Introduction
Local anesthetics (LAs) are widely used to relieve
acute, intraoperative and postoperative chronic pain (1). Ropivacaine is a novel type of amide
LA that blocks fewer motor fibers than sensory fibers, and is less
cardiotoxic and neurotoxic compared with other Las (2). Therefore, ropivacaine is commonly
selected instead of bupivacaine for postoperative analgesia
(3).
The relatively short duration of the effects of a
single LA injection frequently leaves the patient in pain when the
block wears off, particularly for postoperative analgesia;
therefore, prolonging the duration of analgesia is a priority
(4). An increase in the dose
and/or volume of LA administered may prolong the duration of
analgesia but may also increase the risk of neurotoxicity (5). Although continuous catheter-based
nerve blocks can extend postoperative analgesia, their placement
requires additional time, cost and skill (4).
Several perineural adjuvants have been studied with
the goal of prolonging the duration of analgesia, reducing the dose
of LA and improving analgesia with fewer adverse effects (6–8).
Dexmedetomidine is a potent, highly selective
α2-adrenoceptor agonist and its
α2/α1 selectivity is eight times greater than
that of clonidine (9). A study
reported that dexmedetomidine enhances the duration of bupivacaine
anesthesia and analgesia of sciatic nerve block in rats (10). In addition, dexmedetomidine
possesses neuroprotective properties in various experimental models
(11). LAs are generally thought
to be relatively safe and the potential neurotoxicity of LAs has
been investigated for a number of years (12). Ropivacaine has been documented to
be less cardiotoxic and neurotoxic compared with other Las
(13). However, there have been a
number of reports on the neurotoxicity of ropivacaine (14,15).
LA-induced direct nerve injury can occur following the
administration of LAs at clinical concentrations (16), and the mechanism underlying
neurotoxicity induced by LAs is complex and not completely
understood (14,17,18).
Evidence of neuronal apoptosis has been observed in a number of
animal models and the mitochondrial pathway has been demonstrated
to be involved in LA-mediated apoptosis (17). The degree of LA toxicity has been
demonstrated to be concentration- or dose-dependent (19).
A previous study has demonstrated that the addition
of dexmedetomidine to an LA extends the duration of blockade in the
peripheral nerves (20); however,
the effect of dexmedetomidine on neurotoxicity is not completely
understood. The present study aimed to investigate the effects of
dexmedetomidine as an adjuvant to ropivacaine for sciatic nerve
block, to explore whether it can mitigate the toxic effect of LAs
and to identify the mechanisms underlying dexmedetomidine.
Materials and methods
Animal selection and housing
The present study was approved by the Ethics
Committee of the First Hospital of Lanzhou University (approval no.
LDYYLL2019-111)and the procedures were performed according to
routine animal care guidelines. Healthy male adult SPF Wistar rats
(n=40; age 8–10 weeks; weight, 180–220 g) were provided by the
Experimental Animal Center of Gansu University of Traditional
Chinese Medicine (Lanzhou, China). The rats were housed in separate
cages in temperature-controlled rooms (20–24°C; relative humidity,
50–60%) with 12-h light/dark cycles, and free access to food and
water until the time of experimentation.
Groups and treatment
The rats were randomly divided into the following
five groups (n=8): i) Control (group C); ii) sham (group S); iii)
ropivacaine (group R); iv) low-dose dexmedetomidine group (group
D1); and v) high-dose dexmedetomidine group (group D2).
Sciatic nerve block was performed according to the
procedure described by Kim et al (21), but in the present study the drug
was injected into the perineural space under the fascia covering
the nerve. Following anaesthetization with 1.2–1.5% isoflurane in
oxygen using a mask, the bilateral sciatic nerve was exposed by a
gluteal muscle-splitting incision. The rats in group C underwent
bilateral sciatic nerve exposure only. The rats in group S received
a bilateral injection of 0.2 ml 0.9% NaCl. The rats in group R
received a bilateral injection of 0.2 ml 0.5% ropivacaine
hydrochloride (batch number: LBDZ; AstraZeneca). The rats in group
D1 received a bilateral injection of 0.2 ml 0.5% ropivacaine plus 6
µg/kg dexmedetomidine hydrochloride (batch number: 16110732;
Jiangsu Hengrui Medicine Co., Ltd.), whereas rats in group D2
received a bilateral injection of 0.2 ml 0.5% ropivacaine plus 20
µg/kg dexmedetomidine hydrochloride. A nonabsorbable muscle fascia
suture was placed at the midpoint of the injection site as a marker
for subsequent nerve removal. Following the injection, the incision
was sutured layer by layer, penicillin powder was applied to the
surgical wound to prevent infection and the rats were placed into
individual feeding chambers.
Paw withdrawal thermal latency (PWL)
testing
The time at which the righting reflex returned was
recorded to the nearest minute (0 min) and the rats were then
placed in a chamber for the PWL test, which was performed with an
RB-200 intelligent hot plate (Shanghai Uilian, Inc.) at 55±1°C
every 30 min for 330 min. The PWL indicated deficiencies in sensory
function. The mean value of three measurements at each time point
was calculated. A cut-off time of 15 sec was used to avoid tissue
damage. If no withdrawal was observed after 15 sec, the stimulus
was removed and the PWL was recorded as 15 sec.
Extensor postural thrust (EPT)
testing
To measure the rat hindfoot EPT (22), each rat was held upright with its
hindlimb extended such that the body's weight was supported by the
distal limb and toes. The extensor thrust, the force that resisted
contact of the heel with the platform, was measured as the force
applied to the digital platform balance (cat. no. JY303; Shanghai
Puchun Measure Instrument Co., Ltd.). A reduction in this force,
which represented reduced extensor muscle tone, was considered a
deficiency in motor function. Once the sensory and motor function
of each rat had returned to baseline, the rat was returned to its
home cage.
Histopathological evaluation
For histopathological assessment, at 24 h post-drug
administration, the rats were anaesthetized with 1.2–1.5%
isoflurane and the sciatic nerves were removed from the injection
site. Each sciatic nerve was fixed with 10% formaldehyde solution
for 24 h at room temperature, sequentially dehydrated with grade
ethanol (70, 80, 95, 100 and 100%), cleared in xylene and embedded
in paraffin. Sections (thickness, 5 µm) were prepared and
deparaffinized at 40°C in a water bath and rehydrated. Samples were
then washed with distilled water and dried. 2% hematoxylin was
added for 5 min at room temperature and rinsed with water.
Subsequently 1% HCl ethanol solution (1 ml HCl added to 99 ml 70%
ethanol) was added for 10 sec in triplicate to remove excess
haematoxylin. Following this, sections were washed using distilled
water for 25 min, then 0.5% eosin was added at room temperature for
2 min and slices were dehydrated with 95 and 100% ethanol.
Dimethylbenzene (Absin Bioscience, Inc.) was added for 5 min twice
and incubated at 37°C for 24 h. Finally, pathological changes were
observed under an optical microscope (magnification, ×400; Olympus
Corporation). The rats were euthanized by overdose of
isoflurane.
Apoptosis of sciatic nerve cells
TUNEL staining was performed to examine apoptosis.
Samples of sciatic nerve were fixed in 10% formalin for 20 min at
room temperature and embedded in paraffin. Sections (5 µm) were
deparaffinized, rehydrated and incubated for 15 min at 37°C with
proteinase K working solution (Shanghai Xiangsheng Biotechnology
Co., Ltd.). The sections were rinsed twice with PBS (pH 7.4) and
incubated in a 0.3% hydrogen peroxide blocking solution for 15 min
at room temperature. Subsequently, 50 µl TUNEL reaction reagent was
then added to the sections, which were incubated for 60 min at 37°C
in a humidified atmosphere in the dark. Following rinsing with PBS
(pH 7.4) three times, 50 µl converter peroxidase was added to the
sections, which were incubated for 30 min at 37°C, and 50 µl
diaminobenzidine substrate was added to the sections prior to
incubation for 10 min at 25°C. The sections were rinsed with PBS
(pH 7.4) three times and analyzed under a light microscope
(magnification, ×400). According to the distribution of apoptotic
positive cells, three unrepeated visual fields of each section were
observed under a light microscope and the percentage of positive
cells was calculated as the apoptosis rate (number of apoptotic
cells/total number of cells in the field).
Cleaved caspase-3 expression in the
rat sciatic nerve
Cleaved caspase-3 expression was detected via
western blotting. The samples were cut into small pieces (2 mm
long; 2 mm thick) and homogenized in lysis buffer [50 mM Tris HCl
(pH 7.6), 20 mM MgCl2, 150 mM NaCl, 0.5% Triton-X, 5 U/ml
aprotinin, 5 µg/ml leupeptin, 5 µg/ml pepstatin, 1 mM benzamidine,
1 mM phenylmethylsulfonyl fluoride]. Lysate protein levels were
determined using a BCA protein assay kit (Shanghai Qcbio Science
& Technologies Co., Ltd.). Equal amounts of protein (30 µg)
were separated via 10% SDS-PAGE and subjected to gel
electrophoresis. The separated proteins were transferred onto
nitrocellulose membranes, which were blocked with 5% nonfat dried
milk in Tris-buffered saline with Tween 20 (137 mM sodium chloride,
20 mM Tris, 0.1% Tween 20; Absin Bioscience, Inc.) for 1 h at room
temperature. Subsequently, the membranes were incubated with
primary antibodies targeted against cleaved caspase-3 (1:1,000;
cat. no. bsm-33199M; Beijing Biosynthesis Biotechnology Co., Ltd.)
and β-actin (1:1,000; cat. no. bsm-33036M Beijing Biosynthesis
Biotechnology Co., Ltd.) diluted in Tris-buffered saline with Tween
20 overnight at 4°C. The membranes were then incubated with
alkaline phosphatase-conjugated goat anti-mouse IgG secondary
antibodies (1:1,000; cat. no. bs-40296G-HRP; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h at room temperature and the
reactive bands were detected following incubation with nitroblue
tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate
(Sigma-Aldrich; Merck KGaA) for 5 min at room temperature. The
bands were scanned, their densities were assessed using an imaging
densitometer (cat. no. GS-800; Bio-Rad Laboratories, Inc.) and the
optical densities were quantified using Image-Pro Plus software
(version 7.0; Media Cybernetics, Inc.) with β-actin as the loading
control.
Statistical analysis
Data were statistically analysed using SPSS 20.0
software (IBM Corp.). All data are presented as the mean ± (SD) of
five experimental repeats (n=6 in each group). One-way ANOVA
followed by Tukey's post hoc test was performed to determine
overall differences in PWLs and EPTs at each time point, as well as
the differences in the levels of apoptosis and expression of
cleaved caspase-3 among all experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
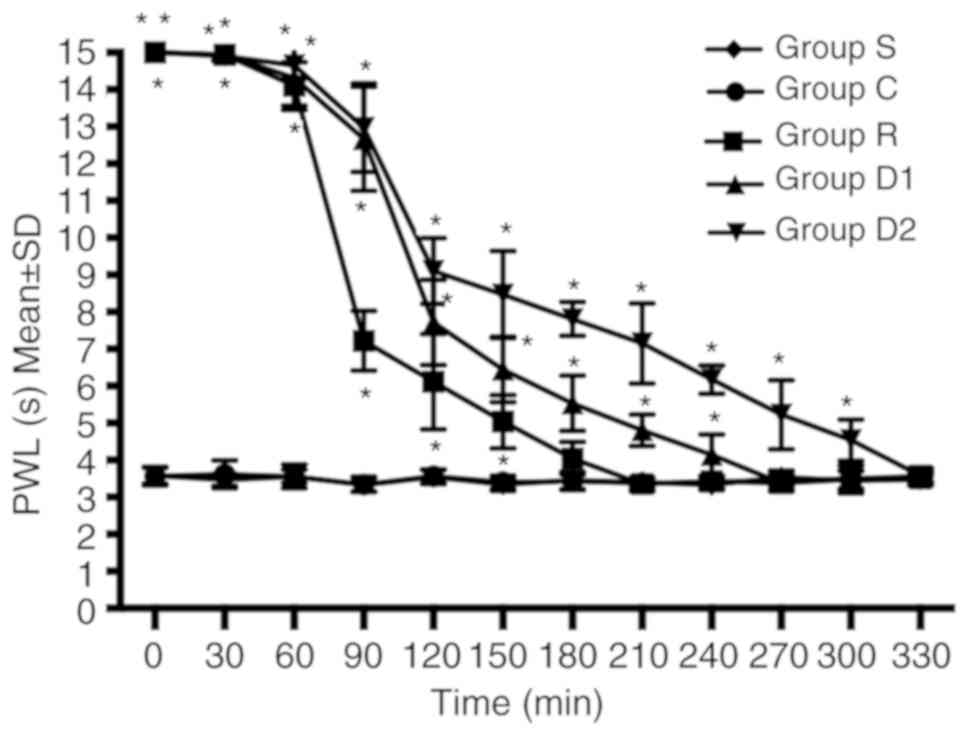
Neurobehavioral evaluation
Compared with group R, groups D1 and D2 displayed an
increased duration of analgesia against a heat stimulus. The time
taken to return to baseline sensory function (defined as P≥0.05
compared with group C) was longer in groups D1 and D2 compared with
group R. The time to return to baseline sensory function was longer
in group D2 compared with group D1. No significant difference was
observed between groups C and S (P>0.05; Fig. 1).
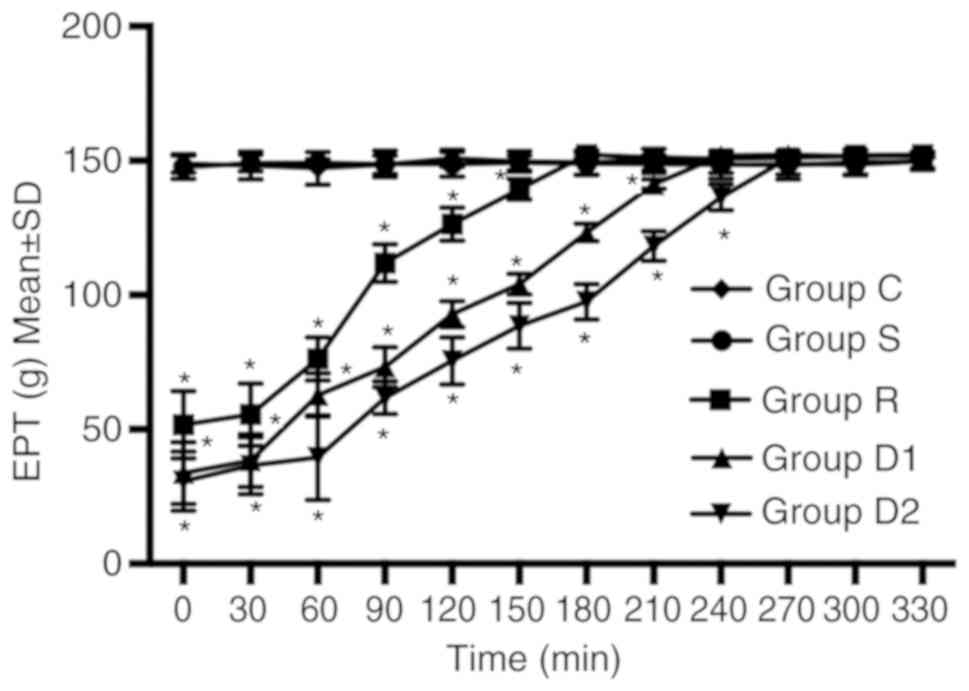
Groups D1 and D2 increased the duration of motor
blockade compared with group R. The time to return to baseline
motor function (defined as P≥0.05 compared with group C) was longer
in groups D1 and D2 compared with group R. The time to return to
baseline motor function was longer in group D2 compared with group
D1. No significant difference was observed between groups C and S
(P>0.05; Fig. 2).
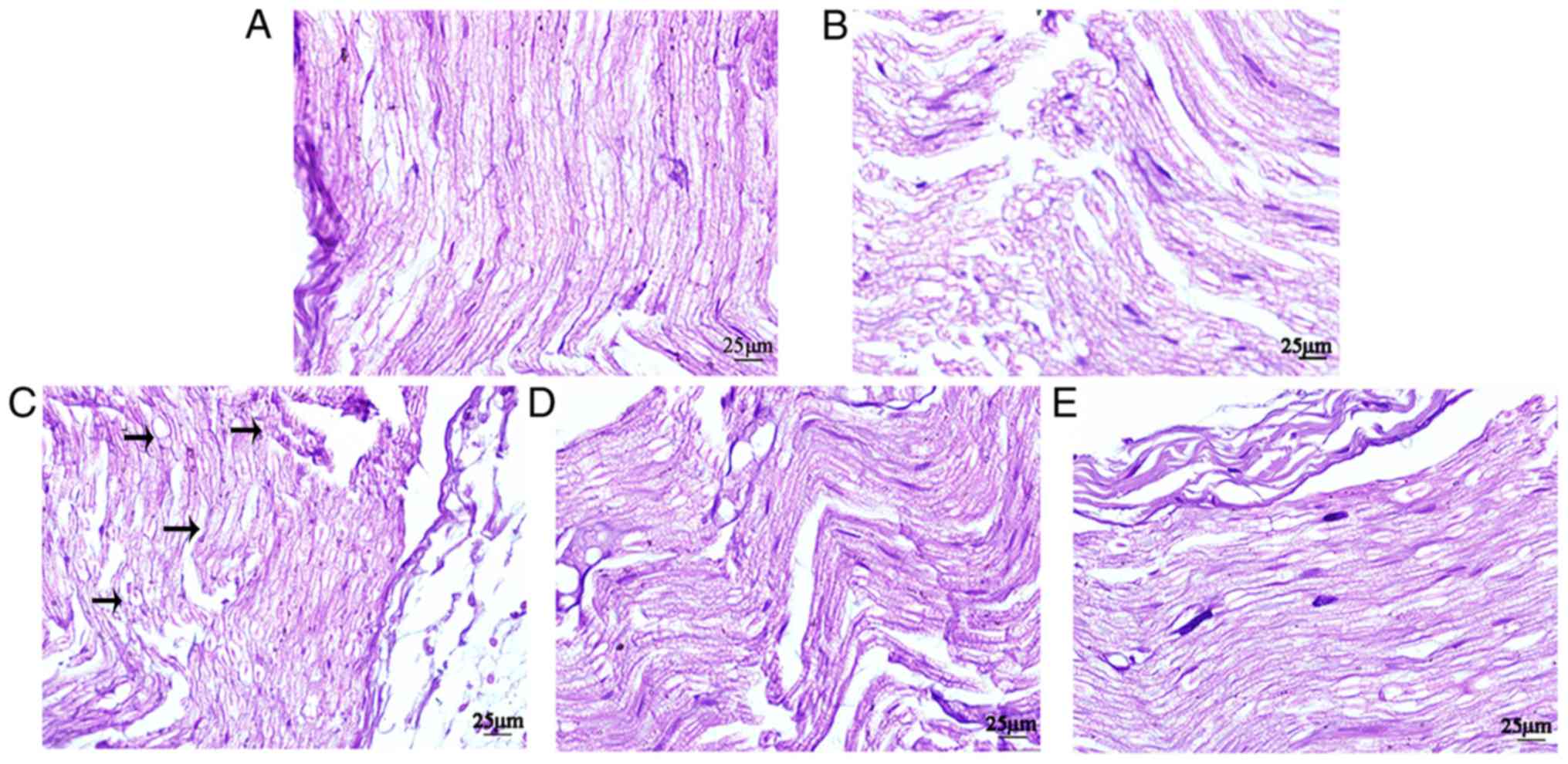
Histopathologic evaluation
Histopathologic analysis indicated that groups D1
and D2 displayed alleviated nerve injury compared with group R. In
groups S and C, the sciatic nerve was intact, the nerve fibers were
arranged tightly and neatly, the morphology was normal, the
staining was uniform and the structures of the axons and myelin
sheath were clear. In group R, certain sciatic nerve fibers were
disordered and interrupted, and certain axons and nerve sheaths
displayed edema. Compared with group R, group D1 displayed markedly
less disorder and discontinuity in the nerve fiber structure, and
axonal and myelin edema in the nerve fibers. Compared with group
D1, the fibers of the sciatic nerve in group D2 were arranged more
neatly, and myelin sheath and axon edema was milder (Fig. 3).
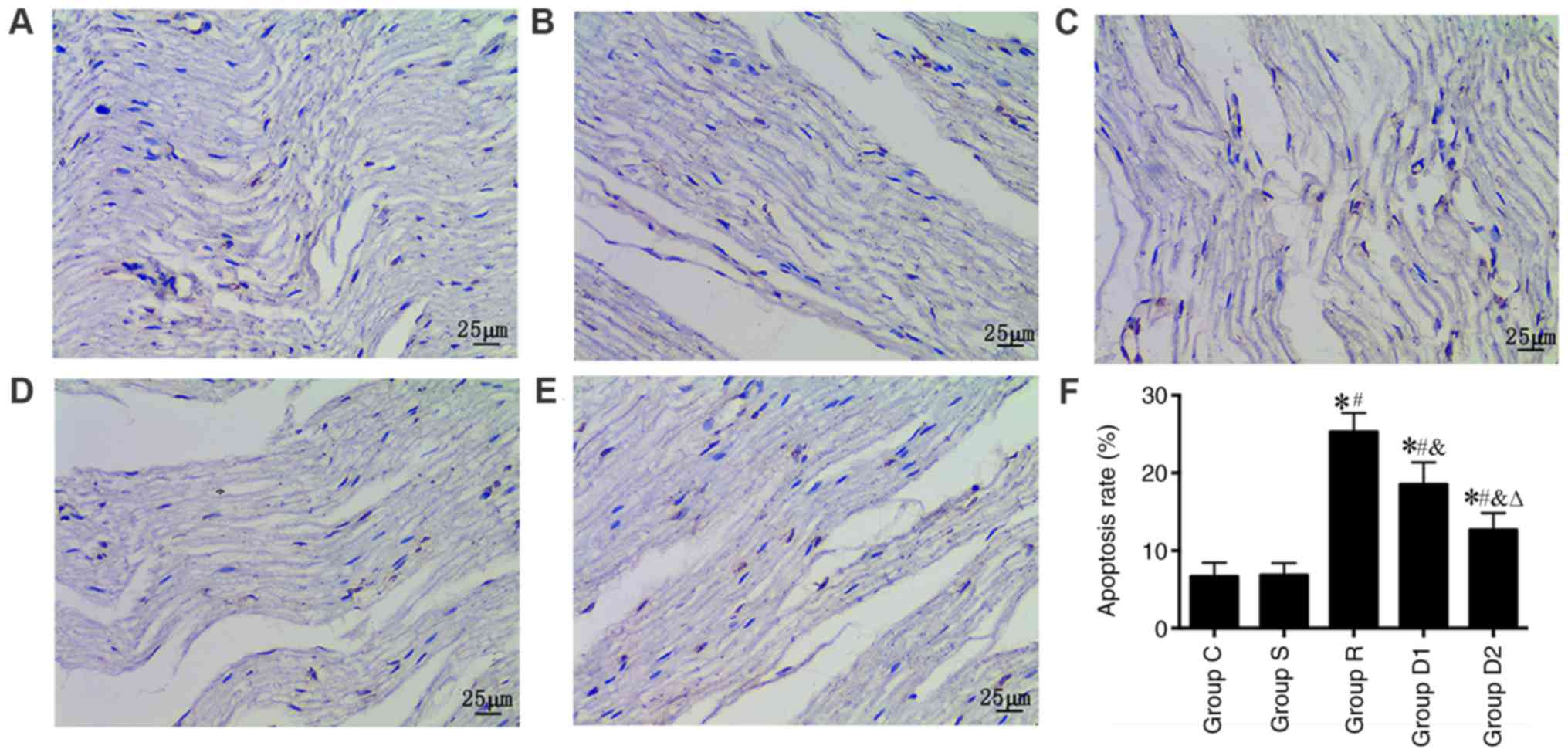
Alterations in apoptosis
The TUNEL assay was used to observe neuronal
apoptosis under a light microscope. The results show that the nerve
cell apoptosis rate was significantly higher in groups R, D1 and D2
compared with group S (P<0.05). Furthermore, the nerve cell
apoptosis rate was significantly lower in groups D1 and D2 compared
with group R (P<0.05). Finally, the nerve cell apoptosis rate
was significantly lower in group D2 compared with group D1
(P<0.05). No significant difference was observed in the nerve
cell apoptosis rate between groups C and S (P>0.05; Fig. 4).
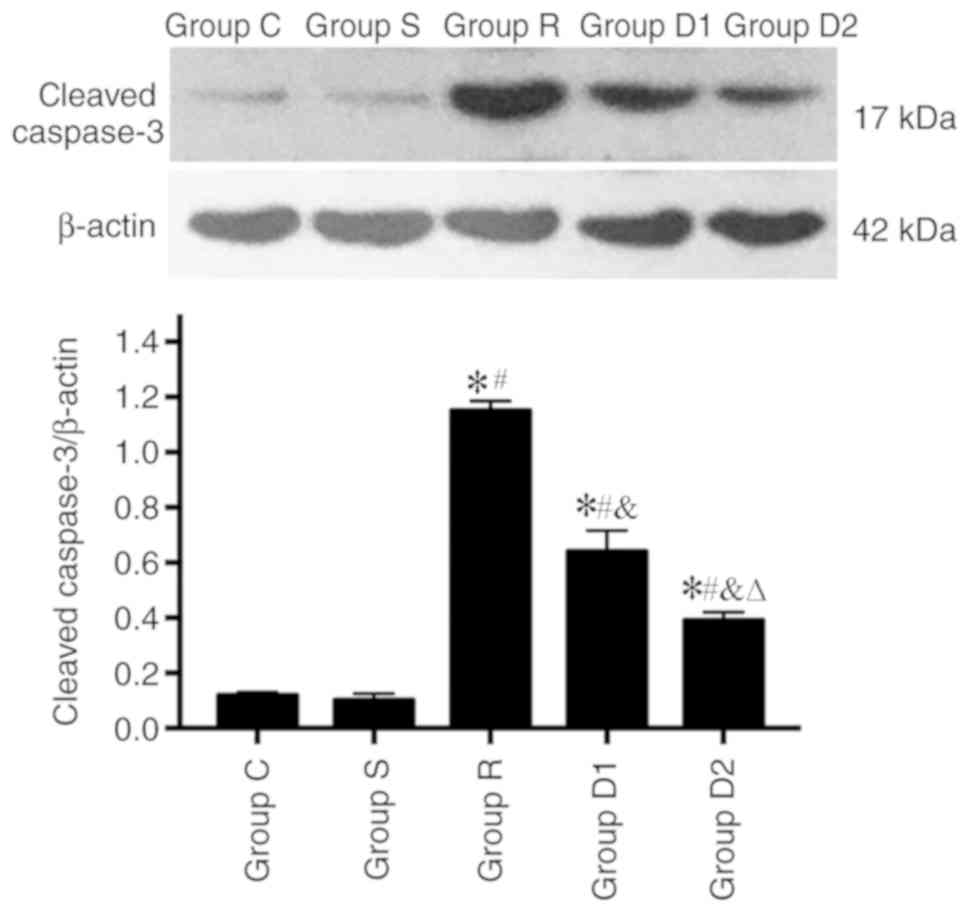
Cleaved caspase-3 expression
Alterations in the expression levels of cleaved
caspase-3 were detected via western blotting. The expression of
cleaved caspase-3 was significantly higher in group R compared with
groups S and C (P<0.05), and the expression of cleaved caspase-3
was significantly lower in groups D1 and D2 compared with group R
(P<0.05). In addition, the expression of cleaved caspase-3 was
significantly lower in group D2 compared with group D1 (P<0.05).
No significant difference was observed in cleaved caspase-3
expression levels between groups C and S (P>0.05; Fig. 5).
Discussion
In the present study, a rat model of sciatic nerve
block was established according to the method described by Kim
et al (21). In the study
conducted by Kim et al (21), 0.5 µg/kg dexmedetomidine was added
to ropivacaine. However, Brummett et al (23) demonstrated that dexmedetomidine
added to ropivacaine increases the duration of sensory blockade in
a dose-dependent manner. The aforementioned study also identified
that the highest dose of dexmedetomidine (20 µg/kg) used in the
study displayed the greatest effects, but there were no significant
differences in motor function between (0.5, 2.0 and 6.0 µg/kg,
respectively) doses of dexmedetomidine. Therefore, in the present
study, 20 mg/kg dexmedetomindin was used in the high dose group and
6.0 µg/kg dexmedetomindin was used in the low dose group. The
present study demonstrated that, compared with group S, the PWL of
group R was increased, whereas the EPT of group R was decreased,
suggesting the successful establishment of a rat model of sciatic
nerve block.
To enhance and prolong the anesthetic and analgesic
effects of LAs, a variety of LA adjuvants have been investigated,
and used in single-shot and continuous peripheral nerve blocks
(24–26). α2-adrenergic receptors
are widely distributed throughout the peripheral and central
nervous systems (27). A previous
study demonstrated that the use of α2-adrenoreceptor
agonists in peripheral nerve blocks is safe and beneficial
(28). Dexmedetomidine, a potent
and highly selective agonist of α2-adrenergic receptors,
has been used as an analgesic and antinociceptive adjuvant
(29).
Previous study has demonstrated that the addition of
dexmedetomidine as an adjuvant for nerve block prolongs the
duration of analgesia and increases the speed of analgesia onset
(30). The present study indicated
that dexmedetomidine prolonged the duration of sciatic nerve block
and enhanced the analgesic efficacy of ropivacaine in rats.
However, the mechanism underlying α2-adrenergic receptor
agonist-induced analgesia and sedation remains to be elucidated. A
previous study demonstrated that dexmedetomidine binds
α2-adrenoceptors on the cell membranes of neurons, which
leads to the activation of G protein-coupled inwardly rectifying
K+ channels and inhibition of Ih channels,
resulting in hyperpolarization of the membrane (31). By contrast, another study
demonstrated that α2 agonists produce analgesia and
sedation by inhibiting substance P release in the nociceptive
pathway at the level of the dorsal root neuron (32). The mechanism underlying
α2 agonist activity is not fully understood but is
probably multifactorial.
In the present study, the addition of
dexmedetomidine to ropivacaine for sciatic nerve block shortened
sensory and motor block onset time, and extended motor and sensory
block duration, particularly sensory duration, compared with
ropivacaine alone. When ropivacaine was combined with 6 µg/kg
dexmedetomidine, the sensory block duration was prolonged from 150
to 240 min, whereas combination treatment with 20 µg/kg
dexmedetomidine prolonged the sensory block duration to 300 min,
suggesting that the effect of 20 µg/kg dexmedetomidine was more
pronounced compared with 6 µg/kg dexmedetomidine.
Although ropivacaine has been considered a
relatively safer LA than bupivacaine in recent years due to its
lower degree of motor block and decreased tendency to cause
neurotoxicity, a previous study demonstrated that ropivacaine at a
clinical concentration (2.5 mg/ml) is neurotoxic to isolated
sensory neurons (33). Ropivacaine
can induce the apoptosis of rabbit annulus fibrosus cells in
vitro, which involved the mitochondrial signaling pathway
(17). The results of the present
study suggested that the nerve cell apoptosis rate was increased
and the expression of cleaved caspase-3 was upregulated in group R
compared group S, which indicated that ropivacaine induced sciatic
nerve toxicity in rats and that the mechanism underlying this
effect may be associated with caspase-3-dependent apoptosis.
A previous study has demonstrated that
dexmedetomidine can serve as a neuroprotective agent against brain
injury via the inhibition of neuronal apoptosis (34). Sun et al (35) identified that dexmedetomidine
confers neuroprotection against spinal cord ischemia-reperfusion
injury via the suppression of spinal cord inflammation and
apoptosis. Another study demonstrated that dexmedetomidine
attenuates neuronal injury induced by maternal propofol anesthesia
in fetal brains (36). The
mechanism underlying dexmedetomidine may be associated with
inhibition of propofol-induced caspase-3 activation and the
microglial response in fetal brains (37). Kim et al (21) demonstrated that dexmedetomidine
added to ropivacaine significantly reduces IL-6 and IL-1β mRNA
levels compared with ropivacaine alone at 60 min post-intraneural
injection. However, the exact effect of dexmedetomidine on the
neurotoxicity of LAs is not completely understood. In the present
study, the effect of dexmedetomidine on the neurotoxicity of
ropivacaine was observed. The results indicated that, compared with
group R, the apoptosis rate and caspase-3 expression levels were
significantly reduced in groups D1 and D2. The results of the
present study also suggested that dexmedetomidine reduced
ropivacaine-induced neurotoxicity for sciatic nerve block and that
the efficacy of 20 µg/kg dexmedetomidine was greater compared with
6 µg/kg dexmedetomidine.
In conclusion, the present study suggested that
dexmedetomidine may serve as a potential LA adjuvant that can
prolong the sensory and motor block time of the sciatic nerve,
enhance the effect of nerve block and reduce ropivacaine-induced
neurotoxicity in rats. Furthermore, the effects of dexmedetomidine
were greatest at a dose of 20 µg/kg. The results of the present
study provided novel insight into the clinical use of
dexmedetomidine, suggesting that dexmedetomidine not only provided
the longest duration of analgesia but also reduced the
neurotoxicity of LAs.
However, the current understanding of the clinical
use of dexmedetomidine is not sufficient. In the present study,
only two doses were studied, thus, whether further increasing the
dose may have a protective effect requires further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81960345) and the
Gansu Province Health Industry Plan (grant no. GSWSKY2017-18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX wrote the manuscript. XX, JF and YL conceived and
designed the present study. XX, JF, XM, YL, XH and JY performed the
experimental procedures. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the First Hospital of Lanzhou University (Gansu,
China; approval no. LDYYLL2019-111).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scott DB: Local anesthetics: Mechanisms of
action and clinical use. Br J Anaesthesia. 48:1031–1032. 1976.
View Article : Google Scholar
|
|
2
|
Hansen TG: Ropivacaine: A pharmacological
review. Expert Rev Neurother. 4:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feldman HS and Covino BG: Comparative
motor-blocking effects of bupivacaine and ropivacaine, a new amino
amide local anesthetic, in the rat and dog. Anesth Analg.
67:1047–1052. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Auyong DB, Cantor DA, Green C and Hanson
NA: The effect of fixation technique on continuous interscalene
nerve block catheter success: A randomized, double-blind trial.
Anesth Analg. 124:959–965. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosen MA, Baysinger CL, Shnider SM, Dailey
PA, Norton M, Curtis JD, Collins M and Davis RL: Evaluation of
neurotoxicity after subarachnoid injection of large volumes of
local anesthetic solutions. Anesth Analg. 62:802–808. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vieira PA, Pulai I, Tsao GC, Manikantan P,
Keller B and Connelly NR: Dexamethasone with bupivacaine increases
duration of analgesia in ultrasound-guided interscalene brachial
plexus blockade. Eur J Anaesthesiol. 27:285–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Candido KD, Hennes J, Gonzalez S,
Mikat-Stevens M, Pinzur M, Vasic V and Knezevic NN: Buprenorphine
enhances and prolongs the postoperative analgesic effect of
bupivacaine in patients receiving infragluteal sciatic nerve block.
Anesthesiology. 113:1419–1426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jarbo K, Batra YK and Panda NB: Brachial
plexus block with midazolam and bupivacaine improves analgesia. Can
J Anaesth. 52:822–826. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaur M and Singh PM: Current role of
dexmedetomidine in clinical anesthesia and intensive care. Anesth
Essays Res. 5:128–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aksu R and Bicer C: Addition of
dexmedetomidine to bupivacaine in supraclavicular brachial plexus
block. Clin Invest Med. 40:E111–E116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma D, Hossain M, Rajakumaraswamy N, Arshad
M, Sanders RD, Franks NP and Maze M: Dexmedetomidine produces its
neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J
Pharmacol. 502:87–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perez-Castro R, Patel S, Garavito-Aguilar
ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ and Xu F:
Cytotoxicity of local anesthetics in human neuronal cells. Anesth
Analg. 108:997–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reiz S, Häggmark S, Johansson G and Nath
S: Cardiotoxicity of ropivacaine-a new amide local anaesthetic
agent. Acta Anaesthesiol Scand. 33:93–98. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen X, Liang H, Li H, Ou W, Wang HB, Liu H
and Li S: In vitro neurotoxicity by ropivacaine is reduced by
silencing Cav3.3 T-type calcium subunits in neonatal rat sensory
neurons. Artif Cells Nanomed Biotechnol. 46:1617–1624.
2018.PubMed/NCBI
|
|
15
|
Sun Z, Liu H, Guo Q, Xu X, Zhong Z and
Wang N: In vivo and in vitro evidence of the neurotoxic effects of
ropivacaine: The role of the Akt signaling pathway. Mol Med Rep.
6:1455–1459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang AZ, Ficklscherer A, Gülecyüz MF,
Paulus AC, Niethammer TR, Jansson V and Müller PE: Cell toxicity in
fibroblasts, tenocytes, and human mesenchymal stem cells-a
comparison of necrosis and apoptosis-inducing ability in
ropivacaine, bupivacaine, and triamcinolone. Arthroscopy.
33:840–848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai XY, Xia Y, Yang SH, Liu XZ, Shao ZW,
Liu YL, Yang W and Xiong LM: Ropivacaine- and bupivacaine-induced
death of rabbit annulus fibrosus cells in vitro: Involvement of the
mitochondrial apoptotic pathway. Osteoarthritis Cartilage.
23:1763–1775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhir S, Ganapathy S, Lindsay P and Athwal
GS: Case report: Ropivacaine neurotoxicity at clinical doses in
interscalene brachial plexus block. Can J Anaesth. 54:912–916.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werdehausen R, Fazeli S, Braun S, Hermanns
H, Essmann F, Hollmann MW, Bauer I and Stevens MF: Apoptosis
induction by different local anaesthetics in a neuroblastoma cell
line. Br J Anaesth. 103:711–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu ZY, Geng J, Li ZQ, Sun YB, Wang SL,
Masters J, Wang DX, Guo XY, Li M and Ma D: Dexmedetomidine enhances
ropivacaine-induced sciatic nerve injury in diabetic rats. Br J
Anaesth. 122:141–149. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim BS, Choi JH, Baek SH and Lee DH:
Effects of intraneural injection of dexmedetomidine in combination
with ropivacaine in rat sciatic nerve block. Reg Anesth Pain Med.
43:378–384. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thalhammer JG, Vladimirova M, Bershadsky B
and Strichartz GR: Neurologic evaluation of the rat during sciatic
nerve block with lidocaine. Anesthesiology. 82:1013–1025. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brummett CM, Padda AK, Amodeo FS, Welch KB
and Lydic R: Perineural dexmedetomidine added to ropivacaine causes
a dose-dependent increase in the duration of thermal
antinociception in sciatic nerve block in rat. Anesthesiology.
111:1111–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Candido KD, Winnie AP, Ghaleb AH, Fattouh
MW and Franco CD: Buprenorphine added to the local anesthetic for
axillary brachial plexus block prolongs postoperative analgesia.
Reg Anesth Pain Med. 27:162–167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mccartney CJ, Duggan E and Apatu E: Should
we add clonidine to local anesthetic for peripheral nerve blockade?
A qualitative systematic review of the literature. Reg Anesth Pain
Med. 32:330–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parrington SJ, O'Donnell D, Chan VW,
Brown-Shreves D, Subramanyam R, Qu M and Brull R: Dexamethasone
added to mepivacaine prolongs the duration of analgesia after
supraclavicular brachial plexus blockade. Reg Anesth Pain Med.
35:422–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosin DL: Distribution of alpha 2A - and
alpha 2C -adrenergic receptor immunoreactivity in the central
nervous system. Methods Mol Biol. 126:475–505. 2000.PubMed/NCBI
|
|
28
|
Singelyn FJ, Gouverneur JM and Robert A: A
minimum dose of clonidine added to mepivacaine prolongs the
duration of anesthesia and analgesia after axillary brachial plexus
block. Reg Anesth. 83:1046–1050. 1996.
|
|
29
|
Nie Y, Tu W, Shen X, Yu W, Yu Y, Song X,
Wang S, Luo A, Cao M, Wu X and Huang S: Dexmedetomidine added to
sufentanil patient-controlled intravenous analgesia relieves the
postoperative pain after cesarean delivery: A prospective
randomized controlled multicenter study. Sci Rep. 8:99522018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brummett CM, Norat MA, Palmisano JM and
Lydic R: Perineural administration of dexmedetomidine in
combination with bupivacaine enhances sensory and motor blockade in
sciatic nerve block without inducing neurotoxicity in rat.
Anesthesiology. 109:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang YC, Meng QT, Pan X, Xia ZY and Chen
XD: Dexmedetomidine produced analgesic effect via inhibition of HCN
currents. Eur J Pharmacol. 740:560–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eisenach JC, De Kock M and Klimscha W:
Alpha(2)-adrenergic agonists for regional anesthesia. A clinical
review of clonidine (1984–1995). Anesthesiology. 85:655–674. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Williams BA, Hough KA, Tsui BY, Ibinson
JW, Gold MS and Gebhart GF: Neurotoxicity of adjuvants used in
perineural anesthesia and analgesia in comparison with ropivacaine.
Reg Anesth Pain Med. 36:225–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang MH, Zhou XM, Cui JZ, Wang KJ, Feng Y
and Zhang HA: Neuroprotective effects of dexmedetomidine on
traumatic brain injury: Involvement of neuronal apoptosis and HSP70
expression. Mol Med Rep. 17:8079–8086. 2018.PubMed/NCBI
|
|
35
|
Sun Z, Zhao T, Lv S, Gao Y, Masters J and
Hao W: Dexmedetomidine attenuates spinal cord ischemia-reperfusion
injury through both anti-inflammation and anti-apoptosis mechanisms
in rabbits. J Transl Med. 16:2092018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei Y, Hu J, Liang Y, Zhong Y, He D, Qin
Y, Li L, Chen J, Xiao Q and Xie Y: Dexmedetomidine pretreatment
attenuates propofol-induced neurotoxicity in neuronal cultures from
the rat hippocampus. Mol Med Rep. 14:3413–3420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Xiong M, Nadavaluru PR, Zuo W, Ye
JH, Eloy JD and Bekker A: Dexmedetomidine attenuates neurotoxicity
induced by prenatal propofol exposure. J Neurosurg Anesthesiol.
28:51–64. 2015. View Article : Google Scholar
|