in RPMI 1640 (HyClone; Cytiva) with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C under 5%
CO2.
Isolation of EXO
Cells were cultured in 1640 medium supplemented with
10% FBS until they reached 70% confluence. After removing the
medium and washing the cells three times with PBS, fresh serum-free
1640 medium (HyClone; Cytiva) was added, and the cells were
cultured for another 48 h at 37°C. The medium was aspirated and
centrifuged for 10 min at 300 × g to remove the dead cells at room
temperature, and then at 9,000 × g for 30 min to remove the cell
debris at room temperature. The supernatant was centrifuged at
100,000 × g for 2 h at 4°C, and the pelleted exosomes were
resuspended in PBS, then ultracentrifuged at 100,000 × g for 2 h at
4°C. The last two steps were repeated, and the purified EXOs were
characterized. The protein concentration of EXOs was determined
using a BCA kit (Pierce; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions.
Electron microscopy
A total of 10 µl purified EXOs (100 µg/ml) was
placed on non-glow-discharged carbon-coated grids (300 mesh;
Beijing XXBR Technology Co., Ltd.) for 10 min with 4%
paraformaldehyde at room temperature and negatively stained with 10
µl 2% uranyl acetate for 1 min at room temperature. The excess
solution was removed by wicking onto filter paper, and dried grids
were viewed under a FEI/Philips CM12 transmission electron
microscope (TEM) operating at 80 KeV (magnification, ×100).
Western blotting
Total protein was extracted from cells using RIPA
buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.). The
protein concentration was determined using a bicinchoninic acid
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts
of cell lysate (WCLs; 20 µg) or exosome lysate (EXOs; 10 µg) were
resolved by Tris-Bis gel electrophoresis using a 4–15% gradient gel
(Bio-Rad Laboratories, Inc.). The protein bands were transferred to
a nitrocellulose membrane and blocked with 5% skimmed milk in PBS
with 0.05% Tween-20 (PBST) at room temperature for 2 h. The blots
were probed with anti-tumor susceptibility gene 101 protein
(Tsg101; cat. no. ab30871; Abcam, 1:800), anti-CD63 (cat. no.
ab68418; Abcam; 1:500), anti-Giantin (GM130; cat. no. ab187514;
Abcam; 1:1,000) and anti-EphA2 (cat. no. ab73254; Abcam; 1:1,000)
antibodies for overnight at 4°C. After washing four times with
PBST, the membranes were incubated with the secondary antibody
(1:5,000) for 1 h at room temperature. Protein bands were
visualized using ECL Western Blotting Detection Reagent (cat. no.
G075; Applied Biological Materials, Inc.). The band intensities
were quantified by ImageJ software (version 1.8.0; National
Institutes of Health).
Wound healing assay
Panc-1 (1×105) and BxPC-3
(5×105) cells were seeded in 6-well plates and cultured
for 24 h to ~100% confluence. The monolayers were gently scratched
with 1 ml pipette tips to create a ‘wound’, and the wells were
gently washed with 1X PBS to remove the dislodged cells. The cells
were cultured for 48 h with 20 µg/ml Panc-1 or
Panc-1EphA2− EXO or 0.5 µg/ml recombinant EphA2 that
were added twice (once each at 0 and 24 h) at 37°C. The scratched
areas were photographed at 0, 24 and 48 h post-wounding using a
confocal microscope (magnification, ×200), and the area was
measured using ImageJ (version 1.8.0; National Institutes of
Health). Both cell lines were maintained in RPMI 1640 with 1% FBS.
The experiment was repeated three times.
Protein identification
The protein extracts were resolved by 15% SDS-PAGE,
and the gel was stained with silver nitrate. The appropriate gel
pieces were cut and digested, and the resulting peptides were
analyzed by positive-ion data-dependent micro-capillary liquid
chromatography-tandem mass spectrometry (LC-MS/MS) using an LTQ 2D
Linear Ion Trap Mass Spectrometer (Thermo Fisher Scientific, Inc.)
as per standard protocol (17).
The initial MS scan was followed by eight further MS/MS scans. The
proteins were identified by aligning the sequences against the
UniProt database (www.uniprot.org) using the Sequest algorithm in
Proteomics Browser software (version 2.3.0; Thermo Fisher
Scientific, Inc.).
EXO internalization
Panc-1 EXOs were labeled with EXO-Red (cat. no.
EXOC300A-1; System Biosciences, LLC) according to the
manufacturer's instructions. BxPC-3 cells were plated at
2×104 cells/well on 8-well chamber slides for 24 h and
supplemented with 20 µg/ml EXO-Red-labeled Panc-1 EXOs. For
microscopy studies, cells were washed three times with PBS,
incubated with 4% paraformaldehyde for 15 min at 25°C, and
incubated in DAPI/PBS solution (1:1,000) for 5 min at 25°C. EXOs
were visualized under a laser scanning confocal microscope
(magnification, ×40; FV-100; Olympus Corporation).
Generation of stable EphA2-knockdown
Panc-1 cell line
The plasmid pGLVH1/GFP+Puro, EphA2_shRNA
(5′-GAACTTCAACACAGCCTGG-3′) and packaging vector PG-P1-VSVG were
prepared by Shanghai GenePharma Co., Ltd., and extracted with high
purity and no endotoxins. 293T cells (density, 60–70%) were
co-transfected with RNAi-mate (Shanghai GenePharma Co., Ltd.).
After 6 h of transfection, they were replaced with a complete
culture medium. After 72 h of culture, the supernatant of cells was
collected with ultracentrifugation at 40,000 × g for 2 h at 4°C and
concentrated to obtain a high titer lentivirus concentrate for
infecting target cells.
Panc-1 cells were transduced with lentivirus
(1×109 TU/ml; dilution, 1:10) and 5 µg/ml polybrene
(Sigma-Aldrich; Merck KGaA) for 24 h at 37°C, then the stable
transfectants were selected in the presence of 10 µg/ml puromycin
(Invitrogen; Thermo Fisher Scientific, Inc.) for 2 days. Decreased
EphA2 protein expression levels were confirmed via western blotting
as aforementioned. The stable EphA2-Panc-1 cells were maintained in
complete RPMI-1640 supplemented with 2 µg/ml puromycin at 37°C.
Patients and specimens
Serum samples were obtained from 40 patients with PC
(median age, 30–70 years; 36 males and 34 females; 20 patients each
at stage I+II and stage III+IV) and 30 healthy controls at the
Tianjin Medical University Cancer Institute and Hospital (Tianjin,
China) between March 2019 and September 2019. The disease was
staged according to the American Joint Committee on Cancer tumor,
node, metastasis classification (18). All experiments involving human
specimens were performed in accordance with the 1964 Declaration of
Helsinki ethical standards and approved by the Research Ethics
Committee of Tianjin Medical University Cancer Institute and
Hospital (approval no. bc2019112). Written informed consent was
obtained from all patients prior to participation.
Quantification of exosomal EphA2 by
ELISA
EXOs were precipitated from 100 µl patient serum,
using ExoQuick (System Biosciences, LLC) according to the
manufacturer's protocols. EXO pellets were then suspended in PBS
and analyzed using an EphA2 ELISA kit (cat. no. DYC3035-2; R&D
Systems, Inc.) according to the manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
of three biological repeats. Multiple groups were compared using
one- or two-way ANOVA with a post hoc Bonferroni test. All
statistical analyses were performed using GraphPad Prism (version
5.0; GraphPad Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
Validation of EXOs derived from PC
cells
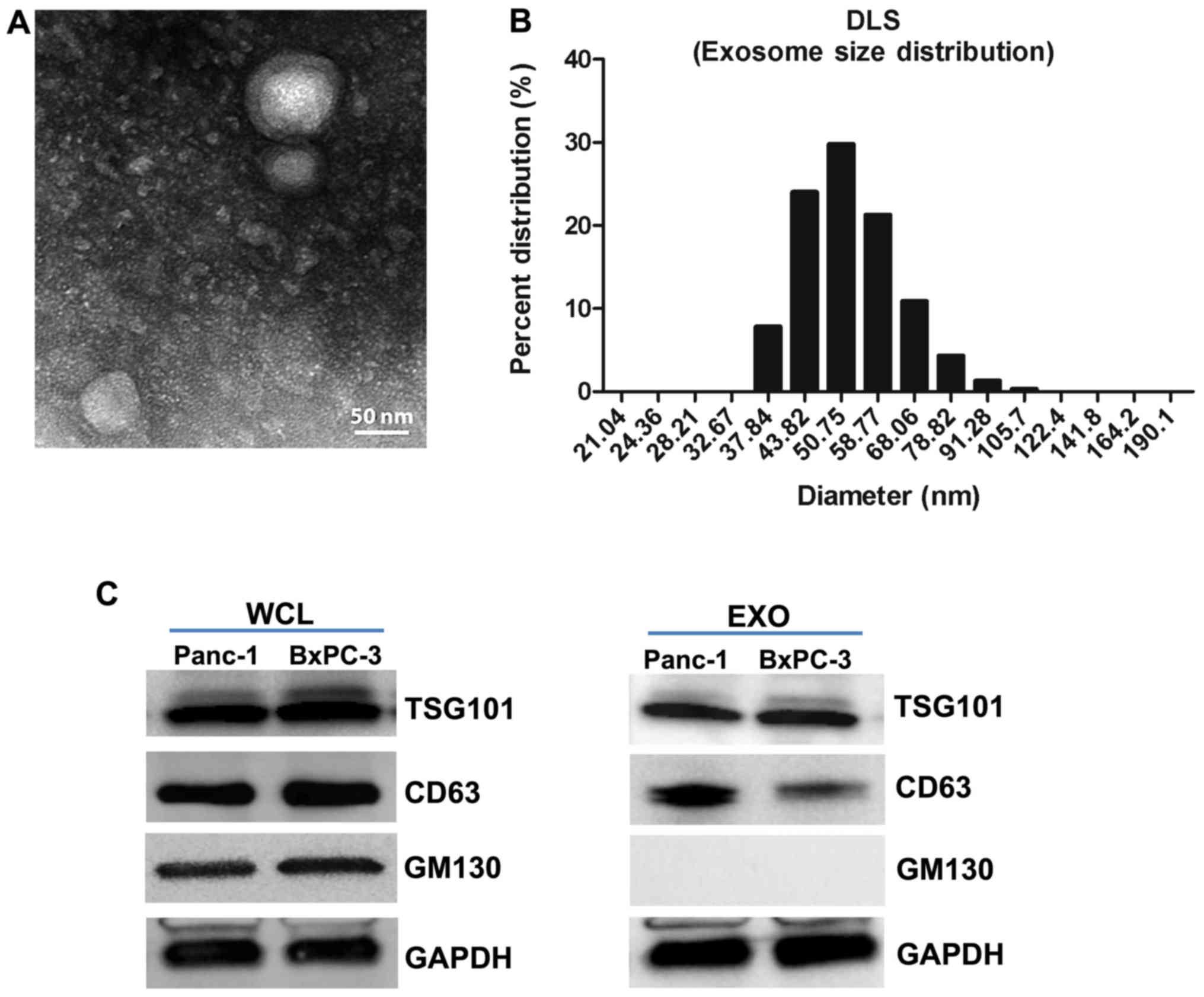
EXOs released from PC cells were purified and
characterized in terms of morphology and exosomal marker expression
levels. The distinctive EXO structure was observed via TEM
(Fig. 1A), and dynamic light
scattering showed a typical size range of 30–100 nm (Fig. 1B). Furthermore, the purified EXOs
expressed Tsg101 and CD63, but not the Golgi protein GM130
(Fig. 1C). Thus, these results
confirmed the purity of EXO preparation.
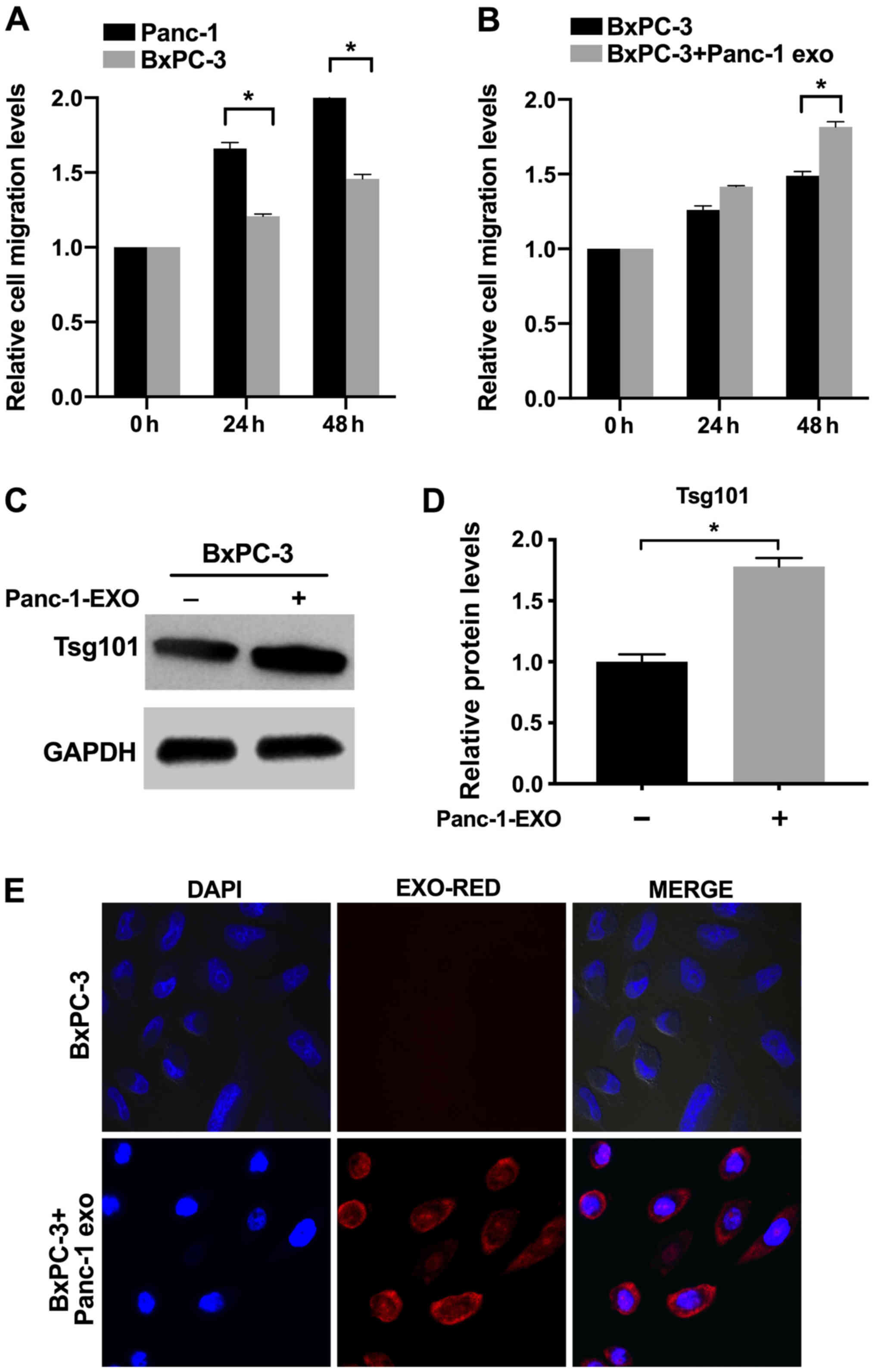
Panc-1 EXOs enhance in vitro migration
of recipient cells
Panc-1 cells exhibited significantly higher in
vitro migration ability compared with BxPC-3 cells (Figs. 2A and S1). In order to determine whether
Panc-1-derived EXOs enhance the migration of BxPC-3 cells, the
latter were incubated with EXOs for 48 h. The percentage of
migrating BxPC-3 cells increased significantly at 48 h of exposure
to Panc-1 EXOs compared with control cells (Figs. 2B and S1). By contrast, EXOs derived from
BxPC-3 cells had no effect on migration (Fig. S2). These results indicated that
EXOs secreted by metastatic PC cells enhanced the migration of
recipient cells, which is likely by transporting relevant
factors.
Panc-1 EXOs are internalized by
recipient cells
BxPC-3 cells incubated with Panc-1 EXOs exhibited
increased expression of Tsg101 (Fig.
2C and D). The internalization of Panc-1 EXOs by BxPC-3 cells
was tracked by labeling EXOs with EXO-Red. Recipient cells
internalized these EXOs within 10 h (Fig. 2E).
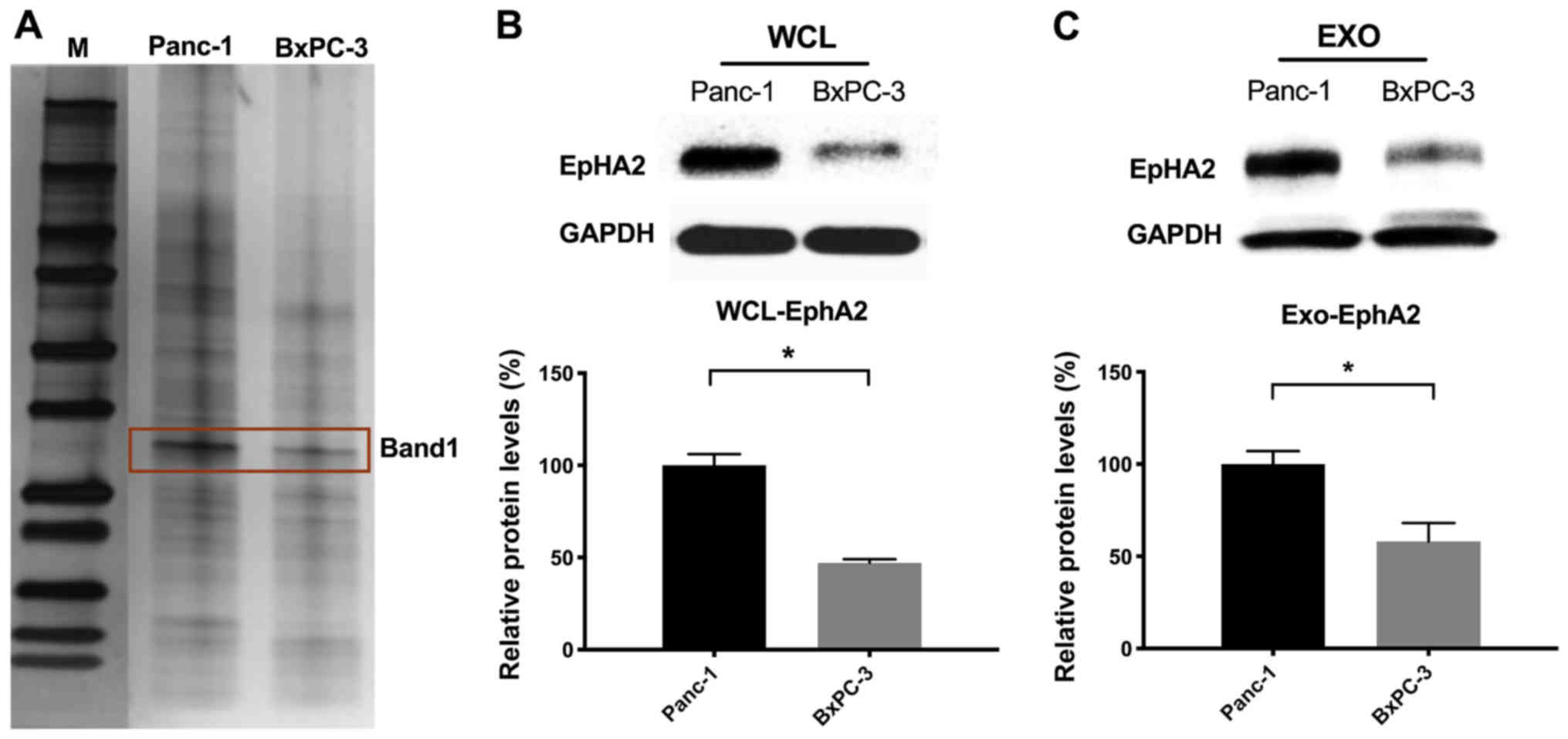
Analysis of exosomal proteins
EXOs mediate intercellular communication via
horizontal transfer of proteins (19). Therefore, it was hypothesized that
EXOs derived from Panc-1 and BxPC-3 cells have distinct proteomes
and are enriched in metastasis-associated proteins. In order to
validate exosomal protein composition, lysates of purified EXOs
derived from Panc-1 and BxPC-3 cells were resolved via SDS-PAGE.
The silver-stained gels showed distinct protein profiles of EXOs
(Fig. 3A), which was confirmed via
LC-MS/MS analysis (Table SI). The
expression levels of EphA2 were significantly higher in both the
lysates and EXOs of Panc-1 compared with BxPC3 cells (Fig. 3B and C) in terms of the LC-MS/MS
score. Taken together, these results indicated that the metastatic
effects of Panc-1 EXOs are likely mediated by EphA2.
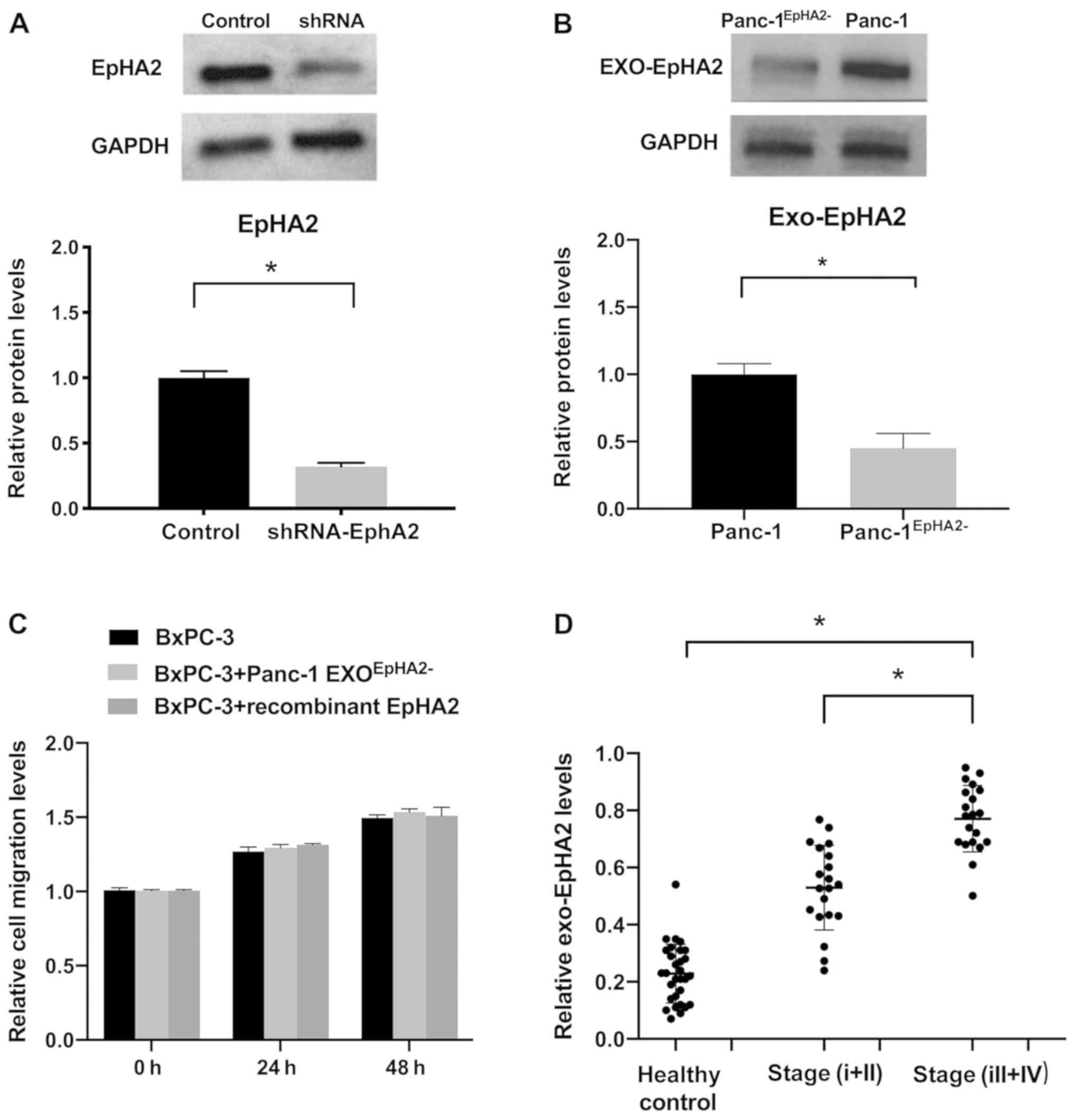
EXO_EphA2 mediates migration of PC
cells in vitro
In order to further validate the metastatic function
of EXO_EphA2, a stable EphA2− Panc-1 cell line was
generated, which exhibited a 70% decrease in EphA2 expression
levels compared with control cells (Fig. 4A). Cell migration was significantly
decreased in Panc-1EphA2− cells compared with Panc-1
cells (Fig. S3). The EXO_EphA2
levels were also significantly decreased in Panc-1EphA2−
cells compared with Panc-1 cells (Fig.
4B). Furthermore, unlike EXOs derived from wild-type Panc-1
cells, Panc-1EphA2− EXOs did not significantly increase
the migration of BxPC-3 compared with control cells. BxPC-3 cells
incubated with recombinant EphA2 did not exhibit increased
migration, indicating that exosomal delivery is essential for the
oncogenic effects of EphA2 (Figs.
4C and S4). These results
indicated that EXO_EphA2 mediates migration of PC cells.
Circulating EXO_EphA2 is a potential
diagnostic biomarker for PC
Tumor-derived EXOs are easily detectable in
circulation, and therefore are promising diagnostic/prognostic
markers for cancer. The levels of circulating EXO_EphA2 were
significantly higher in the serum of patients with PC compared with
those in healthy controls (Fig.
4D). In addition, levels of circulating EXO_EphA2 were
significantly elevated in advanced stage (III+IV) patients compared
with those in the early stages (I+II). These results indicated that
EXO_EphA2 is a potential diagnostic biomarker for PC.
Discussion
Surgical resection is currently the most effective
method for controlling PC. However, only 9–10% of patients are
eligible for surgery due to late diagnosis (20). Therefore, the mechanisms underlying
PC progression and metastasis need to be elucidated in order to
identify novel diagnostic markers (21,22).
There is increasing interest in the molecular
biological function of EXOs in cancer. EXOs are secreted vesicles
that mediate cellular signaling and trafficking, and serve a key
role in cancer progression by transporting oncogenic factors in a
paracrine manner (23,24). Yan et al (25) demonstrated that EXOs released from
stromal cells induce Myc-dependent metabolic reprogramming and
promote tumor growth. Similarly, studies have revealed that
Wnt5b-harboring EXOs trigger cancer cell migration and
proliferation in a paracrine manner (26), and that downregulation of exosomal
C-type lectin domain family 3 member B in hepatocellular carcinoma
promotes metastasis and angiogenesis via the 5′AMP-activated
protein kinase and vascular endothelial growth factor pathways
(27). However, EXO secreted by
different types of PC cells have not yet been fully characterized.
In the present study, EXOs derived from highly metastatic Panc-1
cells enhanced the migratory capacity of low-metastatic BxPC-3
cells, which was associated with high levels of EphA2 expression.
Therefore, it was hypothesized that oncogenic EXOs may also endow
tumor cells with metastatic abilities in a paracrine manner in
situ.
EphA2 is overexpressed in melanoma, as well as
breast and lung cancer, and is associated with increased tumor
progression (28–30). In addition, EXOs released from
senescent cells promote cancer cell proliferation by transporting
EphA2, indicating its potential as a therapeutic target (31). Zhuang et al (32) further demonstrated that EphA2
mediates trastuzumab resistance in breast cancer cells. Consistent
with these previous findings, the present results revealed a
previously unknown pro-metastatic function of EphA2-enriched EXOs
in PC. In the present study, BxPC-3 cells treated with recombinant
EphA2 protein did not exhibit increased cell migration. It was
hypothesized that the fusion of EXOs with the cell membrane
transfers soluble and membrane-associated factors such as EphA2 to
recipient cells, which may not be able to internalize the naked
recombinant EphA2 protein. These results indicate that exosomal
delivery serves a key role in EphA2-mediated cell migration. Since
effective prognostic markers for PC remain elusive (33), the present findings are significant
in demonstrating the diagnostic potential of Exo_EphA2 in PC. To
the best of our knowledge, the present study is one of few to show
that direct exosomal transfer of an oncogenic factor can
phenotypically alter recipient cells. Future studies should analyze
the circulating levels of Exo_EphA2 in patients with PC to validate
its clinical value. Targeting Exo-EphA2 may represent a novel
strategy to diagnose metastasis and invasion in PC.
Although EXO_EphA2 likely plays an important role in
PC metastasis and appear to be a reliable diagnostic biomarker for
patients with PC, there are certain limitations of the present
study. In the wound healing assay, cells were maintained with 1%
FBS to avoid excessive cell death that may influence the
experimental results. Moreover, future studies need to be performed
in larger cohorts of patients with PC to validate the clinical
utility of Exo_EphA2. In addition, prospective studies are required
to further explore the potential prognostic value of EXO_EphA2 for
PC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81702996) and the
National Science Foundation of Tianjin (grant no.
18JCZDJC32600).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QW and LR conceived and designed the present study.
JZ and LW provided the study materials, collected clinical
follow-up data and performed the clinical interpretation. ZL and HF
performed the experiments. QW performed statistical analysis and
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures carried out in studies involving
human participants were approved by the Research Ethics Committee
of Tianjin Medical University Cancer Institute and Hospital
(approval no. bc2019112) and were in accordance with the 1964
Declaration of Helsinki ethical standards. Written informed consent
was obtained from all patients prior to participation, and the
study was approved by the local Ethical Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PC
|
pancreatic cancer
|
|
EphA2
|
receptor tyrosine kinase Eph receptor
A2
|
|
EXO
|
exosome
|
|
TEM
|
transmission electron microscope
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:2941–34. 2019. View Article : Google Scholar
|
|
2
|
Shahrokni A and Saif MW: Metastatic
pancreatic cancer: The dilemma of quality vs. quantity of life.
JOP. 14:391–394. 2013.PubMed/NCBI
|
|
3
|
Farrell TJ, Barbot DJ and Rosato FE:
Pancreatic resection combined with intraoperative radiation therapy
for pancreatic cancer. Ann Surg. 226:66–69. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noel M, O'Reilly EM, Wolpin BM, Ryan DP,
Bullock AJ, Britten CD, Linehan DC, Belt BA, Gamelin EC, Ganguly B,
et al: Phase 1b study of a small molecule antagonist of human
chemokine (C-C motif) receptor 2 (PF-04136309) in combination with
nab-paclitaxel/gemcitabine in first- line treatment of metastatic
pancreatic ductal adenocarcinoma. Invest New Drugs. 38:800–811.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hood JL, San RS and Wickline SA: Exosomes
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Pol E, Boing AN, Harrison P, Sturk
A and Nieuwland R: Classification, functions, and clinical
relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor DD and Gercel-Taylor C:
Exosomes/microvesicles: Mediators of cancer-associated
immunosuppressive microenvironments. Semin Immunopathol.
33:441–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corrado C, Raimondo S, Chiesi A, Ciccia F,
De Leo G and Alessandro R: Exosomes as intercellular signaling
organelles involved in health and disease: Basic science and
clinical applications. Int J Mol Sci. 14:5338–5366. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Min L, Shen J, Tu C, Hornicek F and Duan
Z: The roles and implications of exosomes in sarcoma. Cancer
Metastasis Rev. 35:377–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luga V, Zhang L, Viloria-Petit AM,
Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M and
Wrana JL: Exosomes mediate stromal mobilization of autocrine
Wnt-PCP signaling in breast cancer cell migration. Cell.
151:1542–1556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szajnik M, Czystowska M, Szczepanski MJ,
Mandapathil M and Whiteside TL: Tumor-derived microvesicles induce,
expand and up-regulate biological activities of human regulatory T
cells (Treg). PLoS One. 5:e114692010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demory Beckler M, Higginbotham JN,
Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M,
Liebler DC and Coffey RJ: Proteomic analysis of exosomes from
mutant KRAS colon cancer cells identifies intercellular transfer of
mutant KRAS. Mol Cell Proteomics. 12:343–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D,
Xu X, Zuo Y, Zhao Y, Wei YQ, et al: Exosomal tRNA-derived small RNA
as a promising biomarker for cancer diagnosis. Mol Cancer.
18:742019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma R, Huang X, Brekken RA and Schroit
AJ: Detection of phosphatidylserine-positive exosomes for the
diagnosis of early-stage malignancies. Br J Cancer. 117:545–552.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishihama Y: Proteomic LC-MS systems using
nanoscale liquid chromatography with tandem mass spectrometry. J
Chromatogr A. 1067:73–83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vyas N and Dhawan J: Exosomes: Mobile
platforms for targeted and synergistic signaling across cell
boundaries. Cell Mol Life Sci. 74:1567–1576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie F, Li C, Zhang X, Peng W and Wen T:
MiR-143-3p suppresses tumorigenesis in pancreatic ductal
adenocarcinoma T by targeting KRAS. Biomed Pharmacother.
119:1094242019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamerkar S, LeBleu VS, Sugimoto H, Yang S,
Ruivo CF, Melo SA, Lee JJ and Kalluri R: Exosomes facilitate
therapeutic targeting of oncogenic KRAS in pancreatic cancer.
Nature. 546:498–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riches A, Campbell E, Borger E and Powis
S: Regulation of exosome release from mammary epithelial and breast
cancer cells-a new regulatory pathway. Eur J Cancer. 50:1025–1034.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kucharzewska P, Christianson HC, Welch JE,
Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain
E, Bengzon J and Belting M: Exosomes reflect the hypoxic status of
glioma cells and mediate hypoxia-dependent activation of vascular
cells during tumor development. Proc Natl Acad Sci USA.
110:7312–7317. 2014. View Article : Google Scholar
|
|
25
|
Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu
J, Liu X, Chen CH, Fadare O, Pizzo DP, et al: Cancer-cell-secreted
exosomal miR-105 promotes tumour growth through the MYC-dependent
metabolic reprogramming of stromal cells. Nat Cell Biol.
20:597–609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harada T, Yamamoto H, Kishida S, Kishida
M, Awada C, Takao T and Kikuchi A: Wnt5b-associated exosomes
promote cancer cell migration and proliferation. Cancer Sci.
108:42–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai W, Wang Y, Yang T, Wang J, Wu W and Gu
J: Downregulation of exosomal CLEC3B in hepatocellular carcinoma
promotes metastasis and angiogenesis via AMPK and VEGF signals.
Cell Commun Signal. 17:1132019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brantley-Sieders DM, Zhuang G, Hicks D,
Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E,
Muraoka-Cook RS and Chen J: The receptor tyrosine kinase EphA2
promotes mammary adenocarcinoma tumorigenesis and metastatic
progression in mice by amplifying ErbB2 signaling. J Clin Invest.
118:64–78. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan YC, Srivastava S, Won BM, Kanteti R,
Arif Q, Husain AN, Li H, Vigneswaran WT, Pang KM, Kulkarni P, et
al: EPHA2 mutations with oncogenic characteristics in squamous cell
lung cancer and malignant pleural mesothelioma. Oncogenesis.
8:492019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miao B, Ji Z, Tan L, Taylor M, Zhang J,
Choi HG, Frederick DT, Kumar R, Wargo JA, Flaherty KT, et al: EPHA2
is a mediator of vemurafenib resistance and a novel therapeutic
target in melanoma. Cancer Discov. 5:274–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takasugi M, Okada R, Takahashi A, Virya
Chen D, Watanabe S and Hara E: Small extracellular vesicles
secreted from senescent cells promote cancer cell proliferation
through EphA2. Nat Communication. 8:157292016. View Article : Google Scholar
|
|
32
|
Zhuang G, Brantley-Sieders DM, Vaught D,
Yu J, Xie L, Wells S, Jackson D, Muraoka-Cook R, Arteaga C and Chen
J: Elevation of receptor tyrosine kinase EphA2 mediates resistance
to trastuzumab therapy. Cancer Res. 70:299–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan J, Wei Q, Koay EJ, Liu Y, Ning B,
Bernard PW, Zhang N, Han H, Katz MH, Zhao Z and Hu Y:
Chemoresistance transmission via exosome-mediated EphA2 transfer in
pancreatic cancer. Theranostics. 8:5986–5994. 2018. View Article : Google Scholar : PubMed/NCBI
|