Gastric cancr (GeC) is the fifth most common
neoplasm and the third most deadly type of cancer according to
Globocan 2018 data (1). It is
characterized by high metastatic probability, and diagnosis is
often made at the late stages of disease (2). The majority of patients with early GC
have no overt clinical symptoms (3), though some individuals may experience
nausea and vomiting or upper gastrointestinal symptoms similar to
that seen with ulcers, all of which lack specificity for the
diagnosis of GC (4). In addition,
the majority of patients with GC are already in the advanced stage
of disease at the time of confirmed diagnosis (5). There is still a lack of effective
diagnostic indicators at present, and therefore it is important to
determine effective diagnostic and therapeutic targets for early
detection and treatment of GC.
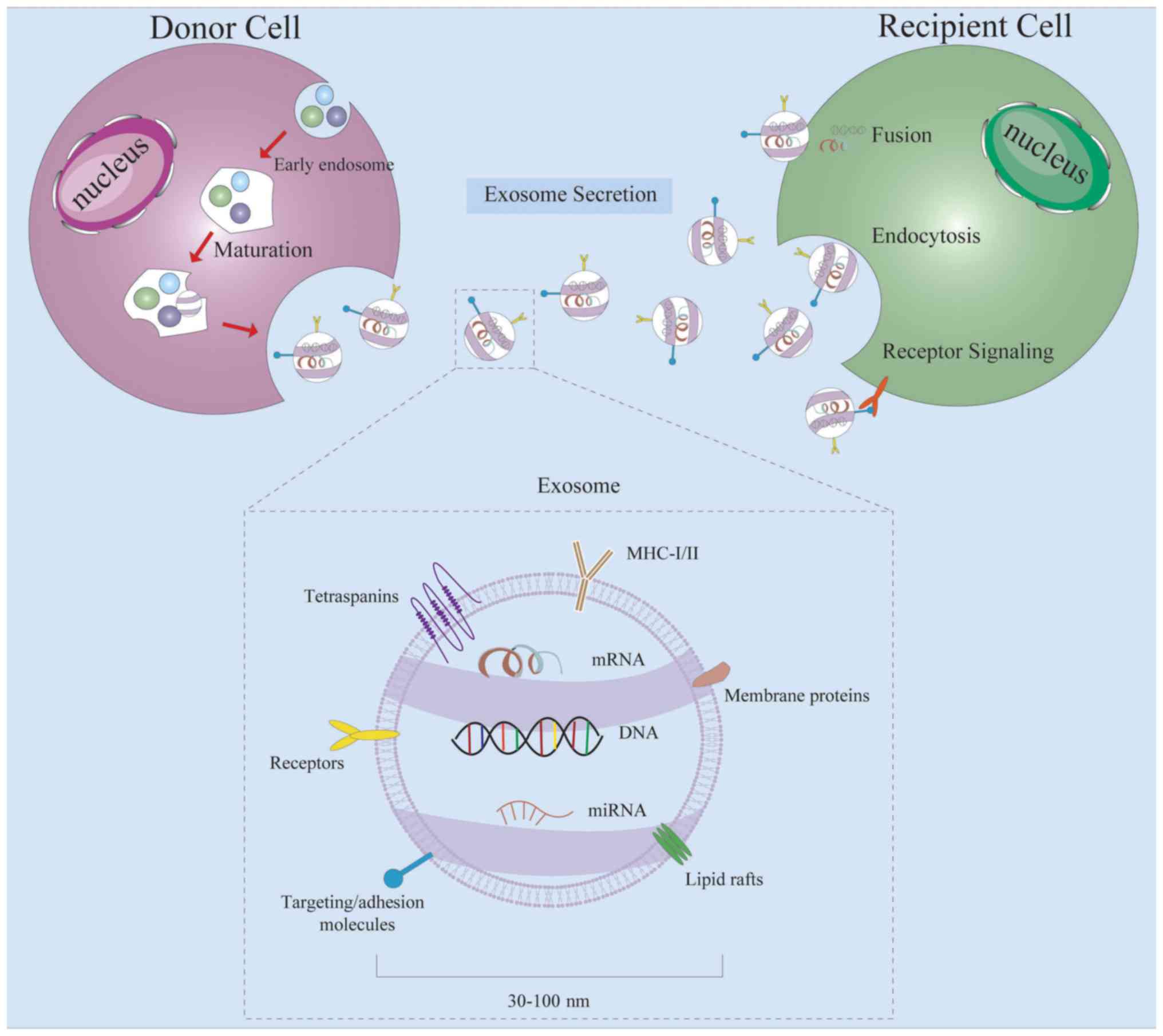
Exosomes are a group of extracellular vesicles with
a diameter of 30–100 nm released from various cell types into body
fluids, including the blood, bile, urine and saliva (6,7).
Exosomes were originally considered to be cell debris and were
therefore underestimated (8). Over
the past decade, increasing attention has been paid to the use of
exosomes as a vessel for transferring proteins, lipids and diverse
RNA molecules (9), or as a key
regulator in the communication of these cargoes with their target
cells (10). Accumulating evidence
has demonstrated that exosomes play important roles in multiple
biological events, such as cell-to-cell communication, cellular
metabolism, tumor metastasis, angiogenesis and immune response
(11).
Non-coding RNAs (ncRNAs) refer to RNAs that can be
transcribed from the genome but with no protein coding capability,
so they can function at their respective RNA levels (12). The majority of ncRNAs are
functional, including small interfering RNAs (siRNAs), antisense
RNAs, microRNAs (miRNAs) and long ncRNAs (lncRNAs) (13). Among them, miRNAs are a type of
non-coding single-stranded RNA molecule with a length of 22
nucleotides encoded by endogenous genes (14). It specifically binds to the
3′untranslated region of the target mRNA, thereby causing
degradation or translation inhibition of the target mRNA molecule
in post-transcriptional gene expression regulation in both animals
and plants (15). lncRNAs are
ncRNAs that are >200 nucleotides in length. Previous studies
have revealed that lncRNAs play important roles in a number of
different life activities, such as dose-compensation effects,
epigenetic regulation, cell cycle regulation and cell
differentiation regulation, and are considered to be a leading
topic in genetic research (16). A
novel group of endogenous ncRNAs, circularRNAs (circRNAs), have
gained increased attention in research (17). circRNAs are a type of ncRNA
molecule that lack the 5′(cap) and 3′(polyadenylation) ends and
form a ring structure with a covalent bond (18). An increasing number of studies have
reported that circRNAs can play key roles in a variety of
physiological or pathological processes, including
epithelial-to-mesenchymal transition (EMT), angiogenesis, tumor
proliferation and tumor metastasis (19–21).
siRNAs, which are occasionally known as short interfering RNA or
silencing RNA, are double-stranded RNAs of 20 to 25 nucleotides in
length. It is currently known that siRNA is primarily involved in
the phenomenon of RNA interference (RNAi), which regulates gene
expression in a specific manner (22). Antisense RNA (asRNA) is a
single-stranded RNA complementary to the transcription product
mRNA. asRNAs can inhibit translation by binding to mRNA (23). In recent years, ncRNAs, especially
miRNAs, lncRNAs and circRNAs, have been suggested to serve as a
novel type of biomarker, which differ from the more conventional
markers, in addition to participating in the development and
progression of different types of cancer (24,25).
Thus, ncRNAs have broad application prospects in the diagnosis and
treatment of diseases.
Exosomes are small membrane vesicles containing
complex RNAs and proteins; specifically, they are discoid vesicles
with a diameter between 30–100 nm (32). They were first found in sheep
reticulocytes in 1983 (33), and
then named ‘exosome’ by Johnstone et al in 1987 (34). A variety of cells can secrete
exosomes under normal and pathological conditions (35). Exosomes are formed by the release
of multivesicular bodies, which are produced as intraluminal
vesicles (ILVs). ILV sorting and final formation process requires
the participation of the endosomal sorting complex required for
transport. In addition, two tetraspanins, CD9 and CD36, have also
been demonstrated to serve regulatory roles in sorting
transmembrane proteins into ILVs, thus promoting its secretion from
the cell. They are the most commonly used exosome-identification
proteins (6).
Routine tumor marker screening may require tissue
biopsy, puncture and other means to obtain living samples from
patients (41); however, this
method requires a solid tumor location and therefore is not
appropriate for disease screening in healthy individuals. At the
same time, tissue biopsy can be harmful to patients (58). Liquid biopsies have emerged as a
non-invasive, rapid and reliable detection method that show great
development potential and application value. There are broad
applications for evaluating the progression of tumor cloning, the
efficacy of chemotherapy, the presence of minimal residual disease
and acquired resistance in real-time. Non-invasive biomarkers have
value in real-time tumor molecular classification and personalized
treatment (59). Tumor
cell-derived exosomes are expected to replace previous tissue
biopsy techniques as a new minimally invasive test (60). The potential utility of miRNAs as
biomarkers in both tissues and blood to assess the response of
5-fluorouracil-based therapies and function as EGFR inhibitors has
been extensively demonstrated in colorectal cancer (61). Several ncRNAs in exosomes have been
demonstrated to be potential diagnostic and predictive biomarkers
for GC (Table I). A previous study
detected total RNA from plasma exosomes of 67 patients with GC and
healthy controls, and revealed that the expression levels of four
exosomal miRNAs were consistent with the serum levels (62). Among these, the expression of
miR-217 showed a significant upward trend, suggesting that miR-217
may contribute to the occurrence of GC. Soon after this study, Zhao
et al (63) reported high
expression levels of lncRNA HOTTIP in exosomes isolated from serum
samples of patients with GC. Compared with conventional GC
biomarkers such as CEA, CA19-9 and CA72-4, exosomal HOTTIP is
expected to become a potential novel target for the diagnosis and
treatment of GC, with improved specificity and sensitivity. At
present, there remains to be a lack of specific minimally invasive
biomarkers to distinguish early-stage GC (EGC) and precancerous
lesions. It has recently been proposed that EGC-specific exosomal
lncUEGC1 and lncUEGC2 could function as non-invasive specific EGC
biomarkers (64). A recent study
performed exosomal long chain RNA sequencing of plasma specimens
from five healthy individuals and 10 patients diagnosed with
first-stage GC, as well as four primary gastric epithelial cells
and four gastric cancer cells. Combining the sequencing results of
plasma samples and culture medium, exosomal lncUEGC1 and lncUEGC2
showed significantly high expression levels and notable changes in
expression (30). This provided a
strong basis for the diagnosis of EGC using these lncRNAs.
In the future, it may be possible to combine
exosomal ncRNAs with circulating tumor DNA (ctDNA) and circulating
tumor cells (CTCs) to provide novel research strategies for liquid
biopsies (29). In view of the
smaller number of CTCs, ctDNA may become a more practical
non-invasive biomarker. ctDNA shows higher accuracy than CTCs in
terms of tumor burden, and can be used as both a diagnostic and
prognostic biomarker. The application of molecular analysis and
mutation identification methods also provide ctDNA with predictive
potential in the evaluation of antitumor therapy (68).
GC is a malignant tumor characterized by a high
incidence, difficult treatment, and easy metastasis and pervasion
(69). Direct spread is one of the
main dispersion methods of GC (70). GC cells are often planted in the
abdominal cavity and pelvic organs, such as the intestine, ovary,
diaphragm, gallbladder and rectal surface, where they often form a
local tumor, producing serous or plasma liquid ascites (71). Wang et al (72) found that high expression levels of
GC cell-derived exosomal miR-27a could translate fibroblasts into
cancer-associated fibroblasts by targeting its downstream target
mRNA cysteine and glycine-rich protein 2. Accordingly, it can
promote the malignant changes of GC, such as accelerating
proliferation and metastasis. Besides blood, ascites can also be
used as the primary medium for liquid biopsy in patients with GC.
Tokuhisa et al (73) used
RNA sequencing to analyze the expression profiles of exosomal
miRNAs in the ascites of patients with GC both before and after
intraperitoneal chemotherapy and patients with non-malignant
disease. They reported that concomitant detection of exosomal
miR-21 and miR-1225-5p in ascites could be used as a prognostic and
diagnostic marker of peritoneal recurrence after surgery and
chemotherapy of patients with GC. Subsequently, another previous
study identified differential characteristics of exosomal miRNA
profiles in ascites between patients with non-malignant disease and
patients with GC before and after intraperitoneal chemotherapy
(74). They suggested that miRNAs
enclosed in exosomes derived from ascites may prove to be
biomarkers for the prognosis of GC peritoneal chemotherapy, thus
providing a novel candidate for the treatment of patients with
peritoneal metastatic GC.
Lymphatic metastasis is the main pathway of
metastasis in GC, and the lymphatic metastasis rate of advanced GC
is as high as 70%, which is positively correlated with the depth of
tumor invasion (75). Thus, there
is an urgent need to find novel therapeutic targets related to
lymphatic metastasis in the diagnosis and treatment of GC. Pan
et al (76) found that
lncRNA ZFAS1 was a highly expressed exosomal ncRNA in the serum
samples of patients with GC. In addition, it was also considered to
be associated with age, Tumor-Node-Metastasis stage and lymphatic
metastasis. The knockdown of ZFAS1 was associated with the increase
of E-cadherin and the decrease of N-cadherin. Inhibition of EMT is
an important mechanism in tumor invasion and metastasis. Exosomal
ZFAS1 was demonstrated to promote the progress of EMT, which in
turn promoted lymphatic metastasis in cancer (76).
EMT refers to the phenotypic transformation of
epithelial cells to Leydig cells under certain physiological or
pathological conditions (77). EMT
is an important biological process underlying the migratory and
invasive abilities of malignant tumor cells derived from epithelial
cells, and is positively correlated with poor prognosis of
malignant tumor cells (78). In
recent years, EMT has been found to be closely associated with
tumor metastasis, which has become the focus of current research
(79). EMT is characterized by the
downregulation of epithelial cell markers, such as E-cadherin and
β-catenin, and the upregulation of mesenchymal phenotypic markers,
including N-cadherin and vimentin (80). Exosomal miR-423-5p has been
reported to promote the migration and proliferation of GC by
restraining the suppressor of fused protein gene, so as to promote
the development of EMT in GC cells (81). High expression levels of miR-191
and let-7a in exosomes also supports the promotion of GC by
inducing EMT (81,82). EMT can not only enhance the ability
of invasion and metastasis of tumor cells, but provides the
characteristics of tumor stem cells and promotes the production of
cancer stem-like cells (CSCs) (83). CSCs have been considered as the
basis of tumor invasion and metastasis (84). The level of CSCs in patients can
indicate the probability of recurrence following treatment
(85,86). A previous study performed miRNA
deep sequencing of exosomes derived from CSCs and screened their
differential cells (DCs), which led to the identification of six
upregulated miRNAs and five downregulated miRNAs (87). These studies observed significant
differences in the type and quantity of miRNAs upregulated in the
exosomes from CSCs and DCs. The data provided by this study can
help improve the current understanding of the predictive role of
CSC-derived exosomal ncRNAs in the development and metastasis of
GC.
Angiogenesis refers to the growth of new capillary
blood vessels derived from existing capillaries and post-capillary
venules (88). Tumor angiogenesis
is an extremely complex process, which generally includes vascular
endothelial matrix degradation, endothelial cell migration,
endothelial cell proliferation, formation of vascular rings, and
formation of a new basement membrane. An increasing number of
studies have demonstrated that benign tumors usually grow slowly
and rarely exhibit angiogenesis, while the majority of malignant
tumors exhibit dense angiogenesis and grow rapidly (89,90).
Therefore, angiogenesis plays an important role in the development
and metastasis of tumors, and the inhibition of this process will
markedly prevent the growth, diffusion and metastasis of tumor
tissue. A previous study demonstrated that tumor-derived exosomes
are involved in the exchange of genetic information between tumor
cells and basal cells, which leads to the formation of ample
neovascularization and promotes the growth and invasion of tumors
(91). In recent years, several
reports have indicated that a variety of exosomal non-coding
molecules derived from cancer serum and cells are major inducers of
angiogenesis both in vivo and in vitro (92–96).
There are also associated angiogenic exosomal ncRNAs that have been
reported in GC. For instance, high expression levels of exosomal
miR-130a in patients with GC was identified to promote tumor
proliferation, migration and tubular formation by targeting c-MYB
directly in vivo and in vitro experiments (97). This provides a novel strategy for
antiangiogenic therapy of GC.
Chemotherapy is one of the most important methods in
the treatment of malignant tumors (21). However, the drug resistance of
tumor cells to chemotherapeutic drugs often leads to the failure of
chemotherapy (98). As an
important topic in recent years, exosomes have been demonstrated to
mediate the drug resistance of tumor cells in a variety of ways
(35). Exosomes exist as a
cell-to-cell communication mediator in the tumor microenvironment
to affect drug resistance (99).
They can also participate in the uptake, metabolism and excretion
of drugs, thus affecting the drug resistance of tumor cells
(100). In addition, drug
resistance of tumor cells can also be mediated by the proteins or
associated genes in exosomes (101). In recent years, there is more
recognition that exosomal miRNAs derived from tumor cells can play
important delivery and regulatory functions in the process of
chemotherapeutic resistance to diseases (102,103). It is currently speculated that
exosomes partially affect the transmission of drug resistance
between resistant cells and parental cells. Recurrent and
metastatic advanced GC requires a chemotherapy-based comprehensive
treatment. Combined use of novel drugs is a new technique in the
treatment of advanced GC. Paclitaxel is considered to be the
optimum natural anticancer drug found thus far, and has been widely
used in the treatment of GC, breast cancer (104), ovarian cancer (105), partial head and neck cancer
(106), and lung cancer (107). However, chemotherapy resistance
of paclitaxel in patients with GC is an issue that needs to be
addressed (108,109). Wang et al (110) identified the delivery mechanism
of exosomal miR-155-5p, through which the drug resistance and EMT
phenotypes could be observed by establishing a paclitaxel-resistant
GC cell line, MGC803R. Adriamycin (ADR) is a member of the
anthracycline family. It is often used in combination with certain
traditional chemotherapeutic drugs, such as fluorouracil,
cisplatin, paclitaxel and mitomycin to treat multiple malignant
tumors including GC (111).
However, drug resistance to doxorubicin remains to be an obstacle
in the treatment of GC. It was found that miR-501 in exosomes
secreted by ADR-resistant GC cell line SGC7901/ADR was higher than
that in exosomes secreted by sensitive SGC7901 cells (112). It was also found that SGC7901
could ingest Cy3-labeled miR-501 in exosomes from SGC7901/ADR.
These experiments on exosomal miR-501 in vitro and in
vivo suggested that drug resistance of patients with GC to
doxorubicin may be associated with enhanced transmission of
exosomal miR-501 by downregulation of BH3-like motif-containing
cell death inducer, and subsequent inactivation of caspase-9/-3 and
activation of AKT phosphorylation. Several studies have revealed
that MSC-derived exosomes have the ability to transmit certain
proteins, including multidrug resistance-associated protein 2,
(113), copper-transporting
ATPase 1 and copper-transporting ATPase 2 (114), as well as certain types of
miRNAs, including miR-100 (115),
miR-222 (116), miR-30a (117) and miR-17 (118). These exosomal cargoes can
activate apoptosis-escaping pathways in ways other than
conventional CaM-Ks/Raf/MEK/ERK signaling pathways in order to
regulate the cell cycle and alter cell apoptosis rates, thus
decreasing the sensitivity of GC cells to 5-fluorouracil (113). Cisplatin is one of the most
commonly used classical drugs for chemotherapy and in vitro
drug sensitivity tests in patients with GC (119). Its antitumor toxicity and
effectiveness have been confirmed, however, in previous years, the
emergence of cisplatin resistance has decreased the efficacy of
cisplatin, and even led to the failure of chemotherapy for GC,
which limits its clinical application (120). A recent study (121) reported that miR-214
overexpression affected the invasion and metastasis of GC cells,
resulting in poor prognosis and resistance to apoptosis. It was
reported that drug sensitivity to cisplatin in patients with
refractory GC could be restored by the mechanism of
exosomal-anti-miR-214 delivery to GC cells (121). Studies have revealed that in
SGC7901/DDP cells, c-Met siRNA delivered by exosomes reversed the
resistance to cisplatin and increased the rate of apoptosis
(122). Exosomal miR-21 derived
from M2 macrophage is also involved in the mechanism of
chemotherapy resistance in GC (123). Exosomal miR-21 can decrease the
expression levels of PTEN mRNA and protein. By means of delivering
exosomal miR-21, GC cell chemoresistance and the antiapoptotic
ability can be enhanced through regulation of the PTEN/PI3K/AKT
signaling pathway.
Exosomes, as a nano-sized biological transport
carrier, can protect the ncRNAs that they contain against external
damage (124). They can promote
the exchange of genetic material through the communication of
ncRNAs between target cells. In the present review, the mechanisms
of ncRNAs carried by exosomes from GC and stromal cells were
briefly summarized, with the aim that the information provided
herein is conducive to an improved understanding of the position
and role of exosomes in the development and progression of GC.
Studies on the activities of exosomal ncRNAs in the proliferation,
metastasis, angiogenesis and drug resistance of GC are still under
progress. These results provide novel research directions and
potential therapeutic targets for tumorigenesis. In addition, the
potential of exosomal ncRNAs as tumor markers for early liquid
biopsies should not be underestimated (125). There is an urgent need to develop
a standard method for the rapid, simple and specific separation of
exosomes, and detect abnormal exosomes of ncRNA quickly and
inexpensively. To the best of our knowledge, the role of known
exosomal ncRNAs in GC has not yet been confirmed, and their value
in clinical application remains to be investigated. With the
gradual increase in research that focuses on exosomes, the
application of exosomal ncRNAs in the research and treatment of GC
may become a reality.
Not applicable.
The present review was supported by the Jiangsu
Provincial Funds for Six Categories of Top Talents (grant no.
WS-066); the Research project of Jiangsu Provincial Health and
Family Planning Commission (grant no. H201526); and the Nantong
Technology Project (grant no. MS12017008-1).
Not applicable.
XL conceived, designed and wrote the manuscript. YZ,
GX and YD were involved in writing and critically reviewing the
manuscript, and also designed the figures. HC and SX checked and
modified the language of the manuscript. All authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aichler M, Luber B, Lordick F and Walch A:
Proteomic and metabolic prediction of response to therapy in
gastric cancer. World J Gastroenterol. 20:13648–13657. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barreto SG and Windsor JA: Redefining
early gastric cancer. Surg Endosc. 30:24–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi JH, Kim ES, Lee YJ, Cho KB, Park KS,
Jang BK, Chung WJ, Hwang JS and Ryu SW: Comparison of quality of
life and worry of cancer recurrence between endoscopic and surgical
treatment for early gastric cancer. Gastrointest Endosc.
82:299–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bobrie A, Colombo M, Raposo G and Thery C:
Exosome secretion: Molecular mechanisms and roles in immune
responses. Traffic. 12:1659–1668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo W, Gao Y, Li N, Shao F, Wang C, Wang
P, Yang Z, Li R and He J: Exosomes: New players in cancer (Review).
Oncol Rep. 38:665–675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caruso S and Poon IKH: Apoptotic
cell-derived extracellular vesicles: More Than just debris. Front
Immunol. 9:14862018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo N, Akiyoshi K and Shiku H:
Exosome-mediated regulation of tumor immunology. Cancer Sci.
109:2998–3004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simons M and Raposo G: Exosomes-vesicular
carriers for intercellular communication. Curr Opin Cell Biol.
21:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng G and Sui G: Noncoding RNA in
oncogenesis: A new era of identifying key players. Int J Mol Sci.
14:18319–18349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WT, Han C, Sun YM, Chen TQ and Chen
YQ: Noncoding RNAs in cancer therapy resistance and targeted drug
development. J Hematol Oncol. 12:552019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iqbal MA, Arora S, Prakasam G, Calin GA
and Syed MA: MicroRNA in lung cancer: Role, mechanisms, pathways
and therapeutic relevance. Mol Aspects Med. 70:3–20. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michlewski G and Caceres JF:
Post-transcriptional control of miRNA biogenesis. RNA. 25:1–16.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XZ, Liu H and Chen SR: Mechanisms of
long non-coding rnas in cancers and their dynamic regulations.
Cancers (Basel). 12:12452020. View Article : Google Scholar
|
|
17
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Y, Liu Y and Jiang Z: CircRNAs: A new
perspective of biomarkers in the nervous system. Biomed
Pharmacother. 128:1102512020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu M, Xia W, Chen R, Wang S, Xu Y, Ma Z,
Xu W, Zhang E, Wang J, Fang T, et al: The Circular RNA circPRKCI
promotes tumor growth in lung adenocarcinoma. Cancer Res.
78:2839–2851. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calatayud D, Dehlendorff C, Boisen MK,
Hasselby JP, Schultz NA, Werner J, Immervoll H, Molven A, Hansen CP
and Johansen JS: Tissue MicroRNA profiles as diagnostic and
prognostic biomarkers in patients with resectable pancreatic ductal
adenocarcinoma and periampullary cancers. Biomarker Res. 5:82017.
View Article : Google Scholar
|
|
21
|
Ho YJ and Yeh CK: Concurrent anti-vascular
therapy and chemotherapy in solid tumors using drug-loaded acoustic
nanodroplet vaporization. Acta Biomater. 49:472–485. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kesharwani P, Gajbhiye V and Jain NK: A
review of nanocarriers for the delivery of small interfering RNA.
Biomaterials. 33:7138–7150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CH, Tsai ZT and Wang D: Role of
antisense RNAs in evolution of yeast regulatory complexity.
Genomics. 102:484–490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong CM, Tsang FH and Ng IO: Non-coding
RNAs in hepatocellular carcinoma: Molecular functions and
pathological implications. Nat Rev Gastroenterol Hepatol.
15:137–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng JF, Zhuang YY, Huang FT and Zhang SN:
Noncoding RNAs and pancreatic cancer. World J Gastroenterol.
22:801–814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Valadi H, Ekstrom K, Bossios A, Sjostrand
M, Lee JJ and Lotvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie Y, Dang W, Zhang S, Yue W, Yang L,
Zhai X, Yan Q and Lu J: The role of exosomal noncoding RNAs in
cancer. Mol Cancer. 18:372019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skotland T, Sandvig K and Llorente A:
Lipids in exosomes: Current knowledge and the way forward. Prog
Lipid Res. 66:30–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin LY, Yang L, Zeng Q, Wang L, Chen ML,
Zhao ZH, Ye GD, Luo QC, Lv PY, Guo QW, et al: Tumor-originated
exosomal lncUEGC1 as a circulating biomarker for early-stage
gastric cancer. Mol Cancer. 17:842018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X, Wang S, Wang H, Cao J, Huang X,
Chen Z, Xu P, Sun G, Xu J, Lv J and Xu Z: Circular RNA circNRIP1
acts as a microRNA-149-5p sponge to promote gastric cancer
progression via the AKT1/mTOR pathway. Mol Cancer. 18:202019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajagopal C and Harikumar KB: The origin
and functions of exosomes in cancer. Front Oncol. 8:662018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan BT and Johnstone RM: Fate of the
transferrin receptor during maturation of sheep reticulocytes in
vitro: Selective externalization of the receptor. Cell. 33:967–978.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
35
|
Milman N, Ginini L and Gil Z: Exosomes and
their role in tumorigenesis and anticancer drug resistance. Drug
Resist Updat. 45:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan BT, Teng K, Wu C, Adam M and Johnstone
RM: Electron microscopic evidence for externalization of the
transferrin receptor in vesicular form in sheep reticulocytes. J
Cell Biol. 101:942–948. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jan AT, Rahman S, Khan S, Tasduq SA and
Choi I: Biology, pathophysiological role, and clinical implications
of exosomes: A Critical Appraisal. Cells. 8:992019. View Article : Google Scholar
|
|
39
|
Ferguson SW and Nguyen J: Exosomes as
therapeutics: The implications of molecular composition and
exosomal heterogeneity. J Control Release. 228:179–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hannafon BN, Carpenter KJ, Berry WL,
Janknecht R, Dooley WC and Ding WQ: Exosome-mediated microRNA
signaling from breast cancer cells is altered by the
anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer.
14:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Z, Yang Z, Dai Y, Zhu Q and Chen LA:
Update on liquid biopsy in clinical management of non-small cell
lung cancer. Onco Targets Ther. 12:5097–5109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sancho-Albero M, Navascues N, Mendoza G,
Sebastián V, Arruebo M, Martín-Duque P and Santamaría J: Exosome
origin determines cell targeting and the transfer of therapeutic
nanoparticles towards target cells. J Nanobiotechnology. 17:162019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai J, Xie X, Lei Y, An G, He L and Chen
R: Consideration of dual characters of exosomes in the tumour
immune response. Cell Biol Int. 38:538–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arima Y, Liu W, Takahashi Y, Nishikawa M
and Takakura Y: Effects of localization of antigen proteins in
antigen-loaded exosomes on efficiency of antigen presentation. Mol
Pharm. 16:2309–2314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Shi H, Yuan X, Jiang P, Qian H
and Xu W: Tumor-derived exosomes induce N2 polarization of
neutrophils to promote gastric cancer cell migration. Mol Cancer.
17:1462018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lloret-Llinares M, Karadoulama E, Chen Y,
Wojenski LA, Villafano GJ, Bornholdt J, Andersson R, Core L,
Sandelin A and Jensen TH: The RNA exosome contributes to gene
expression regulation during stem cell differentiation. Nucleic
Acids Res. 46:11502–11513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Singh R, Pochampally R, Watabe K, Lu Z and
Mo YY: Exosome-mediated transfer of miR-10b promotes cell invasion
in breast cancer. Mol Cancer. 13:2562014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Di C, Zhang Q, Wang Y, Wang F, Chen Y, Gan
L, Zhou R, Sun C, Li H, Zhang X, et al: Exosomes as drug carriers
for clinical application. Artif Cells Nanomed Biotechnol. 46
(sup3):S564–S570. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Izadpanah M, Seddigh A, Ebrahimi Barough
S, Fazeli SAS and Ai J: Potential of extracellular vesicles in
neurodegenerative diseases: Diagnostic and therapeutic indications.
J Mol Neurosci. 66:172–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dhondt B, Van Deun J, Vermaerke S, de
Marco A, Lumen N, De Wever O and Hendrix A: Urinary extracellular
vesicle biomarkers in urological cancers: From discovery towards
clinical implementation. Int J Biochem Cell Biol. 99:236–256. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roy S, Hochberg FH and Jones PS:
Extracellular vesicles: The growth as diagnostics and therapeutics;
a survey. J Extracell Vesicles. 7:14387202018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Akers JC, Gonda D, Kim R, Carter BS and
Chen CC: Biogenesis of extracellular vesicles (EV): Exosomes,
microvesicles, retrovirus-like vesicles, and apoptotic bodies. J
Neurooncol. 113:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang X, Yao X, Xie T, Chang Z, Guo Y and
Ni H: Exosome-derived uterine miR-218 isolated from cows with
endometritis regulates the release of cytokines and chemokines.
Microb Biotechnol. 13:1103–1117. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mathieu M, Martin-Jaular L, Lavieu G and
Thery C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Cell
Biol. 21:9–17. 2019.
|
|
55
|
Klingeborn M, Dismuke WM, Bowes Rickman C
and Stamer WD: Roles of exosomes in the normal and diseased eye.
Prog Retin Eye Res. 59:158–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xitong D and Xiaorong Z: Targeted
therapeutic delivery using engineered exosomes and its applications
in cardiovascular diseases. Gene. 575:377–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang XX, Sun C, Wang L and Guo XL: New
insight into isolation, identification techniques and medical
applications of exosomes. J Control Release. 308:119–129. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lopez-Beltran A, Cheng L, Gevaert T,
Blanca A, Cimadamore A, Santoni M, Massari F, Scarpelli M,
Raspollini MR and Montironi R: Current and emerging bladder cancer
biomarkers with an emphasis on urine biomarkers. Expert Rev Mol
Diagn. 20:231–243. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Normanno N, Cervantes A, Ciardiello F, De
Luca A and Pinto C: The liquid biopsy in the management of
colorectal cancer patients: Current applications and future
scenarios. Cancer Treat Rev. 70:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Buscail E, Maulat C, Muscari F, Chiche L,
Cordelier P, Dabernat S, Alix-Panabières C and Buscail L: Liquid
biopsy approach for pancreatic ductal adenocarcinoma. Cancers.
11:8522019. View Article : Google Scholar
|
|
61
|
Boussios S, Ozturk MA, Moschetta M,
Karathanasi A, Zakynthinakis-Kyriakou N, Katsanos KH, Christodoulou
DK and Pavlidis N: The developing story of predictive biomarkers in
colorectal cancer. J Pers Med. 9:122019. View Article : Google Scholar
|
|
62
|
Li W and Gao YQ: MiR-217 is involved in
the carcinogenesis of gastric cancer by down-regulating CDH1
expression. Kaohsiung J Med Sci. 34:377–384. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao R and Zhang Y, Zhang X, Yang Y, Zheng
X, Li X, Liu Y and Zhang Y: Exosomal long noncoding RNA HOTTIP as
potential novel diagnostic and prognostic biomarker test for
gastric cancer. Mol Cancer. 17:682018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang G, Liu W, Zou Y, Wang G, Deng Y, Luo
J, Zhang Y, Li H, Zhang Q, Yang Y and Chen G: Three isoforms of
exosomal circPTGR1 promote hepatocellular carcinoma metastasis via
the miR449a-MET pathway. EBioMedicine. 40:432–445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li J, Li Z, Jiang P, Peng M, Zhang X, Chen
K, Liu H, Bi H, Liu X and Li X: Circular RNA IARS (circ-IARS)
secreted by pancreatic cancer cells and located within exosomes
regulates endothelial monolayer permeability to promote tumor
metastasis. J Exp Clin Cancer Res. 37:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang W, Fu K, Sun H, Rong D, Wang H and
Cao H: CircRNA microarray profiling identifies a novel circulating
biomarker for detection of gastric cancer. Mol Cancer. 17:1372018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zarkavelis G, Boussios S, Papadaki A,
Katsanos KH, Christodoulou DK and Pentheroudakis G: Current and
future biomarkers in colorectal cancer. Ann Gastroenterol.
30:613–621. 2017.PubMed/NCBI
|
|
69
|
Vedeld HM, Goel A and Lind GE: Epigenetic
biomarkers in gastrointestinal cancers: The current state and
clinical perspectives. Semin Cancer Biol. 51:36–49. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shah M, Prasad A, Rajan D, Tan CB, Shah M,
Raghavan P and Mustacchia P: Direct liver invasion from a gastric
adenocarcinoma as an initial presentation of extranodal tumor
spread. Case Rep Med. 2012:6512322012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ruggieri V, Russi S, Zoppoli P, La Rocca
F, Angrisano T, Falco G, Calice G and Laurino S: The Role of
MicroRNAs in the regulation of gastric cancer stem cells: A
meta-analysis of the current status. J Clin Med. 8:6392019.
View Article : Google Scholar
|
|
72
|
Wang J, Guan X, Zhang Y, Ge S, Zhang L, Li
H, Wang X, Liu R, Ning T, Deng T, et al: Exosomal miR-27a derived
from gastric cancer cells regulates the transformation of
fibroblasts into cancer-associated fibroblasts. Cell Physiol
Biochem. 49:869–883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tokuhisa M, Ichikawa Y, Kosaka N, Ochiya
T, Yashiro M, Hirakawa K, Kosaka T, Makino H, Akiyama H, Kunisaki C
and Endo I: Exosomal miRNAs from peritoneum lavage fluid as
potential prognostic biomarkers of peritoneal metastasis in gastric
cancer. PLoS One. 10:e01304722015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hu Y, Qi C, Liu X, Zhang C, Gao J, Wu Y,
Yang J, Zhao Q, Li J, Wang X and Shen L: Malignant ascites-derived
exosomes promote peritoneal tumor cell dissemination and reveal a
distinct miRNA signature in advanced gastric cancer. Cancer Lett.
457:142–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang ZK, Lin JX, Li P, Xie JW, Wang JB, Lu
J, Chen QY, Cao LL, Lin M, Tu RH, et al: Higher risk of lymph node
metastasis in young patients with early gastric cancer. J Cancer.
10:4389–4396. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pan L, Liang W, Fu M, Huang ZH, Li X,
Zhang W, Zhang P, Qian H, Jiang PC, Xu WR and Zhang X:
Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes
gastric cancer progression. J Cancer Res Clin Oncol. 143:991–1004.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bagger SO, Hopkinson BM, Pandey DP, Bak M,
Brydholm AV, Villadsen R, Helin K, Rønnov-Jessen L, Petersen OW and
Kim J: Aggressiveness of non-EMT breast cancer cells relies on
FBXO11 activity. Mol Cancer. 17:1712018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Colella B, Faienza F and Di Bartolomeo S:
EMT regulation by autophagy: A new perspective in glioblastoma
biology. Cancers. 11:3122019. View Article : Google Scholar
|
|
79
|
Aiello NM, Maddipati R, Norgard RJ, Balli
D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, et al:
EMT subtype influences epithelial plasticity and mode of cell
migration. Dev Cell. 45:681–695.e684. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pastushenko I and Blanpain C: EMT
Transition States during Tumor Progression and Metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang H, Fu H, Wang B, Zhang X, Mao J, Li
X, Wang M, Sun Z, Qian H and Xu W: Exosomal miR-423-5p targets SUFU
to promote cancer growth and metastasis and serves as a novel
marker for gastric cancer. Mol Carcinog. 57:1223–1236. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tian W, Liu S and Li B: Potential role of
exosomes in cancer metastasis. Biomed Res Int. 2019:46497052019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rodriguez-Aznar E, Wiesmuller L, Sainz B
Jr and Hermann PC: EMT and stemness-key players in pancreatic
cancer stem cells. Cancers. 11:11362019. View Article : Google Scholar
|
|
84
|
Lee IC, Fadera S and Liu HL: Strategy of
differentiation therapy: effect of dual-frequency ultrasound on the
induction of liver cancer stem-like cells on a HA-based multilayer
film system. J Mater Chem B. 7:5401–5411. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Park SY, Choi JH and Nam JS: Targeting
cancer stem cells in triple-negative breast cancer. Cancers
(Basel). 11:9652019. View Article : Google Scholar
|
|
86
|
Peitzsch C, Tyutyunnykova A, Pantel K and
Dubrovska A: Cancer stem cells: The root of tumor recurrence and
metastases. Semin Cancer Biol. 44:10–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sun ZP, Li AQ, Jia WH, Ye S, Van Eps G, Yu
JM and Yang WJ: MicroRNA expression profiling in exosomes derived
from gastric cancer stem-like cells. Oncotarget. 8:93839–93855.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Viallard C and Larrivee B: Tumor
angiogenesis and vascular normalization: alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dong H, Weng C, Bai R, Sheng J, Gao X, Li
L and Xu Z: The regulatory network of miR-141 in the inhibition of
angiogenesis. Angiogenesis. 22:251–262. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bikfalvi A: History and conceptual
developments in vascular biology and angiogenesis research: A
personal view. Angiogenesis. 20:463–478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhao S, Li J, Zhang G, Wang Q, Wu C, Zhang
Q, Wang H, Sun P, Xiang R and Yang S: Exosomal miR-451a functions
as a tumor suppressor in hepatocellular carcinoma by targeting
LPIN1. Cell Physiol Biochem. 53:19–35. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang ZF, Liao F, Wu H and Dai J: Glioma
stem cells-derived exosomal miR-26a promotes angiogenesis of
microvessel endothelial cells in glioma. J Exp Clin Cancer Res.
38:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wu XG, Zhou CF, Zhang YM, Yan RM, Wei WF,
Chen XJ, Yi HY, Liang LJ, Fan LS, Liang L, et al: Cancer-derived
exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in
cervical squamous cell carcinoma. Angiogenesis. 22:397–410. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hu HY, Yu CH, Zhang HH, Zhang SZ, Yu WY,
Yang Y and Chen Q: Exosomal miR-1229 derived from colorectal cancer
cells promotes angiogenesis by targeting HIPK2. Int J Biol
Macromol. 132:470–477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Matsuura Y, Wada H, Eguchi H, Gotoh K,
Kobayashi S, Kinoshita M, Kubo M, Hayashi K, Iwagami Y, Yamada D,
et al: Exosomal miR-155 derived from hepatocellular carcinoma cells
under hypoxia promotes angiogenesis in endothelial cells. Dig Dis
Sci. 64:792–802. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang H, Zhang H, Ge S, Ning T, Bai M, Li
J, Li S, Sun W, Deng T, Zhang L, et al: Exosome-Derived miR-130a
activates angiogenesis in gastric cancer by targeting C-MYB in
vascular endothelial cells. Mol Ther. 26:2466–2475. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hida K, Kikuchi H, Maishi N and Hida Y:
ATP-binding cassette transporters in tumor endothelial cells and
resistance to metronomic chemotherapy. Cancer Lett. 400:305–310.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang HD, Jiang LH, Hou JC, Zhong SL, Zhu
LP, Wang DD, Zhou SY, Yang SJ, Wang JY, Zhang Q, et al: Exosome: A
novel mediator in drug resistance of cancer cells. Epigenomics.
10:1499–1509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Qu Z, Wu J, Wu J, Luo D, Jiang C and Ding
Y: Exosomes derived from HCC cells induce sorafenib resistance in
hepatocellular carcinoma both in vivo and in vitro. J Exp Clin
Cancer Res. 35:1592016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhang W, Cai X, Yu J, Lu X, Qian Q and
Qian W: Exosome-mediated transfer of lncRNA RP11838N2.4 promotes
erlotinib resistance in non-small cell lung cancer. Int J Oncol.
53:527–538. 2018.PubMed/NCBI
|
|
102
|
Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng
L, Ding L, Zhang Y, Zhang L, Li N, et al: Exosomes derived from
gemcitabine-resistant cells transfer malignant phenotypic traits
via delivery of miRNA-222-3p. Mol Cancer. 16:1322017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X,
Shen B, Liu S, Yan D and Feng J: Cisplatin-resistant lung cancer
cell-derived exosomes increase cisplatin resistance of recipient
cells in exosomal miR-100-5p-dependent manner. Int J Nanomedicine.
12:3721–3733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xie F, Chen R, Zhang L, Yin Z, Zhu Q, You
S, Jiang C, Li Y, Li S, Zha X and Wang J: Efficacy of two-weekly
nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy
for breast cancer. Nanomedicine (Lond). 14:1595–1603. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Vergote I, Scambia G, O'Malley DM, Van
Calster B, Park SY, Del Campo JM, Meier W, Bamias A, Colombo N,
Wenham RM, et al: Trebananib or placebo plus carboplatin and
paclitaxel as first-line treatment for advanced ovarian cancer
(TRINOVA-3/ENGOT-ov2/GOG-3001): A randomised, double-blind, phase 3
trial. Lancet Oncol. 20:862–876. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Colevas AD: Systemic therapy for
metastatic or recurrent squamous cell carcinoma of the head and
neck. J Natl Compr Canc Netw. 13:e37–e48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Socinski MA, Okamoto I, Hon JK, Hirsh V,
Dakhil SR, Page RD, Orsini J, Yamamoto N, Zhang H and Renschler MF:
Safety and efficacy analysis by histology of weekly nab-paclitaxel
in combination with carboplatin as first-line therapy in patients
with advanced non-small-cell lung cancer. Ann Oncol. 24:2390–2396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Guo Z, Wang X, Lin R, Chen L, Fan N, Chen
Y, Lin J and Yu J: Paclitaxel-based regimens as first-line
treatment in advanced gastric cancer. J Chemother. 27:94–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang D and Fan D: Multidrug resistance in
gastric cancer: Recent research advances and ongoing therapeutic
challenges. Expert Rev Anticancer Ther. 7:1369–1378. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, Tan
C, Zhu W and Shen B: Paclitaxelresistant gastric cancer MGC803
cells promote epithelialtomesenchymal transition and
chemoresistance in paclitaxelsensitive cells via exosomal delivery
of miR1555p. Int J Oncol. 54:326–338. 2019.PubMed/NCBI
|
|
111
|
Hultman B, Mahteme H, Sundbom M, Ljungman
M, Larsson R and Nygren P: Benchmarking of gastric cancer
sensitivity to anti-cancer drugs ex vivo as a basis for drug
selection in systemic and intraperitoneal therapy. J Exp Clin
Cancer Res. 33:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu X, Lu Y, Xu Y, Hou S, Huang J, Wang B,
Zhao J, Xia S, Fan S, Yu X, et al: Exosomal transfer of miR-501
confers doxorubicin resistance and tumorigenesis via targeting of
BLID in gastric cancer. Cancer Lett. 459:122–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan
Y, Wang M, Zhu W, Qian H and Xu W: Exosomes derived from human
mesenchymal stem cells confer drug resistance in gastric cancer.
Cell Cycle. 14:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Panfoli I, Ravera S, Podestà M, Cossu C,
Santucci L, Bartolucci M, Bruschi M, Calzia D, Sabatini F,
Bruschettini M, et al: Exosomes from human mesenchymal stem cells
conduct aerobic metabolism in term and preterm newborn infants.
FASEB J. 30:1416–1424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol
(Dordr). 40:457–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bliss SA, Sinha G, Sandiford OA, Williams
LM, Engelberth DJ, Guiro K, Isenalumhe LL, Greco SJ, Ayer S, Bryan
M, et al: Mesenchymal stem cell-derived exosomes stimulate cycling
quiescence and early breast cancer dormancy in bone marrow. Cancer
Res. 76:5832–5844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang H, Wang Y, Yang G, Yu H, Zhou Z and
Tang M: MicroRNA-30a regulates chondrogenic differentiation of
human bone marrow-derived mesenchymal stem cells through targeting
Sox9. Exp Ther Med. 18:4689–4697. 2019.PubMed/NCBI
|
|
118
|
Xin H, Liu Z, Buller B, Li Y, Golembieski
W, Gan X, Wang F, Lu M, Ali MM, Zhang ZG and Chopp M: MiR-17-92
enriched exosomes derived from multipotent mesenchymal stromal
cells enhance axon-myelin remodeling and motor electrophysiological
recovery after stroke. J Cereb Blood Flow Metab. 2020.(Ahead of
print). View Article : Google Scholar
|
|
119
|
Cheng Q, Li X and Liu J, Ye Q, Chen Y, Tan
S and Liu J: Multiple myeloma-derived exosomes regulate the
functions of mesenchymal stem cells partially via modulating miR-21
and miR-146a. Stem Cells Int. 2017:90121522017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ho GY, Woodward N and Coward JI: Cisplatin
versus carboplatin: comparative review of therapeutic management in
solid malignancies. Crit Rev Oncol Hematol. 102:37–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang X, Zhang H, Bai M, Ning T, Ge S, Deng
T, Liu R, Zhang L, Ying G and Ba Y: Exosomes serve as nanoparticles
to deliver anti-miR-214 to reverse chemoresistance to cisplatin in
gastric cancer. Mol Ther. 26:774–783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang Q, Zhang H, Ning T, Liu D, Deng T,
Liu R, Bai M, Zhu K, Li J, Fan Q, et al: Exosome-delivered c-met
sirna could reverse chemoresistance to cisplatin in gastric cancer.
Int J Nanomedicine. 15:2323–2335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie
G, Ma Y and Shen L: Exosomal transfer of tumor-associated
macrophage-derived miR-21 confers cisplatin resistance in gastric
cancer cells. J Exp Clin Cancer Res. 36:532017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
van der Pol E, Boing AN, Harrison P, Sturk
A and Nieuwland R: Classification, functions, and clinical
relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Palmirotta R, Lovero D, Cafforio P, Felici
C, Mannavola F, Pellè E, Quaresmini D, Tucci M and Silvestris F:
Liquid biopsy of cancer: A multimodal diagnostic tool in clinical
oncology. Ther Adv Med Oncol. 10:17588359187946302018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ren W, Zhang X, Li W, Feng Q, Feng H, Tong
Y, Rong H, Wang W, Zhang D, Zhang Z, et al: Exosomal miRNA-107
induces myeloid-derived suppressor cell expansion in gastric
cancer. Cancer Manag Res. 11:4023–4040. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang N, Wang L, Yang Y, Gong L, Xiao B and
Liu X: A serum exosomal microRNA panel as a potential biomarker
test for gastric cancer. Biochem Biophys Res Commun. 493:1322–1328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K
and Mochizuki T: Let-7 microRNA family is selectively secreted into
the extracellular environment via exosomes in a metastatic gastric
cancer cell line. PLoS One. 5:e132472010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cai C, Zhang H, Zhu Y, Zheng P, Xu Y, Sun
J, Zhang M, Lan T, Gu B, Li S and Ma P: Serum exosomal long
noncoding RNA pcsk2-2:1 as a potential novel diagnostic biomarker
for gastric cancer. Onco Targets Ther. 12:10035–10041. 2019.
View Article : Google Scholar : PubMed/NCBI
|