Introduction
Influenza A infection may cause central nervous
system complications, including multiple sclerosis, febrile
seizures, encephalopathy and Reye's syndrome, as well as other
neurological abnormalities, high mortality, poor prognosis and
sequelae in the majority of survivors (1–5).
Vaccination is the most effective method of preventing and
controlling influenza. However, due to certain factors, including
the immunological characteristics of the influenza vaccine itself,
a small number of influenza vaccination subjects may develop
diseases, including Guillain-Barré syndrome and narcolepsy, while
obtaining immunoprotection. At present, to the best of our
knowledge, the causes of these serious adverse reactions remain
unclear (6,7).
In our previous study, 84 monoclonal antibodies
(mAbs) against hemagglutinin (HA) were prepared. When identifying
their characteristics, it was revealed that the H1-84mAb not only
binds to the HA antigen, but also cross-reacts with human brain
tissue, suggesting that H1N1 influenza virus HA and human brain
tissue have a heterophilic antigen (8). Heterophilic antigens are a class of
common antigens that are unrelated between species, and exist in
humans, animals and microorganisms (9). When studying microbial infection
immunity, it has been revealed that E. coli O14
lipopolysaccharide and human colon mucosa possess heterophilic
antigens, leading to the occurrence of ulcerative colitis (10). Antibodies against the enterovirus
Coxsackie VP1 protein may cross-react with mitochondrial proteins
of β-islet cells, and this may be associated with infection-induced
diabetes (11).
The presence of heterophilic antigens between
influenza HA and human brain tissue may be an important factor
affecting the safety of the influenza A vaccine. Therefore, it is
important to find and identify heterophilic antigens recognised by
H1-84mAb. It has been previously identified that H1-84mAb
recognises a nine-peptide linear epitope of influenza HA (12). The present study used H1-84mAb as a
research tool to confirm its cross-reactivity with heterophilic
antigens from brain tissue and to provide experimental data for
subsequent studies investigating the pathogenic mechanism involving
these antigens.
Materials and methods
Experimental materials
A total of 5 Male Sprague Dawley (SD) rats (weight,
250–300 g) were purchased from the Experimental Animal Centre of
the Fourth Military Medical University (Xi'an, China) in order to
prepare paraffin sections and total protein extracts of rat brain
tissues. The 6–8-week SD rats received humane care and were raised
in the same clean environment, with ambient temperature at 26°C,
humidity of 50±5%, and a 12-h light/dark cycle. In addition, the
standard food and water available ad libitum to the animals
was sterilized. Following the experiments, the animals were
anesthetized with ether, and clinical manifestations included loss
of consciousness, loss of systemic pain, inhibition of reflexes,
and skeletal muscle relaxation. The animals were euthanized by
cervical dislocation. Cell culture supernatant of the H1-84mAb
against influenza virus hemagglutinin was maintained in our
laboratory (titre, 1:1,000; https://doi.org/10.1007/s12250-019-00100-9). A
horseradish peroxidase-labelled goat anti-mouse secondary antibody
(cat. no. B141027) and a tissue immunohistochemical staining kit
(cat. no. QN2755) were purchased from OriGene Technologies, Inc.
Bovine serum (cat. no. 16000-044) for cell cultures was purchased
from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.
RIPA lysis buffer (cat. no. P0013C) and BeyoECL Plus (cat. no.
P0018S) were purchased from Beyotime Institute of Biotechnology.
The SP2/0 hybridoma cells were purchased from Hangzhou Lianke
Meixun Biomedical Technology Co., Ltd. (cat. no. YB-ATCC-2224).
BL21(DE3)pLysS competent cells, >106 cfu/µg, were purchased from
Promega Corporation (cat. no. L1191). Protein A/G PLUS agarose
(cat. no. GS4780) was purchased from Santa Cruz Biotechnology, Inc.
The total RNA extraction kit (cat. no. DP433), cDNA first-strand
synthesis kit (cat. no. KR104) and bicinchoninic acid (BCA) protein
assay kit (cat. no. P0012S) were purchased from Tiangen Biotech
Co., Ltd. PCR polymerase (cat. no. C10966-018), the pMD19-T vector
(cat. no. 6013) and DM5000 DNA Marker (cat. no. 116899) were
purchased from Takara Biotechnology Co., Ltd., and a prokaryotic
expression vector was kept at our laboratory. Primer synthesis and
sequencing were performed by Beijing Liuhe Huada Gene Technology
Co., Ltd.
Preparation and identification of
mAbs
mAbs against the H1N1 influenza virus HA protein,
including H1-84mAb, were prepared in our laboratory. The titre of
the antibody was determined using the indirect ELISA method, and
the reactivity of the antibody with the HA antigen was determined
by western blotting (8). It has
been previously determined that H1-84mAb binds to a nine-peptide
linear epitope (191-LVLWGIHHP-199) on HA (12).
Immunohistochemistry
In brief, SD rat brain tissues were obtained to
generate paraffin sections. Immunohistochemical staining was
performed according to the kit instructions. Paraffin sections were
dewaxed in xylene, rehydrated with alcohol at gradient
concentration, and finally soaked in distilled water. Citrate
buffer (pH 6.0) was used for antigen retrieval at 60°C microwave.
Subsequently, 3% hydrogen peroxide was used to block endogenous
peroxidase activity at room temperature for 20 min, followed by
blocking in 3% sheep serum at 37°C for 30 min. The sections were
incubated with H1-84mAb (dilution, 1:50) at 4°C overnight.
Subsequently, the sections were rewarmed to room temperature for 60
min, followed by three washes with phosphate-buffered saline (PBS).
The horseradish peroxidase-labelled goat anti-mouse secondary
antibody (dilution, 1:500) was added and incubated at 37°C for 40
min, followed by three washes with PBS. Colour development was
performed with diaminobenzidine and haematoxylin counterstaining at
room temperature for 10 min. A conventional dehydrated transparent
neutral gum mounting medium was used. Negative control was
established.
Immunoprecipitation and mass
spectrometry
As previously described (13), total protein was extracted from rat
brain tissues using RIPA lysis buffer, and lysed on ice or at 4°C
for 30 min. The supernatant was collected following centrifugation
at 12,000 × g for 30 min at 4°C. The protein content was determined
using a BCA protein assay kit. A total of 500 µg of the collected
protein was transferred to a 1.5-ml microcentrifuge tube, and 2 µg
H1-84mAb was added and incubated at 4°C for 1 h. Next, 20 µl of a
resuspended volume of protein A/G PLUS agarose was added. This was
incubated overnight at 4°C with gentle agitation. Following
immunoprecipitation, the samples were centrifuged at 1,500 × g for
5 min at 4°C. The protein A/G beads were centrifuged to the bottom
of the tube, the supernatant was carefully aspirated, the protein
A/G beads were washed 3–4 times with 1 ml lysis buffer, and finally
15 µl 2X SDS loading buffer was added and the samples were boiled
for 10 min. Samples were then analysed by SDS-PAGE, western
blotting and mass spectrometry.
Western blotting
The sample was mixed with SDS electrophoresis sample
buffer and the protein was transferred onto a nitrocellulose
membrane following SDS-PAGE on a 12% separation gel. Then the
protein gel was transferred to the NC film and blocked overnight
with 5% skimmed milk at 4°C. H1-84mAb was used as the primary
antibody (dilution, 1:100). A goat anti-mouse enzyme-labelled
secondary antibody, as mentioned above, (dilution, 1:2,500) was
used. Enhanced chemiluminescence (ECL) was used to develop the
colour.
Cloning of heterogeneous nuclear
ribonucleoprotein (hnRNP)A1 and hnRNPA2/B1
As previously described (14), total RNA was extracted from brain
tissue total protein and then reverse transcribed into cDNA.
Subsequently, PCR was used to amplify hnRNPA1 and hnRNPA2/µl. The 6
µl purified PCR product was subcloned into the 2 µl
pGEM®-T vector (concentration ratio 3:1). The correctly
sequenced plasmid was converted into the pET-28a-SUMO vector (SUMO
tag size, 15 kDa) and then was transformed into BL21(DE3)pLysS
competent cells by thermal shock for 90 sec at 42°C. Recombinant
hnRNPA1 and hnRNPA2/B1 were expressed by adding isopropyl
β-d-1-thiogalactopyranoside (IPTG; 0.5 mM) to an E. coli
strain, purified using Ni-NTA and verified on Coomassie-stained
gels.
Segmental expression and
identification of hnRNPA1 and A2/B1
The antigenicity of hnRNPA1 and hnRNPA2/B1 were
analyzed theoretically. Using hnRNPA1 and hnRNPA2/B1 as templates,
PCR was used to amplify truncated hnRNPA1 and hnRNPA2/B1, using the
aforementioned steps. Truncated hnRNPA1 and hnRNPA2/B1 were
expressed by adding IPTG (0.5 mM) to an E. coli strain,
purified using Ni-NTA and verified on Coomassie-stained gels. The
expression product was identified by SDS-PAGE and western
blotting.
Statistical analysis
The results were analyzed using SPSS 19.0
statistical software (IBM Corp., Armonk, NY, USA). Data are
expressed as the mean ± SEM. One-way analysis of variance, followed
by Tukey's post hoc test, was used to determine the
significance of differences in multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cross-reactivity of influenza virus HA
mAb with rat brain tissue
In our previous study, 84 mAbs against influenza
virus HA were obtained and screened using a human tissue
microarray. H1-84mAb cross-reacted with human brain tissue, and
western blotting revealed that H1-84mAb bound to the HA antigen
(8).
Due to the limitations of medical ethics, the
present study used rat brain tissues instead of human brain tissues
for subsequent experiments. To further confirm the cross-reactivity
of H1-84mAb, immunohistochemical staining of paraffin sections of
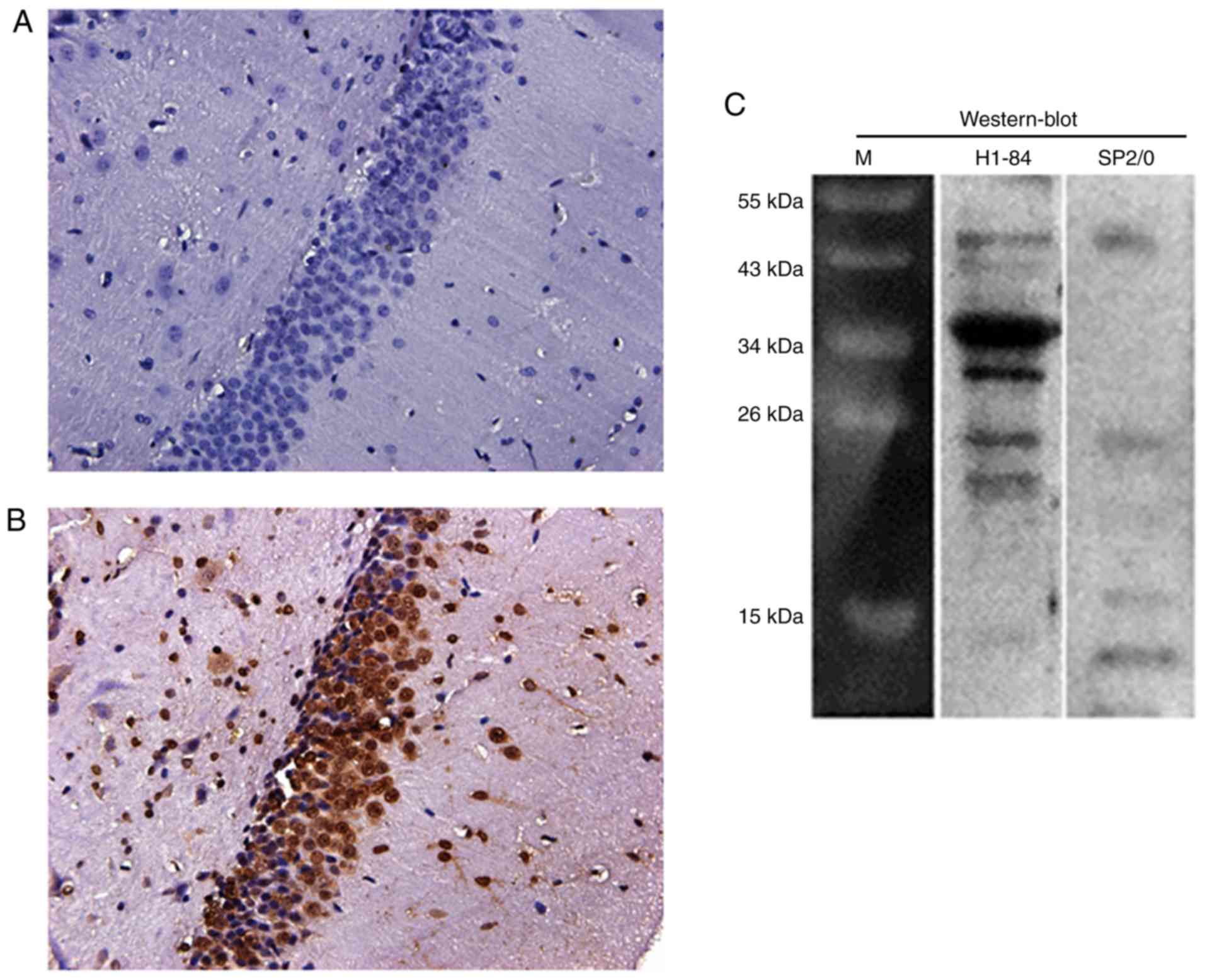
rat brain tissues was performed. The results revealed that the
control antibody (cell culture supernatant of the SP2/0 hybridoma)
was negative (Fig. 1A).
Additionally, H1-84mAb reacted with the rat brain (Fig. 1B). Furthermore, western blotting
demonstrated that H1-84mAb reacted with the protein components of
brain tissue. The molecular weight of the reactive protein was ~35
kDa (Fig. 1C).
Immunoprecipitation and mass
spectrometry
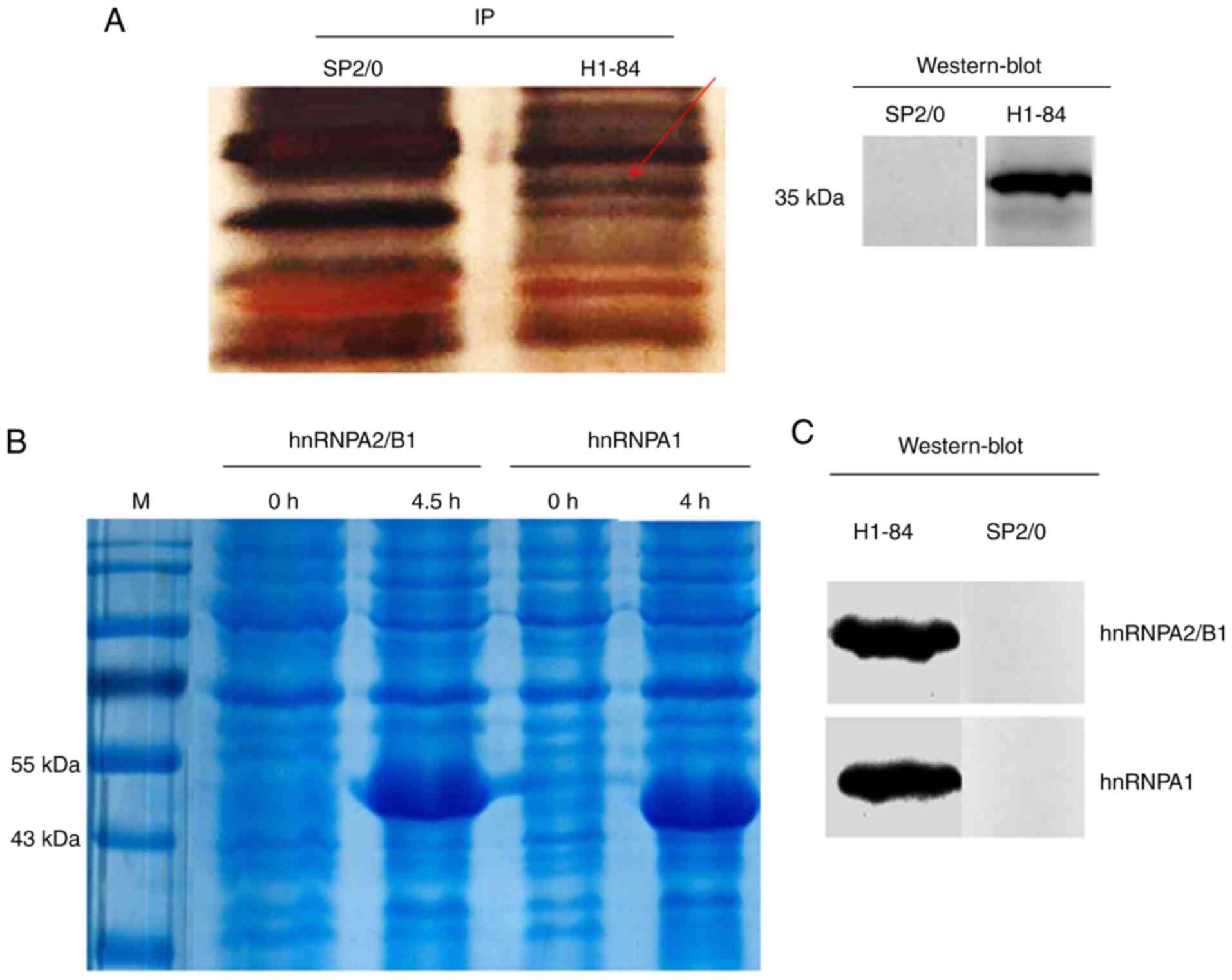
H1-84mAb bound to the protein in rat brain tissue in
immunoprecipitation experiments. The cell culture supernatant of
the SP2/0 hybridoma served as a negative control. Western blotting
revealed that the molecular weight of the target antigen reacting
with H1-84mAb was ~35 kDa (Fig.
2A). Specific reaction bands were excised from the SDS-PAGE gel
and analysed by mass spectrometry.
Verification of cross reactivity
Immunoprecipitation combined with mass spectrometry
indicated that H1-84mAb bound to hnRNPA1 and hnRNPA2/B1 from brain
tissues. The two proteins were expressed separately using an E.
coli expression system (Fig.
2B). Subsequently, the cross-reactivity of the expressed
proteins with H1-84mAb was demonstrated by western blotting. The
results revealed that H1-84mAb cross-reacted with the two purified
recombinant proteins (Fig.
2C).
Fine localisation of H1-84mAb binding
to brain tissue proteins
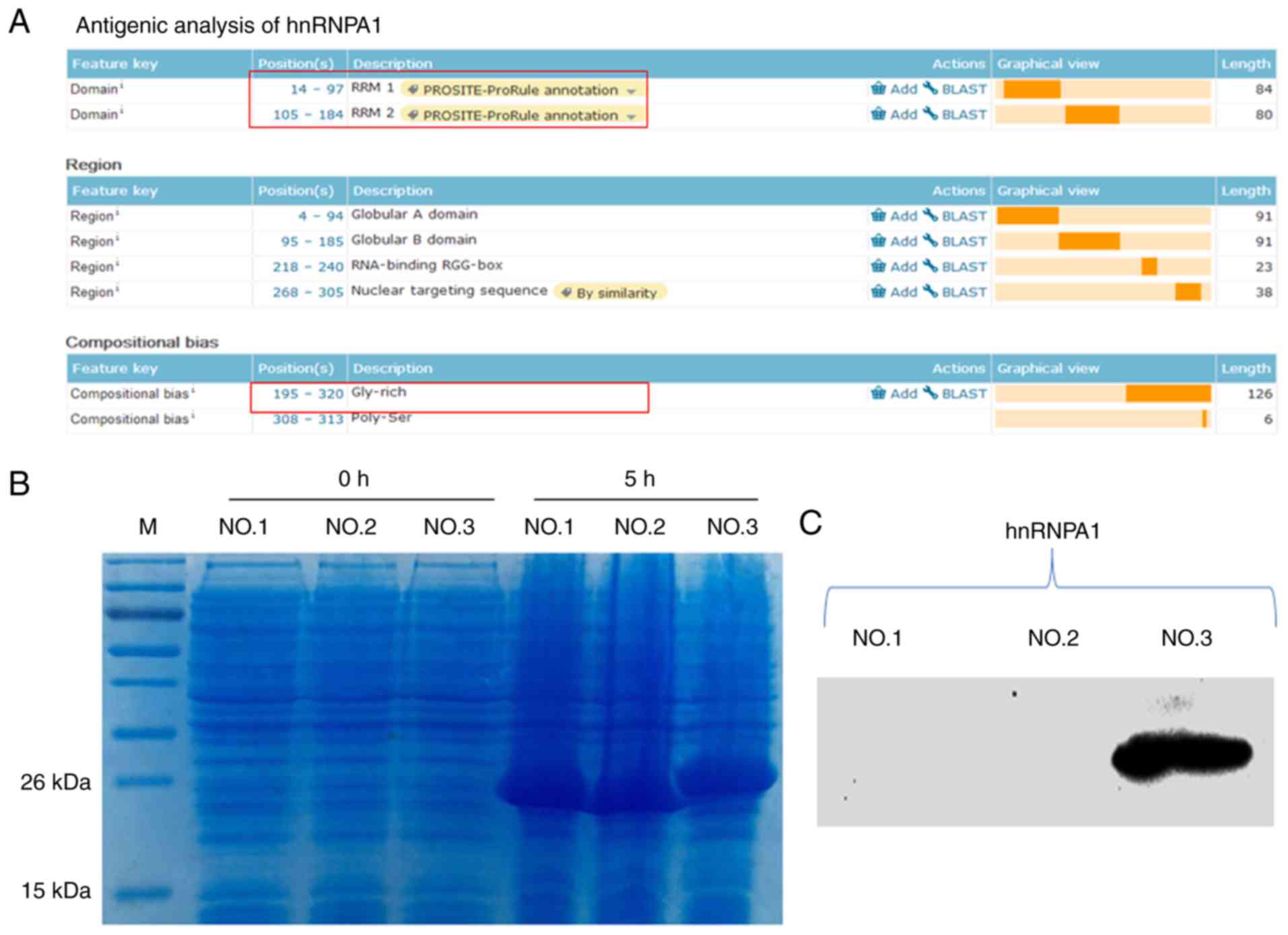
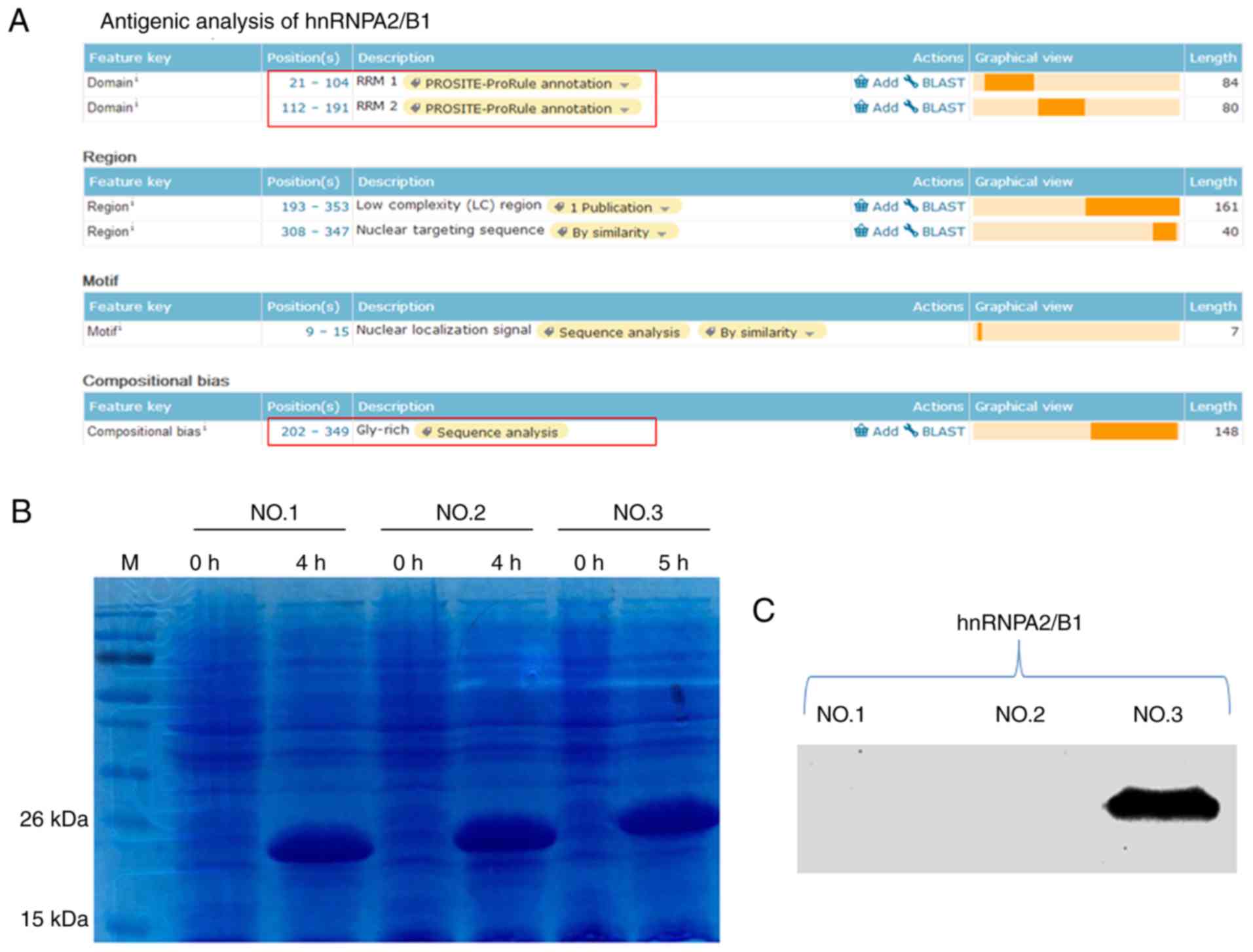
The functional regions of the hnRNPA1 and hnRNPA2/B1
proteins were analysed (Figs. 3A
and 4A); they were divided into
three segments and prokaryotic expression was performed (Figs. 3B and 4B). Subsequently, western blot analysis
was used to identify them. The results revealed that the third
segments [the glycine (Gly)-rich domain] of these two proteins were
the antigens that H1-84 cross-reacted with in brain tissues
(Figs. 3C and 4C). Fig.
3C demonstrated that hnRNPA1-NO.3 (195aa-320aa Gly-rich domain)
contained 126 amino acids. Fig. 4C
demonstrated that hnRNPA2/B1-NO.3 (202aa-349aa Gly-rich domain)
contained 148 amino acids. These two parts were H1-84mAb that
recognized specific antigens on hnRNPA1 and hnRNPA2/B1.
Verification of H1-84mAb combined with
brain tissue antigens
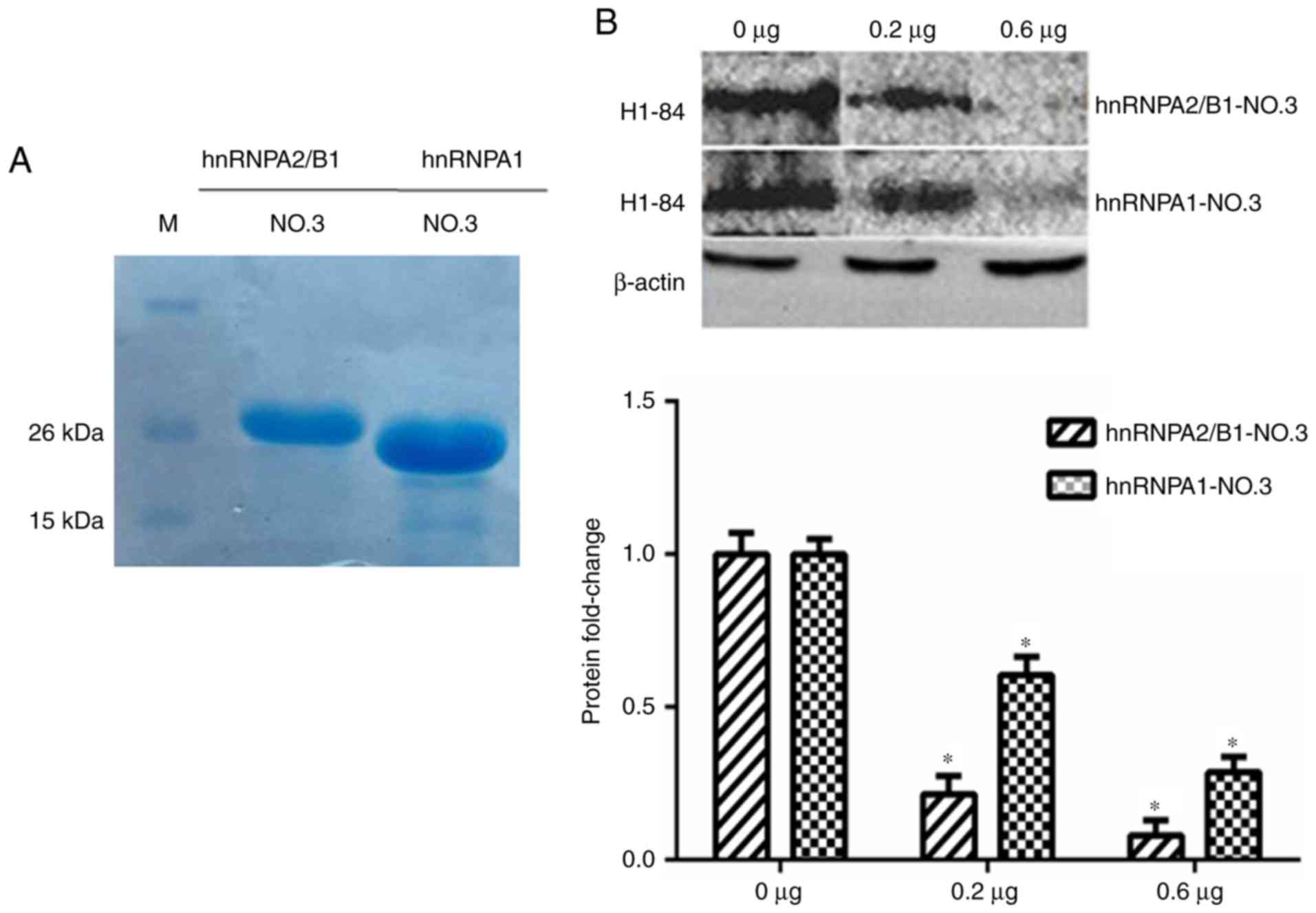
The hnRNPA1-NO.3 and hnRNPA2/B1-NO.3 were purified
(Fig. 5A) and then these two
antigens were used to block the binding of H1-84 to brain tissue
proteins, respectively. The same batch of brain tissue proteins was
sampled for western blot analyses, and the consistency of the
sample volume was monitored with β-actin. The H1-84mAb was
pre-incubated with 0, 0.2 and 0.6 µg of the glycine (Gly)-rich
domain of these two proteins, respectively, and then reacted with
the brain tissue proteins on the NC membrane, *P<0.05 vs. 0 µg
group. It was verified that these two partial antigens may block
H1-84mAb binding to brain tissue (Fig.
5B).
Discussion
Influenza A vaccination may induce neurological
adverse reactions in a small number of individuals, indicating that
there is a problem with the safety of the influenza A vaccine
(3,15). At present, the mechanism of this
remains unclear, which limits the efficiency of clinical prevention
and control, and seriously affects the treatment of patients. In
2015, Ahmed et al (16)
reported that the nucleoproteins of influenza A vaccines stimulate
the body to produce cross-reactive antibodies against hypothalamic
receptor 2. This antibody blocks the hypothalamic receptors of
nerve cells, leading to a sleep-wake regulation disorder, which in
turn causes narcolepsy (17,18).
Our previous study demonstrated that influenza virus
H1-84mAb not only bound to the HA antigen, but also reacted with
human brain tissue (8). Combined
with the concept of heterophilic antigens, this indicated that
there was a heterotropic antigen between influenza virus HA and
human brain tissue. This is an important factor affecting the
safety of the vaccine. Using molecular biology and immunological
methods, the present study demonstrated that the influenza virus
H1-84mAb cross-reacted with heterophilic antigens in brain tissues.
The heterophilic antigens were the Gly-rich domains of hnRNPA1
(195aa-320aa) and hnRNPA2/B1 (202aa-349aa) by prokaryotic
expression. Although the protein expressed in the bacterial system
cannot simulate the natural protein in nuclear organisms, the
current research field mostly uses prokaryotic expression methods
to identify heterophilic antigens. Sun et al (13) used a prokaryotic expression method
to report that the anti-influenza virus H1-50mAb recognizes target
antigen inhibin on pancreatic islet cells. Furthermore, an article
published in Nature Medicine confirmed that an antibody produced by
human T lymphotropic virus type 1 (HTLV-1) infection reacts with
hnRNPA1 to cause myelopathy/tropical paraplegia (HAM/TSP) by
prokaryotic expression (14).
Therefore, the present study used the prokaryotic expression method
to verify heterophilic antigens.
hnRNP is a multifunctional protein family molecule
that is mainly localised in the nucleus and may be combined with
newly-synthesised heterogeneous nuclear RNA. It regulates a series
of important processes, including the splicing of mRNA precursors,
as well as mRNA nuclear transport, translation and degradation
(19). In studies investigating
how viral diseases cause neurological diseases, there have been
various reports on spontaneous host antibodies, and a number of
studies have focused on the pathogenicity of hnRNP antibodies.
Levin et al (20) revealed
that autoantibodies to hnRNPA1 may cause neurodegenerative changes.
Animal experiments have demonstrated that hnRNPA1 antibodies are
associated with multiple sclerosis (21). Sueoka et al (22) detected antibodies in the
cerebrospinal fluid of 35 patients with multiple sclerosis and
reported that 32 of them had antibodies against the hnRNPB1
protein. The present experiments revealed that the H1-84mAb of
influenza virus HA binds to Gly-rich domains of hnRNPA1 and
hnRNPA2/B1. The proteins hnRNPA1 and hnRNPA2/B1 were screened using
the IP method and were highly expressed in neurons. The Gly-rich
domain of these two proteins may block the binding of H1-84mAb to
brain tissue.
The Gly-rich domain includes the RGG domain and the
M9 shuttle domain, which are two important functional regions of
hnRNP. Antibodies against these two functional domains may cause
nervous system damage (23).
Therefore, the specific epitope of H1-84 binding to the Gly-rich
domain and the pathogenesis of neurological diseases caused by
H1-84mAb binding to the Gly-rich domains of these proteins should
be studied.
Although hnRNP is widely distributed in various
cells and tissues of different species, H1-84mAb specifically binds
to brain tissue. Furthermore, Sun et al (13) reported that anti-influenza virus
H1-50mAb recognises a target antigen on islet cells, which has been
revealed to be prohibitin. Prohibitin is an antiproliferative
protein that is widely distributed in various cell types in
different species, and serves an important regulatory role in cell
metabolism, growth, differentiation, senescence and apoptosis
(24).
The present study compared the similarity between
the HA of influenza virus and the Gly-rich domain of hnRNPA1 and
hnRNPA2/B1; and they were found to have no homologous sequences.
Sun et al (13) revealed
that an influenza virus mAb (H1-50) cross-reacts with islet cells,
but there is no homologous sequence between HA and prohibitin.
Previous studies (14,25) revealed that an antibody produced by
human T lymphotropic virus type 1 infection reacts with hnRNPA1 to
cause myelopathy/tropical paraplegia. Although there is no
homologous sequence between the dominant epitope (KHFRETEV) and
hnRNPA1, autoantibodies against hnRNPA1 may still cause neuronal
damage. In a mouse model of viral-induced myocarditis, a
cross-reaction was found between myocardial myosin and Coxsackie B3
virus neutralizing antibody, but no molecularly mimicked sequence
was found (26). Not all
cross-reactions can find homologous sequences, as they may be
conformational fit.
H1-84mAb may recognize HA antigens of human
2009H1N1, seasonal H1N1, H3N2, avian influenza H5N1 and H9N2. It
was reported that H1-84mAb can recognize the epitope
191-LVLWGIHHP-199 of human influenza and avian influenza HA
(12). Antibodies that recognize
this epitope have also been detected in patients' sera following
vaccination, and our recent studies have reported that H1-84mAb may
mediate nervous system damage (study under submission). Therefore,
it is theoretically feasible to mutate the 191/199 epitope of HA to
avoid the production of H1-84-like pathogenic antibodies, thereby
improving vaccine safety.
In the future, the association between H1-84mAb,
Gly-rich domains, neurological diseases and the safety of the
vaccine will be investigated further. This will provide
experimental data for designing a safe influenza vaccine and will
be of great significance in preventing influenza virus
infection.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Research and Development Program of China (grant no.
2016YFD0500700) and the Incubation Fund Program of Shaanxi
Provincial People's Hospital (grant no. 2019YXQ-12).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
CG, LS, SH and XH designed the study and performed
critical revisions; CG, HH, DL, QF, YL and YF performed the
laboratory measurements; CG, XX and JH performed the data
collection and analysis; and CG drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments performed in the present
study were approved by the Medical School of Xi'an Jiaotong
University Biomedical Ethics Committee and fully meet the
requirements of the animal ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mahmud SM, Bozat-Emre S, Mostaço-Guidolin
LC and Marrie RA: Registry cohort study to determine risk for
multiple sclerosis after vaccination for pandemic influenza A(H1N1)
with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 24:1267–1274.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuda M, Yoshida T, Moroki M, Hirayu N,
Nabeta M, Nakamura A, Uzu H and Takasu O: Influenza A with
hemorrhagic shock and encephalopathy syndrome in an adult: A case
report. Medicine (Baltimore). 98:e150122019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegers JY, van de Bildt MWG, Lin Z,
Leijten LM, Lavrijssen RAM, Bestebroer T, Spronken MIJ, De Zeeuw
CI, Gao Z, Schrauwen EJA, et al: Viral factors important for
efficient replication of influenza A viruses in cells of the
central nervous system. J Virol. 93:e02273–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ninove L, Daniel L, Gallou J, Cougard PA,
Charpentier A, Viard L, Roquelaure B, Paquis-Flucklinger V, de
Lamballerie X, Zandotti C and Charrel RN: Fatal case of Reye's
syndrome associated with H3N2 influenza virus infection and
salicylate intake in a 12-year-old patient. Clin Microbiol Infect.
17:95–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francis JR, Richmond P, Robins C, Lindsay
K, Levy A, Effler PV, Borland M and Blyth CC: An observational
study of febrile seizures: The importance of viral infection and
immunization. BMC Pediatr. 16:2022016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cárdenas G, Soto-Hernández JL, Díaz-Alba
A, Ugalde Y, Mérida-Puga J, Rosetti M and Sciutto E: Neurological
events related to influenza A (H1N1) pdm09. Influenza Other Respir
Viruses. 8:339–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tin SS and Wiwanitkit V: Neurological
complications after H1N1 influenza vaccination. Arq Neuropsiquiatr.
72:9772014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo CY, Tang YG, Qi ZL, Liu Y, Zhao XR,
Huo XP, Li Y, Feng Q, Zhao PH, Wang X, et al: Development and
characterization of a panel of cross-reactive monoclonal antibodies
generated using H1N1 influenza virus. Immunobiology. 220:941–946.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto M, Cid E and Yamamoto F:
Molecular genetic basis of the human Forssman glycolipid antigen
negativity. Sci Rep. 2:9752012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JJ, Yang GX, Zhang WC, Lu L,
Tsuneyama K, Kronenberg M, Véla JL, Lopez-Hoyos M, He XS, Ridgway
WM, et al: Escherichia coli infection induces autoimmune
cholangitis and anti-mitochondrial antibodies in non-obese diabetic
(NOD).B6 (Idd10/Idd18) mice. Clin Exp Immunol. 175:192–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coppieters KT and von Herrath M: Antibody
cross-reactivity and the viral aetiology of type 1 diabetes. J
Pathol. 230:1–3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo CY, Zhang HX, Zhang JJ, Sun LJ, Li HJ,
Liang DY, Feng Q, Li Y, Feng YM, Xie X and Hu J: Localization
analysis of heterophilic antigen epitopes of H1N1 influenza virus
hemagglutinin. Virol Sin. 34:306–314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun L, Li H, Sun J, Guo C, Feng Y, Li Y,
Zhao X, Xie X and Hu J: Antibodies against H1N1 influenza virus
hemagglutinin cross-react with prohibitin. Biochem Biophys Res
Commun. 513:446–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levin MC, Lee SM, Kalume F, Morcos Y,
Dohan FC Jr, Hasty KA, Callaway JC, Zunt J, Desiderio D and Stuart
JM: Autoimmunity due to molecular mimicry as a cause of
neurological disease. Nat Med. 8:509–513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, Tu JL and Xiong YS:
Influenza-related central nervous system damage. Chin J Neurol.
43:737–738. 2010.
|
|
16
|
Ahmed SS, Volkmuth W, Duca J, Corti L,
Pallaoro M, Pezzicoli A, Karle A, Rigat F, Rappuoli R, Narasimhan
V, et al: Antibodies to influenza nucleoprotein cross-react with
human hypocretin receptor 2. Sci Transl Med. 7:294ra1052015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarkanen TO, Alakuijala APE, Dauvilliers
YA and Partinen MM: Incidence of narcolepsy after H1N1 influenza
and vaccinations: Systematic review and meta-analysis. Sleep Med
Rev. 38:177–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wekerle H: Vaccination and narcolepsy:
Immune link found? Sci Transl Med. 7:1–3. 2015. View Article : Google Scholar
|
|
19
|
Krecic AM and Swanson MS: hnRNP complexes:
Composition, structure, and function. Curr Opin Cell Biol.
11:363–371. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levin MC, Lee SM, Gardner LA, Shin Y,
Douglas JN and Salapa H: Autoantibodies to heterogeneous nuclear
ribonuclear protein A1 (hnRNPA1) cause altered ‘ribostasis’ and
neurodegeneration; the legacy of HAM/TSP as a model of progressive
multiple sclerosis. J Neuroimmunol. 304:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Douglas JN, Gardner LA, Salapa HE, Lalor
SJ, Lee S, Segal BM, Sawchenko PE and Levin MC: Antibodies to the
RNA-binding protein hnRNP A1 contribute to neurodegeneration in a
model of central nervous system autoimmune inflammatory disease. J
Neuroinflammation. 13:1782016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sueoka E, Yukitake M, Iwanaga K, Sueoka N,
Aihara T and Kuroda Y: Autoantibodies against heterogeneous nuclear
ribonucleoprotein B1 in CSF of MS patients. Ann Neurol. 56:778–786.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee S, Xu L, Shin Y, Gardner L, Hartzes A,
Dohan FC, Raine C, Homayouni R and Levin MC: A potential link
between autoimmunity and neurodegeneration in immune-mediated
neurological disease. J Neuroimmunol. 235:56–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mishra S, Murphy LC and Murphy LJ: The
prohibitins: Emerging roles in diverse functions. J Cell Mol Med.
10:353–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SM, Dunnavant FD, Jang H, Zunt J and
Levin MC: Autoantibodies that recognize functional domains of
hnRNPA1 implicate molecular mimicry in the pathogenesis of
neurological disease. Neurosci Lett. 401:188–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gauntt CJ, Arizpe HM, Higdon AL, Wood HJ,
Bowers DF, Rozek MM and Crawley R: Molecular mimicry,
anti-coxsackievirus B3 neutralizing monoclonal antibodies, and
myocarditis. J Immunol. 154:2983–2995. 1995.PubMed/NCBI
|