Introduction
Breast cancer is one the most frequently diagnosed
cancer types in most countries and is the leading cause of
cancer-associated death in >100 countries (1). Chemotherapy is a major treatment
approach for breast cancer. However, the development of cancer cell
resistance to different drugs, known as multidrug resistance (MDR),
remains a challenge of cancer treatment (2). Various cellular pathways may be
involved in drug resistance, including the prevention of the
intracellular accumulation of anticancer drugs via transport
proteins that pump drugs out of cells (3). Ample evidence suggests that the
expression of ATP-binding cassette (ABC) transporters, especially
P-glycoprotein (P-gp) encoded by ABC subfamily B member 1
(ABCB1), can confer resistance to cytotoxic and targeted
chemotherapy (4–6). In vitro studies have shown
that ABC transporters confer resistance to numerous drugs used to
treat breast cancer, such as anthracyclines, taxanes and vinca
alkaloids (7). However, the
mechanisms underlying the increased expression and/or activation of
P-gp in breast cancer cells remains unclear.
Paired-related homeobox 1 (PRRX1) promotes EMT in
breast (8), colon (9), pancreatic (10) and gastric (11) cancer. Tumor cells undergoing EMT
are characterized by increased motility and invasiveness, which
promotes dissemination to distant sites and the formation of
metastases. In addition, tumor cells show decreased apoptosis and
increased resistance to antitumor drugs, they contribute to
immunosuppression and act as cancer stem-like cells (12). However, it is not clear whether
PRRX1 promotes MDR in breast cancer cells.
The present study aimed to determine the effects of
PRRX1 overexpression on MDR in MCF-7 cells and the underlying
mechanism of the process.
Materials and methods
Cell culture and transient
transfection
MCF-7 breast cancer cells (The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences) were
cultured in DMEM/high glucose (HyClone; Cytiva) supplemented with
10% fetal bovine serum (Biological Industries) at 37°C with 5%
CO2 and 98% relative humidity in a culture incubator.
Cells were separated and inoculated in 24-well plates after
reaching 80% confluence. The cells in the experimental group
(referred to as the PRRX1+ group) were transfected with a
recombinant plasmid (1 µg/ml) carrying the PRRX1 gene
(pEX-3/PRRX1; Shanghai GenePharma Co., Ltd.) to generate MCF-7
cells with transient PRRX1 overexpression. Cells in the
negative control group (N.C group) were transfected with an empty
pEX-3 vector (GenePharma), and the blank control group (the B.C
group) included untreated MCF-7 cells.
The culture medium was replaced the day before
transfection, and transfection was performed using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
reagent according to the manufacturer's instructions. The
morphology and transfection efficiency were observed under an
inverted fluorescence microscope (magnification, ×200; Olympus
Corporation) 48 h after transfection. A total of three fluorescence
images were captured randomly, and the percentage of transfected
cells were calculated using the following equation: (Number of
GFP+ cells/total number of cells) ×100. Cells
(~9×105 cells/well) in the three groups were harvested
for mRNA and protein analyses.
Reverse transcription-quantitative
(RT-q)PCR assay
Total RNA was extracted from cultured MCF-7 cells
from the three groups using TRIzol® reagent (Beijing
Solarbio Science & Technology Co., Ltd.). A reverse
transcription kit and Trans Start Probe RT-PCR Super Mix (both
purchased from Transgene SA) were used to reverse transcribe RNA
into template cDNA. Transcript data were obtained using the Bio-Rad
One-Step Plus system (CFX96 Touch; Bio-Rad Laboratories, Inc.).
Primer sequences are shown in Table
I. The relative mRNA expression levels of E-cadherin, vimentin,
PTEN, MDR1 and PRRX1 were determined, and normalized
to the interval reference gene GAPDH. The reaction conditions were
as follows: Pre-denaturation at 95°C for 30 sec, followed by 40
cycles of denaturation at 98°C for 10 sec, annealing at 60°C for 30
sec and 95°C for 1 min, followed by cooling at 40°C. TaqMan
(Transgene SA) probes were used. The relative mRNA expression
levels were calculated using the Pfaffl method (13).
 | Table I.Primer sequences for PRRX1, PTEN,
MDR1, N-cadherin, vimentin and GAPDH. |
Table I.
Primer sequences for PRRX1, PTEN,
MDR1, N-cadherin, vimentin and GAPDH.
| Gene | Primer sequences
(5′→3′) |
|---|
| PRRX1 | F:
GCACAGGCGGATGAGAAC |
|
| R:
TCTTCTGAGTTCAGCTGGTCAT |
| PTEN | F:
AGTTCCCTCAGCCGTTACCT |
|
| R:
ATTTGACGGCTCCTCAACTG |
| MDR1 | F: CCCATCATTGCA
ATAGCAGG |
|
| R:
TGTTCAAACTTCTGCTCCTGA |
| N-cadherin | F:
CCTTTCAAACACAGCCACGG |
|
| R:
TGTTTGGGTCGGTCTGGATG |
| Vimentin | F:
TTGACAATGCGTCTCTGGCA |
|
| R:
CGTGAGGTCAGGCTTGGAAA |
| GAPDH | F:
GGAGCCAAAAGGGTCATCATCT |
|
| R:
AGGGGCCATCCACAGTCTTCT |
Simple western blotting analysis
Simple western blotting was performed as described
previously (14,15). MCF-7 cells were lysed in
radioimmunoprecipitation assay buffer (Beijing Solarbio Science
& Technology Co., Ltd.) supplemented with a protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA) on ice for 1 h and centrifuged
at 8,000 × g for 20 min at 4°C. The supernatant was collected, and
the protein concentration was measured using a bicinchoninic acid
assay (Beyotime Institute of Biotechnology). The protein lysate was
diluted with 0.1X sample buffer (cat. no. 042–195; ProteinSimple)
and the final concentration of the protein sample was adjusted to
0.2 mg/ml. The samples and biotinylated ladder were denatured at
95°C for 5 min and stored on ice. A primary antibody against PRRX1
(cat. no. ab171502) was purchased from Abcam, and primary
antibodies against N-Cadherin (cat. no. 13116), Vimentin (cat. no.
5741), P-gp (cat. no. 13342), PTEN (cat. no. 9188), phosphorylated
(p-)AKT (cat. no. 4060), AKT (cat. no. 4691), p-PI3K (cat. no.
4228), PI3K (cat. no. 4249) and β-actin (cat. no. 3700) were
purchased from CST Biological Reagents Co., Ltd. All these primary
antibodies were diluted with Antibody Diluent 2 (1:50; cat. no.
042-203; ProteinSimple). The secondary antibodies used were as
follows: Anti-rabbit secondary antibody (cat. no. 042-206;
ProteinSimple) and anti-mouse secondary antibody (cat. no. 042-205;
ProteinSimple). The secondary horseradish peroxidase conjugate
(Streptavidin-HRP; cat. no. 042-414; ProteinSimple) was provided in
the detection module and was ready to use. In total, ~200 µl
Lumino-S and 200 µl of peroxide supplied in the detection module
(cat. no. DM-TP01; ProteinSimple) were mixed in a microcentrifuge
tube, gently pipetted and stored on ice. All reagents were added to
the wells of the plate and loaded into a Wes system (ProteinSimple)
for automatic western blotting assays and a capillary cartridge was
inserted into the cartridge holder. Data were analyzed using
Compass (version 3.1.7 1205.1438; ProteinSimple).
Cell viability analysis
Three groups of MCF-7 cells (4,000 cells/well) in
the logarithmic growth phase were inoculated into 96-well plates.
After the cells adhered to the wall, various chemotherapeutic drugs
were added. The final concentrations of docetaxel (Beijing Solarbio
Science & Technology Co., Ltd.) were 0.2,0.4, 0.8, 1.0 and 1.2
µmol/l and the final concentrations of cis-platinum (Beijing
Solarbio Science & Technology Co., Ltd.) complexes were 0.5,
1.0, 1.5, 2.0, 2.5 and 3.0 µmol/l. Three replicate wells were set
at each concentration. Cells in the B.C group were used as control
wells. Blank wells without any cells were filled with equal volume
(100 µl) of DMEM (cat. no. 01-052-1ACS; Biological Industries).
After 24 h of drug treatment, Cell Counting Kit (CCK)-8 (cat. no.
ab228554, Abcam) reagent was added at 100 µl per well and mixed,
followed by incubation for 1 h at room temperature according to the
manufacturer's protocols. Finally, the optical density (OD) at 450
nm was determined for each well using an enzyme-linked
immunosorbent assay detection system (HBS-1096c; Nanjing Detielab
Experimental Equipment Co., Ltd.). Cell viability and the half
maximal inhibitory concentration (IC50) of the two drugs
in different groups were calculated using GraphPad Prism version 6
(GraphPad Software).
Statistical analysis
Data were obtained from three independent
experiments and results are presented as mean ± standard deviation.
Data were analyzed using one-way ANOVA followed by Tukey's post hoc
test using GraphPad Prism version 6 (GraphPad Software). P<0.05
was considered to indicate a statistically significant
difference.
Results
Transfection of MCF-7 cells with PRRX1
promotes the EMT
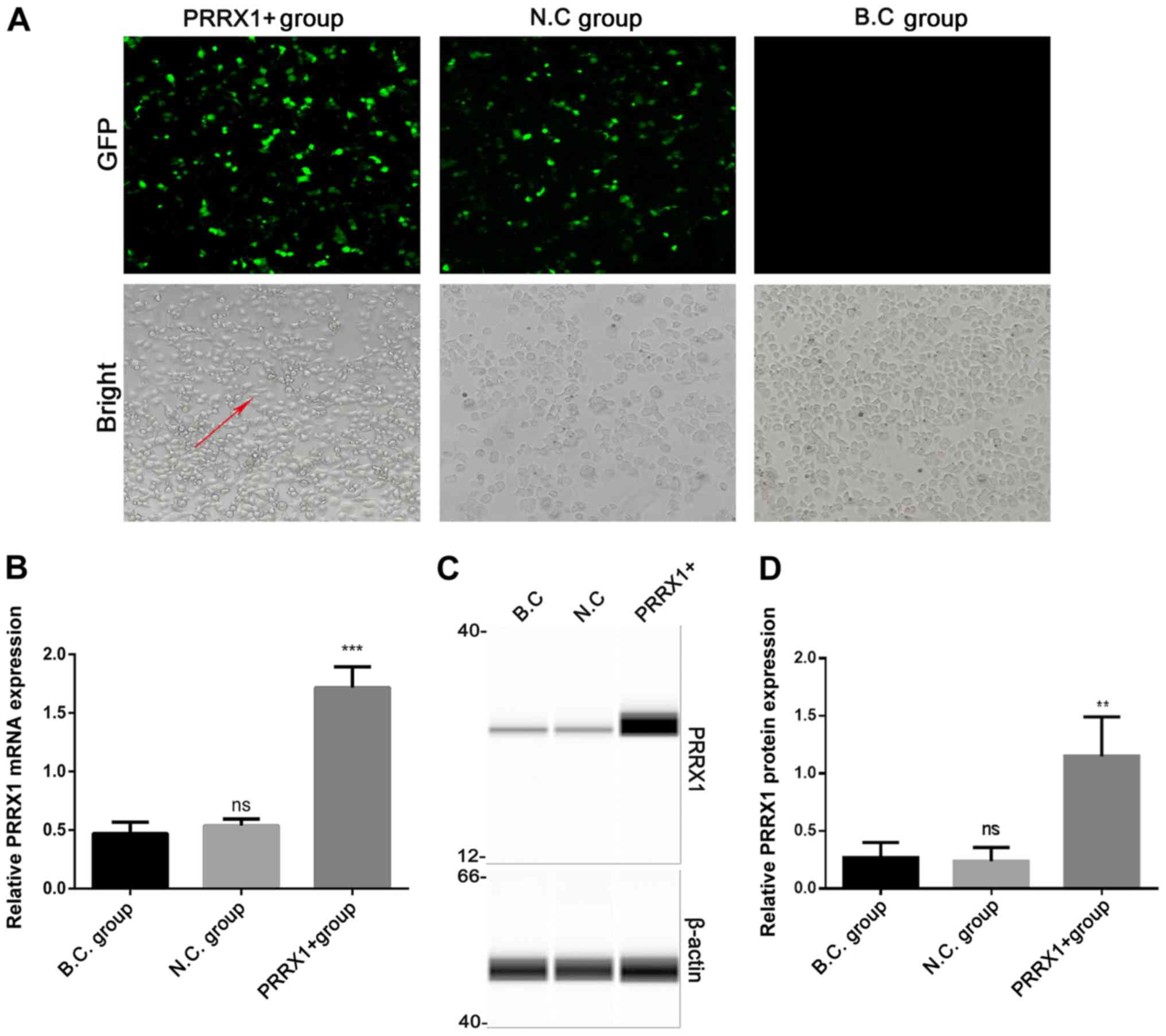
Cells in the three groups were observed under an
inverted fluorescence microscope 48 h after transfection (Fig. 1A). The expression of PRRX1 and EMT
markers were quantified using RT-qPCR and western blotting 48 h
after transfection. Green fluorescent protein expression revealed
that >80% of the cells had been successfully infected. The mRNA
and protein levels of PRRX1 were significantly higher in the PRRX1+
group compared with those in both control groups (P<0.001 and
P<0.01; Fig. 1B and D,
respectively). These results demonstrated that transfection was
effective.
Cells in the N.C and B.C groups exhibited a paving
stone shape and were tightly clustered, whereas cells in the PRRX1+
group showed a spindle morphology with loose connections among
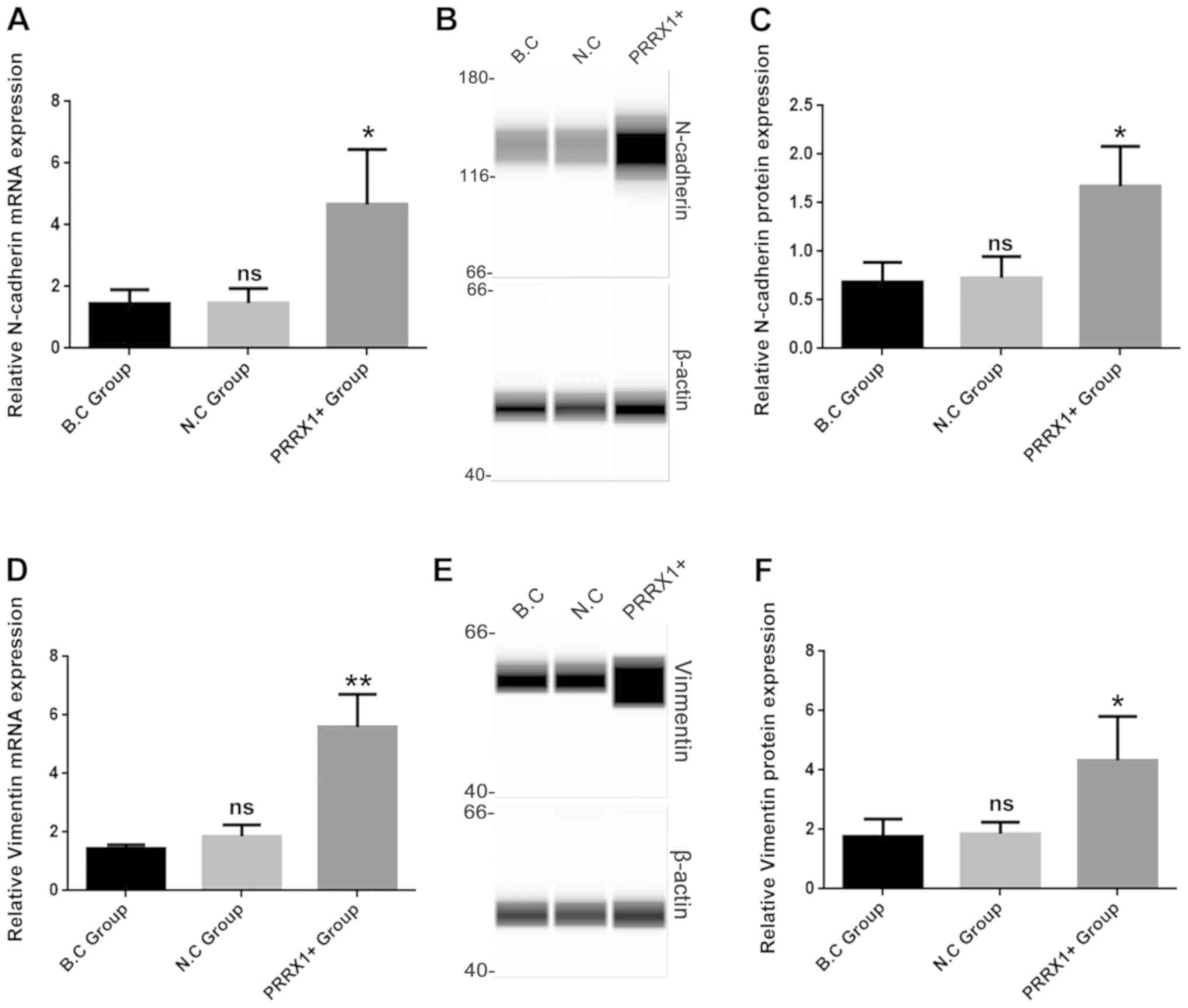
cells (bright field microscopy images, Fig. 1A). The mRNA and protein levels of
N-cadherin were significantly higher in the PRRX1+ group compared
with those in both control groups (P<0.05; Fig. 2A and C). The mRNA and protein
levels of vimentin were also significantly higher in the PRRX1+
group compared with those in both control groups (P<0.01 and
P<0.05; Fig. 2D and F,
respectively). These results indicated that PRRX1 promotes EMT in
breast cancer cells.
PRRX1 overexpression promotes MDR and
increases P-gp expression
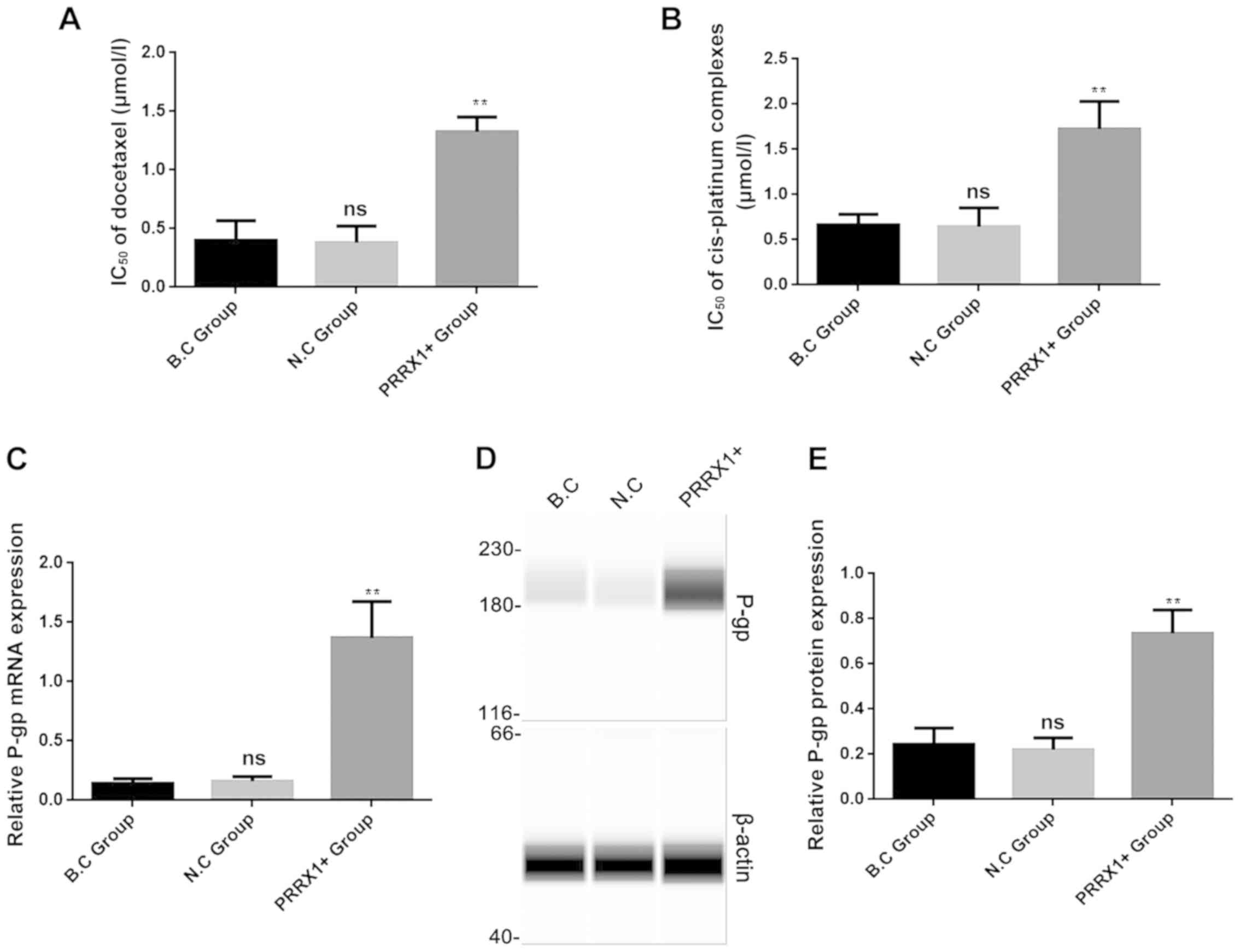
Cell proliferation was analyzed in the three groups
using a CCK-8 assay after treatment with docetaxel and cis-platinum
complexes for 24 h. The IC50 values of docetaxel and
cis-platinum complexes for cells transfected with PRRX1 were
significantly higher compared with those of the two control groups
(both P<0.01; Fig. 3A and B,
respectively). These results indicated that overexpression of PRRX1
decreased the inhibitory effects of docetaxel and cis-platinum
complexes on proliferation in breast cancer cells.
To further verify the effect of PRRX1 on MDR in
breast cancer, the expression levels of the MDR-associated protein
P-gp were analyzed in the three groups using RT-qPCR and western
blotting. As shown in Fig. 3C and
D, P-gp mRNA and protein levels were significantly higher in
the PRRX1+ group compared with those in both control groups (both
P<0.01). These results revealed that PRRX1 can promote
P-gp-associated MDR in breast cancer cells.
PRRX1 overexpression increases p-PI3K
and AKT expression and decreases PTEN
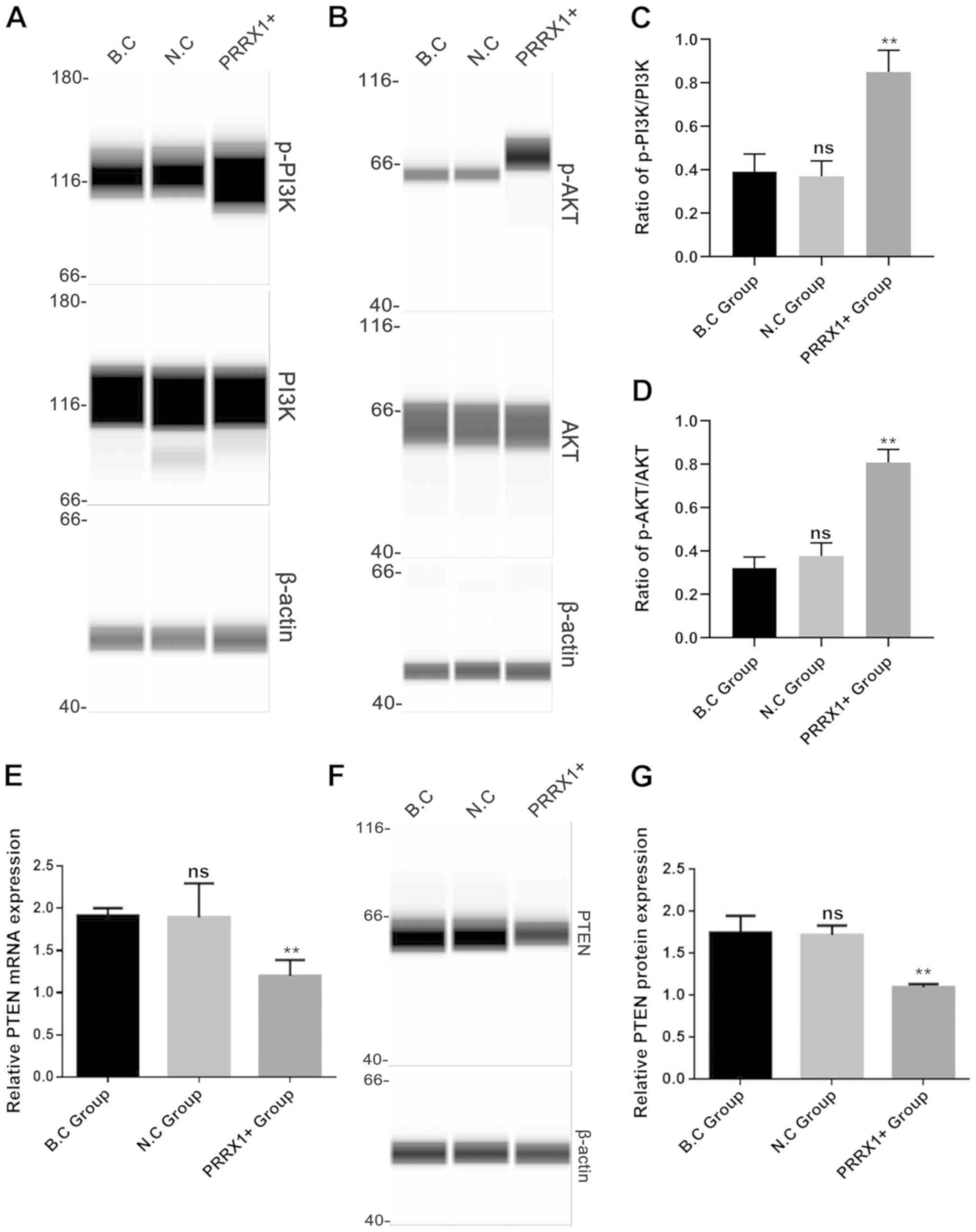
The present study further evaluated the mechanisms
by which PRRX1 promotes MDR by investigating the roles of PI3K and
AKT. The levels of p-PI3K and p-AKT proteins (Fig. 4A and B) and the ratio of
p-PI3K/PI3K and p-AKT/AKT were significantly higher in the PRRX1+
group compared with those in both control groups (P<0.01;
Fig. 4C and D), whereas the
protein expression of total PI3K and AKT in the three groups showed
no difference (Fig. 4A and B). It
has been reported that PTEN acts as a tumor inhibitor gene
by negatively regulating the PI3K/Akt pathway (16). Next, it was explored whether PRRX1
regulates the PI3K and Akt phosphorylation by downregulating PTEN
expression. As shown in Fig. 4E-G,
the mRNA and protein levels of PTEN were significantly lower in the
PRRX1+ group compared with those in the two control groups (both
P<0.01). These results suggested that PRRX1 is a potential
regulator of PI3K/Akt signaling pathway in breast cancer cells.
Discussion
Although several chemotherapy regiments have
contributed to a marked increase in the survival rates of patients
with breast cancer, MDR remains a serious issue with respect to
drug efficacy (17) and is
associated with the risk of relapse and poor prognosis (18). Accordingly, methods to predict and
circumvent MDR are likely to improve chemotherapy outcomes.
EMT is a transitional process accompanied by changes
in cell morphology along with loss of polarity, intercellular
junctions and expression of the EMT markers, such as N-cadherin,
E-cadherin and vimentin (19).
Several studies have shown that there is an association between EMT
and insensitivity to chemotherapeutic agents (20–22).
Fischer et al (23)
reported that EMT induces cyclophosphamide resistance by the
activation of aldehyde dehydrogenase in breast cancer cells. Saxena
et al (24) revealed that
in addition to invasion, EMT inducers also promote drug resistance
by upregulating ABC transporters, which efflux chemotherapeutic
drugs. PRRX1 is a newly identified EMT inducer and confers invasive
properties in cancer cells (8).
The present results, along with the aforementioned studies, showed
that MCF-7 cells undergo EMT in response to PRRX1 overexpression,
exhibiting a spindle-like shape and increased expression levels of
the mesenchymal markers N-cadherin and vimentin.
The ability of PRRX1 to promote MDR in breast cancer
cells has not been investigated, to the best of our knowledge.
Previous studies have shown that PRRX1 promotes EMT and is
associated with metastasis, poor prognosis and radiotherapy
resistance in colorectal cancer (9,25).
However, in lung cancer cells the loss of PRRX1 induces EMT and
induces antiapoptotic ability and resistance to cisplatin (26,27).
The present study demonstrated that PRRX1 decreases the sensitivity
of MCF-7 breast cancer cells to different drugs. These data, along
with the aforementioned studies, suggested that PRRX1 has distinct
functions among different types of cancer. In tumor cell lines, MDR
is often associated with an ATP-dependent decrease in cellular drug
accumulation, which is attributed to the overexpression of certain
ABC transporter proteins (28,29).
Among causes of MDR, the overexpression of P-gp, encoded by
MDR1, plays a notable role in drug recognition and export
before reaching intracellular targets (30). The present study demonstrated that
MDR is increased in PRRX1-overexpressing cells and that P-gp levels
in these cells are higher compared with those in both control
cells. A previous study has shown that PRRX1 expression is higher
in breast cancer tissues compared with adjacent normal tissues and
is associated with the migration and invasion of breast cancer
cells (31). The expression status
of PRRX1 in patients with and without drug resistant should be
analyzed in future research to further investigate the clinical
significance of PRRX1 in drug resistance.
The PI3K/AKT signaling pathway is associated with
various biochemical processes, including EMT (32,33),
MDR (34,35), cell invasion, migration (36,37)
and proliferation (38,39). As a key tumor suppressor gene, PTEN
can inhibit the PI3K/AKT signaling pathway and serves an important
role in the regulation of a series of biological processes. The
loss of PTEN activity leads to uncontrolled PI3K signaling, which
is found in a variety of primary and metastatic tumors, including
breast cancer (40,41). The present study reported that
PRRX1 overexpression in MCF-7 cells resulted in a decrease in PTEN
and increases in the phosphorylation of PI3K and AKT. It was also
demonstrated that PRRX1 may promote MDR through the activation of
the PTEN/PI3K/AKT signaling pathway. Inhibitors of PI3K and AKT
will be applied in future studies to further verify the function of
PI3K/AKT signaling in the PRRX1-induced MDR.
The present study is the first, to the best of our
knowledge, suggesting that PRRX1 promotes MDR in breast cancer
cells. However, only one cell line was used, and no drug resistant
cell lines were constructed, which are limitations of the present
study. Moreover, the reverse effect of PRRX1 downregulation on MDR
and other associated pathways were not investigated. Further
research is needed to evaluate drug resistance through combined
detection of multiple indicators using different tumor cell lines.
Nevertheless, the present findings demonstrated that PRRX1
overexpression induced the EMT process and promoted MDR in MCF-7
breast cancer cells, which may be underpinned by PTEN/PI3K/AKT
signaling. The present study not only provides novel insights into
the role of PRRX1 in MDR, but also highlights the potential of
PRRX1 as a novel target for breast cancer treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 8130-2290 and 81700029) and
the Natural Science Foundation of Shandong Province (grant no.
ZR-2017PH032).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FL, HL and JD designed the study. HL and SC
conducted the experiments and wrote the manuscript. LJ and DJ
analyzed the data. XW and QC collected data, performed data
analysis and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
MDR
|
multidrug resistance
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:3183–424. 2018. View Article : Google Scholar
|
|
2
|
Pastan I and Gottesman M: Multiple-drug
resistance in human cancer. N Engl J Med. 316:1388–1393. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trock BJ, Leonessa F and Clarke R:
Multidrug resistance in breast cancer: A meta-analysis of
MDR1/gp170 expression and its possible functional significance. J
Natl Cancer Inst. 89:917–931. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robey RW, Pluchino KM, Hall MD, Fojo AT,
Bates SE and Gottesman MM: Revisiting the role of ABC transporters
in multidrug-resistant cancer. Nat Rev Cancer. 18:454–464. 2018.
View Article : Google Scholar
|
|
5
|
Battistella C and Klok HA: Reversion of
P-gp-mediated drug resistance in ovarian carcinoma cells with
PHPMA-zosuquidar conjugates. Biomacromolecules. 18:1855–1865. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Vera AA, Gupta P, Lei Z, Liao D,
Narayanan S, Teng Q, Reznik SE and Chen ZS: Immuno-oncology agent
IPI-549 is a modulator of P-glycoprotein (P-gp, MDR1,
ABCB1)-mediated multidrug resistance (MDR) in cancer: In vitro and
in vivo. Cancer Lett. 442:91–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ocaña OH, Córcoles R, Fabra A,
Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A and
Nieto MA: Metastatic colonization requires the repression of the
epithelial-mesenchymal transition inducer Prrx1. Cancer Cell.
22:709–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Akiyoshi S, Eguchi H, Sudo T, et
al: Paired related homoeobox 1, a new EMT inducer, is involved in
metastasis and poor prognosis in colorectal cancer. Br J Cancer.
109:307–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reichert M, Takano S, Von Burstin J, Kim
SB, Lee JS, Ihida-Stansbury K, Hahn C, Heeg S, Schneider G, Rhim
AD, et al: The Prrx1 homeodomain transcription factor plays a
central role in pancreatic regeneration and carcinogenesis. Genes
Dev. 27:288–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo J, Fu Z, Wei J, Lu W, Feng J and Zhang
S: PRRX1 promotes epithelial-mesenchymal transition through the
Wnt/β-catenin pathway in gastric cancer. Med Oncol. 32:3932015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marcucci F, Stassi G and Maria RD:
Epithelial-mesenchymal transition: A new target in anticancer drug
discovery. Nat Rev Drug Discov. 15:311–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fourier A, Escal J, Bernard E, Lachman I,
Perret-Liaudet A, Leblanc P and Quadrio I: Development of an
automated capillary nano-immunoassay-simple western assay-to
quantify total TDP43 protein in human platelet samples. Anal
Bioanal Chem. 411:267–275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dahl JA, Jung I, Aanes H, Greggains GD,
Manaf A, Lerdrup M, Li G, Kuan S, Li B, Lee AY, et al: Broad
histone H3K4me3 domains in mouse oocytes modulate
maternal-to-zygotic transition. Nature. 537:548–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maehama T, Taylor GS and Dixon JE: PTEN
and myotubularin: Novel phosphoinositide phosphatases. Annu Rev
Biochem. 70:247–279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Videira M, Reis RL and Brito MA:
Deconstructing breast cancer cell biology and the mechanisms of
multidrug resistance. Biochim Biophys Acta. 1846:312–325.
2014.PubMed/NCBI
|
|
18
|
Kovalchuk O, Filkowski J, Meservy J,
Ilnytskyy Y, Tryndyak VP, Chekhun VF and Pogribny IP: Involvement
of microRNA-451 in resistance of the MCF-7 breast cancer cells to
chemotherapeutic drug doxorubicin. Mol Cancer Ther. 7:2152–2159.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial- mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mallini P, Lennard TWJ, Kirby JA and
Meeson A: Epithelial-to-mesenchymal transition: What is the impact
on breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rice AJ, Cortes E, Lachowski D, Cheung
BCH, Karim SA, Morton JP and Del Río Hernández A: Matrix stiffness
induces epithelial-mesenchymal transition and promotes
chemoresistance in pancreatic cancer cells. Oncogenesis.
6:e3522017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saxena M, Stephens MA, Pathak H and
Rangarajan A: Transcription factors that mediate
epithelial-mesenchymal transition lead to multidrug resistance by
upregulating ABC transporters. Cell Death Dis. 2:e1792011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin SM, Xia Q, Zhang YQ, Sun AM and Chen
LH: miR-124 regulates radiosensitivity of colorectal cancer cells
by targeting PRRX1. Nan Fang Yi Ke Da Xue Xue Bao. 36:1110–1116.
2016.(In Chinese). PubMed/NCBI
|
|
26
|
Zhu H and Sun G: Loss of PRRX1 induces
epithelial-mesenchymal transition and cancer stem cell-like
properties in A549 cells. Am J Transl Res. 9:1641–1650.
2017.PubMed/NCBI
|
|
27
|
Zhu H, Sun G, Dong J and Fei L: The role
of PRRX1 in the apoptosis of A549 cells induced by cisplatin. Am J
Transl Res. 9:396–402. 2017.PubMed/NCBI
|
|
28
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: An
overview. Adv Drug Del Rev. 55:3–29. 2003. View Article : Google Scholar
|
|
30
|
Meng H, Liong M, Xia T, Li Z, Ji Z, Zink
JI and Nel AE: Engineered design of mesoporous silica nanoparticles
to deliver doxorubicin and P-glycoprotein siRNA to overcome drug
resistance in a cancer cell line. ACS Nano. 4:4539–4550. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv Z, Kong B, Liu X, Jin LY, Dong Q, Li FN
and Wang HB: miR-655 suppresses epithelial-to-mesenchymal
transition by targeting Prrx1 in triple-negative breast cancer. J
Cell Mol Med. 20:864–873. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perumal E, Youn KS, Sun S, Seung-Hyun J,
Suji M, Jieying L and Yeun-Jun C: PTEN inactivation induces
epithelial-mesenchymal transition and metastasis by intranuclear
translocation of β-catenin and snail/slug in non-small cell lung
carcinoma cells. Lung Cancer. 130:25–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan R, Wang Y, Shi M, Xiao Y, Liu L, Liu L
and Guo B: Regulation of PTEN/AKT/FAK pathways by PPARγ impacts on
fibrosis in diabetic nephropathy. J Cell Biochem. Jan 16–2019.(Epub
ahead of print).
|
|
34
|
Gao C, Yuan X, Jiang Z, Gan D, Ding L, Sun
Y, Zhou J, Xu L, Liu Y and Wang G: Regulation of AKT
phosphorylation by GSK3β and PTEN to control chemoresistance in
breast cancer. Breast Cancer Res Treat. 176:291–301. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Lin N and Li Y: The PI3K/AKT
signaling pathway regulates ABCG2 expression and confers resistance
to chemotherapy in human multiple myeloma. Oncol Rep. 41:1678–1690.
2019.PubMed/NCBI
|
|
36
|
Zhang Y, Sui R, Chen Y, Liang H, Shi J and
Piao H: Long noncoding RNA MT1JP inhibits proliferation, invasion,
and migration while promoting apoptosis of glioma cells through the
activation of PTEN/Akt signaling pathway. J Cell Physiol.
234:19553–19564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen SR, Cai WP, Dai Xj, Guo AS, Chen HP,
Lin GS and Lin RS: Research on miR-126 in glioma targeted
regulation of PTEN/PI3K/Akt and MDM2-p53 pathways. Eur Rev Med
Pharmacol Sci. 23:3461–3470. 2019.PubMed/NCBI
|
|
38
|
Feng H, Zhang Z, Qing X, French SW and Liu
D: miR-186-5p promotes cell growth, migration and invasion of lung
adenocarcinoma by targeting PTEN. Exp Mol Pathol. 108:105–113.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang R, Wang F, Cao H and Yang JY: miR-223
regulates proliferation and apoptosis of IL-22-stimulated HaCat
human keratinocyte cell lines via the PTEN/Akt pathway. Life Sci.
230:28–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|