Yes-associated protein (YAP) is a transcriptional
co-activator in the Hippo signaling pathway, a pathway that is
universally regarded as a key regulator of organ size and tissue
homeostasis by controlling cell proliferation and apoptosis. A
recent study revealed that Hippo is even able to reprogram
different cell types into their corresponding tissue-specific stem
cells (1). In the upstream part of
the Hippo signaling pathway, Ste20 family kinases [macrophage
stimulating 1 (Mst1) and Mst2] are activated and cause a kinase
cascade reaction. Furthermore, these two proteins phosphorylate
tumor suppressor homolog large tumor suppressor kinases (Lats), and
Lats kinases then phosphorylate YAP (2–5).
Phosphorylated YAP associates with 14-3-3 protein and remains in
the cytoplasm while unphosphorylated YAP controls the gene
expression by binding to transcriptional factor TEA domain (TEAD)
in the nucleus (6–8). The Hippo signaling pathway may be
affected by various factors, including mechanical stress, cell
polarity, extracellular stimulus and interaction of upstream
regulating factors. Furthermore, the promotion of osteogenic
differentiation activity was observed in response to mechanical
stimuli using magnetically responsive coatings, as reported in
previous studies by our group (9,10);
the literature search and analysis for the present review aimed to
identify whether Hippo is the major signaling pathway in
controlling this process.
YAP is also a regulator in the Wnt signaling pathway
by binding to β-catenin, another transcriptional co-activator in
Wnt. This pathway serves an important role in homeostatic
mechanisms, and β-catenin expression regulates various biological
processes, such as cell proliferation, differentiation,
adipogenesis and aging (11,12).
A recent study revealed that in neural stem cells, the binding of
β-catenin-YAP has a stronger function compared with YAP-TEAD
(13). Furthermore, elucidation of
the linking role of YAP of the Hippo and Wnt signaling pathways may
facilitate the understanding of the regulation and homeostasis and
may have a clinical significance for future treatment
strategies.
Since YAP does not have any DNA-binding domains, it
acts via target transcriptional factors to stimulate gene
expression. TEAD is one of these transcriptional factors and
chromatin immunoprecipitation-on-chip experiments indicated that
TEAD occupies a more similar set of gene promoters to YAP compared
with other transcriptional factors (6,14).
The same results have also been demonstrated via gene set
enrichment analysis, suggesting that this is not a random
event.
In the Wnt signaling pathway, YAP is a downstream
effector of the non-canonical Wnt axis. Previously, it was
indicated that YAP and β-catenin are recruited to genes via the
TEAD and T cell factor (TCF)/lymphoid enhancer factor-1 family of
transcriptional factors, respectively (7). The present understanding is that YAP
is involved in regulating tissue differentiation and migration of
tumor cells by binding to β-catenin (15). In cells where the Wnt signaling
pathway is inactivated, YAP binds to the destruction complex
together with β-catenin in the cytoplasm, which is not only useful
for β-catenin's localization in the cytoplasm but also essential in
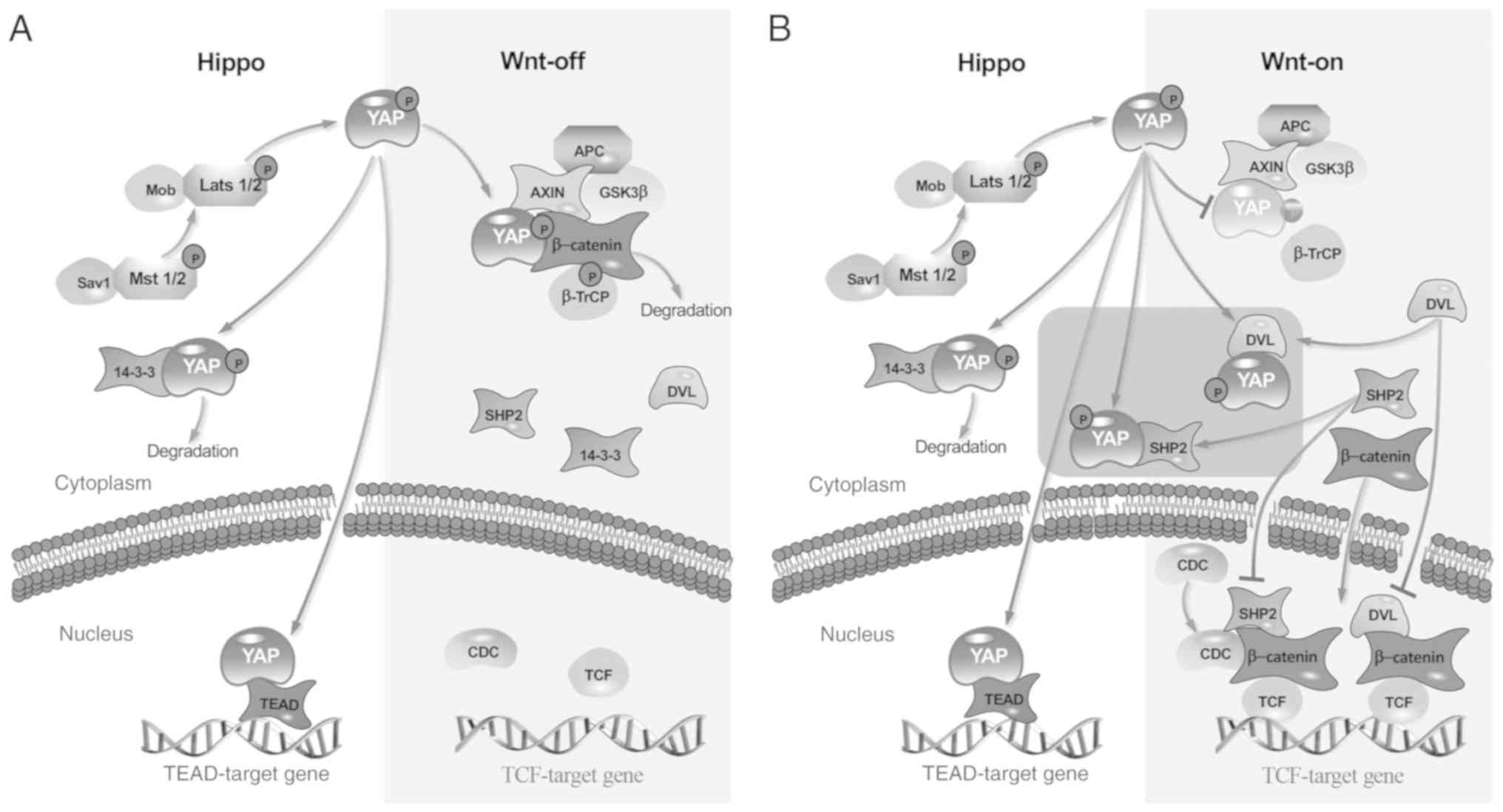
β-catenin inactivation (Fig. 1A).
This process does not have any effect on the stability of β-catenin
but suppresses its transcriptional activity (15). In cells where the Wnt signaling
pathway is activated, YAP is released from the destruction complex,
and without guidance from the destruction complex, YAP migrates
from the cytoplasm to the nucleus and serves its role to activate
the Hippo pathway (Fig. 1B)
(16,17).
YAP induces cell proliferation, which is of great
significance for tissue growth and organ development. In humans,
connective tissue growth factor (CTGF) is a YAP-regulated target
gene and the interaction between YAP and TEAD is a necessity to
induce it. It is reported that blocking CTGF by mutating TEAD
causes Sveinsson's chorioretinal atrophy (6). In Drosophila, large eyes were
observed in subjects that overexpressed both the YAP homolog Yorkie
and the TEAD homolog Scalloped, and animals only expressing Yorkie
have small eyes (6). Another
experiment in Drosophila demonstrated that YAP is involved
in organ size control via the Wnt signaling pathway, and mutation
of YAP leads to excessive proliferation of imaginal discs (8). Furthermore, Wnt controls cell
proliferation and apoptosis, and is involved in tissue and organ
development. Silencing of YAP attenuates the effect of β-catenin,
suggesting that YAP effectively modulates the transcriptional
activation of Wnt/β-catenin signaling molecules (18). In mice, nuclear β-catenin
accumulation causes cystic kidneys (19), and transgenic mice that expressed
an activated mutant of β-catenin developed cysts (20). Therefore, it was indicated that YAP
may be essential for the normal functioning of Wnt/β-catenin.
Only a small number of previous studies have
investigated whether YAP-TEAD or YAP-β catenin has the more
significant function, as they are influenced and modulated by each
other. That is to say, the role of YAP in the body, as a
transcriptional co-regulator or a regulator, requires further
studies. It has been indicated that the cell type is the major
factor determining the role of YAP. For instance, in neural stem
cells, the binding activity of YAP-β-catenin was reported to have a
stronger function on neuronal differentiation compared with TEAD
(13), which is different from
previous results obtained with mesenchymal stem cells (21). Furthermore, these results are
surprising because YAP is a well-known transcriptional co-activator
that binds to TEAD in the Hippo pathway, but it acts as a regulator
of the Wnt signaling pathway. Whether the cell is healthy is also
an influencing factor. It was revealed that YAP knockout is able to
overcome the rapid demise of mice with adenomatous polyposis coli
(APC) deficiency, and it was suggested that both the Wnt and the
Hippo pathway may be involved in this process (16). In healthy cells, β-catenin/Wnt
controls homeostasis and YAP acts as a regulator. In cells exposed
to different conditions, such as APC deficiency, tumorigenesis or
regeneration, YAP prefers the transcriptional co-regulatory
activity in the Hippo pathway. Therefore, whether YAP acts as a
transcriptional co-activator in the Hippo pathway or a regulator in
the Wnt pathway depends on the cell type. While the underlying
mechanisms remain elusive, it was proved that rigid matrix
stiffness promotes the nuclear localization and activity of YAP
(22). It was speculated that
different cell types may activate different mechanisms according to
matrix stiffness, thereby determining the role of YAP. Therefore,
further studies are required to elucidate the underlying
mechanisms.
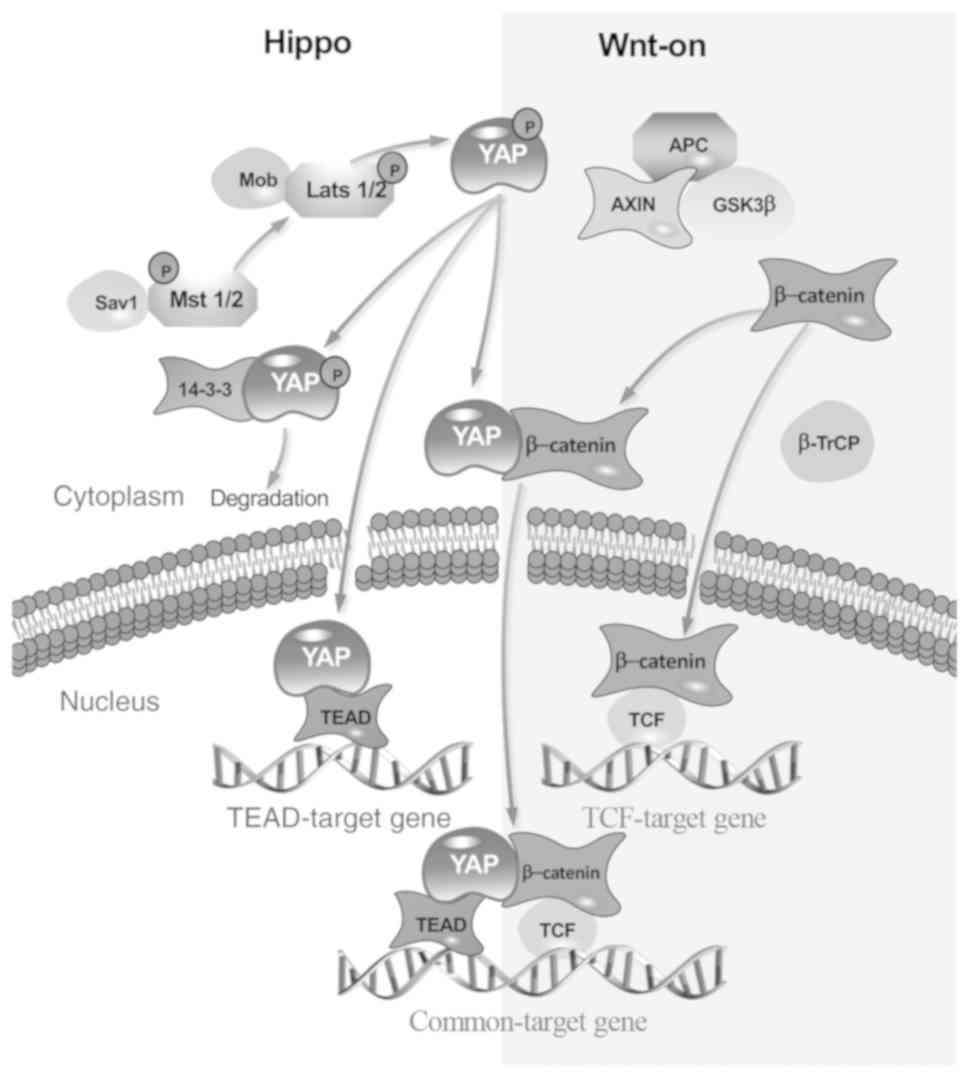
While YAP-TEAD and YAP-β-catenin are discussed
separately, they may occasionally bind to each other. YAP is the
target of Wnt/β-catenin, and it is able to directly bind to the
N-terminal domain of β-catenin. The TCF-β-catenin-YAP-TEAD complex
then transfers into the nuclear and locates to the common gene of
TEAD and TCF (Fig. 2). A previous
study revealed that the β-catenin-induced localization to the Oct4
distal enhancer is TEAD-dependent and also demonstrated the
occurrence of the YAP-TEAD-β-catenin trimeric complex (23). Thus, the crosstalk between Hippo
and Wnt is complex.
It has been reported that cytoplasmic YAP has a
negative regulatory effect on the Wnt signaling pathway (24). YAP is phosphorylated by upstream
factors in the Hippo pathway. When Wnt is activated, phosphorylated
YAP and β-catenin are dislodged from the complex and two routes are
involved in the regulation of the Wnt signaling pathway. One of
these routes is that the phosphorylated YAP may impede nuclear
β-catenin localization by combining and sequestering Dishevelled 2
in the cytoplasm (8,25). Alternatively, Src homology 2 domain
tyrosine phosphatase (SHP2), an amino acid phosphatase, promotes
the transcription of β-catenin after it translocates into the
nucleus. Thus, once YAP locates SHP2 in the cytoplasm, Wnt
signaling pathway activation is dampened (Fig. 1B) (26–28).
A previous study revealed that in osteoblast-lineage
cells, reduced β-catenin protein expression was present in
YAP-deficiency cells in culture and in vivo, and the
expression of YAP increases the expression of β-catenin (29). Thus, YAP has a positive effect on
the Wnt signaling pathway. Similar results were reported in other
studies. For instance, using an in vitro model of mouse
chondrogenic cells, YAP was demonstrated to upregulate β-catenin to
control chondrocyte differentiation (30). In an in vivo study, YAP was
indicated to act via a β-catenin-dependent mechanism in muscle
cells to control neuromuscular junction formation and regeneration
(31). The observed positive
regulation was different from those of previous studies, as the
experimental subject was nuclear YAP. As mentioned above, the roles
of YAP may differ depending on the cell type, and there may be cell
type-dependent differences in terms of whether the nucleus or
cytoplasm is the site for YAP to regulate β-catenin. It was
speculated that in the nucleus, YAP and β-catenin act together,
while they are negatively associated in the cytoplasm, which is
based on the result that YAP in the nucleus promotes SHP2
translocation into the nucleus, which activates the transcription
of β-catenin (27). However, as
the positive regulatory interaction between the two signaling
pathways has only been discovered in recent years, future
investigations are required.
Fibrosis is a pathological process that results from
the deposition and insufficient resorption of extracellular matrix
(ECM). The Wnt signaling pathway serves a pivotal role in
fibroblast activation and the epithelial-to-mesenchymal transition
process (32), contributing to the
dynamic deposition of ECM and an increase of ECM stiffness. Thus,
Wnt has been proposed as a target of fibrosis treatment. ICG-001
(33), Klotho (34) and poricoic acid (35) have all been indicated to attenuate
Wnt and reverse fibrosis. In recent years, the Hippo pathway has
been suggested to be involved in the development of fibrosis
(36). YAP may be considered as a
novel target for the treatment of fibrosis, as the diminishment of
nuclear YAP localization is associated with inhibition of the Wnt
signaling pathway. Previous studies aimed to control nuclear YAP
localization to treat fibrosis (37,38).
Furthermore, it was demonstrated that highly specific inhibitors
may be developed based on the protein-protein interactions located
at the intersection of the Hippo and Wnt signaling pathways, which
may be used as a novel target (39).
In cancer stroma, fibrotic processes occur to
various degrees, which may lead to fibrosis, and there is a close
association between fibrosis and cancer. The Wnt and Hippo
signaling pathways modulate the features of cancer cells (40–42),
and identifying targets on these two signaling pathways has been a
hot research topic. Certain previous studies have also focused on
both pathways simultaneously (43–45).
The Wnt signaling pathway has been indicated to be
involved in cancer regulation via persistent activation of
β-catenin signaling (12,46). The involvement of the YAP-β-catenin
complex is important. For instance, in colon cancer, the
β-catenin-YAP1-T-box transcription factor 5 complex was identified
to promote the survival and transformation of β-catenin-active
cancer cell lines. By examining the top 50 genes in proliferating
β-catenin-active cells, a significant enrichment of proteins
associated with YAP was identified (47). In hepatoblastoma cells, only the
combination of β-catenin and YAP caused rapid cell proliferation
and then induced cancer, but this effect was not observed with
β-catenin or YAP alone (46).
Based on these results, Ras association domain family 1A gene was
used to inhibit the binding of YAP (23), and ICG-001, a small molecule
inhibitor, was used as a target for head and neck cancer by
regulating β-catenin/Wnt (48).
YAP binds to TEAD to induce proliferation while
entering the nucleus, and is degraded when binding to 14-3-3
protein in the cytoplasm (25);
this is also the mechanism in the Hippo signaling pathway resulting
in dysregulated cell proliferation. In previous studies, different
medicines, such as verteporfin or protoporphyrin IX (49,50),
were considered to be possible treatments for various cancer types
(51,52). The mechanism of action of these
medicines is to inhibit the Hippo pathway by altering or disrupting
the binding interface of YAP and TEAD (53). In addition, certain studies are
aiming to utilize the role of Hippo in tumor immunity to treat
non-small cell lung cancer (54)
and malignant pleural mesothelioma (55); however, this is a novel area of
research and requires further investigation.
A new direction for future cancer treatment has been
discovered by studying the crosstalk between Wnt and Hippo. Based
on the crosstalk between these two signaling pathways, it has been
proposed that overexpression of cytoplasmic YAP may restrict the
Wnt signal and thus reduce the proliferation of cancer cells
(56,57). Furthermore, aberrant oncogenic Wnt
signaling has been demonstrated to be detrimental for the abnormal
proliferation of cancer cells, but had no obvious effect on normal
stem cells; thus, it is a favorable strategy for future cancer
therapy (16). As this mechanism
has only been investigated in cells or mouse models, additional
research is necessary prior to clinical use.
In conclusion, YAP not only serves a role in the
Hippo pathway to act as a co-regulator of transcription activity
but also acts as a regulator in the Wnt signaling pathway, and
knockdown of YAP causes metabolic disorders and results in abnormal
organ size. Depending on the cell type, YAP exerts different roles
by activating different mechanisms. Nuclear YAP upregulates
β-catenin and cytoplasmic YAP negatively modulates the Wnt
signaling pathway. As these two signaling pathways are involved in
physiological metabolism and homeostasis, YAP may be used as a
novel target for the treatment of fibrosis and cancer. While this
has been recently investigated in the laboratory, the crosstalk
between the Hippo and Wnt signaling pathways is complex and further
research is required in the future.
Not applicable.
This work was supported by the National Natural
Science Foundation of China (grant no. 81970978), the Zhejiang
Province Natural Science Foundation of China (grant no.
LQ18H140004) and the Zhejiang Provincial Medical Health &
Hygienic Science and Technology Project of China (grant nos.
2018256920 and 2019RC156).
Not applicable.
LJ and JuaL conceived and designed the study. LJ, CZ
and YS searched the literature and drafted the manuscript. JuaL and
JunL critically revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Panciera T, Azzolin L, Fujimura A, Di
Biagio D, Frasson C, Bresolin S, Soligo S, Basso G, Bicciato S,
Rosato A, et al: Induction of expandable tissue-specific
stem/progenitor cells through transient expression of YAP/TAZ. Cell
Stem Cell. 19:725–737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mauviel A, Nallet-Staub F and Varelas X:
Integrating developmental signals: A Hippo in the (path)way.
Oncogene. 31:1743–1756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishioka N, Inoue K, Adachi K, Kiyonari H,
Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N,
et al: The Hippo signaling pathway components Lats and Yap Pattern
TEAD4 activity to distinguish mouse trophectoderm from inner cell
mass. Dev Cell. 16:398–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xin M, Kim Y, Sutherland LB, Murakami M,
Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, et
al: Hippo pathway effector Yap promotes cardiac regeneration. Proc
Natl Acad Sci USA. 110:13839–13844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stampouloglou E and Varelas X:
Phosphatidic acid signals via the Hippo pathway. Mol Cell.
72:205–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heallen T, Zhang M, Wang J,
Bonilla-Claudio M, Klysik E, Johnson RL and Martin JF: Hippo
pathway inhibits Wnt signaling to restrain cardiomyocyte
proliferation and heart size. Science. 332:458–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varelas X, Miller BW, Sopko R, Song S,
Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill
H, et al: The Hippo pathway regulates Wnt/β-catenin signaling. Dev
Cell. 18:579–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang J, Lin S, Dong L, Cheng K and Weng
W: Magnetically actuated mechanical stimuli on
Fe3O4/mineralized collagen coatings to
enhance osteogenic differentiation of the MC3T3-E1 cells. Acta
Biomater. 71:49–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuang J, Lin S, Dong L, Cheng K and Weng
W: Magnetically assisted electrodeposition of aligned collagen
coatings. ACS Biomater Sci Eng. 4:52018.
|
|
11
|
Regimbald-Dumas Y and He X: Wnt
signalling: What the X@# is WTX? EMBO J. 30:1415–1417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
MacDonald BT, Tamai K and He X:
Wnt/β-Catenin signaling: Components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sebastian R, Michael SK, Katerina G,
Sanjay K and David VS: Dynamics of mechanosensitive neural stem
cell differentiation. Stem Cells. 35:497–506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Zhao B, Wang P, Chen F, Dong Z, Yang
H, Guan KL and Xu Y: Structural insights into the YAP and TEAD
complex. Genes Dev. 24:235–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imajo M, Miyatake K, Iimura A, Miyamoto A
and Nishida E: A molecular mechanism that links Hippo signalling to
the inhibition of Wnt/β-catenin signalling. EMBO J. 31:1109–1122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Azzolin L, Panciera T, Soligo S, Enzo E,
Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V,
et al: YAP/TAZ incorporation in the β-catenin destruction complex
orchestrates the Wnt response. Cell. 158:157–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J and Martin JF: Hippo pathway: An
emerging regulator of craniofacial and dental development. J Dent
Res. 96:1229–1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng F, Peng L, Li Z, Tan G, Liang E, Chen
S, Zhao X and Zhi F: YAP triggers the Wnt/β-catenin signalling
pathway and promotes enterocyte self-renewal, regeneration and
tumorigenesis after DSS-induced injury. Cell Death Dis. 9:1532018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sansom OJ, Griffiths DF, Reed KR, Winton
DJ and Clarke AR: Apc deficiency predisposes to renal carcinoma in
the mouse. Oncogene. 24:8205–8210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saadi-Kheddouci S, Berrebi D, Romagnolo B,
Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A and Perret C: Early
development of polycystic kidney disease in transgenic mice
expressing an activated mutant of the beta-catenin gene. Oncogene.
20:5972–5981. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dupont S, Morsut L, Aragona M, Enzo E,
Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M,
Bicciato S, et al: Role of YAP/TAZ in mechanotransduction. Nature.
474:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuroda M, Wada H, Kimura Y, Ueda K and
Kioka N: Vinculin promotes nuclear localization of TAZ to inhibit
ECM stiffness-dependent differentiation into adipocytes. J Cell
Science. 130:989–1002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papaspyropoulos A, Bradley L, Thapa A,
Leung CY, Toskas K, Koennig D, Pefani DE, Raso C, Grou C, Hamilton
G, et al: RASSF1A uncouples Wnt from Hippo signalling and promotes
YAP mediated differentiation via p73. Nat Commun. 9:4242018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park HW, Kim YC, Yu B, Moroishi T, Mo JS,
Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al: Alternative
Wnt signaling activates YAP/TAZ. Cell. 162:780–794. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Du S, Lei T, Wang H, He X, Tong R
and Wang Y: Multifaceted regulation and functions of YAP/TAZ in
tumors (Review). Oncol Rep. 40:16–28. 2018.PubMed/NCBI
|
|
26
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsutsumi R, Masoudi M, Takahashi A, Fujii
Y, Hayashi T, Kikuchi I, Satou Y, Taira M and Hatakeyama M: YAP and
TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2
function. Dev Cell. 26:658–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi A, Tsutsumi R, Kikuchi I, Obuse
C, Saito Y, Seidi A, Karisch R, Fernandez M, Cho T, Ohnishi N, et
al: SHP2 tyrosine phosphatase converts parafibromin/cdc73 from a
tumor suppressor to an oncogenic driver. Mol Cell. 43:45–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan J, Xiong L, Zhao K, Zeng P, Wang B,
Tang FL, Sun D, Guo HH, Yang X, Cui S, et al: YAP promotes
osteogenesis and suppresses adipogenic differentiation by
regulating β-catenin signaling. Bone Res. 6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang B, Sun H, Song F, Yu M, Wu Y and Wang
J: YAP1 negatively regulates chondrocyte differentiation partly by
activating the β-catenin signaling pathway. Cell. 87:104–113.
2017.
|
|
31
|
Zhao K, Shen C, Lu Y, Huang Z, Li L, Rand
CD, Pan J, Sun XD, Tan Z, Wang H, et al: Muscle Yap is a regulator
of neuromuscular junction formation and regeneration. J Neurosci.
37:3465–3477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Urban ML, Manenti L and Vaglio A:
Fibrosis-a common pathway to organ injury and failure. N Engl J
Med. 373:95–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henderson WR Jr, Chi EY, Ye X, Nguyen C,
Tien YT, Zhou B, Borok Z, Knight DA and Kahn M: Inhibition of
Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses
pulmonary fibrosis. Proc Natl Acad Sci USA. 107:14309–14314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Satoh M, Nagasu H, Morita Y, Yamaguchi TP,
Kanwar YS and Kashihara N: Klotho protects against mouse renal
fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol.
303:F1641–F1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang M, Chen DQ, Chen L, Liu D, Zhao H,
Zhang ZH, Vaziri ND, Guo Y, Zhao YY and Cao G: Novel RAS inhibitors
poricoic acid ZG and poricoic acid ZH attenuate renal fibrosis via
a Wnt/β-catenin pathway and targeted phosphorylation of smad3
signaling. J Agric Food Chem. 66:1828–1842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Noguchi S, Saito A and Nagase T: YAP/TAZ
signaling as a molecular link between fibrosis and cancer. Int J
Mol Sci. 19:36742018. View Article : Google Scholar
|
|
37
|
Haak AJ, Kostallari E, Sicard D, Ligresti
G, Choi KM, Caporarello N, Jones DL, Tan Q, Meridew J, Diaz
Espinosa AM, et al: Selective YAP/TAZ inhibition in fibroblasts via
dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med.
11:eaau62962019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Toyama T, Looney AP, Baker BM, Stawski L,
Haines P, Simms R, Szymaniak AD, Varelas X and Trojanowska M:
Therapeutic targeting of TAZ and YAP by dimethyl fumarate in
systemic sclerosis fibrosis. J Invest Dermatol. 138:78–88. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu H, Ge T, Pan Y and Zhang S: Advanced
role of Hippo signaling in endometrial fibrosis: Implications for
intrauterine adhesion. Chin Med J (Engl). 130:2732–2737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Verma S, Yeddula N, Soda Y, Zhu Q, Pao G,
Moresco J, Diedrich JK, Hong A, Plouffe S, Moroishi T, et al:
BRCA1/BARD1-dependent ubiquitination of NF2 regulates Hippo-YAP1
signaling. Proc Natl Acad Sci USA. 116:7363–7370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zanconato F, Forcato M, Battilana G,
Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M
and Piccolo S: Genome-wide association between YAP/TAZ/TEAD and
AP-1 at enhancers drives oncogenic growth. Nat Cell Biol.
17:1218–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moya IM and Halder G: Hippo-YAP/TAZ
signalling in organ regeneration and regenerative medicine. Nat Rev
Molr Cell Biol. 20:211–226. 2019. View Article : Google Scholar
|
|
43
|
Sulaiman A, McGarry S, Li L, Jia D, Ooi S,
Addison C, Dimitroulakos J, Arnaout A, Nessim C, Yao Z, et al: Dual
inhibition of Wnt and Yes-associated protein signaling retards the
growth of triple negative breast cancer in both mesenchymal and
epithelial states. Mol Oncol. 12:423–440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu J, Gao C, Chen G and Gao X: Abstract
LB-310: PYK2 as a therapeutic target for pancreatic cancer. Cancer
Res. 77:3102017.
|
|
45
|
Szalmás A, Tomaić V, Basukala O, Massimi
P, Mittal S, Kónya J and Banks L: The PTPN14 tumor suppressor is a
degradation target of human papillomavirus E7. J Virol.
91:e00057–00017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tao J, Calvisi DF, Ranganathan S, Cigliano
A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger
S, et al: Activation of β-Catenin and Yap1 in human hepatoblastoma
and induction of hepatocarcinogenesis in mice. Gastroenterology.
147:690–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rosenbluh J, Nijhawan D, Cox AG, Li X,
Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et
al: β-catenin-driven cancers require a YAP1 transcriptional complex
for survival and tumorigenesis. Cell. 151:1457–1473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kartha VK, Alamoud KA, Sadykov K, Nguyen
BC, Laroche F, Feng H, Lee J, Pai SI, Varelas X, Egloff AM, et al:
Functional and genomic analyses reveal therapeutic potential of
targeting β-catenin/CBP activity in head and neck cancer. Genome
Med. 10:542018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The Hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu-Chittenden Y, Huang B, Shim JS, Chen
Q, Lee SJ, Anders RA, Liu JO and Pan D: Genetic and pharmacological
disruption of the TEAD-YAP complex suppresses the oncogenic
activity of YAP. Genes Dev. 26:1300–1305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rozengurt E, Sinnett-Smith J and Eibl G:
Yes-associated protein (YAP) in pancreatic cancer: At the epicenter
of a targetable signaling network associated with patient survival.
Signal Transduct Target Ther. 3:112018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stampouloglou E, Cheng N, Federico A,
Slaby E, Monti S, Szeto GL and Varelas X: Yap suppresses T-cell
function and infiltration in the tumor microenvironment. PLoS Biol.
18:e30005912020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Crawford JJ, Bronner SM and Zbieg JR:
Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: A
patent review. Expert Opin Ther Pat. 28:867–873. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsu PC, Jablons DM, Yang CT and You L:
Epidermal growth factor receptor (EGFR) pathway, Yes-associated
protein (YAP) and the regulation of programmed death-ligand 1
(PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci.
20:38212019. View Article : Google Scholar
|
|
55
|
Hsu PC, Yang CT, Jablons DM and You L: The
role of Yes-associated protein (YAP) in regulating programmed
death-ligand 1 (PD-L1) in thoracic cancer. Biomedicines. 6:1142018.
View Article : Google Scholar
|
|
56
|
Alam M, Bouillez A, Tagde A, Ahmad R,
Rajabi H, Maeda T, Hiraki M, Suzuki Y and Kufe D: MUC1-C represses
the crumbs complex polarity factor CRB3 and downregulates the Hippo
pathway. Mol Cancer Res. 14:1266–1276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|