Introduction
Breast cancer is one of the most common malignancies
in women worldwide (1). According
to incomplete statistics, ~1.7 million new breast cancer cases are
reported each year, accounting for 11.6% of all new cancer cases
(1,2). Based on this increasing rate, it has
been estimated that the number of breast cancer cases and deaths
worldwide will reach 2.64 million and 1.7 million in 2030,
respectively (3). Although the
diagnosis and treatment of breast cancer have markedly improved in
recent years, a number of patients experience post-treatment
recurrence and metastasis (4), and
the mechanism underlying breast cancer is not completely
understood. Therefore, identifying novel strategies to prevent the
recurrence and metastasis of breast cancer, and to further improve
the survival rate and quality of life of patients with breast
cancer is important.
Cyclin-dependent kinases (CDKs), a member of the
serine/threonine protein kinase family, can coordinate critical
regulatory events during the cell cycle and transcription (5). Uncontrolled cell division is one of
the hallmarks of cancer, and alterations in at least one CDK
regulator or effector have been identified in almost all types of
cancer (6). Moreover, inhibiting
the cell cycle has been reported as a successful therapeutic
strategy in oncology (7,8). CDK8, as a member of the CDK family,
serves an important role in gene transcription (9). Specifically, CDK8 has been reported
to regulate the cell cycle and proliferation at the
post-transcriptional level, and promote the development of various
tumors, such as melanoma, acute myeloid leukemia (AML) and breast
cancer (10,11).
Previous studies have indicated that CDK8 impacts
various signaling pathways, including the β-catenin (12), p53 (13) and Notch1 (13,14)
signaling pathways. A recent studies has revealed that CDK8 and the
Wnt/β-catenin signaling pathway serve key roles in breast cancer
(15). Aberrant activation of the
Wnt/β-catenin signaling pathway causes β-catenin accumulation in
the nucleus and can induce breast cancer (12). Firestein et al (12) demonstrated that CDK8 can increase
the level of β-catenin in the cytoplasm, promote its translocation
to the nucleus and binding to the TCF/LEF element, activate certain
oncogenes, and promote the unrestricted proliferation of primary
cells by unrestricted transcription and translation, which
eventually leads to tumorigenesis. Additionally, it has been
reported that CDK8 gene knockout can inhibit the activation of
β-catenin and its downstream signaling, thereby inhibiting tumor
cell proliferation, invasion and metastasis (16). Collectively, the aforementioned
studies indicated that CDK8 may serve as a potential therapeutic
target for breast cancer.
Capsaicin, an active ingredient extracted from chili
pepper, has been reported to display multiple pharmacological
effects, including analgesic and anticancer effects (17). Capsaicin can be absorbed into the
blood circulation via the digestive system and is eventually
eliminated by the liver (18).
Studies have demonstrated that capsaicin, if formed into liposomes
or encapsulated in nanocapsules, can be accurately delivered to
tumor tissue (18,19). Additionally, it has been reported
that capsaicin can inhibit B16-F10 melanoma cell migration by
inhibiting the PI3K/Akt/Rac family small GTPase 1 (Rac1) signaling
pathway (20). Although the
aforementioned studies demonstrated the anticancer effects of
capsaicin, the studies did not clearly explain the underlying
mechanisms. Therefore, the present study investigated the antitumor
effect of capsaicin on MDA-MB-231 breast cancer cells and explored
the potential anticancer mechanism underlying capsaicin via
inhibition of the CDK8/PI3K/Akt/Wnt/β-catenin signaling
pathway.
Materials and methods
Cell culture
The MDA-MB-231 breast cancer cell line and the
MCF10A healthy breast cell line were purchased from the American
Type Culture Collection. Cells were cultured in L-15 medium
(Nanjing KeyGen Biotech Co., Ltd.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 U/ml streptomycin (Nanjing KeyGen Biotech Co., Ltd.) in
humidified 5% CO2 at 37°C.
Drugs and reagents
The primary antibodies targeted against CDK8 (cat.
no. 17395); p-PI3K (cat. no. 17366), PI3K (cat. no. 4255), p-Akt
(cat. no. 4060), Akt (cat. no. 4685), β-catenin (cat. no. 8480) and
Wnt (cat. no. 2721) were purchased from Cell Signaling Technology,
Inc. The primary antibody targeted against GAPDH (cat. no.
14-9523-37) was purchased from Sigma-Aldrich (Merck KGaA).
Capsaicin was purchased from Sigma-Aldrich (Merck KGaA), diluted in
DMSO at 100 mM and stored at −20°C. LY294002 and Senexin A were
purchased from MedChemExpress. FBS were purchased from Gibco
(Thermo Fisher Scientific, Inc.). Cell Cycle and Apoptosis Analysis
Kit (cat. no. C1052) was purchased from Beyotime Institute of
Biotechnology. All other chemicals were purchased from
Sigma-Aldrich (Merck KGaA).
Cell viability assay
Cell viability was assessed by performing MTT
assays. Briefly, MDA-MB-231 cells were seeded (1×104
cells/well) into a 96-well plate and cultured for 24 h. Cells were
then incubated with different concentrations of capsaicin (0, 10,
50, 100 or 200 µM) for 48 h at 37°C with 5% CO2.
Subsequently, 20 µl MTT solution (5 mg/ml) was added to each well
for 4 h at 37°C with 5% CO2. The supernatant was removed
and 100 µl DMSO was added to each well to dissolve the formazan
crystal. Absorbance was measured at a wavelength of 450 nm using an
ELISA microplate reader (PerkinElmer, Inc.). Cell viability is
presented as the mean ± SD of three independent experiments.
Wound healing assay
Cells were seeded (1×106 cells/well) into
a 6-well plate and cultured to 40–50% confluence. Subsequently,
cells were incubated with different concentrations of capsaicin (0,
10, 50, 100 and 200 µM) for 24 h at 37°C with 5% CO2
until the cell monolayer reached 100% confluence. The medium was
then replaced with serum-free medium. The cell monolayer was
scratched with a 10-µl pipette tip and washed three times with PBS
to remove cell debris. The width of the wound was observed at 0 and
48 h using an inverted light microscope and calculated using ImageJ
software (version 1.8.0; National Institutes of Health). The rate
of cell migration was calculated according to the following
formula: Experimental group migration distance/control group
migration distance. The wound healing assay was performed in
triplicate.
Cell cycle analysis
Cells in the logarithmic growth phase were seeded
(2×105 cells/well) into a 6-well plate and incubated at
37°C for 24 h. The medium was replaced with serum-free medium.
Cells were incubated with or without capsaicin (0, 10, 50, 100 and
200 µM) for 48 h at 37°C. Subsequently, cells were collected,
washed with pre-cooled PBS and fixed with 70% ethanol overnight at
4°C. After washing with PBS, cells were incubated with 10 µg/ml
RNase at 37°C for 30 min. Subsequently, cells were incubated with 2
mg/ml propidium iodide (PI; final concentration, 10 µg/ml) for 30
min at room temperature in the dark. Cell cycle distribution was
analyzed using a FACSCalibur analyzer (BD Biosciences). Flow
cytometry was performed in triplicate.
Cell transduction
MDA-MB-231 cells were seeded (1×106
cells/well) into a 6-well plate for 24 h at 37°C. Lentiviral
vectors [LVs; LV-CDK8-short hairpin (sh)RNA or LV-negative control
(NC)-shRNA] (empty vector) were purchased from OBiO Technology
(Shanghai) Corp., Ltd. Cells were transduced with
3.0×1010 PFU/ml of LV-CDK8-shRNA or LV-NC-shRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 48 h
post-infection, cells were used for subsequent experiments.
Western blotting
Cells were seeded (1×106 cells/well) into
the 6-well plate and treated with or without capsaicin (10, 50, 100
and 200 µM) for 24 h at 37°C. Subsequently, cells were collected
and total protein was extracted using RIPA lysis buffer (Thermo
Fisher Scientific, Inc.). Total protein was quantified using a BCA
Protein assay (Thermo Fisher Scientific, Inc.). A total of 40 µg of
proteins was loaded and separated by 10% SDS-PAGE and
electrophoretically transferred onto polyvinylidene fluoride
membranes (EMD Millipore). The membranes were blocked with 5%
bovine serum albumin for 1 h at room temperature, probed with with
primary antibodies targeted against: CDK8 (1:1,000), phosphorylated
(p)-PI3K (1:1,000), PI3K (1:1,000), p-Akt (1:1,000), Akt (1:1,000),
Wnt (1:1,000), β-catenin (1:1,000) and GAPDH (1:8,000) overnight at
4°C and then incubated with horseradish peroxide-conjugated
secondary antibodies [goat anti-rabbit IgG (H+L); cat. no. 31460 or
goat anti-mouse IgG (H+L); cat. no. 31430; 1:5,000; Chemicon
International; Thermo Fisher Scientific, Inc.] for 2 h at room
temperature. Protein bands were visualized using enhanced
chemiluminescence reagents (PerkinElmer, Inc.) and the Gel Doc™ XR,
170-8170 Molecular Imager. Protein expression levels were
semi-quantified using Quantity One software (version 4.6.5; Bio-Rad
Laboratories, Inc.) with GAPDH as the loading control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the All-in-One™ miRNA Q-PCR
Detection kit (GeneCopoeia, Inc.). The temperature protocol of the
reverse transcription step was as follows: 30°C for 10 min, 42°C
for 30 min, 99°C for 5 min and 4°C for 5 min). Subsequently, qPCR
was performed using SYBR-Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and a 7500 Fast Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for qPCR: 95°C for 10
min; followed by 40 cycles at 95°C for 10 sec, 57°C for 20 sec and
72°C for 15 sec. The following primers were used for qPCR: CDK8
forward, 5′-TCACCTTTGAAGCCTTTAGC-3′ and reverse,
5′-CTGATGTAGGAAGTGGGTCT-3′; and GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. mRNA expression levels were
quantified using the 2−∆∆Cq method (21) and normalized to the internal
reference gene GAPDH. RT-qPCR was performed in triplicate.
Statistical analysis
Statistical analyses were performed using GraphPad
Instat software (version 7.0; GraphPad Software, Inc.). Comparisons
among multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. Data are presented as the mean ± SD of the
three independent experiments. P<0.05 was considered to indicate
a statistically significant difference.
Results
CDK8 mRNA and protein expression
levels are significantly increased in MDA-MB-231 breast cancer
cells
Previous studies have demonstrated that CDK8 serves
a key role in increasing β-catenin expression levels and promoting
cell proliferation in various types of cancer, such as prostate
(22) and colorectal (23) cancer. In the present study, the
mRNA and protein expression levels of CDK8 were measured in
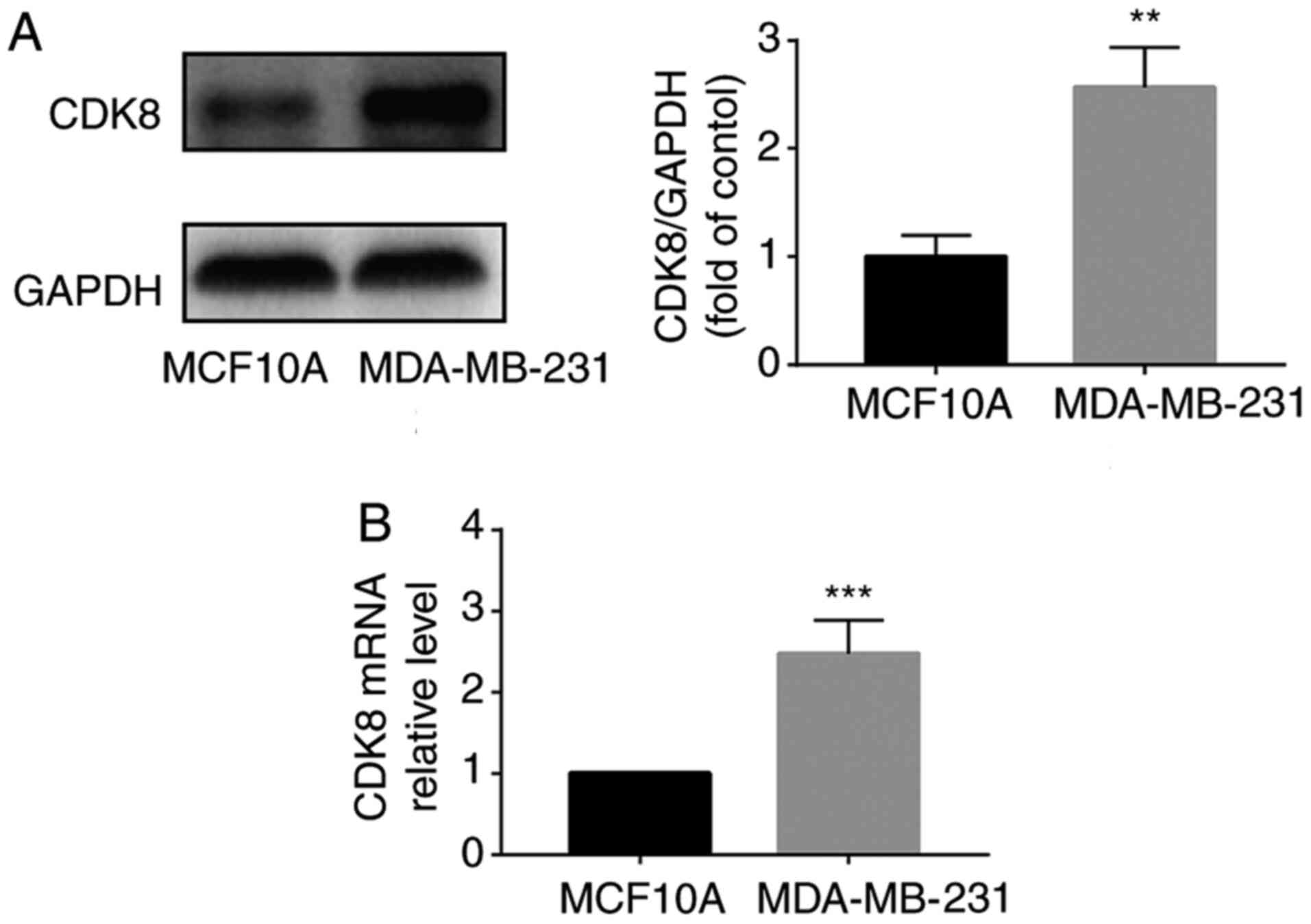
MDA-MB-231 breast cancer cells. The results suggested that CDK8
protein expression levels were significantly increased in
MDA-MB-231 cells compared with MCF10A healthy breast cells
(Fig. 1A). CDK8 mRNA expression
levels were also significantly higher in MDA-MB-231 breast cancer
cells compared with MCF10A healthy breast cells (Fig. 1B). These results suggested that
CDK8 was upregulated in MDA-MB-231 breast cancer cells.
CDK8 enhances MDA-MB-231 breast cancer
cell viability and migration
Previous studies have indicated that CDK8 functions
as a transcriptional regulator and serves an important role in the
development of melanoma, AML, prostate cancer and breast cancer
(11,24). The aforementioned results indicated
that CDK8 was significantly upregulated in MDA-MB-231 cells;
therefore, whether breast cancer cell viability was dependent on
CDK8 was investigated. MDA-MB-231 cells were infected with
LV-CDK8-shRNA and the control group was infected with LV-NC-shRNA.
Subsequently, MTT and wound healing assays were performed to
examine cell viability and migration in vitro, respectively.
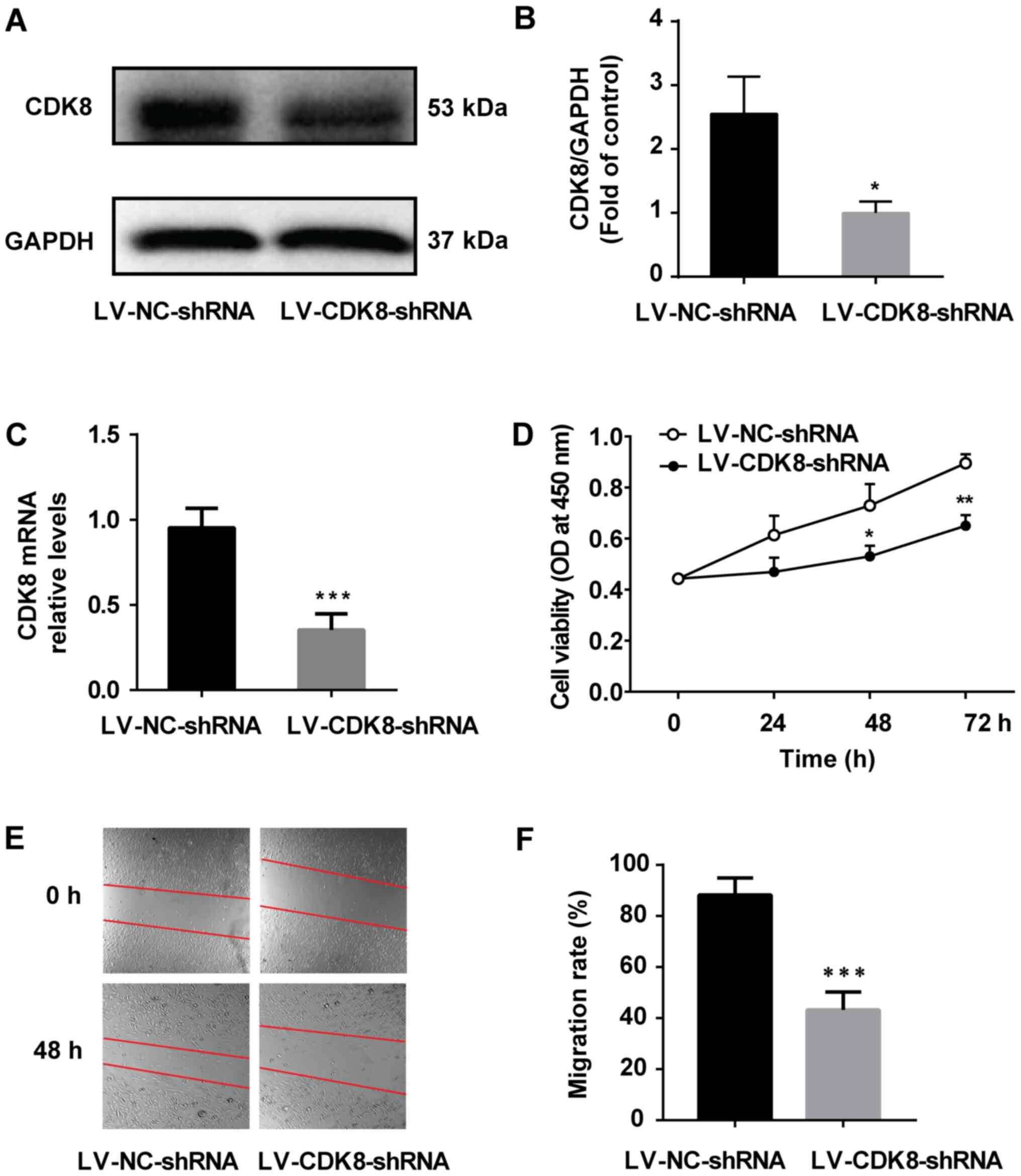
The western blotting and RT-qPCR results indicated that CDK8
expression was significantly decreased by LV-CDK8-shRNA compared
with LV-NC-shRNA (Fig. 2A-C).
Moreover, MDA-MB-231 cell viability (Fig. 2D) and migration (Fig. 2E and F) in the LV-CDK8-shRNA group
were significantly lower compared with those in the LV-NC-shRNA
group. The results indicated that MDA-MB-231 breast cancer cell
viability and migration were dependent on CDK8.
Capsaicin inhibits breast cancer cell
viability by reducing CDK8
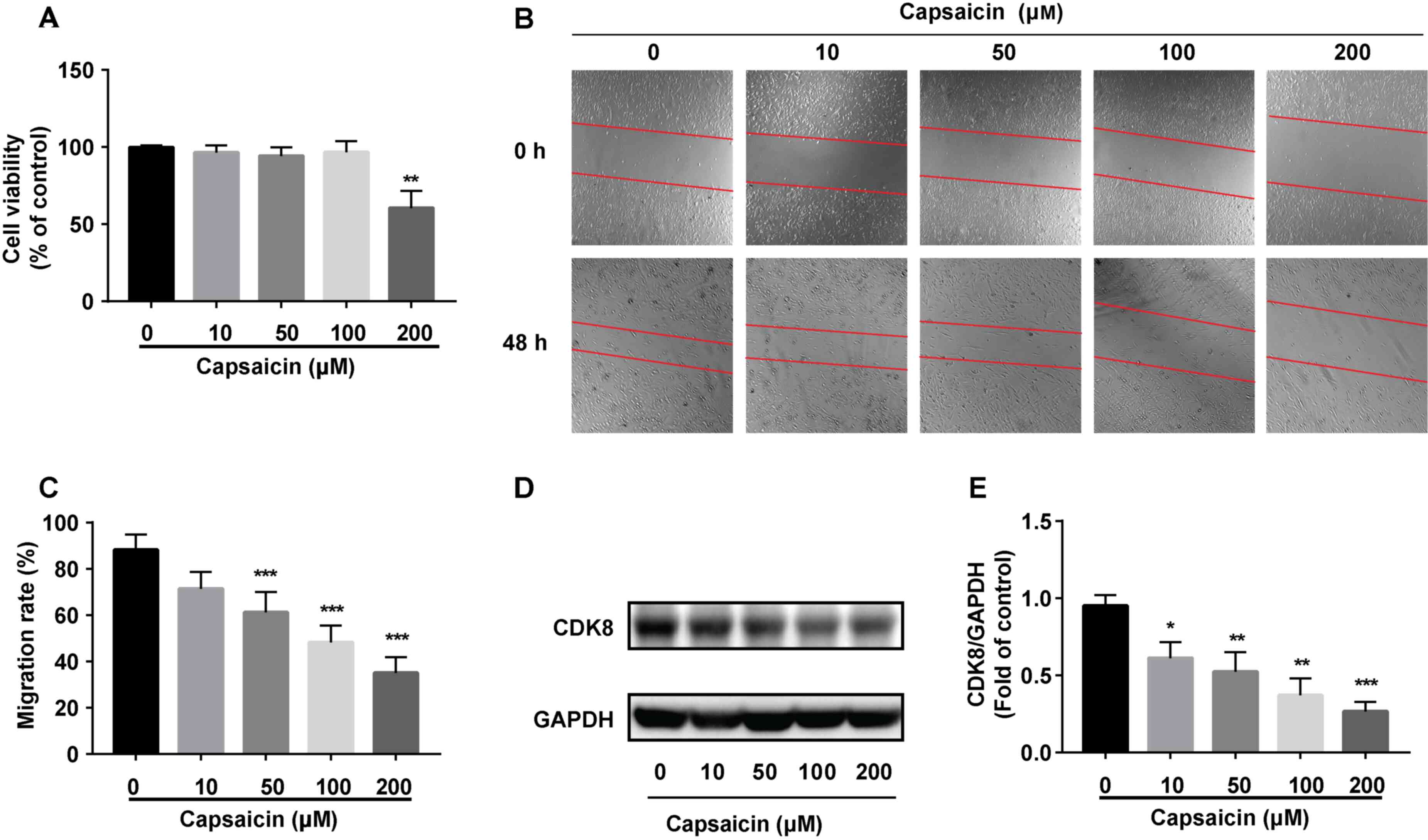
The MTT assay was performed to assess the antitumor
effects of capsaicin in MDA-MB-231 breast cancer cells. The results
indicated that low concentrations of capsaicin (10, 50 and 100 µM)
did not significantly alter cell viability, whereas 200 µM
capsaicin significantly reduced MDA-MB-231 breast cancer cell
viability compared with the 0 µM capsaicin group (Fig. 3A). The effects of capsaicin on
MDA-MB-231 breast cancer cell migration were assessed. Capsaicin
(>10 µM) significantly inhibited MDA-MB-231 cell migration in
vitro compared with the 0 µM capsaicin group (Fig. 3B and C). Moreover, capsaicin
significantly decreased the expression of CDK8 in MDA-MB-231 cells
compared with the 0 µM capsaicin group (Fig. 3D and E). Collectively, the results
suggested that capsaicin had a potent inhibitory effect on
MDA-MB-231 breast cancer cells.
Capsaicin induces G2/M cell
cycle arrest in MDA-MB-231 breast cancer cells
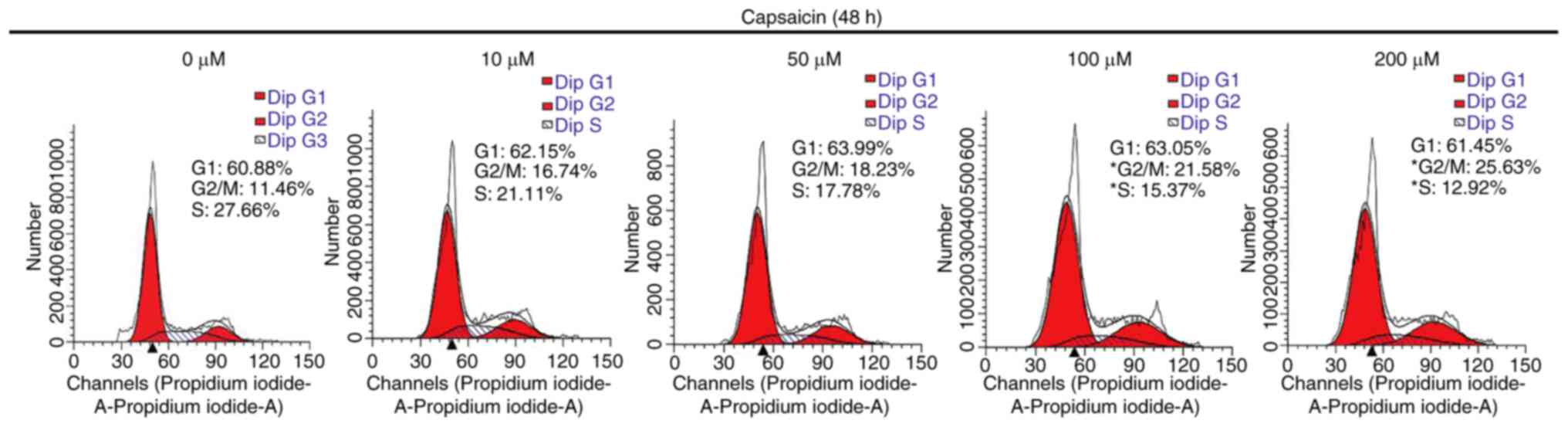
To explore the potential anticancer mechanisms
underlying capsaicin, capsaicin-induced alterations in the cell
cycle distribution were assessed. MDA-MB-231 breast cancer cells
were treated with or without capsaicin for 48 h, and the cell cycle
distribution was evaluated via flow cytometry. The results
demonstrated that capsaicin induced cell cycle arrest at the
G2/M phase (Fig. 4).
The proportion of MDA-MB-231 cells at the G2/M phase
increased from 11.46% in the 0 µM capsaicin group to 16.74, 18.23,
21.58 and 25.63% in the 10, 50, 100 and 200 µM capsaicin groups,
respectively (Fig. 4). The results
indicated that capsaicin could induce G2/M phase cell
cycle arrest in MDA-MB-231 breast cancer cells.
Capsaicin inhibits breast cancer cell
viability by downregulating the CDK8/PI3K/Akt signaling
pathway
A previous study demonstrated the potent inhibitory
effect of capsaicin on B16-F10 malignant melanoma cells and its
potential relation to the PI3K/Akt/Rac1 signaling pathway (20). To assess the association between
the antimigratory effects of capsaicin and the PI3K/Akt signaling
pathway, the expression levels of the proteins involved in the
signaling pathway were measured via western blotting. The results
suggested that >10 µM capsaicin significantly reduced the
expression levels of p-PI3K and p-Akt in MDA-MB-231 breast cancer
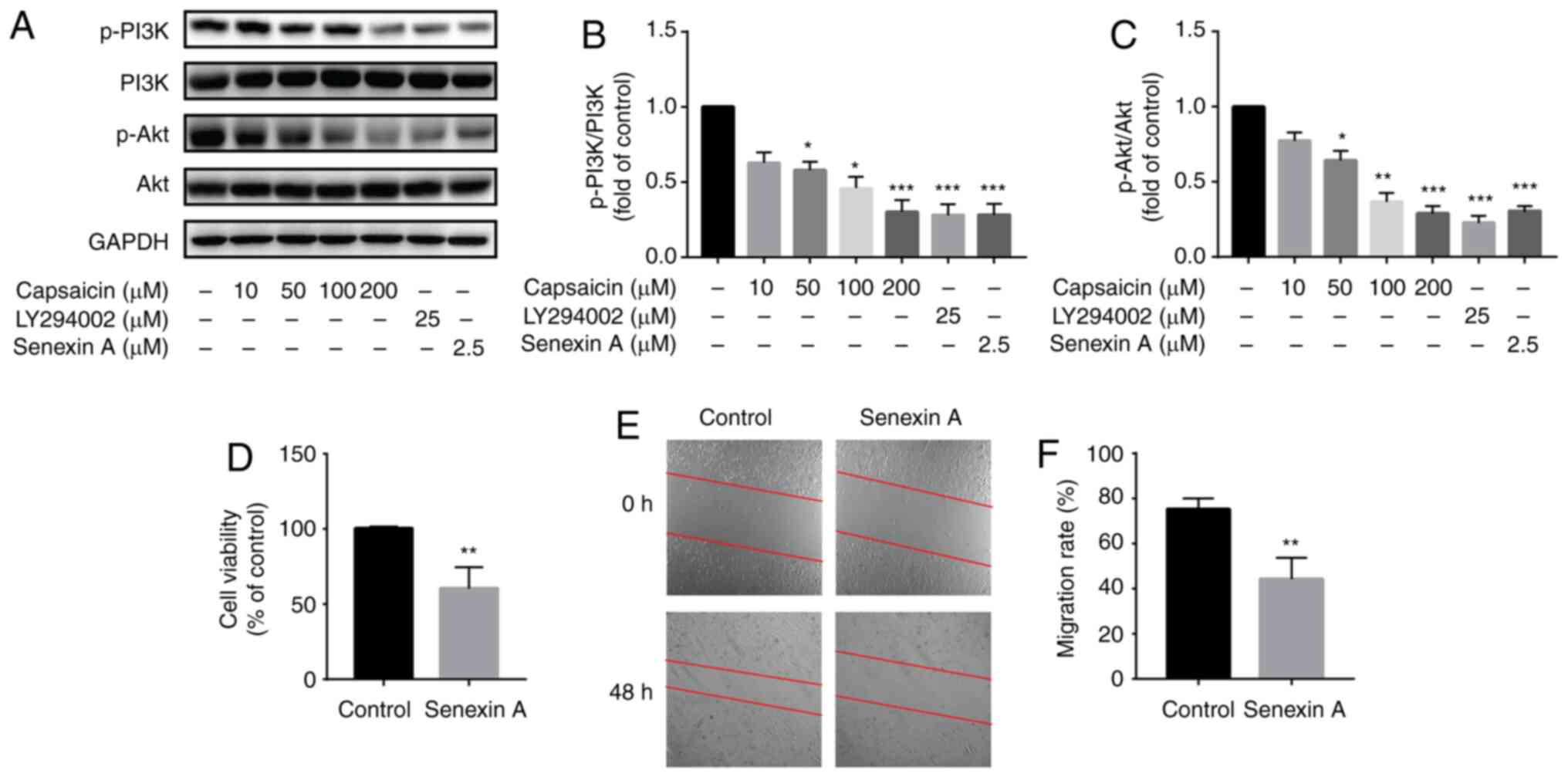
cells compared with the control group (Fig. 5A-C). Furthermore, a specific
inhibitor of PI3K (LY294002; 25 µM) was used to study the
antimigratory effects of capsaicin. Pretreatment with LY294002 for
24 h significantly decreased the expression levels of p-PI3K and
p-Akt in vitro compared with the control group (Fig. 5A-C). CDK8 inhibitor (Senexin A; 2.5
µM) also significantly reduced the expression levels of p-PI3K and
p-Akt in vitro compared with the control group (Fig. 5A-C). Moreover, pretreatment with
Senexin A also significantly inhibited MDA-MB-231 breast cancer
cell viability and migration in vitro compared with the
control group (Fig. 5D-F). The
results indicated that the inhibitory effect of capsaicin on breast
cancer cell viability was associated with suppressing the
CDK8/PI3K/Akt signaling pathway.
Capsaicin significantly inhibits
Wnt/β-catenin signaling in MDA-MB-231 breast cancer cells
Previous studies have indicated that the canonical
Wnt signaling pathway serves an important role in cell
proliferation and differentiation (25,26).
The aforementioned results indicated that capsaicin inhibited cell
viability and migration. Therefore, whether capsaicin inhibited
Wnt/β-catenin signaling in MDA-MB-231 breast cancer cells was
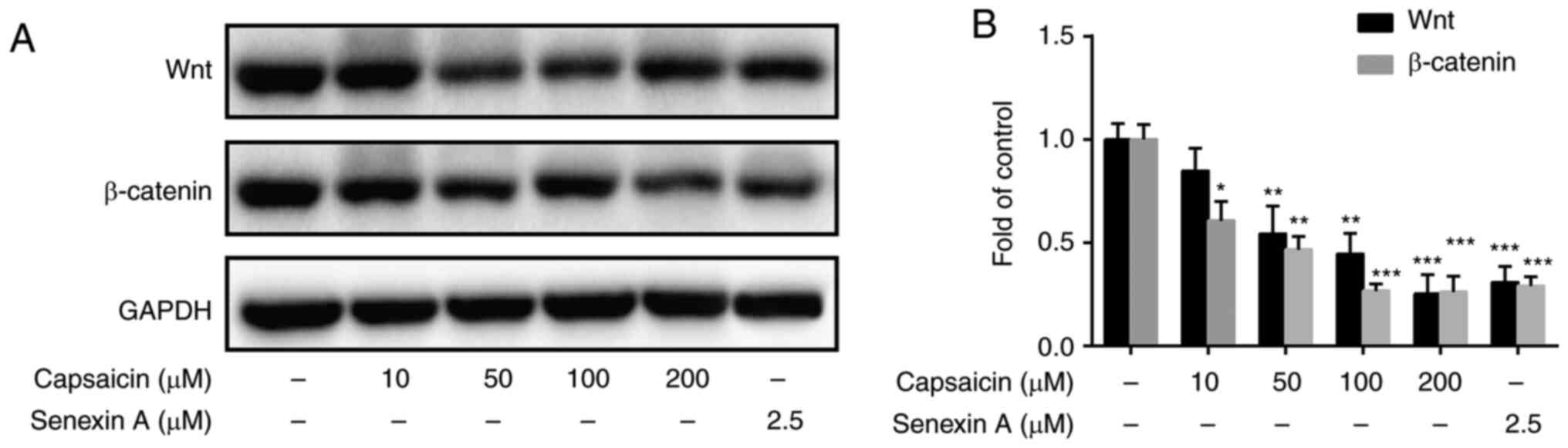
investigated. MDAMB-231 cells were treated with or without
capsaicin for 24 h. The results suggested that 50, 100 and 200 µM
capsaicin significantly decreased the expression levels of Wnt and
β-catenin in MDA-MB-231 cells compared with the control group
(Fig. 6A and B). Moreover,
pretreatment with the CDK8 inhibitor (Senexin A; 2.5 µM) also
significantly decreased the expression levels of Wnt and β-catenin
in vitro compared with the control group (Fig. 6A and B). Collectively, the results
indicated that capsaicin inhibited breast cancer cell migration
potentially via suppressing the CDK8/Wnt/β-catenin signaling
pathway.
Discussion
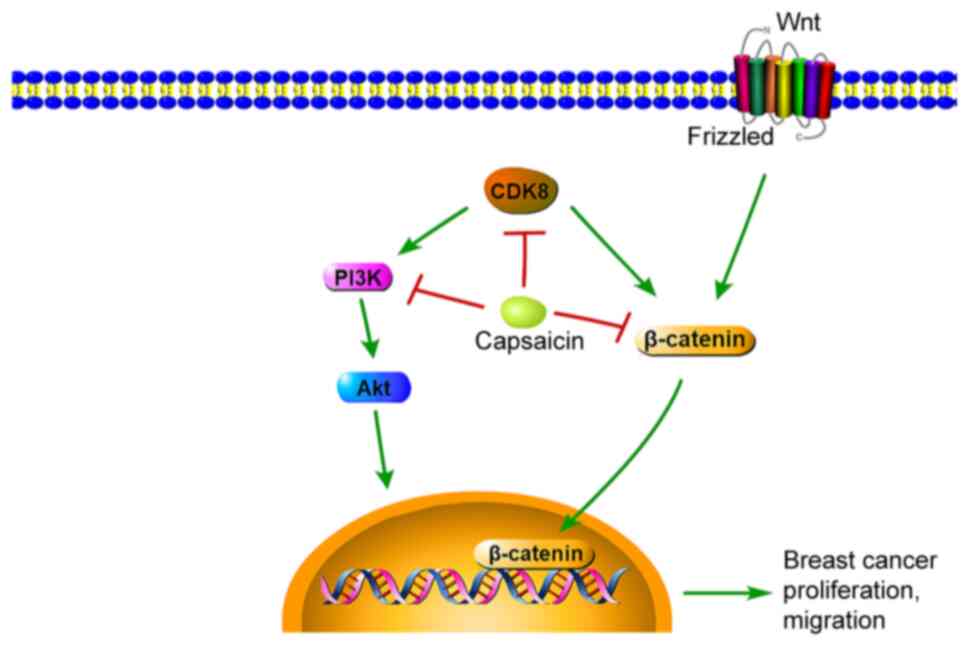
The present study demonstrated that CDK8 was
significantly upregulated in breast cancer cells compared with
healthy breast cells. In addition, the results indicated that,
compared with the control group, capsaicin significantly inhibited
breast cancer cell viability and migration by suppressing CDK8
expression and the PI3K/Akt/Wnt/β-catenin signaling pathway in
vitro (Fig. 7).
CDKs are cell cycle regulator kinases, which can
interact with cyclins and alter CDK inhibitor-mediated cell
cycling. Under stable conditions, CDKs primarily regulate the cell
cycle at two restriction points, G0/G1 and
G2/M phases. However, abnormal CDK expression leads to
loss of control of the two regulatory points, resulting in
proliferating cells continuously entering the cell cycle, thereby
disturbing proliferation, differentiation and apoptosis, and
leading to the occurrence of malignant tumors (5). CDK8 is a member of the CDK family
that promotes cell cycle phase transition, initiates DNA synthesis
and regulates cellular transcription. Moreover, CDK8 also serves an
important role in regulating the cell cycle and cell proliferation
at the transcriptional level (27). Cai et al (28) demonstrated that CDK8 is a key
factor in the development of cervical cancer, and the expression of
CDK8 was gradually increased with the severity of the lesion. In
addition, CDK8 has been reported to affect the occurrence and
development of colorectal cancer metastasis and malignant melanoma
(29). In colorectal cancer, the
β-catenin signaling pathway is usually activated, and CDK8, as the
upstream signaling molecule of β-catenin, promotes not only its
accumulation in the cytoplasm, but also its nuclear transfer
(12). Moreover, Kapoor et
al (30) reported that CDK8
knockout significantly inhibited the proliferation and metastasis
of malignant melanoma cells. In the present study, the mRNA and
protein expression levels of CDK8 were significantly increased in
MDA-MB-231 breast cancer cells compared with MCF10A healthy breast
cells. Furthermore, CDK8 knockdown significantly inhibited breast
cancer cell viability and migration compared with the LV-NC-shRNA
group. Based on the results of the aforementioned studies and the
present study, it was hypothesized that CDK8 may serve as a
therapeutic target for breast cancer, and suppressing CDK8 may
inhibit breast cancer cell proliferation and metastasis.
Capsaicin, a natural compound, displays various
pharmacological properties, including antibacterial, analgesic and
antitumor effects (31).
Clinically, capsaicin has been used as an analgesic in topical
ointments and dermal patches to relieve pain, typically at
concentrations of 0.025–0.1% (32). High-concentration capsaicin patches
(Qutenza) have been approved by the Food and Drug Administration
for the treatment of post-herpetic neuralgia, HIV-neuropathy and
diabetic neuropathy (33). In
recent years, the antitumor activity of capsaicin has been
investigated, and increasing evidence has demonstrated that
capsaicin could inhibit NF-κB and Akt/mTOR signaling pathways in
pancreatic and colon cancer (34,35).
Moreover, previous studies have indicated that capsaicin could
induce breast cancer cell apoptosis via mitochondrial dysfunction
(36). An analogue of capsaicin,
MRS1477, a positive allosteric modulator of transient receptor
potential cation channel subfamily V member 1, has been shown to
reduce MCF7 breast cancer cell viability (37). To further explore the mechanisms
underlying capsaicin in breast cancer, MDA-MB-231 cells were
selected in the present study. The results indicated that, compared
with the control group, capsaicin significantly inhibited
MDA-MB-231 cell viability and migration by inducing G2/M
cell cycle arrest, which may serve as one of the key mechanisms
underlying inhibition of breast cancer cell proliferation. Although
previous studies have reported that capsaicin can induce cell cycle
arrest at the G0/G1 phase (38), other studies have indicated that
capsaicin and its analogs induce cell cycle arrest at the
G2/M phase (39,40).
The inconsistencies between the studies may be due to varied tumor
cell types or inconsistent detection timing.
Breast cancer is a complex disease caused by a
variety of factors that activate multiple signaling pathways,
including the PI3K/Akt/mTOR, RAF/MEK/ERK and endoplasmic reticulum
(ER) stress signaling pathways (41–43).
PI3K is the main intracellular factor in the transmission of cell
migration signals (44). In
addition, Akt, one of the major downstream targets of PI3K,
promotes cancer cell motility and migration in the tumor
microenvironment (45). It has
also been reported that targeting the PI3K/Akt/mTOR signaling
pathway is promising for the treatment of breast cancer (46). In the present study, capsaicin
decreased the expression levels of p-PI3K and p-Akt in MDA-MB-231
cells compared with the control group. Pretreatment with LY294002
or a CDK8 inhibitor also significantly decreased the expression
levels of p-PI3K and p-Akt in vitro. Collectively, these
results indicated that capsaicin-mediated inhibition of breast
cancer cell viability was associated with suppression of the
CDK8/PI3K/Akt signaling pathway.
Wnt signaling is an important signaling pathway
involved in the regulation of cell proliferation, differentiation
and morphogenesis in different organs (47). β-catenin, an important signaling
molecule of the Wnt/β-catenin signaling pathway, moves freely
within cells, contributes to cell-cell adhesions in the membrane
and functions as a transcriptional activator in the nucleus
(12). When the Wnt signal is
activated, β-catenin can be translocated into the nucleus and
participate in normal development or tumorigenesis by regulating
multiple cellular functions (26).
It has previously been demonstrated that CDK8 may act as a positive
regulator of Wnt/β catenin signaling, which can be increased in
several types of human cancer, such as lung, prostate, breast,
liver and colon cancer (48).
Moreover, it has been reported that CDK8 can not only directly
induce the activation of β-catenin-mediated transcription targets
(49), but also indirectly
activate β-catenin-dependent transcription targets by inhibiting
E2F transcription factor 1 (50).
In the present study, compared with the control group, capsaicin
significantly reduced the expression levels of β-catenin in
MDA-MB-231 cells, and pretreatment with CDK8 inhibitor also
markedly decreased the expression of β-catenin in breast cancer
cells.
The present study indicated that capsaicin induced
G2/M cell cycle arrest and inhibited breast cancer cell
viability compared with the control group. In addition, compared
with the control group, capsaicin decreased the expression of CDK8,
and reduced breast cancer cell viability and migration by
inhibiting the PI3K/Akt/Wnt/β-catenin signaling pathway. In
summary, the results of the present study identified a role for
capsaicin in inhibiting breast cancer cell viability by suppressing
the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and HJ designed the study and performed the
experiments. DW, HJ and ZZ collected and analyzed the data. SL
conceptualized the study, drafted the work and revised it
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burney IA, Furrukh M and Al-Moundhri MS:
What are our options in the fight against breast cancer? Sultan
Qaboos Univ Med J. 14:e149–e151. 2014.PubMed/NCBI
|
|
3
|
Akarolo-Anthony SN, Ogundiran TO and
Adebamowo CA: Emerging breast cancer epidemic: Evidence from
Africa. Breast Cancer Res. 12 (Suppl 4):S82010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan H, Gray R, Braybrooke J, Davies C,
Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, et
al: 20-Year risks of breast-cancer recurrence after stopping
endocrine therapy at 5 years. N Engl J Med. 377:1836–1846. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roskoski R Jr: Cyclin-dependent protein
serine/threonine kinase inhibitors as anticancer drugs. Pharmacol
Res. 139:471–488. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asghar U, Witkiewicz AK, Turner NC and
Knudsen ES: The history and future of targeting cyclin-dependent
kinases in cancer therapy. Nat Rev Drug Discov. 14:130–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leake R: The cell cycle and regulation of
cancer cell growth. Ann N Y Acad Sci. 784:252–262. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarita Rajender P, Ramasree D, Bhargavi K,
Vasavi M and Uma V: Selective inhibition of proteins regulating
CDK/cyclin complexes: Strategy against cancer-a review. J Recept
Signal Transduct Res. 30:206–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rzymski T, Mikula M, Wiklik K and Brzozka
K: CDK8 kinase-an emerging target in targeted cancer therapy.
Biochim Biophys Acta. 1854:1617–1629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xi M, Chen T, Wu C, Gao X, Wu Y, Luo X, Du
K, Yu L, Cai T, Shen R and Sun H: CDK8 as a therapeutic target for
cancers and recent developments in discovery of CDK8 inhibitors.
Eur J Med Chem. 164:77–91. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pelish HE, Liau BB, Nitulescu II,
Tangpeerachaikul A, Poss ZC, Da Silva DH, Caruso BT, Arefolov A,
Fadeyi O, Christie AL, et al: Mediator kinase inhibition further
activates super-enhancer-associated genes in AML. Nature.
526:273–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Firestein R, Bass AJ, Kim SY, Dunn IF,
Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, et al: CDK8
is a colorectal cancer oncogene that regulates beta-catenin
activity. Nature. 455:547–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donner AJ, Ebmeier CC, Taatjes DJ and
Espinosa JM: CDK8 is a positive regulator of transcriptional
elongation within the serum response network. Nat Struct Mol Biol.
17:194–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trakala M and Malumbres M: Cyclin C
surprises in tumour suppression. Nat Cell Biol. 16:1031–1033. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Philip S, Kumarasiri M, Teo T, Yu M and
Wang S: Cyclin-dependent kinase 8: A new hope in targeted cancer
therapy? J Med Chem. 61:5073–5092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McDermott MS, Chumanevich AA, Lim CU,
Liang J, Chen M, Altilia S, Oliver D, Rae JM, Shtutman M, Kiaris H,
et al: Inhibition of CDK8 mediator kinase suppresses estrogen
dependent transcription and the growth of estrogen receptor
positive breast cancer. Oncotarget. 8:12558–12575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu M, Yu X, Zheng Z, Huang J, Yang X and
Shi H: Capsaicin suppressed activity of prostate cancer stem cells
by inhibition of Wnt/β-catenin pathway. Phytother Res. 34:817–824.
2020. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rollyson WD, Stover CA, Brown KC, Perry
HE, Stevenson CD, McNees CA, Ball JG, Valentovic MA and Dasgupta P:
Bioavailability of capsaicin and its implications for drug
delivery. J Control Release. 196:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linderoth L, Peters GH, Madsen R and
Andresen TL: Drug delivery by an enzyme-mediated cyclization of a
lipid prodrug with unique bilayer-formation properties. Angew Chem
Int Ed Engl. 48:1823–1826. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin DH, Kim OH, Jun HS and Kang MK:
Inhibitory effect of capsaicin on B16-F10 melanoma cell migration
via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp
Mol Med. 40:486–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Menzl I, Witalisz-Siepracka A and Sexl V:
CDK8-Novel therapeutic opportunities. Pharmaceuticals (Basel).
12:922019. View Article : Google Scholar
|
|
23
|
He L, Lu N, Dai Q, Zhao Y, Zhao L, Wang H,
Li Z, You Q and Guo Q: Wogonin induced G1 cell cycle arrest by
regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in
human colorectal cancer carcinoma cells. Toxicology. 312:36–47.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu D, Li CF, Zhang X, Gong Z, Chan CH, Lee
SW, Jin G, Rezaeian AH, Han F, Wang J, et al: Skp2-macroH2A1-CDK8
axis orchestrates G2/M transition and tumorigenesis. Nat Commun.
6:66412015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li K, Pan WT, Ma YB, Xu XL, Gao Y, He YQ,
Wei L and Zhang JW: BMX activates Wnt/β-catenin signaling pathway
to promote cell proliferation and migration in breast cancer.
Breast Cancer. 27:363–371. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galbraith MD, Donner AJ and Espinosa JM:
CDK8: A positive regulator of transcription. Transcription. 1:4–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai WS, Shen F, Feng Z, Chen JW, Liu QC,
Li EM, Xu B and Cao J: Downregulation of CDK-8 inhibits colon
cancer hepatic metastasis by regulating Wnt/β-catenin pathway.
Biomed Pharmacother. 74:153–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu W and Ji JY: Dysregulation of CDK8 and
cyclin C in tumorigenesis. J Genet Genomics. 38:439–452. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kapoor A, Goldberg MS, Cumberland LK,
Ratnakumar K, Segura MF, Emanuel PO, Menendez S, Vardabasso C,
Leroy G, Vidal CI, et al: The histone variant macroH2A suppresses
melanoma progression through regulation of CDK8. Nature.
468:1105–1109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Tharakan ST, Sung B and Anand P: Potential of spice-derived
phytochemicals for cancer prevention. Planta Med. 74:1560–1569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fattori V, Hohmann MS, Rossaneis AC,
Pinho-Ribeiro FA and Verri WA: Capsaicin: Current understanding of
its mechanisms and therapy of pain and other pre-clinical and
clinical uses. Molecules. 21:8442016. View Article : Google Scholar
|
|
33
|
Sharma SK, Vij AS and Sharma M: Mechanisms
and clinical uses of capsaicin. Eur J Pharmacol. 720:55–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bessler H and Djaldetti M: Capsaicin
modulates the immune cross talk between human mononuclears and
cells from two colon carcinoma lines. Nutr Cancer. 69:14–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang JH, Lai FJ, Chen H, Luo J, Zhang RY,
Bu HQ, Wang ZH, Lin HH and Lin SZ: Involvement of the
phosphoinositide 3-kinase/Akt pathway in apoptosis induced by
capsaicin in the human pancreatic cancer cell line PANC-1. Oncol
Lett. 5:43–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang HC, Chen ST, Chien SY, Kuo SJ, Tsai
HT and Chen DR: Capsaicin may induce breast cancer cell death
through apoptosis-inducing factor involving mitochondrial
dysfunction. Hum Exp Toxicol. 30:1657–1665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nazıroğlu M, Çiğ B, Blum W, Vizler C,
Buhala A, Marton A, Katona R, Jósvay K, Schwaller B, Oláh Z and
Pecze L: Targeting breast cancer cells by MRS1477, a positive
allosteric modulator of TRPV1 channels. PLoS One. 12:e01799502017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qian K, Wang G, Cao R, Liu T, Qian G, Guan
X, Guo Z, Xiao Y and Wang X: Capsaicin suppresses cell
proliferation, induces cell cycle arrest and ROS production in
bladder cancer cells through FOXO3a-mediated pathways. Molecules.
21:14062016. View Article : Google Scholar
|
|
39
|
Lin CH, Lu WC, Wang CW, Chan YC and Chen
MK: Capsaicin induces cell cycle arrest and apoptosis in human KB
cancer cells. BMC Complement Altern Med. 13:462013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de-Sá-Júnior PL, Pasqualoto KFM, Ferreira
AK, Tavares MT, Costa Bernstorff Damião MCF, de Azevedo RA, Dias
Câmara DA, Pereira A, de Souza DM and Filho RP: RPF101, a new
capsaicin-like analogue, disrupts the microtubule network
accompanied by arrest in the G2/M phase, inducing apoptosis and
mitotic catastrophe in the MCF-7 breast cancer cells. Toxicol Appl
Pharmacol. 266:385–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma VR, Gupta GK and Sharma AK, Batra
N, Sharma DK, Joshi A and Sharma AK: PI3K/Akt/mTOR intracellular
pathway and breast cancer: Factors, mechanism and regulation. Curr
Pharm Des. 23:1633–1638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nougarede A, Popgeorgiev N, Kassem L,
Omarjee S, Borel S, Mikaelian I, Lopez J, Gadet R, Marcillat O,
Treilleux I, et al: Breast cancer targeting through inhibition of
the endoplasmic reticulum-based apoptosis regulator Nrh/BCL2L10.
Cancer Res. 78:1404–1417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi L, Wu Z, Miao J, Du S, Ai S, Xu E,
Feng M, Song J and Guan W: Adenosine interaction with adenosine
receptor A2a promotes gastric cancer metastasis by enhancing
PI3K-AKT-mTOR signaling. Mol Biol Cell. 30:2527–2534. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ghosh JC, Seo JH, Agarwal E, Wang Y,
Kossenkov AV, Tang HY, Speicher DW and Altieri DC: Akt
phosphorylation of mitochondrial Lonp1 protease enables oxidative
metabolism and advanced tumor traits. Oncogene. 38:6926–6939. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo Y and Pei X: Tetrandrine-induced
autophagy in MDA-MB-231 triple-negative breast cancer cell through
the inhibition of PI3K/AKT/mTOR signaling. Evid Based Complement
Alternat Med. 2019:75174312019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kleszcz R: The canonical Wnt pathway.
Postepy Biochem. 65:183–192. 2019.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim MY, Han SI and Lim SC: Roles of
cyclin-dependent kinase 8 and β-catenin in the oncogenesis and
progression of gastric adenocarcinoma. Int J Oncol. 38:1375–1383.
2011.PubMed/NCBI
|
|
49
|
Firestein R, Shima K, Nosho K, Irahara N,
Baba Y, Bojarski E, Giovannucci EL, Hahn WC, Fuchs CS and Ogino S:
CDK8 expression in 470 colorectal cancers in relation to
beta-catenin activation, other molecular alterations and patient
survival. Int J Cancer. 126:2863–2873. 2010.PubMed/NCBI
|
|
50
|
Broude EV, Gyorffy B, Chumanevich AA, Chen
M, McDermott MSJ, Shtutman M, Catroppo JF and Roninson IB:
Expression of CDK8 and CDK8-interacting genes as potential
biomarkers in breast cancer. Curr Cancer Drug Targets. 15:739–749.
2015. View Article : Google Scholar : PubMed/NCBI
|