Introduction
Diabetes is one of the most prevalent and costly
chronic diseases worldwide, and has increased the morbidity of
cardiovascular, cerebrovascular and peripheral arterial diseases
(1,2). The prevalence of adults with diabetes
around the world in 2014 was 8.5% and in 2016, diabetes results in
1.6 million deaths (3,4). Due to the increasingly aggressive and
accelerated course of atherosclerosis in diabetes, patients have a
greater probability of having strokes. In 2010, stroke was the
second leading cause of death in patients >60 years old and the
fifth leading cause of death in people aged 15–59 years worldwide
(5,6). As a result, diabetic patients with
ischemic stroke undergo more revascularization procedures compared
with the general population (7).
Vascular interventions have several advantages, including
microtrauma, short procedural duration and quick recovery, compared
with carotid endarterectomy surgery, making them an essential
treatment for carotid artery stenosis or occlusion (7). However, neointimal hyperplasia
following the procedure, including balloon angioplasty and stenting
is a common problem in patients with diabetes (8–10).
Insulin affects neointimal hyperplasia via distinct
signaling pathways (10–12). In vascular tissues, insulin
stimulates two major signaling pathways: The phosphatidylinositol
3-kinase (PI3K)/protein kinase B (Akt) and mitogen-activated
protein kinase (MAPK)/extracellular signal-regulated kinase (ERK)
pathways (13). PI3K activation is
essential for insulin-mediated glucose uptake, cell survival and
nitric oxide (NO) production, while MAPK activation stimulates
cellular proliferation and migration, and prothrombotic and
proinflammatory responses (8). In
the state of insulin resistance following balloon or stent injury,
excess insulin stimulates signaling from the PI3K/Akt pathway to
the MAPK/ERK pathway, and is involved in the endothelial production
of NO and vascular smooth muscle cell (VSMC) proliferation and
migration (11,14). Furthermore, a higher ratio of
phosphorylated (p-)ERK/total ERK to p-Akt/total Akt was associated
with increased neointimal hyperplasia following vascular injury
(11). Thus, it is evident that
insulin is, at least in part, responsible for enhanced neointimal
hyperplasia in insulin-resistant or type 2 diabetic models
secondary to MAPK activation and/or PI3K impairment. However, given
the different metabolic environments exhibited by patients with
type 1 vs. type 2 diabetes, the detailed signaling pathways
regulating neointimal hyperplasia in type 1 diabetes remain
unclear.
Thus, we hypothesized that the PI3K/Akt or MAPK/ERK
pathway regulated neointimal hyperplasia following arterial injury
in type 1 diabetes, with or without insulin therapy. The
preferential signaling along the PI3K/Akt pathway of insulin action
in response to insulin deficiency may be involved. The current
study performed in vitro cellular experiments and
constructed an in vivo rat model of neointimal hyperplasia
in type 1 diabetes, in which the roles of the PI3K/Akt and MAPK/ERK
pathways were investigated. The present study provided a novel
approach for the reduction of neointimal hyperplasia in type 1
diabetes.
Materials and methods
Animal model
The rats used in the current study were from the
same strain as those used in our previous studies on type 1
diabetes (15,16). A total of 30 male Sprague-Dawley
(age, 11 weeks; weight, ~300 g) rats were maintained at the Animal
Centre of Jinling Hospital (Nanjing, China). Rats were housed at
room temperature with 12-h light/dark cycles, 60±5% relative
humidity, and free access to food and water in a pathogen-free
animal facility. Rats were randomly selected for a single
intraperitoneal injection of streptozotocin (STZ; Sigma-Aldrich;
Merck KGaA; 60 mg/kg dissolved in pH 4.2 citrate buffer; n=22) or
citrate buffer alone (control group; n=8) (15). A total of 19 STZ-treated rats
(19/22) with a fasting blood glucose >16.67 mmol/l (300 mg/dl),
which typically exhibits within 5 days of STZ injection, were
considered as type 1 diabetic rats (8,15).
Rats (n=3) with a low blood glucose (<16.67 mmol/l) within 7
days of STZ injection were excluded from the subsequent
experiments. A subset of the type 1 diabetic rats (9/19; at random)
received insulin glargine (Sanofi SA; 3 units; STZ + I group;
controlled type 1 diabetes; n=9) daily via subcutaneous injection
once hyperglycemia was detected. This treatment was continued daily
for days prior to the establishment of the rat carotid injury model
(8,10). Insulin therapy in this group was
continued for the remaining 2 weeks following surgery, with each
rat receiving daily insulin administration for a total of 21 days.
The other subset of the type 1 diabetic rats (10/19) without
insulin administration were considered as uncontrolled type 1
diabetes (STZ group; n=10).
Animal surgery
At ~day 14 following STZ injections, all 27 rats
underwent surgery for the carotid artery balloon injury model. All
animal procedures were performed according to the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health (NIH; publication no. 85–23; 1996) and
approved by the Institutional Animal Care and Use Committee of
Nanjing University (Nanjing, China). Rats were anesthetized with
inhaled isoflurane (2–3% induction; 0.5–1.5% maintenance) (8,17).
Atropine was administered subcutaneously (0.1 mg/kg) to decrease
airway secretions. The neck was shaved and prepped with betadine
and alcohol (75%). Following a midline neck incision, the rat
carotid artery balloon injury model was performed using a 2F
Fogarty catheter (Edwards Lifesciences), as previously described
(8,12,18).
Following injury and restoration of blood flow, the neck incision
was closed. A total of 5 rats (1 in the control group and 2 in the
STZ and STZ + I groups) died of cardiopulmonary arrest during
surgery. Additionally, 4 rats (1 in the control group, 2 in the STZ
group and 1 in the STZ + I group) died of systemic embolism or
serious infection during the follow-up period. A total of 18
surviving rats were euthanized at day 14 post-surgery by exposure
to CO2 for 5 min (displacement rate, 20% of home cage
volume/minute). Following this, cervical dislocation (rats weighing
<200 g) or decapitation (>200 g) were performed under
CO2 anesthesia. Presumed death was confirmed based on
unambiguous signs of death, including cardiopulmonary arrest and/or
fixed dilated pupils.
Morphometric analysis
Carotid arteries harvested at 2 weeks post-surgery
were examined histologically for evidence of neointimal hyperplasia
using routine hematoxylin-eosin staining. Briefly, the vessels were
fixed with 4% paraformaldehyde at 4°C overnight. Then, the fixed
vessels were embedded in paraffin, and 5-µm thick sections were cut
and mounted on slides. The paraffin sections were stained with
hematoxylin and eosin as previously described (15). Representative images of the aorta
from the rats were observed under a light microscope
(magnifications, ×10 and ×20). Both the intimal and medial areas
were measured using ImageJ software (version 1.46r; National
Institutes of Health) with uniform arbitrary units for subsequent
calculation of the intima-to-media area (I/M) ratios.
Proliferation assay
VSMCs, isolated from rat carotid arteries as
previously described (19), were
characterized by smooth muscle cell morphology (multilayer sheets;
‘hills and valleys’) and smooth muscle α-actin expression (19). A VSMC proliferation assay was
conducted according to previous studies (19,20).
Briefly, primary VSMCs were plated in 12-well plates
(5×104 cells/well) and cultured at 37°C for 24 h in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). The cells were then
exposed to serum-free media containing tritiated (3H)
thymidine (1 µCi/ml; China Institute of Atomic Energy,), glucose
and/or bovine insulin for an additional 24 h. Exposing VSMCs to
normal or high glucose (5 or 25 mM) and/or normal insulin (100 nM)
concentrations for 24 h mimicked starved, normal, uncontrolled or
controlled type 1 diabetes, respectively, as per previous studies
(8,21) (Table
SI). [3H]thymidine incorporation into
trichloroacetic acid-precipitated DNA was quantified by
scintillation counting using a liquid scintillation counter
(Beckman LS6500; Beckman Coulter, Inc.). Sorbitol (25 mM) was used
as an osmotic control for all experiments.
Immunoblotting
Immunoblotting was performed as previously described
(22,23). Briefly, total proteins were
extracted from the carotid arteries or VSMCs treated without
[3H thymidine using RIPA Lysis Buffer (Beyotime
Institute of Biotechnology). Protein concentrations were determined
using the BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Samples containing 50 µg protein or 10 µl pre-stained molecular
weight marker (cat. no. P0076; Beyotime Institute of Biotechnology)
were separated via 10% SDS-PAGE and electroblotted onto
nitrocellulose membranes (Bio-Rad Laboratories). The membranes were
blocked overnight with 5% nonfat dry milk in PBS-T [0.05% Tween-20
in 10 mmol/l PBS] at 4°C with constant shaking. The blots were
probed with primary monoclonal rabbit anti-rat ERK (1:800; cat. no.
4695), Akt (1:800; cat. no. 4685), p-ERK (1:600; cat. no. 4370),
p-Akt (1:600; cat. no. 4060) or GAPDH (1:1,000; cat. no. 5174)
antibodies (all Cell Signaling Technology) overnight at 4°C.
Subsequently, the membranes were incubated with polyclonal
IRDye® 800CW-labeled goat anti-rabbit IgG secondary
antibodies (1:10,000; cat. no. 102673-300; LI-COR Biosciences) for
1 h at room temperature in dark. Proteins were visualized using an
infrared imaging system (LI-COR Biosciences). The density of each
sample was calculated using Odyssey software (version 3.0; LI-COR
Biosciences). The ratio of p-ERK/ERK to p-Akt/Akt in the carotid
arteries was also calculated.
Blood chemistry assay
Blood glucose levels were screened on alternate days
for 1 week following injection of STZ and twice weekly thereafter.
Blood glucose was measured with a standardized, portable glucometer
(Johnson & Johnson) via puncture of the tail vein. Serum
samples were collected from non-fasted animals at death and frozen
at −20°C until assay. Insulin levels were determined by
radioimmunoassay with an antibody (cat. no. SRI-13K; 1:1; Linco;
EMD Millipore) made specifically against rat insulin. The rat
insulin antibody had 100% cross-reactivity with human insulin.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 25.0; IBM Corp.). Data are expressed as the mean
± standard deviation. Comparisons between groups were performed
using one-way analysis of variance (ANOVA) followed by Duncan's and
Tukey's (>3 groups) post-hoc tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Metabolic parameters of SD rats
A total of 18, or two-thirds, of the rats survived
until euthanasia, which was consistent with a published model of
arterial injury in diabetic rats (10). The type 1 diabetic rats without
exogenous insulin administration exhibited significant weight loss,
increased blood glucose and decreased insulin. Blood glucose was
also significantly increased at 72 h after STZ treatment in the STZ
+ I group compared with the control group. The sample size, age,
weight, blood glucose and insulin levels in each group are
presented in Table I.
 | Table I.Metabolic parameters of rats
(n=6). |
Table I.
Metabolic parameters of rats
(n=6).
| Characteristic | Control | STZ | STZ + I |
|---|
| Initial age,
weeks | 11 | 11 | 11 |
| Initial weight,
g | 313±10 | 315±8 | 310±8 |
| Final weight,
g | 415±21 | 289±9a | 413±16 |
| Glucose, 72 h after
STZ treatment, mM | 6.1±0.6 |
20.8±3.0a |
20.4±3.3a |
| Glucose, 2 weeks
after surgery, mM | 6.6±0.4 |
21.1±2.2a | 7.3±0.5 |
| Insulin, 2 weeks
after surgery, ng/ml | 4.32±0.47 |
0.83±0.15a | 3.93±0.19 |
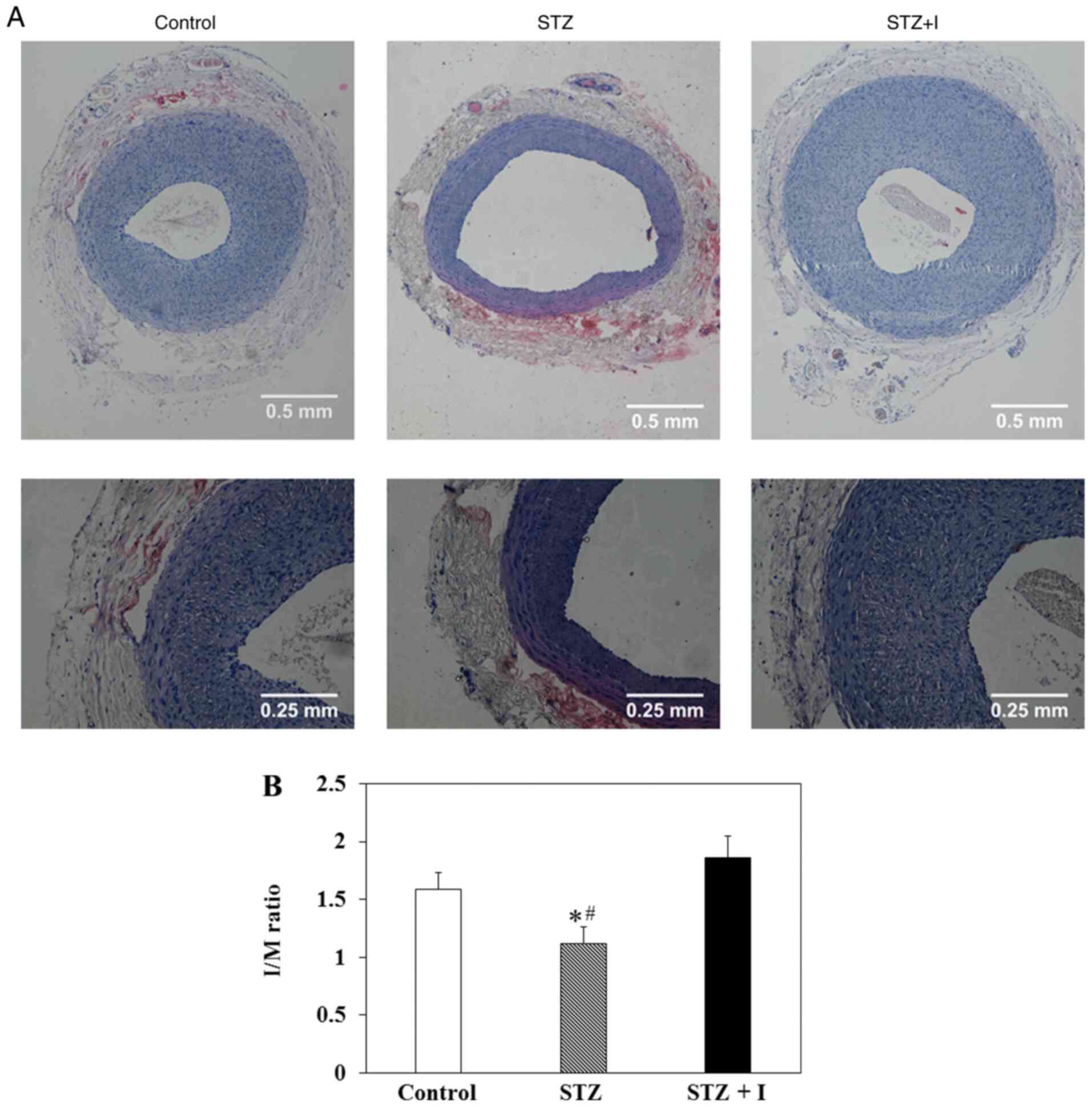
Enhanced neointimal hyperplasia in STZ
+ I rats
The representative images of balloon-injured carotid
artery in SD rats treated with controls, STZ and STZ + I stained
with hematoxylin-eosin at day 14 post-surgery are presented in
Fig. 1A. The upper and lower
images were observed under a microscope at magnifications of ×10
and ×20, respectively, exhibiting the neointimal hyperplasia
following arterial injury (Fig.
1A). The results demonstrated that the I/M ratios were
significantly decreased in the carotid arteries of the STZ rats
compared with those in the controls (Fig. 1B). These levels were recovered and
slightly increased in STZ + I rats (Fig. 1B); however, this increase was not
significant.
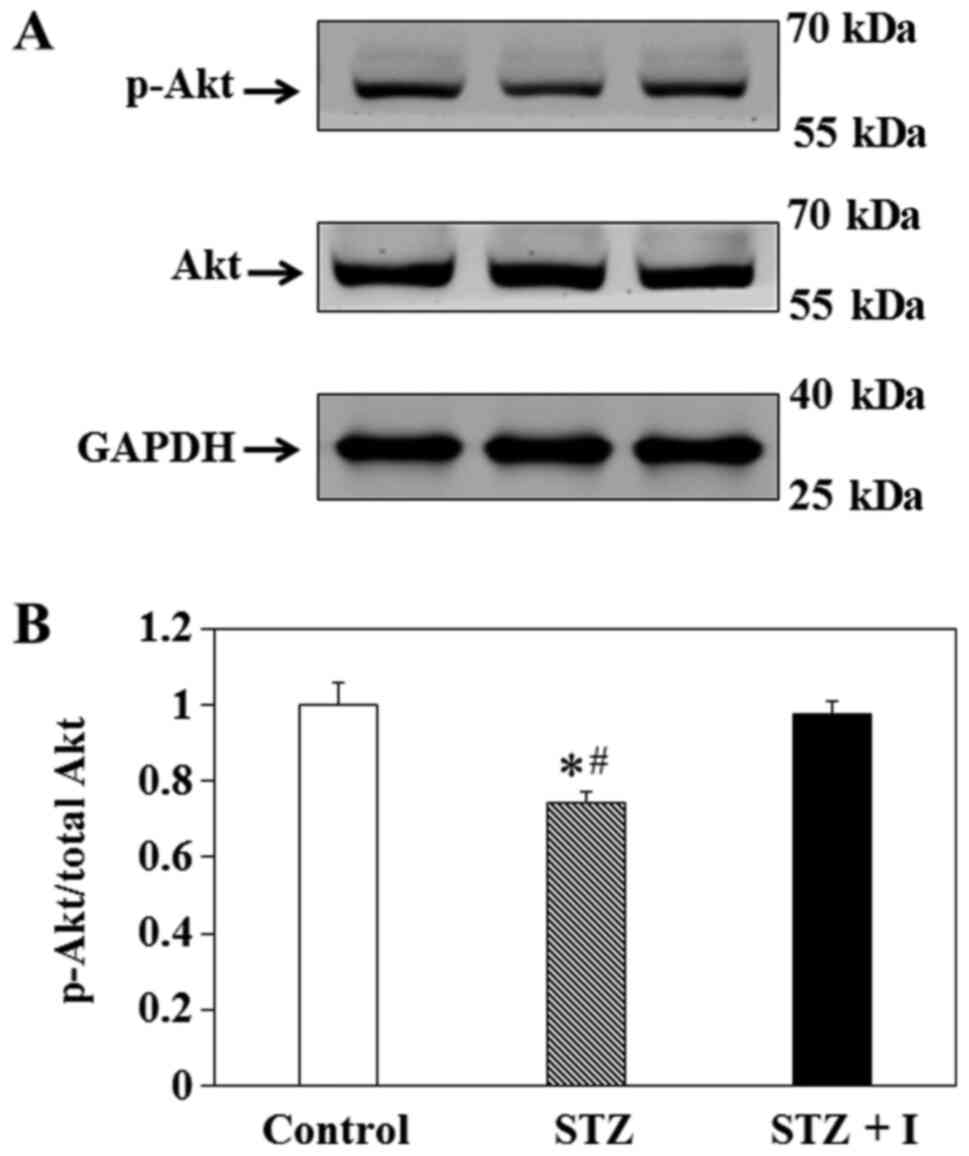
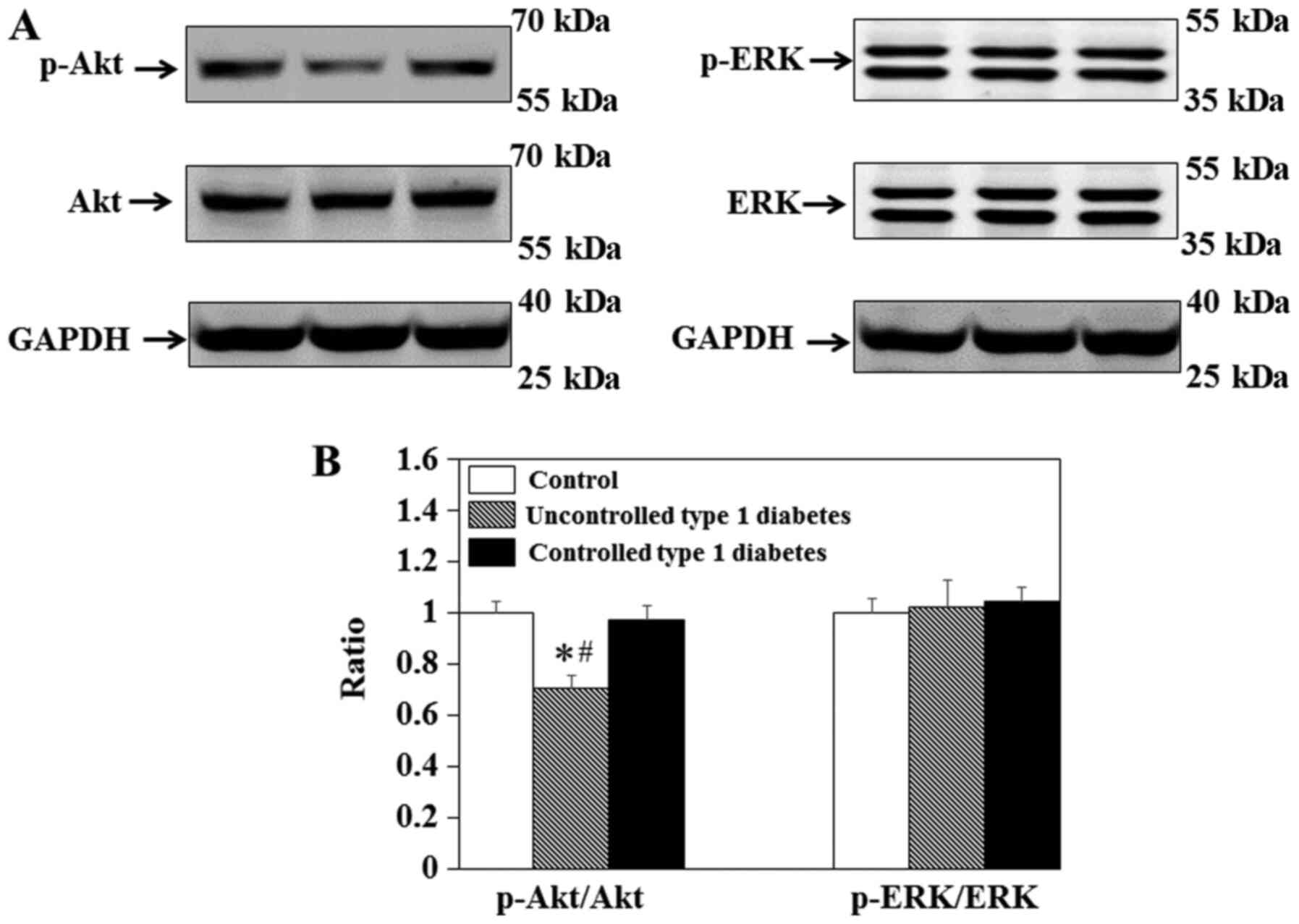
Effect of insulin on Akt expression in
balloon-injured carotid arteries
To elucidate the effect of insulin on the PI3K/Akt
pathway in vivo, immunoblotting was used to measure the
levels of p-Akt and total Akt in the carotid arteries of the rats
(Fig. 2A). The results
demonstrated that p-Akt/total Akt in the carotid arteries of STZ
rats was significantly decreased compared with that in the control
(Fig. 2B). This effect was
ameliorated by insulin in the STZ + I group (Fig. 2B).
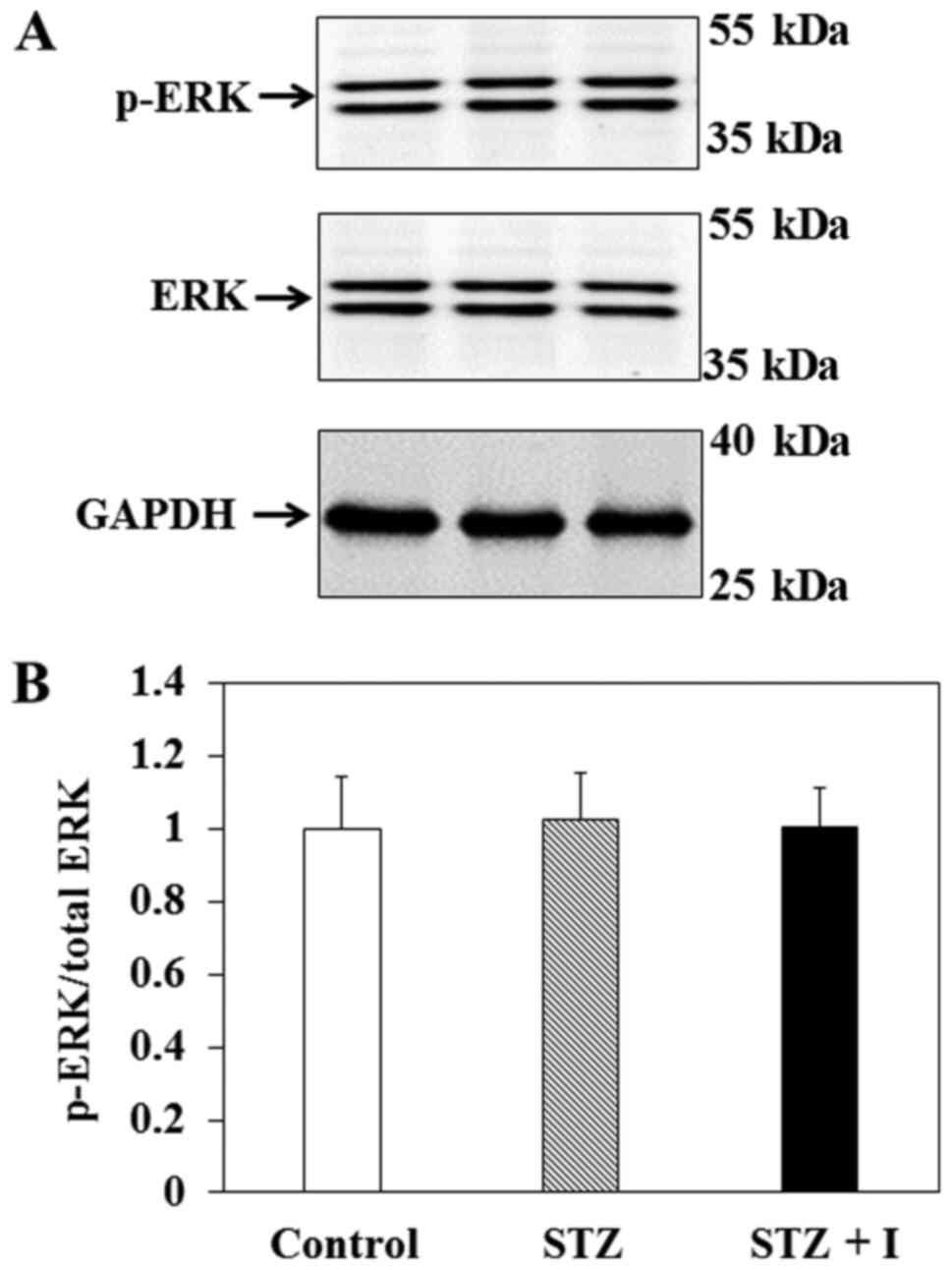
Effect of insulin on ERK expression in
balloon-injured carotid arteries
To elucidate the influence of insulin on the
MAPK/ERK pathway in vivo, immunoblotting was performed to
measure the levels of p-ERK and total ERK in the carotid arteries
of the rats (Fig. 3A). The results
revealed that p-ERK/ERK in the carotid arteries of SD rats were not
significantly different between groups (Fig. 3B).
Representative migration patterns of the pre-stained
molecular weight marker and target proteins, including Akt, p-Akt,
ERK, p-ERK and GAPDH, as determined by immunoblotting, are
presented in Fig. S1.
Association between the effect of
insulin and the ratio of p-ERK/ERK to p-Akt/Akt
To further clarify the effect of insulin on the
MAPK/ERK and/or PI3K/Akt pathway, the ratio of p-ERK/ERK to
p-Akt/Akt in the carotid arteries was also calculated. The results
demonstrated that the ratio of p-ERK/ERK to p-Akt/Akt was
significantly increased in the STZ group compared with that in the
control group (Fig. S2).
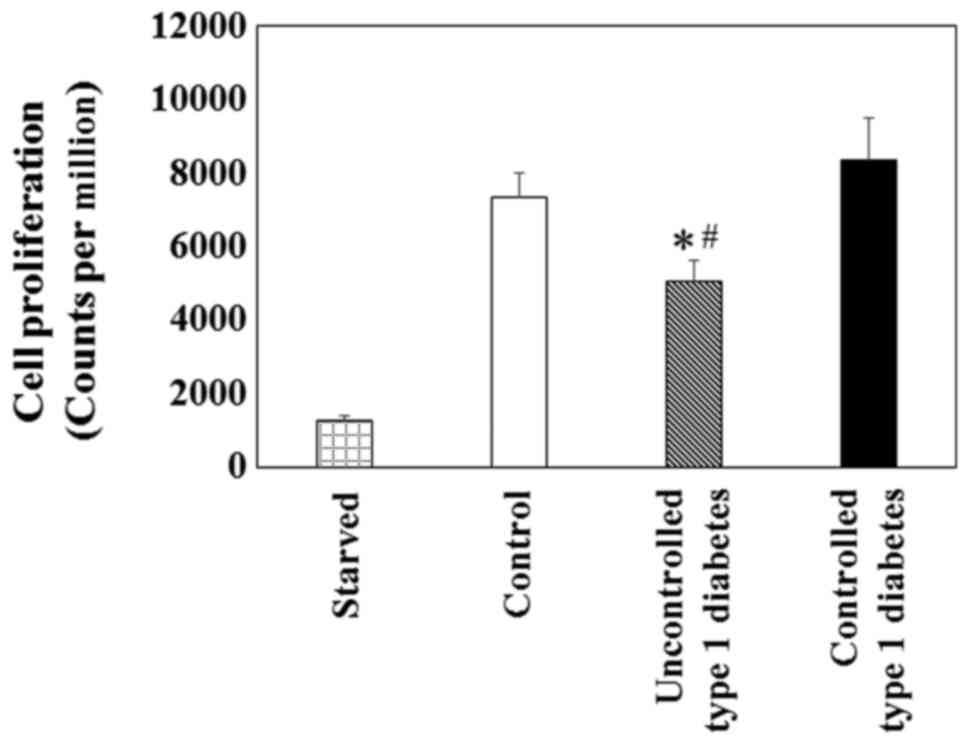
Cell proliferation and signaling
proteins in VSMCs
To investigate VSMC proliferation in the state of
uncontrolled or controlled type 1 diabetes, an in vitro
assay was conducted using [3H]thymidine incorporation to
serve as a surrogate for cell proliferation. Primary VSMCs exposed
to the high glucose (25 mM) in the uncontrolled type 1 diabetes
group demonstrated a significant reduction in proliferation
compared with the low glucose (5 mM) in the control or controlled
type 1 diabetes groups (Fig. 4).
Furthermore, the results demonstrated that the p-Akt/total Akt in
the uncontrolled type 1 diabetes group was significantly decreased
compared with the control or controlled type 1 diabetes groups
(Fig. 5). However, the p-ERK/total
ERK in VSMCs was not significantly different between groups.
Discussion
The results of the current study demonstrated that,
compared with controls, in uncontrolled type 1 diabetes both the
neointimal hyperplasia and expression of p-Akt were significantly
decreased, but were recovered following exogenous insulin
administration in controlled type 1 diabetes. However, the
difference in the expression of p-ERK was not significant between
groups. Furthermore, the cellular results of the in vitro
experiments were consistent with those from the in vitro
animal experiments. These findings indicated that high glucose may
inhibit VSMC proliferation by inhibiting the PI3K/Akt signaling
pathway in type 1 diabetes with low insulin levels, which can be
improved by exogenous insulin supplementation.
Consistent with previous studies, the results of the
current study demonstrated that PI3K/Akt signaling was impaired in
uncontrolled type 1 diabetes, compared with controls, most likely
due to low insulin and subsequent high glucose (11,14,24).
The present study indicated that once the insulin and glucose
levels normalized, the impairment was recovered. Unexpectedly,
neointimal hyperplasia was slightly increased, but not significant,
in controlled type 1 diabetes compared with the controls. A
possible explanation is that Akt activation by abundant insulin in
VSMCs may be necessary for maintaining a quiescent, fully
differentiated phenotype rather than a migratory, proliferative one
(14,21). Furthermore, endothelial production
of NO induced by Akt activation may result in vascular protective
effects (25,26). However, in the setting of insulin
resistance, the balance that normally regulates VSMC
proliferation/migration is disrupted by differentially shunted
signaling from Akt to ERK activation (11). Furthermore, Akt inhibition coupled
with excess insulin may lead to endothelial reduced production of
NO and increased expression of cellular adhesion molecules, such as
CD11a or ICAM-1, with increased monocyte rolling and arrest
(27).
Additionally, the results of the present study
reported that MAPK/ERK signaling was not significantly different in
uncontrolled or controlled type 1 diabetes, with low or normal
insulin levels, respectively. However, as described previously, in
the setting of insulin resistance, the MAPK/ERK signaling pathway
may be overactive, promoting proliferation and migration;
therefore, a higher ratio of p-ERK/ERK to p-Akt/Akt was associated
with increased neointimal hyperplasia following vascular injury
(8,11,14).
The results of the current study demonstrated that the increased
ratio of p-ERK/ERK to p-Akt/Akt did not produce increased
neointimal hyperplasia following vascular injury in the setting of
uncontrolled type 1 diabetes with low insulin. Nevertheless, the
increased ratio of p-ERK/ERK to p-Akt/Akt in controlled type 1
diabetic rats compared with control rats with normal insulin levels
may, at least in part, be associated with enhanced neointimal
hyperplasia following vascular injury, which is consistent with
previously published studies (10,11,14,28).
The current study had limitations. Data were both
similar to (8,28) and different from (10,29)
published studies, indicating that glucose can stimulate and
inhibit VSMC proliferation (8,10,28,29).
Regardless, the current study also observed a direct association
between in vitro and in vivo experiments with regard
to VSMC proliferation and neointimal hyperplasia in type 1 diabetes
with insulin therapy. Notably, the results reported that PI3K/Akt
signaling served a further important role in VSMC proliferation in
the type 1 diabetic model with different insulin levels.
Additionally, the insulin levels in type 1 diabetic rats indicated
the damage to the pancreatic tissue induced by STZ, irrespective of
insulin administration. However, the pancreatic tissue should be
examined to confirm β cell damage in future studies.
The preferential signaling along the PI3K/Akt
pathway of insulin action in response to insulin restoration may
contribute to neointimal hyperplasia. The present study provided a
novel method for further treatment of atherosclerosis in type 1
diabetes.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81400332, 81571148
and 81701229).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC conceived the current study, analyzed data and
wrote the manuscript. HW and HS acquired and analyzed data. RY, YC,
KL and ZQ performed the experiments. MJ, YX, RG and QL analyzed
data and revised the manuscript. XL conceived the current study and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were performed according the
Guide for the Care and Use of Laboratory Animals published by the
NIH (publication no. 85-23; 1996) and approved by the Institutional
Animal Care and Use Committee of Nanjing University, Nanjing,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Akt
|
protein kinase B
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
VSMC
|
vascular smooth muscle cell
|
References
|
1
|
Narayan KM, Boyle JP, Thompson TJ,
Sorensen SW and Williamson DF: Lifetime risk for diabetes mellitus
in the United States. JAMA. 290:1884–1890. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beckman JA, Creager MA and Libby P:
Diabetes and atherosclerosis: Epidemiology, pathophysiology, and
management. JAMA. 287:2570–2581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Yu C, Wang Y, Bi Y, Liu Y and Zhang
ZJ: Trends in the incidence and mortality of diabetes in China from
1990 to 2017: A joinpoint and age-period-cohort analysis. Int J
Environ Res Public Health. 16:1582019. View Article : Google Scholar
|
|
4
|
NCD Risk Factor Collaboration (NCD-RisC),
. Worldwide trends in diabetes since 1980: A pooled analysis of 751
population-based studies with 4.4 million participants. Lancet.
387:1513–1530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang G, Wang Y, Zeng Y, Gao GF, Liang X,
Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, et al: Rapid health
transition in China, 1990–2010: Findings from the global burden of
disease study 2010. Lancet. 381:1987–2015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Wang D, Wong KS and Wang Y: Stroke
and stroke care in China: Huge burden, significant workload, and a
national priority. Stroke. 42:3651–3654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai N and Sakai C: Current indication of
carotid diseases, CEA vs CAS. Nihon Geka Gakkai Zasshi. 111:75–78.
2010.(In Japanese). PubMed/NCBI
|
|
8
|
Varu VN, Ahanchi SS, Hogg ME,
Bhikhapurwala HA, Chen A, Popowich DA, Vavra AK, Martinez J, Jiang
Q, Saavedra JE, et al: Insulin enhances the effect of nitric oxide
at inhibiting neointimal hyperplasia in a rat model of type 1
diabetes. Am J Physiol Heart Circ Physiol. 299:H772–H779. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Y, Wang S, Sun W, Dai Q, Li W, Cai J,
Fan X, Zhu W, Xiong Y, Han Y, et al: Prediction of favorable
outcome by percent improvement in patients with acute ischemic
stroke treated with endovascular stent thrombectomy. J Clin
Neurosci. 38:100–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SH, Marso SP, Zhou Z, Foroudi F,
Topol EJ and Lincoff AM: Neointimal hyperplasia after arterial
injury is increased in a rat model of non-insulin-dependent
diabetes mellitus. Circulation. 104:815–819. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jonas M, Edelman ER, Groothuis A, Baker
AB, Seifert P and Rogers C: Vascular neointimal formation and
signaling pathway activation in response to stent injury in
insulin-resistant and diabetic animals. Circ Res. 97:725–733. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahanchi SS, Varu VN, Tsihlis ND, Martinez
J, Pearce CG, Kapadia MR, Jiang Q, Saavedra JE, Keefer LK, Hrabie
JA and Kibbe MR: Heightened efficacy of nitric oxide-based
therapies in type II diabetes mellitus and metabolic syndrome. Am J
Physiol Heart Circ Physiol. 295:H2388–H2398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
White MF: Insulin signaling in health and
disease. Science. 302:1710–1711. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CC, Gurevich I and Draznin B: Insulin
affects vascular smooth muscle cell phenotype and migration via
distinct signaling pathways. Diabetes. 52:2562–2569. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sang H, Qiu Z, Cai J, Lan W, Yu L, Zhang
H, Li M, Xie Y, Guo R, Ye R, et al: Early increased bradykinin 1
receptor contributes to hemorrhagic transformation after ischemic
stroke in type 1 diabetic rats. Transl Stroke Res. 8:597–611. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sang H, Liu L, Wang L, Qiu Z, Li M, Yu L,
Zhang H, Shi R, Yu S, Guo R, et al: Opposite roles of bradykinin B1
and B2 receptors during cerebral ischaemia-reperfusion injury in
experimental diabetic rats. Eur J Neurosci. 43:53–65. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kenny JD, Chemali JJ, Cotten JF, Van Dort
CJ, Kim SE, Ba D, Taylor NE, Brown EN and Solt K: Physostigmine and
methylphenidate induce distinct arousal states during isoflurane
general anesthesia in rats. Anesth Analg. 123:1210–1219. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shears LL II, Kibbe MR, Murdock AD,
Billiar TR, Lizonova A, Watkins SC and Tzeng E: Efficient
inhibition of intimal hyperplasia by adenovirus-mediated inducible
nitric oxide synthase gene transfer to rats and pigs in vivo. J Am
Coll Surg. 187:295–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Yu C, Han Y, Ren H, Shi W, Fu C, He
D, Huang L, Yang C, Wang X, et al: Inhibitory effect of D1-like and
D3 dopamine receptors on norepinephrine-induced proliferation in
vascular smooth muscle cells. Am J Physiol Heart Circ Physiol.
294:H2761–H2768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Shi W, Luo H, Yue R, Wang Z, Wang
W, Liu L, Wang WE, Wang H and Zeng C: Inhibitory effect of D1-like
dopamine receptors on neuropeptide Y-induced proliferation in
vascular smooth muscle cells. Hypertens Res. 38:807–812. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torella D, Iaconetti C, Tarallo R, Marino
F, Giurato G, Veneziano C, Aquila I, Scalise M, Mancuso T,
Cianflone E, et al: miRNA regulation of the hyperproliferative
phenotype of vascular smooth muscle cells in diabetes. Diabetes.
67:2554–2568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai J, Han Y, Ren H, Chen C, He D, Zhou L,
Eisner GM, Asico LD, Jose PA and Zeng C: Extracellular
vesicle-mediated transfer of donor genomic DNA to recipient cells
is a novel mechanism for genetic influence between cells. J Mol
Cell Biol. 5:227–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: LincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis, and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong Z, Han Y, Wu L, Xia T, Ren H, Yang D,
Gu D, Wang H, Hu C, He D, et al: Translocator protein 18 kDa ligand
alleviates neointimal hyperplasia in the diabetic rat artery injury
model via activating PKG. Life Sci. 221:72–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng G, Nystrom FH, Ravichandran LV, Cong
LN, Kirby M, Mostowski H and Quon MJ: Roles for insulin receptor,
PI3-kinase, and Akt in insulin-signaling pathways related to
production of nitric oxide in human vascular endothelial cells.
Circulation. 101:1539–1545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuboki K, Jiang ZY, Takahara N, Ha SW,
Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ and King
GL: Regulation of endothelial constitutive nitric oxide synthase
gene expression in endothelial cells and in vivo: A specific
vascular action of insulin. Circulation. 101:676–681. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montagnani M, Chen H, Barr VA and Quon MJ:
Insulin-stimulated activation of eNOS is independent of
Ca2+ but requires phosphorylation by Akt at Ser(1179). J
Biol Chem. 276:30392–30398. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Indolfi C, Torella D, Cavuto L, Davalli
AM, Coppola C, Esposito G, Carriero MV, Rapacciuolo A, Di Lorenzo
E, Stabile E, et al: Effects of balloon injury on neointimal
hyperplasia in streptozotocin-induced diabetes and in
hyperinsulinemic nondiabetic pancreatic islet-transplanted rats.
Circulation. 103:2980–2986. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuang D, Pu Q, Ceacareanu B, Chang Y,
Dixit M and Hassid A: Chronic insulin treatment amplifies
PDGF-induced motility in differentiated aortic smooth muscle cells
by suppressing the expression and function of PTP1B. Am J Physiol
Heart Circ Physiol. 295:H163–H173. 2008. View Article : Google Scholar : PubMed/NCBI
|