Introduction
Radiation therapy serves a key role in the treatment
of patients with head and neck cancer; however, it causes long-term
side effects on the surrounding healthy tissues (1). The bone is one of the most commonly
irradiated normal tissues, and osteoradionecrosis (ORN) of the jaw
and temporal bone represents one of the most frequent
radiation-induced head and neck cancer treatment complications
(2,3). ORN, a type of post-radiation bone
damage that includes devitalization and devascularization, is
described as a chronic disease that, if left untreated,
spontaneously results in disrupted bone homeostasis, with occurs in
5–15% of patients in the first 3 years after radiotherapy (4,5).
Preventing radiation-induced bone tissue damage remains a challenge
in clinical radiotherapy.
Macroautophagy, also referred to as autophagy, is a
highly evolutionarily conserved catabolic process characterized by
the formation of a double-membrane autophagosome with recruited
lysosomes in the outer membrane, which degrades damaged molecules
for recycling (6). Autophagy plays
an important role in maintaining cellular homeostasis during
environmental or intracellular stress (7). Previous studies have indicated that
autophagy is associated with radiotherapy, in vitro and
in vivo (8–11). In response to stress induced by
radiation, autophagy is activated to promote cell survival by
removing damaged cellular components (9). However, successive autophagy
activation could induce cell death through the degradation of
constitutive cellular components (10). Autophagic pathways behave as either
cytoprotective or cytotoxic depending on the cellular context in
normal and cancer cells (8–11).
Autophagy has been suggested to regulate bone
homeostasis by modulating osteoblast differentiation and
mineralization, as well as adjusting osteoclast differentiation and
function (12,13). Recent studies have reported the
cytoprotective effects of autophagy on osteoblast survival,
differentiation and mineralization (13–15).
However, it is currently unclear whether radiological
stress-induced autophagy is actually cytoprotective and which
molecular mechanisms are involved. The present study aimed to
determine the role of autophagy in osteoblasts under radiotherapy
stress using doxycycline (DOX) to inhibit autophagy by suppressing
autophagy-related 5 (Atg5) expression in a pre-osteoblastic
MC3T3-E1 cell line (16,17).
Materials and methods
Cell culture
The mouse pre-osteoblastic MC3T3-E1 cell line was
obtained from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. Cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Animal Blood Ware, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin. The complete medium
was replaced every 2–3 days. The cells were maintained at 37°C in a
humidified atmosphere with 5% CO2. In MC3T3-E1 cells,
Atg5 expression was suppressed by 2.5 nM DOX for at least 48
h before subsequent experiments. An MTT cell viability assay
confirmed that 2.5 nM DOX exerted no significant effects on cell
viability. For mineralization, the cells were cultured in complete
medium supplemented with dexamethasone (10 nM), β-glycerophosphate
(10 mM) and ascorbic acid (50 mg/l) for 3 weeks in a humidified
atmosphere with 5% CO2 at 37°C (18).
Radiation
The cells were divided into the control (without
irradiation), DOX (2.5 nM DOX for 48 h immediately after
irradiation), irradiation and irradiation + DOX (2.5 nM DOX for 48
h immediately after irradiation) groups. For irradiation treatment,
MC3T3-E1 cells were irradiated using a PRIMUS linear accelerator
radiotherapeutic machine (Siemens AG). The cells were treated with
X-ray irradiation doses of 0.25, 0.5, 1, 2 or 4 Gy, with the final
irradiation dose reaching 4 Gy by being successively superimposed
at a rate of 6 MV/min and a distance of 100 cm. To ensure the
irradiation efficiency of cells, before irradiation, the medium was
replaced with 1.5 ml osteogenic medium (complete culture containing
10 nM dexamethasone, 10 mM β-glycerophosphate and 50 mg/l ascorbic
acid) (18). After irradiation,
the osteogenic medium was supplemented to 3 ml. The cells were
incubated at 37°C with 5% CO2 for up to 72 h. The medium
was replaced every 24 h.
Clonogenic assay
A total of 1×103 cells were plated in
60-mm culture dishes in triplicate. Irradiation was performed as
described above. Following irradiation, the cells were further
incubated for 2 weeks to form visible colonies. The cells were
fixed with 100% methanol at room temperature for 20 min, and 0.5%
Giemsa staining (Merck KGaA) was performed at room temperature for
30 min. The culture dishes were observed under white light and
photographed. All experiments were repeated three times. Colonies
that contained >50 cells were counted. ImageJ software (version
1.52j; National Institutes of Health) was used for quantification
of the colonies.
Alizarin red staining
MC3T3-E1 cells were cultured in 12-well plates.
After cells reached 60% confluence, the medium was replaced with
osteogenic medium for 3 weeks. Subsequently, mineralization was
analyzed by alizarin red staining. Briefly, cells were washed with
PBS and fixed with 95% ethanol at room temperature for 15 min.
After washing with deionized water, 0.2% Alizarin Red S (Beijing
Solarbio Science & Technology Co., Ltd.) was added to the
wells, followed by incubation at room temperature for 30 min.
Plates were rinsed three times with deionized water and allowed to
air dry, and images were captured for analysis. All experiments
were repeated three times. ImageJ software (version 1.52j) was used
for quantification of the grey-scale values of Alizarin red
staining.
Alkaline phosphatase (ALP) activity
assay
The MC3T3-E1 cell culture supernatant was used to
detect ALP activity, which is one of the earliest markers expressed
during osteoblast differentiation (19). ALP activity was measured by
colorimetric assay using a commercial ALP assay kit (cat. no.
A059-1-1; Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's instructions. All experiments were repeated
three times.
Autophagic vesicle detection by
monodansylcadaverine (MDC) staining
MC3T3-E1 cells (5,000) were seeded on coverslips in
24-well plates, with or without DOX after irradiation at 37°C with
5% CO2 for 72 h. Cells were washed twice with PBS and
incubated with 50 µM MDC (cat. no. D4008; Sigma-Aldrich; Merck
KGaA) for 15 min at 37°C. Following incubation, the cells were
washed three times with PBS, and the coverslips were analyzed using
fluorescence microscopy (IX-81; Olympus Corporation). ImageJ
software (version 1.52j) was used to quantify fluorescence
intensity.
Hoechst 33342 staining
MC3T3-E1 cells (5,000) were seeded on coverslips in
24-well plates, with or without DOX after irradiation at 37°C with
5% CO2 for 72 h. For Hoechst 33342 staining, the cells
of each group were washed with PBS twice and fixed with 4%
paraformaldehyde for 15 min at room temperature, then stained with
1 mM Hoechst 33342 for 15 min at 37°C in the dark. Apoptotic cells
with clear condensation and small bright nuclei were evaluated
using a fluorescence microscope.
Caspase-3 activity assay
The Caspase-3 Activity Assay kit (cat. no. G015-1-3;
Nanjing Jiancheng Bioengineering Institute) was used to detect cell
apoptosis. As aforementioned, after 72 h of treatment with DOX or
irradiation, proteins were extracted from each group. After
operating according to the instructions of the kit, the absorbance
was detected at 405 nm by using a microplate reader (Tecan Group,
Ltd.).
Western blot analysis
Whole cell lysates were collected using RIPA lysis
buffer (Beyotime Institute of Biotechnology). The protein
concentration of the cell lysates was determined using the
Bicinchoninic Acid Protein Assay kit (CWBio). In the irradiation
and irradiation + DOX groups, 50 µg protein from each sample was
resolved via SDS-PAGE on a 12% gel for protein separation.
Fractionated proteins were transferred to polyvinylidene difluoride
membranes (EMD Millipore). Following blocking using 5% non-fat milk
(BD Biosciences) for 1 h at room temperature, the membranes were
incubated with the following rabbit polyclonal primary antibodies:
β-actin (1:1,000; cat. no. 20536-1-AP), ATG5 (1:1,000; cat. no.
10181-2-AP), Beclin-1 (1:1,000; cat. no. 11306-1-AP), P62 (1:1,000;
cat. no. 18420-1-AP), microtubule-associated protein 1 light chain
3β (LC3; 1:500; cat. no. 14600-1-AP), Bcl-2 (1:1,000; cat. no.
26593-1-AP), BAX (1:1,000; cat. no. 50599-2-Ig), caspase-3
(1:1,000; cat. no. 19677-1-AP), P38 (1:1,000; cat. no. 14064-1-AP)
(all from ProteinTech Group, Inc.), runt-related transcription
factor 2 (RUNX2; 1:1,000; cat. no. ab76956; Abcam) and p-P38
(1:1,000; cat. no. 4631; Cell Signaling Technology, Inc.) at 4°C
overnight. Goat anti-rabbit IgG secondary antibody conjugated to
horseradish peroxidase (1:2,500; cat. no. TA130023; OriGene
Technologies, Inc.) for 1 h at 37°C. Enhanced chemiluminescent
reagents (EMD Millipore) were used for chemiluminescence
development. β-actin was used as an internal control. All
experiments were repeated three times. The band intensities were
determined by gray value using Quantity One software (version
4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Calculations and statistical tests were performed using SPSS 22.0
(IBM Corp). One-way analysis of variance with Bonferroni post hoc
test was conducted to evaluate differences between the irradiation
and control groups; between the irradiation + DOX and DOX control
groups; and between the irradiation + DOX and irradiation groups
under same irradiation dose. P<0.05 was considered to indicate a
statistically significant difference.
Results
DOX inhibits autophagy in irradiated
MC3T3-E1 cells
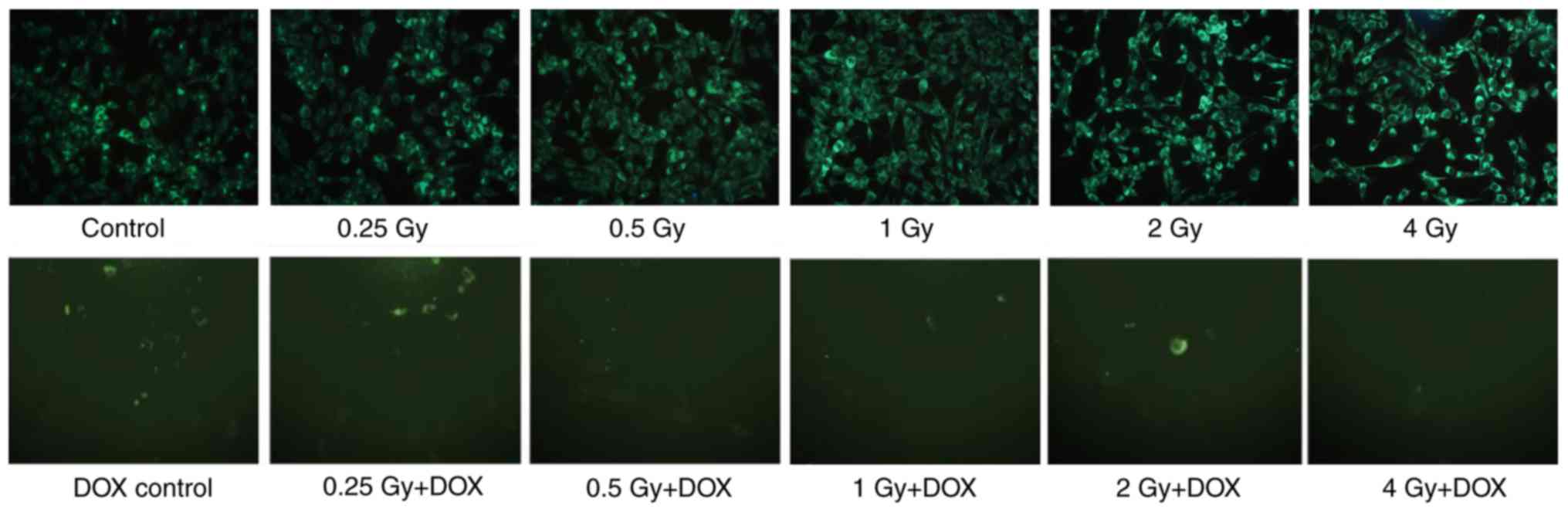
Autophagy was observed in irradiated MC3T3-E1 cells
by MDC fluorescence staining at 72 h post-irradiation. The results
demonstrated that the visible fluorescence intensity of the
irradiation groups increased as the irradiation dose increased. In
the irradiation + DOX groups, only a limited number of MDC-stained
cells were observed (Fig. 1).
DOX-mediated inhibition of autophagy
attenuates the irradiation-induced dose-dependent decrease in
MC3T3-E1 cell colony formation and mineralization
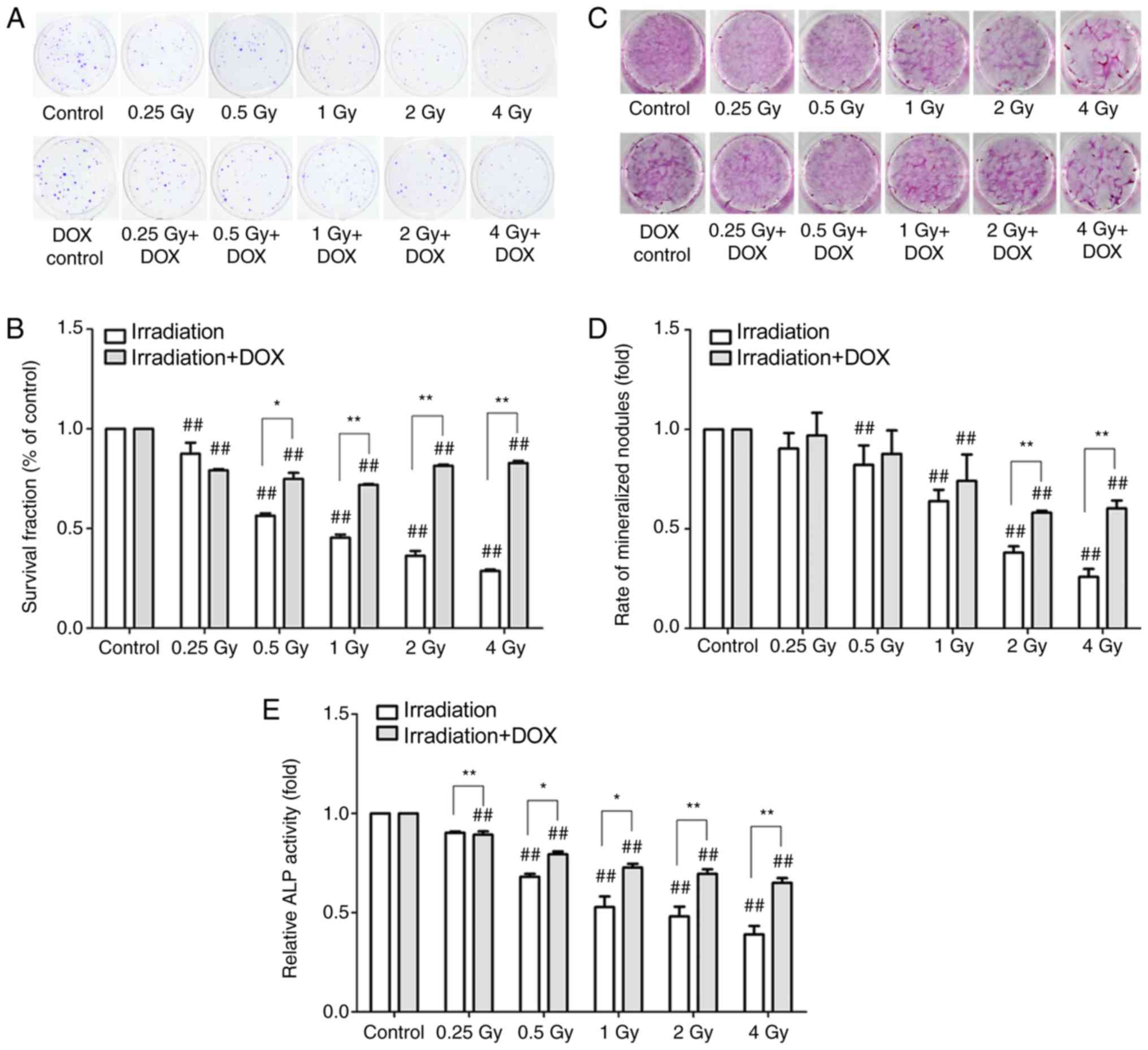
The results of the clonogenic assay demonstrated
that the colony formation rate of the irradiation groups decreased
gradually in a dose-dependent manner at 2 weeks post-irradiation
(P<0.01; Fig. 2A and B). In the
irradiation with DOX treatment groups, the clone formation rates of
MC3T3-E1 cells were significantly higher compared with those of the
corresponding irradiation groups (P<0.05), with the exception of
the 0.25 Gy-treated groups (Fig. 2A
and B).
Alizarin red staining was used to observe the
effects of irradiation on the mineralization ability of MC3T3-E1
osteoblasts at 3 weeks post-irradiation. The results demonstrated
that the number of mineralized nodules decreased significantly with
the increase in radiation dose (P<0.01; Fig. 2C and D). In the irradiation + DOX
groups, the number of mineralized nodules significantly increased
in the 2 and 4 Gy-treated groups compared with the respective
irradiation groups (P<0.05; Fig. 2C
and D). The ALP activity of MC3T3-E1 osteoblasts was detected
by the ALP kit 72 h post-irradiation. The results demonstrated that
the ALP activity was significantly decreased in the irradiation
group compared with that in the control group at a radiation dose
of 0.5–4 Gy (P<0.01). In the irradiation with DOX treatment
groups, the ALP activity levels were higher compared with those in
the irradiation groups at all radiation doses (P<0.05; Fig. 2E).
DOX-mediated inhibition of autophagy
significantly enhances the dose-dependent increase in cell
apoptosis in irradiated MC3T3-E1 cells
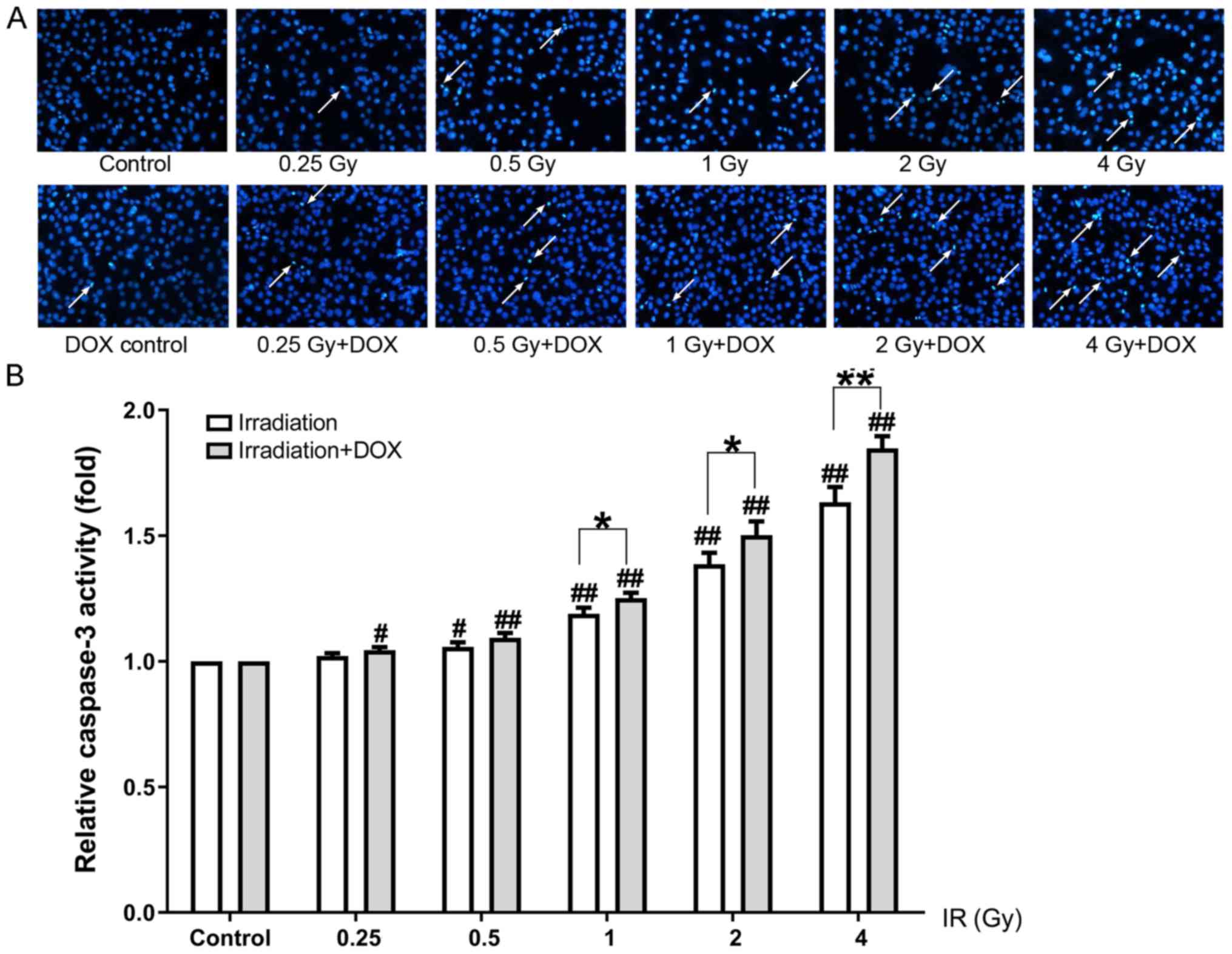
Hoechst 33342 staining and a caspase-3 activity
assay were used to detect apoptosis 72 h post-irradiation. Hoechst
33342 staining demonstrated that irradiation of MC3T3-E1
osteoblasts increased the number of nuclear agglomerations and
apoptotic bodies, which increased as the irradiation dose
increased. Compared with the irradiation groups, an increased
number of apoptotic bodies was observed in the irradiation + DOX
groups under the same radiation dose treatment conditions (Fig. 3A). The results of the caspase-3
activity assay revealed that caspase-3 activity was significantly
increased in a dose-dependent manner in the irradiation groups; in
the irradiation + DOX groups, caspase-3 activity levels were higher
compared with those in the irradiation groups under the same
radiation dose treatment conditions (P<0.05; Fig. 3B).
Effects of DOX-mediated inhibition of
autophagy on protein expression in irradiated MC3T3-E1 cells
Western blot analysis was performed to detect
autophagy- and apoptosis-associated protein expression levels in
all groups at 72 h post-irradiation (Fig. 4A-B). Compared with control group,
the expression levels of autophagy pathway-associated proteins in
the irradiation groups were significantly altered; increases in the
LC3-II/LC3-I ratio, ATG5 and beclin-1 expression levels, and
decreases in P62 expression levels were observed (Fig. 4C-F). In addition, the expression
levels of mitochondrial apoptosis pathway-associated proteins in
the irradiation groups were significantly different compared with
those in the control group, with the ratio of Bcl-2/BAX decreased
and the expression of pro-caspase-3 increased (Fig. 4G and H). The expression of the bone
development-related transcription factor RUNX2 was significantly
decreased in the irradiation groups compared with that in the
control group (Fig. 4I). Compared
with the irradiation groups, the changes in the expression levels
of the autophagy pathway-associated proteins were attenuated, with
the exception of P62, and the radiation-induced alterations in the
expression of the mitochondrial apoptosis pathway-associated
proteins were enhanced in the irradiation with DOX treatment groups
(Fig. 4C-H). However, the
expression of RUNX2 in the irradiation with DOX treatment groups
was higher compared with that in the respective irradiation groups.
Compared with the DOX control group, the expression of RUNX2
increased significantly at 0.25 Gy and then gradually decreased
with increasing radiation doses in the irradiation with DOX
treatment groups (Fig. 4I). These
results indicated that DOX inhibited autophagy in irradiated
MC3T3-E1 cells, which may have contributed to the increased
apoptosis and cell mineralization.
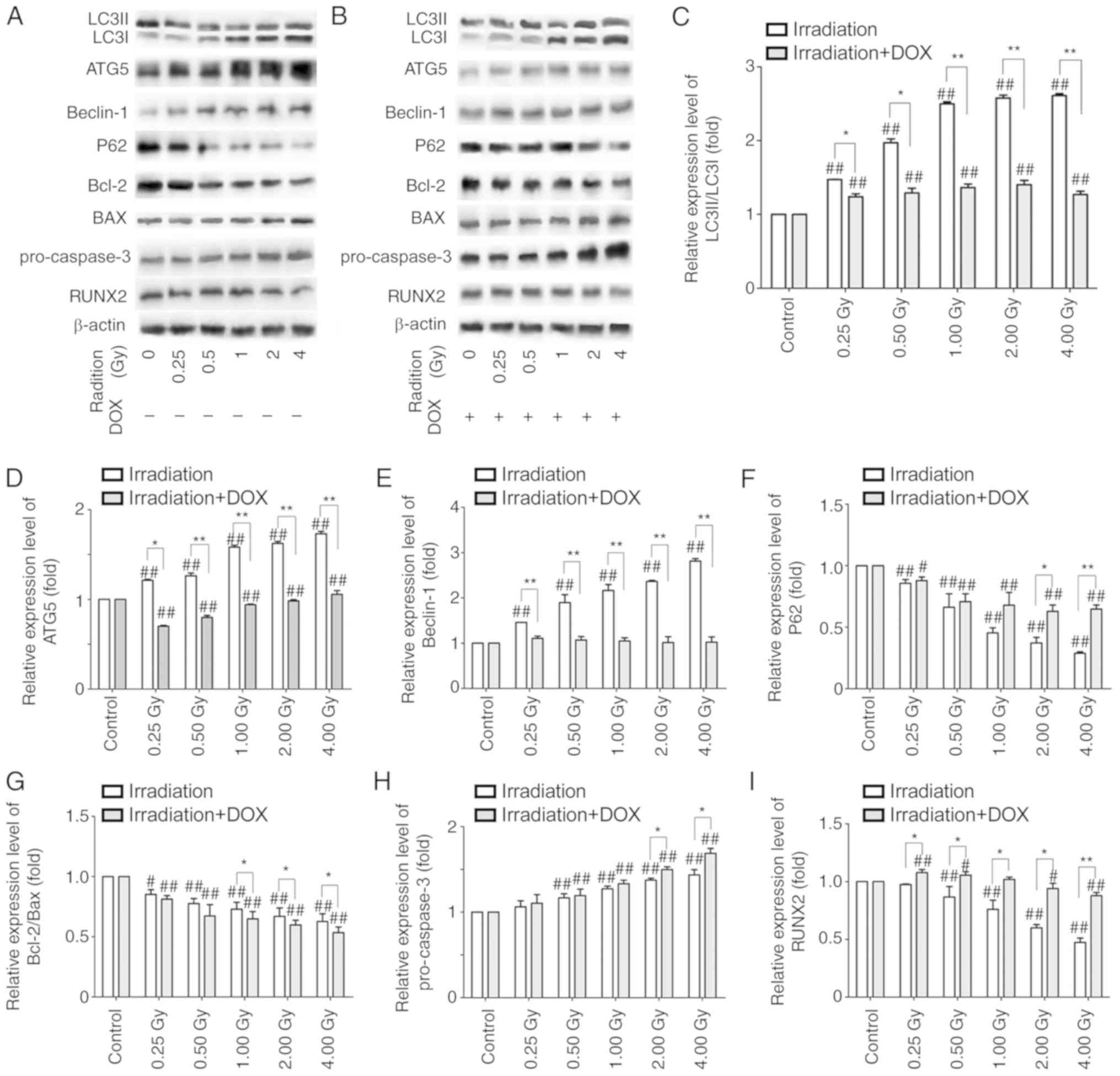 | Figure 4.Western blot detection of the
expression levels of proteins in the irradiation and irradiation +
DOX groups. (A and B) Representative blots of the (A) irradiation
and (B) irradiation + DOX group. (C-I) Protein expression levels of
(C) LC3II/LC3I, (D) ATG5, (E) beclin-1, (F) P62, (G) Bcl-2/BAX, (H)
pro-caspase-3 and (I) RUNX2, as assessed by western blot analysis
in the irradiation and irradiation + DOX groups. β-actin was used
as an internal control. n=3. #P<0.05 and
##P<0.01 vs. control or control + DOX; *P<0.05 and
**P<0.01 vs. the irradiation group. LC3, microtubule-associated
protein 1 light chain 3β; ATG5, autophagy related 5; Bcl-2, B-cell
lymphoma 2; BAX, Bcl-2 associated X protein; RUNX2, Runt-related
transcription factor 2; DOX, doxycycline. |
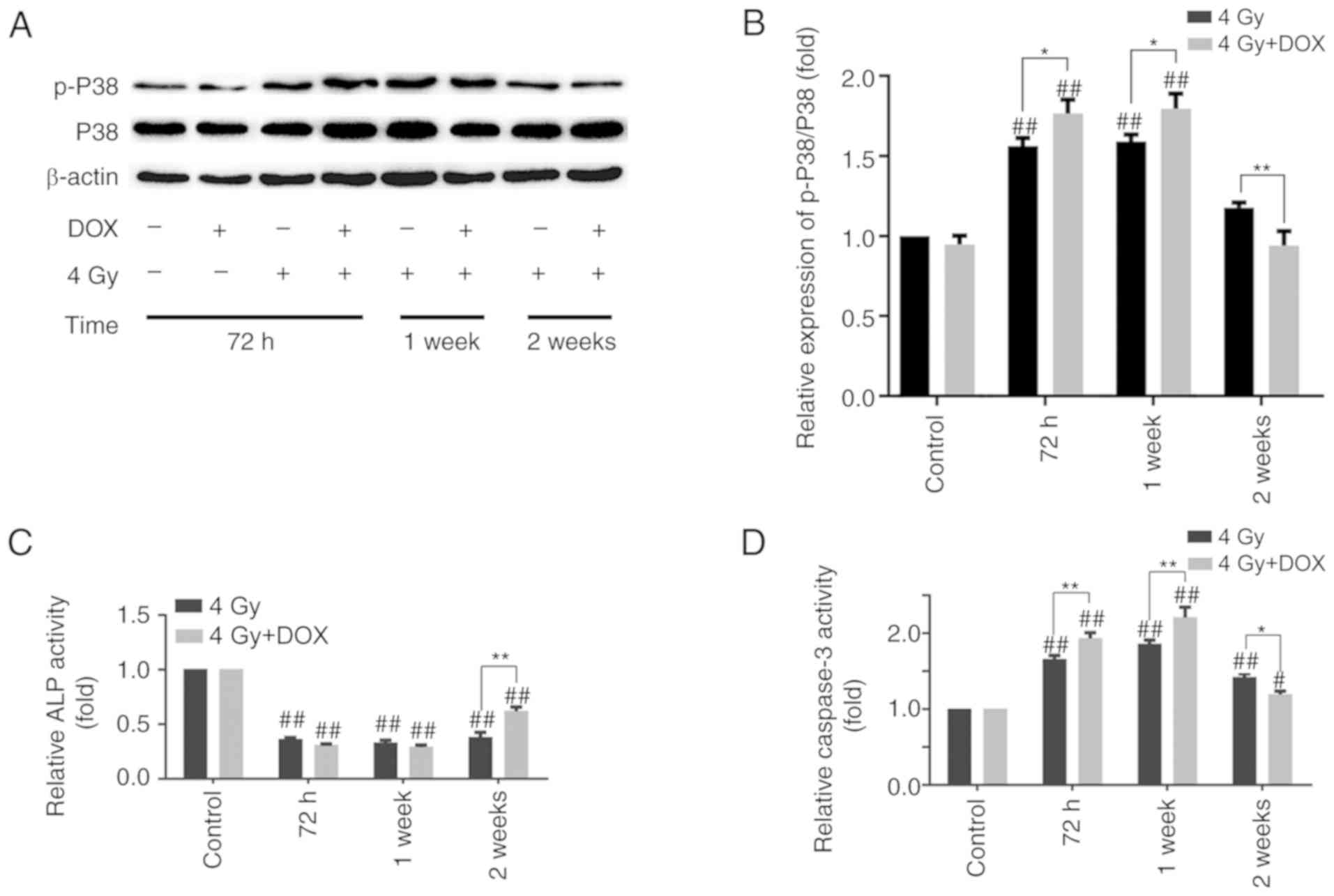
Effects of DOX-mediated autophagy
inhibition on MC3T3-E1 cells at different time points
The aforementioned results showed that cell
apoptosis was increased at 72 h, whereas mineralization and clone
formation were also increased at 2 and 3 weeks after DOX inhibited
autophagy. These results seem to be contradictory, but perhaps this
phenomenon can be explained when considering the different
detection time points of each experiment. The P38 and p-P38
expression, ALP and caspase-3 activity levels were further examined
at 72 h, 1 and 2 weeks post-4 Gy irradiation with or without DOX,
and the results demonstrated that the ratio of p-P38/P38 and
caspase-3 activity were increased, whereas ALP activity was
decreased in the irradiation with DOX treatment group compared with
those in the irradiation group at 72 h. This trend was maintained
at 1 week post-irradiation. However, the ratio of p-P38/P38,
caspase-3 and ALP activity levels were reversed at 2 weeks
post-irradiation, with lower levels observed in the irradiation +
DOX group compared with those in the irradiation group (Fig. 5A-D). These results may explain why
DOX-inhibited autophagy promoted apoptosis at 72 h
post-irradiation, and cell mineralization and clone formation at 2
and 3 weeks post-irradiation, respectively.
Discussion
ORN of the jaw is a common late complication of
radiotherapy in patients with head and neck malignancies (2,3). A
radiation dose of >70 Gy is the main cause of ORN (4,5).
Previous studies have suggested that radiation-induced vascular
injury and fibrosis may comprise the pathophysiology of ORN, but
the mechanism is still being explored (2,20).
In addition, radiation-induced autophagy plays an important role in
cell survival and bone homeostasis (12,13).
In the present study, the effects of autophagy in irradiated
MC3T3-E1 cells were explored.
Autophagy is considered to be a protective cell
response to stress conditions, such as ionizing radiation, toxic
stimulation and chemotherapy (9,11).
However, autophagy is also the mechanism of cell death in certain
contexts (10). According to Kim
et al (10), concurrent
induction of apoptosis and autophagy leads to a lower cell survival
rate and higher radiosensitivity compared with activation of
apoptosis alone in H460 lung cancer cells and transplanted tumor
models. The positive effect of autophagy on radiosensitization may
be the primary mechanism underlying the promotion of cell death
(11). Therefore, it is important
to distinguish between the autophagy that protects cells and the
autophagy that accelerates cell death (11). Beclin-1 induces autophagy
initiation and nucleation (6,11).
The combination of ATG5 complexes and the autophagosome membrane
subsequently promotes the recruitment of LC3 to autophagy vacuoles
(6,11). LC3 is an autophagy precursor and a
protein marker of autophagy (6).
P62 is a ubiquitin-binding protein, and aggregation of
ubiquitinated protein is used as a marker for autolysosome
degradation and autophagy flux. P62 expression is negatively
associated with autophagy, and damaged autophagy is often
accompanied by P62 accumulation (6,7). In
the present study, the autophagy level was observed by MDC staining
to be increased in irradiated MC3T3-E1 cells compared with the
non-irradiated control group. The results demonstrated that the
fluorescence intensity increased as the irradiation dose increased.
DOX was added to inhibit autophagy, and the fluorescence intensity
appeared to be decreased in each group compared with the respective
irradiation group. The results of western blot analysis revealed
that the ratio of LC3 II/LC3 I as well as the levels of ATG5 and
beclin-1 were increased, and those of P62 protein expression were
decreased with the increase in the radiation dose in the
irradiation groups compared with those in the control group. In
addition, compared with the irradiation groups, the changes in the
ratio of LC3-II/LC3-I, ATG5 and P62 expression were attenuated, and
P62 exhibited a certain degree of accumulation in the irradiation
with DOX treatment groups. Beclin-1 acts as a core protein in the
phosphatidylinositol-3-kinase complex, which is required for
vesicle nucleation (6,11). The expression of beclin-1 did not
change with increasing radiation doses in the irradiation with DOX
treatment groups, which may have occurred due to DOX suppressing
the expression of ATG5 rather than beclin-1. These results
suggested that DOX inhibited the radiation-induced increases in
autophagy and obstructed the autophagy process in irradiated
MC3T3-E1 cells.
The results of the present study also demonstrated
that the clone formation rate, mineralization ability and ALP
secretion decreased in the irradiation groups with the increase in
the radiation dose. However, those trends were attenuated following
autophagy inhibition by DOX. In addition, the expression levels of
RUNX2, which is a key transcription factor in osteoblast
differentiation (12,21), were downregulated following
irradiation compared with those in the control group, which may
have impaired cell mineralization. However, DOX-inhibited autophagy
reversed the expression of RUNX2 in irradiated MC3T3-E1 cells.
Taken together, these results suggested that irradiation inhibited
osteoblast differentiation and mineralization in MC3T3-E1 cells,
and that DOX-inhibited autophagy appeared to protect MC3T3-E1 cells
from radiation damage. Kook et al (21) have suggested that
irradiation-mediated ALP levels are decreased in MC3T3-E1 cells
compared with non-irradiated cells, and that the oxidative
stress-mediated nuclear factor erythroid 2-related factor 2/heme
oxygenase 1 pathway under irradiation is responsible for the
inhibition of mineralization and differentiation. However, previous
studies have reported that low-dose irradiation promotes
mineralization (22), and
autophagy promotes the differentiation and mineralization of
osteoblasts (13), which
contradicts the results observed in the present study. This
contradiction may be associated with the different response of
osteoblasts to radiation stimulation in different environments. For
example, RUNX2 mRNA expression was increased in osteoblast
precursors after 2 or 4 Gy radiation treatment, whereas its
expression was decreased in osteoblasts under the same treatment
conditions (23). In osteoblastic
MC3T3-E1 cells, reactive oxygen species production was decreased by
low dose radiation (1.5 mGy), but increased by high dose radiation
(15 mGy) (24). Thus, the role of
radiation-induced autophagy in osteoblasts should be further
studied.
The mitochondrial apoptosis pathway has been
reported to be mediated by oxidative stress under irradiation in
osteoblasts (25,26). In the present study, increased
caspase-3 activity levels and numbers of apoptotic bodies were
observed in irradiated MC3T3-E1 cells, as demonstrated by Hoechst
33342 staining and the caspase-3 activity assay. Western blot
analysis revealed that irradiation decreased the ratio of Bcl-2/BAX
but increased the expression levels of caspase-3 compared with
those in the control group, which suggested that irradiation
promoted MC3T3-E1 apoptosis. Additionally, the treatment of
irradiated MC3T3-E1 cells with DOX enhanced the increased number of
apoptotic bodies, decreased the ratio of Bcl-2/BAX and upregulated
caspase-3. Collectively, these findings suggested that
irradiation-induced apoptosis in MC3T3-E1 cells was aggravated by
the autophagy inhibitor DOX.
The aforementioned results suggested that autophagy
was activated, mineralization and proliferation were decreased, and
the apoptotic rate was increased in irradiated MC3T3-E1 cells
compared with the control group, which was reversed by
DOX-inhibited autophagy with the exception of apoptosis. The
apoptotic, mineralization and clonogenic assays were performed at
72 h, 2 and 3 weeks post-cell treatment, respectively; the
different detection time points may be responsible for the
different results regarding the increases in cell mineralization,
proliferation and apoptosis in the irradiation with DOX treatment
groups compared with the irradiation groups.
Alwood et al (27) have reported that the expression of
osteogenic genes in bone marrow stem cells or the bone is higher at
the early compared with the late stage of irradiation. Liu et
al (13) have revealed that
the effects of autophagy on osteoblast differentiation are observed
at a later stage, but not at the early stage. Different stages of
autophagy have also exhibited different effects on MC3T3-E1
apoptosis; autophagy inhibits apoptosis during intracellular
autophagosome formation, but promotes apoptosis after
autophagosomes are fully formed (28). Furthermore, oxidative stress has
been reported to serve a crucial role in irradiation-induced cell
damage (29,30). P38 MAPK can be activated in
response to oxidative stress and is associated with apoptosis
(29–31). p-P38 MAPK serves important roles in
MC3T3-E1 cell function (29–31).
The results of the present study demonstrated that irradiation
increased the ratio of p-P38/P38 in MC3T3-E1 cells 1 week
post-irradiation, and the ratio of p-P38/P38 was higher in the
irradiation + DOX groups compared with that in the respective
irradiation groups. However, at 2 weeks post-irradiation, the ratio
of p-P38/P38 was observed to be lower in the irradiation with DOX
treatment group compared with that in the irradiation group. The
fluctuation in the p-P38/P38 ratio may indicate that the
DOX-mediated inhibition of autophagy promoted apoptosis in the
early stage of irradiation. Further induction of DOX reversed the
radiation-induced apoptosis, promoting cell proliferation and
maintaining the proliferation and mineralization of MC3T3-E1 cells
at a later stage after irradiation.
In conclusion, the results of the present study
indicated that autophagy was activated, cell mineralization and
proliferation were decreased, and apoptosis was increased in
irradiated MC3T3-E1 cells compared with the non-irradiated
controls. Following the inhibition of autophagy by DOX, the
decreases in cell mineralization and proliferation were alleviated,
whereas the increase in the apoptosis rate was aggravated. p-P38
MAPK may be involved in the effects of DOX-inhibited autophagy at
different time points after irradiation in MC3T3-E1 cells. The
above molecular mechanisms may help understand the pathogenesis of
ORN and provide a new target for the treatment of ORN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360141), the
Postdoctoral Science Foundation of China (grant no. 22019M653474),
the Natural Science Foundation of Gansu Province (grant no.
18JR3RA277), Youth Science and Technology Fund of Gansu Province
(grant no. 17JR5RA231) and Scientific Research Projects of Health
of Gansu Province (grant no. GSWSKY2017-58).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and BL designed the experiments and revised the
manuscript. RL, WY and XH performed the experiments and analyzed
the data. RL and WY drafted the manuscript. DZ, KH and CW analyzed
the data and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DOX
|
doxycycline
|
|
ALP
|
alkaline phosphatase
|
|
MDC
|
monodansylcadaverine
|
|
RUNX2
|
runt-related transcription factor
2
|
|
LC3
|
microtubule-associated protein 1 light
chain 3
|
|
ORN
|
osteoradionecrosis
|
References
|
1
|
Moding EJ, Kastan MB and Kirsch DG:
Strategies for optimizing the response of cancer and normal tissues
to radiation. Nat Rev Drug Discov. 12:3473–542. 2013. View Article : Google Scholar
|
|
2
|
Cheriex KC, Nijhuis TH and Mureau MA:
Osteoradionecrosis of the jaws: A review of conservative and
surgical treatment options. J Reconstr Microsurg. 29:69–76. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chronopoulos A, Zarra T, Ehrenfeld M and
Otto S: Osteoradionecrosis of the jaws: Definition, epidemiology,
staging and clinical and radiological findings. A concise review.
Int Dent J. 68:22–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rice N, Polyzois I, Ekanayake K, Omer O
and Stassen LFA: The management of osteoradionecrosis of the jaws-a
review. Surgeon. 13:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendenhall WM, Suárez C, Genden EM, de
Bree R, Strojan P, Langendijk JA, Mäkitie AA, Smee R, Eisbruch A,
Lee AWM, et al: Parameters associated with mandibular
osteoradionecrosis. Am J Clin Oncol. 41:1276–1280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ktistakis NT and Tooze SA: Digesting the
expanding mechanisms of autophagy. Trends Cell Biol. 26:624–635.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang BL: Autophagy in response to
environmental stresses: New monitoring perspectives. Ecol Indic.
60:453–459. 2016. View Article : Google Scholar
|
|
8
|
Ko A, Kanehisa A, Martins I, Senovilla L,
Chargari C, Dugue D, Mariño G, Kepp O, Michaud M, Perfettini JL, et
al: Autophagy inhibition radiosensitizes in vitro, yet reduces
radioresponses in vivo due to deficient immunogenic signalling.
Cell Death Differ. 21:92–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu C and Xie C: Radiation-induced
autophagy promotes esophageal squamous cell carcinoma cell survival
via the LKB1 pathway. Oncol Rep. 35:3559–3565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KW, Moretti L, Mitchell LR, Jung DK
and Bo L: Combined Bcl-2/mammalian target of rapamycin inhibition
leads to enhanced radiosensitization via induction of apoptosis and
autophagy in non-small cell lung tumor xenograft model. Clin Cancer
Res. 15:6096–6105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ondrej M, Cechakova L, Durisova K, Pejchal
J and Tichy A: To live or let die: Unclear task of autophagy in the
radiosensitization battle. Radiother Oncol. 119:265–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nollet M, Santucci-Darmanin S, Breuil V,
Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S,
Cailleteau L, et al: Autophagy in osteoblasts is involved in
mineralization and bone homeostasis. Autophagy. 10:1965–1977. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Fang F, Yuan H, Yang D, Chen Y,
Williams L, Goldstein SA, Krebsbach PH and Guan JL: Suppression of
autophagy by FIP200 deletion leads to osteopenia in mice through
the inhibition of osteoblast terminal differentiation. J Bone Miner
Res. 28:2414–2430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Y, Zhang L, Xing Y, Zhang L, Chen X,
Tang P and Chen Z: Autophagy relieves the function inhibition and
apoptosis-promoting effects on osteoblast induced by
glucocorticoid. Int J Mol Med. 41:800–808. 2018.PubMed/NCBI
|
|
15
|
Li Y, Su J, Sun W, Cai L and Deng Z:
AMP-activated protein kinase stimulates osteoblast differentiation
and mineralization through autophagy induction. Int J Mol Med.
41:2535–2544. 2018.PubMed/NCBI
|
|
16
|
Hosokawa N, Hara Y and Mizushima N:
Generation of cell lines with tetracycline-regulated autophagy and
a role for autophagy in controlling cell size. FEBS Lett.
580:2623–2629. 2007. View Article : Google Scholar
|
|
17
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Wei S, Liu C, Song M, Wu H and Yang
Y: Regulation of the osteogenic and adipogenic differentiation of
bone marrow-derived stromal cells by extracellular uridine
triphosphate: The role of P2Y2 receptor and ERK1/2 signaling. Int J
Mol Med. 37:63–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang S, Wang S, Bian C, Yang Z, Zhou H,
Zeng Y, Li H, Han Q and Zhao RC: Upregulation of miR-22 promotes
osteogenic differentiation and inhibits adipogenic differentiation
of human adipose tissue-derived mesenchymal stem cells by
repressing HDAC6 protein expression. Stem Cells Dev. 21:2531–2540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian L, He LS, Soni B and Shang HT:
Myofibroblasts and their resistance to apoptosis: A possible
mechanism of osteoradionecrosis. Clin Cosmet Investig Dent.
4:21–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kook SH, Kim KA, Ji H, Lee D and Lee JC:
Irradiation inhibits the maturation and mineralization of
osteoblasts via the activation of Nrf2/HO-1 pathway. Mol Cell
Biochem. 410:255–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu W, Xu L, Chen M, Mao YT, Xie ZG, Wu SL
and Dong QR: The effects of low dose x-irradiation on osteoblastic
MC3T3-E1 cells in vitro. BMC Musculoskelet Disord. 13:942012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang B, Tang Q, Post J, Zhou H, Huang XB,
Zhang XD, Wang Q, Sun YM and Fan FY: Effect of radiation on the
notch signaling pathway in osteoblasts. Int J Mol Med. 31:698–706.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pramojanee SN, Pratchayasakul W,
Chattipakorn N and Chattipakorn SC: Low-dose dental irradiation
decreases oxidative stress in osteoblastic MC3T3-E1 cells without
any changes in cell viability, cellular proliferation and cellular
apoptosis. Arch Oral Biol. 57:252–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Blough E, Dai X, Olajide O,
Driscoll H, Leidy JW, July M, Triest WE and Wu M: Protective
effects of cerium oxide nanoparticles on MC3T3-E1 osteoblastic
cells exposed to X-ray irradiation. Cell Physiol Biochem.
38:1510–1519. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szymczyk KH, Shapiro IM and Adams CS:
Ionizing radiation sensitizes bone cells to apoptosis. Bone.
34:148–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alwood JS, Shahnazari M, Chicana B,
Schreurs AS, Kumar A, Bartolini A, Shirazi-Fard Y and Globus RK:
Ionizing radiation stimulates expression of pro-osteoclastogenic
genes in marrow and skeletal tissue. J Interferon Cytokine Res.
35:480–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Meng H and Yang M: Autophagy
protects osteoblasts from advanced glycation end products-induced
apoptosis through intracellular reactive oxygen species. J Mol
Endocrinol. 56:291–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yumoto H, Hirao K, Tominaga T, Bando N,
Takahashi K and Matsuo T: Electromagnetic wave irradiation promotes
osteoblastic cell proliferation and up-regulates growth factors via
activation of the ERK1/2 and p38 MAPK pathways. Cell Physiol
Biochem. 35:601–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao Y, Liu C, Huang J, Gu Y, Li H, Yang Z,
Liu J, Wang W and Li R: Ghrelin protects against depleted
uranium-induced apoptosis of MC3T3-E1 cells through oxidative
stress-mediated p38-mitogen-activated protein kinase pathway.
Toxicol Appl Pharmacol. 290:116–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kralova J, Dvorak M, Koc M and Kral V: p38
MAPK plays an essential role in apoptosis induced by
photoactivation of a novel ethylene glycol porphyrin derivative.
Oncogene. 27:3010–3020. 2008. View Article : Google Scholar : PubMed/NCBI
|