Introduction
Retinoblastoma (Rb) is one of the most common types
of intraocular malignant tumor to occur in children; the annual
global incidence rate in children <15 years is 3.5 cases per
million children (1). It
demonstrates a high invasive and metastatic ability, which
corresponds with a poor prognosis and visual impairment in
children, endangering lives (2–4).
Previous studies have demonstrated that tumor invasion and
metastasis may derive from epithelial-mesenchymal transition (EMT),
a basic physiological phenomena which is characterized by the
transition of epithelial cells into active mesenchymal cells
capable of moving freely between cell substrates (5–7).
Currently, several therapeutic options exist for Rb, including
ophthalmectomy, or radiation, chemical, drug, laser
photocoagulation, photodynamic, thermo-, freezing and gene
therapies (8–11). Notably, due to its targeting effect
and minor toxicity, gene therapy has become the focus of numerous
studies (12,13).
Long non-coding RNAs (lncRNAs) are non-protein
coding RNAs of >200 nucleotides in length, which are found in
abundance in eukaryotic and mammalian cells (14). Emerging evidence has revealed that
lncRNAs serve an important role in the migration, invasion and EMT
of tumors, including breast cancer, colorectal cancer,
hepatocellular carcinoma, prostate cancer and Rb (15–17).
Interestingly, lncRNAs have been previously used as therapeutic
targets and prognostic markers in numerous types of tumor,
including glioblastoma, as well as gastric and colorectal cancer
(18,19). FEZ family zinc finger 1 antisense
RNA 1 (FEZF1-AS1) is a more recently discovered lncRNA of 2,564 bp
in length, located on chromosome 7 (20). A previous study demonstrated that
the expression levels of FEZF1-AS1 were upregulated in Rb cell
lines and tissues, whereby these upregulated expression levels of
FEZF1-AS1 were discovered to be an independent unfavorable
prognostic factor that promoted the proliferation, invasion and
migration of Rb cells (21).
MicroRNAs (miRs/miRNAs) are non-coding, single-
stranded RNA molecules of ~22 nucleotides in length, encoded by
endogenous genes (22). The
abnormal expression of miRNAs has been reported in various types of
cancer, such as hepatocellular carcinoma, gastric cancer and
retinoblastoma (23,24). Notably, previous studies have
demonstrated that miR-1236-3p inhibited the migration and invasion
of A549 and ovarian cancer cells by targeting Kruppel-like factor 8
or zinc finger E-box-binding homeobox 1 (25,26).
However, to the best of our knowledge, studies on the effects of
miR-1236-3p in Rb have not been reported. In the present study,
LncBase software was used to predict that FEZF1-AS1 may bind to
miR-1236-3p. Therefore, it was hypothesized that FEZF1-AS1 may
interact with miR-1236-3p to regulate the viability, invasion,
migration and EMT of Rb cells.
Materials and methods
Cell culture and transfection
Normal retinal epithelial ARPE-19 cells (control)
and the human Rb cell line Y79 were purchased from BeNa Culture
Collection; Beijing Beina Chuanglian Biotechnology Research
Institute. The human Rb cell line SO-Rb50 was obtained from
Qincheng Biotechnology, the human Rb cell line Weri-Rb-1 was
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences and the human Rb cell line RBL-13 was
purchased from the American Type Culture Collection. All cells were
cultured in RPMI-1640 medium (American Type Culture Collection),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
and maintained in a humidified incubator at 37°C with 5%
CO2.
Synthetic sequences of short hairpin RNA (shRNA)
targeting FEZF1-AS1 (shRNA-FEZF1-AS1; Shanghai GenePharma Co.,
Ltd.) and non-targeting shRNA (shRNA-NC) were inserted into
pGPU6/Neo vector (Shanghai GenePharma Co., Ltd.). 2×104
cells/well were seed into a 24-well plate, then 0.8 µg shRNA was
added into each well at confluence of 40–60% using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The miR-1236-3p mimic and negative control (NC;
miR-NC mimic), miR-1236-3p inhibitor and miR-NC inhibitor were
generated from Shanghai GenePharma Co., Ltd., 1×105
cells/well were seed into a 6-well plate, 100 nM miR-1236-3p
mimic/miR-NC mimic or 200 nM miR-1236-3p inhibitor/miR-NC inhibitor
was transfected into Y79 cells at confluence of 40–60% using
Lipofectamine® 2000 reagent. Following 48 h of
transfection at 37°C, the transfection efficiency was validated
using reverse transcription-quantitative PCR (RT-qPCR). All
sequences are listed in Table
I.
 | Table I.Oligonucleotide sequences used for
the transfection experiments. |
Table I.
Oligonucleotide sequences used for
the transfection experiments.
| Name | Sequence
(5′→3′) |
|---|
| miR-1236-3p
mimic | F:
CGCGGATCCCTGGCCCTCACTTACCTC |
|
| R:
CCGAATTCCCATCTACATTCCAACTTGGAG |
| miR-NC mimic | F:
UUCUCCGAACGUGUCACGUTT |
|
| R:
ACGUGACACGUUCGGAGAATT |
| miR-1236-3p
inhibitor |
CUGGAGAGACAAGGGGAAGAGG |
| miR-NC
inhibitor |
CAGUACUUUUGUGUAGUACAA |
| shRNA-FEZ family
zinc | F:
CCGGCCCACGAAGTTTAAAGCATAACTCGAGTTATGCTTTAAACTTCGTGGGTTTTTG |
| finger 1 antisense
RNA 1 | R:
AATTCAAAAACCCACGAAGTTTAAAGCATA ACTCGAGTTATGCTTTAAACTTCGTGGG |
| shRNA-NC | F:
CCGGTTCTCCGAACGTGTCACGTAACTCGAGTTACGTGACACGTTCGGAGAATTTTTG |
|
| R:
AATTCAAAAATTCTCCGAACGTGTCACGTAACTCGAGTTACGTGACACGTTCGGAGAA |
Bioinformatics analysis
LncBase V.2 software (carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex)
was used to predict interactions between FEZF1-AS1 and miR-1236-3p
by searching for the binding sites between the two sequences.
RT-qPCR
Total RNA was extracted from transfectedcells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). PrimeScript™ RT reagent kit (Takara Bio, Inc.) was used for
cDNA generation of FEZF1-AS1, using the following reaction
conditions: 42°C for 15 min followed by 3 cycles and 85°C for 5
sec. For miR-1236-3p, a TaqMan MicroRNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.) was used for cDNA generation using
the following reaction conditions: 50°C for 5 min and 80°C for 2
min. Subsequently, the reaction templates were mixed in tubes
according to the manufacturer's instructions of SYBR Premix Ex Taq™
II kit (Takara Bio, Inc.), centrifuged gently (111 × g) for 5 sec
at 4°C and run through a 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Amplification
condition were: 95°C for 10 sec, followed by 40 cycles of 5 sec at
95°C and 30 sec at 60°C. The following primer sequences were used
for the qPCR: FEZF1-AS1 forward, 5′-TTAGGAGGCTTGTTCTGTGT-3′ and
reverse, 5′-GCGCAGGTACTTAAGAAAGA-3′; GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′;
miR-1236-3p forward, 5′-CCAATCAGCCTCTTCCCCTT-3′ and reverse,
5′-TATGGTTGTTCACGACTCCTTCAC-3′; and U6 forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. The relative expression levels of
miR-1236-3p and FEZF1-AS1 were calculated using the
2−ΔΔCt method (27),
and FEZF1-AS1 expression levels were normalized to GAPDH and
miR-1236-3p expression levels to U6.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology), according to
the manufacturer's protocol. Total protein was quantified by BCA
Protein Assay kit (Beyotime Institute of Biotechnology) and
equivalent amount of 20 µg protein samples were separated by 10%
SDS-PAGE (Beyotime Institute of Biotechnology). The separated
proteins were subsequently transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% non-fat milk for 2 h at room
temperature. The membranes were then incubated with the following
primary antibodies at 4°C overnight: Anti-Vimentin (1:1,000; cat.
no. 3932; Cell Signaling Technology, Inc.), anti-Snail (1:1,000;
cat. no. 3879; Cell Signaling Technology, Inc.), anti-Slug
(1:1,000; cat. no. 9585; Cell Signaling Technology, Inc.),
anti-Claudin-1 (1:1,000; cat. no. 4933; Cell Signaling Technology,
Inc.), anti-β-catenin (1:500; cat. no. sc-59737; Santa Cruz
Biotechnology, Inc.), anti-N-cadherin (1:500; cat. no. sc-59987;
Santa Cruz Biotechnology, Inc.), anti-E-cadherin (1:500; cat. no.
sc-8426; Santa Cruz Biotechnology, Inc.), anti-matrix
metalloproteinase (MMP) 2 (1:1,000; cat. no. 10373–2-AP;
ProteinTech Group, Inc.), anti-MMP9 (1:1,000; cat. no. 10375-2-AP;
ProteinTech Group, Inc.) and anti-GAPDH (1:1,000; cat. no.
SAB5600208; Sigma-Aldrich; Merck KGaA). Subsequently, the membranes
were incubated with the following secondary antibodies: Horseradish
peroxidase (HRP)-conjugated Affinipure Goat Anti-Mouse IgG (H+L)
(1:10,000; cat. no. SA00001-1; ProteinTech Group, Inc.) and
HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H+L) (1:10,000;
cat. no. SA00001-2; ProteinTech Group, Inc.). Protein bands were
visualized via Immobilon Western Chemilum HRP Substrate (cat. no.
WBKLS0100; EMD Millipore) and the expression levels of each protein
were analyzed using Image Lab software version 4.1 (Bio-Rad
Laboratories, Inc.).
Cell Counting Kit-8 (CCK-8) assay
Y79 cells (1×103 cells/well) were seed
into a 96-well plate in a humidified incubator at 37°C for 24 h.
Cell viability was determined using a CCK-8 assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. A total of 10 µl CCK-8 reagent was added into each well
and incubated for 1 h at 37°C. The absorbance was analyzed at 450
and 490 nm (reference wavelength) using a Multiskan™ GO microplate
spectrophotometer (Thermo Fisher Scientific, Inc.).
Wound healing assay
The cell migratory ability was analyzed using a
wound healing assay. Briefly, 5×105 cells/well were
plated into a six-well plate and cultured to 100% confluence. A
200-µl pipette tip was used to scratch a wound in the cell
monolayer and then the cells were cultured in serum-free RPMI-1640
medium for 24 h at 37°C. The width of the wound in each group was
photographed at 0 and 24 h using a light microscope (magnification,
×100, Olympus Corporation) and analyzed using Image J software
version 1.8.0 (National Institutes of Health).
Transwell Matrigel assay
Transwell chambers (Costar; Corning, Inc.) were used
to determine the invasive ability of the cells. Matrigel (BD
Biosciences) was diluted with serum-free RPMI-1640 medium at a 1:9
ratio and then used to precoat the membrane of the upper chambers
at 37°C for 2 h. PBS and serum-free RPMI-1640 medium were used once
each to wash Y79 cells and then 2×105 transfected cells
were suspended in 1 ml serum-free RPMI-1640 medium, 200 µl cell
suspension was added into the upper chambers of the Transwell
plates. RPMI-1640 medium supplemented with 10% FBS was plated in
the lower chambers. Following incubation for 24 h at 37°C, the
Transwell chamber was removed and the invasive cells in the lower
chamber were washed twice with PBS, prior to being fixed with 4%
formaldehyde for 30 min at room temperature and stained with 0.1%
crystal violet for 60 min at room temperature. Stained cells were
counted at least six random microscopic fields (magnification,
×100) using a light microscope (Olympus Corporation).
Dual-luciferase reporter assay
The binding sites between the two sequences of
FEZF1-AS1 and miR-1236-3p were predicted via LncBase V.2 software.
The 3′untranslated region (UTR) fragments from FEZF1-AS1 cDNA
containing the predicted miR-1236-3p-binding sites were synthesized
and inserted downstream of the luciferase gene in the pGL3 Basic
vector (Promega Corporation), to create FEZF1-AS1-wild-type (WT)
vectors. The FEZF1-AS1-mutant (MUT) vectors (Promega Corporation)
were synthesized using mutant sequences of FEZF1-AS1. Y79 cells
(1×105) were co-transfected with 0.6 µg FEZF1-AS1-WT or
FEZF1-AS1-MUT vectors and 100 nM miR-1236-3p mimic or miR-NC mimic
using Lipofectamine® 2000 reagent. Firefly
luciferase activity was analyzed via a Dual Luciferase Assay kit
(Promega Corporation) and normalized to Renilla luciferase
activity after 48 h of transfection at 37°C.
RNA immunoprecipitation (RIP)
assay
RIP was performed using the Magna RIP RNA-Binding
Protein Immunoprecipitation kit (EMD Millipore), according to the
manufacturer's protocol. Briefly, 2×107 cells were
washed with cold PBS twice, then incubated with 100 µl RIP Lysis
Buffer (EMD Millipore) for 5 min at 4°C, the obtained cell lysate
was centrifugated (43,512 × g) at 4°C for 10 min, and incubated
with magnetic beads that were conjugated with either an
anti-Argonaute 2 antibody (Ago2; cat. no. 2897; Cell Signaling
Technology) or anti-IgG antibody (cat. no. PP64B; EMD Millipore).
IgG group served as a negative control. Proteinase K (EMD
Millipore) was used to digest proteins at 55°C for 30 min prior to
the isolation of immunoprecipitated RNA. The expression levels of
FEZF1-AS1 and miR-1236-3p in the immunoprecipitated RNA were
measured by RT-qPCR.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.) and data are presented as the mean ± SEM.
Statistical differences between 2 groups were determined using an
unpaired Student's t-test, whereas statistical differences between
multiple groups were determined using an one-way ANOVA, followed by
a Tukey's post hoc test. Each experiment was performed ≥3 times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
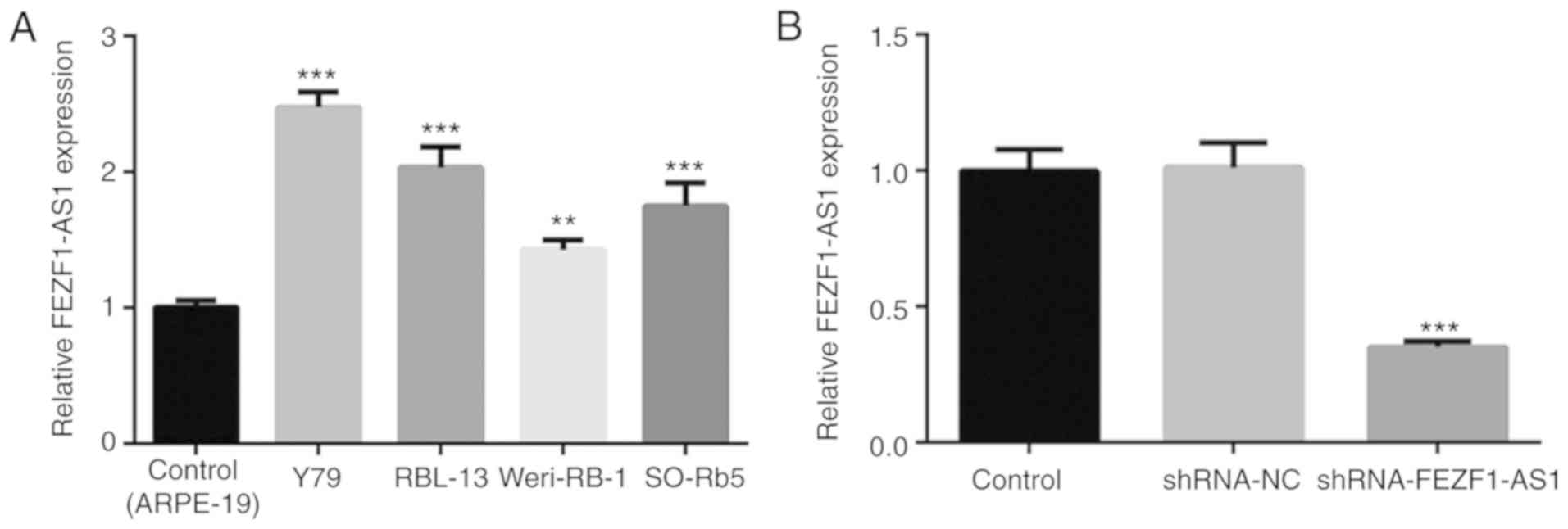
FEZF1-AS1 expression levels are
upregulated in Rb cells
To investigate the role of FEZF1-AS1 in Rb
progression, the mRNA expression levels of FEZF1-AS1 in four Rb
cell lines were determined. The results revealed that FEZF1-AS1
expression levels were significantly upregulated in Rb cell lines,
particularly in Y79 cells, compared with the control ARPE-19 cells
(Fig. 1A). Thus, Y79 cells were
chosen for subsequent experiments. To investigate the function of
FEZF1-AS1 in Rb cells, shRNA-FEZF1-AS1 was transfected into Y79
cells. The transfection efficiency was confirmed by RT-qPCR, which
demonstrated a significant downregulation of FEZF1-AS1 expression
levels in the shRNA-FEZF1-AS1-transfected cells compared with the
shRNA-NC group (Fig. 1B). Thus,
the results suggested that FEZF1-AS1 expression levels may be
upregulated in Rb cells and shRNA-FEZF1-AS1 was successful in
silencing FEZF1-AS1 expression in vitro.
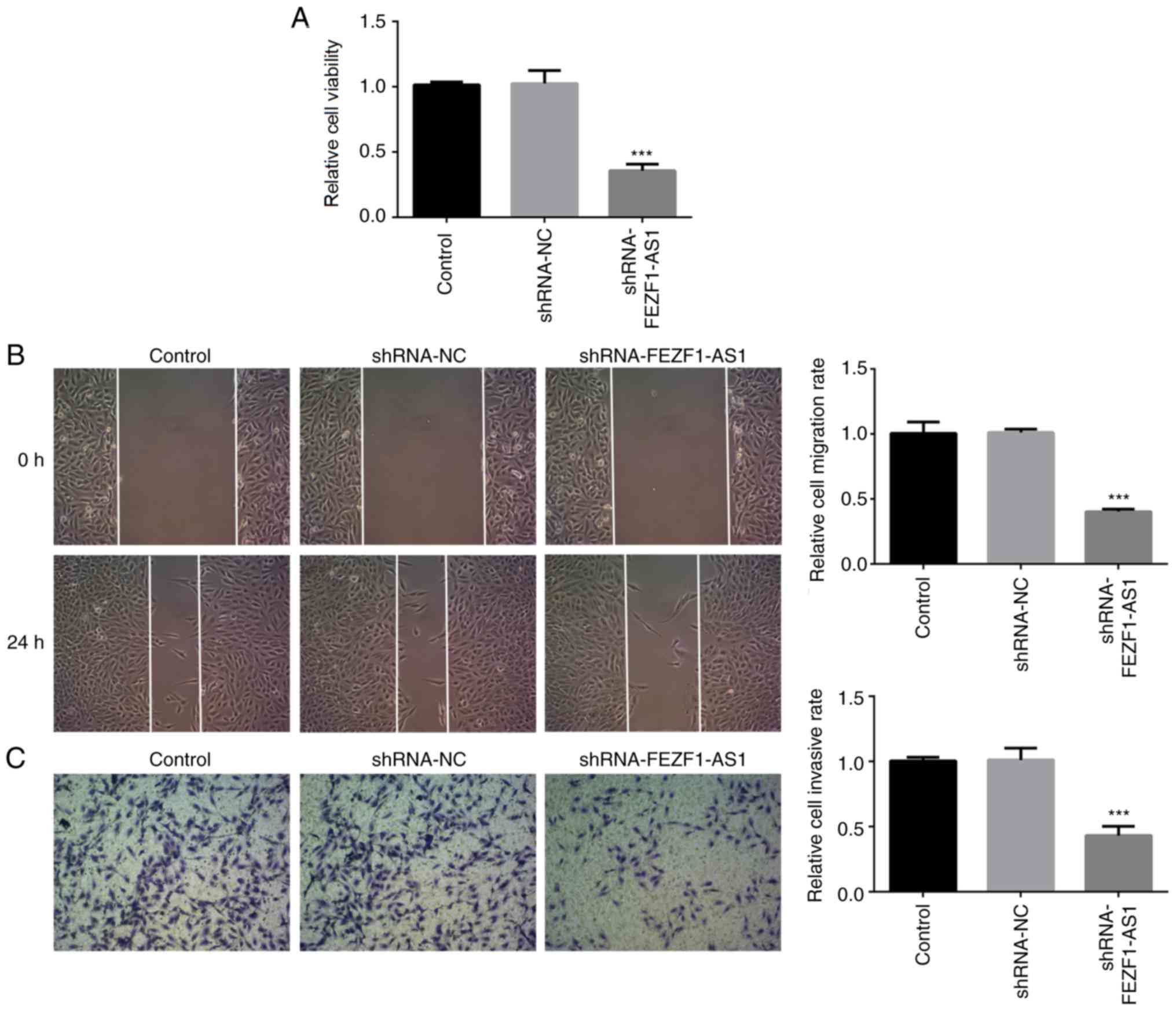
Silencing FEZF1-AS1 inhibits cell
viability, migration and invasion
Subsequently, a CCK-8 assay was performed to
determine the function of shRNA-FEZF1-AS1 on cell viability. The
data indicated that shRNA-FEZF1-AS1 significantly decreased the
cell viability compared with the shRNA-NC group (Fig. 2A). In addition, the wound healing
assay revealed that the genetic knockdown of FEZF1-AS1
significantly reduced the cell migration rate compared with the
shRNA-NC group (Fig. 2B). The
results of the cell invasion assay were consistent with those of
the migration assay; the cell invasive rate was significantly
decreased in the shRNA-FEZF1-AS1-transfected cells compared with
the shRNA-NC group (Fig. 2C).
Collectively, these data suggested that FEZF1-AS1 may regulate the
viability, migration and invasion of Rb cells.
EMT of Rb cells is suppressed by
shRNA-FEZF1-AS1
EMT has a critical role in tumor invasion and
metastasis, thus serving an important role in tumor progression
(5–7). In the present study, western blotting
was used to analyze the expression levels of EMT-related proteins,
including cytoskeletal proteins (Vimentin, Snail, Slug and
β-catenin), cell-cell surface junction proteins (N-cadherin,
E-cadherin and Claudin-1) and cell-extracellular matrix proteins
(MMP2 and MMP9). The expression levels of Vimentin, Snail and Slug
were significantly downregulated in cells transfected with
shRNA-FEZF1-AS1, whereas those of β-catenin were significantly
upregulated, compared with the shRNA-NC-transfected cells (Fig. 3A). In addition, the expression
levels of N-cadherin were also observed to be significantly
downregulated in shRNA-FEZF1-AS1-transfected cells, while the
expression levels of E-cadherin and Claudin-1 were significantly
upregulated, compared with the shRNA-NC-transfected cells (Fig. 3B). Finally, the protein expression
levels of ECM proteins, MMP2 and MMP9, in the cells transfected
with shRNA-FEZF1-AS1 were significantly downregulated compared with
the shRNA-NC group (Fig. 3C).
Overall, these results indicated that the genetic knockdown of
FEZF1-AS1 may inhibit the EMT of Y79 cells.
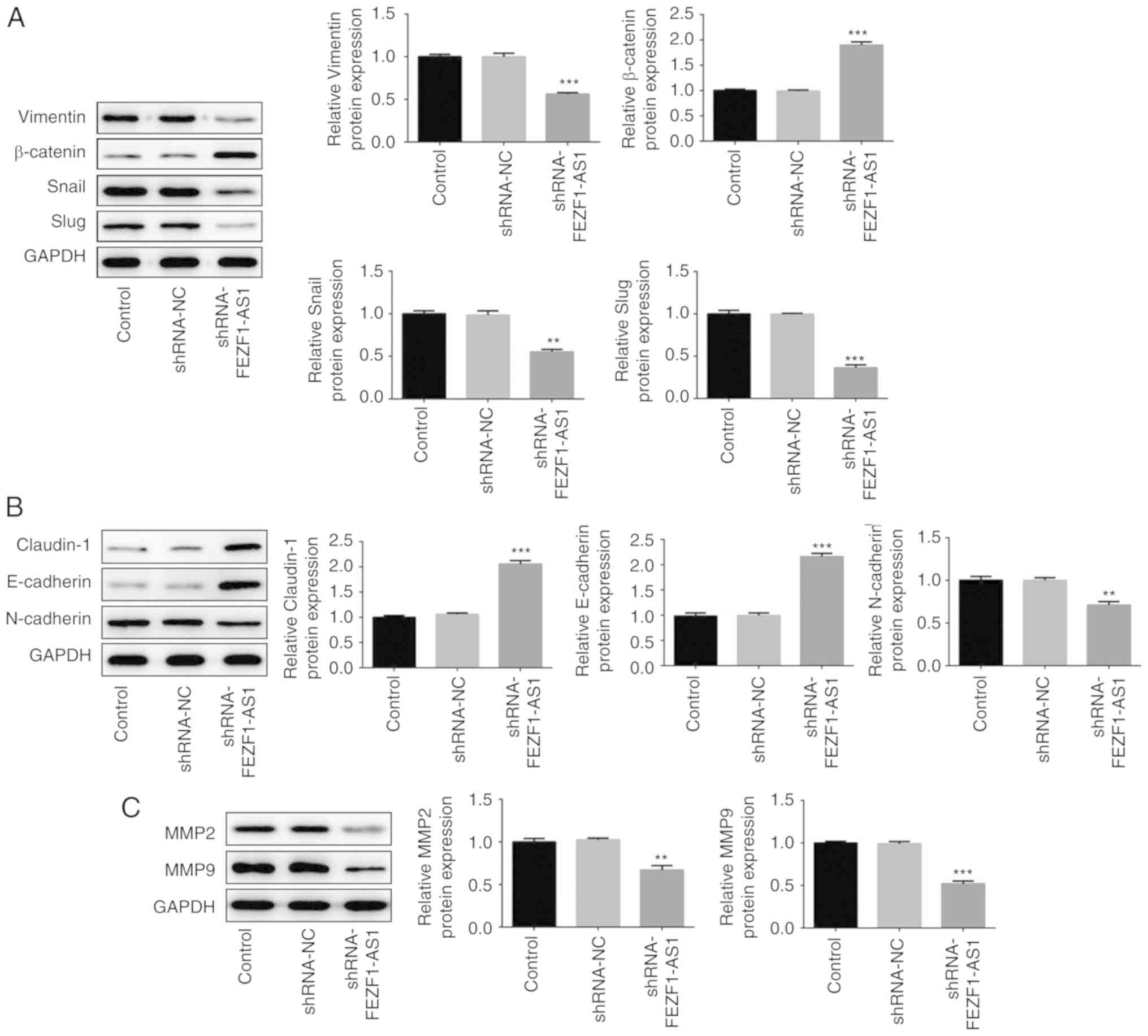 | Figure 3.Epithelial-mesenchymal transition of
retinoblastoma cells is suppressed by shRNA-FEZF1-AS1. Western
blotting was used to analyze the protein expression levels of (A)
Vimentin, Snail, Slug and β-catenin, (B) N-cadherin, E-cadherin,
Claudin-1 and (C) MMP2 and MMP9 in Y79 cells transfected with
shRNA-NC or shRNA-FEZF1-AS1. All data are expressed as the mean ±
SEM. **P<0.01, ***P<0.001 vs. shRNA-NC group. FEZF1-AS1, FEZ
family zinc finger 1 antisense RNA 1; shRNA, short hairpin RNA; NC,
negative control; MMP, matrix metalloproteinase. |
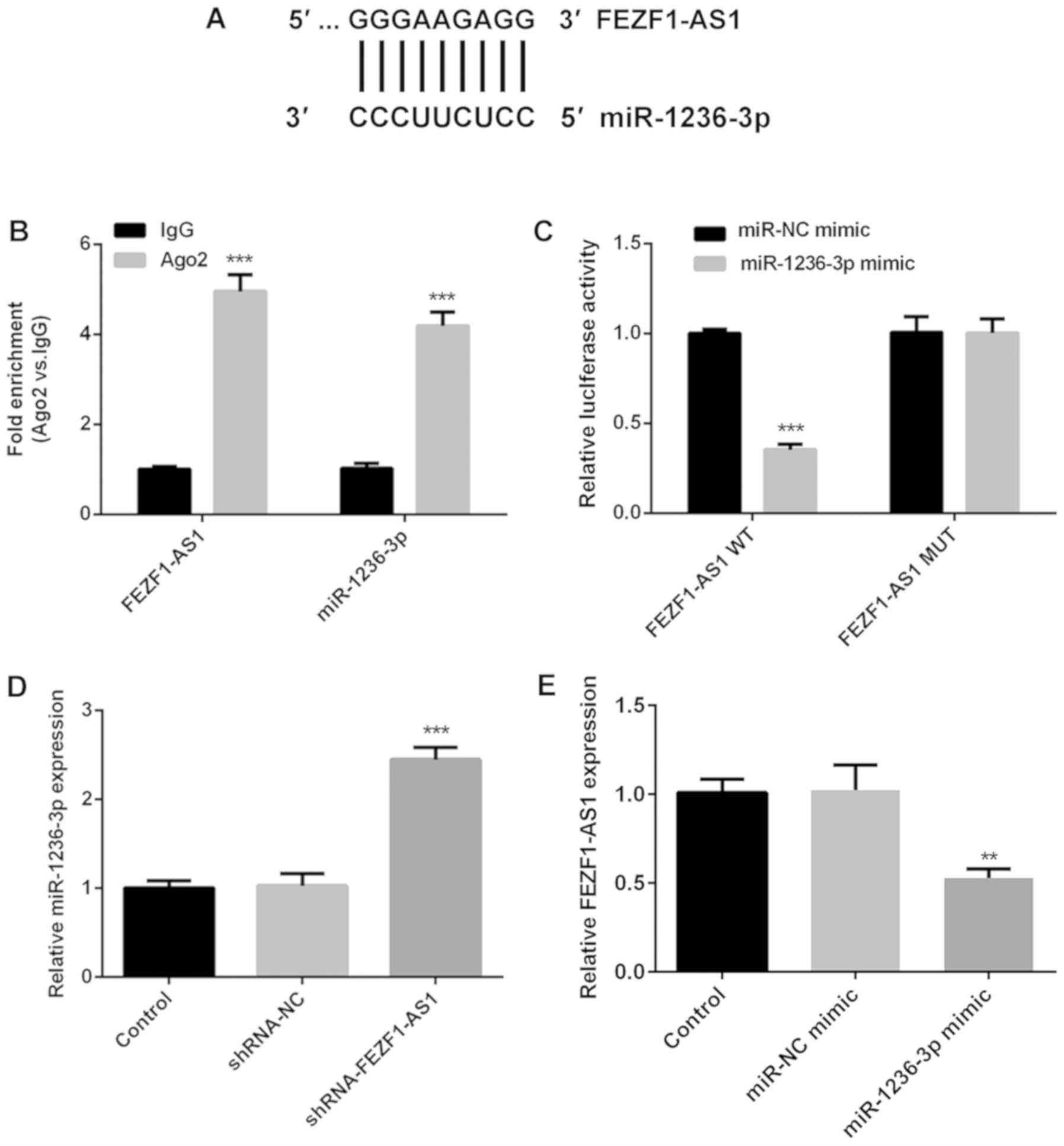
miR-1236-3p is a direct target of
FEZF1-AS
lncRNAs contain binding sites that are complementary
to miRNAs, which permits them to serve as miRNA ‘sponges’ (28,29).
The potential binding site between miR-1236-3p and FEZF1-AS1 was
predicted using LncBase v2 (Fig.
4A). The RIP assay revealed that both FEZF1-AS1 and miR-1236-3p
expression levels were significantly increased in the anti-Ago2
groups compared with their respective anti-IgG groups, indicating
that FEZF1-AS1 may serve as a sponge for miR-1236-3p (Fig. 4B). The expression levels of
miR-1236-3p in Y79 cells were significantly upregulated following
the transfection of the miR-1236-3p mimic compared with the miR-NC
mimic (Fig. S1A). Subsequently, a
dual-luciferase reporter assay revealed that luciferase activity
was notably decreased in Y79 cells co-transfected with FEZF1-AS1-WT
vector and miR-1236-3p mimic, while no significant differences were
observed in the other three groups (Fig. 4C), validating that miR-1236-3p may
directly bind to FEZF1-AS1.
Further experiments were conducted to determine the
association between FEZF1-AS1 and miR-1236-3p. The genetic
silencing of FEZF1-AS1 resulted in the significant upregulation of
miR-1236-3p expression levels compared with the
shRNA-NC-transfected cells (Fig.
4D), while the miR-1236-3p mimic-transfected cells were
identified to have significantly downregulated expression levels of
FEZF1-AS1 compared with miR-NC mimic-transfected cells (Fig. 4E), suggesting that FEZF1-AS1 may
negatively regulate the expression of miR-1236-3p. Taken together,
these results indicated that FEZF1-AS1 may directly target
miR-1236-3p in Y79 cells.
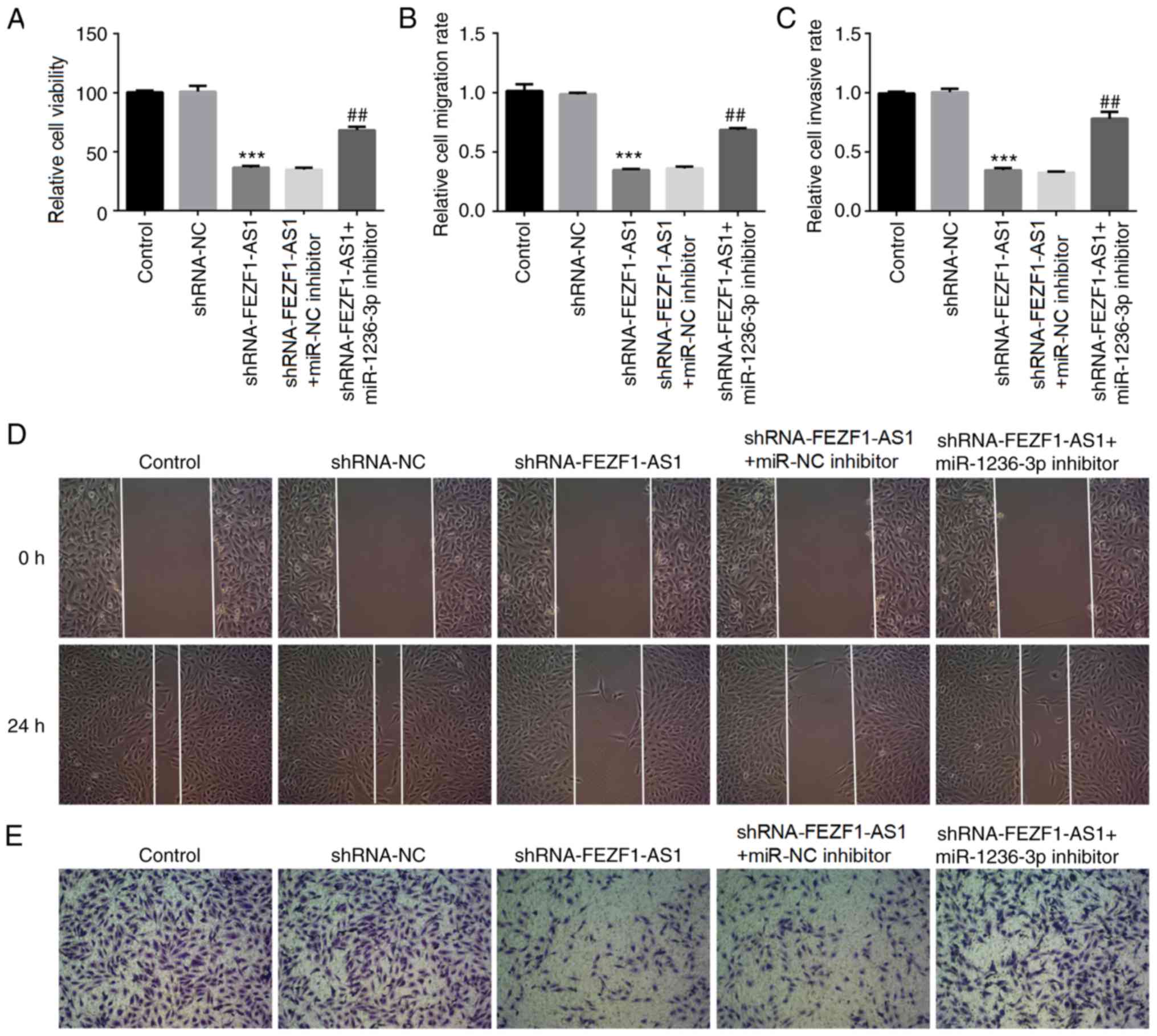
miR-1236-3p inhibitor reverses
FEZF1-AS1-induced cell viability, migration and invasion
To investigate whether miR-1236-3p was involved in
the effects regulated by FEZF1-AS1 in Rb cells, a miR-1236-3p
inhibitor was transfected into Y79 cells. The expression levels of
miR-1236-3p in Y79 cells were significantly downregulated following
the transfection with the miR-1236-3p inhibitor compared with the
miR-NC inhibitor-transfected cells (Fig. S1B). It was subsequently
demonstrated that the cell viability was significantly increased in
cells co-transfected with shRNA-FEZF1-AS1 and miR-1236-3p inhibitor
compared with cells co-transfected with shRNA-FEZF1-AS1 and miR-NC
inhibitor (Fig. 5A). Similar
results were observed in the cell migration and invasion assays
(Fig. 5B-E). These results further
suggested that miR-1236-3p may be required for the effects of
FEZF1-AS1 on cell viability, migration and invasion.
Inhibition of miR-1236-3p reverses the
effects of shRNA- FEZF1-AS1 on EMT
The expression levels of EMT-related proteins were
subsequently determined using western blotting. The shRNA-FEZF1-AS1
group was identified to have significantly upregulated expression
levels of β-catenin compared with the shRNA-NC group; however, the
expression levels were partially reversed by the co-transfection
with the miR-1236-3p inhibitor. The levels of Vimentin, Snail and
Slug showed an opposite trend with β-catenin (Fig. 6A). In addition, the expression
levels of N-cadherin were significantly downregulated at the
protein level in Y79 cells transfected with shRNA-FEZF1-AS1
compared with the shRNA-NC-transfected cells, while the miR-1236-3p
inhibitor was discovered to partially weaken the effect of
shRNA-FEZF1-AS1 (Fig. 6B). The
protein levels of E-Cadherin and Claudin-1 presented an opposite
trend with N-cadherin. Moreover, the co-transfection of the
miR-1236-3p inhibitor with shRNA-FEZF1-AS1 reversed the
downregulation of MMP2 and MMP9 expression levels mediated by
shRNA-FEZF1-AS1 (Fig. 6C).
Collectively, these results suggested that the inhibition of
miR-1236-3p may reverse the modulatory effects of shRNA-FEZF1-AS1
on EMT in Y79 cells.
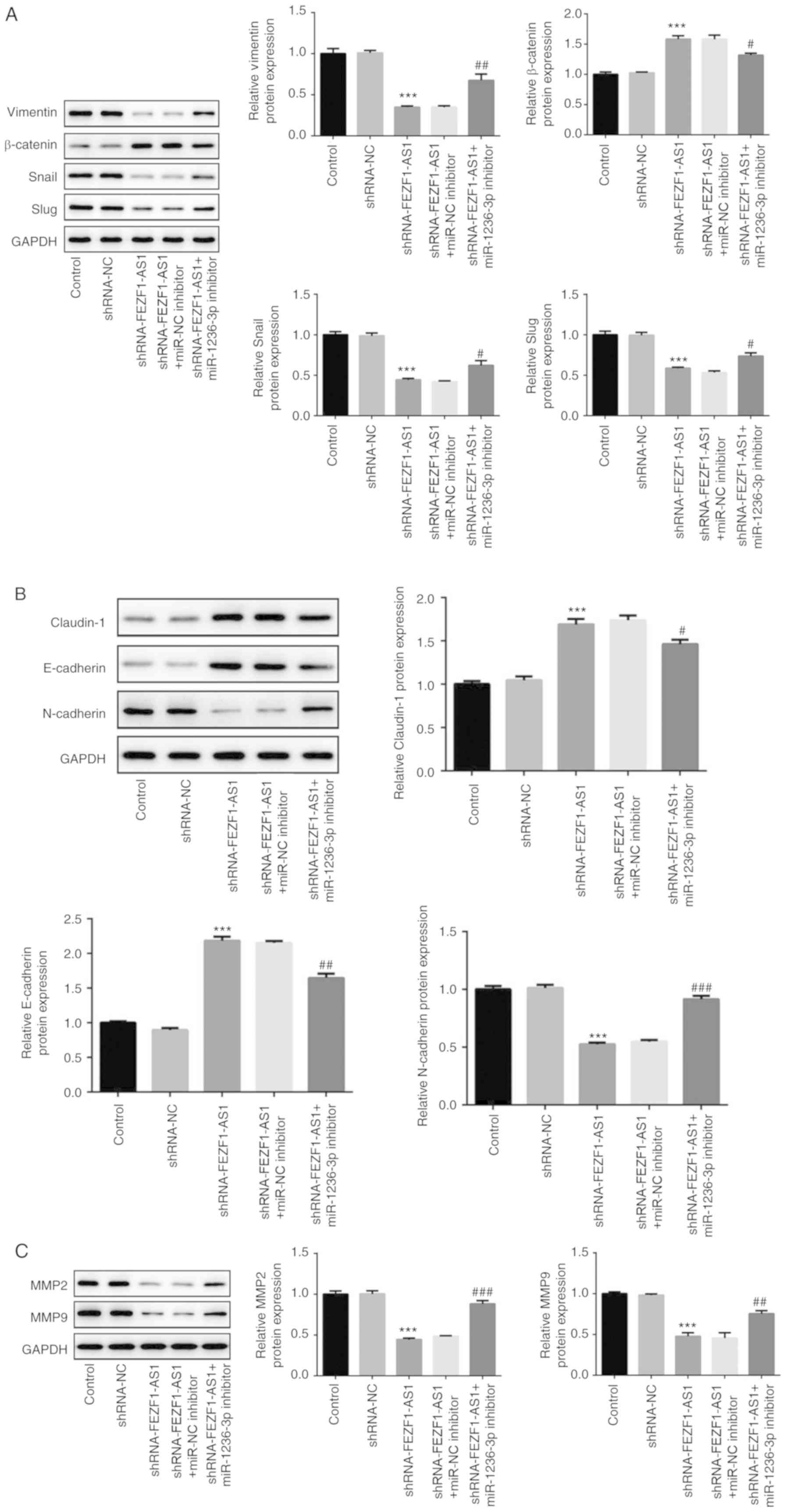 | Figure 6.Continued. Inhibition of miR-1236-3p
reverses the effects of shRNA-FEZF1-AS1 on epithelial-mesenchymal
transition. (A) Western blotting was used to analyze the protein
expression levels of (A) Vimentin, Snail, Slug and β-catenin, (B)
N-cadherin, E-cadherin and Claudin-1, and (C) MMP2 and MMP9 in Y79
cells transfected with shRNA-NC or shRNA-FEZF1-AS1 with or without
miR-1236-3p inhibitor or miR-NC inhibitor. All data are expressed
as the mean ± SEM. ***P<0.001 vs. shRNA-NC group;
#P<0.05, ##P<0.01,
###P<0.001 vs. shRNA-FEZF1-AS1 + miR-NC inhibitor
group. FEZF1-AS1, FEZ family zinc finger 1 antisense RNA 1; shRNA,
short hairpin RNA; NC, negative control; miR, microRNA; MMP, matrix
metalloproteinase. |
Discussion
Dysregulated lncRNA profiles have been revealed to
serve as both oncogenes or tumor suppressor genes, where they have
been widely reported to be involved in the initial metastasis of
tumors by controlling cellular processes, such as migration and
invasion (30). Although there are
a number of studies investigating lncRNAs, to the best of our
knowledge, few lncRNAs have been functionally clarified. Antisense
RNAs, as one part of lncRNAs, which are transcribed from the
antisense strand, are known to have specific functions (31), including exerting significant
modulatory effects and regulate gene translation (32,33).
FEZF1-AS1 is a newly discovered antisense RNA that was identified
to be overexpressed in numerous types of tumor. For example, one
study reported that the expression levels of FEZF1-AS1 were
associated with a poor prognosis and the dysregulation of FEZF1-AS1
contributed to the progression of lung adenocarcinoma (34). In addition, Gong et al
(29) reported that FEZF1-AS1
served as an oncogene in hepatocellular carcinoma. Upregulated
FEZF1-AS1 was also observed in breast cancer tissues (35). FEZF1-AS1 also promoted
tumorigenesis via the activation of the Wnt signaling pathway in
gastric cancer, which subsequently predicted a poor prognosis
(36).
A previous study reported that the overexpression of
miR-1236-3p significantly inhibited the invasion, metastasis and
progression of EMT in gastric cancer by targeting
metastasis-associated protein MTA2 (37). In lung cancer cells, miR-1236-3p
reversed cisplatin resistance by modulating
translationally-controlled tumor protein and
serine/threonine-protein kinase pim-3 (38). Another study revealed that
miR-1236-3p served an important role in regulating colorectal
cancer progression (39).
Additionally, miR-1236-3p inhibited non-small-cell lung carcinoma
cell growth by upregulating p21 expression (40). The aforementioned studies suggest a
wide variety of functions for miR-1236-3p in various types of
tumor; however, to the best of our knowledge, the role of
miR-1236-3p in Rb and the interaction between miR-1236-3p and
FEZF1-AS1 has not been fully elucidated.
The present study focused on FEZF1-AS1 and revealed
its function in Rb cells. The result demonstrated that FEZF1-AS1
expression levels were significantly upregulated in human Rb cell
lines, especially Y79 cells. The genetic silencing of FEZF1-AS1 was
discovered to inhibit the cell viability, and invasive and
migratory ability of Y79 cells, in addition to the EMT process.
Furthermore, the binding sites of miR-1236-3p on the FEZF1-AS1
sequence were identified. Notably, the miR-1236-3p inhibitor
reversed the inhibitory effects of shRNA-FEZF1-AS1 on cell
viability, invasion, migration and EMT, indicating a potential
therapeutic target for treating Rb.
The subcellular locations of β-catenin and
E-cadherin determine the status of the EMT process; thus, a
limitation of the present study was that the subcellular locations
of these proteins were not investigated. Moreover, only in
vitro experiments were included in the present study,
therefore, further verification using tumor tissues from patients
with Rb or tumor-bearing animals will be necessary to validate the
current findings.
In conclusion, the results of the present study
demonstrated that the expression levels of FEZF1-AS1 were
significantly upregulated in Rb cell lines, which indicated that
FEZF1-AS1 may be considered as an oncogene. In addition, the
findings of the present study indicated that FEZF1-AS1 may promote
the cell viability, invasion, migration and EMT of Rb cells, while
these effects were inhibited by transfecting with miR-1236-3p.
Thus, lncRNA-FEZF1-AS1 may promote the viability, invasion,
migration and EMT of Rb cells via regulating miR-1236-3p.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ and WY conceived and designed the study and
performed the experiments. DL and XL analyzed and interpreted the
data, JH and RH drafted the manuscript and analyzed and interpreted
the data. JL performed the experiments and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: Retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dimaras H and Corson TW: Retinoblastoma,
the visible CNS tumor: A review. J Neurosci Res. 97:29–44. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kivelä TT and Hadjistilianou T: Neonatal
retinoblastoma. Asia Pac J Oncol Nurs. 4:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh L and Kashyap S: Update on pathology
of retinoblastoma. Int J Ophthalmol. 11:2011–2016. 2018.PubMed/NCBI
|
|
5
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaliki S, Shields CL and Retinoblastoma:
Achieving new standards with methods of chemotherapy. Indian J
Ophthalmol. 63:103–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao J, Zeng J, Guo B, He W, Chen J, Lu F
and Chen D: Clinical presentation and treatment outcome of
retinoblastoma in children of south western China. Medicine
(Baltimore). 95:e52042016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JW, Abramson DH and Dunkel IJ: Current
management strategies for intraocular retinoblastoma. Drugs.
67:2173–2185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez-Galindo C, Chantada GL, Haik BG
and Wilson MW: Treatment of retinoblastoma: Current status and
future perspectives. Curr Treat Options Neurol. 9:294–307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang M and Wei W: Long non-coding RNAs in
retinoblastoma. Pathol Res Pract. 215:1524352019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benavente CA and Dyer MA: Genetics and
epigenetics of human retinoblastoma. Annu Rev Pathol. 10:547–562.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing YH, Bai Z, Liu CX, Hu SB, Ruan M and
Chen LL: Research progress of long noncoding RNA in China. IUBMB
Life. 68:887–893. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang JY, Lee JC, Chang YT, Hou MF, Huang
HW, Liaw CC and Chang HW: Long noncoding RNAs-related diseases,
cancers, and drugs. ScientificWorldJournal. 2013:9435392013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heery R, Finn SP, Cuffe S and Gray SG:
Long non-coding RNAs: Key regulators of epithelial-mesenchymal
transition, tumour drug resistance and cancer stem cells. Cancers
(Basel). 9:382017. View Article : Google Scholar
|
|
18
|
Hao F, Mou Y, Zhang L, Wang S and Yang Y:
lncRNA AFAP1-AS1 is a prognostic biomarker and serves as oncogenic
role in retinoblastoma. Biosci Rep. 38:BSR201803842018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamang S, Acharya V, Roy D, Sharma R,
Aryaa A, Sharma U, Khandelwal A, Prakash H, Vasquez KM and Jain A:
SNHG12: An lncRNA as a potential therapeutic target and biomarker
for human cancer. Front Oncol. 9:9012019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi C, Sun L and Song Y: FEZF1-AS1: A
novel vital oncogenic lncRNA in multiple human malignancies. Biosci
Rep. 39:BSR201912022019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quan LJ and Wang WJ: FEZF1-AS1 functions
as an oncogenic lncRNA in retinoblastoma. Biosci Rep.
39:BSR201907542019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solé C and Lawrie CH: MicroRNAs and
metastasis. Cancers (Basel). 12:962019. View Article : Google Scholar
|
|
24
|
Liu J and Tao C: Overexpression of
miRNA-125a-5p inhibits the growth and angiogenesis of
hepatocellular carcinoma by regulating the expression of VEGF-A.
Biotechnol Biotechnol Equip. 33:1116–1125. 2019. View Article : Google Scholar
|
|
25
|
Bian T, Jiang D, Liu J, Yuan X, Feng J, Li
Q, Zhang Q, Li X, Liu Y and Zhang J: miR-1236-3p suppresses the
migration and invasion by targeting KLF8 in lung adenocarcinoma
A549 cells. Biochem Biophys Res Commun. 492:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Yan S, Liu X, Zhang W, Li Y, Dong
R, Zhang Q, Yang Q, Yuan C, Shen K and Kong B: miR-1236-3p
represses the cell migration and invasion abilities by targeting
ZEB1 in high-grade serous ovarian carcinoma. Oncol Rep.
31:1905–1910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Zhang S, Wu L and Pei M: Interaction
between LncRNA-ROR and miR-145 contributes to
epithelial-mesenchymal transition of ovarian cancer cells. Gen
Physiol Biophys. 38:461–471. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong J, Wang J, Liu T, Hu J and Zheng J:
lncRNA FEZF1-AS1 contributes to cell proliferation, migration and
invasion by sponging miR-4443 in hepatocellular carcinoma. Mol Med
Rep. 18:5614–5620. 2018.PubMed/NCBI
|
|
30
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long non-coding RNA in the pathogenesis of cancers. Cells.
8:10152019. View Article : Google Scholar
|
|
31
|
Pelechano V and Steinmetz LM: Gene
regulation by antisense transcription. Nat Rev Genet. 14:880–893.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Halley P, Kadakkuzha BM, Faghihi MA,
Magistri M, Zeier Z, Khorkova O, Coito C, Hsiao J, Lawrence M and
Wahlestedt C: Regulation of the apolipoprotein gene cluster by a
long noncoding RNA. Cell Rep. 6:222–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carrieri C, Cimatti L, Biagioli M, Beugnet
A, Zucchelli S, Fedele S, Pesce E, Ferrer I, Collavin L, Santoro C,
et al: Long non-coding antisense RNA controls Uchl1 translation
through an embedded SINEB2 repeat. Nature. 491:454–457. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, Zhao P, Han Y and Lu S: lncRNA
FEZF1-AS1 is associated with prognosis in lung adenocarcinoma and
promotes cell proliferation, migration, and invasion. Oncol Res.
27:39–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, Sun L, Zhang Y, Lu G, Li Y and
Wei Z: Long non-coding RNA FEZF1-AS1 promotes breast cancer
stemness and tumorigenesis via targeting miR-30a/Nanog axis. J Cell
Physiol. 233:8630–8638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu X, Zhang P, Zhu H, Li S, Chen X and Shi
L: Long noncoding RNA FEZF1-AS1 indicates a poor prognosis of
gastric cancer and promotes tumorigenesis via activation of Wnt
signaling pathway. Biomed Pharmacother. 96:1103–1108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An JX, Ma MH, Zhang CD, Shao S, Zhou NM
and Dai DQ: miR-1236-3p inhibits invasion and metastasis in gastric
cancer by targeting MTA2. Cancer Cell Int. 18:662018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Liu L, Guo X, Guo C and Wang W:
microRNA-1236-3p regulates DDP resistance in lung cancer cells.
Open Med (Wars). 14:41–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng W, Gong H, Wang Y, Zhu G, Xue T, Wang
Y and Cui G: circIFT80 functions as a ceRNA of miR-1236-3p to
promote colorectal cancer progression. Mol Ther Nucleic Acids.
18:375–387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Ge Q, Liu J, Zhang Q, Wang C, Cui K
and Chen Z: Effects of miR-1236-3p and miR-370-5p on activation of
p21 in various tumors and its inhibition on the growth of lung
cancer cells. Tumour Biol. 39:10104283177108242017. View Article : Google Scholar : PubMed/NCBI
|