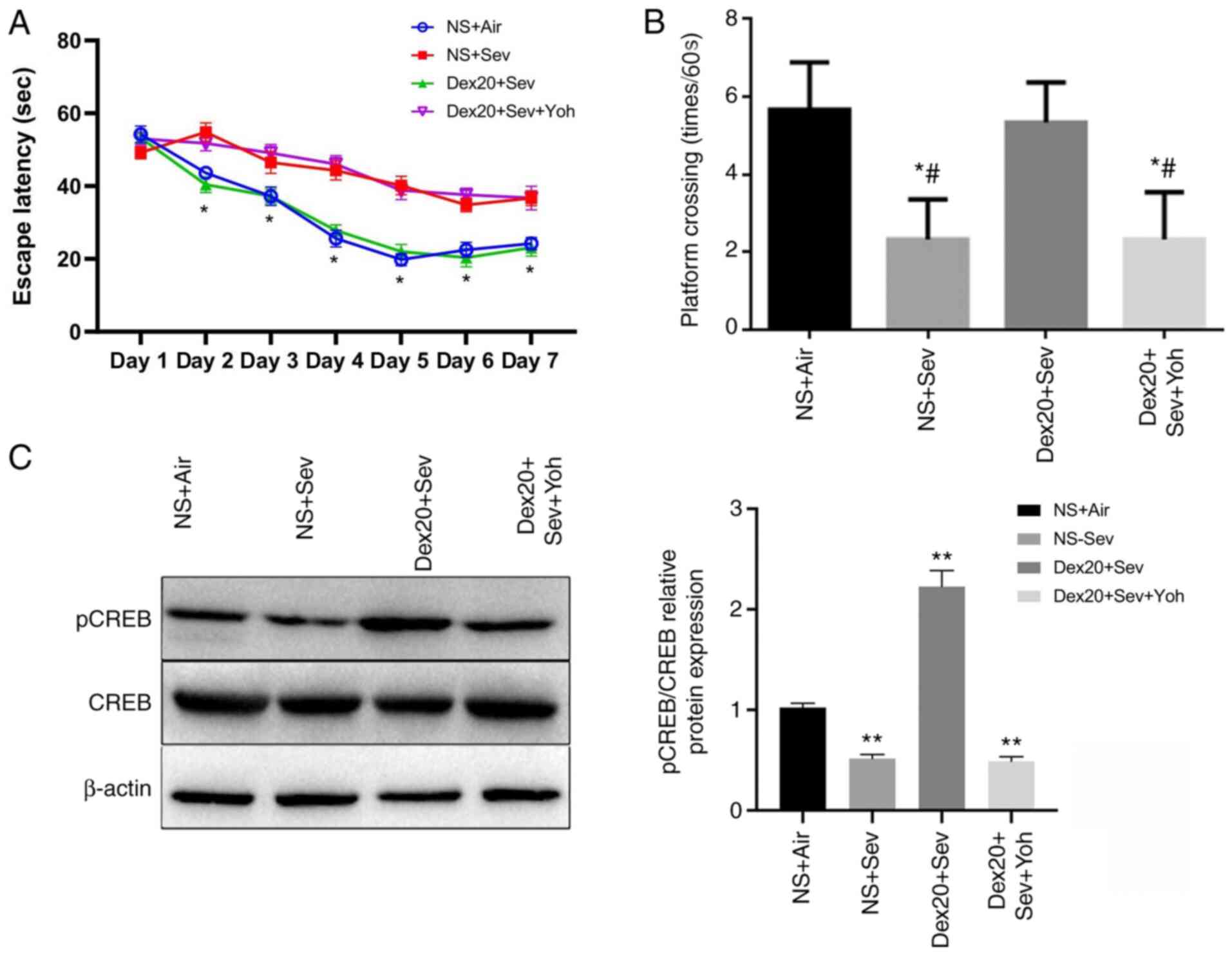
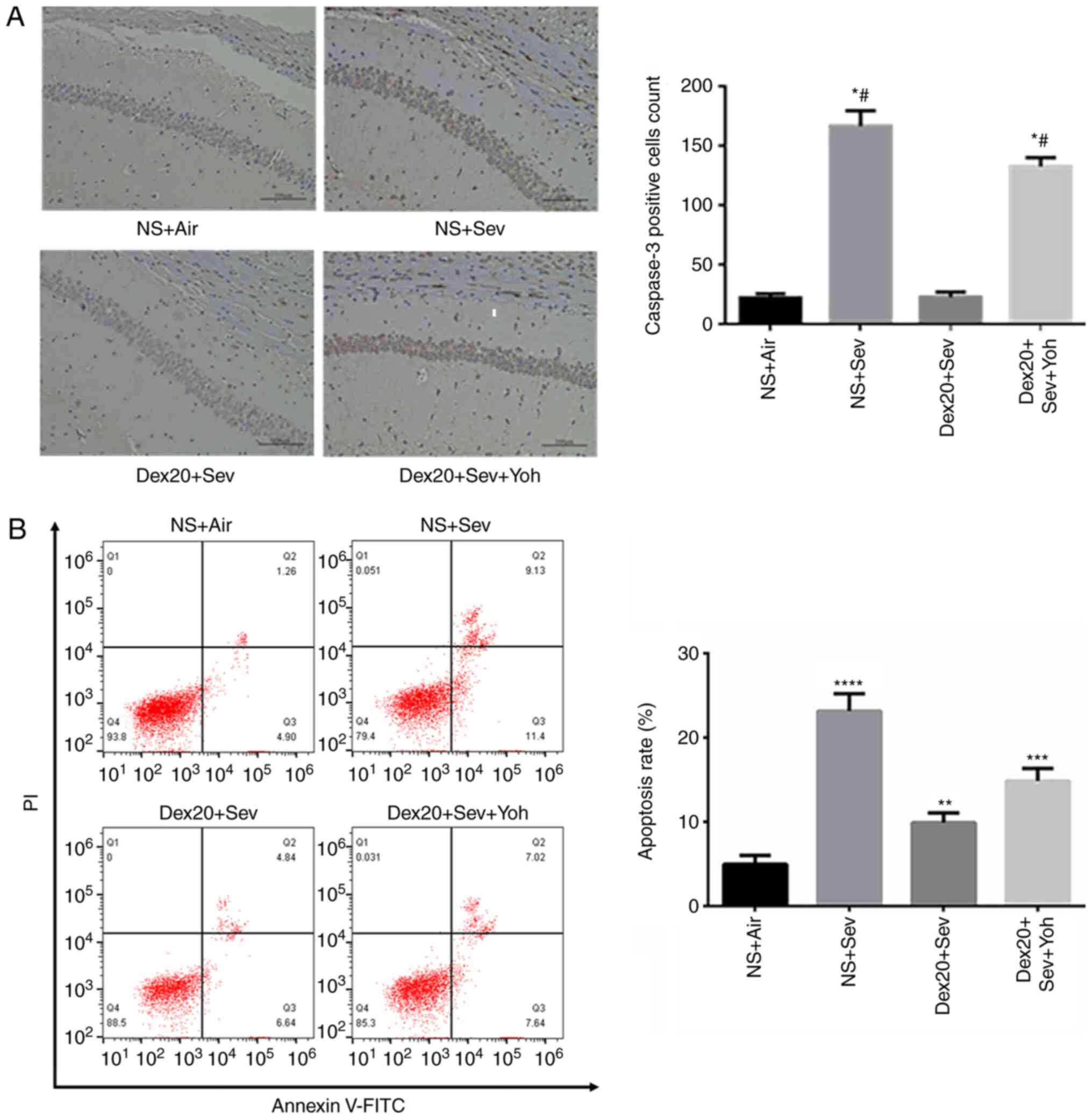
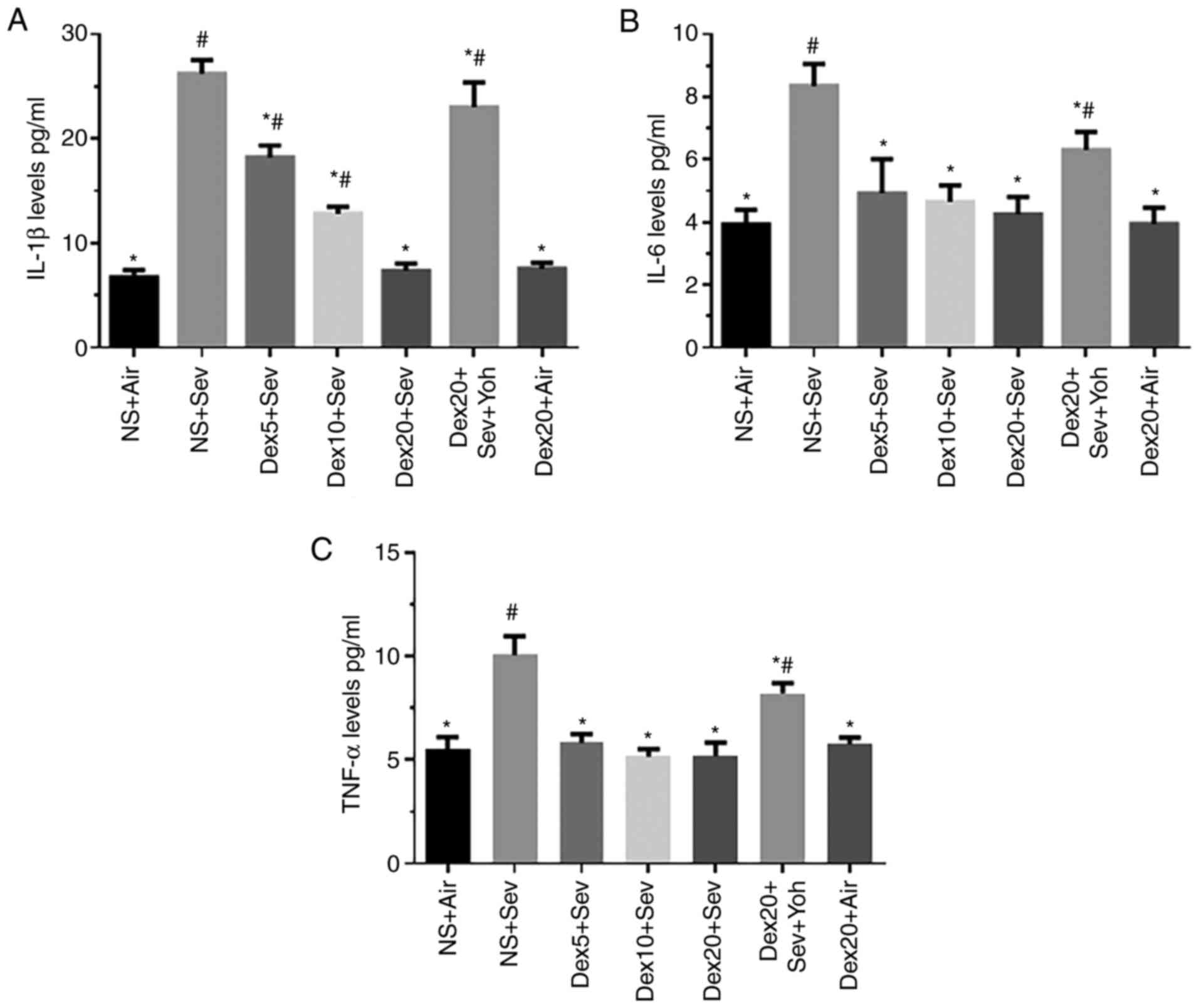
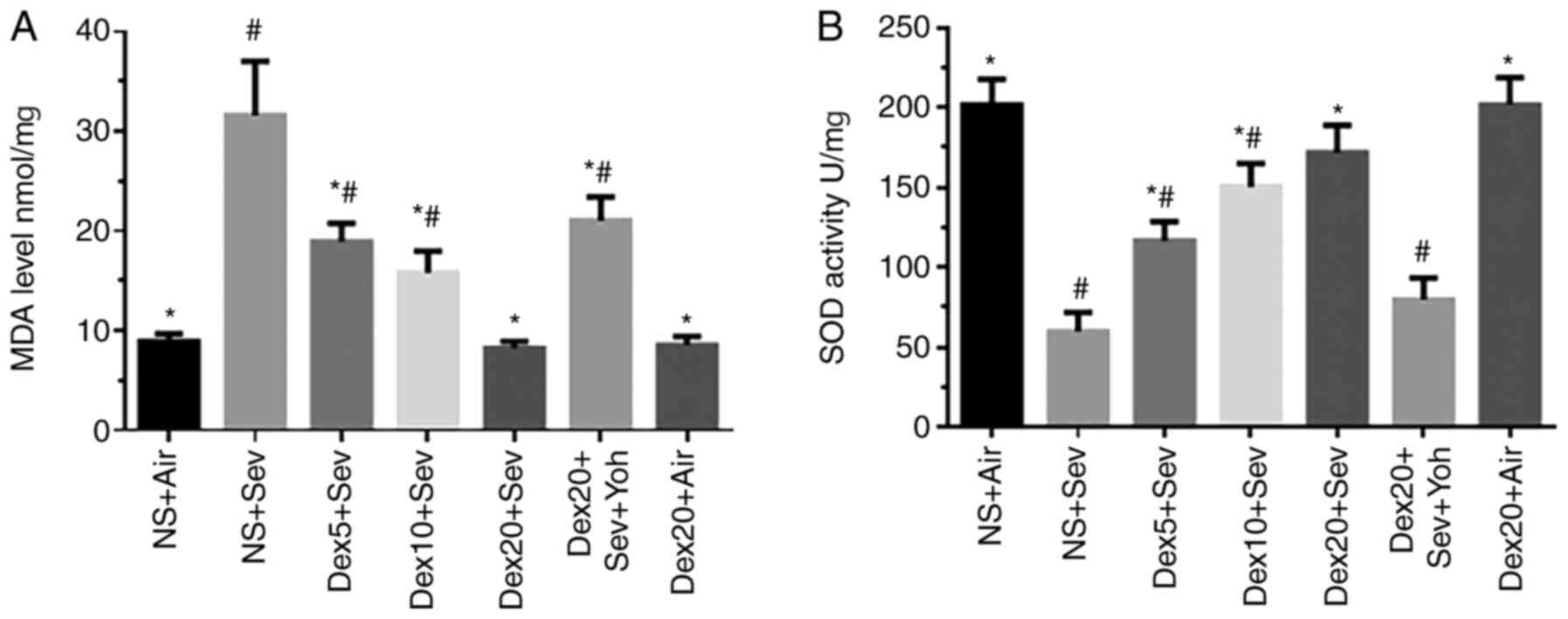
|
1
|
Brioni JD, Varughese S, Ahmed R and Bein
B: A clinical review of inhalation anesthesia with sevoflurane:
From early research to emerging topics. J Anesth. 31:764–778. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lerman J, Sikich N, Kleinman S and Yentis
S: The pharmacology of sevoflurane in infants and children.
Anesthesiology. 80:814–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devroe S, Van de Velde M and Rex S:
General anesthesia for caesarean section. Curr Opin Anaesthesiol.
28:240–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen X, Liu Y, Xu S, Zhao Q, Guo X, Shen R
and Wang F: Early life exposure to sevoflurane impairs adulthood
spatial memory in the rat. Neurotoxicology. 39:45–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu J, Shi P, Mao W, Zhao Y, Liu W and
Wang Y: Effect of apoptosis in neural stem cells treated with
sevoflurane. BMC Anesthesiol. 15:252015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Rainosek SW, Frisch-Daiello JL,
Patterson TA, Paule MG, Slikker W Jr, Wang C and Han X: Potential
adverse effects of prolonged sevoflurane exposure on developing
monkey brain: From abnormal lipid metabolism to neuronal damage.
Toxicol Sci. 147:562–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nie H, Peng Z, Lao N, Dong H and Xiong L:
Effects of sevoflurane on self-renewal capacity and differentiation
of cultured neural stem cells. Neurochem Res. 38:1758–1767. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Zhang J, Yang L, Dong Y, Zhang Y
and Xie Z: Isoflurane and sevoflurane increase interleukin-6 levels
through the nuclear factor-kappa B pathway in neuroglioma cells. Br
J Anaesth. 110 (Suppl 1):i82S–i91S. 2013. View Article : Google Scholar
|
|
10
|
Zhang Y, Dong Y, Zheng H, Shie V, Wang H,
Busscher JJ, Yue Y, Xu Z and Xie Z: Sevoflurane inhibits
neurogenesis and the Wnt-catenin signaling pathway in mouse neural
progenitor cells. Curr Mol Med. 13:1446–1154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weerink MAS, Struys MMRF, Hannivoort LN,
Barends CRM, Absalom AR and Colin P: Clinical pharmacokinetics and
pharmacodynamics of dexmedetomidine. Clin Pharmacokinet.
56:893–913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Cesare Mannelli L, Micheli L, Crocetti
L, Giovannoni MP, Vergelli C and Ghelardini C: α2 Adrenoceptor: A
target for neuropathic pain treatment. Mini Rev Med Chem.
17:95–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun L, Guo R and Sun L: Dexmedetomidine
for preventing sevoflurane-related emergence agitation in children:
A meta-analysis of randomized controlled trials. Acta Anaesthesiol
Scand. 58:642–650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan D, Xia H, Sun S and Wang F: Effect of
ancillary drugs on sevoflurane related emergence agitation in
children undergoing ophthalmic surgery: A Bayesian network
meta-analysis. BMC Anesthesiol. 19:1382019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winzer-Serhan U and Leslie F: Expression
of alpha2A adrenoceptors during rat neocortical development. J
Neurobiol. 38:259–269. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Philipp M, Brede ME, Hadamek K, Gessler M,
Lohse MJ and Hein L: Placental alpha (2)-adrenoceptors control
vascular development at the interface between mother and embryo.
Nat Genet. 31:311–315. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Q, Lu R, Zhao J and Limbird LE:
Arrestin serves as a molecular switch, linking endogenous
α2-adrenergic receptor to SRC-dependent, but not SRC-independent,
ERK activation. J Biol Chem. 281:25948–25955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dahmani S, Paris A, Jannier V, Hein L,
Rouelle D, Scholz J, Gressens P and Mantz J: Dexmedetomidine
increases hippocampal phosphorylated extracellular signal-regulated
protein kinase 1 and 2 content by an
alpha2-adrenoceptor-independent mechanismevidence for the
involvement of imidazoline I1 receptors. Anesthesiology.
108:457–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma D, Hossain M, Rajakumaraswamy N, Arshad
M, Sanders RD, Franks NP and Maze M: Dexmedetomidine produces its
neuroprotective effect via the α2A-adrenoceptor subtype. Eur J
Pharmacol. 502:87–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cibelli M, Fidalgo AR, Terrando N, Ma D,
Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS
and Maze M: Role of interleukin-1beta in postoperative cognitive
dysfunction. Ann Neurol. 68:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cruickshank AM, Fraser WD, Burns HJ, Van
Damme J and Shenkin A: Response of serum interleukin-6 in patients
undergoing elective surgery of varying severity. Clin Sci (Lond).
79:161–165. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan H, Bi J, Wang Y, Zhang J and Zuo Z:
Transfusion of old RBCs induces neuroinflammation and cognitive
impairment. Crit Care Med. 43:e276–e286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terrando N, Eriksson LI, Ryu JK, Yang T,
Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K
and Maze M: Resolving postoperative neuroinflammation and cognitive
decline. Ann Neurol. 70:986–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan Y, Xu J, Ma D, Zeng Y, Cibelli M and
Maze M: Postoperative impairment of cognitive function in rats: A
possible role for cytokine-mediated inflammation in the
hippocampus. Anesthesiology. 106:436–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv Q, Guo Y, Zhu M, Geng R, Cheng X, Bao
C, Wang Y, Huang X, Zhang C, Hao Y, et al: Predicting individual
responses to lithium with oxidative stress markers in drug-free
bipolar disorder. World J Biol Psychiatry. 20:778–789. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soltesz I and Losonczy A: CA1 pyramidal
cell diversity enabling parallel information processing in the
hippocampus. Nat Neurosci. 21:484–493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crawley JN: Behavioral phenotyping
strategies for mutant mice. Neuron. 57:809–818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Chen K and Shen X: Environmental
enrichment attenuated sevoflurane-induced neurotoxicity through the
PPAR-γ signaling pathway. Biomed Res Int. 2015:1071492015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shan Y, Yang F, Tang Z, Bi C, Sun S, Zhang
Y and Liu H: Dexmedetomidine ameliorates the neurotoxicity of
sevoflurane on the immature brain through the BMP/SMAD signaling
pathway. Front Neurosci. 12:9642018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu D, Li L and Yuan W: Neonatal anesthetic
neurotoxicity: Insight into the molecular mechanisms of long-term
neurocognitive deficits. Biomed Pharmacother. 87:196–199. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Z, Dong Y, Wang H, Culley DJ,
Marcantonio ER, Crosby G, Tanzi RE, Zhang Y and Xie Z: Peripheral
surgical wounding and age-dependent neuroinflammation in mice. PLoS
One. 9:e967522014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Block ML: Neuroinflammation: Modulating
mighty microglia. Nat Chem Biol. 10:988–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiu LL, Ji MH, Zhang H and Yang JJ, Sun
XR, Tang H, Wang J, Liu WX and Yang JJ: NADPH oxidase 2-derived
reactive oxygen species in the hippocampus might contribute to
microglial activation in postoperative cognitive dysfunction in
aged mice. Brain Behav Immun. 51:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sriram N, Kalayarasan S and Sudhandiran G:
Enhancement of antioxidant defense system by
epigallocatechin-3-gallate during bleomycin induced experimental
pulmonary fibrosis. Biol Pharm Bull. 31:1306–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Del RD, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu F, Armstrong R, Urrego D, Qazzaz M,
Pehar M, Armstrong JN, Shutt T and Syed N: The mitochondrial
division inhibitor Mdivi-1 rescues mammalian neurons from
anesthetic-induced cytotoxicity. Mol Brain. 9:352016. View Article : Google Scholar : PubMed/NCBI
|