Introduction
Keloids, characterized by excessive proliferation of
fibroblasts, are one of the most common and refractory diseases in
dermatology (1). Keloids are
abnormal tissue scars that form when fibroblasts over accumulate,
resulting in a large amount of collagen fibrin and extracellular
matrix deposition (2). Patients
with keloids frequently present with pain, itching and occasionally
loss of shin function, which affect the physical and psychological
quality of life of patients (3–5).
Despite the development of clinical therapies, such as interferon,
radiation, surgical resection and hormones, the treatment of
keloids is still limited due to insufficient research on the
underlying pathogenic mechanism (3,6,7).
Therefore, exploring the key genes and microRNAs (miRNAs/miRs)
associated with keloid pathogenesis might be useful for the
development of novel therapeutic targets and for the theoretical
guidance of keloids.
miRNAs/miRs are a group of essential regulatory
factors that negatively regulate the expression of their target
genes to modulate a number of biological processes (8–12).
Increasing evidence has suggested that abnormally expressed miRNAs
are associated with diverse cellular processes, including cell
apoptosis, differentiation, proliferation and extracellular matrix
production (13–16). A previous study demonstrated that
miRNA profiles in keloid fibroblasts were different compared with
those in normal fibroblasts via microarray analysis (17). Liu et al (18) also demonstrated that the expression
of miRNAs was altered in keloid tissues, which might contribute to
the development of keloids via regulating signaling pathways
associated with scar wound healing.
Nuclear receptor subfamily 2 group F member 2
(NR2F2) is a member of the steroid/thyroid nuclear hormone receptor
superfamily (18). Aberrant
activation of NR2F2 is related to the occurrence of cancer,
including lung, breast and prostate cancer (19–21).
NR2F2 overexpression augmented angiogenesis, tumor growth and
malignant progression by restraining the TGF-β-induced growth
barrier in prostate cancer (22).
However, the effect of NR2F2 on the development of keloids, benign
skin tumors, is not completely understood.
The present study aimed to investigate the roles of
miR-194-5p and NR2F2 in keloids using bioinformatics analysis and
cell functional experiments. Our findings may be useful to provide
a novel therapeutic target for keloids.
Materials and methods
Microarray analysis
The GSE7890 dataset (23) from the Gene Expression Omnibus
database (www.ncbi.nlm.nih.gov/gds) demonstrated the gene
profiling of keloid fibroblasts. The GSE7890 dataset included five
keloid fibroblasts samples and five healthy fibroblasts samples.
Differentially expressed genes (DEGs) with an adjusted P-value
<0.05 were selected out and uploaded to Search Tool for the
Retrieval of Interacting Genes/Proteins (STRING; string-db.org) and Metascape (metascape.org/gp/index.html#/main/step1) to
perform Gene Ontology (GO) and pathway enrichment analyses.
Cell culture and cell
transfection
The human normal skin fibroblast (HSF) cell line was
purchased from BeNa Culture Collection (cat. no. 338008) and the
human keloid fibroblast (KEL-FIB) cell line was purchased from
American Type Culture Collection (cat. no. CRL-1762). Cells were
maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2. miR-194-5p mimic, miR-194-5p
inhibitor, small interfering (si)RNA targeting NR2F2 (si-NR2F2),
miR-mimic negative control (NC) and miR-inhibitor NC plasmids were
purchased from Guangzhou RiboBio Co., Ltd. At 30% confluence,
KEL-FIB cells were transfected with miR-194-5p mimic (100 nM),
miR-194-5p inhibitor (100 nM), si-NR2F2 (50 nM) or 50 nM miR-mimic
and 50 nM miR-inhibitor NC using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in serum-free DMEM.
Following incubation for 4 h at 37°C, serum-free medium was
replaced with fresh DMEM supplemented with 10% FBS. At 48 h
post-transfection, transfection efficiency was assessed via reverse
transcription-quantitative PCR (RT-qPCR). The sequences of plasmid
are presented in Table SI.
RT-qPCR
Total RNA was isolated from HSF and KEL-FIB cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (1 µg) was reverse transcribed into cDNA using the
GoScript Reverse Transcription System kit (cat. no. A5001; Promega
Corporation) at 70°C for 5 min, 25°C for 5 min, 42°C for 60 min and
70°C for 15 min. Subsequently, qPCR was performed using a C1000
Thermal Cycler (Bio-Rad Laboratories, Inc.) and SYBR Premix Ex Taq
II (cat. no. RR820A; Takara Biotechnology Co., Ltd.). The PCR
conditions were specifically shown as: Pre-denaturation at 95°C for
30 sec, and 40 cycles of denaturation at 95°C for 5 sec and
annealing at 60°C for 30 sec. The sequences of primers used in the
present study are presented in Table
I. miRNA and mRNA expression levels were normalized to the
internal reference genes U6 and GAPDH, respectively. The relative
expression of miRNA and mRNA was calculated with the
2−∆∆Cq method (24).
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′→3′) |
|---|
| miR-194-5p | F:
ACACTCCAGCTGGGTGTAACAGAGCAACTCC |
|
| R:
TGGTGTCGTGGAGTCG |
| NR2F2 | F:
GGCAATGGTAGTCAGCACG |
|
| R:
ACCCATGATGTTGTTGGGCT |
| GAPDH | F:
GTCAAGGCTGAGAACGGGAA |
|
| R:
AAATGAGCCCCAGCCTTCTC |
| U6 | F:
TGCGGGTGCTCGCTTCGGCAGC |
|
| R:
CCAGTGCAGGGTCCGAGGT |
Cell proliferation assay
Transfected KEL-FIB cells were seeded
(3×105 cells/well) into a 96-well plate containing 100
µl DMEM per well. Following incubation for 24, 48 or 72 h, 10 µl
Cell Counting Kit-8 (CCK-8) reagent (Dojindo Molecular
Technologies, Inc.) in 100 µl DMEM medium was added to each well
for 2 h at 37°C. The optical density values were measured at a
wavelength of 450 nm using a microplate reader.
Flow cytometry analysis of cell
apoptosis
To assess cell apoptosis, the Annexin V-FITC
Apoptosis Staining/Detection kit (cat. no. ab14085; Abcam) was used
according to the manufacturer's protocol. Then, cell apoptosis was
detected by flow cytometry on a FACSCalibur Flow Cytometer (BD
Biosciences), and the apoptosis rate was analyzed by the CellQuest
Pro software (version 5.1; Becton-Dickinson). Transfected KEL-FIB
cells (1×104) were collected and stained with Annexin
V-FITC and PI. The rate of apoptosis was calculated as the sum of
late apoptosis (Q1-UR) and early apoptosis (Q1-LR).
Wound healing assay
Transfected KEL-FIB cells were plated
(1×106 cells/well) in 6-well plates. At 90% confluence
(Fig. S1), cells were cultured in
serum-free medium. After 12 h, a scratch in the center of the cell
monolayer was made using a pipette tip. Cells were washed three
times with PBS to remove cell fragments. Subsequently, cells were
cultured in serum-free DMEM. Images of the wounds were captured at
0, 24 and 48 h under a light microscope (magnification, ×100; Leica
Microsystems GmbH). The wound width was measured using ImageJ
software version 1.46 (National Institutes of Health) to assess
cell migration. The rate of cell migration was calculated as the
ratio of the migration distance at 24 or 48 h to the wound width at
0 h.
Transwell invasion assay
Transwell upper chambers were pre-coated with 5
mg/ml Matrigel at 4°C overnight (Corning, Inc.). Subsequently,
transfected KEL-FIB cells were seeded (2×105 cells/well)
in serum-free DMEM into the upper chambers. DMEM supplemented with
10% FBS was plated in the lower chambers. Following incubation for
48 h, the medium was removed, and invading cells were fixed in
ice-cold 100% methanol for 5 min at 4°C, stained with 0.1% crystal
violet for 3 min at room temperature and washed with PBS. Invading
cells on the lower surface of the membrane were observed in 5–7
randomly selected fields of view using a light microscope (Nikon
Corporation) at ×100 magnification.
Dual-luciferase reporter assay
The binding site between NR2F2 3′-untranslated
region (UTR) and miR-194-5p was predicted using TargetScan Human
(http://www.targetscan.org/vert_72/).
The wild-type (WT) and mutated (MUT) KEL-FIB cells derived NR2F2
3′UTR vectors were constructed by Sangon Biotech Co., Ltd. Briefly,
the full length WT NR2F2 3′UTR containing the binding site of
miR-194-5p was amplified via PCR and cloned into the pmirGLO vector
(Promega Corporation) between the Xhol and Notl sites
to construct the pGLO-NR2F2-WT vector. DNA Polymerase was purchased
from Invitrogen, Ltd. The thermocycling conditions were as follows:
Initial denaturation at 94°C for 1 min, 35 cycles of 94°C for 15
sec, annealing/elongation at 60°C for 30 sec and a final extension
at 72°C for 30 sec. As for mutated NR2F2 3′UTR, the binding site of
NR2F2 3′UTR was mutated using a site-directed mutagenesis kit
(Takara Bio, Inc.) to 5′-GGAUGAAUCAA-3′ and amplified via PCR. The
mutated NR2F2 3′UTR was inserted between the Xhol and
Notl sites of the pmirGLO vector to construct the
pGLO-NR2F2-MUT vector. KEL-FIB cells (1×105) were
co-transfected with 0.8 µg pGLO-NR2F2-WT or 0.8 µg pGLO-NR2F2-MUT
and 50 nM miR-194-5p mimic or NC using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At 48 h
post-transfection, luciferase activities were measured using the
Dual-Luciferase Reporter Assay System (cat. no. E1910; Promega
Corporation). Firefly luciferase activities were normalized to
Renilla luciferase activities. Luciferase activity was
averaged from three replicates. The primers used to perform
mutagenesis are presented in Table
SII.
Western blotting
Total protein was isolated from KEL-FIB cells using
RIPA lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd.), and the protein concentration was detected using BCA Protein
Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Total proteins
(30 µg/lane) were separated via 10% SDS-PAGE and transferred to
PVDF membranes, which were then blocked with 5% skimmed milk for 1
h at room temperature. Subsequently, the membranes were incubated
at 4°C overnight with primary antibodies targeted against NR2F2
(1:1,000, cat. no. ab211777; Abcam) and GAPDH (1:1,000, cat. no.
ab181602; Abcam). Following primary incubation, the membranes were
incubated in Horseradish peroxidase (HRP)-conjugated secondary
antibody (1:5,000, cat. no. ab205718; Abcam) for 2 h at room
temperature. The membranes were washed three times with TBST
containing 0.1% of Tween-20. Protein bands were visualized using
ECL (EMD Millipore) and the ChemiDoc Touch Imaging system (Bio-Rad
Laboratories, Inc.). Protein expression levels were semi-quantified
using FluorChem FC2 system version 6.0.2 (Alpha Innotech
Corporation) with GAPDH as the loading control.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 7.0; GraphPad Software, Inc.). Data are
presented as the mean ± SD from at least three independent
experiments. Comparisons between two groups were analyzed using the
unpaired Student's t-test. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Dunnett's or Tukey's post
hoc test. The correlation between NR2F2 mRNA expression and
miR-194-5p expression was analyzed using Pearson's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
NR2F2 is a key gene related to keloid
pathology
The GSE7890 dataset was analyzed to identify key
genes related to keloid pathology. A total of 104 DEGs with an
adjusted P-value <0.05 were selected to perform GO and pathway
enrichment analyses. The STRING analysis indicated that ‘regulation
of cell population proliferation’, including 21 genes, was the most
important biological process (Fig.
1A). The Metascape analysis of the 104 DEGs indicated that
‘negative regulation of cell proliferation’ and ‘negative
regulation of cell migration’ were the key biological processes
(Fig. 1B). The five overlapping
genes (insulin like growth factor binding protein 5, pleiotrophin,
T-box transcription factor 5, NR2F2 and DLC1 Rho GTPase activating
protein) were selected from STRING and Metascape (Fig. 1C). Subsequently, we found that,
NR2F2 was upregulated in keloid biopsies after literature review
(23) so that NR2F2 was identified
as the key gene closely associated with keloid pathology. The
results also indicated that miR-194-5p might directly target NR2F2
(Fig. 1D).
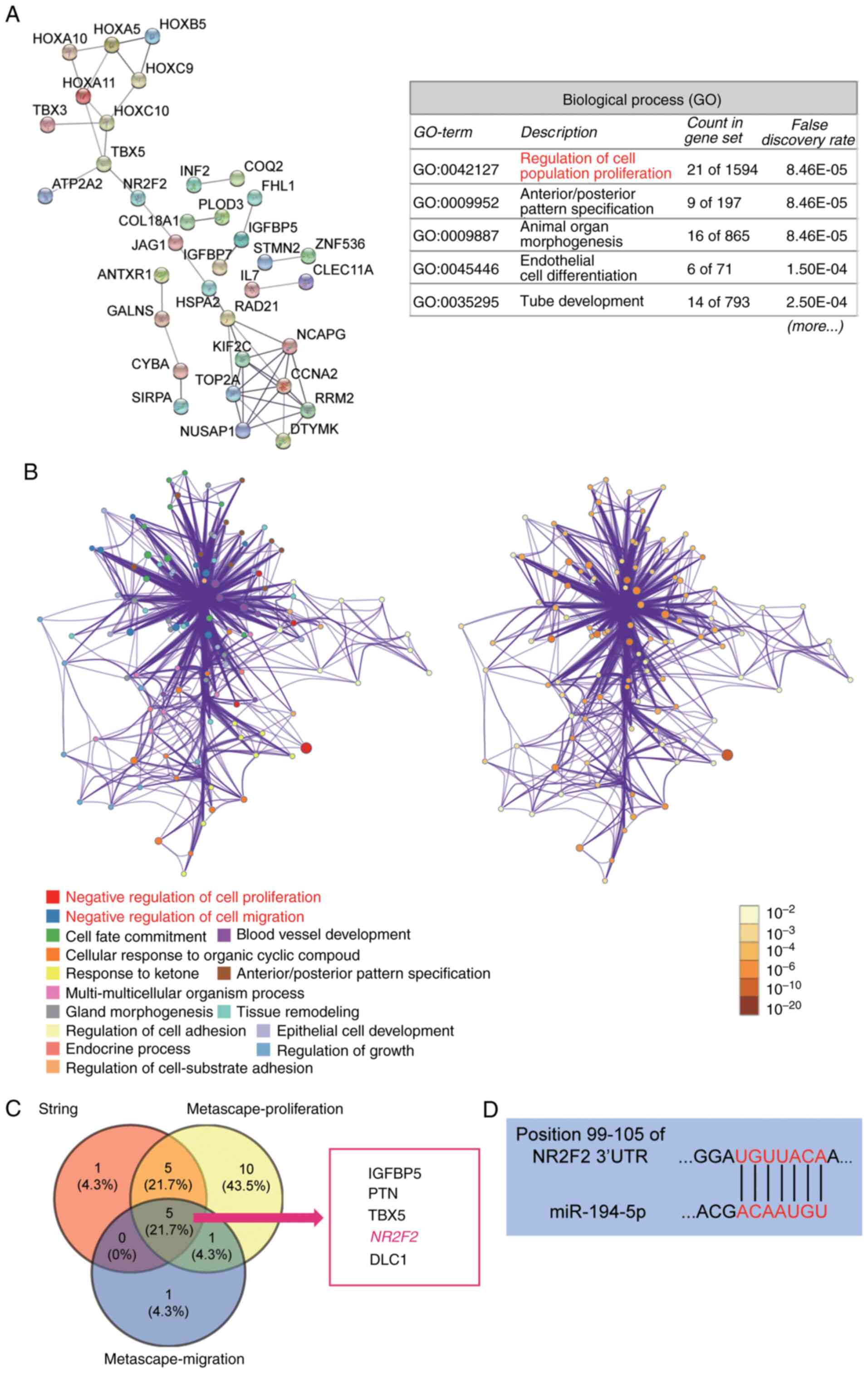 | Figure 1.NR2F2 and miR-194-5p are the key mRNA
and miRNA of interest in keloids, respectively. (A) The biological
process analysis of 104 DEGs using STRING. A total of 104 DEGs were
selected from the GSE7890 dataset, which was downloaded from Gene
Expression Omnibus. STRING (string-db.org) is a database for functional
enrichment analysis. (B) The GO and pathway analysis of 104 DEGs
using Metascape. Metascape (metascape.org) is a visualized tool for the gene
annotation and analysis. (C) The five overlapping genes (IGFBP5,
PTN, TBX5, NR2F2 and DLC1) from STRING and Metascape analyses. (D)
TargetScan Human was used to predict the binding site between NR2F2
mRNA 3′UTR and miR-194-5p. NR2F2, nuclear receptor subfamily 2
group F member 2; miR, microRNA; DEG, differentially expressed
genes; STRING, Search Tool for the Retrieval of Interacting
Genes/Proteins; GO, Gene Ontology; IGFBP5, insulin like growth
factor binding protein 5; PTN, pleiotrophin; TBX5, T-box
transcription factor 5; DLC1, DLC1 Rho GTPase activating protein;
UTR, untranslated region. |
miR-194-5p inhibits cell proliferation
but promotes cell apoptosis in keloid fibroblasts
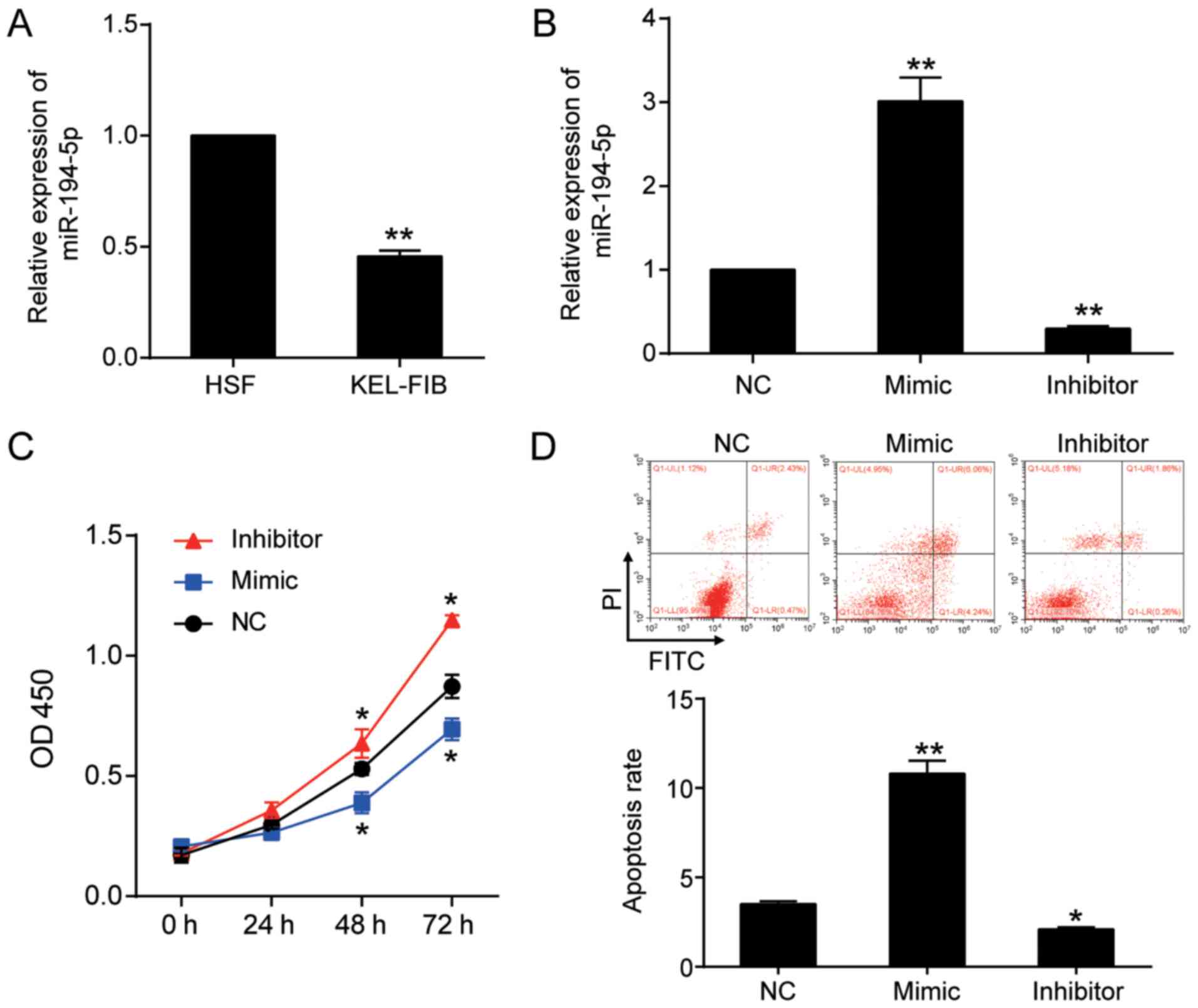
The RT-qPCR results indicated that miR-194-5p
expression was decreased by 50% in in KEL-FIB cells compared with
HSF cells (Fig. 2A). Subsequently,
KEL-FIB cells were transfected with miR-194-5p mimic and miR-194-5p
inhibitor to further assess the function of miR-194-5p (Fig. 2B). The effects of miR-194-5p in
KEL-FIB cells were investigated. The CCK-8 assay results suggested
that, compared with the NC group, miR-194-5p overexpression
significantly inhibited cell proliferation, whereas miR-194-5p
knockdown significantly increased KEL-FIB cell proliferation
(Fig. 2C). Compared with the NC
group (3.14±0.23), the rate of apoptosis was increased by 3.44-fold
in the miR-194-5p mimic group (10.80±0.73), whereas the rate of
apoptosis was decreased by 46% in the miR-194-5p inhibitor group
(2.10±0.11; Fig. 2D).
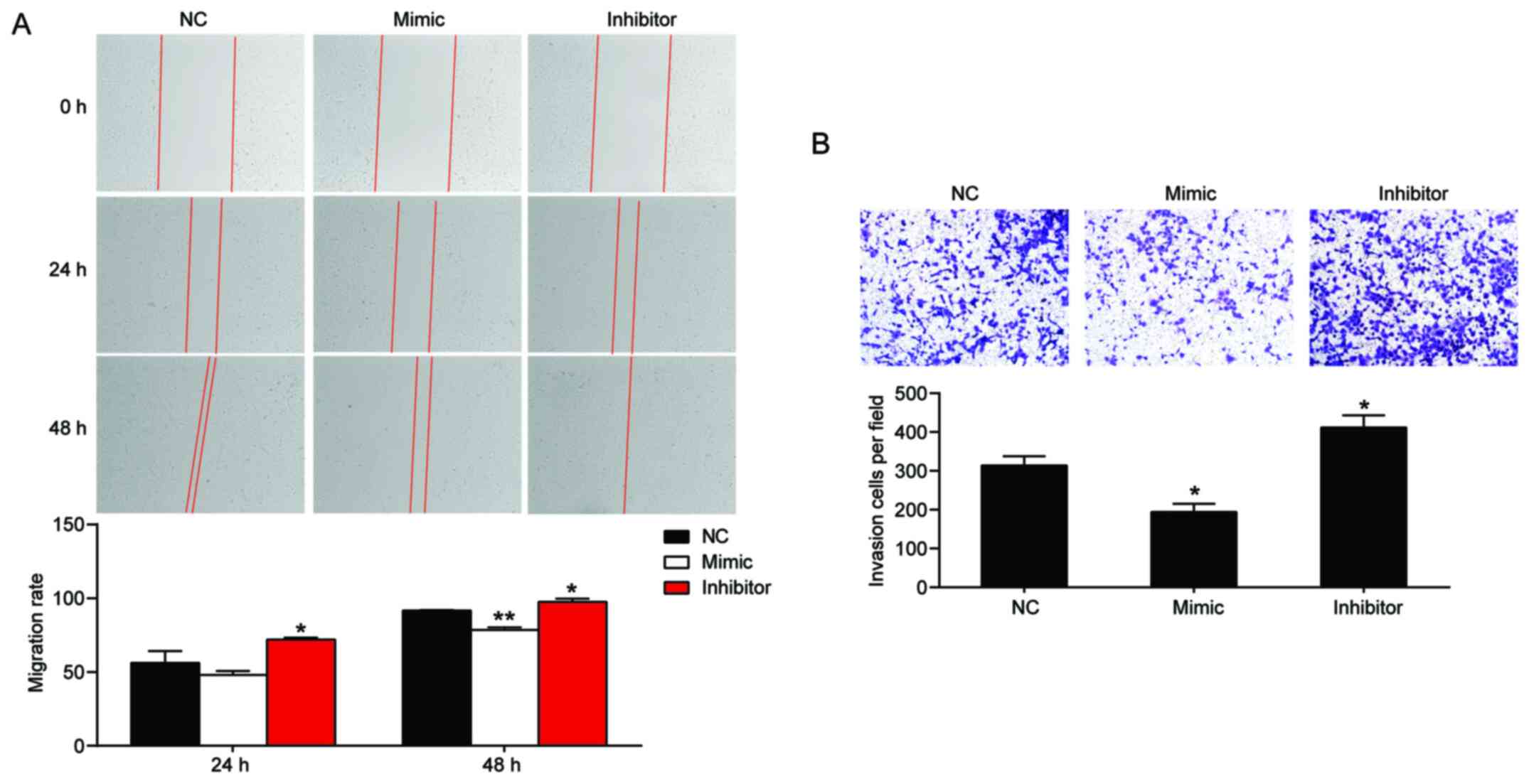
miR-194-5p inhibits keloid fibroblast
migration and invasion
Following transfection with miR-194-5p mimic and
miR-194-5p inhibitor, the effect of miR-194-5p on cell migration
and invasion was assessed by performing wound healing and Transwell
invasion assays, respectively. The results indicated that
miR-194-5p overexpression decreased KEL-FIB cell migration by 14%,
whereas miR-194-5p knockdown increased KEL-FIB cell migration by
1.1-fold compared with the NC group (Fig. 3A). Similarly, the Transwell invasion
assay results indicated that miR-194-5p mimic decreased KEL-FIB
cell invasion, whereas miR-194-5p inhibitor increased KEL-FIB cell
invasion compared with the NC group (Fig. 3B).
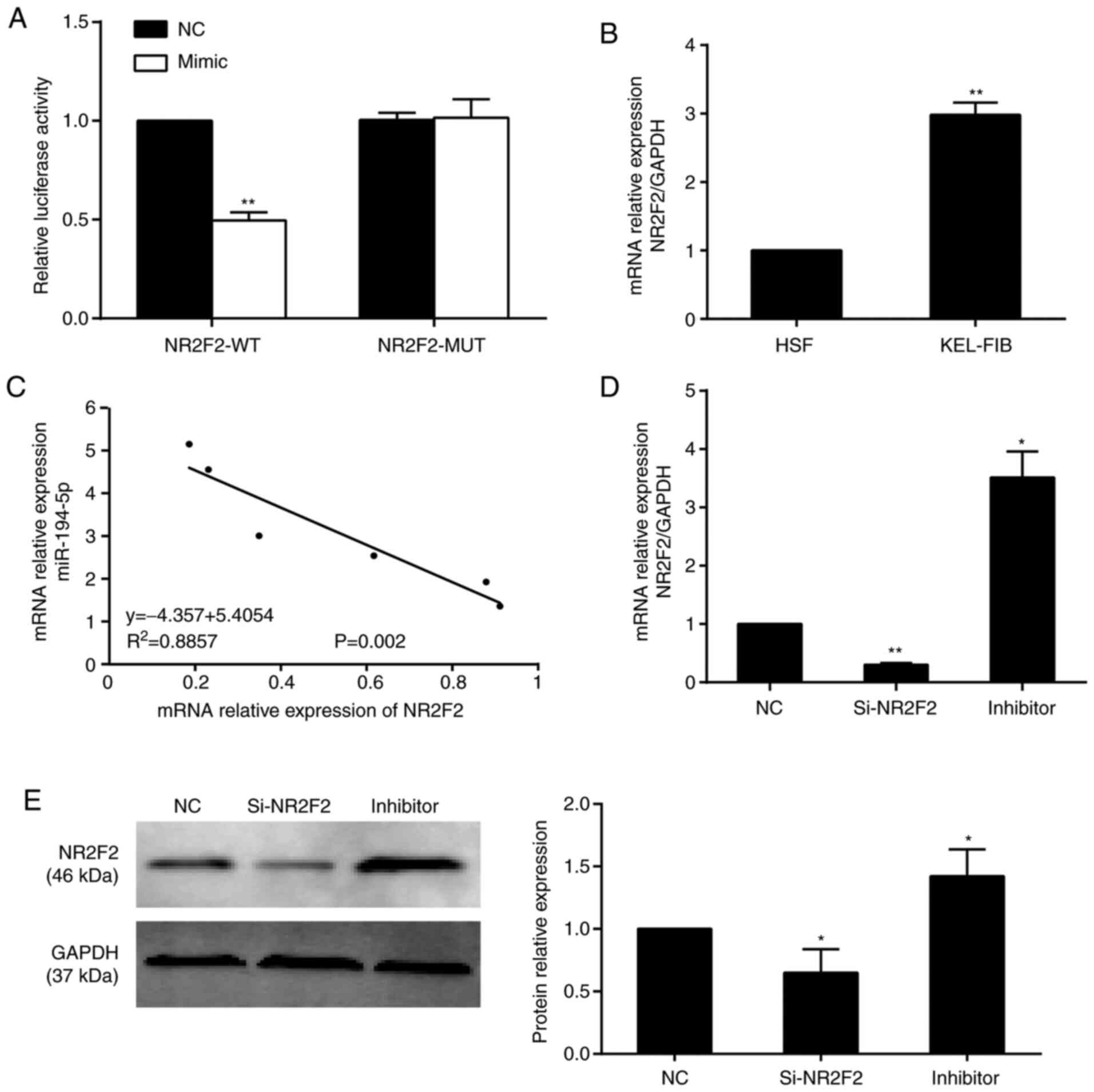
Validation of NR2F2 as a direct target
of miR-194-5p
The dual-luciferase reporter assay results indicated
that miR-194-5p overexpression decreased the luciferase activity of
NR2F2-WT by 50% compared with the NC group, whereas miR-194-5p
overexpression did not significantly alter the luciferase activity
of NR2F2-MUT (Fig. 4A). In
addition, NR2F2 expression levels were increased by 3-fold in
KEL-FIB cells compared with HSF cells (Fig. 4B). Pearson's correlation analysis
indicated that NR2F2 and miR-194-5p expression levels were
negatively correlated in KEL-FIB cells (Fig. 4C). Compared with the NC group, NR2F2
knockdown decreased NR2F2 expression, whereas miR-194-5p inhibitor
increased NR2F2 expression at both the mRNA (Fig. 4D) and protein (Fig. 4E) levels.
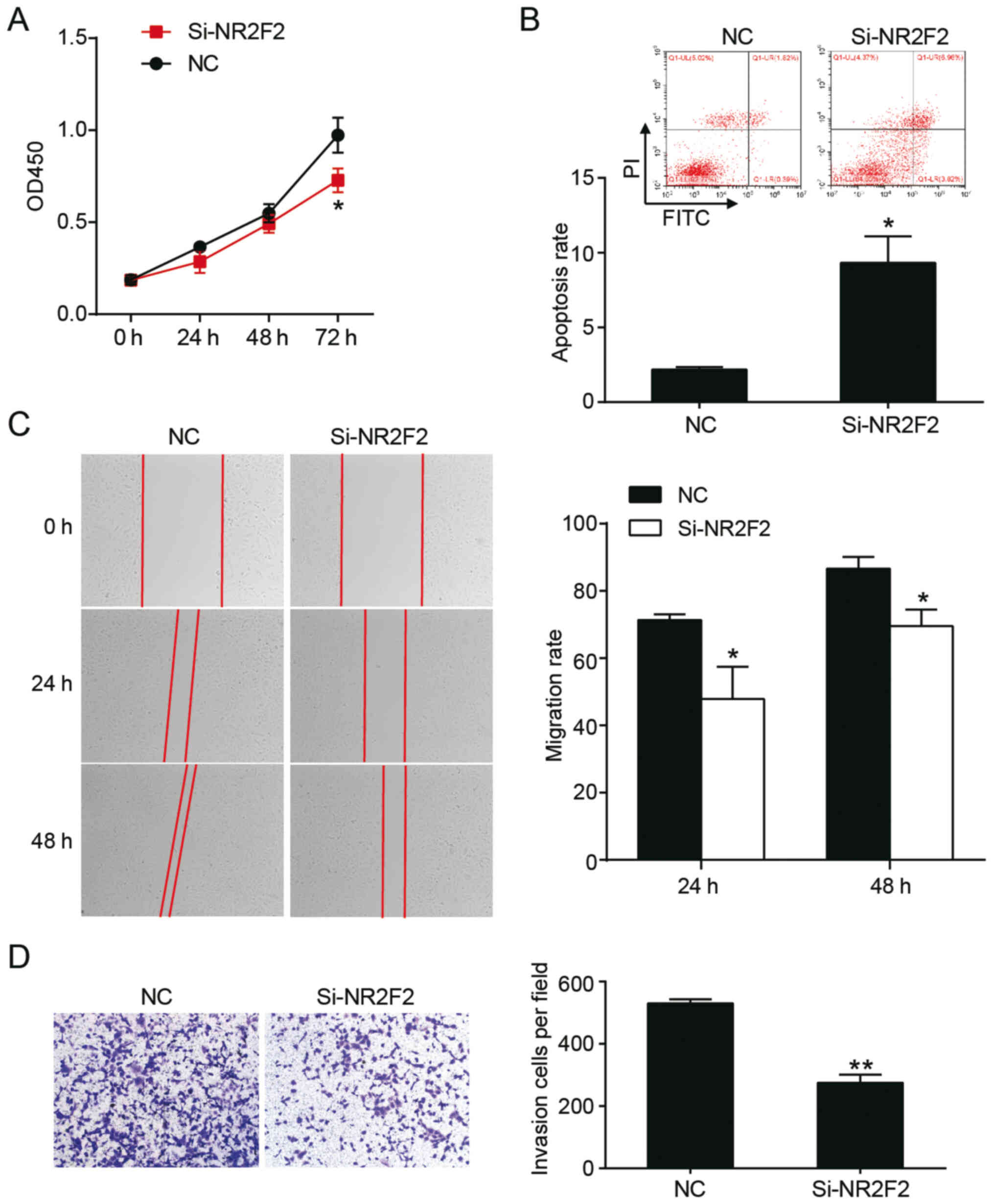
NR2F2 knockdown alters the aggressive
phenotypes of keloid fibroblasts
To investigate the effect of NR2F2 on keloids in
vitro, the CCK-8 assay was performed. The results indicated
that NR2F2 knockdown significantly decreased KEL-FIB cell
proliferation at 72 h compared with the NC group (Fig. 5A). The rate of apoptosis was
determined via flow cytometry, and the results suggested that the
rate of apoptosis in the si-NR2F2 group was increased by 4.4-fold
compared with the NC group (Fig.
5B). The migratory abilities of cells were evaluated by
performing a wound healing assay. Compared with the NC group, cell
migration in the si-NR2F2 group was decreased at 24 (48±9.6 vs.
71±1.7%) and 48 h (70±5.0 vs. 87±3.5%) post-transfection (Fig. 5C). The Transwell invasion assay
indicated that si-NR2F2 decreased the number of invading cells
compared with the NC group (Fig.
5D). Collectively, the results indicated that NR2F2 knockdown
suppressed keloid progression in vitro.
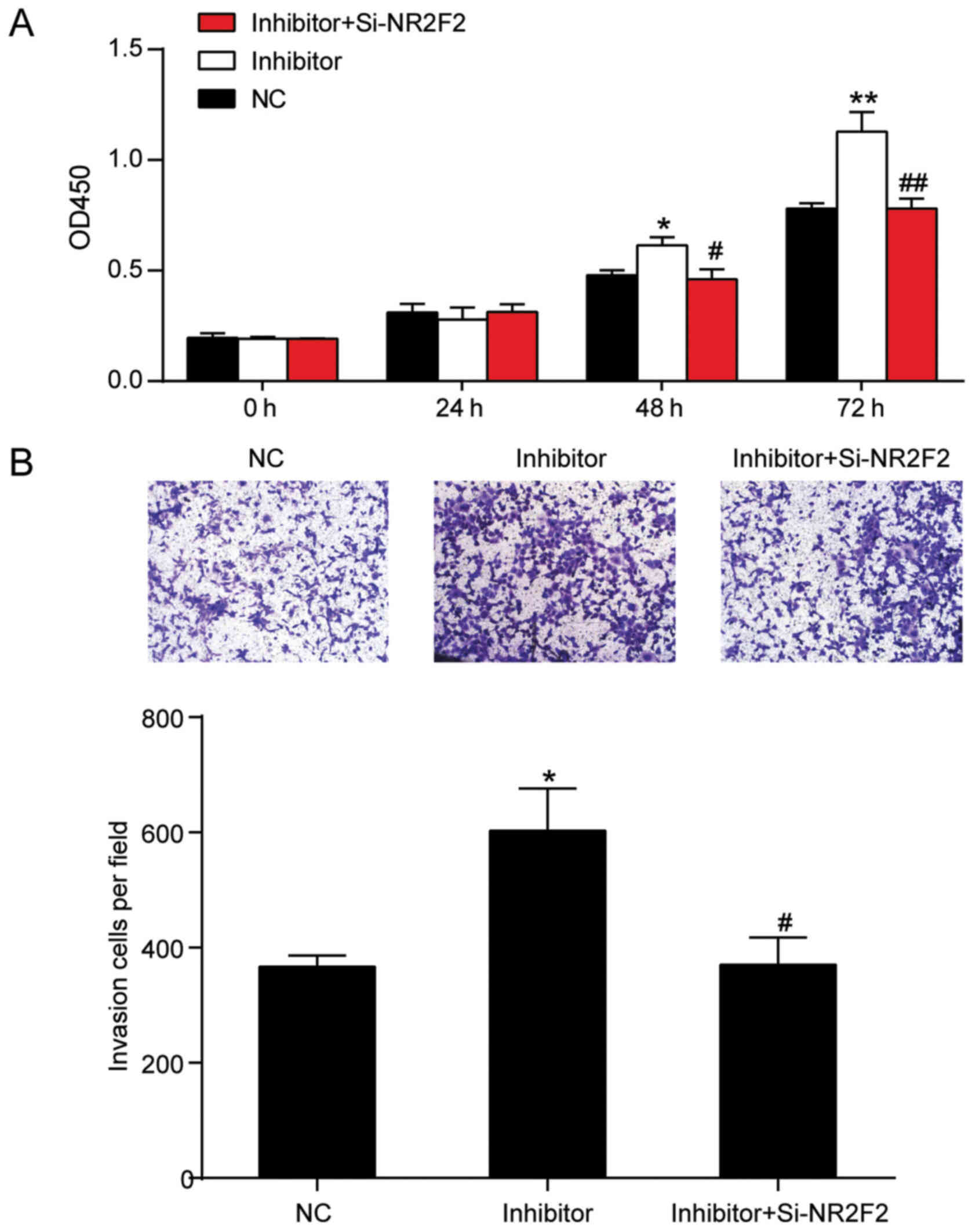
NR2F2 knockdown reverses the influence
of miR-194-5p inhibitor on keloid fibroblasts
To identify whether miR-194-5p regulated keloid
fibroblasts by targeting NR2F2, a rescue experiment was performed.
Co-transfection of miR-194-5p inhibitor and si-NR2F2 resulted in
similar proliferation and invasion phenotypes to the NC group. The
results suggested that NR2F2 knockdown reversed miR-194-5p
inhibitor-mediated induction of KEL-FIB cell proliferation and
invasion (Fig. 6A and B). Moreover,
the CCK-8 assay results indicated that NR2F2 knockdown abolished
the effects of miR-194-5p inhibitor at 48 and 72 h. Similarly, the
Transwell invasion assay results suggested that the number of
invading cells in the co-transfection group was decreased by 38.7%
(603±73) compared with the miR-194-5p inhibitor group (370±48;
Fig. 6B). Therefore, the results
indicated that miR-194-5p inhibited keloid progression by targeting
NR2F2 in vitro.
Discussion
Keloids are a wound healing reaction due to abnormal
skin injury, characterized by collagen deposition, as well as
persistent fibrosis and inflammation (25,26).
The key pathogenic mechanism underlying keloids is not completely
understood. In the present study, the results indicated that
miR-194-5p expression was significantly decreased in KEL-FIB cells
compared with HSF cells. Compared with the NC group, miR-194-5p
overexpression inhibited the aggressive phenotypes of keloid
fibroblasts in vitro. Moreover, miR-194-5p was identified as
a direct regulator of NR2F2 in KEL-FIB cells. Compared with the NC
group, NR2F2 knockdown also inhibited the aggressive phenotypes of
keloid fibroblasts, and promoted KEL-FIB cell apoptosis.
Collectively, the results indicated that miR-194-5p suppressed
keloid progression by targeting NR2F2.
miRNAs, consisting of 18–25 nucleotides, are highly
conserved non-coding RNAs that can induce the degradation or
translational inhibition of mRNA by binding to the 3′UTR of target
genes (27,28). miRNAs have received increasing
attention in medical research and have been reported to serve as
tumor suppressor genes or proto-oncogenes (29). Previous studies have demonstrated
that miRNAs are closely associated with the formation and
development of keloids, which are benign tumors. In addition, Zhang
et al (30) reported that
miR-637 inhibited aggressive phenotypes by targeting Smad3 in human
keloid fibroblast cells. In the present study, human keloid
fibroblast cells were used to investigate the effects of miR-194-5p
on the aggressive phenotypes of keloid fibroblast cells. The
results indicated that miR-194-5p was downregulated KEL-FIB cells
compared with HSF cells, and miR-194-5p overexpression inhibited
the aggressive phenotypes of keloid fibroblast cells compared with
the NC group.
NR2F2, a member of the steroid/thyroid receptor
superfamily, was screened out and predicted to be a miR-194-5p
target gene via bioinformatics analysis in the present study.
Previously, the biological significance of NR2F2 in prostate cancer
was reported, demonstrating that NR2F2 was a therapeutic target and
prognostic marker for prostate cancer via patient sample analysis
(22). In vivo, NR2F2
knockdown suppressed pancreatic β tumor cell invasion due to a
defect in the angiogenic switch (31). Bao et al (32) demonstrated that NR2F2 overexpression
was important for colorectal cancer metastasis, and promoted cell
migration and metastasis in association with snail family
transcriptional repressor 1 in vivo and in vitro. In
line with the aforementioned previous studies, the present study
investigated the hypothesis that NR2F2 might serve as a promoter in
keloids. The results suggested that NR2F2 was upregulated in
KEL-FIB cells compared with HSF cells, and NR2R2 knockdown
effectively inhibited keloid fibroblast cell proliferation,
migration and invasion, but promoted cell apoptosis compared with
the NC group. In addition, the results suggested that NR2F2 3′UTR
was regulated by miR-194-5p, and NR2F2 knockdown significantly
reversed miR-194-5p inhibitor-mediated effects on the aggressive
phenotypes of keloid fibroblasts.
However, the present study had a number of
limitations that should be addressed in future studies. First, the
KEL-FIB cell line was derived from a 35-year-old black female,
whereas the HSF cell line was established in China, suggesting that
the HSF cell line and KEL-FIB cell line might be derived from
individuals with different ethnicities. A previous study reported
that keloid formation in Caucasians was often accompanied by
erythema and telangiectasia and pigmentation compared with keloid
formation in African Americans (33); however, to the best of our
knowledge, no previous study has investigated the difference in
keloids between Asian and American individuals. In the present
study, the HSF cell line (a normal skin fibroblast cell line) was
used as a control cell line to assess the relative expression level
of NR2F2 in the KEL-FIB cell line (a keloid fibroblast cell line).
All functional experiments were performed using the KEL-FIB cell
line to study the effects of NR2F2 on keloid fibroblasts. The HSF
cell line was selected as the control cell line because, firstly,
it has been widely used in previous keloid studies (34–36),
and secondly, ATCC did not provide a normal skin fibroblast cell
line. The lack of cell lines derived from individuals of the same
ethnicity is a key limitation of the present study. Whether the
miR-194-5p/NR2F2 interactome could serve as a diagnostic and
therapeutic target for keloids also requires further investigation
in vivo and validation using additional clinical
characteristics data. Both in vivo assays and clinical-level
correlation analyses should be conducted. In addition, the
mechanism underlying the effect of NR2F2 in keloid fibroblasts
should be investigated in future studies.
In summary, the present study indicated that
miR-194-5p inhibited cell proliferation, migration and invasion,
but promoted cell apoptosis in keloids. In addition, the results
suggested that the expression of NR2F2 was downregulated by the
upstream regulator miR-194-5p in keloid fibroblasts. Therefore, the
present study indicated that the miR-194-5P/NR2F2 axis may serve as
a potential biomarker or novel treatment strategy for keloids in
the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wuhan
University Young Teachers Funding Project (grant no.
2042018kf0145).
Availability of data and materials
The datasets used and or/analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SJ designed the study and prepared the manuscript.
QX supervised the study and interpreted the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel PA, Bailey JK and Yakuboff KP:
Treatment outcomes for keloid scar management in the pediatric burn
population. Burns. 38:767–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yao X, Cui X, Wu X, Xu P, Zhu W, Chen X
and Zhao T: Tumor suppressive role of miR-1224-5p in keloid
proliferation, apoptosis and invasion via the TGF-β1/Smad3
signaling pathway. Biochem Biophys Res Commun. 495:713–720. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seifert O and Mrowietz U: Keloid scarring:
Bench and bedside. Arch Dermatol Res. 301:259–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finnerty CC, Jeschke MG, Branski LK,
Barret JP, Dziewulski P and Herndon DN: Hypertrophic scarring: The
greatest unmet challenge after burn injury. Lancet. 388:1427–1436.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slemp AE and Kirschner RE: Keloids and
scars: A review of keloids and scars, their pathogenesis, risk
factors, and management. Curr Opin Pediatr. 18:396–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berman B, Maderal A and Raphael B: Keloids
and hypertrophic scars: Pathophysiology, classification, and
treatment. Dermatol Surg. 43 (Suppl 1):S3–S18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu W, Wu X, Yang B, Yao X, Cui X, Xu P
and Chen X: miR-188-5p regulates proliferation and invasion via
PI3K/Akt/MMP-2/9 signaling in keloids. Acta Biochim Biophys Sin
(Shanghai). 51:185–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi R, O'Carroll D, Pasolli HA, Zhang Z,
Dietrich FS, Tarakhovsky A and Fuchs E: Morphogenesis in skin is
governed by discrete sets of differentially expressed microRNAs.
Nat Genet. 38:356–362. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chau BN and Brenner DA: What goes up must
come down: The emerging role of microRNA in fibrosis. Hepatology.
53:4–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Yu X, Shen J, Chan MT and Wu WK:
MicroRNA in intervertebral disc degeneration. Cell Prolif.
48:278–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu X, Li Z and Liu J: MiRNAs in primary
cutaneous lymphomas. Cell Prolif. 48:271–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Yu X, Shen J and Jiang Y: MicroRNA
dysregulation in uveal melanoma: A new player enters the game.
Oncotarget. 6:4562–4568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren L, Zhang J, Wang J, Wei J, Liu J, Li
X, Zhu Y, Li Y, Guo C, Duan J, et al: Silica nanoparticles induce
spermatocyte cell apoptosis through microRNA-2861 targeting death
receptor pathway. Chemosphere. 228:709–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao MJ, Xie J, Shu WJ, Wang HY, Bi J,
Jiang W and Du HN: MiR-15b and miR-322 inhibit SETD3 expression to
repress muscle cell differentiation. Cell Death Dis. 10:1832019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan L, Duan D, Wei W, Sun Z, Xu H, Guo L
and Wu X: MiR-19b-3p attenuates IL-1β induced extracellular matrix
degradation and inflammatory injury in chondrocytes by targeting
GRK6. Mol Cell Biochem. 459:205–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Bai Y, Liu H, Zuo X, Yao H, Xu Y and
Cao M: Comparative study of microRNA profiling in keloid fibroblast
and annotation of differential expressed microRNAs. Acta Biochim
Biophys Sin (Shanghai). 45:692–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Yang D, Xiao Z and Zhang M: miRNA
expression profiles in keloid tissue and corresponding normal skin
tissue. Aesthetic plastic surgery. 36:193–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Navab R, Gonzalez-Santos JM, Johnston MR,
Liu J, Brodt P, Tsao MS and Hu J: Expression of chicken ovalbumin
upstream promoter-transcription factor II enhances invasiveness of
human lung carcinoma cells. Cancer Res. 64:5097–5105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Litchfield LM, Appana SN, Datta S and
Klinge CM: COUP-TFII inhibits NFkappaB activation in
endocrine-resistant breast cancer cells. Mol Cell Endocrinol.
382:358–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Liu J, Qiu J, Fu X, Tang Q, Yang
F, Zhao Z and Wang H: MicroRNA-382 inhibits prostate cancer cell
proliferation and metastasis through targeting COUP-TFII. Oncol
Rep. 36:3707–3715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin J, Wu SP, Creighton CJ, Dai F, Xie X,
Cheng CM, Frolov A, Ayala G, Lin X, Feng XH, et al: COUP-TFII
inhibits TGF-β-induced growth barrier to promote prostate
tumorigenesis. Nature. 493:236–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith JC, Boone BE, Opalenik SR, Williams
SM and Russell SB: Gene profiling of keloid fibroblasts shows
altered expression in multiple fibrosis-associated pathways. J
Invest Dermatol. 128:1298–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sidgwick GP and Bayat A: Extracellular
matrix molecules implicated in hypertrophic and keloid scarring. J
Eur Acad Dermatol Venereol. 26:141–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang C and Ogawa R: The link between
hypertension and pathological scarring: Does hypertension cause or
promote keloid and hypertrophic scar pathogenesis? Wound Repair
Regen. 22:462–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rutnam ZJ, Wight TN and Yang BB: miRNAs
regulate expression and function of extracellular matrix molecules.
Matrix Biol. 32:74–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meltzer PS: Cancer genomics: Small RNAs
with big impacts. Nature. 435:745–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Price C and Chen J: MicroRNAs in cancer
biology and therapy: Current status and perspectives. Genes Dis.
1:53–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Guo B, Hui Q, Li W, Chang P and
Tao K: Downregulation of miR-637 promotes proliferation and
metastasis by targeting Smad3 in keloids. Mol Med Rep.
18:1628–1636. 2018.PubMed/NCBI
|
|
31
|
Qin J, Chen X, Yu-Lee LY, Tsai MJ and Tsai
SY: Nuclear receptor COUP-TFII controls pancreatic islet tumor
angiogenesis by regulating vascular endothelial growth
factor/vascular endothelial growth factor receptor-2 signaling.
Cancer Res. 70:8812–8821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang
X, Shi Q, Cui G, Yu H, Tang C and Deng A: COUP-TFII regulates
metastasis of colorectal adenocarcinoma cells by modulating Snail1.
Br J Cancer. 111:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naylor MC and Brissett AE: Current
concepts in the etiology and treatment of keloids. Facial Plast
Surg. 28:504–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou Z, Fan F and Liu P: BTXA regulates the
epithelial-mesenchymal transition and autophagy of keloid
fibroblasts via modulating miR-1587/miR-2392 targeted ZEB2. Biosci
Rep. 39:BSR201906792019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin J, Zhai HF, Jia ZH and Luo XH: Long
non-coding RNA HOXA11-AS induces type I collagen synthesis to
stimulate keloid formation via sponging miR-124-3p and activation
of Smad5 signaling. Am J Physiol Cell Physiol. 317:C1001–C1010.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin J, Jia ZH, Luo XH and Zhai HF: Long
non-coding RNA HOXA11-AS accelerates the progression of keloid
formation via miR-124-3p/TGFβR1 axis. Cell Cycle. 19:218–232. 2020.
View Article : Google Scholar : PubMed/NCBI
|