Introduction
Chronic rhinosinusitis (CRS) is a multifunctional
inflammatory disease of the nasal cavity and sinus mucosa (1) that often lasts >12 weeks (2). CRS is often accompanied by chronic
sinus inflammation (3). Some forms
of the disease are driven by allergy, often in association with
asthma (4). According to nasal
endoscopy and whether nasal polyps (NPs) are present, CRS can be
divided into two heterogeneous phenotypes: i) CRS with nasal
polyposis (CRSwNP); and ii) CRS without nasal polyposis (CRSsNP)
(5). CRSsNP is mainly
characterized by nasal obstruction, anosmia, drainage, headaches
and facial pain or pressure (6).
CRSwNP is more closely associated with obstruction of the nasal
cavity and anosmia (7).
Clinically, CRSwNP is frequently resistant to medical therapy
(8). Recently, it has been
demonstrated that epithelial-to-mesenchymal transition (EMT) and
its effects on nasal epithelial cells may result in the tissue
remodeling of nasal polyps (9).
However, the regulation of EMT by microRNAs (miRNAs or miRs) in CRS
has not been investigated to the same extent and requires further
investigation.
miRNAs are single-strand and non-coding RNAs 18–25
nucleotides in length that regulate related gene expression by
binding to the 3′-untranslated regions (3′-UTR) of mRNA targets and
induce mRNA destruction or translation inhibition (10). A previous study revealed that
miRNAs may serve a vital role in the progression of inflammation
and CRSwNP remodeling (11).
Furthermore, miR-142-3p may regulate inflammatory responses by
regulating tumor necrosis factor α (11). Interactions between miR-4492 and
interleukin 10 associated with Janus kinase/signal transducers and
activators of the transcription signaling pathway may be one of the
key mechanisms in CRSwNP (8).
miR-155-5p has also been reported to be targeted by circular RNA
nuclear receptor subfamily 1 group H member 4 in the pathological
process of renal injury regulation in salt-sensitive hypertensive
mice (12). Moreover, miR-155-5p
has been revealed to inhibit vascular smooth muscle cell viability
by targeting proto-oncogene c-Fos and Zic family member 3 to
promote aneurysm formation (13).
As the underlying biological functions and molecular
mechanisms of miR-155-5p in CRS development and progression remain
unclear, the present study investigated the role of miR-155-5p on
EMT in CRS related to sirtuin 1 (SIRT1) to establish the molecular
mechanisms of miR-155-5p in CRS development.
Materials and methods
Ethical statement
The Ethics Committee of Zhujiang Hospital, Southern
Medical University, Guangzhou, P.R. China approved the present
study (approval no. EBYHK20190503) and written informed consent was
obtained from all patients.
Study population
A total of 35 patients who attended Zhujiang
Hospital, Southern Medical University for treatment from June 2019
to December 2019 were enrolled in the present study and were
divided into the following groups: i) The CRSsNP group (n=14), from
whom mucosa samples originating from the middle turbinates were
collected; ii) The CRSwNP group (n=11), from whom polyp tissue
samples were collected from the middle meatus; and iii) The control
group (n=10), which was comprised of patients who had received
septoplasty or rhinoseptoplasty with anatomical variations without
sinus disease and who had inferior turbinate samples collected.
Patients with CRS were diagnosed based on clinical
symptoms, examinations, nasal endoscopy and sinus-computed
tomography (CT) scans in accordance with the criteria from the
European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS;
2012) (5). Generally, patients
included in the study were examined for: i) Cystic fibrosis; ii)
Gross immunodeficiency; iii) Congenital mucociliary problems; iv)
Non-Invasive fungal sinusitis; v) Invasive fungal disease; vi)
Systemic vasculitis; and vii) Granulomatous diseases as listed by
EPOS. None of the patients received antihistamines,
antileukotrienes, oral or intranasal decongestants, or intranasal
anticholinergics 2 weeks prior to the present study. Moreover, drug
treatments, including steroids, antibiotics and antihistamines,
were terminated for >1 month prior to the present study.
Paranasal sinus CT scans were scored using the Lund-Mackay scoring
method (14) and nasal endoscopic
quantitative evaluation was performed using the Lund-Kennedy
scoring method (15). Moreover,
patients were scored using a visual analog scale (16). Patients were excluded if they had
immune deficiencies, primary ciliary dyskinesia, fungal sinusitis,
cystic fibrosis, antrochoanal polyps, vasomotor rhinitis, asthma or
a history of sinus surgery.
Demographic and clinical characteristics of all the
patients are presented in Table I.
Fresh samples of nasal tissues were collected during surgery and
stored at −80°C for subsequent experiments.
 | Table I.Clinical characteristics of the
patients with CRS. |
Table I.
Clinical characteristics of the
patients with CRS.
|
Characteristics | Controls | CRSsNP | CRSwNP |
|---|
| No. of
patients | 10 | 14 | 11 |
| Median age
(IQR) | 32 (13–61) | 42 (18–63) | 38 (21–59) |
| Sex |
|
|
|
|
Male | 4 | 5 | 4 |
|
Female | 6 | 9 | 6 |
| Median VAS score
(IQR) | 0 | 4 (2–9) | 8 (6–12) |
| Median CT score
(IQR) | 0 | 5 (3–8) | 8 (4–9) |
| Median endoscopy
score (IQR) | 0 | 10.5 (8–14) | 13 (10–15) |
| Positive skin prick
test | 0 | 2 | 1 |
Skin prick tests
The patients underwent skin prick tests by specially
trained nurses with a panel of 13 common allergen, including alder
pollen, birch pollen, Timothy grass pollen, smooth meadow grass
pollen, Festuca pratensis pollen, mugwort pollen, dandelion
pollen, dog dander, cat dander, horse dander, cow dander and house
dust mites Dermatophagoides farinae and Dermatophagoides
pteronyssinus. Saline was used as negative control and
histamine as positive control. Skin prick test responses were
considered positive if the allergen caused a wheal with a diameter
>3 mm.
Cell culture and treatment
Primary human nasal epithelial cells (HNEpCs) were
obtained from PromoCell GmbH. Cells were grown in a RPMI-1640
medium (cat. no. 31870082, Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.) in a humidified incubator at 37°C with 5% CO2.
HNEpCs were cultured in a 96-well plate
(3×105 cells/ml) for 24 h. miR-155-5p inhibitor
(5′-ACCCCUAUCACGAUUAGCAUUAA-3′) and inhibitor control
(5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from Sigma-Aldrich,
Merck KGaA. The medium in the plates was replaced with a fresh one
following culturing for 24 h and the HNEpCs were transfected with
50 nM miR-155-5p inhibitor and inhibitor control using
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.). The cells were harvested at 48 h
post-transfection for subsequent studies.
To explore the effect of TGF-β1 on HNEpC morphology
and EMT, HNEpCs were stimulated with 5 ng/ml transforming growth
factor (TGF)-β1 (cat. no. 34-8348-82; Invitrogen; Thermo Fisher
Scientific, Inc.) in the presence of miR-155-5p inhibitor or
inhibitor control. HNEpC morphology was observed under a DM500
light microscope (Leica Microsystems, Inc.). Furthermore, to
explore the function of SIRT1, an SIRT1 inhibitor, Splitomicin
(cat. no. S4068; Sigma-Aldrich; Merck KGaA) at final concentration
of 100 µM was incubated with the cells for 48 h at 37°C.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was determined using a CCK-8 kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The cells were cultured at 37°C with 95%
O2 and 5% CO2 in an incubator for 24, 48 or
72 h. The CCK-8 solution was then added and cultured for another 2
h at 37°C. Optical density values were measured at 450 nm using a
microplate reader (model 680; Bio-Rad Laboratories, Inc.).
Western blotting
Protein expression was detected by western blotting
as previously described (17).
Briefly, HNEpCs were cultured in a 24-well plate (3×105
cells/wells) for 24 h. Total proteins from HNEpCs and nasal samples
(CRSsNP, CRSwNP and control groups) were lysed and extracted using
RIPA buffer (cat. no. P0013C; Beyotime Institute of Biotechnology)
and protein concentrations were measured using a BCA protein kit
(Sigma-Aldrich; Merck KGaA). Subsequently, 30 µg protein sample
lysates were electrophoresed by 12% SDS-PAGE (cat. no. P0012A;
Beyotime Institute of Biotechnology) and transferred to PVDF
membranes (cat. no. FFP28; Beyotime Institute of Biotechnology).
Membranes were then blocked with 5% non-fat milk for 2 h at room
temperature and then incubated with the primary antibodies
anti-TGF-β1 (mouse; 1:2,000; cat. no. ab27969), anti-E-cadherin
(mouse; 1:1,000; product code ab1416), anti-α-smooth muscle actin
(SMA; rabbit; 1:1,000; cat. no. ab5694), anti-fibronectin (rabbit;
1:1,000; cat. no. ab2413), anti-vimentin (rabbit; 1:1,000; cat. no.
ab92547), anti-SIRT1 (mouse; 1:1,000; cat. no. ab110304) and
anti-β-actin (mouse; 1:1,000; cat. no. ab8226) at 4°C overnight.
β-actin served as an internal reference. Membranes were
subsequently incubated with secondary horseradish peroxidase
(HRP)-combined antibodies goat anti-rabbit immunoglobulin (Ig) G
H&L (goat; 1:2,000; cat. no. ab205718) and goat anti-mouse IgG
H&L (HRP; goat; 1:2,000; cat. no. ab205719) at room temperature
for 1 h and then washed with TBST three times. All antibodies were
purchased from Abcam. Protein bands were obtained and developed
using an ECL kit (EMD Millipore). Grey values were further
collected and calculated using ImageJ software (version 5.0;
Bio-Rad Laboratories, Inc.).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
HNEpCs were cultured in a 96-well plate
(3×105 cells/ml) for 24 h at 37°C. Total RNA was
extracted from HNEpCs and nasal samples (CRSsNP, CRSwNP and control
groups) using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), preserved in a refrigerator at −80°C. Total RNA
concentration was detected and quantified using a biological
spectrometer (Nano Drop 2000; Thermo Fisher Scientific, Inc.). cDNA
was synthesized from 1 µg of total RNA using a First-strand cDNA
Synthesis kit (cat. no. E6300L; New England BioLabs, Inc.),
according to the manufacturer's protocol. RT-qPCR was conducted
using a SYBR Premix Ex Taq II kit (cat. no. RR820L; Takara, Bio,
Inc.) and a Touch real-time PCR Detection system (cat. no. CFX96;
Bio-Rad Laboratories, Inc.), according to the manufacturer's
protocol. The thermocycling conditions were as follows:
Denaturation at 95°C for 30 sec, 95°C for 5 sec and 60°C for 1 min
for a total of 40 cycles. Primer sequences are presented in
Table II. β-actin and U6 were
used as internal references. Relative gene expression was
quantified using the 2−ΔΔCq method (18).
 | Table II.Primers used for reverse
transcription-quantitative PCR. |
Table II.
Primers used for reverse
transcription-quantitative PCR.
| Genes | Primers |
|---|
| miR-155-5p |
|
|
Forward |
5′-GGGTGTCGTATCCAGTGCAA-3′ |
|
Reverse |
5′-GTCGTATCCAGTGCGTGTCG-3′ |
| SIRT1 |
|
|
Forward |
5′-AAATGCTGGCCTAATAGAGTGG-3′ |
|
Reverse |
5′-TGGCAAAAACAGATACTGATTACC-3′ |
| U6 |
|
|
Forward |
5′-GAGAAAGTTAGCACGGCTTCTG-3′ |
|
Reverse |
5′-CAAAATATGGAATGCTTCAAAGAG-3′ |
| β-actin |
|
|
Forward |
5′-ATTGGCAATGAGCGGTTC-3′ |
|
Reverse |
5′-GGATGCCACAGGACTCCA-3′ |
Target gene prediction and
dual-luciferase reporter assay
Target genes and potential binding sites of
miR-155-5p and SIRT1 were predicted using TargetScan software
(version 7.2; targetscan.org) and subsequently
confirmed by a dual-luciferase reporter assay.
Wild-type (WT) or mutant (MUT) SIRT1 sequences were
cloned into pMirGLO reporter vectors (Promega Corporation) to
generate SIRT1-WT and SIRT1-MUT vectors. HNEpCs were cultured in
96-well plates (5×103 cells/well) for 24 h at 37°C and
co-transfected with SIRT1-WT or SIRT1-MUT and miR-155-5p inhibitor
(I group) or inhibitor control using Lipofectamine® 2000
Transfection reagent (Thermo Fisher Scientific, Inc.) at 37°C.
Cells were harvested at 48 h post-transfection and subjected to a
dual-luciferase reporter assay system (cat. no. E1910; Promega
Corporation). Luciferase activity was normalized to Renilla
luciferase.
Statistical analysis
All experiments were performed in triplicate. Data
are expressed as the mean ± standard deviation. Statistical
analysis was performed using SPSS software (version 21.0; IBM
Corp.). Data were analyzed using an unpaired Student's t-test and
one-way ANOVA followed by Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Epithelial markers are downregulated
and TGF-β1, mesenchymal markers and miR-155-5p are upregulated in
CRSwNP
To determine whether EMT occurs in CRS, the protein
expression of TGF-β1 and EMT-associated markers (E-cadherin,
vimentin, α-SMA, and fibronectin) in the CRSwNP, CRSsNP and control
groups was determined by western blotting. The protein expression
of E-cadherin was downregulated and TGF-β1, vimentin, α-SMA, and
fibronectin were upregulated in the CRSsNP and CRSwNP groups
compared with the control (Fig.
1A; P<0.001), indicating that epithelial marker expression
was downregulated and the expression of TGF-β1 and mesenchymal
markers were upregulated in the CRSsNP and CRSwNP group.
Furthermore, changes in E-cadherin, TGF-β1, vimentin, α-SMA, and
fibronectin expression were greater in the CRSwNP group than in the
CRSsNP group.
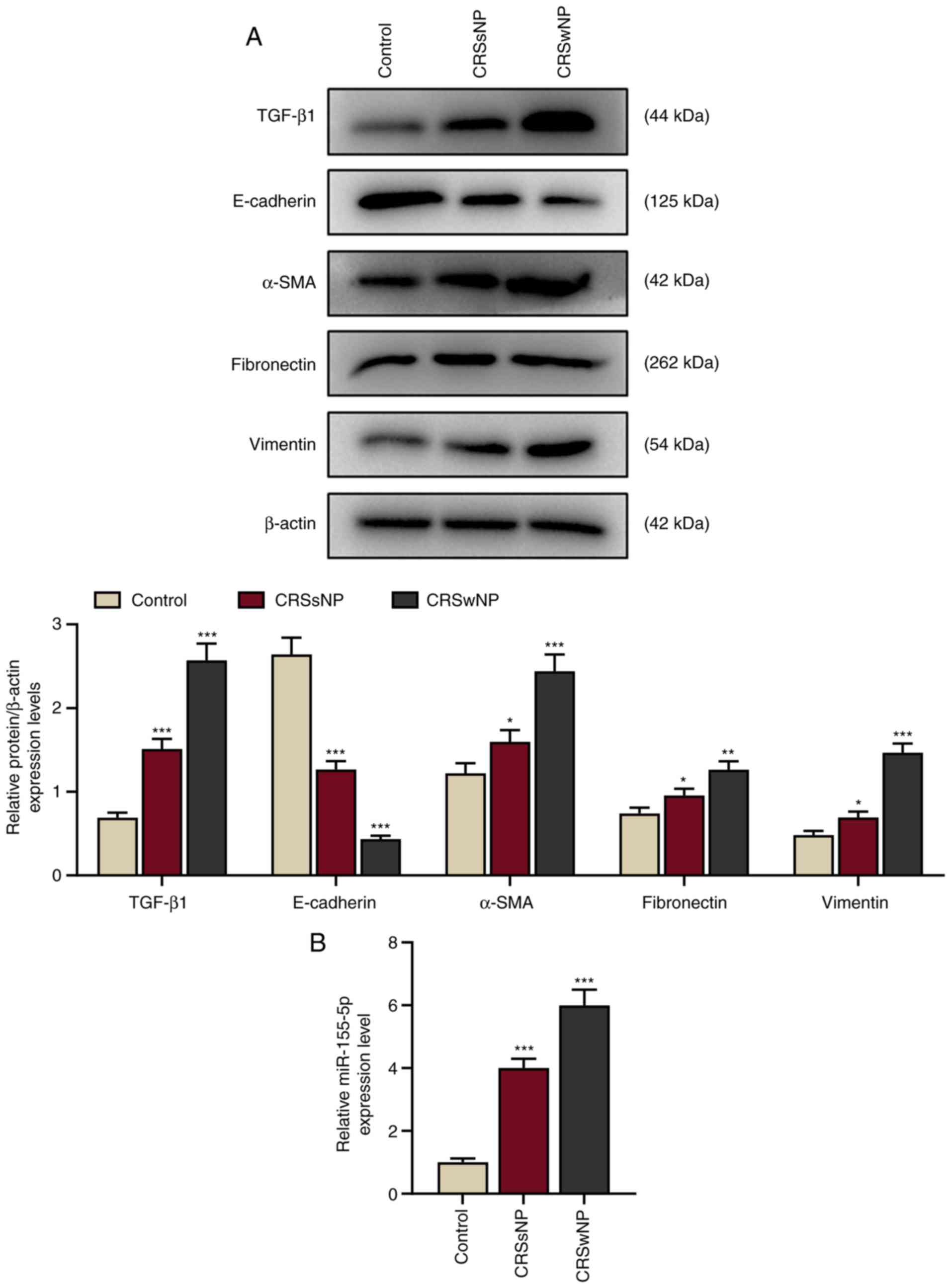 | Figure 1.Epithelial markers are downregulated
and TGF-β1, mesenchymal markers and miR-155-5p are upregulated in
patients with CRSwNP. (A) Relative protein/β-actin expression
levels of TGF-β1 and epithelial-to-mesenchymal
transition-associated proteins (E-cadherin, α-SMA, fibronectin and
vimentin) in the control, CRSsNP and CRSwNP groups were detected by
western blotting. β-actin served as the internal control. (B)
Relative miR-155-5p expression in the control, CRSsNP and CRSwNP
groups was assessed by reverse transcription-quantitative PCR. U6
served as the internal control. Experiments were performed in
triplicate. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001 vs. control. TGF-β1,
transforming growth factor β1; α-SMA, α-smooth muscle actin;
CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP,
chronic rhinosinusitis with nasal polyps; miR, microRNA. |
Additionally, the expression of miR-155-5p was
determined to investigate the role of miR-155 in CRS. The
expression of miR-155-5p was significantly increased in the CRSwNP
and CRSsNP groups compared with the control (P<0.001; Fig. 1B), indicating that miR-155-5p may
be a therapeutic target for CRS.
TGF-β1 upregulates miR-155-5p
expression and induces EMT in HNEpCs, which is reversed by
miR-155-5p inhibitors
To determine the function of miR-155-5p in HNEpCs,
HNEpCs were transfected with miR-155-5p inhibitors to knockdown
miR-155-5p expression. Control and NC groups were established and
the transfection rate of miR-155-5p was measured by RT-qPCR. The
results demonstrated that miR-155-5p expression in the I group was
downregulated compared with the control (Fig. 2A; P<0.05 vs. NC), indicating
that miR-155-5p inhibitors reduced miR-155-5p expression.
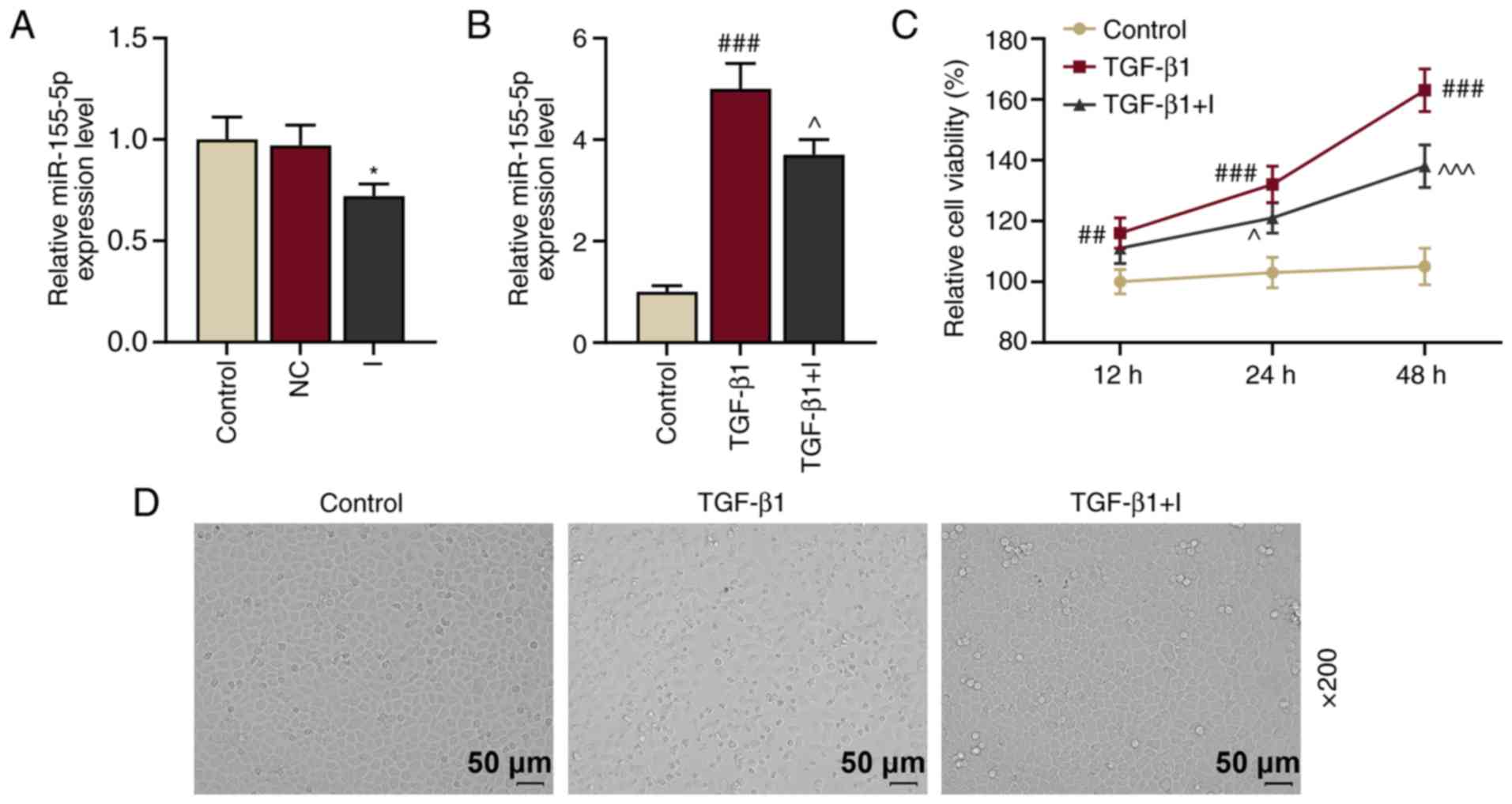 | Figure 2.TGF-β1 upregulates miR-155-5p
expression and induces epithelial-to-mesenchymal transition of
HNEpCs, which is reversed by miR-155-5p inhibitors. (A) Relative
miR-155-5p expression in the control, NC and I groups were measured
using RT-qPCR. U6 served as the internal control. *P<0.05 vs.
NC. (B) Relative miR-155-5p expression levels in the control,
TGF-β1 and TGF-β1+I groups were determined by RT-qPCR. U6 served as
the internal control. ###P<0.001 vs. control;
^P<0.05 vs. TGF-β1. (C) Cell viability in the HNEpC
control, TGF-β1 and TGF-β1+I groups was assessed using a Cell
Counting Kit-8 assay. ##P<0.01 and
###P<0.001 vs. control; ^P<0.05 and
^^^P<0.001 vs. TGF-β1. (D) Morphological changes on
HNEpCs were observed under a microscope. Experiments were performed
in triplicate. Data are presented as the mean ± standard deviation.
TGF-β1, transforming growth factor β1; miR, microRNA; HNEpCs, human
nasal epithelial cells; NC, negative control; I, miR-155-5p
inhibitor; RT-qPCR, reverse transcription-quantitative PCR. |
miR-155-5p expression has been reported to be
upregulated by TGF-β1 (19).
Therefore, miR-155-5p expression in HNEpCs prior to or following 5
ng/ml TGF-β1 treatment was examined. The results demonstrated that
miR-155-5p expression was significantly upregulated following 5
ng/ml TGF-β1 treatment (P<0.001 vs. the control; Fig. 2B); however, this expression was
inhibited by miR-155-5p inhibitors (P<0.05 vs. TGF-β1).
Furthermore, cell viability was determined via CCK-8 assay and the
results demonstrated that viability in HNEpCs was promoted by
TGF-β1 (P<0.001 vs. the control) and inhibited by miR-155-5p
inhibitors (P<0.05 vs. TGF-β1; Fig.
2C).
A previous study revealed that TGF-β1 induced EMT in
primary airway epithelial cells (20). In the present study, HNEpCs were
stimulated by TGF-β1 to observe whether TGF-β1 induced EMT in
HNEpCs. TGF-β1 treatment in HNEpCs induced transition HNEpCs from
normal epithelial morphology with cobblestone-like appearance into
migratory mesenchymal morphology with an abnormally elongated
appearance (Fig. 2D). However,
these changes were inhibited following the transfection of
miR-155-5p inhibitors. These results indicated that TGF-β1
treatment induced EMT in HNEpCs. Immunohistochemical staining and
transepithelial resistance could be performed in future studies to
examine cell-cell contact adhesion.
During EMT development, cell-to-cell adhesion,
polarity of epithelial cells and transformation of epithelial cells
into mesenchymal cells gradually decline (21). The protein expression levels of
EMT-related proteins (E-cadherin, α-SMA, fibronectin, and vimentin)
were assessed using western blotting. In accordance with changes in
cell morphology, TGF-β1 treatment significantly downregulated
E-cadherin protein expression and significantly upregulated α-SMA,
fibronectin and vimentin protein expression in HNEpCs (P<0.01
and P<0.001 vs. the control; Fig.
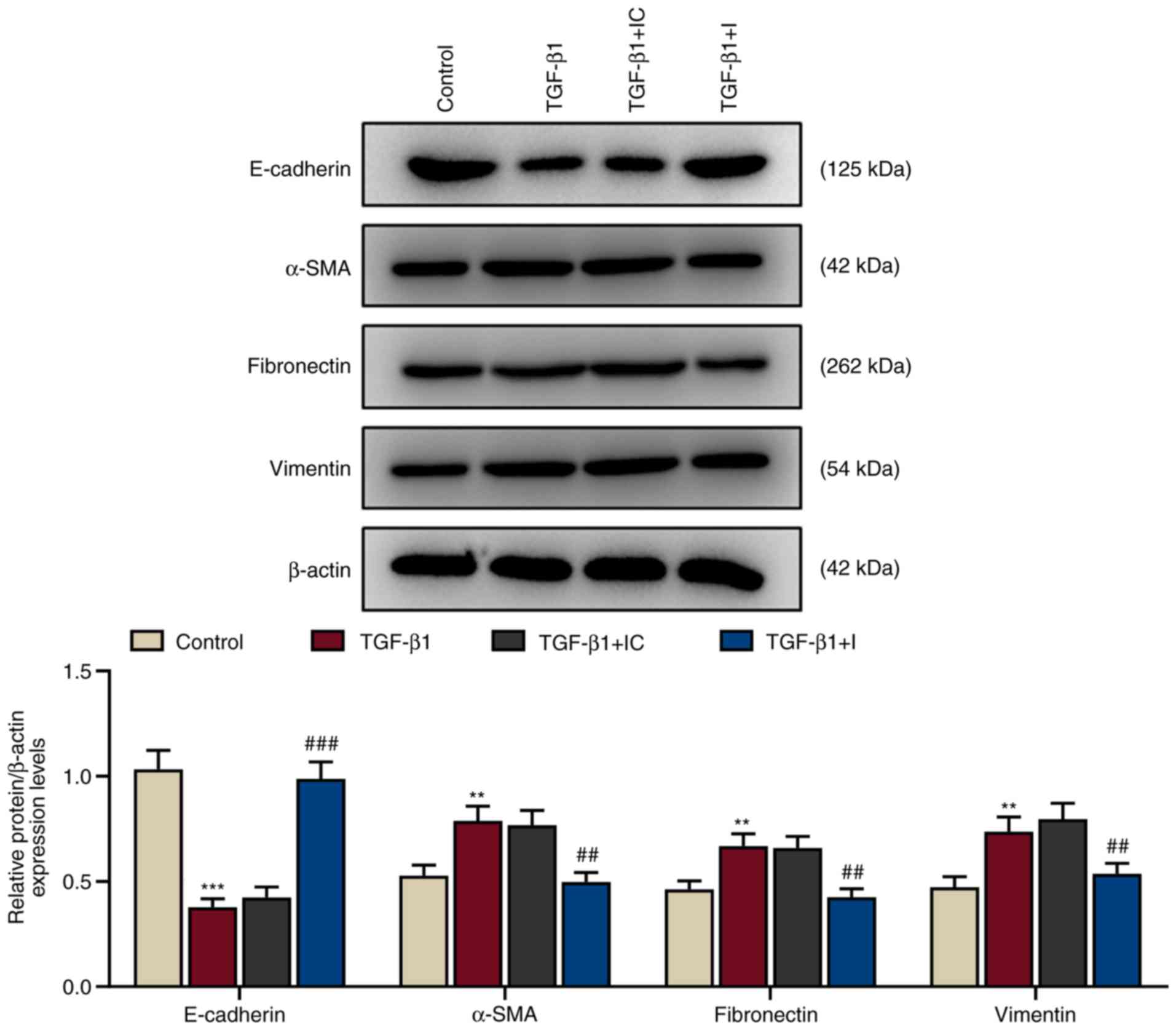
3). These expression levels were reversed by miR-155-5p
inhibitors (P<0.01 and P<0.001 vs. TGF-β1+IC), indicating
that TGF-β1 induced EMT in HNEpCs.
miR-155-5p mediates TGF-β1-induced EMT
in HNEpCs by regulating SIRT1 expression
miRNAs regulate gene expression by binding to the
3′-UTR of target mRNAs (22). The
online database TargetScan was used to successfully predict that
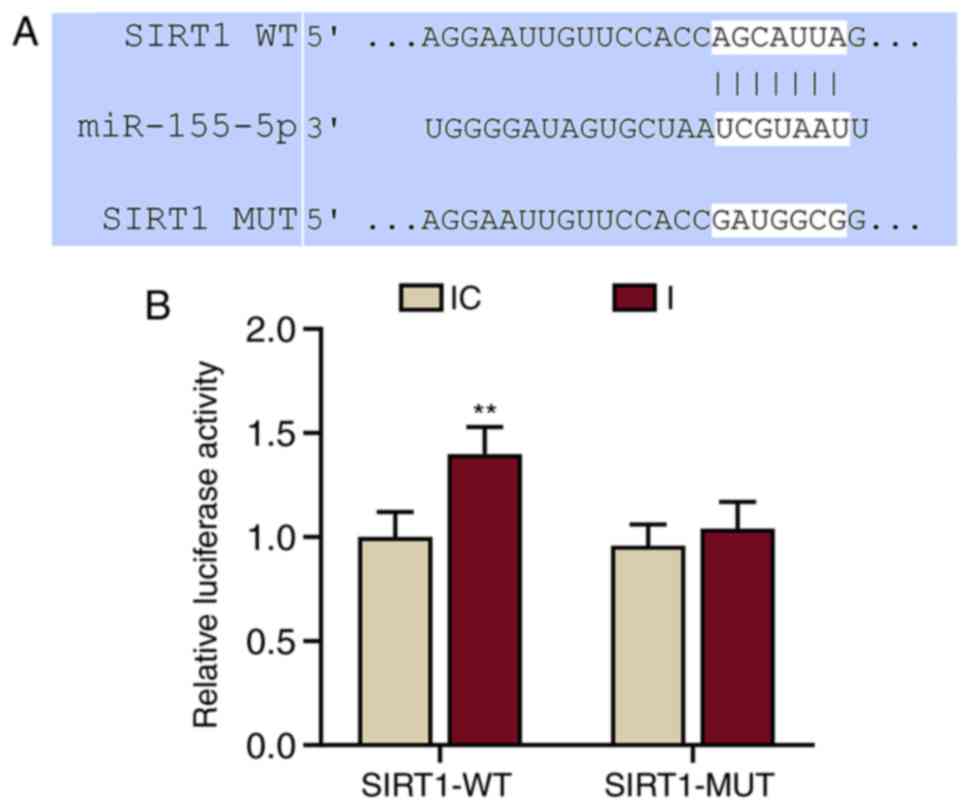
SIRT1 was a possible target of miR-155-5p (Fig. 4A). This prediction was confirmed by
dual-luciferase reporter assays (Fig.
4B). The results demonstrated that the relative luciferase
activity of the SIRT1-WT group was significantly increased compared
with the IC group (P<0.001 vs. IC). miR-155-5p inhibitors did
not affect the luciferase activity in the SIRT1-MUT group. These
results demonstrated that SIRT1 was a target of miR-155-5p.
To further determine the role of SIRT1 in CRS, the
protein and mRNA expression levels of SIRT1 in CRSsNP, CRSwNP and
control groups were detected by western blotting and RT-qPCR. The
results demonstrated that the protein and mRNA expression levels of
SIRT1 were downregulated in the CRSsNP and CRSwNP groups compared
with the control (Fig. 5A and B;
P<0.01 and P<0.001 vs. the control).
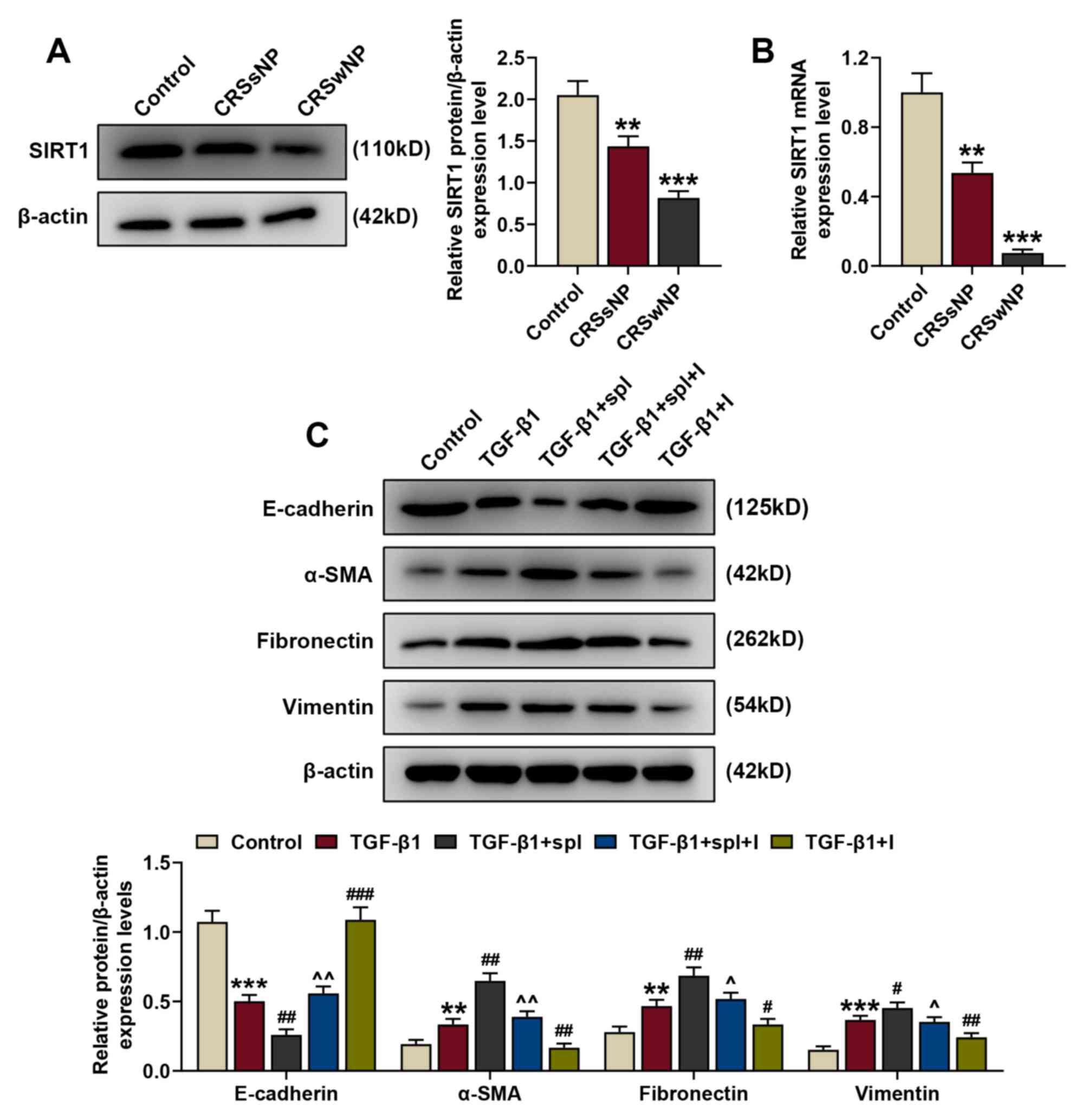 | Figure 5.miR-155-5p mediates TGF-β1-induced
EMT of human nasal epithelial cells by regulating SIRT1 expression.
(A) Relative SIRT1/β-actin expression in the control, CRSsNP and
CRSwNP groups was measured by western blotting. β-actin served as
the internal control. (B) Relative SIRT1 mRNA expression in the
control, CRSsNP and CRSwNP groups was measured by reverse
transcription-quantitative PCR. β-actin served as the internal
control. (C) Relative protein/β-actin expressions of EMT-related
proteins (E-cadherin, α-SMA, fibronectin and vimentin) were
assessed by western blotting. β-actin served as the internal
control. **P<0.01 and ***P<0.001 vs. the control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. TGF-β1; ^P<0.05 and
^^P<0.01 vs. TGF-β1+spl. Experiments were performed
in triplicate. Data are presented as the mean ± standard deviation.
miR, microRNA; TGF-β1, transforming growth factor β1; EMT,
epithelial-to-mesenchymal transition; SIRT1, sirtuin 1; CRSsNP,
chronic rhinosinusitis without nasal polyps; CRSwNP, chronic
rhinosinusitis with nasal polyps; α-SMA, α-smooth muscle actin;
spl, splitomicin; I, miR-155-5p inhibitor. |
Following treatment with 5 ng/ml TGF-β1 and 100 µM
splitomicin treatment, the protein expression of EMT-associated
markers (E-cadherin, α-SMA, fibronectin and vimentin) in HNEpCs was
assessed by western blotting to detect the association between
miR-155-5p and SIRT1, and their effects on EMT. Consistent with
previous results, following TGF-β1 treatment, the protein
expression of E-cadherin was downregulated and the expression
levels of α-SMA, fibronectin and vimentin were upregulated compared
with the control (Fig. 5C;
P<0.01 and P<0.001 vs. the control). Splitomicin treatment
further enhanced the effect of TGF-β1 on protein expression
(P<0.01 and P<0.001, vs. TGF-β1), indicating that SIRT1
inhibition promoted EMT. Furthermore, these changes in protein
expression were reversed by miR-155-5p inhibitors (P<0.05 and
P<0.01 vs. TGF-β1+spl). This suggested that miR-155-5p
downregulation mediated TGF-β1-induced EMT in HNEpCs by regulating
SIRT1 expression.
Discussion
The present study revealed that miR-155-5p mediated
EMT in HNEpCs by regulating SIRT1 expression in response to TGF-β1.
To the best of knowledge, the present study is the first to report
that miR-155-5p served an essential role in EMT in CRS. Although
miRNA dysfunction has been widely discussed in various
pathophysiological processes, such as coronary heart disease
(23), atherosclerosis (24) and cancer (25), their biological roles and molecular
mechanisms in CRS development and progression remain unclear.
miR-155 upregulation and TGF-β1 overexpression accelerate EMT and
promote cell migration and invasion, thereby inducing
hepatocellular carcinoma progression (26). Furthermore, TGF-β1 has been
reported to upregulate miR-155 expression to alter osteoclast
differentiation (27). Based on
these findings, the present study hypothesized that miR-155
induction by TGF-β1 may serve a potential role in CRS
pathogenesis.
CRS is an inflammatory upper respiratory tract
disease characterized by a persistent inflammatory state and
paranasal sinuses, with a minimum duration of 12 weeks (28,29).
Based on the presence or absence of polyposis, CRS can be divided
into CRSwNP and CRSsNP (5). Though
the occurrence of CRSwNP is infrequent, medical intervention is
unsuccessful for a large proportion of patients with CRSwNP
(8). In addition to inflammation,
CRSwNP and CRSsNP are characterized by remodeling of structural
components, particularly in the epithelium (30). Remodeling under normal conditions
is defined as an essential reconstruction process in response to
minor inflammatory conditions; however, dysregulated remodeling,
which may be induced by severe or chronic inflammation, can result
in pathological reconstruction and formation of pathological
tissues during healing and repairing (31). Depending on the disease and its
severity, inflammation often causes different degrees of tissue
injury, indicating that remodeling may occur during inflammatory
diseases, including CRSwNP (17).
Recently, the role of remodeling in upper airway diseases, such as
CRS, has received considerable attention, partly since remodeling
begins at an early disease stage and contributes to later
development and progression (31).
To date, the mechanisms of tissue remodeling have
not been fully elucidated, however, accumulating evidence has
indicated that EMT may be involved (32–34).
In multiple progressive fibrotic diseases, EMT is seen as a point
of convergence between inflammation and pathological remodeling
(31). EMT is a process by which
differentiated epithelial cells undergo phenotypic transformation
and acquire the mesenchymal cell phenotype (35). A previous study reported that
ongoing EMT may result in the progression of numerous diseases,
such as cancer (36) and chronic
kidney disease (37), particularly
in CRSwNP (1). Following injury,
the expression of junctional proteins, including E-cadherin, are
downregulated and the expression of mesenchymal proteins, such as
α-SMA, vimentin and fibronectin, are upregulated in epithelial
cells, which then undergo EMT and alter morphology (38). In accordance with previous studies,
the present study suggested that E-cadherin expression was reduced,
while expression of α-SMA, vimentin and fibronectin were increased
in CRSwNP and CRSsNP tissues compared with control groups. CRSsNP
is characterized by fibrosis, a thickened membrane of basement and
excessive goblet cells (39).
TGF-β1, a significant regulator of fibrosis promotion and airway
remodeling, was demonstrated to be upregulated in CRSsNP compared
with control. This result was consistent with a previous study
(40).
TGF-β1 is a multifunctional peptide regulating
various cellular functions, including cell growth, differentiation,
apoptosis and migration (41).
TGF-β1 is implicated in the pathogenesis of airway diseases,
including chronic obstructive pulmonary disease (42) and CRS (1). TGF-β1 serves a key role in fibrosis
and EMT, and induces epithelial cell transition, contributes to
tissue remodeling and CRSwNP pathogenesis (43). The present results revealed that
TGF-β1 expression was upregulated in the CRSwNP group compared with
the control. Furthermore, TGF-β1 altered HNEpCs morphology and
regulated the expression of EMT-related proteins. These expression
levels were reversed by downregulating the expression of
miR-155-5p.
miR-155-5p is associated with various diseases. For
example, miR-155-5p affects Wilms' tumor cell proliferation and
apoptosis by targeting CREB1 (44). Moreover,
p53/miR-155-5p/SIRT1-mediated autophagic processes have been
implicated in diabetic kidney injury (45). miR-155-5p has also been
demonstrated to mediate the TGF-β1 signaling pathway and EMT
(26). Zhang et al
(46) reported that miR-155-5p
regulated cardiac fibrosis induced by glucose via the TGF-β1
signaling pathway. Wang et al (47) demonstrated that miR-155-5p
regulated TGF-β-induced EMT in human coronary artery endothelial
cells by targeting c-Ski to affect cardiac fibrosis (47). The results of the present study
revealed that miR-155-5p expression was increased in the CRSwNP
group compared with the control and that upregulated miR-155-5p
expression induced by TGF-β1 caused EMT in HNEpCs. Furthermore,
these results were reversed by miR-155-5p downregulation.
A previous study reported that SIRT1 attenuates
nasal polypogenesis through EMT suppression (48). Furthermore, it has been
demonstrated that SIRT1 is a target gene of miR-155-5p (49). Huang et al (49) reported that miR-155 downregulation
stimulated cardioprotection against myocardial ischemia/reperfusion
injury by binding to SIRT1. However, the role of miR-155-5p in
inhibiting EMT has not been extensively reported. The present study
demonstrated that miR-155 downregulation attenuated CRS by
inhibiting EMT via targeting of SIRT1.
However, the present study still had some
limitations. Firstly, the number of samples in the experimental
groups were not equal and an animal model was not established.
Secondly, there was a lack of immunohistochemical data. Further
studies could perform Transwell assays to confirm the ETM state of
the HNEpCs cells.
In conclusion, the results of the present study
revealed that miR-155 may be a potential therapeutic target for CRS
treatment by suppressing EMT. Furthermore, miR-155 inhibitors may
be a novel anti-polyp drug in the regulation of SIRT1 expression in
HNEpCs and miR-155 downregulation may be a potential method for
treating CRS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Clinical
Medical Technology Innovation Guide Project of Hunan Province
(grant no. 2017SK51405).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NY and HC conceptualized and designed the present
study, and drafted and critically revised the manuscript for
intellectual content. QM, XZ and MX performed data acquisition,
analysis and interpretation. All authors agreed to be accountable
in ensuring that enquiries related to the accuracy and/or integrity
of the present work are appropriately investigated and resolved.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Zhujiang Hospital, Southern
Medical University, Guangzhou, China approved the present study
(approval no. EBYHK20190503) and written informed consent was
obtained from all patients. Furthermore, all procedures involving
human participants were in accordance with the ethical standards of
the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schleimer RP: Immunopathogenesis of
chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol.
12:331–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee S and Lane AP: Chronic rhinosinusitis
as a multifactorial inflammatory disorder. Curr Infect Dis Rep.
13:159–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savovic S, Buljcik-Cupic M, Jovancevic L,
Kljajic V, Lemajic-Komazec S and Dragicevic D: Frequency and
intensity of symptoms in patients with chronic rhinosinusitis.
Srpski Arhiv Za Celokupno Lekarstvo. 147:132018.
|
|
4
|
Hull BP and Chandra RK: Refractory chronic
rhinosinusitis with nasal polyposis. Otolaryngol Clin North Am.
50:61–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: European position paper on rhinosinusitis and nasal polyps
2012. Rhinol Suppl. 23:32012.PubMed/NCBI
|
|
6
|
Piromchai P, Kasemsiri P, Laohasiriwong S
and Thanaviratananich S: Chronic rhinosinusitis and emerging
treatment options. Int J Gen Med. 6:453–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koskinen A, Numminen J, Markkola A,
Karjalainen J, Karstila T, Seppälä M, Julkunen A, Lemmetyinen R,
Pekkanen J, Rautiainen M, et al: Diagnostic accuracy of symptoms,
endoscopy, and imaging signs of chronic rhinosinusitis without
nasal polyps compared to allergic rhinitis. Am J Rhinol Allergy.
32:121–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Feng J, Zhang D, Yong J, Wang Y, Yao
J and Huang R: Differential expression of miR-4492 and IL-10 is
involved in chronic rhinosinusitis with nasal polyps. Exp Ther Med.
18:3968–3976. 2019.PubMed/NCBI
|
|
9
|
Konnecke M, Burmeister M, Pries R, Boscke
R, Bruchhage KL, Ungefroren H, Klimek L and Wollenberg B:
Epithelial-mesenchymal transition in chronic rhinosinusitis:
Differences revealed between epithelial cells from nasal polyps and
inferior turbinates. Arch Immunol Ther Exp (Warsz). 65:157–173.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qing X, Zhang Y, Peng Y, He G, Liu A and
Liu H: miR-142-3p regulates inflammatory response by contributing
to increased TNF-α in chronic rhinosinusitis with nasal polyposis.
Ear Nose Throat J. 9:1455613198479722019.
|
|
12
|
Lu C, Chen B, Chen C, Li H, Wang D, Tan Y
and Weng H: CircNr1h4 regulates the pathological process of renal
injury in salt-sensitive hypertensive mice by targeting miR-155-5p.
J Cell Mol Med. 24:1700–1712. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L, Ouyang Y, Bai Y, Gong J and Liao
H: miR-155-5p inhibits the viability of vascular smooth muscle cell
via targeting FOS and ZIC3 to promote aneurysm formation. Eur J
Pharmacol. 853:145–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thwin M, Weitzel EK, McMains KC,
Athanasiadis T, Psaltis A, Field J and Wormald PJ: Validating the
use of report-derived lund-macKay scores. Am J Rhinol Allergy.
23:33–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lund VJ and Kennedy DW: Quantification for
staging sinusitis. The staging and therapy group. Ann Otol Rhinol
Laryngol Suppl. 167:17–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Linder A: Symptom scores as measures of
the severity of rhinitis. Clin Allergy. 18:29–37. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Li C, Zhu G, Yuan W and Xiao ZA:
TGF-β1 induces epithelial-mesenchymal transition of chronic
sinusitis with nasal polyps through MicroRNA-21. Int Arch Allergy
Immunol. 179:304–319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu F, Kong X, Lv L and Gao J: TGF-β1 acts
through miR-155 to down-regulate TP53INP1 in promoting
epithelial-mesenchymal transition and cancer stem cell phenotypes.
Cancer Lett. 359:288–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park IH, Kang JH, Shin JM and Lee HM:
Trichostatin A inhibits epithelial mesenchymal transition induced
by TGF-β1 in airway epithelium. PLoS One. 11:e01620582016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Z, Shen Y, Zeng Q, Liu J, Yang L, Fu R
and Hu G: MiR-150-5p regulates EGR2 to promote the development of
chronic rhinosinusitis via the DC-Th axis. Int Immunopharmacol.
54:188–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu ZW, Liu YF, Wang S and Li B: miRNA-146a
induces vascular smooth muscle cell apoptosis in a rat model of
coronary heart disease via NF-κB pathway. Genet Mol Res.
14:18703–18712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Canfrán-Duque A, Rotllan N, Zhang X,
Fernández-Fuertes M, Ramírez-Hidalgo C, Araldi E, Daimiel L, Busto
R, Fernández-Hernando C and Suárez Y: Macrophage deficiency of
miR-21 promotes apoptosis, plaque necrosis, and vascular
inflammation during atherogenesis. EMBO Mol Med. 9:1244–1262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Haidari AA, Syk I and Thorlacius H:
miR-155-5p positively regulates CCL17-induced colon cancer cell
migration by targeting rhoA. Oncotarget. 8:14887–14896. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li DP, Fan J, Wu YJ, Xie YF, Zha JM and
Zhou XM: MiR-155 up-regulated by TGF-β promotes
epithelial-mesenchymal transition, invasion and metastasis of human
hepatocellular carcinoma cells in vitro. Am J Transl Res.
9:2956–2965. 2017.PubMed/NCBI
|
|
27
|
Zhao H, Zhang J, Shao H, Liu J, Jin M,
Chen J and Huang Y: Transforming growth factor β1/Smad4 signaling
affects osteoclast differentiation via regulation of miR-155
expression. Mol Cells. 40:211–221. 2017.PubMed/NCBI
|
|
28
|
Koennecke M, Benecke F, Masche A, Linke R,
Bruchhage KL, Pries R, Klimek L and Wollenberg B: Increased
phosphorylation of eNOS in nasal polyps of chronic rhinosinusitis
patients can be diminished by 1,8-cineol. Nitric Oxide. 78:89–94.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
DeConde AS and Soler ZM: Chronic
rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol
Allergy. 30:134–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hupin C, Gohy S, Bouzin C, Lecocq M,
Polette M and Pilette C: Features of mesenchymal transition in the
airway epithelium from chronic rhinosinusitis. Allergy.
69:1540–1549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee HM, Kang JH, Shin JM, Lee SA and Park
IH: Chemical chaperone of endoplasmic reticulum stress inhibits
epithelial-mesenchymal transition induced by TGF-β1 in airway
epithelium via the c-src pathway. Mediators Inflamm.
2017:81232812017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pu Y, Liu Y, Liao S, Miao S, Zhou L and
Wan L: Azithromycin ameliorates OVA-induced airway remodeling in
Balb/c mice via suppression of epithelial-to-mesenchymal
transition. Int Immunopharmacol. 58:87–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirota N and Martin JG: Mechanisms of
airway remodeling. Chest. 144:1026–1032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian X, Tian X, Huo R, Chang Q, Zheng G,
Du Y, Chen Y and Niu B: Bacillus calmette-guerin alleviates airway
inflammation and remodeling by preventing TGF-β1 induced
epithelial-mesenchymal transition. Hum Vaccin Immunother.
13:1758–1764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cruz-Solbes AS and Youker K: Epithelial to
mesenchymal transition (EMT) and endothelial to mesenchymal
transition (EndMT): Role and implications in kidney fibrosis.
Results Probl Cell Differ. 60:345–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Title AC, Hong SJ, Pires ND, Hasenöhrl L,
Godbersen S, Stokar-Regenscheit N, Bartel DP and Stoffel M: Genetic
dissection of the miR-200-Zeb1 axis reveals its importance in tumor
differentiation and invasion. Nat Commun. 9:46712018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu L, Zhu J, Zhang Y, Wang Y, Zhang S and
Xia A: Febuxostat inhibits TGF-β1-induced epithelial-mesenchymal
transition via downregulation of USAG-1 expression in madin-darby
canine kidney cells in vitro. Mol Med Rep. 19:1694–1704.
2019.PubMed/NCBI
|
|
38
|
Sisto M, Lisi S and Ribatti D: The role of
the epithelial-to-mesenchymal transition (EMT) in diseases of the
salivary glands. Histochem Cell Biol. 150:133–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khalmuratova R, Park JW and Shin HW:
Immune cell responses and mucosal barrier disruptions in chronic
rhinosinusitis. Immune Netw. 17:60–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen L, Xiao L, Liu J, Shen Y, Ke X, Huang
J, Hu G and Yang Y: Differential expression of the aryl hydrocarbon
receptor and transforming growth factor beta 1 in chronic
rhinosinusitis with nasal polyps with allergic rhinitis. ORL J
Otorhinolaryngol Relat Spec. 79:295–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim SI and Choi ME: TGF-β-activated
kinase-1: New insights into the mechanism of TGF-β signaling and
kidney disease. Kidney Res Clin Pract. 31:94–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mahmood MQ, Reid D, Ward C, Muller HK,
Knight DA, Sohal SS and Walters EH: Transforming growth factor
(TGF) β1 and Smad signalling pathways: A likely key to
EMT-associated COPD pathogenesis. Respirology. 22:133–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ryu NH, Shin JM, Um JY, Park IH and Lee
HM: Wogonin inhibits transforming growth factor β1-induced
extracellular matrix production via the p38/activator protein 1
signaling pathway in nasal polyp-derived fibroblasts. Am J Rhinol
Allergy. 30:128–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao XS, Han B, Zhao JX, Tao N and Dong
CY: miR-155-5p affects wilms' tumor cell proliferation and
apoptosis via targeting CREB1. Eur Rev Med Pharmacol Sci.
23:1030–1037. 2019.PubMed/NCBI
|
|
45
|
Wang Y, Zheng ZJ, Jia YJ, Yang YL and Xue
YM: Role of p53/miR-155-5p/sirt1 loop in renal tubular injury of
diabetic kidney disease. J Transl Med. 16:1462018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang D, Cui Y, Li B, Luo X, Li B and Tang
Y: miR-155 regulates high glucose-induced cardiac fibrosis via the
TGF-β signaling pathway. Mol Biosyst. 13:215–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, He W, Xu X, Guo L, Zhang Y, Han S
and Shen D: The mechanism of TGF-β/miR-155/c-Ski regulates
endothelial-mesenchymal transition in human coronary artery
endothelial cells. Biosci Rep. 37:BSR201606032017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee M, Kim DW, Yoon H, So D, Khalmuratova
R, Rhee CS, Park JW and Shin HW: Sirtuin 1 attenuates nasal
polypogenesis by suppressing epithelial-to-mesenchymal transition.
J Allergy Clin Immunol. 137:87–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang G, Hao F and Hu X: Downregulation of
microRNA-155 stimulates sevoflurane-mediated cardioprotection
against myocardial ischemia/reperfusion injury by binding to SIRT1
in mice. J Cell Biochem. 120:15494–15505. 2019. View Article : Google Scholar : PubMed/NCBI
|