Introduction
Cisplatin is a typical chemotherapeutic drug that is
widely used in the treatment of a variety of solid tumors (1,2).
However, the dose-limiting side effects of high concentrations of
cisplatin, such as renal damage, neurotoxicity and ototoxicity can
restrict its clinical application (3–5).
Among patients that receive cisplatin chemotherapy, >90% may end
up with ear-associated adverse effects (3,6).
However, currently no effective treatment exists to avoid or reduce
ototoxicity induced by cisplatin (5,7–9).
Therefore, the prevention of ototoxicity induced by cisplatin has
become a major focus in tumor therapy.
To the best of our knowledge, the mechanism of the
aforementioned process has not yet been elucidated. However,
additional evidence has demonstrated that overproduction of
reactive oxygen species (ROS) serves a key role in this process
(10,11). High accumulation of cisplatin in
cochlear tissues may reduce the expression levels of nuclear factor
erythroid 2-related factor 2 (Nrf2), a well-known transcription
factor responsible for the antioxidant-pro-oxidant balance
(12,13). This is caused by modulation of the
expression levels of various antioxidant enzymes, including heme
oxygenase 1 (HO-1), NAD(P)H quinone dehydrogenase 1 (NQO1) and
glutamate-cysteine ligase catalytic (GCLC) (14–16).
This pathological process disrupts the balance between ROS
production, and antioxidant enzyme activity and expression, leading
to auditory cell injury and apoptosis (17). Therefore, upregulation of Nrf2
expression and the increase in antioxidant enzyme expression has
become an important target for treating cisplatin-induced
ototoxicity.
Panax notoginseng Saponins (PNS) are
compounds that are extracted from Panax notoginseng (Sanqi).
Panax notoginseng is routinely used to treat acute cerebral
infarction and acute myocardial infarction (18,19).
A number of previous reports have demonstrated that PNS can inhibit
cisplatin-induced nephrotoxicity and neurotoxicity (20–23).
However, whether PNS can inhibit cisplatin-induced ototoxicity
remains unknown. A previous study indicated that PNS could regulate
Nrf2 antioxidant signaling via the AKT signaling pathway (24). Therefore, the present study
examined whether PNS could protect against ototoxicity induced by
cisplatin via activation of the AKT/Nrf2 signaling pathway. HEI-OC1
cells were incubated with cisplatin with or without pretreatment of
PNS and subsequently cell viability and apoptotic rate were
determined. Additionally, the associated signaling mechanisms were
examined.
Materials and methods
Chemicals and materials
PNS was obtained from Chengdu Manst Biotechnology
Co., Ltd. (www.cdmust.com). and dissolved in DMSO
(cat. no. A1222; purity ≥98%). House Ear Institute-Organ of Corti 1
(HEI-OC1) cell lines derived from the auditory organ of a
transgenic mouse were obtained from House Ear Institute. The
antibodies for active caspase 3, Nrf2, NQO1 and Bcl-2 were obtained
from Abcam. The antibodies for HO-1, GCLC, AKT, and
phosphorylated-AKT (p-AKT) were obtained from Abcam. The Hoechst
33258 staining kit was obtained from Biyuntian Biotechnology Co.,
Ltd. Cisplatin and the propidium iodide (PI) staining kit were
obtained from Sigma-Aldrich; Merck KGaA. The oxygen species assay
kit was purchased from Nanjing KeyGen Biotech Co., Ltd. The p-AKT
inhibitor LY294002 was purchased from Selleck Chemicals. Cells were
treated with 25 µmol/l LY294002 for 24 h at room temperature before
transfection. The culture media were obtained from Gibco; Thermo
Fisher Scientific, Inc.
Cell culture and cell viability
assay
The HEI-OC1 cell line is a frequently used model of
ototoxicity assessment and expresses several molecular
characteristics of Corti sensory cells (25,26).
This cell line was selected for use in the present study. The cells
were cultivated in complete medium containing high-glucose DMEM
(Beijing Solarbio Science & Technology Co., Ltd.) supplemented
with 10% FBS (Beijing Solarbio Science & Technology Co., Ltd.)
and 50 U/ml interferon-γ without antibiotics. Cells were cultured
at 33°C in the presence of 10% CO2 in a humidified
incubator (permissive conditions). Cell toxicity was assessed as
follows: A total of 5×103 HEI-OC1 cells/well in 100 µl
complete medium were seeded in a 96-well plate and cultured at 33°C
in the presence of 10% CO2. The cells were pretreated
with different concentrations of PNS (0, 25, 50, 100, 200, 400 and
800 µg/ml) for 2 h at room temperature. Subsequently, 20 µM
cisplatin was added to each well of the plate. Following a 24-h
cultivation, 10 µl Cell Counting Kit-8 (CCK-8; cat. no. CK-4;
Dojindo Molecular Technologies, Inc.) solution was added to each
well and incubated for an additional 30 min, according to the
manufacturer's protocol. A microplate reader (Lab Systems Multiskan
Ascent 354; MTX Lab Systems, Inc.) was used to detect cell
viability at 450 nm. Finally, the appropriate doses of PNS were
selected for further in vitro studies depending on the
initial toxicity data.
Hoechst 33258 and PI double
staining
A total of 1×105 HEI-OC1 cells were
diluted in 4 ml complete medium and incubated in 6-well plates with
100 µg/ml PNS for 2 h prior to treatment with cisplatin (20 µM) at
33°C in a humidified 10% CO2 environment for 24 h.
Following two washes with PBS, the cells were fixed with 4%
paraformaldehyde for 30 min at 4°C, and stained with 2 µg/ml
Hoechst 33258 and 1 µg/ml PI for 20 min in the dark at 4°C.
Finally, following two washes with PBS, the cells were visualized
using a Leica DMi8 fluorescence microscope (magnification, ×200;
Leica Microsystems, Inc.). The highest emission was recorded at
~460 nm for PI staining and at 485 nm for Hoechst dyes. The amount
of dead cells was quantified using ImageJ software version 1.8.0
(National Institutes of Health) and calculated as follows: Amount
of Hoechst-positive cells/amount of PI-positive cells.
TUNEL staining
To assess the DNA fragmentation using TUNEL, cells
were fixed with 1% formaldehyde for 10 min at room temperature.
Following incubated with 100 µg/ml PNS for 2 h prior to treatment
with cisplatin (20 µM) at 33°C in a humidified 10% CO2
environment for 24 h, a total of 1×105 HEI-OC1 cells
were incubated with 20 µg/ml proteinase K for 60 min at room
temperature. The slides were then rinsed with PBS for 3 min, dried
and incubated in 20 µl TUNEL reaction mixture for 1 h at room
temperature. Subsequently, cells were treated with 2%
H2O2 for 30 min at room temperature. After
washing with PBS, sections were incubated with 150 U/ml
anti-digoxigenin peroxidase conjugate for another 30 min at room
temperature in a humidified atmosphere. Finally, sections were
stained with 10 µg/ml diaminobenzene in the dark for 10 min at room
temperature and counterstained with 20 µg/ml hematoxylin at room
temperature for 10 min. Cell nuclei were stained with 0.1 µg/ml
DAPI at 37°C for another 10 min. After being washed with PBS for 3
min, 1% ammonia (cat. no. SJ00815F17008; Sigma-Aldrich; Merck
KGaA), and rehydrated using different concentration of alcohol, the
sections were sealed and detected with a fluorescent microscope
(magnification, ×200) with VECTASHIELD mounting media (Vector
Laboratories, inc.) for fluorescence microscope. TUNEL positive
(apoptotic) cells were quantified by counting amber-colored cells
in 10 fields (6,000 µm2/field). The specimens were
visualized using a Leica confocal laser scanning microscope and
processed with Photoshop CS6 (Adobe Systems, Inc.).
Determination of intracellular
ROS
A total of 1×105 HEI-OC1 cells were
diluted in 4 ml complete medium and cultured in 6-well plates. The
cells were pre-incubated with 100 µg/ml PNS for 2 h prior to
incubation with cisplatin (20 µM) at 33°C in a humidified 10%
CO2 environment for 24 h. Subsequently, the cells were
washed with cold PBS twice and incubated with
2,7-dichlorofluorescein diacetate (DCFH-DA; Invitrogen; Thermo
Fisher Scientific, Inc.) for 20 min at room temperature. The cells
were harvested into 1.5 ml tubes using trypsin-EDTA and washed with
PBS twice.
Flow cytometry analysis
The quantification of cell death was determined via
flow cytometry using the Annexin V-FITC apoptosis detection kit
according to the manufacturer's protocol (BD Pharmingen; BD
Bioscience) on a BD FACSCalibur flow cytometer (BD Biosciences) at
an excitation and emission wavelength of 488 and 525 nm,
respectively. Briefly, (10,000 events per sample) DCFH-DA-treated
cells were seeded into each Petri dish (size, 30 mm) and after a 24
h incubation, various concentrations of the test compound (20 µM
CP, 100 µg/ml PNS or 20 µM CP + 100 µg/ml PNS) were added and
incubated for 24, 48 and 72 h at room temperature. The cells were
washed with PBS, suspended in Annexin V binding buffer and then
added to an Annexin V-FITC solution and propidium iodide (PI) for
10 min at room temperature. The percentage of apoptotic cells was
calculated using CellQuest software version 5.1 (Becton, Dickinson
and Company). Data are presented as the percentage of Annexin
V-stained cells, and all experiments were performed in triplicate.
Morphological changes were imaged under a phase contrast microscope
(magnification, ×200; Olympus Corporation).
Western blot analysis
Total protein was extracted from cells using the
Cell Total Protein Extraction kit (Sigma-Aldrich; Merck KGaA). The
concentration of the proteins was measured using the bicinchoninic
acid method. The 20 µg/lane protein samples were separated by 10%
SDS-PAGE and analyzed by electrophoresis. The proteins were
transferred to PVDF membranes and blocked in 3% BSA (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Subsequently, the
membranes were incubated at 4°C overnight with the following
primary antibodies: Active caspase-3 (dilution, 1:2,000; rabbit;
cat. no. ab214430; Abcam), Bcl-2 (dilution, 1:1,000; rabbit; cat.
no. ab182858; Abcam), GCLC (dilution, 1:2,000; rabbit; cat. no.
ab190685; Abcam), NQO1 (dilution, 1:2,000; rabbit; cat. no.
ab80588; Abcam), HO-1 (dilution, 1:10,000; rabbit; cat. no.
ab68477; Abcam), Nrf2 (dilution, 1:2,000; rabbit; cat. no. ab62352;
Abcam), AKT (dilution, 1:2,000; rabbit; cat. no. ab179463; Abcam),
p-AKT (dilution, 1:2,000; rabbit; cat. no. ab192623; Abcam) or
GAPDH (dilution, 1:3,000; rabbit; cat. no. ab181602; Abcam). The
next morning, the membranes were incubated with secondary
antibodies conjugated to horseradish peroxidase IgG (dilution,
1:2,000; cat. no. ab181658; Abcam) for 1 h. Finally, the proteins
were visualized using the WesternBright™ ECL kit (cat. no.
E-IR-R301; Elabscience, Inc.) and analyzed using ImageJ software
version 1.8.0 (National Institutes of Health).
Statistical analysis
SPSS v22.0 software (IBM Corp.) was used for the
statistical analysis and GraphPad Prism 6 software (GraphPad
Software, Inc.) was used for the generation of statistical charts.
All data are presented as the mean ± standard error of the mean.
All the experiments in this study were conducted in triplicate.
Statistical analysis was performed by one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
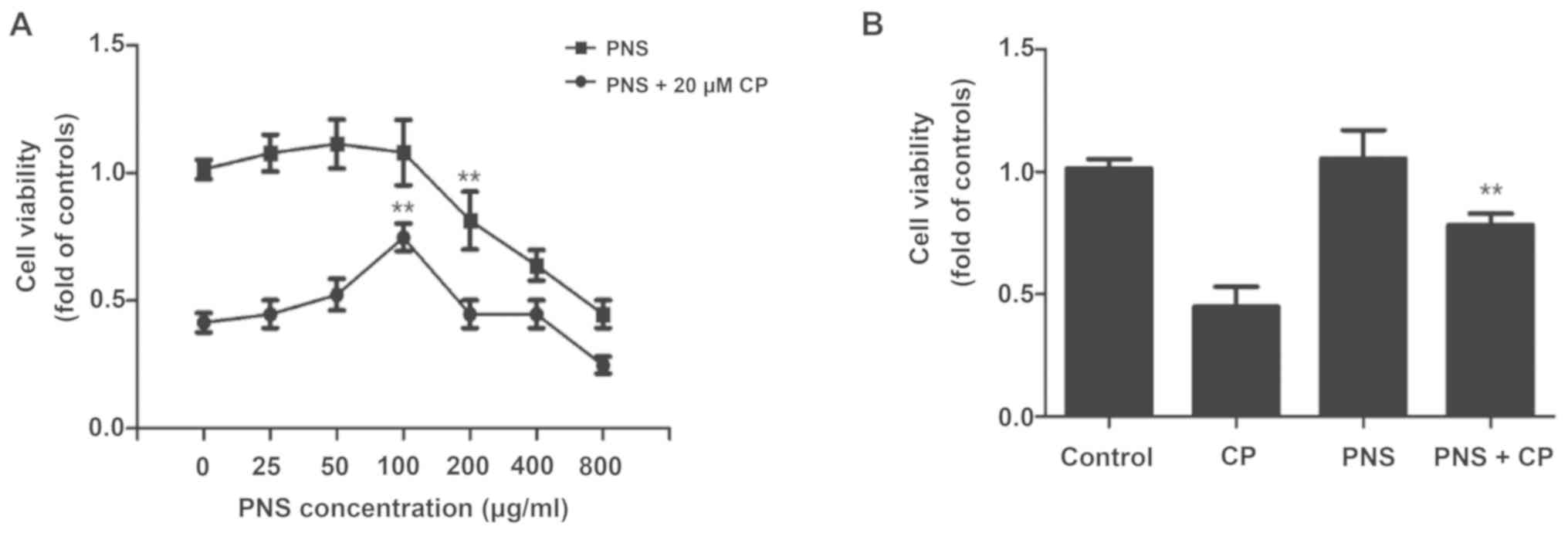
PNS prevents cisplatin-induced
cytotoxicity
As shown in Fig.
1A, at concentrations up to 100 µg/ml PNS, the cell viability
of HEI-OC1 was not significantly affected. However, there was a
significant variation between 100 and 200 µg/ml PNS. The results of
the cell viability assay indicated that in the presence or absence
of cisplatin, the viability of HEI-OC1 cells was not affected by
pretreatment with PNS concentrations <100 µg/ml. Therefore, 100
µg/ml PNS could be used in the subsequent experiments in order to
avoid toxicity. Furthermore, after treated with 20 µM CP, it was
found that 100 µg/ml PNS was able to prevent cisplatin-induced
cytotoxicity in HEI-OC1 cells (Fig.
1B; 0.42 ± 0.11 vs. 0.71 ± 0.19; P<0.01). Therefore, this
concentration (100 µg/ml PNS and 20 µM/l CP) was selected for use
in subsequent experiments.
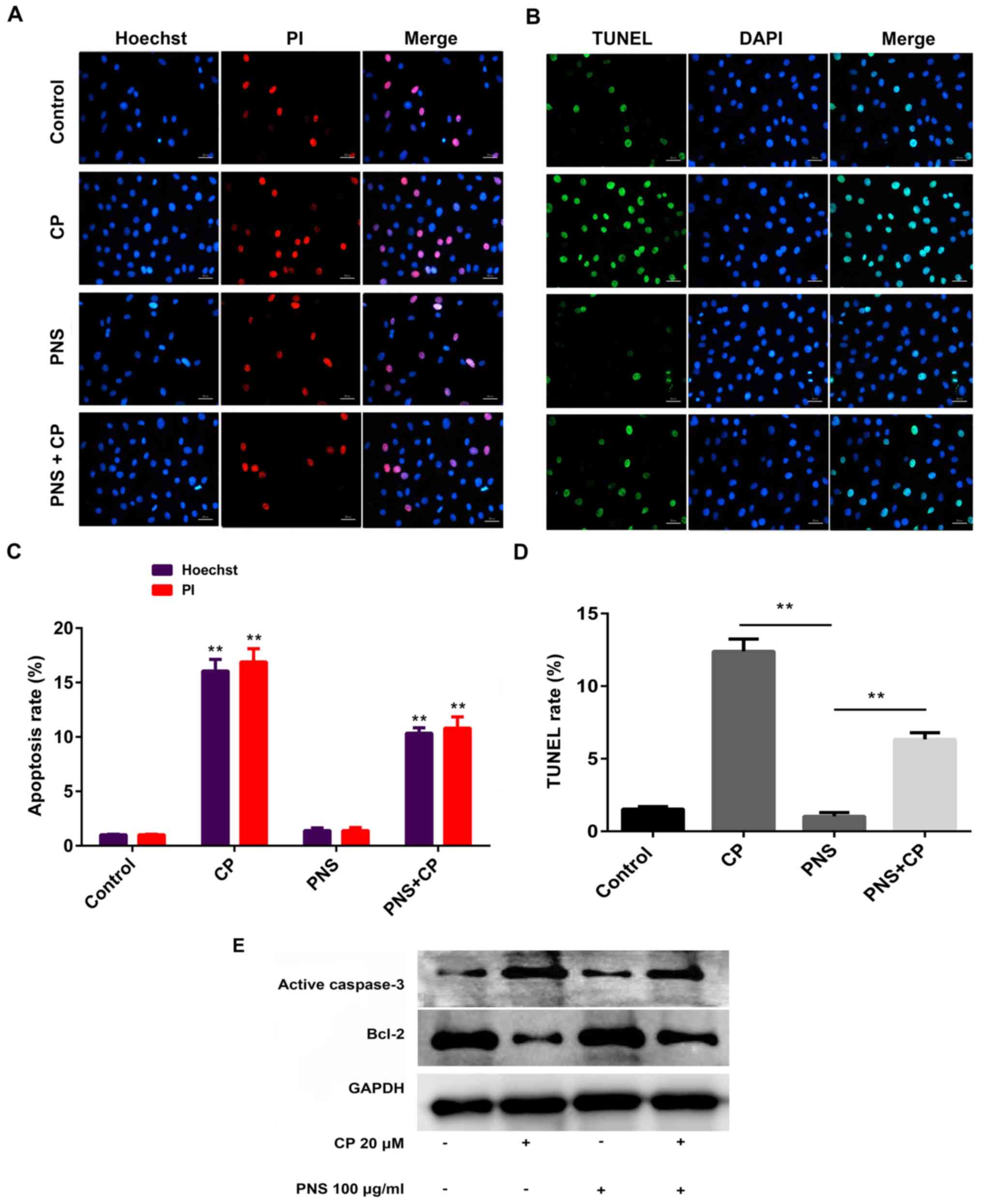
PNS reduces cisplatin-mediated
apoptosis in HEI-OC1 cells
The ImageJ software version 1.8.0 (National
Institutes of Health) was used to measure the rate of cell
apoptosis. The induction of HEI-OC1 cell apoptosis was not evident
in the control group. Following exposure to cisplatin, the number
of HEI-OC1 cells that had undergone apoptosis was significantly
increased compared with control or PNS group (Fig. 2A-D). This effect was significantly
relieved following pretreatment of the cells with PNS (Fig. 2A-D). To further verify the
anti-apoptotic effect of PNS, western blotting was used to assess
the expression levels of apoptosis-associated proteins and the
results indicated that HEI-OC1 cells treated with cisplatin
exhibited increased expression levels of active caspase-3 and
reduced Bcl-2 expression levels compared with the corresponding
levels noted in the control group (Fig. 2E).
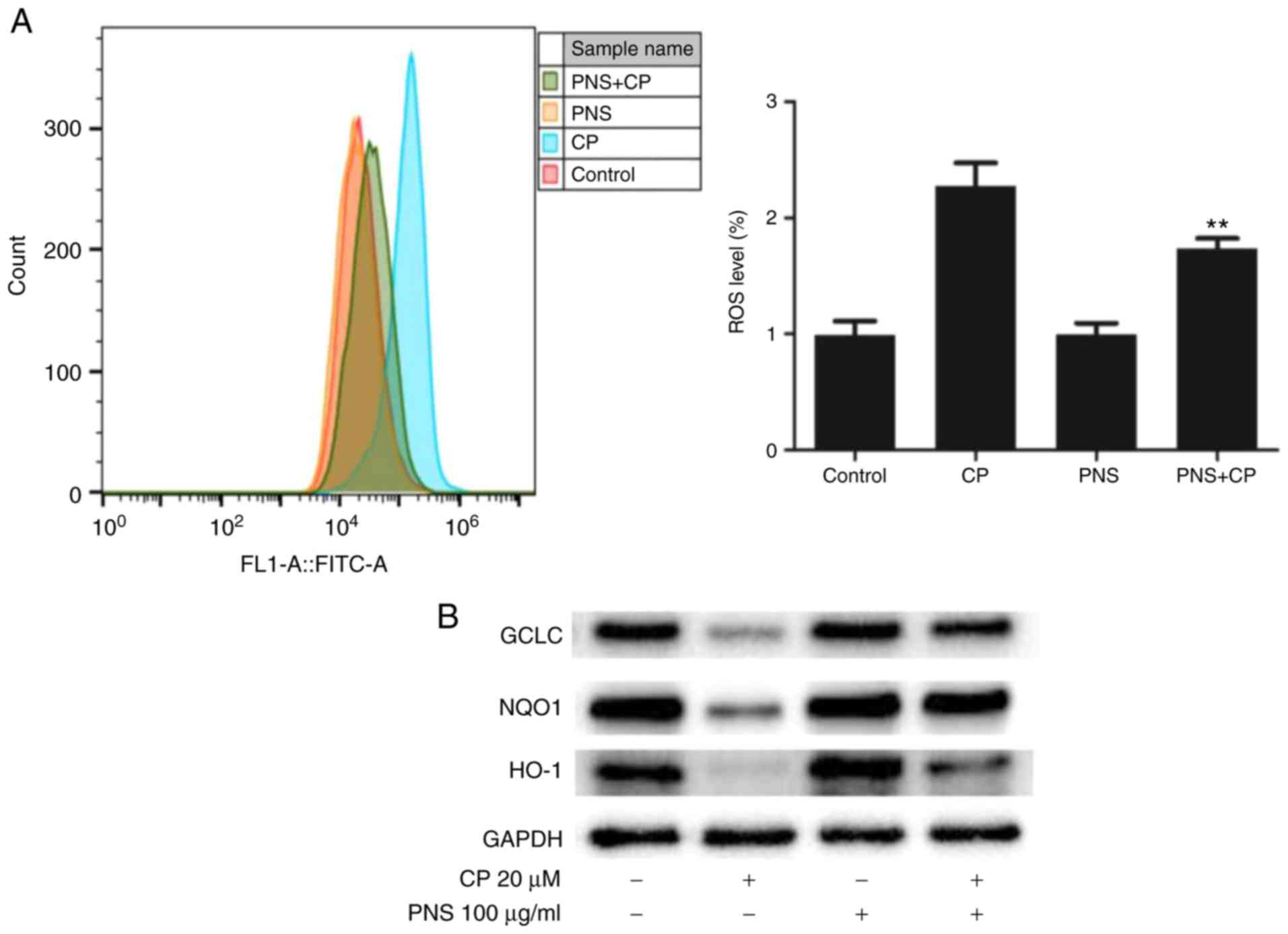
PNS attenuates cisplatin-mediated ROS
production and increases antioxidant enzyme expression
To further investigate the mechanism by which PNS
attenuated apoptosis induction by cisplatin in HEI-OC1 cells,
changes in the production of ROS and in the expression levels of
antioxidant enzymes were examined. The ROS levels in the CP group
was higher compared with the PNS + CP, PNS or control groups
(Fig. 3). This suggested that PNS
could significantly reduce the increase in ROS levels caused by
cisplatin. Western blot analysis indicated that cisplatin could
reduce the expression levels of GCLC, NQO1 and HO-1 (Fig. 3B). These results indicated that PNS
could alleviate cisplatin-induced cell damage and apoptosis by
restoring the balance between oxidative stress and the expression
levels of antioxidant enzymes.
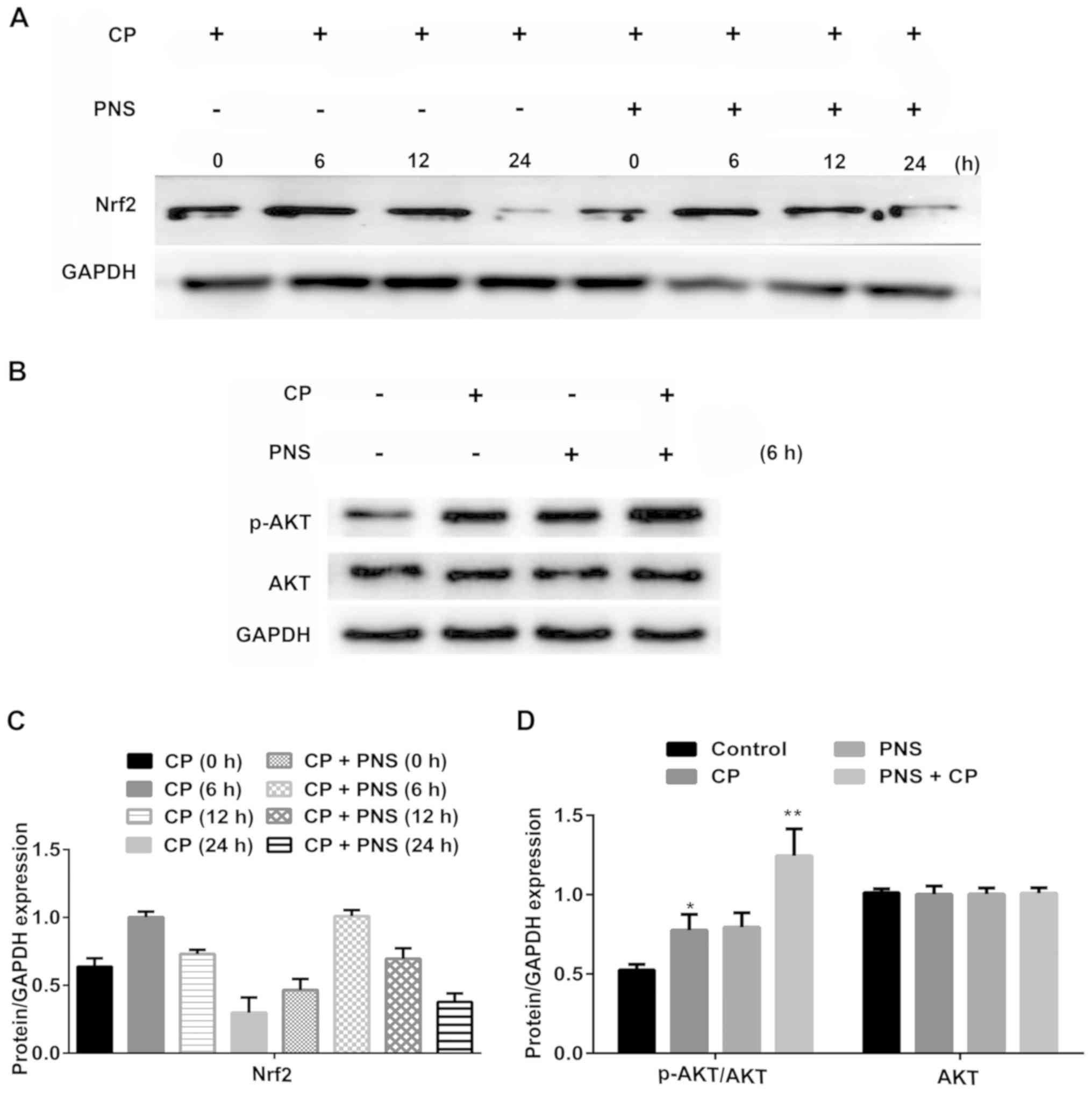
PNS increases Nrf2 expression by
upregulating AKT phosphorylation in HEI-OC1 cells
A recent study has demonstrated that the AKT-Nrf2
axis regulates the expression of antioxidant enzymes (27). Initially, the effects of PNS
pretreatment on Nrf2 expression were examined. Following cisplatin
administration, Nrf2 expression in HEI-OC1 cells has no significant
variation at 6 h, whereas it was markedly decreased at 12 and 24 h
(Fig. 4A and C). However, HEI-OC1
cells pretreated with PNS expressed lower levels of Nrf2 at 6 and
12 h compared with the cisplatin group. In addition, the
phosphorylation levels of AKT were examined after 6 h of PNS
treatment of cells (Fig. 4B and
D). PNS treatment increased AKT phosphorylation levels induced
by cisplatin at 6 h. These results indicated that the protective
effect of PNS on cisplatin-induced cytotoxicity may be achieved via
the AKT/Nrf2 axis.
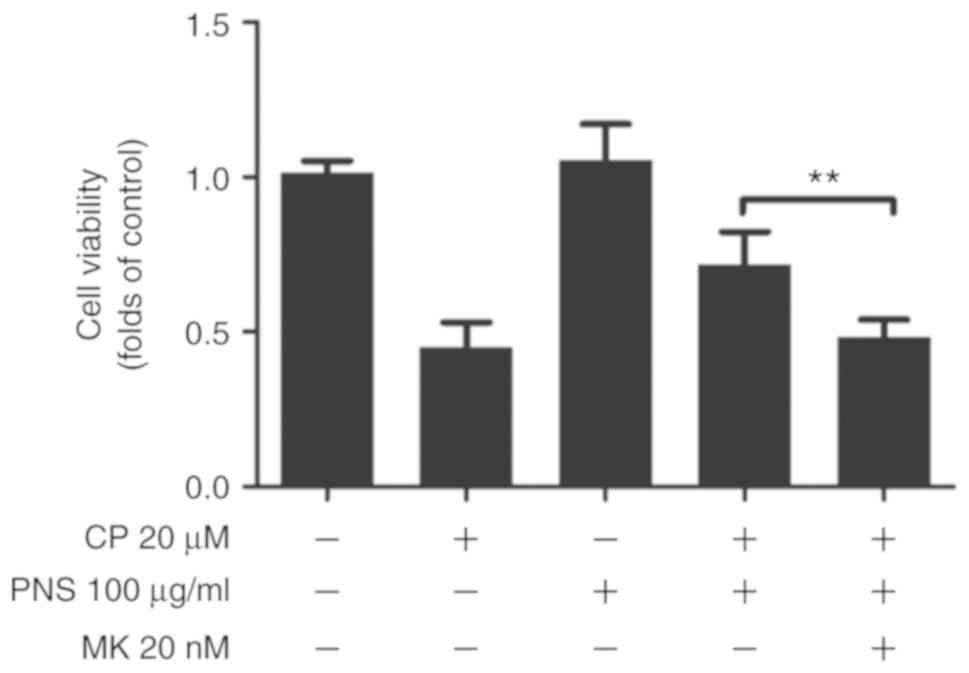
Protective effects of PNS on HEI-OC1
cells are blocked by AKT inhibition
To demonstrate that the inhibition of
cisplatin-induced cytotoxicity by PNS was mediated via the AKT/Nrf2
axis, the AKT inhibitor LY294002 was used. Treatment of the cells
with MK2002 blocked the protective effect of PNS on
cisplatin-induced cytotoxicity (0.43±0.16 vs. 0.76±0.17, P<0.01;
Fig. 5).
Discussion
Cisplatin is a complex of heavy metal platinum and
was officially approved for clinical chemotherapy in the United
States in 1972 (28,29). As one of the most widely used
broad-spectrum antitumor drugs in clinical settings, cisplatin
exerts potent antitumor activity and is widely used to treat
malignant tumors of the head and neck (1,30,31).
However, cisplatin causes ototoxicity that mainly manifests as
deafness (2,3,32).
This adverse effect limits its clinical application (2,3,32).
Deafness is usually the most apparent cause of bilateral hearing
impairment (33). As the dose of
cisplatin increases, hearing loss can gradually develop from the
low frequency region and affect the language frequency, eventually
leading to permanent hearing loss (31). Accumulating evidence has
demonstrated that cisplatin primarily destroys hearing by inducing
auditory cell apoptosis (31–33).
In the present study, the auditory HEI-OC1 cell line was used.
Following culture with cisplatin, the viability of HEI-OC1 cells
was reduced and the apoptotic rate was increased, which was
consistent with observations from other studies (34,35).
PNS is an active ingredient extracted from Panax
notoginseng (Sanqi). It contains >20 types of dammarane-type
saponins and is prepared as a Xueshuantong capsule injection
(36,37). PNS is commonly used to treat acute
cerebral infarction and acute myocardial infarction (18,19).
Previous studies have demonstrated that PNS can inhibit
cisplatin-induced nephrotoxicity and neurotoxicity (20–23).
However, whether PNS can inhibit ototoxicity induced by cisplatin
has not yet been elucidated. The present study demonstrated that
PNS attenuated apoptosis induced by cisplatin in HEI-OC1 cells and
increased their viability, which was consistent with the changes in
the apoptosis-associated proteins Bcl-2 and caspase-3. The results
indicated that PNS exhibited a therapeutic effect on ototoxicity
induced by cisplatin.
At present, the underlying mechanisms of ototoxicity
induced by cisplatin have not been fully demonstrated, whereas the
excessive induction of oxidative stress and auditory cell apoptosis
caused by increased free radical production has been widely
accepted (3,10,38).
Previous studies have reported that cisplatin can produce an excess
of free radicals and deplete the activity of antioxidant enzyme
systems in the cochlea, resulting in DNA, lipid and protein damage,
as well as hair cell apoptosis, via the mitochondrial signaling
pathway (38–40). Therefore, the restoration of the
balance between oxidative stress and the expression of antioxidant
enzymes is considered an important way to treat cisplatin-induced
ototoxicity (4). Previous studies
have demonstrated that PNS is dependent on potent free radical
scavenging and antioxidant action in order to exert its therapeutic
effects on cardiovascular and cerebrovascular conditions (36,41,42).
In the present study, PNS administration could inhibit ROS
production in HEI-OC1 cells exposed to cisplatin. Further
experiments revealed that the protein expression levels of the
antioxidant enzymes, including HO-1, NQO1 and GCLC, were markedly
increased following PNS pretreatment, which indicated that
remission of HEI-OC1 cell apoptosis may be associated with
PNS-induced restoration of ROS balance.
Nrf2 is a transcription factor that exerts its
function by regulating the redox homeostatic gene network (43). A previous study demonstrated that
the activated Nrf2 protein binds to the antioxidant response
element and increases the transcription of various cytoprotective
genes that encode antioxidant proteins, including NQO1, HO-1 and
GCLC (27). The increase in the
expression levels of antioxidant enzymes, including GCLC, NQO1 and
HO-1, could counteract the induction of oxidative stress (11–14).
These enzymes reduce the intracellular ROS levels and, therefore,
protect cells from oxidative damage (44,45).
Additionally, the Nrf2 protein can upregulate the expression levels
of Bcl-2 in order to prevent cell apoptosis (31). In the present study, PNS increased
the levels of AKT phosphorylation and Nrf2 expression. These
effects were reversed by treatment of the cells with an AKT
inhibitor. These results suggested that PNS protected against
ototoxicity induced by cisplatin via the activation of the
AKT/Nrf2/ signaling-mediated redox pathway.
However, in vivo experiments should be
conducted in the future in order to further discover the underlying
effects of PNS on regulation of cisplatin-induced ototoxicity in
auditory cells, which is considered to be a limitation of the
present study.
In conclusion, the present study indicated the
potential mechanism of the anti-cytotoxic and anti-apoptotic
effects of PNS against cisplatin-induced ototoxicity. This
mechanism was mediated by activation of the AKT/Nrf2 signaling
pathway. The results indicated that PNS may function as a promising
candidate in the prevention and treatment of cisplatin-induced
ototoxicity.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81670931),
a grant from the Six Talent Peaks Project of Jiangsu Province
(grant no. WSW-075), the Medical Science and Technology Development
Key Foundation Nanjing Department of Health (grant no. ZKX17019)
and the Jiangsu Provincial Key Medical Discipline (grant no.
ZDXKB2016015), China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BF, ZBL, LSX, LYL, WYZ, JL, YHD and WDS made
substantial contributions to conception, design and acquisition of
data. BF, LYL, WYZ and JL contributed to manuscript drafting,
analysis and interpretation of data. YHD and WDS were involved in
manuscript revising, general supervision. All authors read and
approved the final manuscript, and agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy of integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanjing Drum Tower Hospital, The Affiliated Hospital
of Nanjing University Medical School and in accordance with the
Declaration of Helsinki. Written consent was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:3533–584.
2007. View
Article : Google Scholar
|
|
2
|
Chovanec M, Abu Zaid M, Hanna N, El-Kouri
N, Einhorn LH and Albany C: Long-term toxicity of cisplatin in
germ-cell tumor survivors. Ann Oncol. 28:2670–2679. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rybak LP, Mukherjea D, Jajoo S and
Ramkumar V: Cisplatin ototoxicity and protection: Clinical and
experimental studies. Tohoku J Exp Med. 219:177–186. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheth S, Mukherjea D, Rybak LP and
Ramkumar V: Mechanisms of cisplatin-induced ototoxicity and
otoprotection. Front Cell Neurosci. 11:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kursunluoglu G, Taskiran D and Kayali HA:
The investigation of the antitumor agent toxicity and capsaicin
effect on the electron transport chain enzymes, catalase activities
and lipid peroxidation levels in lung, heart and brain tissues of
rats. Molecules. 23:32672018. View Article : Google Scholar
|
|
6
|
Breglio AM, Rusheen AE, Shide ED,
Fernandez KA, Spielbauer KK, McLachlin MM, Hall MD, Amable L and
Cunningham LL: Cisplatin is retained in the cochlea indefinitely
following chemotherapy. Nat Commun. 8:16542017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moroso MJ and Blair RL: A review of
cis-platinum ototoxicity. J Otolaryngol. 12:365–379.
1983.PubMed/NCBI
|
|
8
|
Sakamoto M, Kaga K and Kamio T: Extended
high-frequency ototoxicity induced by the first administration of
cisplatin. Otolaryngol Head Neck Surg. 122:828–833. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waissbluth S and Daniel SJ:
Cisplatin-induced ototoxicity: Transporters playing a role in
cisplatin toxicity. Hear Res. 299:37–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravi R, Somani SM and Rybak LP: Mechanism
of cisplatin ototoxicity: Antioxidant system. Pharmacol Toxicol.
76:386–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rybak LP, Ravi R and Somani SM: Mechanism
of protection by diethyldithiocarbamate against cisplatin
ototoxicity: Antioxidant system. Fundam Appl Toxicol. 26:293–300.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
So H, Kim H, Kim Y, Kim E, Pae HO, Chung
HT, Kim HJ, Kwon KB, Lee KM, Lee HY, et al: Evidence that
cisplatin-induced auditory damage is attenuated by downregulation
of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res
Otolaryngol. 9:290–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vomund S, Schäfer A, Parnham MJ, Brüne B
and Knethen A: Nrf2, the master regulator of anti-oxidative
responses. Int J Mol Sci. 18:27722017. View Article : Google Scholar
|
|
14
|
Kopke RD, Liu W, Gabaizadeh R, Jacono A,
Feghali J, Spray D, Garcia P, Steinman H, Malgrange B, Ruben RJ, et
al: Use of organotypic cultures of Corti's organ to study the
protective effects of antioxidant molecules on cisplatin-induced
damage of auditory hair cells. Am J Otol. 18:559–571.
1997.PubMed/NCBI
|
|
15
|
Kim SJ, Park C, Han AL, Youn MJ, Lee JH,
Kim Y, Kim ES, Kim HJ, Kim JK, Lee HK, et al: Ebselen attenuates
cisplatin-induced ROS generation through Nrf2 activation in
auditory cells. Hear Res. 251:70–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Na HK and Surh YJ: Oncogenic potential of
Nrf2 and its principal target protein heme oxygenase-1. Free Radic
Biol Med. 67:353–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vriend J and Reiter RJ: The
Keap1-Nrf2-antioxidant response element pathway: A review of its
regulation by melatonin and the proteasome. Mol Cell Endocrinol.
401:213–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Xu T, Zhou P, Zhang J, Guan G, Zhang
H, Ling X, Li W, Meng F, Liu G, et al: Post-marketing safety
surveillance and re-evaluation of Xueshuantong injection. BMC
Complement Altern Med. 18:2772018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Guo B, Shi B, Gao Q and Zhou Q:
Chinese herbal medicine xueshuantong enhances cerebral blood flow
and improves neural functions in Alzheimer's disease mice. J
Alzheimers Dis. 63:1089–1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang X, Yang Y, Huang Z, Zhou J, Li Y and
Zhong X: Panax notoginseng saponins mitigate cisplatin
induced nephrotoxicity by inducing mitophagy via HIF-1α.
Oncotarget. 8:102989–103003. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Huang Z, Zou X, Yang Y, Qiu Y and
Wen Y: Panax notoginseng saponins attenuates
cisplatin-induced nephrotoxicity via inhibiting the mitochondrial
pathway of apoptosis. Int J Clin Exp Pathol. 7:8391–8400.
2014.PubMed/NCBI
|
|
22
|
Liu X, Huang Z, Zou X, Yang Y, Qiu Y and
Wen Y: Possible mechanism of PNS protection against
cisplatin-induced nephrotoxicity in rat models. Toxicol Mech
Methods. 25:347–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SJ and Zhou SW: Panax
notoginseng saponins attenuated cisplatin-induced
nephrotoxicity. Acta Pharmacol Sin. 21:257–260. 2000.PubMed/NCBI
|
|
24
|
Hu S, Wu Y, Zhao B, Hu H, Zhu B, Sun Z, Li
P and Du S: Panax notoginseng saponins protect cerebral
microvascular endothelial cells against oxygen-glucose
deprivation/reperfusion-induced barrier dysfunction via activation
of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules.
23:27812018. View Article : Google Scholar
|
|
25
|
Kalinec G, Thein P, Park C and Kainec F:
HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear
Res. 335:105–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalinec GM, Webster P, Lim DJ and Kalinec
F: A cochlear cell line as an in vitro system for drug ototoxicity
screening. Audiol Neurootol. 8:177–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Unoki T, Akiyama M, Kumagai Y, Gonçalves
FM, Farina M, da Rocha JBT and Aschner M: Molecular pathways
associated with methylmercury-induced Nrf2 modulation. Front Genet.
9:3732018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hill JM, Loeb E, MacLellan A, Hill NO,
Khan A and King JJ: Clinical studies of platinum coordination
compounds in the treatment of various malignant diseases. Cancer
Chemother Rep. 59:647–659. 1975.PubMed/NCBI
|
|
29
|
Hill JM and Speer RJ: Organo-platinum
complexes as antitumor agents (review). Anticancer Res. 2:173–186.
1982.PubMed/NCBI
|
|
30
|
Sun CY, Zhang QY, Zheng GJ and Feng B:
Phytochemicals: Current strategy to sensitize cancer cells to
cisplatin. Biomed Pharmacother. 110:518–527. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cepeda V, Fuertes MA, Castilla J, Alonso
C, Quevedo C and Pérez JM: Biochemical mechanisms of cisplatin
cytotoxicity. Anticancer Agents Med Chem. 7:3–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trendowski MR, El Charif O, Dinh PC Jr,
Travis LB and Dolan ME: Genetic and modifiable risk factors
contributing to cisplatin-induced toxicities. Clin Cancer Res.
25:1147–1155. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Travis LB, Fossa SD, Sesso HD, Frisina RD,
Herrmann DN, Beard CJ, Feldman DR, Pagliaro LC, Miller RC, Vaughn
DJ, et al: Chemotherapy-induced peripheral neurotoxicity and
ototoxicity: New paradigms for translational genomics. J Natl
Cancer Inst. 106:dju0442014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Im GJ, Chang J, Lee S, Choi J, Jung HH,
Lee HM, Ryu SH, Park SK, Kim JH and Kim HJ: Protective role of
edaravone against cisplatin-induced ototoxicity in an auditory cell
line. Hear Res. 330:113–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SJ, Park C, Lee JN, Lim H, Hong GY,
Moon SK, Lim DJ, Choe SK and Park R: Erdosteine protects HEI-OC1
auditory cells from cisplatin toxicity through suppression of
inflammatory cytokines and induction of Nrf2 target proteins.
Toxicol Appl Pharmacol. 288:192–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui
F, Li C, Tang L and Wang Z: Traditional uses, botany,
phytochemistry, pharmacology and toxicology of Panax
notoginseng (Burk.) F.H. Chen: A review. J Ethnopharmacol.
188:234–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao H, Han Z, Li G, Zhang S and Luo Y:
Therapeutic potential and cellular mechanisms of Panax
notoginseng on prevention of aging and cell
senescence-associated diseases. Aging Dis. 8:721–739. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rybak LP, Mukherjea D and Ramkumar V:
Mechanisms of cisplatin-induced ototoxicity and prevention. Semin
Hear. 40:197–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Callejo A, Sedó-Cabezón L, Juan ID and
Llorens J: Cisplatin-induced ototoxicity: Effects, mechanisms and
protection strategies. Toxics. 3:268–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Op de Beeck K, Schacht J and Van Camp G:
Apoptosis in acquired and genetic hearing impairment: The
programmed death of the hair cell. Hear Res. 281:18–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang PF, Song XY and Chen NH: Advances in
pharmacological studies of Panax notoginseng saponins on
brain ischemia-reperfusion injury. Yao Xue Xue Bao. 51:1039–1046.
2016.(In Chinese).
|
|
42
|
Su P, Wang L, Du SJ, Xin WF and Zhang WS:
Advance in studies of Panax notoginseng saponins on
pharmacological mechanism of nervous system disease. Zhongguo Zhong
Yao Za Zhi. 39:4516–4521. 2014.(In Chinese). PubMed/NCBI
|
|
43
|
Ooi BK, Goh BH and Yap WH: Oxidative
stress in cardiovascular diseases: Involvement of Nrf2 antioxidant
redox signaling in macrophage foam cells formation. Int J Mol Sci.
18:23362017. View Article : Google Scholar
|
|
44
|
Lourenço Dos Santos S, Petropoulos I and
Friguet B: The oxidized protein repair enzymes methionine sulfoxide
reductases and their roles in protecting against oxidative stress,
in ageing and in regulating protein function. Antioxidants (Basel).
7:1912018. View Article : Google Scholar
|
|
45
|
Machado-Silva A, Cerqueira PG,
Grazielle-Silva V, Gadelha FR, Peloso Ede F, Teixeira SM and
Machado CR: How trypanosoma cruzi deals with oxidative stress:
Antioxidant defence and DNA repair pathways. Mutat Res Rev Mutat
Res. 767:8–22. 2016. View Article : Google Scholar : PubMed/NCBI
|